Abstract
The middle ear muscle reflex has been implicated in modulation of auditory input and protection of the inner ear from acoustic trauma. However, the identification of neurons in the cochlear nuclei participating in this reflex has not been fully elucidated. In the present study, we injected the retrograde transynaptic tracer pseudorabies virus into single tensor tympani (TT) muscles, and identified transynaptically labeled cochlear nucleus neurons at multiple survival times. Motoneurons controlling TT were located ventral to the ipsilateral motor trigeminal nucleus and extended rostrally towards the medial aspect of the lateral lemniscus. Transynaptically-labeled neurons were observed bilaterally in the dorsal and dorso-medial parts of ventral cochlear nuclei as early as 48 h after virus injection, and had morphological features of radiate multipolar cells. After ≥ 69 h, labeled cells of different types were observed in all cochlear nuclei. At those times, labeling was also detected bilaterally in the medial nucleus of the trapezoid body and periolivary cell groups in the superior olivary complex. Based on the temporal course of viral replication, our data strongly suggest the presence of a direct projection of neurons from the ventral cochlear nuclei bilaterally to the TT motoneuron pool in rats. The influence of neurons in the cochlear nuclei upon TT activity through direct and indirect pathways may account for multifunctional roles of this muscle in auditory functions.
Keywords: direct and indirect acoustic reflex pathways, middle ear muscle, transynaptic transport
1. Introduction
The middle ear muscle reflex (MEM) constitutes one of the two major efferent feedback pathways to the auditory periphery, and is often assessed in the audiological evaluation of hearing impairment in humans. Two muscles participate in this reflex, the tensor tympani (TT) and the stapedius muscle. Contraction of these muscles induces a stiffening of the middle ear ossicular chain, hence dampening acoustic input to the cochlea (e.g., Wever et al, 1955; Galambos and Rupert, 1958; Carmel and Starr, 1963; Møller 1965). This effect has been suggested to modulate auditory input in challenging circumstances, such as speech discrimination in noisy environments (e.g., Borg and Zakrisson, 1974; Mahoney et al., 1979), and possibly protect the inner ear from acoustic trauma (Borg et al., 1984; Borg and Counter, 1989). During ultrasonic calling behavior in the rat, the acoustic reflex has been suggested to improve the signal-to-noise ratio under intense low frequency noises (Murata et al., 1986).
Prior studies have delineated the brain structures participating in the acoustic reflex pathways in some mammalian species. Some have described a 4-neuron arc reflex (Borg, 1973; Rouiller et al., 1986, 1989). In this regard, a combination of physiological and lesion experiments showed that output from the ventral cochlear nuclei (VCN) excited the TT motoneurons through the superior olivary complex (SOC) in the rabbit (e.g., Borg, 1973). In rats, Rouiller et al. (1986) showed the consistent presence of transynaptically labeled cells bilaterally in the medial nucleus of the trapezoid body (MNTB) and periolivary cell groups (PO) after injecting the retrograde transynaptic tracer herpes virus suis into unilateral TT muscles. In contrast with those studies, other investigators reported that the TT motoneuronal pool fired spikes monosynaptically with short latency when electrical stimulus was applied to the cochlear nucleus in cats (Ito and Honjo, 1988). In addition, injections of conventional retrograde tracers into the region of the pontine tegmentum containing the TT motoneurons induced labeling bilaterally in both dorsal cochlear nuclei (DCN) and VCN (Itoh et al., 1986; Ito and Honjo, 1988) further supporting that TT reflex may also consist of a 3-neuron chain. Recently in rats, Lee et al. (2006) confirmed the participation of neurons in the VCN in the MEM reflex using surgical transection and focal lesions of the auditory brainstem. To date however, the identification and organization of neurons in the cochlear nuclei controlling the MEM, and in particular the TT muscle, remain to be elucidated. The primary goal of the present investigation was to identify the cell types and topographical organization of cochlear nucleus neurons that participate in the control of single TT muscles. The approach involved injections of PRV-152, a recombinant strain of the parental PRV-Bartha, into individual TT muscles. PRV-Bartha is a swine neurotropic α-herpesvirus, also known to be an attenuated strain of PRV developed as a vaccine (Bartha, 1961). PRV-Bartha has been used widely for transneuronal tracing in the retrograde direction (e.g., Card, 2001; Enquist and Card, 2003 for recent reviews). The TT muscle was studied because it is more accessible of the two MEMs, the location of its motoneuronal pool is well known, and this muscle was shown to contribute equally with the stapedius muscle to the acoustic reflex in rats (van den Berge et al., 1990). In addition to the known acoustic reflex pathways through the superior olivary complex (SOC), the temporal course of viral replication also suggests the presence of a direct projection of neurons from the cochlear nuclei bilaterally onto the TT motoneuronal pool in rats
2. Results
2.1. Motoneurons innervating TT
At 48 h a dense labeling in motoneurons was observed in a region ventral to the ipsilateral motor trigeminal nucleus (at about 4.0 mm from the obex). Labeling extended rostrally towards the medial aspect of the lateral lemniscus. This finding was consistent following injections of PRV-152 and/or the conventional retrograde tracer subunit b of cholera toxin (b-CT) into single TT muscles. The TT motoneuronal pool formed a cluster of cells in the region ventral to the motor trigeminal nucleus, whereas it was organized in a compact column at the level of the lateral lemniscus region. Labeled cells had a triangular shape with a maximum diameter of about 25 μm. At 48h-survival time, a few motoneurons showed signs of early cytopathic damage. This was characterized by the cell nuclei becoming invaginated and containing inclusion bodies. Fig. 1 illustrates examples of motoneurons labeled with PRV-152 or b-CT. The number of labeled motoneurons increased towards about 69-71-hour survival times, and a maximum of 145 labeled motoneurons innervating the TT muscle were counted. The distribution and location of infected neurons revealed by injections of the recombinant strain of PRV (PRV-152) into single TT muscles duplicated the pattern of transport that resulted from individual injections of the parental virus (Rouiller et al., 1986) and/or retrograde monosynaptic tracers (Spangler et al., 1982; Rouiller et al., 1986) into this muscle. At those later times, labeled motoneurons showed advanced signs of cytopathic damage such that the cell bodies became irregularly shaped, and their dendrites became beaded.
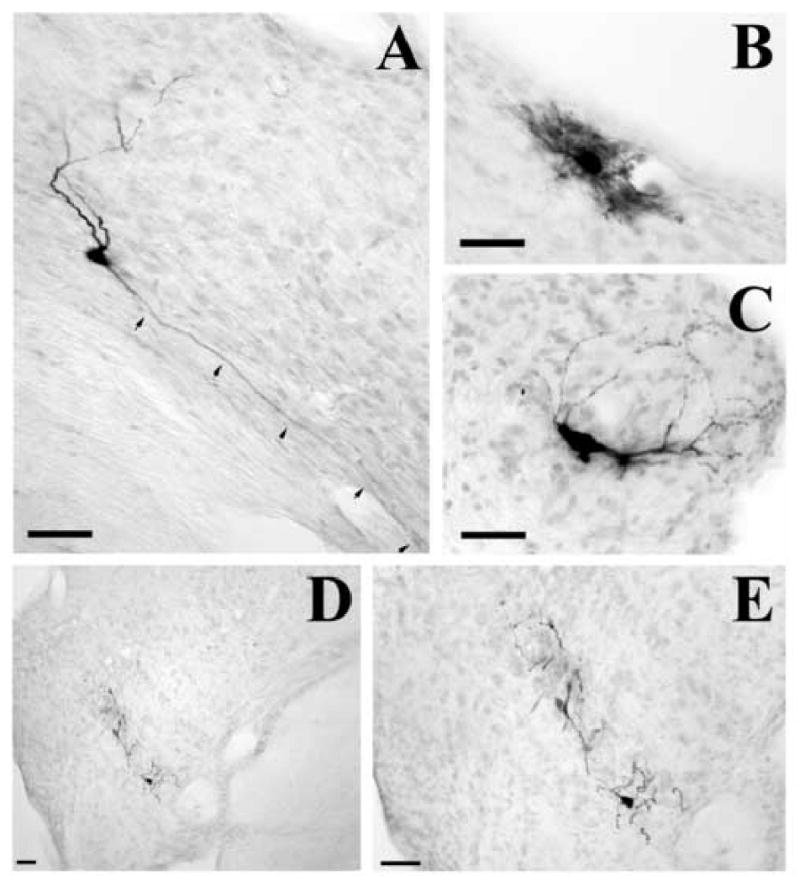
Fig. 1.
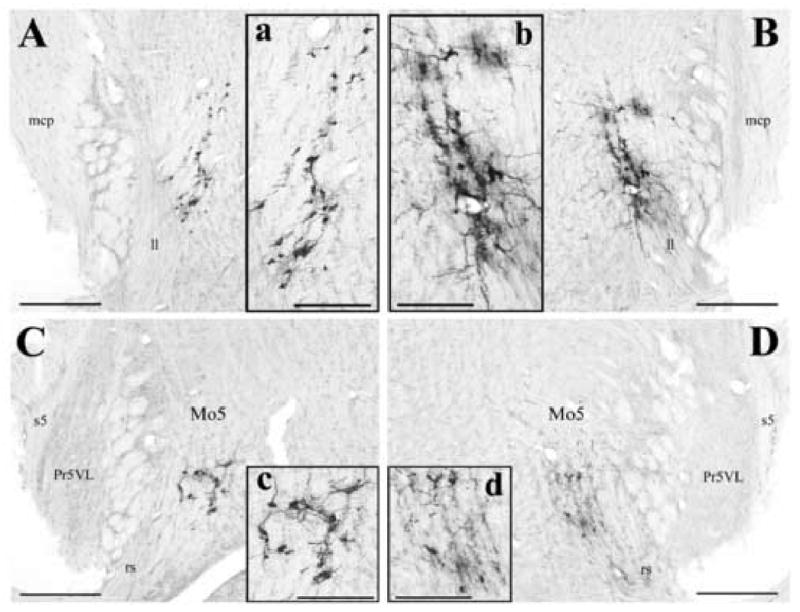
Photomicrographs illustrating examples of labeled motoneurons controlling the tensor tympani muscle located medial to the lateral lemniscus (A and B) and ventral to the motor trigeminal nucleus (C and D). All neurons are ipsilateral to the side of injections, and are located at about 4.0 mm rostral from the obex (C and D), and 5.0 mm rostral from the obex (A and B). Panels A and C show labeled motoneurons after injecting the subunit b of cholera toxin into the left tensor tympani muscle at an 80-h survival time. Panels B and D represent labeled motoneurons after injecting PRV-152 into the right tensor tympani muscle at a 62-h survival time. Panels a, b, c and d are higher magnification of labeled motoneurons of Panels A, B, C and D. Scale bars represent 400 μm in Panels A, B, C, and D, and 200 μm in Panels a, b, c, and d. Abbreviations: ll, lateral lemniscus; mcp, middle cerebellar peduncle; Mo5, motor trigeminal nucleus; Pr5VL, principal sensory 5 nucleus, ventrolateral; rs, rubrospinal tract; s5, sensory root of the trigeminal nerve.
2.2. Neurons in the cochlear nuclei controlling TT after 48-62 hours
As early as 48-62 h a few labeled cells were observed bilaterally in the dorsal part of the magnocellular VCN (see Table 1; mean of 7.5 cells ± 6.5 SD ipsilateral to the injection side versus 5.3 ± 3.9 SD contralateral). Labeling was mostly located in the dorsal and dorso-medial parts of the AVCN, particularly its caudal half. Fig. 2 shows the distribution of labeled neurons in the cochlear nuclei for all cases of 48-62 hour-survival times. Based on the morphology of labeled cells and their location in the VCN, a majority of them corresponded to radiate multipolar cells. Morphological characteristics of these cells included large soma (∼ 20-25 μm in diameter), dendrites that radiated across the cochlear nucleus with some of them extending into the granule cell domain (Doucet and Ryugo, 1997). In one case of 62 h, a few larger cells (∼30 μm in the larger diameter) with elongated nuclei and a single large dendrite were located at the periphery of PVCN and the glia Schwann-cell border of the cochlear nerve. Fig. 3 illustrates the two types of labeled neurons that were observed in the VCN at those early post-inoculation times.
Table 1. Cases of injections of PRV into single tensor tympani muscles.
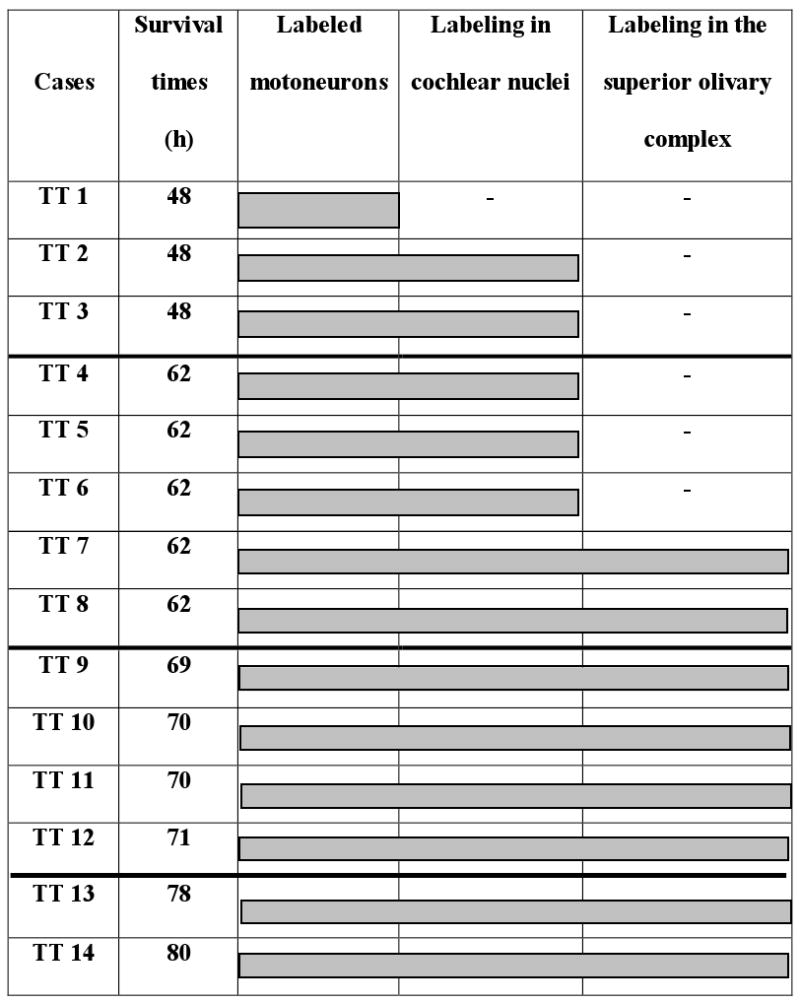 |
Cases are represented with their respective survival times. Shaded areas indicate brain regions in the acoustic reflex pathways that contained labeling.
Fig. 2.
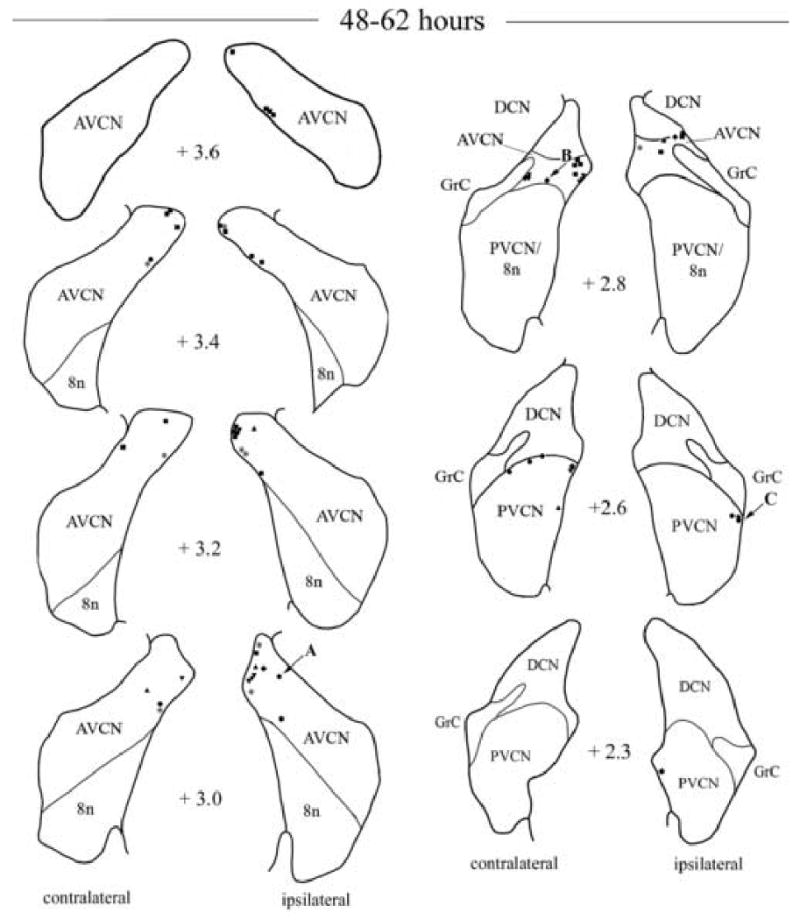
Camera lucida drawings showing the locations of labeled neurons in the ventral cochlear nuclei following injection of PRV into the right tensor tympani muscle at survival times of 48 and 62 h. All cases are presented by a different symbol. The number in between diagrams indicates the relative rostro-caudal distance in mm of the section from the obex. Neurons on the right side of each diagram are located ipsilateral to the PRV injection. Each symbol represents a labeled neuron. Arrows with A, B and C letters indicate the locations of infected neurons shown in Fig. 3. Abbreviations: 8n, vestibulo-cochlear nerve; AVCN, anteroventral cochlear nucleus; DCN, dorsal cochlear nucleus; GrC, granular layer of the cochlear nuclei; PVCN, posteroventral cochlear nucleus.
Fig. 3.
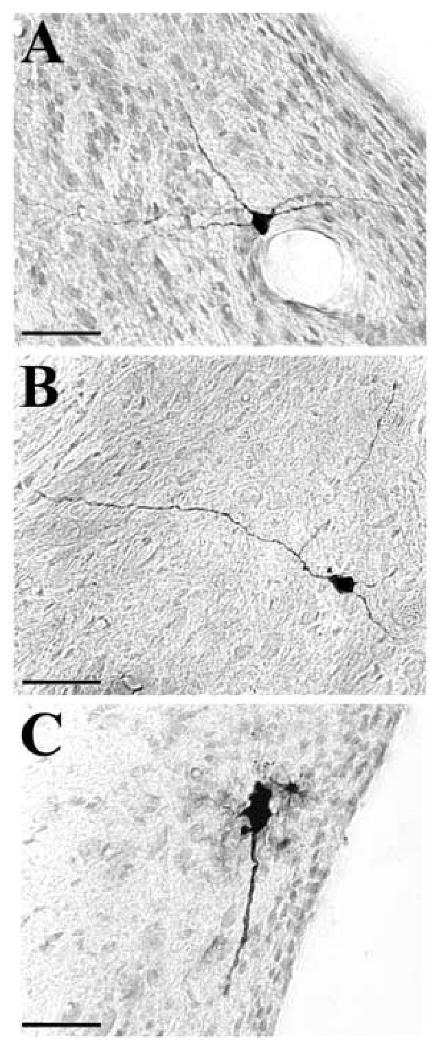
Microphotographs of labeled neurons in the ventral cochlear nuclei infected after 48-62 h following injection of PRV into the right tensor tympani muscle. The different panels illustrate neurons that were identified in Fig. 2 with an arrow. Panels A and B represent radiate type of multipolar cells in the anteroventral cochlear nucleus. Panel C illustrates an example of large cell at the junction of the posteroventral cochlear nucleus and the glial Schwann-cell border of the cochlear nerve. Scale bars represent 50 μm.
2.3. Neurons in the cochlear nuclei controlling TT after ≥ 69 hours
At times of 69-71 h, the number of neurons increased bilaterally in the AVCN (mean of 20.8 ± 10.9 SD ipsilateral versus 29.7 ± 19.8 SD contralateral). Labeling also increased in the PVCN, although not as much as its anterior counterpart (2.3 ± 3.0 ipsilateral versus 7.8 ± 7.7 contralateral). In the DCN, labeling was detected particularly in the dorsal aspect of its rostral half (5.5 ± 5.9 SD ipsilateral versus 30.5 ± 32.3 SD contralateral). A few labeled cells could also be distinguished at the border of the DCN and the PVCN. Fig. 4 illustrates the distribution of labeled cells in the cochlear nuclei in one case of 71 h. Fig. 5A-F shows examples of labeled neurons in the cochlear nuclei at 69-71 h survival times. In the VCN, some labeled radiate-type multipolar cells showed signs of cytopathic damage. In addition to radiate type of multipolar cells, labeled marginal cells with their dendrites oriented dorso-ventrally were particularly distributed at the dorsal and dorso-medial periphery of the magnocellular part of VCN. In one case of labeled marginal cells the dendrite running medial to the AVCN periphery extended over a distance of 1200 μm. Figure 5A shows an example of marginal cell located in the dorso-medial part of the ipsilateral AVCN. In the case of 69 h, labeled planar cells were observed in the contralateral PVCN, forming a band that crossed the lateral-medial axis of the nucleus (Fig. 5D and E). In addition to multipolar types of cells, a few labeled microneurons were scattered in the granule cell domain surrounding the dorsal and dorso-medial aspects of VCN (e.g., Fig. 5B, F), and between the DCN and PVCN. However, the approach used did not allow us to determine which types of microneurons were labeled, i.e., granule, Golgi, chestnut or unipolar brush cells. In one case of 71 h, occasional labeled giant cells were also observed in the latero-ventral part of the contralateral DCN (e.g, Fig. 5C). At longer survival times (≥ 78 h) the labeling pattern was consistent with that described at prior survival times, although smooth and oval-shaped microneurons outnumbered the presence of multipolar-types of neurons and new types of labeled neurons were also detected. Further, many labeled cells showed advanced cytopathic damage. Fig 5G-J shows examples of new labeled cell types observed at longer survival times (78-80 h). In addition to multipolar- and cells in the granule cell domain, there were clusters of labeled cells in the dorsal part of the VCN and DCN (Fig 5G and H). These clusters contained multipolar-type intermixed with other types of cells and/or processes (see Fig 5G, H). However, viral immunoreactivity did not fill sufficiently the cell dendrites and/or the plane of section did not allow accurate characterization of morphological characteristics of those neurons. The presence of microneurons increased dramatically in the granule cell domain, especially in the dorsal parts of the AVCN, DCN and between DCN and PVCN. Other types of cells were observed with much less frequency. For instance, neurons resembling cochlear root neurons were scattered in the acoustic nerve (see e.g., Fig. 5J). Occasionally a few labeled cells were scattered in more ventral parts of the AVCN and more caudal levels of the DCN.
Fig. 4.
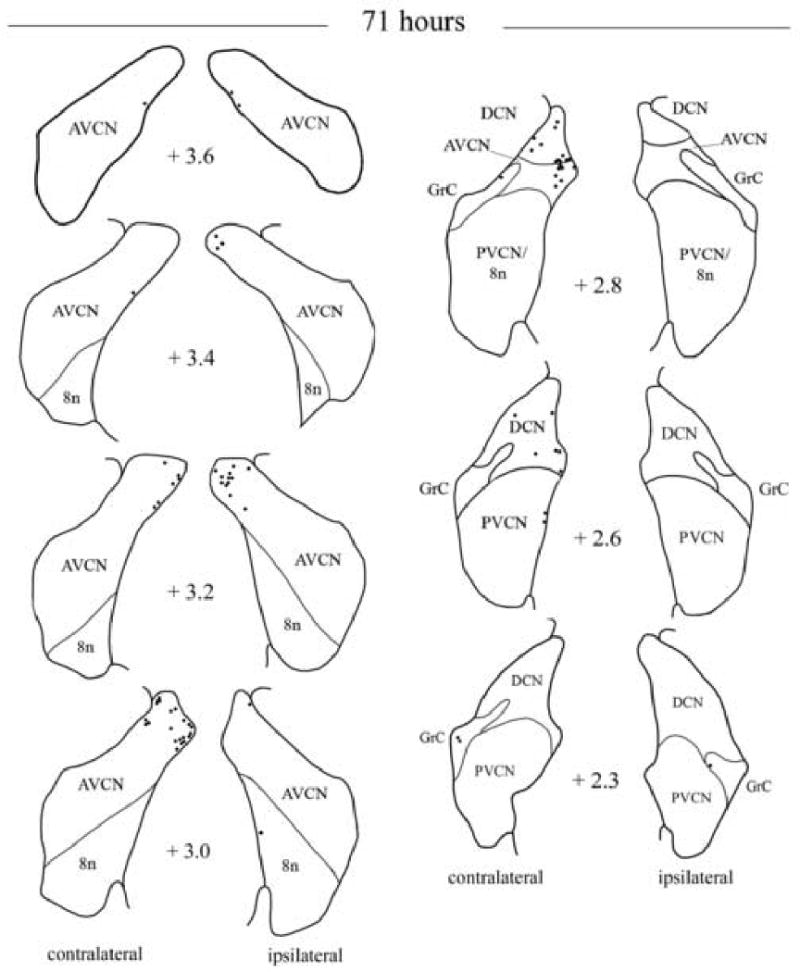
Camera lucida drawings showing the locations of labeled neurons in the cochlear nuclei following injection of PRV into the right tensor tympani after 71 h. The number next to each diagram indicates the relative rostro-caudal distance in mm of the section from the obex. Neurons on the left side of each diagram are located contralateral to the PRV injection. Each dot represents a labeled neuron. Abbreviations are the same as Figure 2.
Fig. 5.
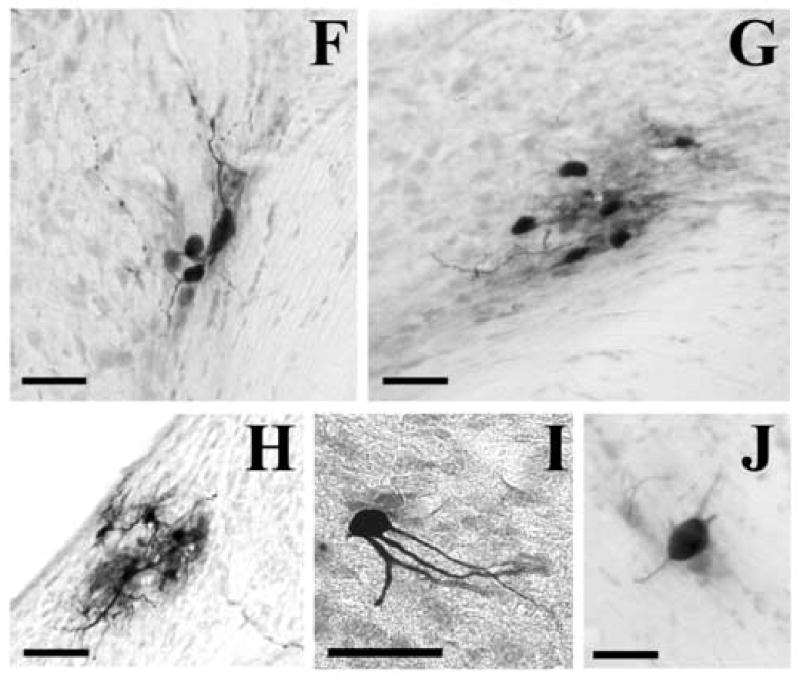
Example of labeled cochlear neurons in the cochlear nuclei infected after > 69 h following injection of PRV into the right tensor tympani. Panel A represents an example of labeled marginal cell at the periphery of the ipsilateral anteroventral cochlear nucleus. Small arrows indicate the dendrite running along the medial aspect of the cochlear nucleus. Panel B shows an example of labeled cell found in the granule cell domain surrounding the ipsilateral anteroventral cochlear nucleus. Panel C illustrates a giant cell observed in the ventro-lateral aspect of the ipsilateral dorsal cochlear nucleus. Panel D shows labeled planar cells forming a band in the lateral-medial direction in the contralateral posteroventral cochlear nucleus. Panel E is an enlargement of the microphotograph shown in Panel D. Panel F is an example of labeled granule cells and another cell in the medial aspect of the contralateral posteroventral cochlear nucleus, near the octopus cell area. Panel G represents a cluster of labeled cells at the dorso-medial aspect of the contralateral anteroventral cochlear nucleus. Panel H shows a cluster of labeled cells in the dorso-lateral part of the contralateral dorsal cochlear nucleus. Panel I is a partial large multipolar cell in the rostral part of the ipsilateral anteroventral cochlear nucleus. Panel J illustrates an example of labeled cochlear root neuron in the ipsilateral auditory nerve. Scale bars represent 50 μm.
2.4. Labeling in the superior olivary complex
In the present study a small number of labeled cells were observed bilaterally in the MNTB of the SOC, with an ipsilateral predominance, and/or the PO as early as 62 h (2 cases out of 5; see Table 1). However, consistent labeling in both the PO and to a lesser extent the MNTB required post-inoculation periods of 69-71 h (37.8 ± 13.9 SD ipsilateral versus 17.5 ± 8.4 SD contralateral). Labeled cells in PO were located in the dorsal, medio-ventral and lateral aspects of the nuclei. Fig. 6 shows templates of SOC on which the distribution of labeled cells was reported after TT injections in two cases of 69-71 h survival times. After 78-80h the number of labeled cells increased drastically, particularly in the PO (mean of 462 ± 331 SD ipsilateral versus 246 ± 66.5 SD contralateral), and were located predominantly ipsilateral to the injection site. The number of labeled cells in the PO, particularly the lateral part, may have been augmented by the number of labeled cells in area A5 which is indistinguishable from the lateral part of PO. At those later times, labeled cells were also detected bilaterally in the superior paraolivary, the rostral periolivary, and the anteroventral periolivary nuclei. At those later survival times, many cells in PO showed cytopathic damage. Fig. 7 illustrates microphotographs of labeled cells in the SOC between 62 and 71 h.
Fig. 6.
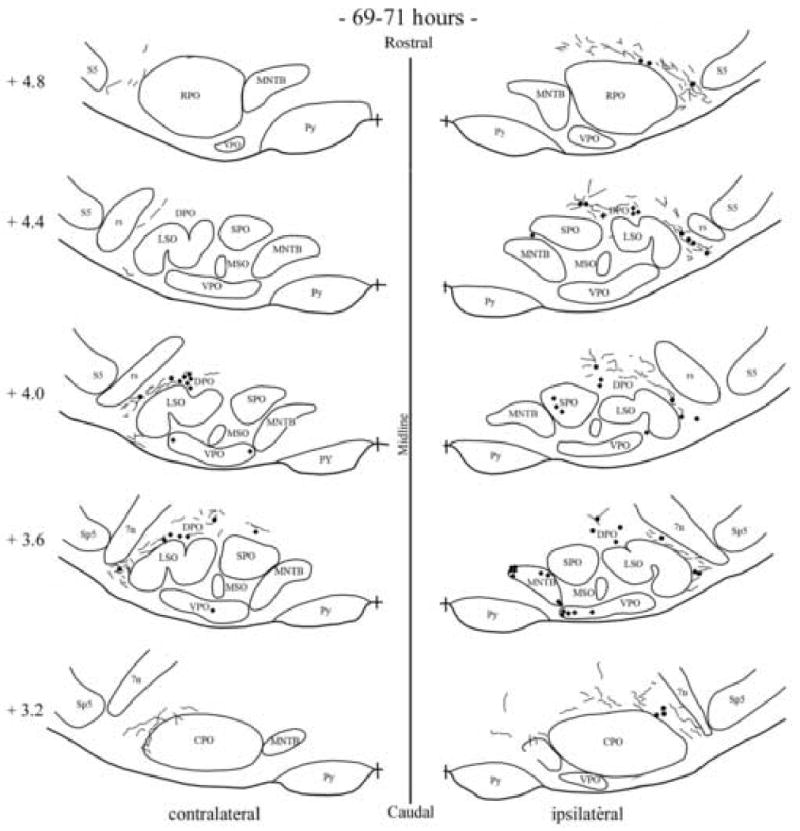
Camera lucida drawings showing the locations of labeled neurons in the superior olivary complex following injection of PRV into the right tensor tympani for two cases of 69-71h. Cases are represented by different symbol (dots and diamonds). Each dot or diamond represents a labeled cell. The number next to each diagram indicates the relative rostro-caudal distance in mm of the section from the obex. Neurons on the left side of each diagram are located contralateral to the PRV injection. Each dot represents a labeled neuron. Abbreviations are the same as in Fig. 1 with the additional: 7n, facial nerve; CPO, caudal periolivary region; DPO, dorsal periolivary group; LSO, lateral superior olive; MNTB, medial nucleus of the trapezoid body; MSO, medial superior olive; rs, rubrospinal tract; sp5, spinal trigeminal tract; SPO, superior paraolivary nucleus; VPO, ventral periolivary nucleus.
Fig. 7.
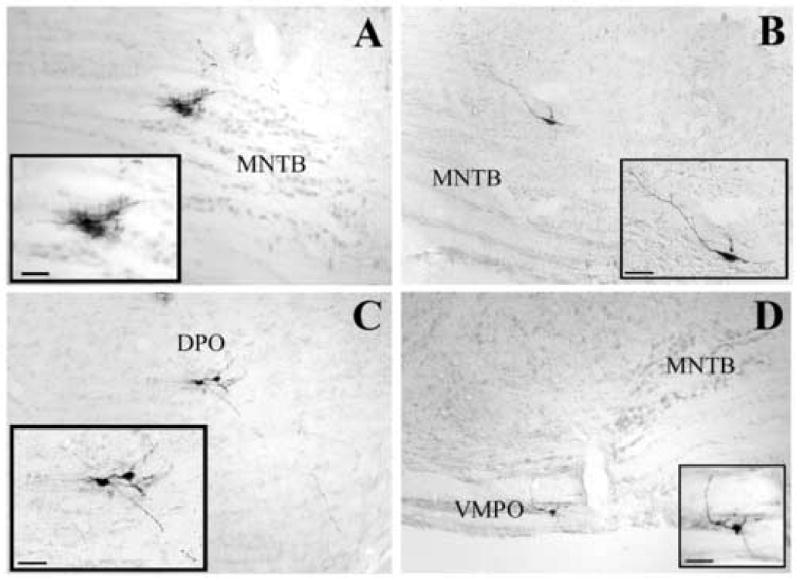
Photomicrographs of labeled cells in the superior olivary complex. Panels A and B illustrate labeled cells in the ipsilateral medial nucleus of the trapezoid body at survival times of 62h and 70h, respectively. Panels C and D show labeled cells in the ipsilateral dorsal (C) and contralateral ventral (D) periolivary group at 71h and 62h, respectively. Scale bars represent 50 μm. Abbreviations are the same as in Fig. 6. MVPO: medioventral periolivary nucleus.
3. Discussion
In the present study, we used the transynaptic transport of PRV to identify the topographical organization and when possible, the morphological features of cochlear nucleus neurons controlling unilateral TT muscles of rats. Importantly, our data provide compelling evidence that neurons in the VCN of both sides project directly to the TT motoneuron pool. This interpretation was based on the temporal delay of viral replication between neurons in the cochlear nuclei (i.e., 48 hours) and those in the SOC (i.e., ≥ 62 hours). The second important finding is that the cells participating in this presumed direct pathway have morphological features resembling the large commissural neurons (i.e., radiate multipolar cells). In contrast, the cells labeled after longer post-inoculation times had a different morphology (e.g., planar and marginal multipolar cells). These neurons may participate in the acoustic reflex pathways through the SOC.
Prior studies on acoustic reflex pathways have reported that output from the cochlear nucleus excites MEM motoneurons directly (cat: Itoh et al., 1986; Ito and Honjo, 1988) as well as through the SOC (rabbit: Borg, 1973; rat: Rouiller et al., 1986, 1989). The present study extends these findings in rats. At short survival times (48-62 hours), labeled neurons were located in the dorsal and/or dorso-medial parts of the AVCN, and to a lesser extent the dorsal part of the PVCN. Based upon the cochleotopical organization of the auditory nerve fibers in the VCN (e.g., Noda and Pirsig, 1974; Fekete et al., 1984) and the tonotopical organization of the VCN (e.g. in rat: Saint-Marie et al., 1999), this result suggests that neurons directly controlling TT may span several isofrequency sheets in the high frequency domain of the VCN. In cats, cells located in a corresponding area called small cell cap of the AVCN that is overlaid by the granule cell layer, were shown to receive primarily auditory nerve fibers of low spontaneous discharge rate and high threshold (Fekete et al., 1984; Liberman, 1991), and possibly encode acoustic stimulus intensity (Ghoshal and Kim, 1996). When viral immunoreactivity sufficiently filled the dendrites to allow morphological characterization of cell types, a majority if not all labeled neurons had features corresponding to radiate multipolar cells. This class of neurons was shown to project to the DCN (e.g., Schofield and Cant, 1996a; Doucet and Ryugo, 1997), and to resemble the large commissural neurons projecting to the cochlear nuclei of the opposite side (e.g., Cant and Gaston, 1982; Wenthold, 1987; Schofield and Cant, 1996a, 1996b; Doucet et al., 1999). Some studies also determined that commissural neurons were glycine-immunoreactive (e.g., Wenthold, 1987; Doucet et al., 1999; Balabian et al., 2002), suggesting that activation of the crossed pathway from one cochlear nucleus to the other may have an inhibitory effect. More recently, Needham and Paolini (2003) demonstrated a fast, monosynaptic inhibitory commissural connection between cochlear nuclei in rats. In the present study, we did not examine the neurochemical phenotypes of those putative commissural neurons in VCN projecting to the TT motoneuronal pool. To our knowledge, no study has identified targets of commissural neurons outside the cochlear nucleus complex, nor provided evidence for a possible inhibitory input in the acoustic reflex pathways of rats.
At longer survival times, our results indicated the participation of a variety of neurons in the dorsal VCN and the DCN in the control of the TT muscle. Most labeled cells had morphological features corresponding to marginal cells, microneurons of the granule cell domain, and others cells that could not be accurately identified with the approach used. The presence of those labeled cells were especially significant in the dorsal VCN at survival times for which labeling was also consistently observed in the PO and the MNTB of both SOC. Prior investigations showed that multipolar-type (i.e., marginal and planar) and bushy-type of cells in the VCN projected to the PO group and the contralateral MNTB (e.g., Friauf and Ostwald, 1988; see Cant and Benson, 2003; see Malmierca, 2003). On the other hand, cells in the PO and MNTB were shown to control the TT motoneuronal pool bilaterally (e.g., Borg, 1973; Rouiller et al., 1986). In our study, a conservative interpretation to explain the labeling in the SOC is that some of if not all neurons labeled during long survival times (such as for example, marginal, planar and the unidentified neurons intermixed with multipolar cells) may control TT muscle through the SOC, and so they may participate in the 4-neuron arc of the acoustic reflex. We cannot rule out however that a portion of labeled cells in the SOC may have also been retrogradely labeled from the labeling of neurons in the cochlear nuclei. In this regard, cells from the PO, particularly in the ventral nucleus of the trapezoid body, were shown to project bilaterally to the cochlear nuclei (e.g., Warr and Beck, 1996; Schofield and Cant, 1999; Thompson and Schofield, 2000), in particular to the granule cell domain (Weedman et al., 1996).
In a prior study, Rouiller et al. (1986) reported that cells in the PO and MNTB provided the main input to the TT motoneurons following injections of the transynaptic retrograde herpes virus suis into unilateral TT muscles of rats. Herpes virus suis, now referred to as PRV-Bartha (Card and Enquist, 1995) is an attenuated strain of PRV that was developed as a vaccine (Bartha, 1961). PRV-Bartha has been widely used as a transynaptic retrograde tracer that highlights multisynaptic central circuitry connected to peripheral targets (e.g., Card, 2001; Enquist and Card, 2003 for recent reviews). Data collected by Rouiller et al. (1986) in the cochlear nuclei and the SOC were based on two animals that were sacrificed after 52 h and 54 h. Hence, their findings focused primarily on the TT acoustic reflex as consisting of a chain of 4 neurons, namely the primary auditory neurons, cochlear nucleus neurons, MNTB or PO neurons and motoneurons. In the present study we used PRV-152, a recombinant strain of the parental PRV-Bartha that was previously shown to produce a similar distribution of labeling (Billig et al., 2000; Card, 2001). Our present data agree with those of Rouiller et al. (1986) regarding the participation of SOC cells in the control of the TT motoneurons. Although cells in MNTB and PO were labeled as early as 62 h (2 cases out of 5), consistent labeling required longer times (i.e., ≥ 69 h). This delayed transynaptic progression of labeling in the SOC compared to Rouiller et al. (1986) likely reflects the slower progression of infection of the TT motoneurons by the recombinant strain PRV-152 compared with PRV-Bartha (Billig et al. 2000). In addition to the study of Rouiller et al. (1986), we showed a consistent labeling in the cochlear nuclei without labeling in the SOC at survival times of 48-62 h. This finding suggests that the TT reflex loop also consists of a chain of 3 neurons in rats, namely the primary auditory neurons, VCN neurons and TT motoneurons. A parsimonious interpretation to explain this difference with Rouiller et al. (1986) is that the range of survival times did not allow them to confirm the 3-neuron arc (i.e., 17h, 52h and 54 h). Hence, it is possible that Rouiller et al. (1986) would have identified the 3-neuron arc if they had used intermediate post-inoculation times. The TT reflex loop as a 3-neuron arc was described in studies conducted in cats using electrophysiology (Ito and Honjo, 1988) and/or conventional retrograde (Itoh et al., 1986; Ito and Honjo, 1988) and anterograde (Itoh et al., 1986) tracing approaches. Interestingly, these studies reported the participation of neurons of the VCN in the 3-neuron arc. In the rat, Pilz et al. (1997) demonstrated that sound attenuation by the MEM reflex increased and latency of the MEM reflex decreased continuously with increasing sound pressure levels (SPL) using chronically implanted electrodes in the middle ears of animals. Importantly, authors showed that there were two components to the MEM reflex, i.e., a short latency and a longer latency part. For instance, cochlear microphonics (CM) increased after a very short latency (6-10 ms) following very intense stimuli (e.g., 110-120 dB SPL), and decreased after a longer latency (10-20 ms) following intense stimuli of 75-100 dB SPL. This feature of the MEM reflex may account for multifunctional roles of MEM in auditory functions. In particular, the very fast latency in the change of CM at very intense SPLs may account for a protective role of the MEM against acoustic trauma of the ear.
At the longest survival times (78-80h), the presence of labeled microneurons was also significant, particularly in the dorsal portion of the DCN and the AVCN, and in the region between DCN and PVCN. The granule cell domain of the cochlear complex was shown to receive projections from a number of auditory and non-auditory nuclei, and from the thin and unmyelinated type II auditory nerve fibers (Brown et al., 1988). Furthermore, Doucet and Ryugo (1997) showed that microneurons of the granule cell domain projected to the DCN. Hence, it cannot be ruled out that some of them may have been labeled through commissural projections and/or microcircuitry in cases of long survival times (e.g., Schofield and Cant, 1996; Weedman et al., 1996; Doucet and Ryugo, 1997). Finally, a few labeled cochlear root neurons were identified in the auditory nerve after injecting PRV into the TT muscle, which may be in general agreement with their participation in the startle reflex (e.g., Lee et al. 1996; Sinex et al., 2001).
In conclusion, our study implicated a variety of auditory neurons in the control of the TT muscle. Importantly, anatomical data strongly suggest a direct projection of neurons in the VCN bilaterally onto TT motoneuronal pool in rats. Those neurons were shown to share morphological characteristics with cells described in prior studies to belonging to pathways connecting the cochlear nuclei of both sides. Based on their location, those cells span the high frequency domain of the VCN. Also, cells located in dorsal part of the AVCN were shown to receive primarily auditory nerve fibers of low spontaneous discharge rate and high threshold (Fekete et al., 1984; Liberman, 1991), and encode acoustic stimulus intensity (Ghoshal and Kim, 1996). Hence, those cells may be good candidates for the afferent limb of the MEM reflex. At longer survival times, we identified other types of multipolar neurons (i.e., marginal and planar) and unidentified types of cells that may influence the TT motoneuronal pool through the SOC. Although this variety of neurons and pathways may account for the potential multi-functionality of the TT muscle in auditory behaviors (i.e., decreasing lower frequency noise, preventing desensitization, protecting ear against acoustic trauma, startle reflex), the extent to which the different pathways and types of neurons in the cochlear nuclei participate in those auditory behaviors remains to be elucidated. In the same manner, it will be interesting to determine the organization and identification of neurons in the cochlear nuclei controlling the stapedius muscle, the other muscle participating in the acoustic reflex. Similarities and differences between the two MEMs may provide information regarding their relative importance in the acoustic reflex.
4. Experimental procedure
Injections were performed in 23 adult male Long Evans rats (weight range: 230-350 g) obtained from Charles Rivers Laboratories (Wilmington, MA, USA). Animals were housed individually and allowed a minimum of 48 h acclimation to the animal facility before surgery. The experiment procedures employed in this study conformed with regulations stipulated in U.S. Department of Health and Human Services publication number CDC 88-8395 and were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
4.1. PRV as a transneuronal tracer
PRV-152, a recombinant strain from the attenuated parental PRV-Bartha, expresses enhanced green fluorescent protein (EGFP). This virus carries an insertion at the gG locus such that EGFP is constitutively expressed using the cytomegalovirus immediate early promoter. Details of its genomic organization have been provided previously (Billig et al., 2000; Card, 2001). PRV was grown in PK-15 cells to a final titer of 108 pfu/ml, cleared of cellular debris by centrifugation, aliquoted at 40 μl per tube, and stored at − 80°C. Individual aliquots of virus were thawed prior to injections. Excess virus was inactivated with bleach and discarded. PRV-152 was graciously provided by Dr. Lynn Enquist from Princeton University (Princeton, NJ).
4.2. Animals injected with PRV
The right TT muscle of twenty-two animals was injected with PRV-152, a recombinant strain from the attenuated parental PRV-Bartha. However, only 14 animals had labeled neurons in the region known to contain the TT motoneuronal pool (rat: Spangler et al., 1982; Rouiller et al., 1986; cat: Mizuno et al., 1982; Keller et al., 1983; Shaw and Baker, 1983; Friauf and Baker, 1985; guinea pig: Mizuno et al., 1982; Spangler et al., 1982; Strutz et al., 1988; rabbit: Borg, 1973; Takahashi et al., 1984; Counter et al., 1993; monkey: Gannon and Eden, 1987). Data from these animals only are discussed in the manuscript. In the other 8 animals, viral infection was restricted to other brain regions, such as autonomic structures, and/or the ipsilateral facial nucleus, and/or the ipsilateral inferior salivatory, and no labeling was observed in the TT motoneuronal pool, the cochlear nuclei and/or regions of the SOC. The autonomic structures included the ipsilateral nucleus of the solitary tract, ambiguus nucleus, caudal raphe nuclei (obscurus and pallidus), area A5, and the ventrolateral medulla of both sides (area A1/C1).
4.3. Animals injected with the Subunit b of cholera toxin
In 3 animals, b-CT (0.25%, List Biological Laboratories), a retrograde monosynaptic tracer, was injected into the left TT muscle, conjointly to the injection of PRV-152 into the right TT muscle. In another animal, b-CT was injected alone into the left TT muscle. Injections of b-CT were performed in order to ascertain the location of motoneurons innervating the TT, and validate the approach used in the present study for injections of PRV.
4.4. Surgical approach
Surgical procedures were performed under aseptic conditions. Anesthesia was induced with a mix of Ketamine (50 mg/kg), xylazine (5 mg/kg) and acepromazine (2 mg/kg) injected i.m., and supplemented with half that dose to maintain areflexia. A retro-auricular incision of the skin and subcutaneous muscle was performed in order to expose the mastoid bulla. The bony wall of the bulla was drilled open so that a portion of the stapedial artery, the promontory, parts of the malleus and incus and TT muscle were clearly visible. The orifice of the Eustachian tube could be visualized by rotating the animal more ventrally and the tendon of the TT could be easily identified at its insertion to the medial process of the malleus handle. The bone covering the TT muscle belly near the Eustachian tube was removed. The thick epimysium and the muscle fibers underneath were then scored. About 3.0 μl of PRV or b-CT was injected at the surface of TT muscle fibers using a 5-μl Hamilton syringe equipped with a 33-gauge needle. Tracers were left in contact with muscle fibers for about 30 min. before being swabbed. In some cases, the tympani bulla was filled with connective tissue, and the skin was sutured close. Animals were maintained under Biosafety level II conditions for the balance of the survival period.
4.5. Tissue processing
Animals injected with PRV were sacrificed after survival times of 48 h (n=3), 62 h (n=5), 69 h (n=1), 70 h (n=2), 71 h (n=1), 78 h (n=1), 80 h (n =1). For the animals injected with b-CT, 3 were sacrificed after 48 h, and another animal was sacrificed after 80 h. At the completion of survival times, animals were deeply anesthetized using sodium pentobarbital (100 mg/kg) i.p., and were perfused transcardially using 250 ml of phosphate buffer saline and 500 ml of the paraformaldehyde-lysine-periodate (PLP) fixative (McLean and Nakane, 1974). The brainstem was removed, postfixed for 4-5 h in PLP at 4°C, and cryoprotected by immersion in a 30% sucrose solution in phosphate buffer saline at 4°C for a couple of days. The tissue was cut into 35-μm thick transverse sections using a freezing microtome, and collected in cryoprotectant (De Olmos et al., 1978). Sections were divided into 6 sequential wells, and stored at − 20°C in cryoprotectant until processed for immunochemical localization of the viral or choleragenoid antigens.
Neurons infected with PRV-152 were visualized using procedures described previously (Billig et al., 2000). Briefly, sections from each animal were incubated in rabbit anti-EGFP polyclonal antibody (Molecular Probes, Eugene, OR, USA; 1: 2000), and processed using the avidin-biotin modification of the peroxidase anti-peroxidase procedure (Hsu et al., 1981), which employed affinity purified donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories) and Vectastain reagents (Vectastain Elite kit; Vector Laboratories). Upon completion of the immunohistochemical processing, the tissue was mounted on gelatin coated slides, dehydrated, cleared and coverslipped with Cytoseal 60 (VWR Scientific). Brainstem sections were counterstained with methyl green so that boundaries of brainstem structures could be determined.
Retrograde labeling of TT motoneurons with b-CT was revealed with a goat polyclonal antiserum (List Biochemicals, 1:50,000). Immunoperoxidase localizations used an affinity-purified secondary antibody donkey anti-goat IgG (Jackson ImmunoResearch Laboratories), and the immunoperoxidase procedure described above as for detecting PRV.
4.6. Tissue analysis
All bins of tissue per animal were examined to determine the distribution of labeled neurons. The spacing between sections analyzed ranged from 35 μm to 210 μm. Prior studies showed that the examination of sections spaced 200 μm apart was sufficient to determine the location of neurons transynaptically labeled by injections of PRV into the rat extraocular muscles (e.g., Billig and Balaban, 2004).
The locations of transynaptically labeled neurons in the cochlear nuclei and SOC were mapped bilaterally by making camera lucida drawings of sections using an Olympus BH-2 microscope equipped with a drawing tube. These maps were then used to produce the schematic diagrams used for illustration. Representative neurons were also photographed using a Nikon E-600 photomicroscope. Digitized photographs were taken using a RT Spot 210 CCD camera (Diagnostic Instruments, Sterling Heights, MI) and an image analysis system (Metamorph Ver. 6.1r4, Universal Corporation, Downingtown, PA). Figures were prepared for publication using Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA). Adjustments were made in the size, contrast, and brightness of each image.
Acknowledgments
Authors wish to thank Dr. David Ryugo for valuable comments on the manuscript, and Jen-Shew Yen for excellent immunocytochemical work. The recombinant strain PRV-152 was the generous gift of Dr. Lynn Enquist of Princeton University. The support of the Pennsylvania Lions Hearing Research Foundation GA-2403 (I.B.), the NIH/NIDCD agency RO3 DC 005911 (I.B) and KO8 DC 006676 (Y.R.) is gratefully acknowledged.
Abbreviations
- AVCN
anteroventral cochlear nucleus
- b-CT
subunit b of cholera toxin
- CM
cochlear microphonics
- DCN
dorsal nuclear nucleus
- MEM
middle ear muscle
- MNTB
medial nucleus of the trapezoid body
- PO
periolivary cell groups
- PRV
pseudorabies virus
- PVCN
posterior ventral cochlear nucleus
- SPL
sound pressure levels
- SOC
superior olivary complex
- TT
tensor tympani
- VCN
ventral cochlear nuclei
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- Balabian AL, Jacomme AV, Doucet JR, Ryugo DK, Rouiller EM. Commissural glycinergic inhibition of bushy and stellate cells in the anteroventral cochlear nucleus. Neuroreport. 2002;13:555–558. doi: 10.1097/00001756-200203250-00038. [DOI] [PubMed] [Google Scholar]
- Bartha A. Experimental reduction of virulence of Aujezky's disease. Magy Allatorv Lapja. 1961;16:42–45. [Google Scholar]
- Billig I, Balaban CD. Zonal organization of the vestibulo-cerebellum in the control of horizontal extraocular muscles using pseudorabies virus. I. Flocculus/ventral paraflocculus. Neurosci. 2004;125:507–520. doi: 10.1016/j.neuroscience.2004.01.051. [DOI] [PubMed] [Google Scholar]
- Billig I, Foris JM, Enquist LW, Card JP, Yates BJ. Definition of neuronal circuitry controlling the activity of phrenic and abdominal motoneurons in the ferret using recombinant strains of pseudorabies virus. J Neurosci. 2000;20:7446–7454. doi: 10.1523/JNEUROSCI.20-19-07446.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg E. On the neuronal organization of the acoustic middle ear reflex. A physiological and anatomical study. Brain Res. 1973;49:101–123. doi: 10.1016/0006-8993(73)90404-6. [DOI] [PubMed] [Google Scholar]
- Borg E, Counter SA. The Middle Ear Muscles. Scientific American. 1989:74–80. doi: 10.1038/scientificamerican0889-74. [DOI] [PubMed] [Google Scholar]
- Borg E, Counter SA, Rosler G. Acoustic reflex: Basic principles and clinical applications. Academic Press; New York: 1984. Theories of the function of the middle ear muscles; pp. 63–99. [Google Scholar]
- Borg E, Zakrisson JE. Stapedius reflex and monaural masking. Acta Otolaryngol. 1974;78:155–161. doi: 10.3109/00016487409126341. [DOI] [PubMed] [Google Scholar]
- Brown MC, Berglund AM, Kiang NYS, Ryugo DK. Central trajectories of type II spiral ganglion neurons. J Comp Neurol. 1988;278:581–590. doi: 10.1002/cne.902780409. [DOI] [PubMed] [Google Scholar]
- Cant NB, Benson CG. Parallel auditory pathways: projection patterns of the different neuronal populations in the dorsal and ventral cochlear nuclei. Brain Res Bull. 2003;60:457–474. doi: 10.1016/s0361-9230(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Cant NB, Gaston KC. Pathways connecting the right and left cochlear nuclei. J Comp Neurol. 1982;212:313–26. doi: 10.1002/cne.902120308. [DOI] [PubMed] [Google Scholar]
- Card P. Pseudorabies virus neuroinvasiveness: A window into the functional organization of the brain. Adv Virus Res. 2001;56:39–71. doi: 10.1016/s0065-3527(01)56004-2. [DOI] [PubMed] [Google Scholar]
- Card JP, Enquist LW. Neurovirulence of pseudorabies virus. Crit Rev Neurobiol. 1995;9:137–162. [PubMed] [Google Scholar]
- Carmel PW, Starr A. Acoustic and non acoustic factors modifying middle ear muscle activity in waking cats. J Neurophysiol. 1963;26:598–616. doi: 10.1152/jn.1963.26.4.598. [DOI] [PubMed] [Google Scholar]
- Counter SA, Aldskogius H, Borg E. Cholera toxin B-HRP and wheat germ agglutinin-HRP tracing of tensor tympani muscle motorneurons and processes in rabbits. Acta Otolaryngol. 1993;113:43–47. doi: 10.3109/00016489309135765. [DOI] [PubMed] [Google Scholar]
- de Olmos J, Hardy H, Lennart H. The Afferent Connections of the Main and the Accessory Olfactory Bulb Formations in the Rat: An Experimental HRP-Study. J Comp Neurol. 1978;181:213–244. doi: 10.1002/cne.901810202. [DOI] [PubMed] [Google Scholar]
- Doucet JR, Ross AT, Gillespie MB, Ryugo DK. Glycine immunoreactivity of multipolar neurons in the ventral cochlear nucleus which project to the dorsal cochlear nucleus. J Comp Neurol. 1999;408:515–531. [PubMed] [Google Scholar]
- Doucet JR, Ryugo DK. Projections from the ventral cochlear nucleus to the dorsal cochlear nucleus in rats. J Comp Neurol. 1997;385:245–264. [PubMed] [Google Scholar]
- Enquist LW, Card JP. Recent advances in the use of neurotropic viruses for circuit analysis. Curr Opin Neurobiol. 2003;13:603–606. doi: 10.1016/j.conb.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Fekete DM, Rouiller EM, Liberman MC, Ryugo DK. The central projections of intracellularly labeled auditory nerve fibers in cats. J Comp Neurol. 1984;229(3):432–450. doi: 10.1002/cne.902290311. [DOI] [PubMed] [Google Scholar]
- Friauf E, Baker R. An intracellular HRP-study of cat tensor tympani motoneurons. Exp Brain Res. 1985;57:499–511. doi: 10.1007/BF00237837. [DOI] [PubMed] [Google Scholar]
- Friauf E, Ostwald J. Divergent projections of physiologically characterized rat ventral cochlear nucleus neurons as shown by intra axonal injection of horseradish peroxidase. Exp Brain Res. 1988;73:263–284. doi: 10.1007/BF00248219. [DOI] [PubMed] [Google Scholar]
- Galambos R, Rupert A. Action of the middle ear muscles in normal cats. J Acoustic Soc Amer. 1959;31:349–355. [Google Scholar]
- Gannon PJ, Eden AR. A specialized innervation of the tensor tympani muscle in Macaca fascicularis. Brain Res. 1987;404:257–262. doi: 10.1016/0006-8993(87)91376-x. [DOI] [PubMed] [Google Scholar]
- Ghoshal S, Kim DO. Marginal shell of the anteroventral cochlear nucleus: intensity coding in single units of the unanesthetized, decerebrate cat. Neurosci Lett. 1996;205:71–74. doi: 10.1016/0304-3940(96)12386-7. [DOI] [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Ito J, Honjo I. Electrophysiological and HRP studies of the direct afferent inputs from the cochlear nuclei to the tensor tympani muscle motoneurons in the cat. Acta Otolaryngol. 1988;105:292–296. doi: 10.3109/00016488809097010. [DOI] [PubMed] [Google Scholar]
- Itoh K, Nomura S, Konishi A, Yasui Y, Sugimoto T, Muzino N. A morphological evidence of direct connections from the cochlear nuclei to tensor tympani motoneurons in the cat: a possible afferent limb of the acoustic middle ear reflex pathways. Brain Res. 1986;375:214–219. doi: 10.1016/0006-8993(86)90979-0. [DOI] [PubMed] [Google Scholar]
- Keller JT, Saunders MC, Ongkiko CM, Johnson J, Frank E, Van Lovera H, Tew JM. Identification of motorneurons innervating the tensor tympani and tensor veli palatini muscles in the cat. Brain Res. 1983;270:209–215. doi: 10.1016/0006-8993(83)90594-2. [DOI] [PubMed] [Google Scholar]
- Lee DJ, De Venecia RK, Guinan JJ, Jr, Brown MC. Central auditory pathways mediating the rat middle ear muscle reflexes. Anat Record. 2006;288A:358–369. doi: 10.1002/ar.a.20296. [DOI] [PubMed] [Google Scholar]
- Lee Y, Lopez DE, Meloni EG, Davis M. A primary acoustic startle pathway: obligatory role of the cochlear root neurons and the nucleus reticularis pontis caudalis. J Neurosci. 1996;16:3775–3789. doi: 10.1523/JNEUROSCI.16-11-03775.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC. Central projections of auditory-nerve fibers of differing spontaneous rate. I. Anteroventral cochlear nucleus. J Comp Neurol. 1991;313(2):240–258. doi: 10.1002/cne.903130205. [DOI] [PubMed] [Google Scholar]
- Mahoney T, Vernon J, Meikle M. Function of the acoustic reflex in discrimination of intense speech. Arch Otolaryngol. 1979;105:119–482. doi: 10.1001/archotol.1979.00790150009003. [DOI] [PubMed] [Google Scholar]
- Malmierca MS. The structure and physiology of the rat auditory system: an overview. Int Rev Neurobiol. 2003;56:147–211. doi: 10.1016/s0074-7742(03)56005-6. [DOI] [PubMed] [Google Scholar]
- McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Mizuno N, Nomura S, Konishi A, Uemura-Sumi M, Takahashi O, Yasui Y, Takada M, Matsushima R. Localization of motoneurons innervating the tensor tympani muscles: an horseredish peroxidase study in the guinea pig and cat. Neurosci Lett. 1982;31:205–208. doi: 10.1016/0304-3940(82)90020-9. [DOI] [PubMed] [Google Scholar]
- Møller AR. An experimental study of the acoustic impedance of the middle ear and its transmission properties. Acta Otolaryngol (Stockh) 1965;60:129–149. doi: 10.3109/00016486509126996. [DOI] [PubMed] [Google Scholar]
- Murata K, Ito S, Horikawa J, Minami S. The acoustic middle ear muscle reflex in albino rats. Hear Res. 1986;23:169–183. doi: 10.1016/0378-5955(86)90014-6. [DOI] [PubMed] [Google Scholar]
- Needham K, Paolini AG. Fast inhibition underlies the transmission of auditory information between cochlear nuclei. J Neurosci. 2003;23:6357–6361. doi: 10.1523/JNEUROSCI.23-15-06357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda Y, Pirsig W. Anatomical projection of the cochlea to the cochlear nuclei of the guinea pig. Arch Oto Rhino Laryng. 1974;208:107–120. doi: 10.1007/BF00453924. [DOI] [PubMed] [Google Scholar]
- Pilz PKD, Ostwald J, Kreiter A, Schnitzler HU. Effect of the middle ear reflex on sound transmission to the inner ear of rat. Hear Res. 1997;105:171–182. doi: 10.1016/s0378-5955(96)00206-7. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Capt M, Dolvio M, De Ribaupierre F. Tensor tympani reflex pathways studied with retrograde horseradish peroxidase and transneuronal viral tracing techniques. Neurosci Lett. 1986;72:247–252. doi: 10.1016/0304-3940(86)90521-5. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Capt M, Dolvio M, De Ribaupierre F. Neuronal organization of the stapedius reflex pathways in the rat: a retrograde HRP and viral transneuronal tracing study. Brain Res. 1989;476:21–28. doi: 10.1016/0006-8993(89)91532-1. [DOI] [PubMed] [Google Scholar]
- Saint Marie RL, Luo L, Ryan AF. Effects of stimulus frequency and intensity on c-fos mRNA expression in the adult rat auditory brainstem. J Comp Neurol. 1999;404:258–270. doi: 10.1002/(sici)1096-9861(19990208)404:2<258::aid-cne9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Schofield BR, Cant NB. Origins and targets of commisural connections between the cochlear nuclei in guinea pigs. J Comp Neurol. 1996a;375:128–146. doi: 10.1002/(SICI)1096-9861(19961104)375:1<128::AID-CNE8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Schofield BR, Cant NB. Projections of the ventral cochlear nucleus to the inferior colliculus and the contralateral cochlear nucleus in guinea pigs. Hear Res. 1996b;102:1–14. doi: 10.1016/s0378-5955(96)00121-9. [DOI] [PubMed] [Google Scholar]
- Schofield BR, Cant NB. Descending Auditory Pathways: Projections From the Inferior Colliculus Contact Superior Olivary Cells That Project Bilaterally to the Cochlear Nuclei. J Comp Neurol. 1999;409:210–223. doi: 10.1002/(sici)1096-9861(19990628)409:2<210::aid-cne3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Shaw MD, Baker R. The locations of stapedius and tensor tympani motoneurons in the cat. J Comp Neurol. 1983;216:10–19. doi: 10.1002/cne.902160103. [DOI] [PubMed] [Google Scholar]
- Sinex DG, Lopez DE, Warr WB. Electrophysiological responses of cochlear root neurons. Hear Res. 2001;158:28–38. doi: 10.1016/s0378-5955(01)00293-3. [DOI] [PubMed] [Google Scholar]
- Spangler KM, Henkel CK, Miller IJ. Localization of the motor neurons to the tensor tympani muscle. Neurosci Lett. 1982;32:23–27. doi: 10.1016/0304-3940(82)90223-3. [DOI] [PubMed] [Google Scholar]
- Strutz J, Munker G, Zollner C. The motor innervation of the tympanic muscles in the guinea pig. Arch Otorhinolaryngol. 1988;245:108–111. doi: 10.1007/BF00481446. [DOI] [PubMed] [Google Scholar]
- Takahashi O, Mizuno N, Mitani A, Takeuchi Y, Matsushima R. Indentification of motoneurons innervating the tensor tympani muscle in the rabbit: a retrograde horseradish peroxidase study. Neurosci Lett. 1984;49:19–23. doi: 10.1016/0304-3940(84)90129-0. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Schofield BR. Afferent Projections of the Superior Olivary Complex. Microsc Res Tech. 2000;51:330–354. doi: 10.1002/1097-0029(20001115)51:4<330::AID-JEMT4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- van den Berge H, Kingma H, Kluge C, Marres EH. Electrophysiological aspects of the middle ear muscle reflex in the rat: latency, rise time and effect on sound transmission. Hear Res. 1990;48:209–219. doi: 10.1016/0378-5955(90)90061-s. [DOI] [PubMed] [Google Scholar]
- Warr WB, Beck JE. Multiple projections from the ventral nucleus of the trapezoid body in the rat. Hear Res. 1996;93:83–101. doi: 10.1016/0378-5955(95)00198-0. [DOI] [PubMed] [Google Scholar]
- Weedman DL, Pongstaporn T, Ryugo DK. Ultrastructural study of the granule cell domain of the cochlear nucleus in rats: mossy fiber endings and their targets. J Comp Neurol. 1996;369:345–360. doi: 10.1002/(SICI)1096-9861(19960603)369:3<345::AID-CNE2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Wenthold RJ. Evidence for a glycinergic pathway connecting the two cochlear nuclei: An immunocytochemical and retrograde transport study. Brain Res. 1987;415:183–187. doi: 10.1016/0006-8993(87)90285-x. [DOI] [PubMed] [Google Scholar]
- Wever EG, Vernon JA, Lawrence M. The maximum strength of the tympanic muscles. Ann Otol (St Louis) 1955;64:383–391. doi: 10.1177/000348945506400205. [DOI] [PubMed] [Google Scholar]


