IL-27 signaling directly into T cells is needed for follicular T helper cell survival, germinal center formation, and the production of T cell–dependent high-affinity antibodies in mice.
Abstract
Maturation and selection of high-affinity B cell clones in the germinal center (GC) relies on support from T follicular helper (TFH) cells. TFH cells are characterized by their localization to the B cell follicle and their high expression of the costimulatory molecules ICOS and PD1 and the cytokine IL-21, which promotes immunoglobulin (Ig) class switching and production by B cells. We show that the heterodimeric cytokine IL-27 is critical for the function of TFH cells and for normal and pathogenic GC responses. IL-27 signaling to T cells results in the production of IL-21, a known autocrine factor for the maintenance of TFH cells, in a STAT3-dependent manner. IL-27 also enhances the survival of activated CD4+ T cells and the expression of TFH cell phenotypic markers. In vivo, expression of the IL-27Rα chain is required to support IL-21 production and TFH cell survival in a T cell–intrinsic manner. The production of high-affinity antibodies is reduced, and pristane-elicited autoantibodies and glomerulonephritis are significantly diminished, in Il27ra−/− mice. Together, our data show a nonredundant role for IL-27 in the development of T cell–dependent antibody responses.
IL-27 is a heterodimeric cytokine consisting of the protein subunits IL27p28 and Epstein Barr virus–induced protein 3 (Pflanz et al., 2002). It signals through a heterodimeric receptor consisting of the ligand-specific IL-27Rα chain and gp130 (Pflanz et al., 2004), which is shared with several other cytokines, including the structurally related cytokine IL-6. Like IL-6, IL-27 signaling involves the activation of Jak1, STAT1, and STAT3 (Batten and Ghilardi, 2007). Despite shared use of the gp130 chain, the contribution of the IL-27Rα subunit makes IL-27 functionally distinct from IL-6 in that it promotes early aspects of TH1 differentiation, such as up-regulation of the transcription factor T-bet and the IL-12 receptor β2 chain while suppressing IL-6–driven T cell proliferation and TH17 differentiation (Batten and Ghilardi, 2007).
In vivo, IL-27 acts to constrain inflammation under most circumstances that have been studied to date (Batten and Ghilardi, 2007). Several possible mechanisms for this immunosuppressive activity have been identified; IL-27 is known to antagonize TH17 development (Batten et al., 2006; Stumhofer et al., 2006), induce IL-10 production (Awasthi et al., 2007; Fitzgerald et al., 2007; Stumhofer et al., 2007; Batten et al., 2008), and suppress IL-6–induced T cell proliferation (Batten et al., 2006). Nevertheless, IL-27 apparently plays a proinflammatory role in some situations. For example, Il27ra−/− mice are protected from proteoglycan-induced arthritis (Cao et al., 2008), which is dependent on both B and CD4+ T cell activity (Banerjee et al., 1992; Hamel et al., 2008). Furthermore, deletion of Il27ra in the MRL/lpr model of lupus results in lower TH1 cytokine production, diminished anti-dsDNA antibodies, and enhanced survival (Shimizu et al., 2005). Together, these results suggest that IL-27 is required in proinflammatory situations that depend on generation of high-affinity antibodies in vivo.
Antibody affinity maturation involves the selection of antigen (Ag)-specific B cell clones that have undergone productive somatic hypermutation. This occurs in germinal centers (GCs) in secondary lymphoid organs and relies on a specialized subset of CD4+ T helper cells termed T follicular helper (TFH) cells (Yu et al., 2009a). Without TFH cells, GCs are short lived and ineffective in generating high-affinity antibodies and B cell memory, whereas aberrant TFH activity can drive autoimmune disease (King, 2009; Yu et al., 2009a). TFH cells express CXCR5 and are thereby attracted to the GC by the B cell chemoattractant CXCL13. Concurrently, they down-regulate CCR7 which would otherwise retain them in the T cell zones. Besides being CXCR5+CCR7lo, TFH cells also express high levels of PD1 and ICOS (Yu et al., 2009a) and low levels of CD127 (IL7R; Lim and Kim, 2007) and CD62L (Fazilleau et al., 2009). Bcl-6 has recently been shown to be a lineage-specific transcription factor, repressing alternative T helper cell differentiation pathways and promoting TFH development (Johnston et al., 2009; Nurieva et al., 2009; Yu et al., 2009b). The requirements for TFH cell differentiation and maintenance are not fully understood but include sustained interactions with B cells (Haynes et al., 2007) and ICOS-ICOSL signaling (Nurieva et al., 2008). Furthermore, TFH-derived IL-21 is known to provide crucial stimulation to B cells (Linterman et al., 2010; Zotos et al., 2010) and can act as an autocrine growth factor for TFH cells (Nurieva et al., 2008; Vogelzang et al., 2008; Eddahri et al., 2009; Linterman et al., 2010). In this paper, we identify IL-27 as an essential cytokine for IL-21 induction, the function of TFH cells and GC responses, and show that the severity of antibody-mediated autoimmune disease is reduced in the absence of IL-27 signaling in a murine model of lupus.
RESULTS
IL-27 induces IL-21 expression in vitro and in vivo
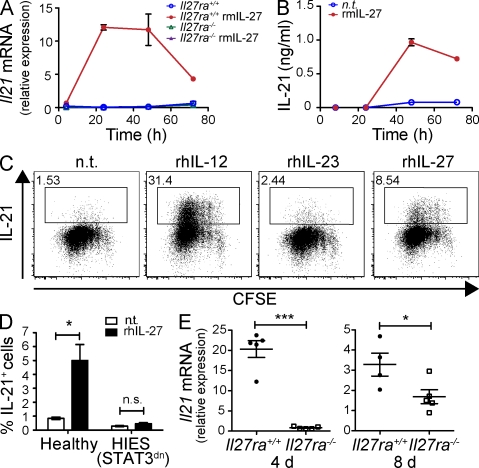
We tested the effect of IL-27 on FACS-purified naive T cells during anti-CD3/anti-CD28 stimulation and found that the addition of IL-27 resulted in greatly elevated Il21 mRNA expression (Fig. 1 A), peaking at ∼24–48 h. This was a specific response to ligand–receptor interaction because no effect was observed on IL-27Rα–deficient (Il27ra−/−) T cells (Fig. 1 A). IL-27 was unable to significantly enhance IL-21 mRNA expression in the presence of the translational repressor cycloheximide (Fig. S1 A), suggesting that translation of intermediate proteins may mediate IL-21 expression. Analysis of the culture supernatants confirmed that IL-27 induces IL-21 protein production by >10-fold compared with untreated cells by 48 h (Fig. 1 B).
Figure 1.
IL-27 induces IL-21 expression. (A) FACS-purified CD4+CD25− T cells isolated from either Il27ra+/+ or Il27ra−/− mice were stimulated with plate-bound anti-CD3 and soluble anti-CD28 under TH0 polarizing conditions and in the presence or absence of 20 ng/ml rmIL-27 for the times indicated. Il21 mRNA was determined by real-time RT-PCR and is presented relative to rpl19 mRNA. Data represent the mean of triplicate experimental samples and error bars indicate standard deviation. (B) CD4+ T cells isolated from C57BL6 spleens were stimulated as in A (n.t., no treatment) and IL-21 protein was measured in the culture supernatant by ELISA. Error bars indicate standard deviation. (C) FACS-purified CD4+CD45RA+CXCR5− cells from human tonsil were labeled with CFSE and stimulated with anti-CD3 + anti-CD28 + anti-CD2 beads (2:1 ratio of beads to cells) in the presence of no additional cytokine, 20 ng/ml rhIL-12 or IL-23, or 50 ng/ml rhIL-27 for 5 d. IL-21 expression was assessed by intracellular staining and flow cytometry and is plotted against CFSE division. The percentage of IL-21–expressing cells is indicated. Similar data were obtained from three individuals. (D) Naive CD4+ T cells were FACS purified from three healthy controls and three AD-HIES patients and stimulated as in C. The mean percentage of IL-21+ cells ± SEM is given. (E) Groups of Il27ra+/+ (filled circles) and Il27ra−/− (open squares) mice were immunized with OVA (30 µg/mouse) in CFA. 4 and 8 d after immunization, CD4+ T cells were isolated from the spleens and IL-21 mRNA was determined by real time RT-PCR (relative to rpl19). Data from individual animals are shown. Bars indicate the mean of five animals ± SEM. *, P < 0.05; ***, P < 0.001 (unpaired Student’s t test). One of three (A and B) or two (E) independent experiments is shown. C shows a single donor representative of six. D shows data combined from three independent donors.
We also found that the addition of rhIL-27 during stimulation induced IL-21 expression by naive CD4+ T cells purified from human tonsil (Fig. 1 C) and peripheral blood (Fig. 1 D). These data suggest that IL-27 has a similar role in human and mouse IL-21 induction. In comparison to IL-27, IL-12 was a more potent IL-21 stimulant in cultures of human CD4+ T cells (Fig. 1 C). Because IL-27 stimulation up-regulated the expression of IL-12Rβ2 (Fig. S1 B), thereby sensitizing the cells to the effects of IL-12, these two cytokines may work together to enhance IL-21 production. Indeed, IL-12 and IL-27 had an additive effect on IL-21 secretion by murine CD4+ T cells (Fig. S1 C).
A prominent feature of IL-27 signaling is the activation of STAT1; however, induction of IL-21 protein expression was not dependent on activation of this transcription factor because IL-27 was able to induce IL-21 expression by STAT1−/− CD4+ T cells (Fig. S1 D). To investigate the importance of STAT3 activation for IL-27–induced IL-21 expression, we used naive CD4+ T cells from autosomal dominant hyper IgE syndrome (AD-HIES) as a result of dominant-negative mutations in STAT3 (Minegishi et al., 2007). The addition of rhIL-27 during the stimulation of AD-HIES CD4+ T cells could no longer induce IL-21 expression (Fig. 1 D), indicating that the induction of IL-21 by IL-27 is STAT3 dependent.
Having established that IL-27 is sufficient to induce IL-21 production in vitro, we next sought to determine whether IL-27 signaling is required for IL-21 induction in vivo. To this end, we immunized WT and Il27ra−/− mice with OVA/CFA and measured Il21 mRNA expression in splenic CD4+ T cells 4 and 8 d after immunization. CD4+ T cells from Il27ra−/− mice contained significantly diminished levels of Il21 mRNA, demonstrating that IL-27 signals are nonredundant for IL-21 expression in vivo (Fig. 1 E). High levels of IL-21 mRNA expression by WT cells at day 4 compared with day 8 may reflect IL-21 expression by recently activated CD4+ T cells in addition to the fully differentiated TFH cells (King, 2009).
TFH cells are reduced in the absence of Il27ra
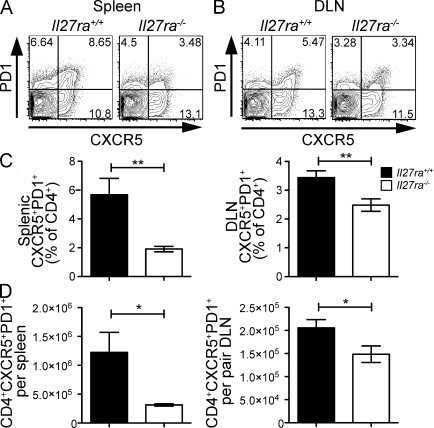
Because Il27ra−/− mice had reduced IL-21 expression, and because IL-21 is both a differentiation factor for and hallmark cytokine of TFH cells (Nurieva et al., 2008; Vogelzang et al., 2008; Eddahri et al., 2009; Yu et al., 2009a; Linterman et al., 2010), we investigated whether these mice had a reduced TFH cell population. To this end, we immunized mice twice with TNP-OVA in Freund’s adjuvant and analyzed CD4+ T cell phenotypes in spleen and LNs 7 d after the second immunization. A statistically significant reduction in both the percentage and absolute number of PD1+CXCR5+CD4+ T cells was observed in the spleens and draining LN (DLN) of Il27ra−/− mice (Fig. 2). To ensure proper discrimination between TFH and activated T cells, we stained with additional markers and found that Il27ra−/− mice have a reduction in CD4+ T cells with a CXCR5+, PD1+, ICOS+, CCR7lo, CD62Llo, and CD127lo cell phenotype (Fig. S2 A), a population matching the published phenotype of TFH cells. Interestingly, more ICOS+ cells were observed in the LN compared with spleen, both in the CXCR5-positive and -negative gates, suggesting that there may be differences in the phenotype or activation level of CD4+ cells in the DLN (Fig. S2 A). Although the percentage of TFH cells was not significantly different between WT and Il27ra−/− mice before immunization (Fig. 2 B), we used a double immunization strategy to ensure that the response to immunization overcame any small baseline differences in TFH cell numbers and to provide sufficient opportunity for the development of high-affinity antibody. However, a similar requirement for Il27ra was observed 7 d after a single immunization (Fig. S2, C and D; and not depicted)
Figure 2.
Il27ra−/− mice contain fewer TFH cells than Il27ra+/+ mice. Groups of Il27ra+/+ and Il27ra−/− mice were immunized twice with TNP-OVA in adjuvant and, 7 d after the second immunization, tissue was collected for analysis. (A and B) Representative flow cytometric analysis of CXCR5 and PD1 expression in the CD4+B220− gate in the spleen (A) and DLN (B). (C) The mean percentage ± SEM of CXCR5+PD1+ cells in each spleen or pair of DLN. (D) The numbers of CXCR5+PD1+ cells per organ were calculated for spleen and DLN by multiplying the percentage obtained by flow cytometry by the total cell count per organ. The mean of at least six animals per group is given and error bars indicate SEM. *, P < 0.05 (unpaired Student’s t test). These data are representative of four individual experiments.
We reproducibly noted a significantly reduced population of TFH cells in both the spleen and DLN of Il27ra−/− mice; however, we consistently saw that the magnitude of the reduction was greatest in the spleen (Fig. 2 and Fig. S2). Inhibition of lymphocyte migration using FTY720 administration did not prevent the tissue-specific effect (Fig. S2 C), suggesting that IL-27–dependent lymphocyte migration is not responsible for these tissue-specific differences. Furthermore, although blockade of IL-21 using a soluble IL21R-Fc molecule diminished the percentage of CXCR5+PD1+ cells in WT mice, particularly in the LN, it was unable to further suppress TFH cell numbers in Il27ra−/− mice (Fig. S2 D), suggesting that these two cytokines work in the same pathway.
Reduced GC activity in the absence of Il27ra
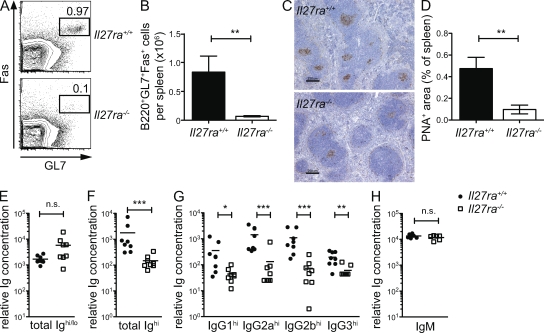
Mouse strains that have a deficiency of IL-21 or TFH cells also display abortive GC reactions (de Vinuesa et al., 2000; Nurieva et al., 2008; Vogelzang et al., 2008). Thus, to confirm that TFH cell function was diminished, we examined the formation of GC in the spleens of immunized Il27ra−/− mice. Flow cytometric analysis showed that significantly fewer Fas+GL7+ GC B cells were present in the absence of Il27ra signaling (Fig. 3, A and B). Histological examination showed that although Il27ra−/− mice did develop some peanut agglutinin (PNA)+ structures resembling GC, these were greatly reduced in size compared with WT controls (Fig. 3, C and D). The PNA-positive GC area per spleen was found to be significantly reduced in Il27ra−/− mice (Fig. 3 D).
Figure 3.
GC dysfunction in Il27ra−/− mice. Groups of Il27ra+/+ and Il27ra−/− mice were immunized twice with TNP-OVA in adjuvant and, 7 d after the second immunization, tissues and sera were collected for analysis. (A) Representative flow cytometric analysis for Fas and GL7 expression in the splenic B220+CD4− cell gate. (B) The number of GL7+Fas+B220+CD4− cells in the spleen of each mouse was calculated by multiplying the percentage obtained by flow cytometry by the total cell count per organ. The mean of at least six animals per group is given and error bars indicate SEM. (C) Representative spleen sections stained with PNA (brown) to detect GC. Bars, 250 µm (D) Slide scanning and image analysis software were used to quantify the PNA+ area in each of eight spleens per genotype. The mean percentage of PNA+ area is given and error bars indicate SEM. (E–G) ELISA using plates coated with 5 µg/ml BSA-TNP28 (E) or BSA-TNP2 (F and G) for analysis of total anti-TNP and high-affinity anti-TNP antibodies, respectively, in the serum of mice immunized as in A. Anti-TNP antibodies were detected with either anti–mouse Ig (E and F) or antibodies against specific mouse Ig isotypes (G). (H) Groups of Il27ra+/+ and Il27ra−/− mice were immunized with 100 µg TNP-Ficoll i.p. and sera were collected 5 d later. Anti–TNP-IgM levels were assessed by ELISA as in E and detected using anti–mouse IgM antibodies. Relative anti-TNP antibody concentration is given for each mouse, and bars indicate the group mean where n = 6–8. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (unpaired Student’s t test). The data are indicative of three individual experiments.
To examine whether this GC defect translated into lower serum levels of high-affinity antibodies, we used an established model (Roes and Rajewsky, 1993) whereby high-affinity antibodies to the immunizing hapten, TNP, can be measured in the serum by virtue of their ability to bind to sparsely haptenated BSA molecules (TNP2-BSA). The level of total (high and low affinity) anti-TNP antibody, as detected using TNP28-BSA, was similar in Il27ra−/− and Il27ra+/+ sera (Fig. 3 E). However, the level of high-affinity anti-TNP antibodies (detected using TNP2-BSA) was reduced in Il27ra−/− mice compared with WT mice (Fig. 3 F), indicating that affinity maturation is compromised in the absence of IL-27 signaling. Il27ra−/− mice had reduced levels of class-switched high-affinity antibodies, including IgG1, IgG2a, IgG2b, and IgG3 (Fig. 3 G), but not IgE (not depicted), which is expected because this isotype is inhibited by the GC transcription factor Bcl-6 (Harris et al., 1999). In general, antibody derived from GC-independent responses appears to be unaffected by the loss of IL-27 signaling. Previous studies showed that Il27ra−/− mice displayed normal levels of total serum Ig, with the exception of IgG2a (Chen et al., 2000; Miyazaki et al., 2005). In line with these observations, we found that the early IgM response to the T cell–independent Ag, TNP-Ficoll, was similar in Il27ra+/+ and Il27ra−/− mice (Fig. 3 H). Collectively, global absence of IL-27 receptor results in a reduction in TFH cells, along with diminished GC formation and selective reduction of high-affinity antibodies, whereas T cell–independent and low-affinity antibodies remain largely unchanged.
Defects in TFH and GC function in Il27ra−/− mice are T cell intrinsic
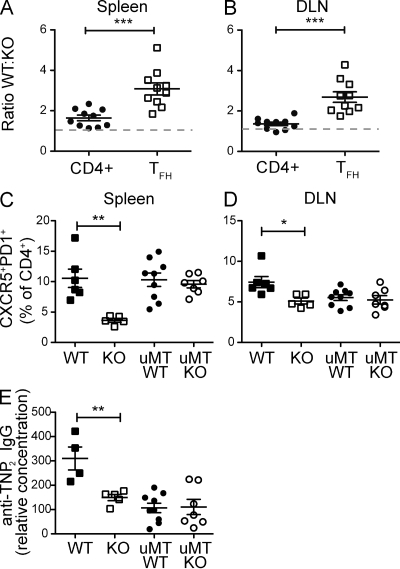
The observation that IL-27 directly promotes IL-21 expression by CD4+ T cells suggested that the defect in TFH cell number and GC function in Il27ra−/− mice could result from a T cell–intrinsic defect. However, other cells, including B cells, express the IL-27 receptor, and thus it remained formally possible that the TFH defect in Il27ra−/− mice was indirect. To discriminate these two possibilities, we generated mixed BM chimeras using Il27ra−/− (CD45.2+ and Thy1.2+) BM mixed at a 1:1 ratio with congenic Il27ra+/+ (CD45.1+ and Thy1.2+) BM and transferred into lethally irradiated Il27ra+/+CD45.1+Thy1.1+ hosts such that Il27ra−/− donors, WT donors, and remnant WT host T cells could each be discriminated in the reconstituted chimeric mice. In such mice, WT and Il27ra−/− T cells have the same exposure to the mixed population of WT and KO Ag-presenting cells and B cells. FACS analysis of the blood of reconstituted chimeric mice revealed a similar contribution of both genotypes to T and B cell compartments before immunization (Fig. S3, A and B), confirming that absence of the Il27ra does not impair repopulation. The chimeric mice were immunized twice, and 7 d after the second immunization the contributions of the WT and Il27ra−/− cells to the total CD4+ and CD4+CXCR5+PD1+ TFH cell populations were analyzed by flow cytometry. In the total CD4+ gate, WT cells contributed with somewhat increased frequency after immunization, with the WT/KO ratio being 1.644 ± 0.14 (mean ± SEM) in the spleen and 1.366 ± 0.088 in the DLN (Fig. 4, A and B). However, WT T cells contributed highly disproportionately to the PD1+CXCR5+ TFH gate, with a WT/KO ratio of 3.08 ± 0.3 in the spleen and 2.689 ± 0.26 in the DLN (Fig. 4, A and B). These data suggest that Il27ra−/− T cells have an intrinsic defect in their follicular helper capacity, and this defect cannot be compensated for by the presence of WT APC and B cells. Because the TFH cell compartment of the mixed chimeric mice was comprised mainly of WT cells, GC function was restored and the levels of high-affinity class-switched Ig were comparable with mice reconstituted with 100% WT cells (Fig. S3 C).
Figure 4.
The GC defect in Il27ra−/− mice is T cell intrinsic. (A and B) WT (CD45.1+):Il27ra−/− (CD45.2+) BM chimeric mice were immunized twice with TNP-OVA, as described, and tissue collected and assessed 7 d after the second injection. The ratio of CD45.1/CD45.2 cells is given for total CD4+ cells and CD4+CXCR5+PD1+ cells in the spleen (A) or DLN (B) for each of 10 chimeric animals. The gray dashed line indicates equivalency of WT and Il27ra−/− cells (ratio of 1). (C and D) BM chimeric mice were reconstituted with BM from WT mice only, Il27ra−/− mice only, WT and µMT mice (1:4 ratio), or Il27ra−/− and µMT mice (1:4 ratio) and immunized twice as described. Tissue was collected 7 d after the second injection and the percentage of CXCR5+PD1+ cells in the CD4+B220− gate in the spleen (C) and DLN (D) was determined by flow cytometry. (E) Serum high-affinity antibody was measured using ELISA plates coated with TNP2-BSA and detected with anti–mouse IgG antibody. Data are given as a relative concentration using pooled serum as a control. Each set of data are indicative of two individual experiments. Bars indicate the mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (unpaired Student’s t test).
To conclusively rule out direct effects of IL-27 on B cells in this model, we constructed BM chimeras using B cell–deficient µMT BM plus either WT or Il27ra−/− BM (at a 80:20 ratio) such that all B cells in the resultant chimeras were derived from either the WT (control) or the Il27ra−/− BM (a B cell–specific Il27ra deletion), whereas the majority of non–B cells were derived from WT BM. The absence of IL-27 signaling in the B cell compartment alone did not affect the development of TFH cells in the spleen or DLN (Fig. 4, C and D). Although the µMT chimeras generally produced lower amounts of high-affinity IgG compared with mice reconstituted with only WT BM, we noted no difference based on the Il27ra genotype of the B cells (Fig. 4 E). Thus, absence of IL-27 signaling in the B cell compartment alone affected neither the development of TFH cells nor the production of high-affinity antibody.
An adoptive transfer system, in which OVA-specific Il27ra+/+ or Il27ra−/− DO11.10 transgenic (Tg) CD4+ T cells were injected into either Il27ra+/+ or Il27ra−/− hosts before immunization, further reinforced the T cell–intrinsic nature of the GC defect. Although complete removal of IL-27Ra from the system recapitulated the Il27ra−/− phenotype, as expected, the percentage of T cells that expressed PD1 and CXCR5 was not significantly changed based on donor Il27ra genotype (Fig. S3 D). These data suggest that the differentiation of TFH cells can occur in the absence of IL-27 signaling. However, lack of Il27ra on the transferred CD4+ T cells significantly reduced their recovery rates (Fig. S3 E), with a corresponding reduction in Fas+GL7+ GC B cells (Fig. S3 F), suggesting that IL-27 supports the survival and/or proliferation of the DO11.10+ cells in a T cell–dependent manner. The effect was most striking when DO11.10+.Il27ra+/+ and DO11.10+.Il27ra−/− cells were transferred into Il27ra−/− recipients (Fig. S3 E), which is consistent with the notion that the transferred DO11.10+.Il27ra+/+ T cells have a greater competitive advantage when the endogenous recipient T cells, which also participate in the response, are unable to respond to IL-27. Alternatively, the increased recovery of DO11.10+.Il27ra+/+ T cells from KO hosts might be the result of increased IL-27 levels in mice that lack expression of the receptor, as is observed in other receptor-deficient mice (Cho et al., 2007). Differences between WT and Il27ra−/− cells were not evident in the LN in the adoptive transfer system (unpublished data). Together, the BM chimera and adoptive transfer data described strongly support a T cell–intrinsic defect in GC responses in Il27ra−/− mice.
IL-27 enhances the expression of TFH cell markers and supports CD4+ T cell survival after Ag activation
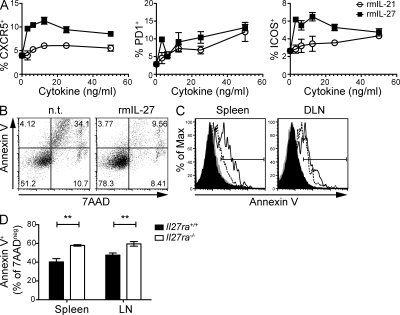
Our data suggest that IL-27 is important for the differentiation and/or maintenance of TFH cells. To test whether IL-27 stimulation of CD4+ T cells directly induces differentiation of TFH cells, we performed in vitro experiments using OT-II Tg T cells. The cells were activated by OVA peptide in the presence of irradiated splenocytes and various concentrations of rmIL-27 or rmIL-21 for 5 d and then restimulated with anti-CD3 for 4 h (RNA expression) or 24 h (protein expression), as described by Nurieva et al. (2008). Under these conditions, the addition of rmIL-27 resulted in a concentration-dependent enhancement of the expression of CXCR5, PD1 and ICOS mRNA, and proteins on the cell surface (Fig. 5 and Fig. S4 A). In our hands, this enhancement was superior to rmIL-21. However, when purified CD4+ T cells were stimulated in the absence of accessory cells, ICOS, but not PD1 or CXCR5, was induced (not depicted; Pot et al., 2009). Moreover, IL-27 had little effect on Bcl-6 mRNA expression (Fig. S4 A), either under the conditions described in this paragraph or after a short-term primary stimulation (4 or 24 h; not depicted), suggesting that IL-27 did not induce full TFH differentiation. In vitro stimulation of OTII TCR Tg T cells in the presence of rmIL-21 resulted in enhanced development of GC in adoptive hosts (Nurieva et al., 2008); however, stimulation with rmIL-27 was not able to enhance B helper activity under these conditions (Fig. S4 B). Although IL-21 is highly up-regulated by IL-27 stimulation, maximal expression appears to reach around 1 ng/ml (Fig. 1 B), which is markedly less than the 50 ng/ml IL-21 used in those in vitro TFH differentiation systems. Moreover, other consequences of IL-27 signaling that diverge from that of IL-21 may also contribute to the differences observed. These data suggest that although IL-27 enhances the expression of TFH effector molecules and cytokines, it is not able to do so in the absence of additional signals and is not sufficient to produce fully differentiated TFH cells.
Figure 5.
IL-27 promotes survival of TFH cells. (A) OTII Tg CD4+ T cells were cultured with irradiated splenic APC plus 0.3 µM OVA323-339 peptide under TH0 conditions and in the presence of either no additional cytokine or various concentrations of rmIL-21 or rmIL-27 for 5 d. After 5 d, CD4+ T cells were restimulated with anti-CD3 for 24 h and CXCR5, PD1, and ICOS expression were assessed by flow cytometry. The positive gate was determined based on histogram plots for each antibody and the mean percentage positive ± SD for triplicate experimental samples is shown. (B) DO11.10 tg.rag2−/− splenocytes were activated with 0.03 µM OVA323-339 in the presence or absence (n.t.) of rmIL27 for 72 h, and viability was assessed by flow cytometry using annexin V and 7AAD staining. The CD4+B220− gate is shown. (C and D) Viability of cells in groups of Il27ra+/+ and Il27ra−/− mice was assessed by flow cytometry 4 d after immunization with TNP-OVA in CFA. (C) Representative plots for annexin V staining in the CD4+B220−7AAD− gate (Il27ra+/+, black fill; Il27ra−/−, gray fill) or CXCR5+PD1+CD4+B220−7AAD− cell gate (Il27ra+/+, dotted line; Il27ra−/−, solid line) in the spleen or DLN. (D) The mean percentage of annexin V+ cells in the CD4+CXCR5+PD1+7AAD− gate. Error bars indicate SEM with n = 5 mice per group. Each set of data are indicative of two individual experiments. **, P < 0.01 (unpaired Student’s t test).
In adoptive transfer experiments, IL-27 signaling influenced the amount of Ag-specific CD4+ T cells that were present after immunization (Fig. S3, D–F). In in vitro culture systems, we noted that the addition of rmIL-27 to Ag-stimulated CD4+ T cells enhanced the survival of these strongly stimulated T cells (Fig. 5 B and Fig. S4 C). The survival advantage conferred to cells by the addition of rmIL-27 was not dependent on IL-21R signaling (Fig. S4 D). To investigate whether the altered cell survival observed in vitro was reflected by changes in TFH cell survival in vivo, we examined the percentage of apoptotic (annexin V+) total CD4+ and TFH cells in immunized Il27ra+/+ and Il27ra−/− mice. We chose a time point of 4 d after immunization to examine early changes in viability and examined the percentage of annexin V (apoptotic) cells in the live (7-aminoactinomycin d [7AAD]−) gate. Although we found that the percentage of annexin V+ cell in the CD4+ T cell pool was similar between WT and Il27ra−/− mice (Fig. 5 C), the percentage of annexin V+ cells in the PD1+CXCR5+ TFH cell gate was significantly higher in Il27ra−/− spleen and LN compared with WT (Fig. 5, C and D). Together, these data suggest that IL-27 is important for the survival of TFH cells as well as enhancing the expression of TFH cell effector proteins.
Loss of IL-27 signaling ameliorates disease in a murine lupus model
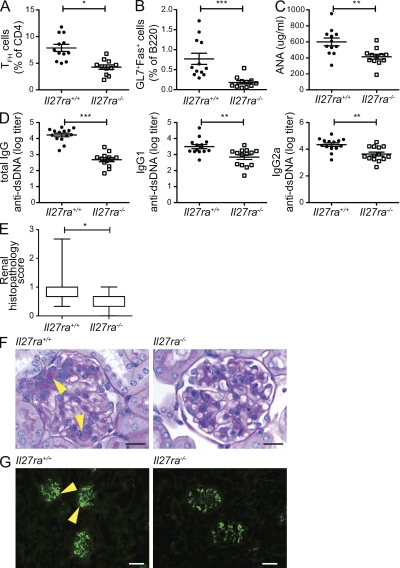
Our data suggest that IL-27 might play a pathogenic role in diseases that are characterized by affinity-matured autoantibody production such as systemic lupus erythematosus (SLE; Wellmann et al., 2005; Mietzner et al., 2008). To test this hypothesis, we used a model in which a single i.p. injection of tetramethylpentadecane (TMPD; commonly known as pristane) results in an SLE-like syndrome characterized by the development of a variety of autoantibodies, polyclonal hypergammaglobulinemia, and glomerulonephritis (Satoh and Reeves, 1994; Reeves et al., 2009). The IgG hypergammaglobulinemia and the development of IgG1 and IgG2a anti-nuclear antibodies appear to be dependent on T cell help (Nacionales et al., 2009). Because genetic factors influence susceptibility to TMPD-induced lupus, we used Il27ra−/− mice on the BALB/c background to study the importance of IL-27 in the development of disease in this model. 42 wk after TMPD injection, the Il27ra−/− displayed significantly reduced populations of TFH and GC B cells (Fig. 6, A and B), along with significantly lower levels of serum anti-nuclear (Fig. 6 C) and anti-dsDNA IgG (Fig. 6 D) antibodies compared with WT controls. Although a global reduction in IgG2a production in Il27ra−/− mice has been noted previously (Chen et al., 2000; Miyazaki et al., 2005), the reduction in anti-dsDNA antibodies was not confined to this isotype because IgG1 anti-dsDNA antibody was also significantly lower in Il27ra−/− serum (Fig. 6 D). Consistent with the reduction in autoantibody production, histological analysis revealed a lower mean renal histopathology score in the Il27ra−/− mice (Fig. 6, E and F) and a reduction in histological evidence of IgG immune complex deposition in the kidney (Fig. 6 G). In both groups, renal lesions consistent with SLE are mild, although there was greater individual variability with occurrence of more severe lesions in the WT group. These data demonstrate that the defects observed in GC function in the absence of IL-27 signaling are relevant in the disease setting.
Figure 6.
The deletion of Il27ra ameliorates pathology in the pristane-induced lupus model. A single 0.5-ml i.p. injection of pristane was administered to groups of Il27ra+/+ and Il27ra−/− mice. 42 wk after induction, mice were euthanized and examined for immune activation parameters and lesions consistent with SLE. (A and B) Flow cytometric analysis of splenocytes. The percentages of CXCR5+PD1+ (TFH-like) cells in the CD4+B220− gate (A) and the percentages of GL7+Fas+ (GC B cells) in the B220+CD4− gate (B) were analyzed. (C) Serum anti-nuclear antibody (ANA). (D) Serum total IgG (left), IgG1 (middle), and IgG2a (right) anti-dsDNA antibody titers. In all graphs bars indicate mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (unpaired Student’s t test). (E and F) Kidney sections were stained with PAS and the glomerulopathy scored by blinded observers. (E) Mean renal lesion scores are depicted as a box plot with max to min whiskers. *, P < 0.05 (unpaired Student’s t test). (F) Representative sections. Arrowheads, lumpy accumulations of PAS-positive material. Bars, 20 µm. (G) Immunofluorescence for IgG immune complex deposition in the kidney. Lumpy IgG deposits (arrowheads) correspond to PAS positive mesangial deposits. Bars, 50 µm. Data represent one experiment with 16 animals per group.
DISCUSSION
The development of TFH cells is not fully understood and represents a topic of intense current interest. Our data position IL-27 as a new factor that is critical for TFH cell function. Stimulation of T cells with IL-27 promoted IL-21 production and expression of the TFH hallmark proteins PD-1, CXCR5, and ICOS. In Il27ra−/− mice, we observed a reduction in IL-21 production, fewer TFH cells, reduced GC formation, and a reduction in high-affinity immunoglobulin production, indicating that IL-27 signaling is required for efficient T cell–dependent humoral immune responses. A functional consequence of IL-27Ra ablation was partial protection in the pristane-induced lupus model, which is characterized by active GC development, production of multiple autoantibodies, and renal pathology. Collectively, these data strongly suggest that IL-27 is required for normal and pathogenic GC responses in vivo.
IL-27 also induced IL-21 expression by human CD4+ T cells, suggesting that the IL-27–IL-21 axis is conserved across species. However, our results differ from recent observations by Schmitt et al. (2009), who observed that rhIL-12, but not rhIL-27, induced IL-21 expression by human PBMC. The reason for this discrepancy remains unresolved at this time but may result from differences in the experimental setup and the strength of TCR stimulation, which we observed to be proportional to IL-21 production (unpublished data). Our data also suggest that although IL-12 and IL-27 can independently induce IL-21 (Fig. 1 C), they can also act in concert to induce maximal production of this cytokine (Fig. S1, B and C). Mechanistically, IL-21 induction was independent of STAT1 in murine T cells (Fig. S1 D) but failed to occur in STAT3-deficient T cells from human AD-HIES patients (Fig. 1 D).
IL-27 was unable to induce the functional TFH phenotype in an in vitro system. This was surprising because IL-27 induced IL-21 production, which by itself is sufficient to induce functional TFH cells (Nurieva et al., 2008). However, IL-21 levels peak only at 48 h and ∼1 ng/ml when induced by IL-27, whereas direct stimulation with recombinant IL-21 was conducted at 50 ng/ml and affected cells from the outset (Nurieva et al., 2008). Consequently, these two in vitro stimulation experiments cannot be directly compared. Moreover, isolated stimulation with a single recombinant cytokine in vitro falls short of mimicking the much more complex stimulation environment in vivo, where IL-27 is clearly required for a functional TFH response.
In addition to inducing hallmark TFH genes, our data also suggest that IL-27 promotes the survival of TFH cells. TFH cells represent a highly activated population, which express high levels of Fas and low levels of IL-7R (Ma et al., 2009) and are exquisitely sensitive to activation-induced cell death (Marinova et al., 2006). In T cell transfer experiments, DO11.10+rag2−/− T cells assumed the TFH phenotype readily and independently of IL-27 signaling (Fig. S3 D). This is perhaps not surprising because the DO11.10+ cells uniformly express a high-affinity Ag-specific TCR and because Ag affinity controls TFH differentiation (Fazilleau et al., 2009). However, DO11.10+rag2−/− T cells that lacked Il27ra were recovered at diminished numbers (Fig. S3 E), suggesting that IL-27 is important for the maintenance of TFH cells. This hypothesis is supported by our observations that recombinant IL-27 enhances the survival of newly activated CD4+ T cells in vitro and that TFH cells undergo increased apoptosis in vivo when Il27ra is genetically ablated. Although the cells studied in vitro were not fully differentiated TFH cells, the death of recently activated CD4+ T cells would likely influence the number of cells that subsequently become TFH cells. IL-27 also induced ICOS, and ICOS signals have been shown to contribute to the survival and expansion of Ag-specific T cells in an immunization system similar to the one used in this paper (Burmeister et al., 2008). Thus, although the relative contributions remain to be determined, ICOS induction, IL-21 production (Nurieva et al., 2008), and other IL-27–elicited effects may all contribute to the enhanced survival of TFH cells.
Somewhat surprisingly, the reduction in TFH cells was more pronounced in spleen compared with the DLN (Fig. 2 and Fig. S2). The transfer of TCR Tg cells confirmed that Ag-specific responses occur in the spleen after immunization in the flank (Fig. S4, C and D). Because inhibition of lymphocyte migration using FTY720 administration did not prevent the tissue-specific effect (Fig. S2 C), we favor a hypothesis in which the proximity of the LN to the site of injection results in increased local exposure to Ag and inflammatory cytokines (Fu et al., 2006). Strong Ag signals promote TFH differentiation (Vogelzang et al., 2008; Fazilleau et al., 2009; Deenick et al., 2010), thus, the dependence on IL-27 signals may be reduced. Despite these possible compensatory effects close to the site of immunization, it is clear that IL-27 signaling is not redundant because significant reductions in TFH cells in the DLN and reduced serum high-affinity antibodies remain.
Our data suggest that the TFH-promoting effect of IL-27 is mediated through direct action on T cells. First, ICOS and IL-21 production were induced in T cell–only systems in vitro (Fig. 1; Pot et al., 2009). Furthermore, in chimeric animals that harbor both WT and Il27ra−/− T cells, the Il27ra−/− T cells are selectively underrepresented in the TFH pool (Fig. 4, A and B). In contrast, no differences in the TFH response or high-affinity Ig levels were noted when Il27ra was selectively ablated on B cells (Fig. 4, C and D), which are known to be functionally responsive to IL-27 in vitro (Yoshimoto et al., 2004; Larousserie et al., 2006). These data do not preclude the possibility that IL-27 influences certain minor aspects of the T cell–dependent humoral response, such as IgG2a class switching (Yoshimoto et al., 2004), through direct activities on B cells. Although the IL-27R is also expressed on myeloid cells, transfer of Il27ra+/+ DO11.10+rag2−/− T cells into Il27ra−/− recipients did not result in a significant decrease of TFH differentiation (Fig. S3, D–F), suggesting that IL-27 signaling into myeloid cells plays no major role in this context.
IL-27 is induced by type I IFN (Pirhonen et al., 2007; Remoli et al., 2007), and Ifnar1−/− mice display a similar phenotype to Il27ra−/− mice with respect to both loss of TFH function (Cucak et al., 2009) and protection from pristane-induced lupus (Nacionales et al., 2007), suggesting that IL-27 is an important downstream effector of type 1 IFN. In human SLE patients, a type 1 IFN signature is readily detectable, and antagonists are currently in clinical trails for the treatment of this disease (Yao et al., 2009; Niewold et al., 2010). Our data suggests that IL-27 might mediate the pathogenic effect of type I IFN and that therapeutic targeting of IL-27 may also be efficacious in SLE and other disorders characterized by excessive GC formation and high-affinity autoantibody production.
MATERIALS AND METHODS
Mice, cells, and reagents.
Il27ra+/+ and Il27ra−/− (Chen et al., 2000) mice (C57BL/6 background, n > 33 unless otherwise stated or, where indicated, BALB/c background, n > 11), OT-II TCR Tg (C57BL6), and Il27ra.DO11.10 TCR Tg/rag2-deficient mice (Il27ra−/−.DO11.10tg.rag2−/− or Il27ra+/+. DO11.10tg.rag2−/− on the BALB/c background) were bred in a specific pathogen-free facility at The Garvan Institute or Genentech. Stat1−/− mice (129Sv/Ev background) and 129Sv/Ev control mice were purchased from Taconic. The TCM (triple congenic) mice, Igha_B6_CD45.1_Cross-B6.SJL, which express congenic markers at the CD45.1, Igha and Thy1.1 loci on the C57BL6 background, and µMT mice (Igh-6tm1Cgn targeted mutation; C57BL/6 background) were bred in a specific pathogen-free facility at Genentech. The rag2−/− animals were purchased from The Jackson Laboratory. All live animal experiments were approved by the Genentech Institutional Animal Care and Use Committee or The Garvan Institute/St. Vincent’s Animal Experimentation Ethics Committee. Human PBMC buffy coats were obtained from the Australian Red Cross Blood Service, and patients with the clinical diagnosis of AD-HIES as a result of mutations in STAT3 were recruited from Immunology Clinics in Canberra and Sydney, Australia. All human experiments were approved by jurisdictional ethics committees in Canberra and Sydney. Unless otherwise indicated, all cytokines were purchased from R&D Systems and all antibodies were from BD. Cycloheximide was purchased from Sigma-Aldrich. FTY720 (Cayman Chemical) was reconstituted in DMSO and injected i.p. in H2O. IL21R-Fc was produced at the Garvan Institute as previously described (Herber et al., 2007).
Isolation of lymphocyte subsets.
Unless otherwise indicated, primary mouse CD4+ T cells were isolated by magnetic depletion using MACS kits (Miltenyi Biotec) according to the manufacturer’s instructions. The purity ranged from 90 to 95%. Where indicated, FACS sorting was performed using a FACSAria instrument (BD) to obtain specific cell populations of >99% purity. After immunization, splenic leukocyte populations were isolated as follows: CD4+ (CD4+B220−), TFH (CD4+CXCR5+PD1+), non-GC B (B220+CD38+), GC B (B220+CD38lo), FDC (B220−CD35hiCD32+), CD11b+ (CD11b+CD11c−B220−), and CD11c+ (CD11c+CD11b−B220−). For cell culture systems that required Ag-presenting cells, splenocyte samples were magnetically depleted of T cells with anti-CD90 (Thy-1.2) MACS MicroBeads (Miltenyi Biotec) and irradiated with a dose of 2,600 rads.
In vitro T cell stimulation.
Primary mouse cells were cultivated in Iscove’s modified Dulbecco’s medium (Invitrogen) supplemented with 10% (volume/volume) heat-inactivated FBS (HyClone PerBio), 1 mM L-glutamine, 1% (volume/volume) penicillin and streptomycin (Invitrogen), and 55 µM 2-mercaptoethanol (MP Biomedicals). Splenic CD4+ T cells from C57BL/6, Il27ra−/−, 129, or Stat1−/− mice were activated for the indicated times in plates coated with 5 µg/ml of anti-CD3 and in the presence of 1 µg/ml anti-CD28. Unfractionated DO11.10tg.rag2−/− or OTIItg splenocytes were stimulated with OVA323–339 peptide at the indicated concentrations. TH0 conditions consist of 5 µg/ml each of hamster anti–mouse IFN-γ (H22), rat anti–mouse IL-4 (BVD4-1D11), and rat anti–mouse IL-12 (C15.6) and 100 ng/ml TGFβRII-Fc (R&D Systems). Where indicated, rmIL-27 was added at a concentration of 20 ng/ml.
Human naive CD4+ T cells were FACS purified from tonsil cell or PBMC preparations based on the CD4+CD45RA+CXCR5− phenotype. Cells were labeled with CFSE and stimulated with T cell activation and expansion beads (Miltenyi Biotec) at a bead/cell ratio of 2:1 in the presence of no additional cytokine, 20 ng/ml rhIL-12, 20 ng/ml rhIL-23, or 50 ng/ml rhIL-27 for 5 d. Where irradiated splenocytes were used as APC, total splenocytes were irradiated with 2,000 rads x-ray irradiation.
Immunization.
For T cell–dependent immunization, groups of age- and sex-matched mice were immunized with TNP14-OVA (30 µg/mouse) or OVA (100 µg/mouse), as indicated, and emulsified in 100 µl CFA (Sigma-Aldrich) by subcutaneous injection into the flank. Where a second immunization was required, this was performed 21 d after the initial injection using the same dose of Ag emulsified in IFA (Sigma-Aldrich) to a volume of 100 µl/mouse and injected subcutaneously into the alternate flank.
For T cell–independent immunization, groups of six mice per genotype were immunized i.p. with 100 µg TNP-aminoethylcarboxymethyl-Ficoll in PBS. Serum was harvested 5 d later.
Flow cytometric analysis.
Cells were treated with Fc blocking Abs (anti-CD16/32 2.4G2) and then surface stained with the specified antibodies. To measure cell viability, cells were stained with FITC-conjugated annexin V and 7-AAD according to the manufacturer’s instructions (BD). Viable cells exclude both stains. IL-21 expression in human T cells was assessed by intracellular staining using Alexa Fluor 647–labeled anti–human IL-21 (eBioscience; clone 3A3-N2). Samples were analyzed using a FACSCanto II or LSR II (BD) and data analyzed using Flow Jo software (Tree Star, Inc.). Contour profiles are presented as 5% probability contours with outliers.
ELISA.
IL-21 was detected in culture supernatants using the mouse IL-21 DuoSet ELISA (R&D Systems) as per the manufacturer’s instructions. To measure the relative amounts of TNP-specific antibodies in mouse serum, plates were coated with 5 µg/ml TNP2-BSA or TNP28-BSA (Biosearch Technology) overnight at 4°C. TNP-specific ELISA was otherwise performed as previously described (Roes and Rajewsky, 1993). To standardize and quantify relative amounts of TNP-specific Ig responses, all experimental samples were compared with a standardized dilution of pooled serum obtained from Il27ra−/− mice immunized with TNP-OVA. This standard was given the arbitrary concentration of 100. In the case of IgM, an anti-TNP monoclonal (BD; clone G155-228) was used as a standard control.
For serum autoantibody measurement, mouse ANA, anti-Sm, and anti-nRNP antibodies were measured using ELISA kits from Alpha Diagnostic International, as per the manufacturer’s instructions. Anti-dsDNA antibodies were measured by coating ELISA plates with 0.01% p-L-Lysine (Sigma-Aldrich) for 1 h at room temperature and then 2.5 µg/ml of calf thymus DNA (Sigma-Aldrich) in PBS at 4°C overnight. After blocking with PBS, 0.5% BSA + 10 ppm Procline, pH 7.4, for 1 h at room temperature, serially diluted mouse serum was added and incubated at room temperature for 2 h. Antibody was detected using 500 ng/ml biotinylated rat anti–mouse IgG, IgG1, or IgG2a (BD) for 1 h at room temperature, followed by HRP-conjugated streptavidin and visualized using TMB substrate. NZBF1 serum was used as a positive control.
BM chimeras.
For mixed BM chimeras, triple congenic mice (CD45.1, Thy1.1, and IgHa) were lethally irradiated (137Cs; gamma source, 1,150 rad) and reconstituted with equal numbers of BM cells from Il27ra+/+ (CD45.1 and Thy1.2) and Il27ra−/− (CD45.2 and Thy1.2) mice by i.v. injection. After 8 wk of reconstitution, the mice were bled to assess reconstitution by flow cytometry and immunized as described in Immunization.
For µMT chimeras, Rag2−/− mice were lethally irradiated (137Cs; gamma source, 1100 rad) and reconstituted with BM from B cell–deficient µMT mice plus either WT or IL-27ra−/− BM (at a 80:20 ratio), such that all B cells in the resultant chimeras were derived from either the WT (control) or the Il27ra−/− BM (a B cell–specific Il27ra deletion), with the majority of non–B cells derived from WT BM. After 8 wk of reconstitution, the mice were bled to assess reconstitution by flow cytometry and immunized as described in Immunization.
Adoptive transfer.
CD4+ T cells from either Il27ra+/+ or −/− DO11.10tg.rag2−/− were isolated by magnetic sorting and 106 CD4+ cells were transferred to either Il27ra+/+ or Il27ra −/− hosts (on a BALB/c genetic background) via tail vein injection. The next day, the mice were immunized with OVA in CFA/IFA as described in Immunization and tissue harvested 7 d after the second immunization. DO11.10 TCR Tg cells were detected using directly fluorochrome-labeled clonotypic KJ1-26 antibody (BD).
Statistical analysis.
Data were analyzed with Prism software (GraphPad Software, Inc.) to calculate an unpaired two-way Student’s t test.
RT-PCR.
Total RNA from FACS-sorted or cultured cells was isolated with the RNeasy kit using on-column DNase I digestion (QIAGEN). Taqman quantitative RT-PCR was performed according to the manufacturer’s instructions (Applied Biosystems). A Lightcycler480 (Roche) instrument was used in the case of human samples. For each sample, triplicate test reactions and a control reaction lacking reverse transcription were analyzed for expression of the gene of interest and results were normalized to those of the housekeeping ribosomal protein L19 mRNA or hGAPDH. Arbitrary units given are the fold change relative to RPL19 (mouse) or GAPDH (human) and multiplied by 1,000. Primer sequences for each target are provided in Table S1.
Histochemical analysis.
For GC quantification, spleens were fixed in 10% neutral buffered formalin and subsequently embedded in paraffin. To detect GC, 5-µm sections were stained with biotin-conjugated PNA followed by visualization with HRP-linked streptavidin and diamino benzidine. The sections were lightly counterstained with hematoxylin. Slides were scanned on a NanoZoomer digital pathology system (Olympus), and Matlab image analysis software (The MathWorks) was used to quantitate the percentage of each spleen that was positive for PNA staining.
For kidney glomerulopathy, tissue collected at necropsy was fixed in formalin, paraffin embedded, sectioned at <4 µm and subjected to Periodic acid-Schiff stain (PAS). Tissue was evaluated by blinded observers using arbitrary severity scores on scales of 0–3 for interstitial nephritis, arteritis/periarteritis and glomerulopathy. Groupwise comparison of mean lesion severity scores was analyzed by ANOVA. For kidney and immune complex deposition, tissue collected at necropsy was snap frozen in OCT, sectioned, and stained by direct immunofluorescence using 10 µg/ml of goat anti–mouse IgG H+L FITC (Jackson ImmunoResearch Laboratories) for 60 min at room temperature.
Pristane-induced lupus.
SLE was induced by a single i.p. injection of 0.5 ml pristane (2,6,10,14-tetramethylpentadecane) to groups of Il27ra+/+ and Il27ra−/− mice on the BALB/c background. Mice were sacrificed 42 wk after induction and examined for lesions consistent with SLE by histology, the presence of autoantibodies in the serum by ELISA, and the presence of TFH and GCB cells in the spleen by flow cytometry.
Online supplemental material.
Fig. S1 shows a requirement for protein translation, but not STAT1 signaling, for IL-21 induction by IL-27, that IL-27 induces Il12RB2 mRNA expression by human T cells, and that IL-12 and IL-27 can additively promote IL-21 production. Fig. S2 further defines the surface marker expression of TFH cells, which are reduced in Il27ra−/− mice, and shows that lack of TFH cells is still evident after inhibition of lymphocyte migration and that blockade of IL-21 signaling does not further suppress TFH generation in Il27ra−/− mice. Fig. S3 shows reconstitution of lymphocyte populations and serum levels of high-affinity antibody in BM chimeric mice as described in Fig. 4, and it shows TFH and GC B cell generation when WT or Il27ra−/− TCR Tg T cells are transferred to WT or Il27ra−/− hosts. Fig. S4 details the effect of IL-27 on TFH differentiation and T cell survival in vitro. Table S1 is a list of primers and probes used for RT-PCR. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20100064/DC1.
Acknowledgments
We thank Rowan Jackson, Ben Torres, and Annie Leung for animal husbandry, Monika Larson for assistance with mouse necropsy, Jeff Eastham-Anderson, Charles Jones, and Shari Lau for histological analysis, and the Garvan Institute and Genentech Inc. flow cytometry facilities for cell sorting.
M. Wong, D.A. Fulcher, M.C. Cook, C. King, S. Tangye, M. Batten, and C. Ma are supported by program and project grants and research fellowships from the Australian National Health and Medical Research Council, The Cancer Institute of New South Wales, and the Juvenile Diabetes Research Foundation.
N. Ghilardi, N. Ramamoorthi, N.M. Kljavin, J.H. Cox, H.S. Dengler, D.M. Danilenko, P. Caplazi, and F.J. de Sauvage are full-time employees at Genentech, a member of the Roche group. The authors declare no additional competing financial interests.
Footnotes
Abbreviations used:
- 7AAD
- 7-aminoactinomycin d
- AD-HIES
- autosomal dominant hyper IgE syndrome
- Ag
- antigen
- DLN
- draining LN
- GC
- germinal center
- PAS
- periodic acid-Schiff
- PNA
- peanut agglutinin
- SLE
- systemic lupus erythematosus
- TFH
- T follicular helper
- Tg
- transgenic
References
- Awasthi A., Carrier Y., Peron J.P., Bettelli E., Kamanaka M., Flavell R.A., Kuchroo V.K., Oukka M., Weiner H.L. 2007. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat. Immunol. 8:1380–1389 10.1038/ni1541 [DOI] [PubMed] [Google Scholar]
- Banerjee S., Webber C., Poole A.R. 1992. The induction of arthritis in mice by the cartilage proteoglycan aggrecan: roles of CD4+ and CD8+ T cells. Cell. Immunol. 144:347–357 10.1016/0008-8749(92)90250-S [DOI] [PubMed] [Google Scholar]
- Batten M., Ghilardi N. 2007. The biology and therapeutic potential of interleukin 27. J. Mol. Med. 85:661–672 10.1007/s00109-007-0164-7 [DOI] [PubMed] [Google Scholar]
- Batten M., Li J., Yi S., Kljavin N.M., Danilenko D.M., Lucas S., Lee J., de Sauvage F.J., Ghilardi N. 2006. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat. Immunol. 7:929–936 10.1038/ni1375 [DOI] [PubMed] [Google Scholar]
- Batten M., Kljavin N.M., Li J., Walter M.J., de Sauvage F.J., Ghilardi N. 2008. Cutting edge: IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. J. Immunol. 180:2752–2756 [DOI] [PubMed] [Google Scholar]
- Burmeister Y., Lischke T., Dahler A.C., Mages H.W., Lam K.P., Coyle A.J., Kroczek R.A., Hutloff A. 2008. ICOS controls the pool size of effector-memory and regulatory T cells. J. Immunol. 180:774–782 [DOI] [PubMed] [Google Scholar]
- Cao Y., Doodes P.D., Glant T.T., Finnegan A. 2008. IL-27 induces a Th1 immune response and susceptibility to experimental arthritis. J. Immunol. 180:922–930 [DOI] [PubMed] [Google Scholar]
- Chen Q., Ghilardi N., Wang H., Baker T., Xie M.H., Gurney A., Grewal I.S., de Sauvage F.J. 2000. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 407:916–920 10.1038/35038103 [DOI] [PubMed] [Google Scholar]
- Cho J.H., Boyman O., Kim H.O., Hahm B., Rubinstein M.P., Ramsey C., Kim D.M., Surh C.D., Sprent J. 2007. An intense form of homeostatic proliferation of naive CD8+ cells driven by IL-2. J. Exp. Med. 204:1787–1801 10.1084/jem.20070740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucak H., Yrlid U., Reizis B., Kalinke U., Johansson-Lindbom B. 2009. Type I interferon signaling in dendritic cells stimulates the development of lymph-node-resident T follicular helper cells. Immunity. 31:491–501 10.1016/j.immuni.2009.07.005 [DOI] [PubMed] [Google Scholar]
- de Vinuesa C.G., Cook M.C., Ball J., Drew M., Sunners Y., Cascalho M., Wabl M., Klaus G.G., MacLennan I.C. 2000. Germinal centers without T cells. J. Exp. Med. 191:485–494 10.1084/jem.191.3.485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deenick E.K., Chan A., Ma C.S., Gatto D., Schwartzberg P.L., Brink R., Tangye S.G. 2010. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity. 33:241–253 10.1016/j.immuni.2010.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddahri F., Denanglaire S., Bureau F., Spolski R., Leonard W.J., Leo O., Andris F. 2009. Interleukin-6/STAT3 signaling regulates the ability of naive T cells to acquire B-cell help capacities. Blood. 113:2426–2433 10.1182/blood-2008-04-154682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazilleau N., McHeyzer-Williams L.J., Rosen H., McHeyzer-Williams M.G. 2009. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat. Immunol. 10:375–384 10.1038/ni.1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald D.C., Zhang G.X., El-Behi M., Fonseca-Kelly Z., Li H., Yu S., Saris C.J., Gran B., Ciric B., Rostami A. 2007. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat. Immunol. 8:1372–1379 10.1038/ni1540 [DOI] [PubMed] [Google Scholar]
- Fu Y.F., Wang J.X., Zhao Y., Yang Y., Tang W., Ni J., Zhu Y.N., Zhou R., He P.L., Li C., et al. 2006. S-adenosyl-L-homocysteine hydrolase inactivation curtails ovalbumin-induced immune responses. J. Pharmacol. Exp. Ther. 316:1229–1237 10.1124/jpet.105.093369 [DOI] [PubMed] [Google Scholar]
- Hamel K., Doodes P., Cao Y., Wang Y., Martinson J., Dunn R., Kehry M.R., Farkas B., Finnegan A. 2008. Suppression of proteoglycan-induced arthritis by anti-CD20 B Cell depletion therapy is mediated by reduction in autoantibodies and CD4+ T cell reactivity. J. Immunol. 180:4994–5003 [DOI] [PubMed] [Google Scholar]
- Harris M.B., Chang C.C., Berton M.T., Danial N.N., Zhang J., Kuehner D., Ye B.H., Kvatyuk M., Pandolfi P.P., Cattoretti G., et al. 1999. Transcriptional repression of Stat6-dependent interleukin-4-induced genes by BCL-6: specific regulation of iepsilon transcription and immunoglobulin E switching. Mol. Cell. Biol. 19:7264–7275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes N.M., Allen C.D., Lesley R., Ansel K.M., Killeen N., Cyster J.G. 2007. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J. Immunol. 179:5099–5108 [DOI] [PubMed] [Google Scholar]
- Herber D., Brown T.P., Liang S., Young D.A., Collins M., Dunussi-Joannopoulos K. 2007. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. J. Immunol. 178:3822–3830 [DOI] [PubMed] [Google Scholar]
- Johnston R.J., Poholek A.C., DiToro D., Yusuf I., Eto D., Barnett B., Dent A.L., Craft J., Crotty S. 2009. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 325:1006–1010 10.1126/science.1175870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C. 2009. New insights into the differentiation and function of T follicular helper cells. Nat. Rev. Immunol. 9:757–766 10.1038/nri2644 [DOI] [PubMed] [Google Scholar]
- Larousserie F., Charlot P., Bardel E., Froger J., Kastelein R.A., Devergne O. 2006. Differential effects of IL-27 on human B cell subsets. J. Immunol. 176:5890–5897 [DOI] [PubMed] [Google Scholar]
- Lim H.W., Kim C.H. 2007. Loss of IL-7 receptor alpha on CD4+ T cells defines terminally differentiated B cell-helping effector T cells in a B cell-rich lymphoid tissue. J. Immunol. 179:7448–7456 [DOI] [PubMed] [Google Scholar]
- Linterman M.A., Beaton L., Yu D., Ramiscal R.R., Srivastava M., Hogan J.J., Verma N.K., Smyth M.J., Rigby R.J., Vinuesa C.G. 2010. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J. Exp. Med. 207:353–363 10.1084/jem.20091738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C.S., Suryani S., Avery D.T., Chan A., Nanan R., Santner-Nanan B., Deenick E.K., Tangye S.G. 2009. Early commitment of naïve human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunol. Cell Biol. 87:590–600 10.1038/icb.2009.64 [DOI] [PubMed] [Google Scholar]
- Marinova E., Han S., Zheng B. 2006. Human germinal center T cells are unique Th cells with high propensity for apoptosis induction. Int. Immunol. 18:1337–1345 10.1093/intimm/dxl066 [DOI] [PubMed] [Google Scholar]
- Mietzner B., Tsuiji M., Scheid J., Velinzon K., Tiller T., Abraham K., Gonzalez J.B., Pascual V., Stichweh D., Wardemann H., Nussenzweig M.C. 2008. Autoreactive IgG memory antibodies in patients with systemic lupus erythematosus arise from nonreactive and polyreactive precursors. Proc. Natl. Acad. Sci. USA. 105:9727–9732 10.1073/pnas.0803644105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi Y., Saito M., Tsuchiya S., Tsuge I., Takada H., Hara T., Kawamura N., Ariga T., Pasic S., Stojkovic O., et al. 2007. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 448:1058–1062 10.1038/nature06096 [DOI] [PubMed] [Google Scholar]
- Miyazaki Y., Inoue H., Matsumura M., Matsumoto K., Nakano T., Tsuda M., Hamano S., Yoshimura A., Yoshida H. 2005. Exacerbation of experimental allergic asthma by augmented Th2 responses in WSX-1-deficient mice. J. Immunol. 175:2401–2407 [DOI] [PubMed] [Google Scholar]
- Nacionales D.C., Kelly-Scumpia K.M., Lee P.Y., Weinstein J.S., Lyons R., Sobel E., Satoh M., Reeves W.H. 2007. Deficiency of the type I interferon receptor protects mice from experimental lupus. Arthritis Rheum. 56:3770–3783 10.1002/art.23023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacionales D.C., Weinstein J.S., Yan X.J., Albesiano E., Lee P.Y., Kelly-Scumpia K.M., Lyons R., Satoh M., Chiorazzi N., Reeves W.H. 2009. B cell proliferation, somatic hypermutation, class switch recombination, and autoantibody production in ectopic lymphoid tissue in murine lupus. J. Immunol. 182:4226–4236 10.4049/jimmunol.0800771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewold T.B., Clark D.N., Salloum R., Poole B.D. 2010. Interferon alpha in systemic lupus erythematosus. J. Biomed. Biotechnol. 2010:948364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva R.I., Chung Y., Hwang D., Yang X.O., Kang H.S., Ma L., Wang Y.H., Watowich S.S., Jetten A.M., Tian Q., Dong C. 2008. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 29:138–149 10.1016/j.immuni.2008.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva R.I., Chung Y., Martinez G.J., Yang X.O., Tanaka S., Matskevitch T.D., Wang Y.H., Dong C. 2009. Bcl6 mediates the development of T follicular helper cells. Science. 325:1001–1005 10.1126/science.1176676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflanz S., Timans J.C., Cheung J., Rosales R., Kanzler H., Gilbert J., Hibbert L., Churakova T., Travis M., Vaisberg E., et al. 2002. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 16:779–790 10.1016/S1074-7613(02)00324-2 [DOI] [PubMed] [Google Scholar]
- Pflanz S., Hibbert L., Mattson J., Rosales R., Vaisberg E., Bazan J.F., Phillips J.H., McClanahan T.K., de Waal Malefyt R., Kastelein R.A. 2004. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J. Immunol. 172:2225–2231 [DOI] [PubMed] [Google Scholar]
- Pirhonen J., Sirén J., Julkunen I., Matikainen S. 2007. IFN-alpha regulates Toll-like receptor-mediated IL-27 gene expression in human macrophages. J. Leukoc. Biol. 82:1185–1192 10.1189/jlb.0307157 [DOI] [PubMed] [Google Scholar]
- Pot C., Jin H., Awasthi A., Liu S.M., Lai C.Y., Madan R., Sharpe A.H., Karp C.L., Miaw S.C., Ho I.C., Kuchroo V.K. 2009. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J. Immunol. 183:797–801 10.4049/jimmunol.0901233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves W.H., Lee P.Y., Weinstein J.S., Satoh M., Lu L. 2009. Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends Immunol. 30:455–464 10.1016/j.it.2009.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remoli M.E., Gafa V., Giacomini E., Severa M., Lande R., Coccia E.M. 2007. IFN-beta modulates the response to TLR stimulation in human DC: involvement of IFN regulatory factor-1 (IRF-1) in IL-27 gene expression. Eur. J. Immunol. 37:3499–3508 10.1002/eji.200737566 [DOI] [PubMed] [Google Scholar]
- Roes J., Rajewsky K. 1993. Immunoglobulin D (IgD)-deficient mice reveal an auxiliary receptor function for IgD in antigen-mediated recruitment of B cells. J. Exp. Med. 177:45–55 10.1084/jem.177.1.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh M., Reeves W.H. 1994. Induction of lupus-associated autoantibodies in BALB/c mice by intraperitoneal injection of pristane. J. Exp. Med. 180:2341–2346 10.1084/jem.180.6.2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt N., Morita R., Bourdery L., Bentebibel S.E., Zurawski S.M., Banchereau J., Ueno H. 2009. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity. 31:158–169 10.1016/j.immuni.2009.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S., Sugiyama N., Masutani K., Sadanaga A., Miyazaki Y., Inoue Y., Akahoshi M., Katafuchi R., Hirakata H., Harada M., et al. 2005. Membranous glomerulonephritis development with Th2-type immune deviations in MRL/lpr mice deficient for IL-27 receptor (WSX-1). J. Immunol. 175:7185–7192 [DOI] [PubMed] [Google Scholar]
- Stumhofer J.S., Laurence A., Wilson E.H., Huang E., Tato C.M., Johnson L.M., Villarino A.V., Huang Q., Yoshimura A., Sehy D., et al. 2006. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat. Immunol. 7:937–945 10.1038/ni1376 [DOI] [PubMed] [Google Scholar]
- Stumhofer J.S., Silver J.S., Laurence A., Porrett P.M., Harris T.H., Turka L.A., Ernst M., Saris C.J., O’Shea J.J., Hunter C.A. 2007. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 8:1363–1371 10.1038/ni1537 [DOI] [PubMed] [Google Scholar]
- Vogelzang A., McGuire H.M., Yu D., Sprent J., Mackay C.R., King C. 2008. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 29:127–137 10.1016/j.immuni.2008.06.001 [DOI] [PubMed] [Google Scholar]
- Wellmann U., Letz M., Herrmann M., Angermüller S., Kalden J.R., Winkler T.H. 2005. The evolution of human anti-double-stranded DNA autoantibodies. Proc. Natl. Acad. Sci. USA. 102:9258–9263 10.1073/pnas.0500132102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Richman L., Higgs B.W., Morehouse C.A., de los Reyes M., Brohawn P., Zhang J., White B., Coyle A.J., Kiener P.A., Jallal B. 2009. Neutralization of interferon-alpha/beta-inducible genes and downstream effect in a phase I trial of an anti-interferon-alpha monoclonal antibody in systemic lupus erythematosus. Arthritis Rheum. 60:1785–1796 10.1002/art.24557 [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Okada K., Morishima N., Kamiya S., Owaki T., Asakawa M., Iwakura Y., Fukai F., Mizuguchi J. 2004. Induction of IgG2a class switching in B cells by IL-27. J. Immunol. 173:2479–2485 [DOI] [PubMed] [Google Scholar]
- Yu D., Batten M., Mackay C.R., King C. 2009a. Lineage specification and heterogeneity of T follicular helper cells. Curr. Opin. Immunol. 21:619–625 10.1016/j.coi.2009.09.013 [DOI] [PubMed] [Google Scholar]
- Yu D., Rao S., Tsai L.M., Lee S.K., He Y., Sutcliffe E.L., Srivastava M., Linterman M., Zheng L., Simpson N., et al. 2009b. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 31:457–468 10.1016/j.immuni.2009.07.002 [DOI] [PubMed] [Google Scholar]
- Zotos D., Coquet J.M., Zhang Y., Light A., D’Costa K., Kallies A., Corcoran L.M., Godfrey D.I., Toellner K.M., Smyth M.J., et al. 2010. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J. Exp. Med. 207:365–378 10.1084/jem.20091777 [DOI] [PMC free article] [PubMed] [Google Scholar]