Treatment with a combination of interferon-α and arsenic trioxide ablates leukemia-initiating activity before reducing primary tumor bulk in a murine model of adult T cell leukemia.
Abstract
Chronic HTLV-I (human T cell lymphotropic virus type I) infection may cause adult T cell leukemia/lymphoma (ATL), a disease with dismal long-term prognosis. The HTLV-I transactivator, Tax, initiates ATL in transgenic mice. In this study, we demonstrate that an As2O3 and IFN-α combination, known to trigger Tax proteolysis, cures Tax-driven ATL in mice. Unexpectedly, this combination therapy abrogated initial leukemia engraftment into secondary recipients, whereas the primary tumor bulk still grew in the primary hosts, only to ultimately abate later on. This loss of initial transplantability required proteasome function. A similar regimen recently yielded unprecedented disease control in human ATL. Our demonstration that this drug combination targeting Tax stability abrogates tumor cell immortality but not short-term growth may foretell a favorable long-term efficiency of this regimen in patients.
Adult T cell leukemia/lymphoma (ATL) is one of the rare human cancers initiated by a transforming retrovirus (Matsuoka and Jeang, 2007). The viral transactivator protein Tax was proposed to be an oncogene in ATL, but the fact that Tax protein expression is undetectable in circulating ATL cells has led to significant controversies (Asquith et al., 2000). However, Tax expression in T cells of transgenic mice or in human CD34+ stem cells induces leukemias with striking ATL-like features, including constitutive NF-κB activation, formally demonstrating that Tax initiates ATL (Portis et al., 2001; Hasegawa et al., 2006; Banerjee et al., 2010). Whether continuous Tax expression is required for maintenance of the transformed phenotype is not known. Antiretroviral agents such as zidovudine and IFN-α have shown some efficacy in ATL patients (Gill et al., 1995; Hermine et al., 1995), with complete clinical and hematological responses, although maintenance therapy is required to avoid relapses. Thus, even though the disease may be controlled, it is not cured by this combination. Indeed, human ATL still carries a dismal long-term prognosis (Bazarbachi et al., 2004). Ex vivo, in HTLV-I (human T cell lymphotropic virus type I)–infected human ATL cell-lines, As2O3 shuts off constitutive NF-κB activation and potentiates the apoptotic effects of IFN-α, at least in part through Tax proteasomal degradation (Bazarbachi et al., 1999; El-Sabban et al., 2000; Nasr et al., 2003). Very recently, a regimen combining As2O3, IFN-α, and zidovudine has resulted in unprecedented disease control in de novo patients with the chronic form of the disease (Kchour et al., 2009). Importantly, this regimen does not induce rapid tumor regression and massive cell death, questioning the cellular mechanisms involved. Leukemia-initiating cells (LICs; Dick, 2008) have the unique property to allow full leukemia development and to self-renew. These rare cells may be quantified by limiting dilutions in secondary transplants, and their therapeutic targeting remains a challenge. Yet, in some mouse or human tumor models, most cells appear to have tumor-initiating activity, questioning the hierarchical model established in myeloid leukemias (Kelly et al., 2007; Quintana et al., 2008). In acute promyelocytic leukemia (APL), degradation of the PML/retinoic acid receptor α (RARA) oncogene by the combined effect of retinoic acid and As2O3 induces very rapid LIC clearance and definitive cures (Nasr et al., 2008; Hu et al., 2009; Kogan, 2009). Yet, this model is complicated by the fact that differentiation concomitantly clears the tumor bulk. Rapid treatment-induced clearance of tumor-initiating activity that does not initially affect the tumor bulk has never been reported. We performed preclinical experiments in a murine transplantation model of ATL that demonstrate that an IFN-α/As2O3 combination abrogates immortal growth and triggers delayed apoptosis, resulting in the cure of ATL recipients. Paradoxically, this drug combination only marginally affects short-term cell proliferation or survival. These results provide a biological basis accounting for the clinical success of IFN-α/As2O3/zidovudine therapy in ATL patients.
RESULTS AND DISCUSSION
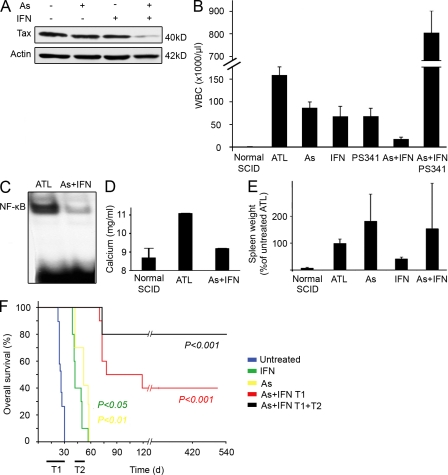
To explore the in vivo efficacy of IFN-α/As2O3, we established ATL transplantation models in which 106 ATL spleen cells from one of three independent Tax transgenic mice (Hasegawa et al., 2006) were inoculated into SCID mice. All recipients rapidly developed massive hyperleukocytosis, splenomegaly, hypercalcemia, and multiple organ invasion, identical to what is observed in transgenics, and they died within 1 mo (unpublished data). These ATL could be serially passaged for years, with very constant time to death in all recipients, pointing to the remarkable stability of this murine ATL model. We first questioned whether murine ATL cells responded ex vivo to the IFN-α/As2O3 combination in the same manner as human ATL cell lines, in which this combination degrades Tax (Fig. 1 A; Bazarbachi et al., 1999; El-Sabban et al., 2000; Nasr et al., 2003). IFN-α or As2O3 triggered apoptosis in <20% Tax transgenic cells. Their combination killed >80% of cells after ex vivo overnight exposure, whereas normal murine lymphocytes were marginally affected (Fig. S1 A). Tax degradation by the proteasome could not be demonstrated in these ATL cells because the baseline level of protein was undetectable by Western blot analysis (Fig. S1 B; Hasegawa et al., 2006), exactly as in primary human ATLs (Matsuoka and Jeang, 2007). Indeed, Tax messenger RNA levels were very similar in primary mouse and human ATL cells, but 1,000-fold higher in HuT-102 cells, in which Tax protein was easily detected (Fig. 1 A and Fig. S1 B).
Figure 1.
A treatment combining IFN-α and As2O3 can cure murine ATL. (A) Western blot analysis of Tax and actin protein expression in human ATL-derived HuT-102 cells after 48 h treatment with As2O3 (As), IFN-α, or a combination thereof. (B–D) Effect, at day 18 after inoculation of SCID mice with Tax transgenic splenocytes, of 3-d IFN-α + As2O3 treatment on circulating leukemia cell numbers (n = 3 for each condition; B). Normal SCID mice were not injected with Tax transgenic splenocytes. NF-κB activation (C) and calcemia (n = 4; D) are shown. ATL denotes untreated animals, and PS-341 denotes the proteasome inhibitor. (E) Effect, at day 12 after inoculation of SCID mice with Tax transgenic splenocytes, of 6-d IFN-α + As2O3 treatment on spleen weight (n = 3). Normal SCID mice were not injected with Tax transgenic splenocytes. (B, D, and E) Error bars show mean ± SD. (F) Kaplan–Meier analysis of overall survival curves of the murine ATL transplant recipients (n = 10 for each condition). P-values are indicated. T1 denotes treatment from day 6 to 30; T2 indicates additional treatment from day 42 to 54. This experiment was reproduced three times with similar results.
Mice with well-established leukemias, which had been inoculated 18 d earlier with ATL cells derived from any one of the three independent transgenic founders, were then treated for 3 d with IFN-α, As2O3, or both (Fig. 1). Such short IFN-α/As2O3 treatment induced a sharp decrease in the number of circulating ATL cells, which, interestingly, was no longer noted when a proteasome inhibitor (PS-341) was concomitantly administered (Fig. 1 B and Fig. S1 C). In contrast, the treatment only marginally affected the spleen weight (Fig. S1 D) and modestly increased baseline apoptosis levels (see Fig. 3 B). Yet, complete reversal of NF-κB activation was observed in ATL spleen cells (Fig. 1 C), as previously observed in human ATL cell lines (El-Sabban et al., 2000), whereas calcium plasma levels returned to normal (Fig. 1 D). Even 6-d IFN-α/As2O3 treatment did not significantly decrease the spleen weight (Fig. 1 E and Fig. S1 E). Thus, in contrast to the ex vivo setting, murine ATLs kept on growing under treatment and did not undergo massive and immediate apoptosis.
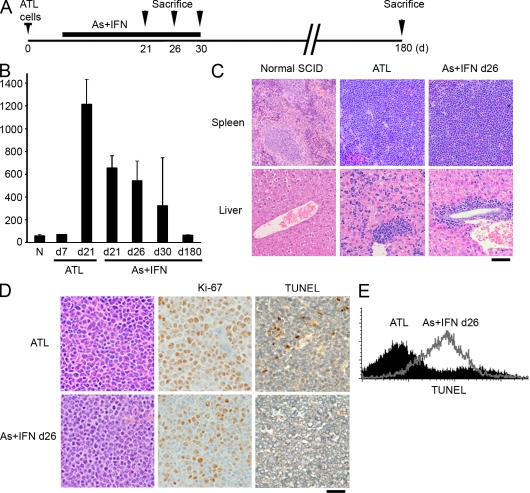
To investigate any survival benefit, mice were treated earlier, from day 6 to 30 after inoculation of ATL cells. A limited advantage was seen in mice receiving IFN-α or As2O3 alone, but a cure (>500-d survival) was noted only among those receiving IFN-α/As2O3, and this occurred more frequently when a second course of therapy was administered (T1 + T2; Fig. 1 F). Protocols in which mice were treated only for 5 d per wk never resulted in a cure (unpublished data), pointing to the importance of continuous drug exposure. To follow the fate of ATL cells in the course of this curative therapy, we sacrificed mice at different time points after treatment initiation (Fig. 2 A). Again, at all time points, treatment only moderately affected the spleen weight (Fig. 2 B) but always resulted in diminished circulating ATL cells (not depicted). Pathology examination demonstrated persistent tissue invasion and proliferation even at day 26. Although the frequency of mitoses in leukemic infiltrates of the spleen and liver decreased (Fig. 2, C and D; and Fig. S2 A), Ki-67 expression was maintained. Even though IFN-α/As2O3 treatment did not clear the disease, it nevertheless significantly reduced micrometastases within sinusoids of the liver parenchyma (Fig. S2 B). At day 30, when treatment was discontinued, half of the examined mice showed residual actively proliferating leukemic cell invasion, whereas the others exhibited large zones of necrosis with only a few remaining tumor cells and regrowth of normal tissue (not depicted), likely distinguishing cured mice from those that would ultimately die (Fig. 1 F). Importantly, blood cell counts remained highly abnormal when IFN-α/As2O3 therapy was discontinued in ultimately cured mice, even in those with rare remaining tumor cells in histological sections (Fig. S2 C). However, at day 180, surviving animals were indistinguishable from noninoculated controls (unpublished data). NF-κB shutoff would be expected to sensitize cells to apoptosis (Portis et al., 2001). Some terminal deoxynucleotidyl transferase–mediated nick end labeling (TUNEL) labeling was noted at days 21 and 26 in vivo (Fig. 2 D and Fig. S2 A) but was massively increased at day 26 ex vivo (Fig. 2 E). Note that TUNEL positivity was always more pronounced in ex vivo than in in vivo labeled cells, possibly because lack of cell to cell contacts precipitates apoptosis. Thus, IFN-α/As2O3 elicits delayed apoptosis, which is most likely responsible for the ultimate ATL eradication. Collectively, the IFN-α/As2O3 combination cures Tax-driven ATLs, but the tumor grows for weeks under therapy, and it is fully cleared only after treatment discontinuation.
Figure 2.
The IFN-α + As2O3 combination elicits a moderate decrease of organ infiltration. (A) Experimental design: mice were treated from day 6 up to day 30 after inoculation of ATL cells and sacrificed at different time points after treatment initiation. (B) Spleen weight at various time points during treatment (N: n = 3; ATL: d7, n = 1; and d21, n = 5; and IFN-α + As2O3 [As]: d21, n = 8; d26, n = 6; d30, n = 6; and d180, n = 2). Error bars show mean ± SD. (C) Histological analysis of the spleen and liver of untreated recipients or at day 26 of IFN-α + As2O3 therapy. (D) Ki-67 and TUNEL labeling of spleen leukemia cell infiltrates of untreated recipients or at day 26 of IFN-α + As2O3 therapy. (E) TUNEL analysis of ATL cells infiltrating the spleen in untreated recipients or after 26 d of IFN-α + As2O3 therapy. Bars: (C) 150 µm; (D) 50 µm.
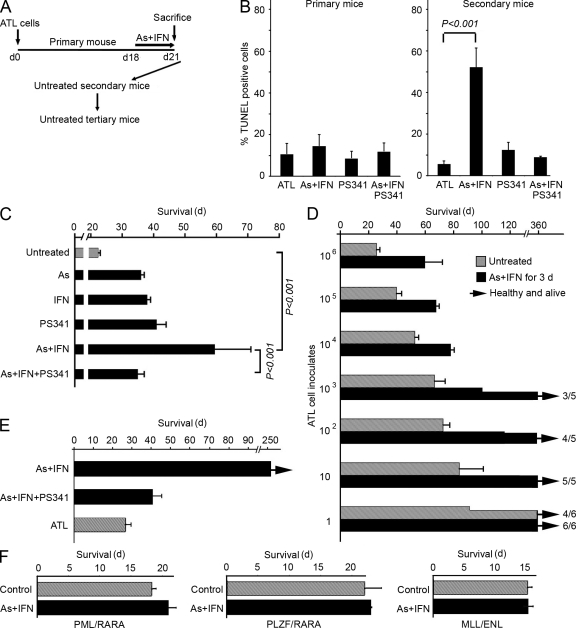
Such a paradoxical pattern of response to therapy in an immunodeficient animal clearly sets this combination aside from most classical cytotoxic anticancer regimens. Thus, we performed serial transplantation experiments in which similar amounts of leukemic spleen cell inoculates from mice untreated or treated for only 3 d with IFN-α and/or As2O3 were injected into secondary recipients, which were not subsequently treated (Fig. 3 A). Even when derived from IFN-α/As2O3-treated mice, cells used for inoculations were all Tax positive (Fig. S3 A) and were still cycling (Fig. S3 B), and their ex vivo TUNEL labeling was only very modestly increased (Fig. 3 B and Fig. S3 D). Yet, survival of secondary recipients inoculated with ATL cells from IFN-α/As2O3-treated primary recipients was dramatically increased with the three independent murine ATLs examined (Fig. 3 C and Fig. S3 C). Comparing the survival of secondary recipients of limiting-dilution inoculates from treated or untreated mice established that IFN-α/As2O3 dramatically decreased the ability of ATL cells to initiate leukemia (Fig. 3 D). Indeed, between 1 and 3 ATL cells from untreated donors allowed ATL development, whereas around 1,000 were required when donors had received IFN-α/As2O3. Critically, no ATL ever developed in 10 tertiary recipients inoculated with ATL cells from secondary mice inoculated with cells from IFN-α/As2O3-treated primary recipients (Fig. 3 E and not depicted).
Figure 3.
Loss of leukemia-initiating activity accounts for the therapeutic effect of the IFN-α + As2O3 combination. (A) Experimental design: serial transplantation of splenocytes from primary mice left untreated or after 3-d IFN-α + As2O3 treatment into secondary and tertiary recipients, which were not subsequently treated. (B) TUNEL staining of splenocytes from primary mice left untreated (ATL) or after 3-d IFN-α + As2O3 treatment (left) and of splenocytes from secondary recipients of ATL cells from untreated (ATL) or treated primary mice (n = 6 for control or IFN-α + As2O3 [As]; n = 3 for PS-341 or IFN-α + As2O3 + PS-341); the p-value is indicated. Secondary recipient cells were analyzed at the time of death of the mice. Secondary mice were not treated. (C) Effect of indicated 3-d treatment of primary donor mice on the survival of secondary recipients of 106 spleen ATL cells (n = 9 for each condition). This experiment was reproduced twice with similar results, as well as with two other independent primary ATLs (Fig. S3 C). (D) Survival of secondary recipient mice inoculated with varying initial amounts of ATL cells from primary mice either untreated or treated with IFN-α + As2O3 for 3 d (n = 10 for each condition). (E) Effect of 3-d treatment of primary mice on the survival of tertiary transplants (n = 10 for each condition). Secondary recipients were inoculated with 106 spleen ATL cells from primary mice. Tertiary recipients were inoculated with 106 spleen ATL cells from untreated secondary recipients. Tertiary mice were not treated. (F) Effect of 3-d treatment of primary mice on the survival of secondary recipients of leukemic cells from PML/RARA, PLZF/RARA APLs, or MLL/ENL leukemia (n = 3 for each condition). (B–F) Error bars show mean ± SD.
When moribund secondary recipients were sacrificed, tumor loads (as determined by spleen weight) were slightly decreased in those that had received inoculates from IFN-α/As2O3-treated primary recipients (unpublished data). Critically, massive apoptosis was constantly noted in all six untreated leukemic secondary recipients of ATL cells from three independent IFN-α/As2O3-treated primary recipients but not in any of the six secondary recipients from untreated donors (Fig. 3 B). Cells derived from infiltrated spleen of these secondary recipients were bona fide ATL cells, as assessed by the fact that they all carried the Tax transgene (Fig. S3 F) and had typical morphology (Fig. S3 G and not depicted). Of note, when proteasome activity was inhibited by PS-341 during the primary recipients’ 3-d IFN-α/As2O3 therapy, enhanced survival of secondary recipients was curtailed (Fig. 3 C and Fig. S3 C). PS-341 also reversed IFN-α/As2O3-induced enhanced apoptosis of ATL cells in the three secondary recipients tested (Fig. 3 B). Finally, all 10 tertiary mice inoculated with ATL cells derived from PS-341/IFN-α/As2O3-treated primary donors rapidly died of ATL (Fig. 3 E), in contrast to those with active proteasome. Collectively, a brief exposure to IFN-α/As2O3 elicits proteasome-mediated and reversible inheritable cellular changes that preclude long-term tumor propagation but does not affect short-term survival or growth. These cellular changes ultimately result in delayed apoptosis and ATL exhaustion. Such a heritable change could reflect genetic alterations, epigenetic modifications, or some other causes that are yet to be investigated.
As control, we evaluated the effect of the IFN-α/As2O3 combination in other leukemias. We found that, in secondary transplant experiments identical to the ones previously performed with ATL cells, PML/RARA-, promyelocytic leukemia zinc finger protein (PLZF)/RARA- or MLL/ENL-transformed cells were marginally (PML/RARA) or not (PLZF/RARA and MLL/ENL) affected by this treatment (Fig. 3 F). This demonstrates that the IFN-α/As2O3 combination does not have a general deleterious activity on LIC self-renewal outside of the context of ATL.
That IFN-α/As2O3 targets immortal growth in Tax-driven murine ATLs could augur a favorable long-term outcome for As2O3/IFN-α/zidovudine-treated chronic ATL patients (Kchour et al., 2009). In that sense, although these patients received a suboptimal 5-d-per-wk treatment, three out of six are still in continuous complete remission 7–18 mo after discontinuing maintenance therapy, whereas five ATL patients previously treated with IFN-α/zidovudine alone all relapsed, on average, in less than 5 mo after treatment discontinuation (Table S1). Similarly, in an ongoing trial of ATL lymphoma patients, all six assessable patients are still in complete remission with 3–84-mo follow up after As2O3/IFN-α consolidation therapy after initial chemotherapy, which is a distinctly uncommon finding (unpublished data). Although preliminary, these observations suggest nonetheless that in patients, As2O3/IFN-α also efficiently targets ATL immortal growth.
To our knowledge, the data presented in this study are the first to decipher the uncoupling between treatment-induced loss of transplantability and continued proliferation of the tumor bulk, which ultimately results in leukemia eradication. Tax-driven murine ATL resembles Myc-driven lymphomas or human melanomas, as most cells can initiate tumor formation (Kelly et al., 2007; Quintana et al., 2008). This suggests that the IFN-α/As2O3 combination initiates irreversible changes that preclude immortal growth but not short-term proliferation. These changes may restore unidentified checkpoints (such as P53), possibly through epigenetic modifications. Such checkpoint activation triggers delayed apoptosis, as shown both by time course analysis (Fig. 2 E) and transplantation experiments (Fig. 3, C and D). That this delayed apoptosis is fully proteasome dependent strongly suggests the contribution of Tax degradation in the process, hence the specificity of IFN-α/As2O3 for ATL cells ex vivo (Bazarbachi et al., 1999; El-Sabban et al., 2000; Nasr et al., 2003) or in vivo (Fig. 3 F). Unfortunately, technical issues of sensitivity preclude the formal demonstration of Tax degradation in vivo and thus definitive conclusion as to the mechanism involved in the effect of therapy. Nevertheless, our data point to the possibility that, in the same manner as in the APL model (Nasr et al., 2008), self-renewal and immortalization require the continuous expression of the driving oncogene, which endows tumor cells with stem cell features (Downing, 2003; Faber and Armstrong, 2007), and the inactivation of which by therapy-induced protein degradation may be a generally applicable strategy.
PML is an IFN-α–induced protein (Stadler et al., 1995) targeted to PML nuclear bodies in an As2O3-dependent manner (Zhu et al., 1997). PML was implicated in normal or leukemia stem cell self-renewal (Ito et al., 2008). Through PML degradation, As2O3 may thus directly affect LIC self-renewal (Ito et al., 2008). Yet, although As2O3 did reduce ATL ability to transplant (Fig. 3 C), it never cleared the disease (Fig. 1 F), possibly because of insufficient exposure. The IFN-α/As2O3 combination sequesters PML partner proteins, including Tax, within PML nuclear bodies (Lallemand-Breitenbach et al., 2001, 2008; unpublished data). Like PML, Tax is both sumoylated and ubiquitinated (Nasr et al., 2006; Lallemand-Breitenbach et al., 2008) and might thus be degraded by a PML-dependent mechanism. In addition, IFN-α forces stem cells into the cell cycle (Essers et al., 2009), possibly rendering them more susceptible to As2O3.
Treatment of multi-relapsed, IFN-α–resistant, ATL patients with an IFN-α/As2O3 regimen has shown some clinical efficacy (Hermine et al., 2004; Ishitsuka et al., 2007). Our latest trial, in which de novo ATL patients were treated with an As2O3/IFN-α/zidovudine combination, using suboptimal 5-d-per-wk As2O3 administration, has nevertheless yielded unprecedented results (Kchour et al., 2009). Interestingly, in these patients, the kinetics of disease clearance were significantly slower than tumor debulking with cytotoxic drugs. Only partial responses were observed on day 30, and complete remissions slowly unfurled over the next month, whereas three of six patients have remained off treatment for months and are still disease free. These clinical observations are fully in line with the delayed apoptosis observed in ATL mice. That IFN-α/As2O3 abrogates immortal growth in Tax-driven murine ATLs, together with the favorable current evolution of many As2O3/IFN-α/zidovudine-treated ATL patients, raises the prospect of a possible cure of ATL.
MATERIALS AND METHODS
Mice.
We used the ATL mouse model of Hasegawa et al. (2006). To test the effect of different targeted therapies, we established a rapid and reproducible model of disease by direct intraperitoneal transfer of 106 spleen cells from Tax transgenic mice into SCID mice (Charles River). Tax expression was detectable only at very low levels by quantitative PCR. PML/RARA and PLZF/RARA APLs were described previously and propagated exactly as before (Nasr et al., 2008). MLL/ENL leukemias were derived from retrovirus infection of primary progenitors from 5-fluorouracyl–treated mice (Lavau et al., 2000). All murine protocols were approved by the Institutional Animal Care and Utilization Committee of the American University of Beirut and the Institut Universitaire d’Hématologie (Université Paris Diderot). All animals were housed in specific pathogen-free facilities. Animals were sacrificed by cervical dislocation after deep anesthesia with isoflurane.
Treatments.
As2O3 was obtained from Sigma-Aldrich, and recombinant human IFN-α (Roferon) was obtained from Roche. The proteasome inhibitor PS-341 or bortezomib (Velcade) was purchased from Millennium Pharmaceuticals. For in vivo experiments, mice received As2O3 (5 µg/g/d) intraperitoneally, IFN-α (106 IU/d) subcutaneously, and bortezomib (0.5 mg/kg/d) through mini-osmotic pumps (Alzet; Charles River). These doses are comparable with those used in other mouse models and predicted to yield plasma concentrations similar to those noted in patients (Lallemand-Breitenbach et al., 2008; Nasr et al., 2008; Kogan, 2009). None of the individual or combination treatment regimens were toxic in normal SCID mice (100% survival for >3 mo; n = 3 for each condition). In ex vivo experiments, malignant cells from Tax transgenic mice were treated in vitro with 1 µM As2O3, 1,000 IU IFN-α, or a combination of both for up to 48 h. Note that the regimen used in ATL mice did not comprise zidovudine because Tax is under the control of the Lck promoter, rather than being expressed from the retrovirus. Treatment protocols for patients with ATL were approved by the ethical committee of Mashhad University of Medical Sciences.
Histopathological and laboratory examination.
Tissues from either treated or untreated mice were fixed in neutral buffer formalin (Sigma-Aldrich), embedded in paraffin, sectioned, stained with hematoxylin and eosin, and examined by light microscopy. Ki-67 expression was assessed by immunohistochemistry using rat anti–mouse monoclonal antibodies. Peripheral blood smears were prepared using Giemsa staining. WBC counts and serum calcium levels were determined using routine clinical laboratory techniques. The TUNEL assay was performed on deparaffinized 5-µm sections treated with 20 µg ml−1 proteinase K for 15 min at room temperature. The number of TUNEL-positive cells was assessed by two pathologists in three different microscopic fields for each organ studied (liver, lung, and spleen) on a microscope (ProvisAX70; Olympus) with wide-field eye-piece number 26.5 at 600 magnification, which provides a field size of 0.244 mm2. Mitoses were counted with the same methods, and the results were expressed as the mean number of apoptotic cells or of mitoses per field at 600 magnification.
Cell cycle analysis and TUNEL assay on isolated splenocytes.
ATL cells infiltrating the spleen were rapidly harvested, filtered, washed twice with cold PBS, fixed in 100% ethanol at −20°C, and kept overnight at −20°C. Subsequently, cells were rinsed with PBS, treated with Tris-HCl buffer, pH 7.4, containing 1% DNase-free RNase A, and stained with propidium iodide (60 mg/ml final). The distribution of cell cycle phases with different DNA contents was determined using a FACScan flow cytometer (BD). In each sample, 10,000 nongated events were acquired. Analysis of cell cycle distribution (including apoptosis) was performed using CellQuest software (BD).
The TUNEL assay was also used to monitor apoptosis of ATL cells infiltrating the spleen. Splenocytes and ATL cells infiltrating the spleen were rapidly collected, filtered, and washed with cold PBS, and the assay was immediately performed according to the manufacturer’s (Roche) recommendations. Fluorescein-conjugated deoxy-UTP incorporated in nucleotide polymers was detected and quantified using flow cytometry. Approximately 10,000 cells per sample were acquired and analyzed using CellQuest software.
Quantitative PCR.
Spleens were harvested, and real-time PCR or RT-PCR using a LightCycler (Roche) was used to quantitate the absolute Tax DNA content or Tax messenger RNA expression using a LightCycler FastStart DNA Master kit or LightCycler RNA Master kit, respectively. We also used the LightCycler software version 4.05 (Roche). Tax primers SK43I (5′-CGGATACCCAGTCTACGTGT-3′) and SK44I (5′-GAGCCGATAACGCGTCCATCG-3′) and probes SK4I-FL (5′-CCCTACTGGCCACCTGTCCAGAGC-FL-3′) and SK4I-LC (5′-LC Red640-TCAGATCACCTGGGACCCCATCPH-3′) were designed in collaboration with TIB-MOLBIOL. We also used circulating leukemia cells from two acute ATL patients after informed consent. For electrophoretic mobility shift assay, nuclear extracts from splenocytes of treated and untreated mice were prepared as described previously (El-Sabban et al., 2000).
Statistical analysis.
Survival curves were calculated according to the method of Kaplan–Meier. Overall survival is defined as the time from injection of ATL cells to death from any cause. Mice that were still alive were censored at the time they were last known to be alive. Analyses were performed using SPSS software version 15.0 (SPSS). The p-value was obtained by log-rank statistical analysis.
Online supplemental material.
Fig. S1 shows the effect of short therapy with IFN-α and As2O3. Fig. S2 shows the effect of prolonged therapy with IFN-α and As2O3. Fig. S3 shows that loss of leukemia-initiating activity accounts for the therapeutic effect of the IFN-α + As2O3 combination. Table S1 lists the follow up of ATL patients in complete remission after stopping treatment. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20101095/DC1.
Acknowledgments
We thank C. Leboeuf (Hôpital Saint Louis, Paris, France) for pathology analyses. We warmly thank J.C. Gluckman for reviewing the manuscript.
This work was supported by the American University of Beirut Medical Practice Plan, the University Research Board, and the Lebanese National Council for Scientific Research. H. El Hajj and R. Nasr were supported by the Lady Tata Memorial Trust. H. Hasegawa was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science and grants from the Ministry of Health, Labour, and Welfare Japan and the Takeda Science Foundation. C. Nicot was supported by National Cancer Institute grants CA106258 and CA115398.
The authors declare no financial conflict of interest.
Footnotes
Abbreviations used:
- APL
- acute promyelocytic leukemia
- ATL
- adult T cell leukemia/lymphoma
- LIC
- leukemia-initiating cell
- PLZF
- promyelocytic leukemia zinc finger protein
- RARA
- retinoic acid receptor α
- TUNEL
- terminal deoxynucleotidyl transferase–mediated nick end labeling
References
- Asquith B., Hanon E., Taylor G.P., Bangham C.R. 2000. Is human T-cell lymphotropic virus type I really silent? Philos. Trans. R. Soc. Lond. B Biol. Sci. 355:1013–1019 10.1098/rstb.2000.0638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee P., Tripp A., Lairmore M.D., Crawford L., Sieburg M., Ramos J.C., Harrington W., Jr., Beilke M.A., Feuer G. 2010. Adult T-cell leukemia/lymphoma development in HTLV-1-infected humanized SCID mice. Blood. 115:2640–2648 10.1182/blood-2009-10-246959 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bazarbachi A., El-Sabban M.E., Nasr R., Quignon F., Awaraji C., Kersual J., Dianoux L., Zermati Y., Haidar J.H., Hermine O., de Thé H. 1999. Arsenic trioxide and interferon-alpha synergize to induce cell cycle arrest and apoptosis in human T-cell lymphotropic virus type I-transformed cells. Blood. 93:278–283 [PubMed] [Google Scholar]
- Bazarbachi A., Ghez D., Lepelletier Y., Nasr R., de Thé H., El-Sabban M.E., Hermine O. 2004. New therapeutic approaches for adult T-cell leukaemia. Lancet Oncol. 5:664–672 10.1016/S1470-2045(04)01608-0 [DOI] [PubMed] [Google Scholar]
- Dick J.E. 2008. Stem cell concepts renew cancer research. Blood. 112:4793–4807 10.1182/blood-2008-08-077941 [DOI] [PubMed] [Google Scholar]
- Downing J.R. 2003. The core-binding factor leukemias: lessons learned from murine models. Curr. Opin. Genet. Dev. 13:48–54 10.1016/S0959-437X(02)00018-7 [DOI] [PubMed] [Google Scholar]
- El-Sabban M.E., Nasr R., Dbaibo G., Hermine O., Abboushi N., Quignon F., Ameisen J.C., Bex F., de Thé H., Bazarbachi A. 2000. Arsenic-interferon-alpha-triggered apoptosis in HTLV-I transformed cells is associated with tax down-regulation and reversal of NF-kappa B activation. Blood. 96:2849–2855 [PubMed] [Google Scholar]
- Essers M.A., Offner S., Blanco-Bose W.E., Waibler Z., Kalinke U., Duchosal M.A., Trumpp A. 2009. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 458:904–908 10.1038/nature07815 [DOI] [PubMed] [Google Scholar]
- Faber J., Armstrong S.A. 2007. Mixed lineage leukemia translocations and a leukemia stem cell program. Cancer Res. 67:8425–8428 10.1158/0008-5472.CAN-07-0972 [DOI] [PubMed] [Google Scholar]
- Gill P.S., Harrington W., Jr., Kaplan M.H., Ribeiro R.C., Bennett J.M., Liebman H.A., Bernstein-Singer M., Espina B.M., Cabral L., Allen S., et al. 1995. Treatment of adult T-cell leukemia-lymphoma with a combination of interferon alfa and zidovudine. N. Engl. J. Med. 332:1744–1748 10.1056/NEJM199506293322603 [DOI] [PubMed] [Google Scholar]
- Hasegawa H., Sawa H., Lewis M.J., Orba Y., Sheehy N., Yamamoto Y., Ichinohe T., Tsunetsugu-Yokota Y., Katano H., Takahashi H., et al. 2006. Thymus-derived leukemia-lymphoma in mice transgenic for the Tax gene of human T-lymphotropic virus type I. Nat. Med. 12:466–472 10.1038/nm1389 [DOI] [PubMed] [Google Scholar]
- Hermine O., Bouscary D., Gessain A., Turlure P., Leblond V., Franck N., Buzyn-Veil A., Rio B., Macintyre E., Dreyfus F., et al. 1995. Brief report: treatment of adult T-cell leukemia-lymphoma with zidovudine and interferon alfa. N. Engl. J. Med. 332:1749–1751 10.1056/NEJM199506293322604 [DOI] [PubMed] [Google Scholar]
- Hermine O., Dombret H., Poupon J., Arnulf B., Lefrère F., Rousselot P., Damaj G., Delarue R., Fermand J.P., Brouet J.C., et al. 2004. Phase II trial of arsenic trioxide and alpha interferon in patients with relapsed/refractory adult T-cell leukemia/lymphoma. Hematol. J. 5:130–134 10.1038/sj.thj.6200374 [DOI] [PubMed] [Google Scholar]
- Hu J., Liu Y.F., Wu C.F., Xu F., Shen Z.X., Zhu Y.M., Li J.M., Tang W., Zhao W.L., Wu W., et al. 2009. Long-term efficacy and safety of all-trans retinoic acid/arsenic trioxide-based therapy in newly diagnosed acute promyelocytic leukemia. Proc. Natl. Acad. Sci. USA. 106:3342–3347 10.1073/pnas.0813280106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitsuka K., Suzumiya J., Aoki M., Ogata K., Hara S., Tamura K. 2007. Therapeutic potential of arsenic trioxide with or without interferon-alpha for relapsed/refractory adult T-cell leukemia/lymphoma. Haematologica. 92:719–720 10.3324/haematol.10703 [DOI] [PubMed] [Google Scholar]
- Ito K., Bernardi R., Morotti A., Matsuoka S., Saglio G., Ikeda Y., Rosenblatt J., Avigan D.E., Teruya-Feldstein J., Pandolfi P.P. 2008. PML targeting eradicates quiescent leukaemia-initiating cells. Nature. 453:1072–1078 10.1038/nature07016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kchour G., Tarhini M., Kooshyar M.M., El Hajj H., Wattel E., Mahmoudi M., Hatoum H., Rahimi H., Maleki M., Rafatpanah H., et al. 2009. Phase 2 study of the efficacy and safety of the combination of arsenic trioxide, interferon alpha, and zidovudine in newly diagnosed chronic adult T-cell leukemia/lymphoma (ATL). Blood. 113:6528–6532 10.1182/blood-2009-03-211821 [DOI] [PubMed] [Google Scholar]
- Kelly P.N., Dakic A., Adams J.M., Nutt S.L., Strasser A. 2007. Tumor growth need not be driven by rare cancer stem cells. Science. 317:337 10.1126/science.1142596 [DOI] [PubMed] [Google Scholar]
- Kogan S.C. 2009. Curing APL: differentiation or destruction? Cancer Cell. 15:7–8 10.1016/j.ccr.2008.12.012 [DOI] [PubMed] [Google Scholar]
- Lallemand-Breitenbach V., Zhu J., Puvion F., Koken M., Honoré N., Doubeikovsky A., Duprez E., Pandolfi P.P., Puvion E., Freemont P., de Thé H. 2001. Role of promyelocytic leukemia (PML) sumolation in nuclear body formation, 11S proteasome recruitment, and As2O3-induced PML or PML/retinoic acid receptor α degradation. J. Exp. Med. 193:1361–1371 10.1084/jem.193.12.1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemand-Breitenbach V., Jeanne M., Benhenda S., Nasr R., Lei M., Peres L., Zhou J., Zhu J., Raught B., de Thé H. 2008. Arsenic degrades PML or PML-RARalpha through a SUMO-triggered RNF4/ubiquitin-mediated pathway. Nat. Cell Biol. 10:547–555 10.1038/ncb1717 [DOI] [PubMed] [Google Scholar]
- Lavau C., Luo R.T., Du C., Thirman M.J. 2000. Retrovirus-mediated gene transfer of MLL-ELL transforms primary myeloid progenitors and causes acute myeloid leukemias in mice. Proc. Natl. Acad. Sci. USA. 97:10984–10989 10.1073/pnas.190167297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka M., Jeang K.T. 2007. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat. Rev. Cancer. 7:270–280 10.1038/nrc2111 [DOI] [PubMed] [Google Scholar]
- Nasr R., Rosenwald A., El-Sabban M.E., Arnulf B., Zalloua P., Lepelletier Y., Bex F., Hermine O., Staudt L., de Thé H., Bazarbachi A. 2003. Arsenic/interferon specifically reverses 2 distinct gene networks critical for the survival of HTLV-1-infected leukemic cells. Blood. 101:4576–4582 10.1182/blood-2002-09-2986 [DOI] [PubMed] [Google Scholar]
- Nasr R., Chiari E., El-Sabban M., Mahieux R., Kfoury Y., Abdulhay M., Yazbeck V., Hermine O., de Thé H., Pique C., Bazarbachi A. 2006. Tax ubiquitylation and sumoylation control critical cytoplasmic and nuclear steps of NF-kappaB activation. Blood. 107:4021–4029 10.1182/blood-2005-09-3572 [DOI] [PubMed] [Google Scholar]
- Nasr R., Guillemin M.C., Ferhi O., Soilihi H., Peres L., Berthier C., Rousselot P., Robledo-Sarmiento M., Lallemand-Breitenbach V., Gourmel B., et al. 2008. Eradication of acute promyelocytic leukemia-initiating cells through PML-RARA degradation. Nat. Med. 14:1333–1342 10.1038/nm.1891 [DOI] [PubMed] [Google Scholar]
- Portis T., Harding J.C., Ratner L. 2001. The contribution of NF-kappa B activity to spontaneous proliferation and resistance to apoptosis in human T-cell leukemia virus type 1 Tax-induced tumors. Blood. 98:1200–1208 10.1182/blood.V98.4.1200 [DOI] [PubMed] [Google Scholar]
- Quintana E., Shackleton M., Sabel M.S., Fullen D.R., Johnson T.M., Morrison S.J. 2008. Efficient tumour formation by single human melanoma cells. Nature. 456:593–598 10.1038/nature07567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler M., Chelbi-Alix M.K., Koken M.H.M., Venturini L., Lee C., Saïb A., Quignon F., Pelicano L., Guillemin M.-C., Schindler C., de Thé H. 1995. Transcriptional induction of the PML growth suppressor gene by interferons is mediated through an ISRE and a GAS element. Oncogene. 11:2565–2573 [PubMed] [Google Scholar]
- Zhu J., Koken M.H.M., Quignon F., Chelbi-Alix M.K., Degos L., Wang Z.Y., Chen Z., de Thé H. 1997. Arsenic-induced PML targeting onto nuclear bodies: implications for the treatment of acute promyelocytic leukemia. Proc. Natl. Acad. Sci. USA. 94:3978–3983 10.1073/pnas.94.8.3978 [DOI] [PMC free article] [PubMed] [Google Scholar]