Diabetes and Cardiovascular Disease Prevention: Framing the Issue
Cardiovascular disease (CVD) is a very common, if not the most common, cause of morbidity and mortality in developed countries, and there has been longstanding recognition that diabetes is a potent risk factor for CVD. 1-3 Individuals with either Type 1 diabetes or Type 2 diabetes manifest CVD rates up to 4-10 times higher than those observed in nondiabetic subjects. Subjects with diabetes also have been shown to have more advanced atherosclerosis, as measured by carotid intima-media thickness measures or coronary artery calcium scores.4-7
The potential pathophysiology of accelerated atherosclerosis and CVD risk in diabetes is complex 8 (Table 1). Patients with Type 2 diabetes commonly have hypertension and manifest a number of abnormalities in systemic lipoprotein metabolism, and in inflammatory and coagulation pathways that are predicted to be proatherogenic and to increase CVD risk based on observational and mechanistic studies conducted in diabetic and nondiabetic experimental models. These abnormalities are related to coexisting insulin resistance in the majority of patients with diabetes and manifest as low HDL cholesterol, increased triglyceride-rich lipoprotein cholesterol, postprandial lipemia, elevated levels of C-reactive protein and other inflammatory markers, and increased levels of plasminogen-activator inhibitor 1 and fibrinogen levels. Insulin resistance in patients with Type 2 diabetes is generally, but not always, related to obesity and may be more specifically linked to central obesity and accumulation of fat in the visceral fat depot. 9, 10
Table 1.
Potential Contributors to Accelerated Atherosclerosis and CVD in Diabetes
| T1DM |
| Hyperglycemia |
| Nephropathy |
| Late-onset central obesity and insulin resistance |
| T2DM |
| Hyperglycemia |
| Nephropathy |
| Central obesity |

|
In patients with Type 1 diabetes, understanding potential pathophysiologies for accelerated atherosclerosis and CVD is complicated by the young age (usually before the second or third decade of life) at age of diagnosis of diabetes in these patients. Because of this young age of diagnosis, many years usually pass between the diagnosis of diabetes and the appearance of clinical CVD in patients with Type 1 diabetes. In addition, at time of diabetes diagnosis, these patients typically manifest none of the proatherogenic changes associated with insulin resistance noted in Type 2 diabetes. Some have suggested that CVD risk in longstanding Type 1 diabetes may be related to weight gain 2, 3 (particularly in the central fat compartment) that may result from many years of sustained peripheral hyperinsulinemia. This accumulation of fat in the central compartment may then produce changes more typical of insulin resistance and the proatherogenic milieu common in Type 2 diabetes. Alternatively, others have argued that higher rates of CVD in subjects with many years of Type 1 diabetes, especially in older studies, really reflect the adverse effects of diabetic microangiopathy, specifically diabetic nephropathy (either proteinuria or azotemia) on CVD risk.2, 3
While the underlying pathophysiology for accelerated atherosclerosis and CVD may not be completely clear, a great deal of information has become available for favorably modifying CVD risk in diabetes over the past two decades. Strict control of blood pressure and treatment with statin-type drugs importantly contribute to CVD risk reduction in patients with diabetes, specifically Type 2, and these have become standard-of-care approaches for managing CVD risk in such patients.1 Even with blood pressure control and statin treatment, however, residual incremental CVD risk in diabetes remains; i.e. in many randomized control trials of blood pressure and statin therapy, actively treated subjects with diabetes continue to manifest higher event rates than actively treated subjects without diabetes.11 In line with this, examination of trends for CVD in diabetes shows decline over time but remain higher than CVD events in non-diabetes.12, 13 Addressing this residual incremental risk for CVD risk in diabetes is not only an important problem from the point of view of the individual patient, but also from the public health perspective because the rates of diabetes are rising dramatically in both developed and developing countries.14, 15 These increasing rates are likely related to aging of the population in developed countries, improved nutrition in developing countries, and increasing rates of obesity in both. In addition to this overall increase in the incidence of diabetes, there has been a shift in the age of diagnosis of Type 2 diabetes to younger-age patients. These patients will, therefore, have many more years of diabetes with resultant increased vulnerability to clinical CVD at a younger age. In view of this ongoing epidemic of diabetes, finding approaches to preventing or delaying its CVD complications remains an important area for investigation.
The complex pathophysiologies for accelerated atherosclerosis and CVD noted above provide many potential high-value therapeutic targets for preventing or delaying CVD in diabetes. Based on pathophysiologic considerations, targeting increased inflammation, pro-coagulation, or disordered lipoprotein metabolism (beyond statin therapy) are all attractive approaches.8, 16, 17 Hyperglycemia, of course, defines diabetes and assessing the effect of glycemic control in preventing or delaying CVD events in diabetes has been considered important for many decades. It has been known for some time that baseline glycemia (measured as a fasting blood sugar or glycohemoglobin) predicts future CVD events, and that this association can extend even into the non-diabetic glycemic range.18, 19 Randomized clinical trials, both large and small, have been performed to evaluate the relationship of glycemic control to CVD in diabetes. These trials have used diverse approaches to improve glycemic control and have assessed both hard and surrogate CVD endpoints. Several large-scale, well-designed, and well-executed trials examining the effect of glycemic control on CVD endpoints in both Type 1 and Type 2 diabetes have been completed in recent years. These trials will be examined in more detail in the subsequent section.
Recent Randomized Clinical Trials to Evaluate the Effect of Glycemic Control on CVD Event Rates in Subjects with Diabetes
A number of large trials have recently been conducted to evaluate the impact of glycemic control on CVD events in diabetes (Table 2 20-24). The Diabetes Control and Complications Trial (DCCT) randomized 1,441 subjects with Type 1 diabetes to intensified or routine blood sugar control with insulin.25 At time of enrollment, the patients were between 13-40 years of age, free of clinical CVD, and without hypertension or hypercholesterolemia. The average follow-up in this trial was 6.5 years. At the completion of the DCCT in 1994, 1,394 subjects agreed to join the Epidemiology of Diabetes Intervention and Complications (EDIC) Study.20 As part of a pre-specified analysis plan, follow-up data on CVD endpoints analyzed by original DCCT treatment assignment group was reported in 2005.
Table 2.
CVD Endpoint Trials of Intensive Glucose Control in Diabetes
| DCCT/EDIC20 | UKPDS21 | ACCORD22 | ADVANCE23 | VADT24 | |
|---|---|---|---|---|---|
| Subjects | 1,394 T1DM | 3,867 T2DM | 10,251 T2DM | 11,140 T2DM | 1,791 T2DM |
| Age (years) | 27 | 53 | 62 | 66 | 60 |
| Follow-up (years) | 17 | 5.0 | 3.4 | 4.9 | 5.6 |
| Targets | glyco < 6.05% | FPG < 6mmol/L vs. Std |
glyco < 6% vs. 7-7.9% |
glyco < 6.5 vs. >6.5% |
glyco < 6%, 1.5% absolute reduction |
| glucose 70-120 pre- meal or <180mg/dl post-meal |
|||||
|
Duration
diabetes (years) |
6 | 0 | 10 | 7 | 10 |
| CVD (%) | 0 | 2 | 35 | 32 | 40 |
|
Baseline BP
mmHg |
115/73 | 135/82 | 136/75 | 145/81 | 132/76 |
| Baseline LDL | 109mg/dl | 3.5mmol/L | 2.7mmol/L | 3.1mmol/L | 2.8mmol/L |
|
Baseline glyco
(%) |
9.1 | 7.1 | 8.3 | 7.5 | 9.4 |
|
Result with
Intensive Control |
42% Reduction CVD (p = 0.02) |
No difference | Increased overall mortality (HR 1.22, p = 0.04) |
No difference | No difference |
At baseline, the subjects in the conventional therapy group had marginally higher systolic blood pressure (115 mmHg compared to 113 mmHg), but no other differences were noted between the conventional and intensive insulin group in other cardiovascular risk factors, including lipid levels, cigarette smoking, or duration of diabetes. At the end of the DCCT and the active intervention period, the conventional treatment group had significantly higher hemoglobin A1c (9.1% vs. 7.4%) and higher prevalence rates for microalbuminuria and albuminuria. The conventional treatment group also manifested a higher prevalence of a creatinine > 2mg/dl during the EDIC follow-up period.
By February 2005, with a total follow-up period of 17 years, 98 CVD events occurred in 52 subjects in the conventional treatment group. By comparison, there were 46 events in 31 subjects in the intensive treatment group. There was a 57% reduction in the risk of a first occurrence of a myocardial infarction, stroke, or CVD death. These differences between the rates of CVD endpoints in the intensive treatment group and conventional treatment group were statistically significant. In further analysis, it was noted that the difference in albuminuria and microalbuminuria contributed to the beneficial effect of intensive insulin therapy on CVD endpoints. However, the treatment effect remained significant even after adjusting for differences in albuminuria and microalbuminuria. The investigators of DCCT/EDIC concluded that intensive glycemic control produced a longterm benefit on CVD in patients with Type 1 diabetes.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) was designed to assess the impact of tight blood sugar control on CVD events in subjects with Type 2 diabetes.22 Over 10,000 patients with a mean age 62.2 years, and a median hemoglobin A1c of 8.1%, were randomly assigned to intensive glucose control (with a hemoglobin A1c goal of < 6%) or standard glucose control (with a hemoglobin A1c goal of 7-7.9%). The median duration of diabetes in this cohort was 10 years in each group, approximately 35% of them had a previous CVD event, and there were no differences in CVD risk factors at baseline. The primary outcome was a composite of non-fatal myocardial infarction, non-fatal stroke, or death from cardiovascular causes. The trial also included arms to evaluate the impact of blood pressure and lipid interventions on CVD in Type 2 diabetes. The ACCORD glycemic control arm was terminated prematurely after a mean follow-up of 3.5 years because of an excess number of deaths in the intensive control group. Attained glycohemoglobin levels were 6.4% and 7.5% in the intensive and control groups respectively. Two hundred fifty-seven subjects in the intensive glucose control group died, compared to 203 in the standard control group (hazard ratio 1.22, 95% CI 1.01-1.46, p=0.04). Three hundred fifty-two patients in the intensive control group, compared to 371 patients in the standard control group, experienced a primary outcome event (hazard ratio 0.90, 95% CI 0.78-1.04, p=0.16). The causes of excess deaths in the ACCORD trial remain to be definitively explained. It is plausible, however, that excess mortality was due to serious hypoglycemia, which was significantly more frequent in the intensive control group. The intensive control group in ACCORD experienced a very rapid rate of decline in hemoglobin A1c (1.4% within 4 months). In addition, 77.3% of subjects in the intensive control group, compared to 55.4% in the standard control group, used any insulin. The rate of bolus insulin usage was 55.3% vs. 35% in these groups, respectively. Hypoglycemic events requiring any assistance occurred in 16.2% in the intensive control group vs. 5.1% in the standard control group. Hypoglycemia requiring medical assistance was 10.5% in the intensive therapy group and 3.5% in the standard therapy group. As a result of their analysis, the ACCORD investigators concluded that intensive glucose lowering produces no benefit in terms of CVD risk reduction, and even produces harm in high-risk patients with Type 2 diabetes. In an analysis of pre-specified subgroups in the ACCORD trial, there was a suggestion that patients who did not have a cardiovascular event before randomization, and with a baseline glycohemoglobin of 8% or less, had fewer fatal or non-fatal cardiovascular events with intensive control compared to standard control.
The Action in Diabetes and Vascular Disease: Preterax in Diamicron Modified Release Controlled Evaluation (ADVANCE) was conduced in 11,140 patients with type 2 diabetes randomized to standard or intensive glucose control.23 Subjects randomized to the latter group all used gliclazid, along with other drugs as required, to achieve a hemoglobin A1c goal of 6.5% or less. The age was 66 years in each group – with a duration of diabetes of 7.9-8 years. Approximately 32% of subjects in each group had a history of myocardial infarction, stroke, or other major macrovascular disease. Median glycohemoglobin at baseline was 7.2% in each group. At the completion of the study (median of 5 years of follow-up), the hemoglobin A1c level was 7.3% in the standard control group and 6.5% in the intensive control group. The primary endpoints in this trial were composites of death from cardiovascular causes, non-fatal myocardial infarction or non-fatal stroke (major macrovascular events), along with new or worsening nephropathy or retinopathy (that define major microvascular events). The trial demonstrated that intensive control led to a significant reduction in combined major macrovascular and microvascular events (18.1% vs. 20%, hazard ratio 0.90, 95% CI 0.82-0.98, p=0.01) and of major microvascular events (9.4% vs. 10.9%, hazard ratio 0.86, 95%CI 0.77-0.97, p=0.01). This reduction was primarily related to a significant reduction in the incidence of nephropathy with intensive control. There was not a significant effect of intensive glucose control on major macrovascular events (hazard ratio 0.94, 95%CI 0.84-1.06, p=0.32), CVD death (hazard ratio 0.88, 95% CI 0.74-1.04, p=0.12), or all-cause mortality (hazard ratio 0.93, 95% CI 0.83-1.06, p=0.28). As in the ACCORD trial, severe hypoglycemia was more common in the intensive control group; however, overall rates of hypoglycemia were much lower compared to ACCORD (2.7% vs. 1.5%, hazard ratio 1.86, 95% CI 1.42-2.40, p<0.001). The decrease in hemoglobin A1c level in ADVANCE was considerably less rapid than that observed in ACCORD (reduction of 0.5% at 6 months, and 0.6% at 12 months).
The Veterans Affairs Diabetes Trial (VADT) enrolled 1,791 veterans with Type 2 diabetes and randomly assigned them to intensive or standard glucose control.24 The primary outcome measure of this trial was time to first occurrence of a composite of myocardial infarction, stroke, cardiovascular death, congestive heart failure, surgery for vascular disease, inoperable coronary disease, and amputation for gangrene. Mean age at time of enrollment was 60.4 years – with a mean duration of diabetes of 11.5 years. Forty percent of the enrollees had a history of a cardiovascular event. Baseline glycohemoglobin in this trial was 9.4%, and there was no difference in cardiovascular risk factors at baseline between the two therapy groups. After a median follow-up of 5.6 years, glycohemoglobin levels in the intensive therapy group were 6.9% and in the standard therapy group 8.4%. There was no significant difference in any component of the primary outcome between the two groups. Rates of hypoglycemia were 24.1% in the intensive therapy group and 17.6% in the standard therapy group. The fall in glycohemoglobin was very rapid, as in the ACCORD trial; from 9.4% to 6.9% in the intensive therapy group by 6 months after randomization. Although there was no significant difference in primary event rate, there was a low overall incidence of primary events in both treatment groups. The predicted event rate was 40% in the standard therapy group, but the observed event rate was 33.5%. The predicted event rate in the intensive therapy group was 31.6%, and the observed event rate was 29.5%. Weight gain was significantly greater in the intensive therapy group.
The United Kingdom Prospective Diabetes Study (UKPDS) is the oldest of the large randomized glycemia control trials and randomized subjects with Type 2 diabetes to intensive blood sugar control vs. standard blood sugar control.21 The trial enrolled patients with newly diagnosed Type 2 diabetes with a median age of 54 years. The goal for intensive therapy was < 6mmol/l of fasting blood sugar. In the standard therapy group drugs were added if the patient experienced symptoms of hyperglycemia or fasting plasma glucose was > 15mmol/l. Hemoglobin A1c over 10 years was 7% in the intensive therapy group, and 7.9% in the standard therapy group. This trial had multiple micro and macrovascular endpoints and demonstrated a significant 25% risk reduction in microvascular endpoints in the intensive therapy group. This study also demonstrated a 16% risk reduction for myocardial infarction with intensive therapy, but this difference did not reach statistical significance (p = 0.052). It is worth recalling that, different from the recently conducted large trials of glycemic control in CVD, the UKPDS trial was conducted before the widespread use of statin therapy in Type 2 diabetes and in subjects with newly diagnosed diabetes and essentially no CVD.
Glycemic Control and CVD Risk in Diabetes. Why is this Still a Question?
The previous section presents results of five large well-designed and well-executed randomized clinical trials examining the impact of tight glycemic control on CVD events in diabetes. In Type 1 diabetes there is evidence for benefit based on results of the DCCT/EDIC. Four trials, including three reported very recently, failed to show a significant benefit of tight glycemic control on CVD rates in Type 2 diabetes. In view of these consistent results in Type 2 diabetes, why is the issue of glycemic control and CVD risk in Type 2 diabetes still worth considering?
The five trials in the previous section were presented in some detail in order to allow a rigorously appropriate interpretation of their results. Based on the results of these trials, it cannot be concluded that glycemic control does not prevent CVD complications in Type 2 diabetes unless this conclusion is substantially qualified. The appropriate qualifiers would include the specific pharmacotherapeutic interventions employed, the patient population studied (especially with respect to age, duration of diabetes and cardiovascular risk factors including pre-existing CVD), the baseline glycemic control, the glycemic goals, duration of the therapeutic intervention, and the period of observation. While randomized clinical trials are justifiably the goal standard for evidence-based practice, the above trial design characteristics (necessary to allow trial feasibility and efficiency) importantly impact the interpretation of trial results. The impact of these factors on the interpretation of trial results needs to be examined carefully when results of randomized clinical trials are not concordant with other types of evidence; for example, observational or pathophysiologic evidence. In addition, and importantly, the difference between results of glycemic control in Type 1 diabetes and Type 2 diabetes on CVD events must be understood and rationalized. Mention has already been made regarding observational evidence that baseline glycemia predicts future CVD events in diabetic and nondiabetic populations. In the next section, we will further examine other types of evidence that bear upon the relationship between tight glycemic control and CVD risk in patients with diabetes.
Mechanistic and Pathophysiologic Studies Using In Vitro and Animal Models
Studies with isolated cells or tissue, or in experimental animal models, cannot unequivocally establish appropriate therapeutic approaches for human disease. However, such studies can provide plausibility and the pathophysiological context for human studies. For example, this approach has provided detailed mechanistic underpinning for observations relating specific native or modified lipoproteins to human atherosclerosis and clinical CVD. Over many years, a consensus has emerged in the literature regarding experimental characteristics in isolated cell studies that are consistent with proatherogenicity.8 Key cellular constituents in the progression from normal vessel wall to atherosclerotic plaque, to vulnerable plaque, to plaque rupture, are thought to be endothelial cells, monocyte-derived macrophages, and arterial smooth muscle cells. In endothelial cells, the increased expression of inflammatory factors or adhesion molecules is considered proatherogenic; as are inhibitors of endothelial cell-mediated vasodilatation or increased endothelial cell death. In macrophages, disturbances in sterol flux and reverse cholesterol transport favoring increased cell sterol retention, the increased expression of adhesion and inflammatory factors, or increased cell death are consistent with proatherogenicity. In arterial smooth muscle cells, factors that increase proliferation or alter the composition of the extracellular matrix are thought to be proatherogenic. The impact of hyperglycemia on the above experimental endpoints, in vitro and on the vessel wall in animals, has been examined (Table 3).
Table 3.
Hyperglycemia and the Vessel Wall
| Cell Studies |
| Endothelial cells |
| Increased inflammatory factor expression |
| Increased adhesion molecule expression |
| Increased cell death |
| Macrophages |
| Increased inflammatory factor expression |
| Increased adhesion molecule expression |
| Increased production ROS |
| Dysregulation of sterol flux |
| Increased cell death |
| Arterial smooth muscle cells |
| Increased cell proliferation |
| Altered matrix production/composition |
| Animal Studies |
| Impaired vasodilatation |
| Increased atherosclerosis |
| AGE protein-mediated |
| Aldose reductase-mediated |
Studies in isolated cells have demonstrated that hyperglycemia increases adhesion of monocytes to endothelium as a result of increased expression of adhesion factors on both endothelial cells and monocyte-macrophages.26 Hyperglycemia also increases expression of NFκB in both endothelial cells and monocyte-derived macrophages, along with increased production of superoxide and reactive oxygen species; thus, producing increased oxidative stress.27-34 Increased oxidative stress can lead to increased production of oxidized LDL in the vessel wall with the subsequent untoward effects experimentally ascribed to this modified lipoprotein. Hyperglycemia has been shown to impair nitric oxide production that is important for endothelial cell-dependent vasodilatation, and to impair endothelial cell-dependent vasodilatation in vivo. Proteins modified by advanced glycosylation end products (AGE-proteins) have been shown to interrupt key steps in reverse cholesterol transport.35 In arterial smooth muscle cells, hyperglycemia supports proliferation and alters composition of arterial smooth muscle cell-derived matrix in a manner predicted to increase retention and subsequent oxidative modification of lipoproteins.36-38
The relationship of hyperglycemia to the vessel wall has also been examined in animal models examining the above-described endpoints, as well as atherosclerosis. Many of these models, however, are confounded because the experimental interventions that produce hyperglycemia also alter lipoprotein pattern. It is uncertain, therefore, whether changes in vessel wall cells or atherosclerosis in these models relate directly to hyperglycemia or to a more atherogenic lipoprotein profile. There are animal models, however, that do lend support to the notion that hyperglycemia is directly injurious to the vessel wall. Goldberg and colleagues studied the effect of increased aldose reductase expression on atherosclerosis in the LDL receptor-deficient atherosclerosis-prone mouse model.39 Aldose reductase mediates the production of cellular toxins from glucose and these investigators reported that in streptozotocin diabetic mice, but not nondiabetic mice, increased expression human aldose reductase leads to more atherosclerosis. Vessel wall cells express receptors that recognize AGE- proteins and signaling via these receptors activate a program of proinflammatory gene expression. Several laboratories have reported that modifying signaling via the receptor for AGE-proteins either by reducing receptor expression, by blocking binding of AGE-protein to the receptor, or reducing formation of advanced glycosylation end products, can reduce atherosclerosis in diabetic mouse models.40-42
Glycemic Control and Surrogate CVD Endpoints in Diabetes
Measurement of carotid intimal-medial thickness (CIMT) by ultrasound, coronary artery calcium (CAC) by CT, or coronary atherosclerosis by coronary intravascular ultrasound (IVUS) have been identified as useful markers for assessing cardiovascular risk and predicting cardiovascular events. They can provide incremental information above that provided by routine cardiovascular risk factor assessment for predicting cardiovascular events in subjects with diabetes.43, 44 Patients with Type 1 and Type 2 diabetes have been shown to have increased CIMT, CAC, and coronary atherosclerosis by IVUS, and progression of these measures has been shown to be more rapid in diabetic subjects compared to nondiabetic subjects. 4-7, 45 Some, but not all, studies have shown that progression of CIMT and CACS are tightly related to measures of glycemic control even after adjustment for routine atherosclerosis risk factors.46-54
In DCCT/EDIC, intensive insulin therapy in Type 1 diabetes led to lower coronary artery calcium burden, and decreased progression of CIMT compared to standard insulin therapy.47, 48 In subjects with Type 1 diabetes, pancreas transplantation has been shown to reduce CIMT independent of changes in lipid, blood pressure, smoking or use of hyperlipidemic drugs.49 In Type 2 diabetes, therapeutic lifestyle changes, metformin, insulin secretagogues, and thiazolidinediones have all been shown to reduce progression of CIMT.50-54 In single trials, reduced progression cannot be clearly related to changes in glycemia. However, an analysis of a large number of trials showed that reductions in rates of progression were closely related to changes in on-trial measures of glycemia.54
Meta-Analysis, Subgroup Analysis and Extended Follow-up of Randomized Controlled Trials
Three separate meta-analyses have recently examined the relationship between glycemic control and CVD events in subjects with Type 2 diabetes (Table 4 55-61). The first of these analyses included UKPDS, ADVANCE, VADT, ACCORD, and PROactive.55 The latter trial randomized subjects with Type 2 diabetes and macrovascular disease to placebo or pioglitazone. The participants in this trial were 62 years of age and had established CVD. Hemoglobin A1c at time of randomization was 7.9%, and the average time of observation was 35.4 months. The trial randomized 5,238 patients. Hemoglobin A1c fell 0.8% in the pioglitazone group and 0.3% in the placebo group from baseline to final visit. The primary endpoint of this trial, which was a composite of hard cardiovascular events and cardiovascular procedures, was not statistically different; however, a secondary endpoint focusing primarily on hard cardiovascular events showed a significant reduction. Including this trial, the five trials in this meta-analysis included 1,497 nonfatal myocardial infarctions, 2,318 coronary heart disease events, 1,127 strokes, and 2,892 all-cause mortality during approximately 163,000 person years of follow-up. The glycohemoglobin concentration was 0.9% lower for the intensive therapy group compared to the standard therapy group. There was an overall significant 17% reduction in nonfatal myocardial infarction, and a 15% reduction in coronary heart disease events. There was no significant difference on stroke or all-cause morality.
Table 4.
Hyperglycemia and CVD in T2DM – Meta-Analyses, Extended Follow-up, and Subgroup Analyses
| Meta-Analyses | Comments |
| Ray et al.55 | 33,040 Subjects |
| UKPDS 3321 & 3456, ACCORD22, ADVANCE23, VADT24, PROactive57 |
Reduction non-fatal MI (17%) Reduced Coronary Heart Disease (15%) No change in mortality Increased hypoglycemia |
| Kelly et al.58 | 27,802 Subjects |
| UKPDS 33 & 34, ACCORD, ADVANCE, VADT |
Reduced CVD events (10%) No change in CV or all-cause mortality Increased hypoglycemia |
| Turnbull et al.59 | 27,049 Subjects |
| UKPDS 33, ACCORD ADVANCE, VADT |
Reduced MI (15%) No change in CV or all-cause mortality Increased hypoglycemia |
| Extended Follow-up (UKPDS) | |
| Holman et al.60 | 3,277 Subjects |
| Reduced CVD events (15%) Reduced all-cause mortality (13%) Total follow-up 17-18 years Glyco 8.1% at start of extended 10-year followup |
|
| Low CAC Subgroup VADT | |
| Reaven et al.61 | 301 Subjects |
| Reduced CVD events (HR 0.08) |
The second meta-analysis included UKPDS, ACCORD, ADVANCE and VADT.58 Including these trials provided information on 27,802 adult subjects and demonstrated that intensive control of glycemia significantly reduced nonfatal myocardial infarction, but did not reduce risk of cardiovascular death or all-cause mortality. Subjects in the intensive therapy group were also at increased risk for severe hypoglycemia. The third meta-analysis also included the ACCORD, ADVANCE, VADT, and UKPDS trials.59 Results of this analysis showed that subjects randomized for more intensive glucose control had a reduced risk of major cardiovascular events by 9% (hazard ratio 0.91 95% CI 0.84-0.99) compared to those randomized to less intensive glucose control. This advantage was primarily the result of a 15% reduction in risk of myocardial infarction. As in previous meta-analyses, there was no difference in overall mortality and subjects in the more intensive control groups had more major hypoglycemic events. An exploratory subgroup analysis suggested that subjects without pre-existing macrovascular disease may have benefited from intensive glycemic control more than subjects with pre-existing disease. Therefore, the results of all three meta-analyses demonstrated a benefit of improved glycemic control on nonfatal myocardial infarction, but no difference in overall mortality. Authors of the meta-analyses point out the limitations of this approach, including use of summary rather than individual data in the analyses by Ray et al55 and Kelly et al.58
The large group sizes incorporated into the design of the recently reported trials of glycemic control on CVD risk in Type 2 diabetes lend themselves to a number of pre-specified subgroup analyses. One of the most provocative of these was conducted in subjects with varying levels of coronary artery atherosclerosis (as quantified by CAC) in the VADT (Table 4).61 Analysis of a subgroup of 301 study participants, with a mean follow-up duration of 5.2 years, indicated that subjects with lower baseline CAC benefited more from intensive glycemic control than those with higher scores. For those subjects with CAC > 100, the multivariable hazard ratio for an endpoint event was 0.74 (95% CI 0.46-1.20, p=0.21) for those randomized to intensive treatment; while it was 0.08 (95% CI 0.008-0.77, p = 0.03) for those randomized to intensive therapy with a CAC ≤ 100.
The results of the UKPDS trial were presented in a previous section, and also were included in each of the meta-analyses described above. As noted previously, there was a 16% reduction in myocardial infarction in UKPDS, which did not reach statistical significance at the time the trial was ended and reported in 1998. At time of trial completion, the majority of patients (78%) continued to be followed, but without differences in therapeutic intervention. Seven aggregate clinical outcomes were pre-specified for analysis on an intention-to-treat basis according to the previous UKPDS randomization group. The differences in glycohemoglobin between intensive therapy and routine therapy group were lost one year after trial completion in 1998, and glycohemoglobin remained essentially identical in the two previously defined treatment groups over the course of a 10-year follow-up. After an additional 10 years of follow-up, the investigators reported a 15% reduction in myocardial infarction in the group previously randomized to intensive therapy.60 This is similar to the percent reduction reported immediately after completion of the trial, but with occurrence of additional events, this reduction was now significant with a p=0.01. Subjects originally randomized to the intensive therapy group also demonstrated 13% less overall mortality (p=0.007) after the additional 10 years of follow-up.
Managing CVD Risk in Diabetes – Glycemic Control in Context
DCCT/EDIC demonstrated that intensive glycemic control in Type 1 diabetes produces benefit for preventing CVD events. This result, along with observational and pathophysiologic data establishes control of glycemia as an integral aspect of managing longterm CVD risk in subjects with Type 1 diabetes. This issue, however, is somewhat more complex for Type 2 diabetes. Several large randomized clinical trials failed to show benefit of intensive glycemic control on overall CVD, and without this data it is impossible to establish an unequivocal recommendation for glycemic control as a means of managing CVD risk in subjects with Type 2 diabetes. There are, however, important considerations that suggest glycemic control can be part of managing CVD risk in this patient population. First, the data above in Type 1 diabetes from DCCT/EDIC provides a reasonable proof-of-concept that hyperglycemia is toxic to the vessel wall in humans. Furthermore, studies in isolated cells and in animals provide several plausible mechanisms for this toxicity. Measures of atherosclerosis in humans by CIMT or CAC are also consistent with vessel wall injury from hyperglycemia and reduced atherosclerosis with better glycemic control. Meta-analysis of the major endpoint trials demonstrates CVD benefit for intensive glycemic control in Type 2 diabetes, and a 10-year extended follow-up of one of the clinical trials (UKPDS) provided evidence of benefit that approximated the benefit predicted by the meta-analyses. This extended follow-up also demonstrated a benefit of intensive glycemic control on overall mortality. Finally, an important subgroup analysis in VADT demonstrated that subjects with less coronary artery disease (as evaluated by amount of coronary artery calcium) had significant benefit from intensive glycemic control with respect to prevention of CVD events.
Several reasonable scenarios could be considered for the inconsistency of individual randomized clinical trial results with other types of data mentioned above. The relationship between glycemic control and CVD could be complicated by an adverse effect of glucose variability on vessel wall homeostasis.62, 63 Further, although hyperglycemia may be toxic to the vessel wall, it may be of overall less importance relative to other factors present in Type 2 diabetes; for example, dyslipidemia, hypertension, inflammation, and pro-coagulation. There is a great deal of pathophysiologic data and observational data suggesting that inflammation and coagulation contribute substantially to CVD risk in subjects with Type 2 diabetes, however, there is as yet very little interventional data. In fact, it could be argued that the data supporting a benefit for managing hyperglycemia is superior to that addressing inflammation or coagulation for primary prevention of CVD in Type 2 diabetes.64-69 On the other hand, randomized clinical trial data to date suggest that statin treatment and managing hypertension will have a more profound effect for reducing CVD event rate in Type 2 diabetes than managing hyperglycemia. The CVD benefits of statin therapy or one of multiple approaches to managing hypertension in Type 2 diabetes have been easily demonstrated in terms of reduction of overall events and reduction of CVD mortality within a 5-year period of intervention/observation in multiple trials.70-73
The two largest randomized clinical trials evaluating the impact of statin therapy on CVD events in subjects with diabetes are the Collaborative Atorvastatin Diabetes Study (CARDS)70, and the Heart Protection Study (HPS)71. In CARDS, 2,838 subjects with Type 2 diabetes, age 40-75, and with no previous history of CVD, were randomized to atorvastatin 10 mg/day or placebo. At baseline, subjects had a glycohemoglobin of 7.8%. At trial entry, LDL cholesterol level was less than 4.14mmol/l and fasting triglyceride was less than 6.78mmol/l. The primary endpoint of the trial was first occurrence of acute coronary heart disease events, coronary revascularization, or stroke. Subjects had a mean follow-up of 3.9 years, and the trial was terminated two years early. At time of trial termination, there was a significant (p = 0.001) 30% reduction in primary endpoint events in the atorvastatin group. Subjects who entered the trial with LDL cholesterol levels above and below 3.1mmol/l benefited similarly from atorvastatin treatment. In addition, there was no influence of baseline glycohemoglobin on benefit. Rates of death were also reduced in the atorvastatin group by 27%, but this did not reach statistical significance at 3.9 years (p = 0.059).
In HPS, 5,963 subjects with diabetes (both Type 1 and Type 2) age 40-80, and with or without preexisting vascular disease, were randomized to simvastatin 40 mg/day or placebo. The primary endpoint of the trial included hard major vascular events. The mean duration of follow-up was 4.8 years, and the baseline LDL cholesterol in this trial was 3.2mmol/l, and baseline fasting triglyceride was 2-2.3mmol/l. Treatment with simvastatin produced a 22% reduction in primary endpoint events (p < 0.0001). Subjects with baseline LDL cholesterol above and below 3.5mmol/l, or glycohemoglobin above or below 7%, benefited similarly from simvastatin treatment.
While the best data for the benefit of aggressive treatment of dyslipidemia in diabetes belongs to the statin class of anti-lipemic drugs, multiple classes of anti-hypertensive drugs have been shown to produce cardiovascular benefit in subjects with diabetes. Reviews of a large number of endpoint trials72, 73 that included use of thiazide diuretics, angiotensin II receptor blockers, angiotensin-converting enzyme inhibitors, calcium channel blockers, and beta-blockers have supported a blood pressure goal of 130-135mmHg and 80mmHg diastolic in diabetes. Overall, analyses fail to support superiority of any one class of drug, and reduction of hard CVD endpoints generally ranged from 20-30% with 3-5 years of follow-up. The overall greater importance of aggressive lipid and blood pressure control compared to glycemic control is also supported by analysis of results of the Steno 2 trial.74 In this trial, a multifactorial approach to managing CVD risk in Type 2 diabetes (including lipid, blood pressure and glucose control) reduced CVD death by over 50% at 13.3 years of follow-up. Subsequent analysis of these results suggested statins and anti-hypertensive treatment provided the largest benefit with glucose control next.75
Other reasons why well-designed and executed randomized clinical trials could fail to demonstrate a benefit relate to the patient population studied, types of intervention, period of intervention and period of observation. For example, a subgroup analysis has already suggested that starting therapy in patients with less established coronary atherosclerosis may be beneficial. A longterm follow-up of UKPDS has suggested that the longer period of observation after institution of glycemic control may be necessary for observing a CVD benefit. Other possibilities include the potential that the drugs currently available for achieving intensive blood sugar control produce untoward effects that counter balance any CVD benefit provided by such control.76 The most obvious of these untoward effects would be hypoglycemia, which is much more common in patients with intensive glycemic control targets. Analysis of the ACCORD data could not establish a relationship between hypoglycemia and increased mortality in the intensive control group.77 A meta-regression analysis, however, did suggest a link between hypoglycemia and cardiovascular mortality.78 Serious hypoglycemic reactions can be nocturnal, frequently go unnoticed, and can produce longterm activation of the sympathetic nervous system. In elderly patients with coronary artery disease, or other similarly vulnerable patients, this physiologic response to hypoglycemia could elevate risk of myocardial infarction, cardiac arrhythmia or death.
In view of all the available data, managing glycemia as an aspect of managing overall CVD risk in subjects with both Type 1 and Type 2 diabetes remains a viable proposition. In addition to all of the considerations above, it is important to recall that intensive glycemic control has been shown to produce a substantial benefit for preventing longterm microvascular complications in both Type 1 and Type 2 diabetes. Although CVD is the major cause of death in subjects with diabetes, microvascular complications produce substantial morbidity. Further, prevention of microvascular complications, specifically nephropathy, could also produce a longterm benefit for prevention of CVD (Fig. 1). Several professional organizations have made recommendations for glycemic targets after consideration of recent data from large randomized clinical trials. In general, glycohemoglobin goals of 7% are believed appropriate; however, it is emphasized that goals need to be individualized for patients. For example, more intensive goals could be appropriate for a young patient with no CVD, whereas less intensive goals appropriate for the elderly or those with established CVD in whom the risk associated with hypoglycemia could be significant. In support of this approach, a recent observational study concluded that better glycemic control is associated with better cardiovascular outcomes in diabetic subjects with fewer comorbidities.79 Recent evidence suggests that the prevalence of diabetes in the United States will approximately double in the next 2-3 decades. While additional randomized clinical trial data examining the relationship of glycemic control to CVD could certainly be of value, the size and duration of such a trial given what we now know (for example, the need to study lower risk patients over a longer period of time) could make its cost prohibitive. Clinicians caring for patients with diabetes need to make decisions regarding optimal management of CVD risk. Currently available evidence makes it reasonable to include glycemic control, with a target individualized to the patient, as part of an overall risk management strategy in this rapidly increasing population of patients.
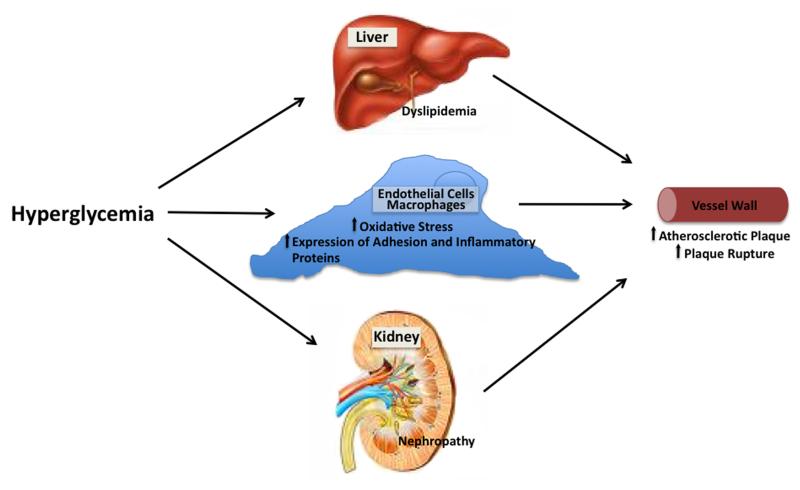
Figure 1.
Hyperglycemia could promote atherosclerosis and CVD events by altering hepatic and peripheral lipoprotein metabolism, by facilitating development of proteinuria and azotemia, or by modulating oxidative stress, inflammation and macrophage-endothelial cell adhesion in the vessel wall.
Acknowledgments
The author thanks Stephanie Thompson for assistance with manuscript preparation, and Dr. Andrea Carnegie for assistance with graphics.
Funding Sources Supported by grants DK71711, HL39653 and UL1RR029879 from the National Institutes of Health.
Footnotes
Disclosures TM is a consultant for Abbott, Merck and Takeda
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Buse JB, Ginsberg HN, Bakris GL, Clark NG, Costa F, Eckel R, Fonseca V, Gerstein HC, Grundy S, Nesto RW, Pignone MP, Plutzky J, Porte D, Redberg R, Stitzel KF, Stone NJ. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2007;115:114–126. doi: 10.1161/CIRCULATIONAHA.106.179294. [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Nathan DM, Abraham K, Brunzell JD, Fradkin JE, Haffner SM, Hsueh W, Rewers M, Roberts BT, Savage PJ, Skarlatos S, Wassef M, Rabadan-Diehl C. Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus. Circulation. 2005;111:3489–3493. doi: 10.1161/CIRCULATIONAHA.104.529651. [DOI] [PubMed] [Google Scholar]
- 3.Orchard TJ, Costacou T, Kretowski A, Nesto RW. Type 1 diabetes and coronary artery disease. Diabetes Care. 2006;29:2528–2538. doi: 10.2337/dc06-1161. [DOI] [PubMed] [Google Scholar]
- 4.Schurgin S, Rich S, Mazzone T. Increased prevalence of significant coronary artery calcification in patients with diabetes. Diabetes Care. 2001;24:335–338. doi: 10.2337/diacare.24.2.335. [DOI] [PubMed] [Google Scholar]
- 5.Sander D, Schulze-Horn C, Bickel H, Gnahn H, Bartels E, Conrad B. Combined effects of hemoglobin A1c and C-reactive protein on the progression of subclinical carotid atherosclerosis: the INVADE study. Stroke. 2006;37:351–357. doi: 10.1161/01.STR.0000199034.26345.bc. [DOI] [PubMed] [Google Scholar]
- 6.Selvin E, Coresh J, Golden SH, Boland LL, Brancati FL, Steffes MW. Glycemic control, atherosclerosis, and risk factors for cardiovascular disease in individuals with diabetes: the atherosclerosis risk in communities study. Diabetes Care. 2005;28:1965–1973. doi: 10.2337/diacare.28.8.1965. [DOI] [PubMed] [Google Scholar]
- 7.Rabago RR, Gomez-Diaz RA, Tanus HJ, Garnica FJ velar, Ramirez SE, Nishimura ME, guilar-Salinas CA, Wacher NH. Carotid intima-media thickness in pediatric type 1 diabetic patients. Diabetes Care. 2007;30:2599–2602. doi: 10.2337/dc07-0922. [DOI] [PubMed] [Google Scholar]
- 8.Mazzone T, Chait A, Plutzky J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. Lancet. 2008;371:1800–1809. doi: 10.1016/S0140-6736(08)60768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sam S, Haffner S, Davidson MH, D’Agostino RB, Feinstein S, Kondos G, Perez A, Mazzone T. Relationship of abdominal visceral and subcutaneous adipose tissue to lipoprotein particle number and size in Type 2 diabetes. Diabetes. 2008;57:2022–2027. doi: 10.2337/db08-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sam S, Haffner S, Davidson MH, D’Agostino RB, Sr., Feinstein S, Kondos G, Perez A, Mazzone T. Relation of abdominal fat depots to systemic markers of inflammation in type 2 diabetes. Diabetes Care. 2009;32:932–937. doi: 10.2337/dc08-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costa J, Borges M, David C, Vaz CA. Efficacy of lipid lowering drug treatment for diabetic and non-diabetic patients: meta-analysis of randomised controlled trials. BMJ. 2006;332:1115–1124. doi: 10.1136/bmj.38793.468449.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox CS, Coady S, Sorlie PD, Levy D, Meigs JB, D’Agostino RB, Sr., Wilson PW, Savage PJ. Trends in cardiovascular complications of diabetes. JAMA. 2004;292:2495–2499. doi: 10.1001/jama.292.20.2495. [DOI] [PubMed] [Google Scholar]
- 13.Preis SR, Hwang SJ, Coady S, Pencina MJ, D’Agostino RB, Sr., Savage PJ, Levy D, Fox CS. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation. 2009;119:1728–1735. doi: 10.1161/CIRCULATIONAHA.108.829176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang ES, Basu A, O’Grady M, Capretta JC. Projecting the future diabetes population size and related costs for the U.S. Diabetes Care. 2009;32:2225–2229. doi: 10.2337/dc09-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 16.Fantuzzi G, Mazzone T. Adipose tissue and atherosclerosis - exploring the connection. Arterioscler Thromb Vasc Biol. 2007;27:996–1003. doi: 10.1161/ATVBAHA.106.131755. [DOI] [PubMed] [Google Scholar]
- 17.Davidson M, Meyer PM, Haffner S, Feinstein S, D’Agostino R, Sr., Kondos GT, Perez A, Chen Z, Mazzone T. Increased high-density lipoprotein cholesterol predicts the pioglitazone-mediated reduction of carotid intima-media thickness progression in patients with type 2 diabetes mellitus. Circulation. 2008;117:2123–2130. doi: 10.1161/CIRCULATIONAHA.107.746610. [DOI] [PubMed] [Google Scholar]
- 18.Selvin E, Coresh J, Golden SH, Brancati FL, Folsom AR, Steffes MW. Glycemic control and coronary heart disease risk in persons with and without diabetes: the atherosclerosis risk in communities study. Arch Intern Med. 2005;165:1910–1916. doi: 10.1001/archinte.165.16.1910. [DOI] [PubMed] [Google Scholar]
- 19.Turner RC, Millns H, Neil HAW, Stratton IM, Manley SE, Matthews DR, Holman RR. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United kingdom prospective diabetes study (UKPDS : 23) Brit Med J. 1998;316:823–828. doi: 10.1136/bmj.316.7134.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with Type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insluin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 22.The Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in Type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The ADVANCE Colloborative Group Intensive blood glucose control and vascular outcomes in patients with Type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 24.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 25.Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 26.Otsuka A, Azuma K, Iesaki T, Sato F, Hirose T, Shimizu T, Tanaka Y, Daida H, Kawamori R, Watada H. Temporary hyperglycaemia provokes monocyte adhesion to endothelial cells in rat thoracic aorta. Diabetologia. 2005;48:2667–2674. doi: 10.1007/s00125-005-0005-6. [DOI] [PubMed] [Google Scholar]
- 27.Piga R, Naito Y, Kokura S, Handa O, Yoshikawa T. Short-term high glucose exposure induces monocyte-endothelial cells adhesion and transmigration by increasing VCAM-1 and MCP-1 expression in human aortic endothelial cells. Atherosclerosis. 2007;193:328–334. doi: 10.1016/j.atherosclerosis.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 28.Yan SD, Schmidt AM, Anderson GM, Zhang JH, Brett J, Zou YS, Pinsky D, Stern D. Enhanced Cellular Oxidant Stress by the Interaction of Advanced Glycation End-Products with Their Receptors Binding-Proteins. J Biol Chem. 1994;269:9889–9897. [PubMed] [Google Scholar]
- 29.Camici GG, Schiavoni M, Francia P, Bachschmid M, Martin-Padura I, Hersberger M, Tanner FC, Pelicci P, Volpe M, Anversa P, Luscher TF, Cosentino F. Genetic deletion of p66(Shc) adaptor protein prevents hyperglycemia-induced endothelial dysfunction and oxidative stress. Proc Natl Acad of Sci. 2007;104:5217–5222. doi: 10.1073/pnas.0609656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quagliaro L, Piconi L, Assaloni R, Da Ros R, Maier A, Zuodar G, Ceriello A. Intermittent high glucose enhances ICAM-1, VCAM-1 and E-selectin expression in human umbilical vein endothelial cells in culture: The distinct role of protein kinase C and mitochondrial. superoxide production. Atherosclerosis. 2005;183:259–267. doi: 10.1016/j.atherosclerosis.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 31.Dasu MR, Devaraj S, Jialal I. High glucose induces IL-1 beta expression in human monocytes: mechanistic insights. Am J Physiol Endocrinol Metab. 2007;293:E337–E346. doi: 10.1152/ajpendo.00718.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Devaraj S, Dasu MR, Rockwood J, Winter W, Griffen SC, Jialal I. Increased TLR2 and TLR4 expression in monocytes from patients with type 1 diabetes: further evidence of a proinflammatory state. J Clin Endocrinol Metab. 2007;93:578–583. doi: 10.1210/jc.2007-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devaraj S, Glaser N, Griffen S, Wang-Polagruto J, Miguelino E, Jialal I. Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes. 2006;55:774–779. doi: 10.2337/diabetes.55.03.06.db05-1417. [DOI] [PubMed] [Google Scholar]
- 34.Venugopal SK, Devaraj S, Yang T, Jialal I. Alpha-tocopherol decreases superoxide anion release in human monocytes under hyperglycemic conditions via inhibition of protein kinase C-alpha. Diabetes. 2002;51:3049–3054. doi: 10.2337/diabetes.51.10.3049. [DOI] [PubMed] [Google Scholar]
- 35.Ohgami N, Miyazaki A, Sakai M, Kuniyasu A, Nakayama H, Horiuchi S. Advanced glycation end products (AGE) inhibits a new crossroad of AGE to cholesterol metabolism. J Atheroscler Thromb. 2003;10:1–6. doi: 10.5551/jat.10.1. [DOI] [PubMed] [Google Scholar]
- 36.Carmody RJ, Chen YH. Nuclear Factor-kappa B: Activation and regulation during toll-like receptor signaling. Cell Mol Immunol. 2007;4:31–41. [PubMed] [Google Scholar]
- 37.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 38.Heickendorff L, Ledet T, Rasmussen LM. Glycosaminoglycans in the Human Aorta in Diabetes-Mellitus - A Study of Tunica Media from Areas with and Without Atherosclerotic Plaque. Diabetologia. 1994;37:286–292. doi: 10.1007/BF00398056. [DOI] [PubMed] [Google Scholar]
- 39.Vikramadithyan RK, Hu Y, Noh HL, Liang CP, Hallam K, Tall AR, Ramasamy R, Goldberg IJ. Human aldose reductase expression accelerates diabetic atherosclerosis in transgenic mice. J Clin Invest. 2005;115:2434–2443. doi: 10.1172/JCI24819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forbes JM, Yee LT, Thallas V, Lassila M, Candido R, Jandeleit-Dahm KA, Thomas MC, Burns WC, Deemer EK, Thorpe SR, Cooper ME, Allen TJ. Advanced glycation end product interventions reduce diabetes-accelerated atherosclerosis. Diabetes. 2004;53:1813–1823. doi: 10.2337/diabetes.53.7.1813. [DOI] [PubMed] [Google Scholar]
- 41.Bucciarelli LG, Wendt T, Qu W, Lu Y, Lalla E, Rong LL, Goova MT, Moser B, Kislinger T, Lee DC, Kashyap Y, Stern DM, Schmidt AM. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E-null mice. Circulation. 2002;106:2827–2835. doi: 10.1161/01.cir.0000039325.03698.36. [DOI] [PubMed] [Google Scholar]
- 42.Soro-Paavonen A, Watson AM, Li J, Paavonen K, Koitka A, Calkin AC, Barit D, Coughlan MT, Drew BG, Lancaster GI, Thomas M, Forbes JM, Nawroth PP, Bierhaus A, Cooper ME, Jandeleit-Dahm KA. Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes. 2008;57:2461–2469. doi: 10.2337/db07-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernard S, Serusclat A, Targe F, Charriere S, Roth O, Beaune J, Berthezene F, Moulin P. Incremental predictive value of carotid ultrasonography in the assessment of coronary risk in a cohort of asymptomatic type 2 diabetic subjects. Diabetes Care. 2005;28:1158–1162. doi: 10.2337/diacare.28.5.1158. [DOI] [PubMed] [Google Scholar]
- 44.Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol. 2004;43:1663–1669. doi: 10.1016/j.jacc.2003.09.068. [DOI] [PubMed] [Google Scholar]
- 45.Wagenknecht LE, Zaccaro D, Espeland MA, Karter AJ, O’Leary DH, Haffner SM. Diabetes and progression of carotid atherosclerosis: the insulin resistance atherosclerosis study. Arterioscler Thromb Vasc Biol. 2003;23:1035–1041. doi: 10.1161/01.ATV.0000072273.67342.6D. [DOI] [PubMed] [Google Scholar]
- 46.Mazzone T, Meyer PM, Kondos GT, Davidson MH, Feinstein SB, D’Agostino RB, Perez A, Haffner SM. Relationship of traditional and non-traditional cardiovascular risk factors to coronary artery calcium in Type 2 diabetes. Diabetes. 2007;56:849–855. doi: 10.2337/db06-0935. [DOI] [PubMed] [Google Scholar]
- 47.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes therapy and carotid intima-media thickness in Type 1 diabetes mellitus. N Engl J Med. 2003;348:2294–2303. doi: 10.1056/NEJMoa022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cleary PA, Orchard TJ, Genuth S, Wong ND, Detrano R, Backlund JY, Zinman B, Jacobson A, Sun W, Lachin JM, Nathan DM. The effect of intensive glycemic treatment on coronary artery calcification in type 1 diabetic participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes. 2006;55:3556–3565. doi: 10.2337/db06-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Larsen JL, Colling CW, Ratanasuwan T, Burkman TW, Lynch TG, Erickson JM, Lyden ER, Lane JT, Mack-Shipman LR. Pancreas transplantation improves vascular disease in patients with type 1 diabetes. Diabetes Care. 2004;27:1706–1711. doi: 10.2337/diacare.27.7.1706. [DOI] [PubMed] [Google Scholar]
- 50.Mazzone T, Meyer PM, Feinstein SB, Davidson MH, Kondos GT, D’Agostino RB, Perez A, Provost JC, Haffner SM. Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes - A randomized trial. JAMA. 2006;296:2572–2581. doi: 10.1001/jama.296.21.joc60158. [DOI] [PubMed] [Google Scholar]
- 51.Esposito K, Giugliano D, Nappo F, Marfella R. Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation. 2004;110:214–219. doi: 10.1161/01.CIR.0000134501.57864.66. [DOI] [PubMed] [Google Scholar]
- 52).Matsumoto K, Sera Y, Abe Y, Tominaga T, Yeki Y, Miyake S. Metformin attenuates progression of carotid arterial wall thickness in patients with type 2 diabetes. Diabetes Res Clin Pract. 2004;64:225–228. doi: 10.1016/j.diabres.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 53.Kim SH, Lee SJ, Kang ES, Kang S, Hur KY, Lee HJ, Ahn CW, Cha BS, Yoo JS, Lee HC. Effects of lifestyle modification on metabolic parameters and carotid intima-media thickness in patients with type 2 diabetes mellitus. Metabolism. 2006;55:1053–1059. doi: 10.1016/j.metabol.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 54.Yokoyama H, Katakami N, Yamasaki Y. Recent advances of intervention to inhibit progression of carotid intima-media thickness in patients with type 2 diabetes mellitus. Stroke. 2006;37:2420–2427. doi: 10.1161/01.STR.0000236632.58323.cd. [DOI] [PubMed] [Google Scholar]
- 55.Ray KK, Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Preiss D, erqou S, Sattar N. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373:1765–1772. doi: 10.1016/S0140-6736(09)60697-8. [DOI] [PubMed] [Google Scholar]
- 56.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 57.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefebvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Koranyi L, Laakso M, Mokan M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 58.Kelly TN, Bazzano LA, Fonseca VA, Thethi TK, Reynolds K, He J. Systematic review: glucose control and cardiovascular disease in type 2 diabetes. Ann Intern Med. 2009;151:394–403. doi: 10.7326/0003-4819-151-6-200909150-00137. [DOI] [PubMed] [Google Scholar]
- 59.Turnbull FM, Abraira C, Anderson RJ, Byington RP, Chalmers JP, Duckworth WC, Evans GW, Gerstein HC, Holman RR, Moritz TE, Neal BC, Ninomiya T, Patel AA, Paul SK, Travert F, Woodward M. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;52:2288–2298. doi: 10.1007/s00125-009-1470-0. [DOI] [PubMed] [Google Scholar]
- 60.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 61.Reaven PD, Moritz TE, Schwenke DC, Anderson RJ, Criqui M, Detrano R, Emanuele N, Kayshap M, Marks J, Mudaliar S, Harsha RR, Shah JH, Goldman S, Reda DJ, McCarren M, Abraira C, Duckworth W. Intensive glucose-lowering therapy reduces cardiovascular disease events in veterans affairs diabetes trial participants with lower calcified coronary atherosclerosis. Diabetes. 2009;58:2642–2648. doi: 10.2337/db09-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 63.Brownlee M, Hirsch IB. Glycemic variability: a hemoglobin A1c-independent risk factor for diabetic complications. JAMA. 2006;295:1707–1708. doi: 10.1001/jama.295.14.1707. [DOI] [PubMed] [Google Scholar]
- 64.Buckley DI, Fu R, Freeman M, Rogers K, Helfand M. C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151:483–495. doi: 10.7326/0003-4819-151-7-200910060-00009. [DOI] [PubMed] [Google Scholar]
- 65.Danesh J, Pepys MB. C-reactive protein and coronary disease: is there a causal link? Circulation. 2009;120:2036–2039. doi: 10.1161/CIRCULATIONAHA.109.907212. [DOI] [PubMed] [Google Scholar]
- 66.Koike T, Kitajima S, Yu Y, Nishijima K, Zhang J, Ozaki Y, Morimoto M, Watanabe T, Bhakdi S, Asada Y, Chen YE, Fan J. Human C-reactive protein does not promote atherosclerosis in transgenic rabbits. Circulation. 2009;120:2088–2094. doi: 10.1161/CIRCULATIONAHA.109.872796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Calvin AD, Aggarwal NR, Murad MH, Shi Q, Elamin MB, Geske JB, Fernandez-Balsells MM, Albuquerque FN, Lampropulos JF, Erwin PJ, Smith SA, Montori VM. Aspirin for the primary prevention of cardiovascular events: a systematic review and meta-analysis comparing patients with and without diabetes. Diabetes Care. 2009;32:2300–2306. doi: 10.2337/dc09-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Antithrombotic Trialists’ Collaboration Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ogawa H, Nakayama M, Morimoto T, Uemura S, Kanauchi M, Doi N, Jinnouchi H, Sugiyama S, Saito Y. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2008;300:2134–2141. doi: 10.1001/jama.2008.623. [DOI] [PubMed] [Google Scholar]
- 70.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 71.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 72.Blood Pressure Lowering Treatment Trialists’ Collaboration Effects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials. Arch Intern Med. 2005;165:1410–1419. doi: 10.1001/archinte.165.12.1410. [DOI] [PubMed] [Google Scholar]
- 73.Vijan S, Hayward RA. Treatment of hypertension in type 2 diabetes mellitus: blood pressure goals, choice of agents, and setting priorities in diabetes care. Ann Intern Med. 2003;138:593–602. doi: 10.7326/0003-4819-138-7-200304010-00018. [DOI] [PubMed] [Google Scholar]
- 74.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 75.Gaede P, Pedersen O. Intensive integrated therapy of type 2 diabetes: implications for long-term prognosis. Diabetes. 2004;53(Suppl 3):S39–S47. doi: 10.2337/diabetes.53.suppl_3.s39. [DOI] [PubMed] [Google Scholar]
- 76.Simpson SH, Majumdar SR, Tsuyuki RT, Eurich DT, Johnson JA. Dose-response relation between sulfonylurea drugs and mortality in type 2 diabetes mellitus: a population-based cohort study. CMAJ. 2006;174:169–174. doi: 10.1503/cmaj.050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bonds DE, Miller ME, Bergenstal RM, Buse JB, Byington RP, Cutler JA, Dudl RJ, Ismail-Beigi F, Kimel AR, Hoogwerf B, Horowitz KR, Savage PJ, Seaquist ER, Simmons DL, Sivitz WI, Speril-Hillen JM, Sweeney ME. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340:b4909. doi: 10.1136/bmj.b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mannucci E, Monami M, Lamanna C, Gori F, Marchionni N. Prevention of cardiovascular disease through glycemic control in type 2 diabetes: a meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis. 2009;19:604–612. doi: 10.1016/j.numecd.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 79.Greenfield S, Billimek J, Pellegrini F, Franciosi M, De BG, Nicolucci A, Kaplan SH. Comorbidity affects the relationship between glycemic control and cardiovascular outcomes in diabetes: a cohort study. Ann Intern Med. 2009;151:854–860. doi: 10.7326/0003-4819-151-12-200912150-00005. [DOI] [PubMed] [Google Scholar]