Abstract
The trifluoromethyl group can dramatically influence the properties of organic molecules, thereby increasing their applicability as pharmaceuticals, agrochemicals, or building blocks for organic materials. Despite the importance of this substituent, no general method exists for its installment onto functionalized aromatic substrates. Current methods either require the use of harsh reaction conditions or suffer from a limited substrate scope. Herein, we report the palladium-catalyzed trifluoromethylation of aryl chlorides under mild conditions, allowing the transformation of a wide range of substrates, including heterocycles, in excellent yields. The process tolerates functional groups such as esters, amides, ethers, acetals, nitriles, and tertiary amines and therefore should be applicable to late-stage modifications of advanced intermediates. We also have prepared all the putative intermediates in the catalytic cycle and demonstrated their viability in the process.
The introduction of the strongly electron-withdrawing trifluoromethyl group into organic molecules can significantly alter their properties, such as lipophilicity, metabolic stability, and bioavailability, that impact the use of these molecules as pharmaceuticals and agrochemicals (1–3). Additionally, trifluoromethylated organic compounds find applications as materials such as liquid crystals (2). Despite the importance of this substituent, no general catalytic method exists for the introduction of the CF3 group onto functionalized aromatic intermediates (4).
Structurally simple benzotrifluorides are accessible by radical chlorination of toluene derivatives and subsequent chlorine-fluorine exchange under harsh conditions (5). The replacement of an aromatic halide by a CF3 group via copper-mediated coupling proceeds under milder reaction conditions, but is mainly limited to aryl iodides (6–16). A catalytic version of this process was recently reported, but only aryl iodides with electron-withdrawing substituents and some heterocycles are good substrates (17).
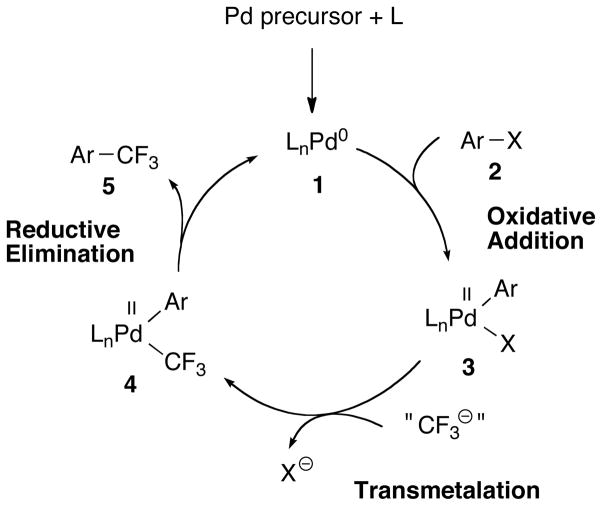
A palladium-catalyzed trifluoromethylation of aryl halides (Fig. 1) has the potential to overcome these limitations: The use of a trifluoromethyl source as transmetalating agent obviates the need for harsh reaction conditions that are required to replace individual substituents on benzylic carbon atoms with fluorine. Additionally, since many known ligands promote oxidative addition even into unactivated aryl chlorides at low temperatures, a wide substrate scope is possible.
Fig. 1.
Generalized catalytic cycle for aryl trifluoromethylation (L = ligand; Ar = aryl; X = Cl, Br, I, triflate).
Mainly due to the high activation barrier for reductive elimination, the development of such a process has so far been unsuccessful. Several complexes 4 with bidentate ligands yield either no (18, 19) or only trace amounts (20) of the benzotrifluoride products 5 even after prolonged heating at 130 °C. The chelating biphosphine ligands dppe and dppp promote the reductive elimination of 4 only at 145 °C to give PhCF3 in 10–60% yield after 64 h (19). On the other hand, the feasibility of fast Ar-CF3 bond formation from a Pd(II) complex under mild conditions was demonstrated by Grushin through quantitative conversion of the complex [XantphosPd(Ph)(CF3)] to PhCF3 upon heating to 80 °C within 3 h (21). However, the replacement of the Xantphos ligand in 3 with trifluoromethyl ions competes with transmetalation to 4, and consequently, no catalytic system with this system was reported (21, 22).
Complexes 4 are typically prepared from complexes 3, where X = Br or I, by treatment with TMSCF3 (TMS is trimethylsilyl) and a fluoride source such as CsF, thereby utilizing the formation of a silicon-fluorine bond as driving force (18–20). The challenge of using trifluoromethylsilanes in the presence of fluoride originates in the fluoride-initiated self-decomposition of R3SiCF3 to give R3SiF and difluorocarbene (23). In a catalytic setting, where elevated temperatures are presumably required to promote reductive elimination from 4 to 5, transmetalation must be significantly faster than this process.
The oxidation of Pd(II)-CF3 complexes with a F+ reagent provides Pd(IV) complexes which readily reductively eliminate benzotrifluorides (20). The catalytic trifluoromethylation of arenes via C-H activation, oxidation of the Pd(II) intermediate with an electrophilic CF3+ source, and final reductive elimination has recently been reported, but is limited to substrates with specific directing groups (24).
Herein, we report the development of a palladium-catalyzed procedure to transform aryl chlorides into their trifluoromethylated analogs using TESCF3 (TES is triethylsilyl) and KF. High functional group tolerance under relatively mild conditions is exhibited, and a wide range of substrates, including heteroaryl ones, can be efficiently converted to the desired products. Mechanistic studies suggest that the reaction proceeds via a classical Pd(0)-Pd(II) catalytic cycle as shown in Fig. 1.
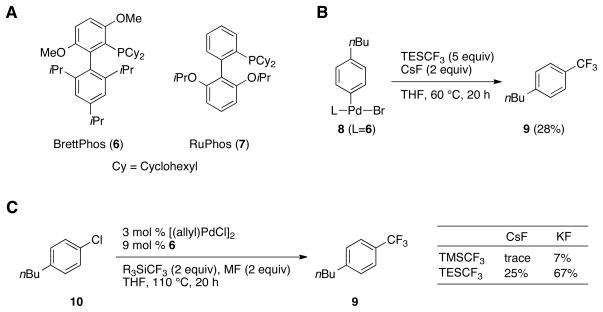
Ligand 6 (BrettPhos) (Fig. 2A) has successfully been employed in challenging amination and fluorination cross-coupling reactions (25, 26). We prepared the oxidative-addition complex 8 and examined numerous trifluoromethyl sources to identify conditions under which both transmetalation and reductive elimination would proceed (Table S1) (27). Although most reagents failed to give product, the mixture of TESCF3 and CsF in THF at 60°C provided 9 in a promising yield of 28% (Fig. 2B). This result confirmed that 6 indeed does promote reductive elimination to form Ar-CF3 bonds, and provided a starting point for the development of a catalytic procedure.
Fig. 2.
(A) Ligands used in this study. (B) Best result from a reagent screen to convert complex 8 into benzotrifluoride 9. (C) Identification of an optimal combination of trifluoromethyl source and activator for the catalytic conversion of 10 to 9.
With 3 mol% [(allyl)PdCl]2 and 12 mol% of ligand 6, benzotrifluoride 9 was formed in 7% yield from aryl chloride 10 with TESCF3 and CsF at 110 °C. We next investigated several combinations of TMSCF3 or TESCF3 with simple fluoride salts and found that the highest yield was obtained using TESCF3 with KF, demonstrating that the catalytic formation of 9 from 10 is possible with these transmetalating agents (Fig. 2C). Full conversion of 10 was achieved by switching the solvent to dioxane and performing the reaction at 120 °C, providing 9 in 80% yield.
We studied the performance of other ligands under these conditions and found that 6 was the best ligand for this transformation (Table S2). Most other monodentate biaryl phosphine ligands gave lower, but still observable amounts between 5% and 20% of product 9. No reaction occurred, however, using Xantphos.
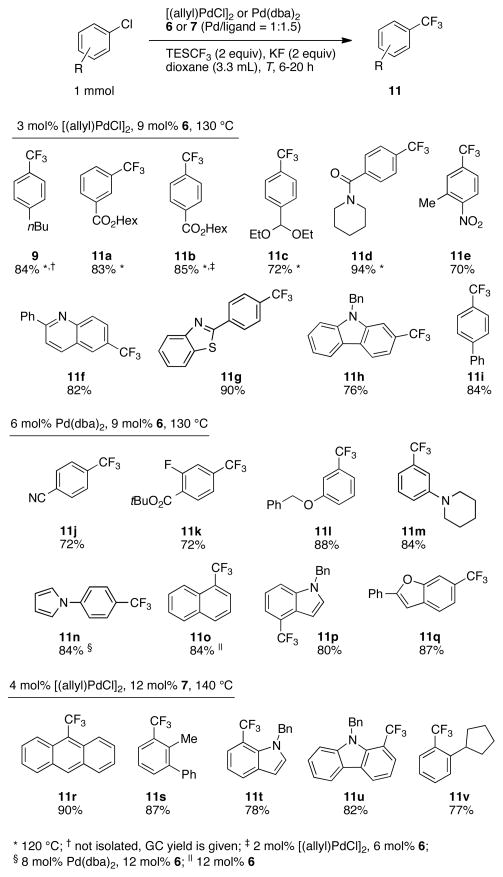
The palladium-catalyzed process expands the scope to aryl chlorides, and exhibits compatibility with a wide range of functional groups (Fig. 3). Both electron-poor and electron-rich aryl chlorides are suitable substrates and provide the trifluoromethylated products in good yields. More importantly, heteroaromatic substrates such as indoles, carbazoles, quinolines, and benzofuranes can be efficiently transformed into their trifluoromethylated analogs. When using ligand 6, we found that ortho-substituted substrates gave the corresponding products only in low yields. Switching to the less bulky ligand RuPhos (7) (Fig. 2A) (28) provided the desired ortho-substituted products 11r-v in excellent yields. Scale-up proved to be straightforward; products 11j and 11b were prepared on a 2 and 5 mmol scale, respectively, in the same yields as those reported for the 1 mmol scale reactions.
Fig. 3.
Scope of the palladium-catalyzed trifluoromethylation of aryl and heteroaryl chlorides. Isolated yields are based upon an average of at least two runs. Minor amounts (2 to 5%) of reduced starting material (Ar-H) were usually observed. In a typical experiment, a solution of the palladium source and ligand 6 or 7 in dioxane was added to spray-dried KF and the aryl chloride. After addition of TESCF3, the reaction was stirred at 120 to 140 °C for 6 to 20 h. Because KF is hygroscopic, all reactions were set up in a nitrogen-filled glovebox in order to prevent the hydrolysis of TESCF3 in the course of the reaction.
Esters, acetals, amides, nitriles, ethers, dialkylamines, and a number of heteroaromatic substituents are tolerated. However, substrates bearing aldehydes or ketones are not suitable. Furthermore, substrates cannot contain unprotected OH or NH groups, presumably because of protonation of the CF3 anion to form fluoroform, reaction at the silicon center of TESCF3, and/or competing coordination to the palladium center.
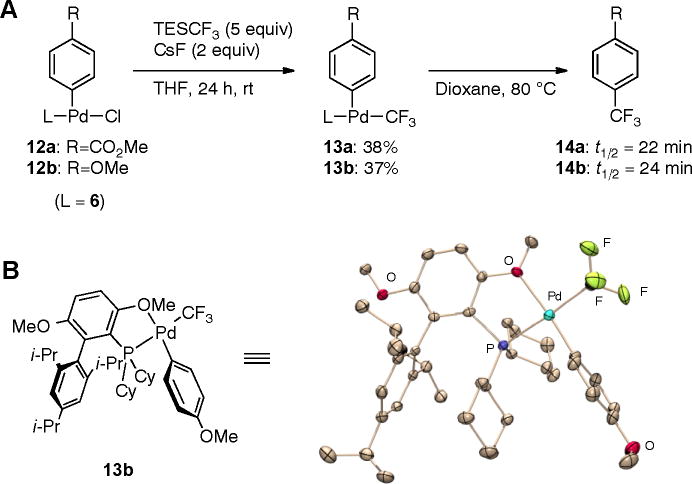
To gain insight into the reaction mechanism, we prepared the presumptive Pd-CF3 intermediates 13 and studied their reductive elimination to yield benzotrifluoride products. Treatment of complexes 12 with TESCF3/CsF at room temperature in THF allowed the isolation of [6•Pd(Ar)(CF3)] complexes 13 (Fig. 4A). The compounds exhibit a characteristic quartet in the 31P-NMR (Nuclear Magnetic Resonance) spectrum and a doublet in the 19F-NMR spectrum with a coupling constant of about 45 Hz. The Pd atom in the crystal structures of 13a (Fig. S1) and 13b (Fig. 4B) is coordinated by the upper ring methoxy group of the ligand 6 and not by the ipso carbon atom of the lower aromatic ring.
Fig. 4.
(A) Formation of and reductive elimination from [6·Pd(Ar)(CF3)] complexes. t1/2 are half-lives of the first-order reductive elimination kinetics (B) X-ray structure of complex 13b. ORTEP (31) drawings at 50% probability; hydrogen atoms are omitted for clarity.
We studied the reductive elimination of complexes 13 in dioxane at 80 °C via 19F-NMR and found first-order decay to give benzotrifluorides 14 in nearly quantitative yield. The rate constants for both the decomposition of 13a and 13b are almost identical (Fig. 4A, Fig. S2, Fig S4). This surprising result is paralleled by DFT calculations that predict an activation energy of ca. 22 kcal mol−1 for both complexes (29)(30). In comparison to the ground states, the calculated Pd-CF3 distance in the transition states is significantly elongated, whereas the distance between the Pd atom and the aryl ring remains essentially unchanged, suggesting that the main contribution to the activation energy is the breaking of the strong Pd-CF3 bond. Since the strength of this bond is only minimally influenced by the substituent on the aryl ring, similar rate constants are observed.
When complex 13a was heated in the presence of excess methyl 4-chlorobenzoate, the oxidative-addition complex 12a was formed in addition to product 14a, thus closing the catalytic cycle. The yield and rate of benzotrifluoride formation were identical in the presence or absence of aryl chloride (Fig S3), which implies that reductive elimination affords a Pd(0) species that then undergoes oxidative addition to form 12a. Therefore, we believe that these reactions proceed via a classical Pd(0)/Pd(II) catalytic cycle as proposed in Fig. 1.
In preliminary experiments, we have demonstrated that this process is applicable, in somewhat lower yields, to aryl bromides and aryl triflates. We are currently seeking to develop a better understanding of the overall reaction mechanism as well as to render this process more generally useful and practical. We hope to accomplish this by broadening its substrate scope, lowering the quantity of catalyst necessary, developing milder reaction conditions, and through the utilization of less expensive and more environmentally friendly trifluoromethylating agents.
Supplementary Material
Acknowledgments
We thank the NIH for financial support of this project (grant GM46059) and Merck, Nippon Chemical, Boehringer Ingelheim, and BASF for gifts of chemicals and additional funds. T.K. thanks the Alexander von Humboldt Foundation for a Feodor Lynen postdoctoral fellowship. The Varian NMR instrument used was supported by the NSF (grants CHE 9808061 and DBI 9729592). We also thank Dr. P. Müller (MIT Chemistry Department, X-Ray Diffraction Facility) for obtaining the crystal structures of 13a and 13b. Cambridge Crystallographic Data Centre (CCDC) 771779 and 771780 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from the CCDC via www.ccdc.cam.ac.uk/data_request/cif.
References
- 1.Cartwright D. In: Fluoroorganic Chemistry. Banks RE, Smart BE, Tatlow JC, editors. Plenum Press; New York: 1994. p. 237. [Google Scholar]
- 2.Kirsch P. Modern Fluoroorganic Chemistry. Wiley-VCH; Weinheim: 2004. [Google Scholar]
- 3.Müller K, Faeh C, Diederich F. Science. 2007;317:1881. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- 4.Thayer M. Chem Eng News. 2006;84:15. [Google Scholar]
- 5.Swarts F. Bull Soc Chim Belg. 1892;24:309. [Google Scholar]
- 6.Mcloughlin VCR, Thrower J. Tetrahedron. 1969;25:5921. [Google Scholar]
- 7.Kobayashi Y, Kumadaki I. Tetrahedron Lett. 1969;10:4095. [Google Scholar]
- 8.Kondratenko NV, Vechirko EP, Yagupolskii LM. Synthesis. 1980:932. [Google Scholar]
- 9.Matsui K, Tobita E, Ando M, Kondo K. Chem Lett. 1981:1719. [Google Scholar]
- 10.Wiemers DM, Burton DJ. J Am Chem Soc. 1986;108:832. [Google Scholar]
- 11.Carr GE, Chambers RD, Holmes TF, Parker DG. J Chem Soc, Perkin Trans. 1988;1:921. [Google Scholar]
- 12.Clark JH, Mcclinton MA, Blade RJ. J Chem Soc, Chem Commun. 1988:638. [Google Scholar]
- 13.Chen QY, Wu SW. J Chem Soc, Chem Commun. 1989:705. [Google Scholar]
- 14.Urata H, Fuchikami T. Tetrahedron Lett. 1991;32:91. [Google Scholar]
- 15.Cottet F, Schlosser M. Eur J Org Chem. 2002:327. [Google Scholar]
- 16.Dubinina GG, Furutachi H, Vicic DA. J Am Chem Soc. 2008;130:8600. doi: 10.1021/ja802946s. [DOI] [PubMed] [Google Scholar]
- 17.Oishi M, Kondo H, Amii H. Chem Commun. 2009:1909. doi: 10.1039/b823249k. [DOI] [PubMed] [Google Scholar]
- 18.Culkin DA, Hartwig JF. Organometallics. 2004;23:3398. [Google Scholar]
- 19.Grushin VV, Marshall WJ. J Am Chem Soc. 2006;128:4632. doi: 10.1021/ja0602389. [DOI] [PubMed] [Google Scholar]
- 20.Ball ND, Kampf JW, Sanford MS. J Am Chem Soc. 2010;132:2878. doi: 10.1021/ja100955x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grushin VV, Marshall WJ. J Am Chem Soc. 2006;128:12644. doi: 10.1021/ja064935c. [DOI] [PubMed] [Google Scholar]
- 22.Naumann D, et al. Z Anorg Allg Chem. 2004;630:746. [Google Scholar]
- 23.Tyrra W, et al. Chem Eur J. 2005;11:6514. doi: 10.1002/chem.200500799. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Truesdale L, Yu J. J Am Chem Soc. 2010;132:3648. doi: 10.1021/ja909522s. [DOI] [PubMed] [Google Scholar]
- 25.Fors BP, Watson DA, Biscoe MR, Buchwald SL. J Am Chem Soc. 2008;130:13552. doi: 10.1021/ja8055358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watson DA, et al. Science. 2009;325:1661. doi: 10.1126/science.1178239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Materials and methods are available as supporting material at Science online.
- 28.Milne JE, Buchwald SL. J Am Chem Soc. 2004;126:13028. doi: 10.1021/ja0474493. [DOI] [PubMed] [Google Scholar]
- 29.Frisch MJ, et al. Gaussian 03, Revision E.01. Gaussian, Inc; Wallingford CT: 2004. [Google Scholar]
- 30.B3LYP/6-311++G(2d,p)/LanL2Dz//B3LYP/6-31G(d)/LanL2Dz.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.