Abstract
Objectives
To evaluate the role of GH in colon carcinogenesis, we examined the formation of aberrant crypt foci (ACFs) and tumor development in wild type (WT) and GH-deficient, spontaneous dwarf rats (SDRs) exposed to the carcinogen azoxymethane (AOM).
Design
ACF were quantified by stereomicroscopy and tumor number and weights were recorded for each animal. Cell proliferation was measured by vincristine metaphase arrest, flow cytometry, and bromode-oxyuridine (BrdU) incorporation. Apoptosis was measured by TUNEL staining and cleaved caspase-3 immunohistochemistry. IGF-I was measured by radioimmunoassay (RIA). Hexokinase activity was measured by spectrophotometric assay. PARP cleavage, and IGF-IR, and p27kip/cip expression were measured by Western blotting.
Results
ACFs detected by stereomicroscopy were markedly reduced (~85%) in SDRs vs. WT rats at 10, 25, and 28 weeks after AOM. Tumor incidence, number, and weight also were reduced in SDR vs. WT animals. AOM treatment increased cell proliferation in the distal colon (where tumors occur) of WT rats but not SDRs, and these changes corresponded to increased ACF and tumor formation. Apoptosis rates were similar in AOM-treated WT and SDRs. Alterations in serum IGF-I levels may contribute to differences in the proliferative response to AOM and decreased ACF formation in SDR vs. WT rats.
Conclusions
We conclude that early neoplastic lesions (ACFs) were reduced in GH-deficient animals. This effect corresponds with differences in AOM-induced proliferation, but not apoptosis. These data indicate that GH is required for the full effect of AOM on colon ACF and tumor development, and that the SDR rat is a promising model for studies regarding the role of GH/IGF system in the initiation and promotion of colon cancer.
Keywords: Aberrant crypt foci, Azoxymethane, Growth hormone, Carcinogenesis
1. Introduction
Growth hormone (GH) and insulin-like growth factor-I (IGF-I), which mediates effects of GH on somatic growth, have been implicated in the etiology of primary cancers of the breast [1], prostate [2], and colon [3]. Importantly, this GH/IGF axis has also been intimately linked to metastatic disease processes as well [4]. Acromegaly, a disease characterized by elevated circulating levels of both GH and IGF-I, is associated with increased risk of colon adenomas [5] and colon cancer [6]. GH polymorphisms that are associated with reduced IGF-I levels have reduced cancer incidence [7]. Conversely, elevated serum IGF-I levels have been associated with increased risk of colonic adenomas [8] while insulin [9] but not IGF-I levels [10] have correlated with colon cancer risk.
The spontaneous dwarf rat (SDR) is homozygous for a point mutation designated dr in the GH gene, which results in undetectable amounts of GH in the pituitary and circulation, markedly reduced circulating levels of IGF-I (~20% normal levels) and growth retardation [11]. The SDR was first identified as a spontaneous mutation in a Sprague-Dawley colony [12]. The dr mutation is located at the 3′ splice site of intron 3 and produces a stop codon and truncated non-functional protein. The dwarf phenotype is inherited in an autosomal recessive manner [13]. Because other pituitary functions appear to be normal, the SDR provides a relatively pure model of growth hormone deficiency. Sonntag and colleagues have shown that the SDR model develops fewer spontaneous neoplasms relative to wild type control rats [14]. To explore the role of the GH-IGF axis in the development of colon cancer, we examined the formation of aberrant crypt foci (ACFs) and tumor development in wild type (WT) and SDR littermates exposed to the carcinogen azoxymethane (AOM).
AOM and its parent compound dimethylhydrazine are alkylating agents that mutate DNA by binding methyl or alkyl groups to guanine residues causing G to A transition mutations [15]. The genetic sequelae of these base pair substitutions are the initiation and maintenance of Ki-ras and β-catenin mutations within early neoplastic lesions that lead to the formation of ACFs, and subsequently, colon tumors.
ACFs are monoclonal collections of abnormal crypts whose formation occurs in a dose-dependent manner in response to carcinogen exposure [16]. Crypt progenitor cells in response to DNA damage undergo apoptosis within 6–8 h post exposure to AOM. Progenitor cells that evade apoptosis subsequently initiate a proliferative response 48–72 h later [17]. These foci of aberrant crypts are monoclonal in origin [18,19] and arise by a process of incomplete crypt fissioning. This process occurs at low levels in the normal murine colon but is increased over 4-fold 4–8 weeks after exposure to carcinogen [20]. Serial sections of ACFs demonstrate that they are incompletely separated from their clonal counterparts at the crypt surface, indicating that impaired fissioning contributes to the formation of these enlarged and abnormal crypts [21]. It is thought that one or more mutational events occurs within a single cell in the proliferative crypt compartment which subsequently is clonally fixed by evading apoptosis, and then goes on to form an ACF by a combination of cell proliferation and incomplete crypt fissioning.
In the present study, we compared the response of SDR and WT rats at specific time points after AOM administration to evaluate the role of growth hormone across a well defined sequence of events linked to malignant transformation of the colon. We speculated that GH might directly or indirectly regulate either the apoptosis cascade, including PARP and caspase-3, and/or the proliferative response to AOM treatment. Since most ACFs and cancers (~70%) develop within the distal colon in both rat and mouse in response to a systemically administered carcinogen, we also compared AOM-induced apoptosis and proliferation in proximal and distal colon segments and the axial expression of growth factor receptor proteins in WT and SDRs.
2. Materials and methods
2.1. Reagents
Azoxymethane was obtained from the NCI Chemical Carcinogen Reference Standard Repository, Midwest Research Institute (Kan-sas City, MO). Schiff’s reagent, vincristine, bromodeoxyuridine (BrdU), and all other biochemical reagents were from Sigma Chemical Co. (St. Louis, MO). Buffered formalin, xylene, and alcohols (histology grade) were from Fisher Scientific (Pittsburgh, PA). The PARP (#9542) antibodies were purchased from Cell Signaling (Beverly, MA); actin (sc-8342) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Male Sprague-Dawley and SDR littermates were bred at UIC.
2.2. Animal care mating and genotyping
Rats were allowed free access to water and Harlan Teklad 8640 diet (Harlan Teklad, Madison, WI). The Institutional Animal Care and Use Committees of the University of Illinois at Chicago and the Jesse Brown VA Medical Center approved this study. The SDR mutation was genotyped using the PCR/RFLP method of Nogami et al. [22]. Heterozygous (Gh+/dr) were mated and only Gh+/+ and Ghdr/dr littermates animals were used in the current studies.
2.3. Carcinogen administration, drug dosing, and observation protocol
Male SDR rats and wild type Sprague-Dawley littermates were studied at 8 weeks of age. Rats received 15 mg/kg of AOM subcutaneously (s.c.) twice 7 days apart (total dose 30 mg/kg) at 8 and 9 weeks of age. Rats were weighed weekly and euthanized by CO2 asphyxiation at 10, 25, or 28 weeks after receiving their initial dose of AOM. Two hours prior to sacrifice, rats received vincristine 1 mg/kg or 50 mg/kg BrdU by intraperitoneal (i.p.) injection, to assess colon epithelial cell proliferation. Six animals of each genotype received 30 mg/kg AOM and were sacrificed 8 h or 72 h post carcinogen, when the maximal apoptotic or proliferative response to AOM occurs [17].
2.4. Analysis of ACFs and tumors
ACFs were identified using the criteria of McLellan and Bird [23], and their positions recorded. To be considered an ACF, each structure had to have at least 4 of the 5 following criteria: crypts that were 2–3 times larger than normal; a thickened layer of epithelial cells; an increased pericryptal area; slit shaped lumina; and be microscopically elevated above the plane of normal crypts in the preparation [23]. Tumors from the opened colon were excised from the colon with a scalpel and their “wet” weight determined on a Mettler precision balance. All tissues were formalin-fixed and paraffin-embedded according to standard Armed Forces Institute of Pathology protocol [24].
2.5. Quantitative metaphase crypt analysis
The number of proliferating colonic crypt cells was determined by counting vincristine-arrested cellular metaphases from freshly dissected crypts according to the method of Alferez and Goodlad [25]. Metaphase nuclei from 20 separate crypts were identified and counted at 400×.
2.6. BrdU and cleaved caspase-3 immunohistochemistry
The antibody employed was a rabbit polyclonal antibody to BrdU incorporated into DNA of proliferating cells in S phase [26]. Proliferation was determined from colon specimens fixed in methyl-Carnoy’s solution using methods of McGinley et al. [27], which ensures maximum immunoreactivity with the BrdU antibody. Immunohistochemistry was performed using an automated two-stage modification of an immunoperoxidase technique we have previously described [28].
To visualize cleaved caspase-3, paraffin-embedded tissue sections were pretreated for antigen recovery by microwave heating in 0.01 M citrate (pH 6.0) and incubated with rabbit polyclonal cleaved Caspase-3 antibody (Asp-175, Cell Signaling Technology, Beverly, MA, USA) at 1:100 dilution as recommended by the manufacturer. The sections were incubated for 1 h with FITC-conjugated anti-mouse IgG (Fab specific 1:100, Sigma Chemical Co., St. Louis, MO). The slides were examined with an Olympus BX-50 fluorescence microscope with appropriate excitation and barrier filters.
2.7. Crypt apoptosis
Cellular apoptosis in response to AOM was determined using the DeadEnd Colorimetric Apoptosis Detection System (Promega, Madison, WI), a modified terminal dUTP nick-end labeling (TUNEL) assay. Apoptotic cells per crypt (HPF, 400×) were counted and averaged for a minimum of 3 crypts/slide.
2.8. IGF-I RIA and hexokinase assay
Serum IGF-I levels were measured using a radioimmunoassay kit (Nichols Institute Diagnostics, San Clemente, CA). To remove IGF binding proteins, briefly centrifuged serum samples were acidified with 0.5 N HCl. The acidified sample was loaded onto a pre-washed C-18 Sep-Pak column and IGF-I was eluted and dried under vacuum prior to assay as per the manufacturer’s instructions. Hexokinase (HK) activity was measured as the total glucose phosphorylating of fresh whole tissues lysates using a standard glucose-6-phosphate dehydrogenase-coupled spectrophotometric assay as described previously [30] with minor modifications. HK specific activity was measured as described by Taneja et al. [29].
2.9. Western Immunoblot for IGF-I receptor and p27Kip/Cip1
Tissue lysates were prepared from sequential 2 cm sections of rat colon minced and dounced followed by sonication in a polytron homogenizer (Brinkman Instruments, Westbury, NY) for 30 s in cold lysis buffer. Protein preparations were heated in Laemmli sample buffer, resolved by 10% SDS/PAGE, and then electrotransferred to nitrocellulose. Membranes were incubated overnight at 4 °C blocked in 5% nonfat dry milk and 0.05% Tween in TBS. Blots were treated with primary antibody for 1.5 h at 21 °C, followed by secondary antibody conjugated to horseradish peroxidase for 1 h. Finally, the blots were developed on Kodak Biomax film using a luminal enhanced chemiluminescence kit (Santa Cruz Biotechnology, Santa Cruz, CA). Quantitation of band intensity was performed using Kodak 1D image analysis software (Eastman Kodak, Rochester, NY).
2.10. Statistical analysis
Statistical analyses were performed using STATA, version 7.0 software (College Station, TX) and Microsoft Excel. Differences between SDR and WT rats with respect to ACF number, proliferation, apoptosis, and protein levels were evaluated by non-paired two tailed student’s t-test. Fischer’s exact test was used to evaluate tumor and adenoma incidence.
3. Results
3.1. Growth and AOM response in wild type and SDR rats
Body weights were reduced in SDR rats by over 50% in adult 8-week-old males compared to age-matched WT littermates, and serum IGF-I levels also were greatly reduced in SDR vs. WT rats (9 ± 4 ng/ml vs. 592 ± 148 ng/ml; P < 0.01), consistent with previous reports [11,30]. The length of the colons were reduced by 25% in 8-week-old SDR rats compared to WT animals (13 ± 1.2 cm vs. 17 ± 2.1 cm, P = 0.001) and colon length did not change significantly in WT or SDR rats over the course of the 28-week experiment. These results also are consistent with previous studies in the SDR [31]. AOM treatment caused a modest reduction in weight (<10%) in both WT and SDR rats, which was maximal 10 weeks after treatment and resolved 3 weeks later, after which, the growth of WT and SDRs were unaffected by AOM treatment. AOM treatment had no significant effect on IGF-I serum levels in either SDR or WT animals at 28 weeks (data not shown).
3.2. Tumor characteristics in SDR and WT rats
As shown in Table 1, tumor number, tumor multiplicity, and tumor weight were all reduced in the SDR and these differences reached or approached significance 28 weeks after carcinogen exposure. Reductions in tumor weight were directly proportional to animal weight (mean tumor wt/animal wt = 0.22 ± 0.9 in both SDR and WT rats. Reduced tumor multiplicity in SDR rats was proportional to the reduction in colon length (Table 1). These differences were also significant. However, the percentage of SDR animals with tumors at 28 weeks (25% vs. 45%) and adenomas at 25 weeks (10% vs. 25%) were ~50% lower in the AOM-treated SDR rat (not proportional to length or weight) but these differences did not reach significance (Table 1). Tumors from both strains were assayed for hexokinase activity and stained for β-catenin. Mucinous carcinoma was found in a proximal location in a single WT and SDR animals. All other tumors were moderately well differentiated. We analyzed 12 tumor blocks for β-catenin expression which was cytoplasmically expressed but did not differ significantly between SDR and WT tumors but was more strongly over expressed in mucinous tumor of both animals. Hexokinase activity was slightly increased within tumors from the background colon but did not differ between animal genotypes (data not shown). The distribution of tumors was similar in both genotypes, with 71% and 75% of colon tumors located in the distal colon (defined as the first 6 cm of colon from the anal verge) of SDR and WT rats, respectively.
Table 1.
Tumors in AOM-treated wild type (WT) and spontaneous dwarf (SDR) rats at 28 weeks.
| Number of rats | Number of tumors | Tumor multiplicity | Tumor incidence | Tumor weight (mg) |
Adenoma incidence | ||
|---|---|---|---|---|---|---|---|
| Mean ± SEM | Range | ||||||
| WT | 20 | 17 | 1.8 | 45% | 117 ± 38 | 31–392 | 25% |
| SDR | 20 | 7 | 1.1 | 25% | 35 ± 10 | 2.5–86 | 10% |
| P value | 0.06 | 0.05 | 0.11 | 0.04 | 0.21 | ||
3.3. ACF formation in SDR and WT rats
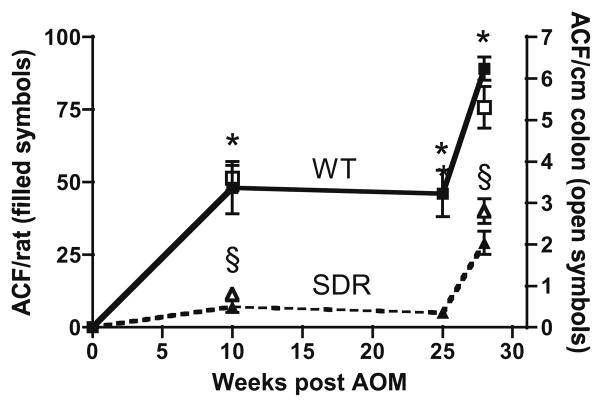
ACFs are the earliest neoplastic lesion identified after AOM carcinogen exposure [23] and are an early and predictive marker of subsequent tumor development [32]. As shown in Fig. 1, the number of ACFs was significantly reduced in SDR vs. WT rats at all time points measured after AOM treatment. No ACFs were detected in any animal of either genotype not exposed to AOM. Ten weeks after AOM exposure, WT rats had increased ACF number per colon (48 ± 9, mean ± SEM) compared to SDR rats treated with equivalent doses of AOM (7 ± 2, P = 0.01; Fig. 1). Large ACF (≥4 crypts per foci), an early marker of tumor susceptibility [33], were also more markedly increased in WT animals (15 ± 2.0 vs. 0.7 ± 0.5 P = 0.002). These differences persisted 25 weeks after AOM exposure with ACF number remaining markedly reduced in SDR compared to WT rats (5 ± 0.5 vs. 46 ± 8, respectively, P = 0.01; Fig. 1). The number of ACFs per colon increased in SDR 28 weeks after AOM exposure, but remained less than WT (29 ± 4 vs. 89 ± 4, respectively, P = 0.006; Fig. 1). WT rats had 4-fold more ACF per unit colon length than their SDR littermates at 10 and 25 weeks (data combined) (3.6 ± 0.3 ACF/cm vs. 0.8 ± 0.1 ACF/cm, P < 0.02), though this difference per unit length declined to <2-fold in WT vs. SDR rats at 28 weeks (5.3 ± 0.5 ACF/cm vs. 2.8 ± 0.3 ACF/cm, respectively, P = 0.056; Fig. 1). The majority of ACF identified in WT and SDRs were found in the distal 6 cm of colon, consistent with previous reports in the Sprague-Dawley strain [34].
Fig. 1.
Atypical crypt foci (ACFs) in WT and SDR rats. Filled symbols connected by lines represent total number of ACF/total number animals at each time point in AOM-treated WT and SDRs. Data represented by open symbols represent total ACF/total length of colon. An asterisk (*) indicates significant difference in ACFs/animals in WT and SDR rats. A section mark (§) indicates significant difference in ACFs/cm in WT and SDR rats.
3.4. Crypt proliferation over time in control and AOM-treated rats
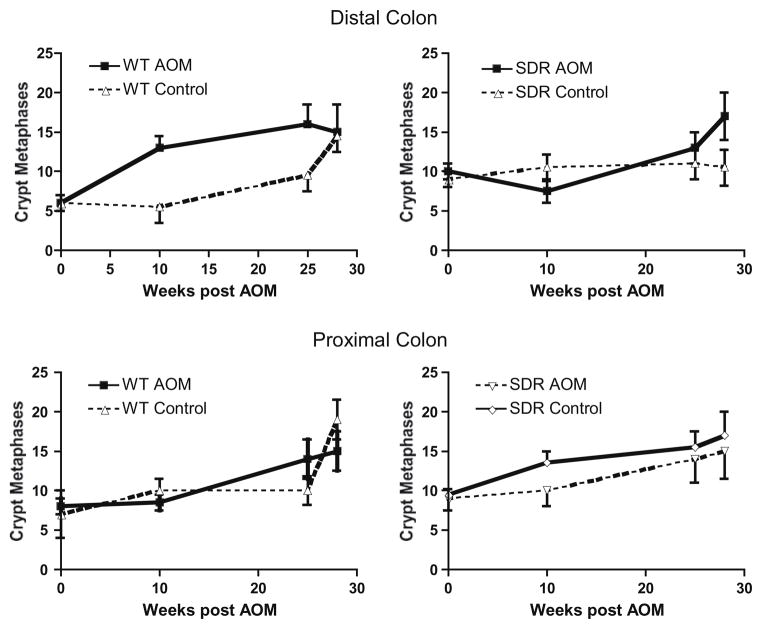
Previous studies have shown that there is an increase in the number of proliferating crypt cells and an apical shift in the distribution of proliferating cells in the crypts in the distal colon of rats treated with AOM [20]. Here, we evaluated cellular proliferation by counting vincristine metaphase arrested cells in whole crypt preparations from WT and SDR animals before (8 weeks old) and 10, 25, and 28 weeks after injection with AOM or carrier alone (Fig. 2). As shown in the two upper panels of Fig. 2, cellular proliferation in the distal colon increases with age in WT animals treated with carrier alone (control). Treatment with AOM further stimulates cellular proliferation in WT animals 10 and 25 weeks after the first injection of AOM compared to control. Interestingly, baseline cellular proliferation in the distal colon is increased ~2-fold in SDR rats compared to WT, before injection of AOM or carrier (P < 0.01), and there is no further increase in proliferation with time in SDR rats treated with carrier alone. In contrast to WT, cellular proliferation declines in SDR rats 10 weeks after AOM treatment, and increased proliferation is seen only at later time points in SDRs. As shown in the lower panels of Fig. 2, differences in cellular proliferation are less apparent in the crypts of the proximal colon in SDR vs. WT rats both at baseline and over time.
Fig. 2.
Metaphase analysis of cell proliferation in proximal and distal colon in control and AOM-treated WT and SDR rats. Proliferation was assessed by counting vincristine-arrested crypt metaphases in 20 dissected crypts from the distal and proximal colon of animals receiving AOM and their respective controls at each time point. Asterisks (*) indicate significant differences between AOM-treated and control WT rats. Section marks (§) indicate significant differences between AOM-treated and control SDR rats.
3.5. Early effects of AOM on apoptosis and cellular proliferation
Previous studies have identified a defined sequence of cellular events that occurs in response to AOM has been described within the colonic crypt [17]. DNA damage is followed 6–8 h later by a wave of apoptosis within the base of the crypt and then by a proliferative response that peaks 48–72 h later [17]. Interestingly, differences in the magnitude of this proliferative response predict the sensitivity of individual murine strains to the formation of AOM-induced tumors [35].
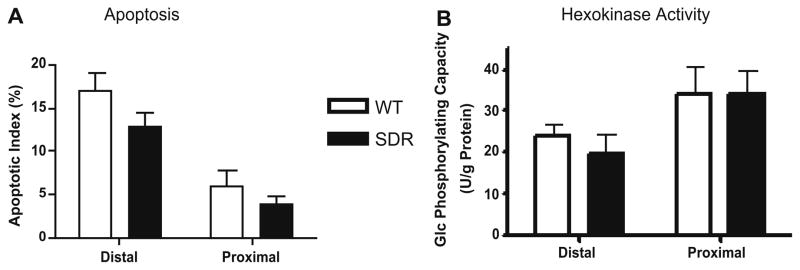
To assess early events that may contribute to differences in AOM-induced ACF and tumor formation in WT and SDRs, we examined apoptosis and cellular proliferation in colonic crypts 8 and 72 h after AOM exposure. Since GH-regulated growth factors can suppress apoptosis, we considered the possibility that increased sensitivity of the colonic epithelium to AOM-induced apoptosis might contribute to reduced formation of ACFs in GH-deficient SDR rats. Apoptotic cells were identified by TUNEL assay in the proliferative compartments of both the proximal and distal colon of both WT and SDR rats 8 h after treatment with AOM. Apoptotic cells were only rarely detected in the distal and proximal colon of SDR and WT rats treated with carrier alone (data not shown). Following treatment with AOM, apoptotic (TUNEL positive) cells were more abundant in the distal colon of both WT and SDR animals compared to the proximal colon, corresponding to the increase in AOM-induced ACFs and tumors that occur in this region of the colon in both genotypes (Fig. 3A). The percent of apoptotic cells detected tended to be reduced in SDR rats compared WT in both the proximal and distal colon, but this difference did not achieve statistical significance.
Fig. 3.
Apoptosis and hexokinase assay in WT and SDR rats. Panel A: TUNEL assay was performed 8 h after AOM treatment, and results expressed as the percent positively stained cells in 3–5 crypts from each animal. No significant difference in apoptotic (TUNEL positive) cells in AOM-treated SDR and WT rats. Panel B: Hexokinase activity was measured as total glucose phosphorylating activity as detailed in Section 2. No significant difference in hexokinase activity in distal vs. proximal colon of AOM-treated SDR or WT rats (P < 0.05).
Since hexokinase activity has been linked to resistance to apoptosis in diverse tissues, suggesting a link between energy metabolism and cell survival (reviewed in [36]), we also assessed hexokinase activity in the proximal and distal colon of SDR and WT rats. As shown in Fig. 3B, hexokinase activity was similar in SDR vs. WT rats in the proximal colon, and hexokinase activity was significantly higher in the proximal colon of both AOM-treated WT and SDR rats compared to the distal colon, where AOM-induced apoptosis was most pronounced (Fig. 3A).
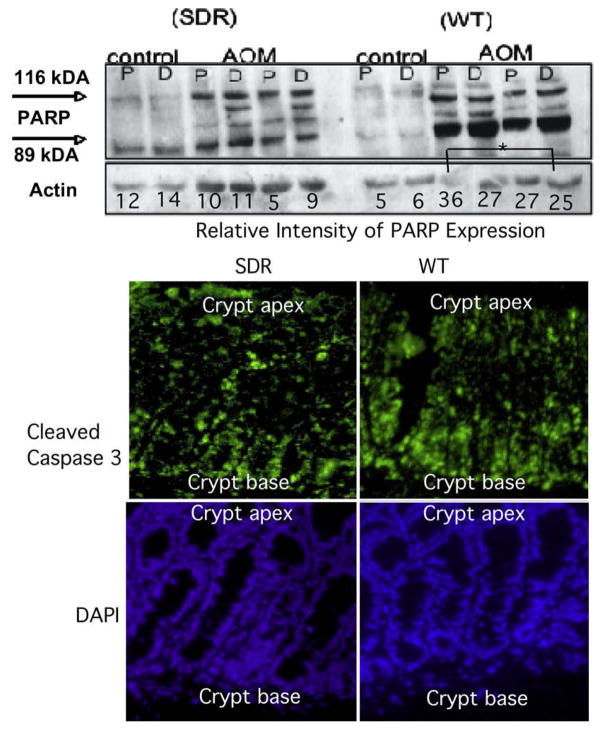
We also examined pro-apoptotic effects of AOM treatment in the distal colon of SDR and WT rats by measuring poly (ADP-ribose) polymerase (PARP) levels and cleavage by Western blotting, and caspase-3 activation by immunohistochemistry. As shown in the top panel of Fig 4, Western blotting showed that levels of PARP protein increased ~5-fold in the colon of WT rats 8 h post AOM exposure. Levels of PARP protein were not increased in SDR rats. Analysis of cleaved caspase-3 by fluorescent immunohistochemistry (bottom panel of Fig. 4) confirmed that the initiation of apoptosis was concentrated in the basal (proliferating) compartment of the distal colon crypt in both SDR and WT AOM-treated animals (Fig. 4), and similar to the PARP expression, suggested that caspsase-3 cleavage may be reduced in SDR compared to WT rats. Together, these results indicate that the reduction in ACF and tumor formation in SDR rats is not due to an increase in the susceptibility of SDR rats to AOM-induced apoptosis.
Fig. 4.
PARP and caspsase-3 cleavage. Top panel. Western blotting for poly (ADP) ribose protein (PARP). Whole cell lysates were prepared from proximal (P) or distal (D) colons from control or AOM-treated SDR or WT rats. Western blotting was conducted with an antibody that recognizes both uncleaved (116 kDA) PARP-1 as well as the large fragment (89 kDa) produced by caspase cleavage. Asterisks (*) indicate significant differences in cleaved PARP in WT rats treated with AOM (P < 0.05). Data shown for two controls and one treated animal. Bottom panel. Cleavage of caspase-3 in tissue sections. Fluorescent immunohistochemistry was performed to detect cleaved caspase-3 in the distal colon of SDR (left) and WT (right) rats 6 h after injection with AOM. Caspase-3 cleavage was detected in a gradient with highest levels in colonic epithelial cells at the base of the crypts in distal WT and SDR colon. Caspase-3 antibody binding was visualized using FITC (green), and nuclei were stained with DAPI (blue).
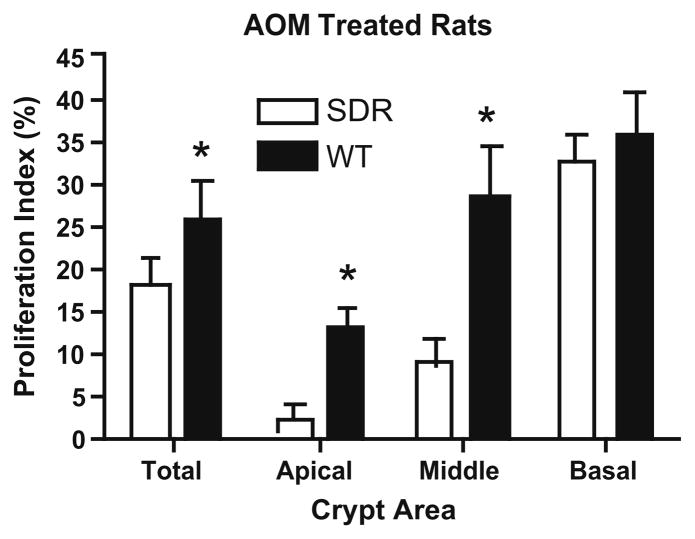
We next used the S-phase marker, BrdU labeling [37], to evaluate the proliferation of crypt cells 72 h after AOM treatment. Control animals treated with carrier alone showed that baseline cellular proliferation within individual crypts was greater in SDR compared to WT rats in the distal colon, consistent with results obtained at later time points by vincristine arrest (Fig. 2). Cellular proliferation increased in the colon of WT rats 72 h after AOM treatment, and this response was most marked in the distal colon, where ACF and tumor formation is favored. Interestingly, the majority of this increase occurred in the apical and middle 1/3 of the colonic crypt, while the increase in the basal 1/3 (the proliferative compartment in the resting crypt) was more modest. In contrast, AOM treatment failed to stimulate cell proliferation in the apical or middle region of the colon of SDR rats, and proliferation in these crypt compartments tended to decline (Fig. 5). These results indicate that the proliferative response to AOM treatment was attenuated in the colonic epithelium in GH-deficient animals, and suggest that impaired ability to stimulate cellular proliferation may limit the formation of ACFs in SDRs.
Fig. 5.
Cellular proliferation in distal crypts 72 h after AOM. Bar graphs summarize results for the proliferation index (percent cells BrdU positive) in the entire crypt (total), or in the basal, middle and apical portions of crypts in WT (black bars)and SDR rats (open bars) treated with AOM (black bars) or carrier alone (open bars). Results for a total of 10 crypts from three animals in each group are shown. Asterisks (*) indicate significant differences in the percent of cells labeled in the entire crypt or apical or middle compartments in WT rats treated with AOM (P < 0.05).
3.6. Axial distribution of selected proteins within the colon
Given that the proliferative response to AOM was altered at 72 h, and the differences in baseline proliferation identified in SDR vs. WT rats; we evaluated baseline expression of IGF-I receptor proteins whose expression would potentially be altered in GH-deficient animals and p27Kip/Cip, a cell cycle protein, implicated in the regulation of the proliferative zone of the colonic crypt. Since AOM induces cell proliferation and ACF in an asymmetric manner (distal > proximal colon), we also examined the axial distribution of these protein along the length of the colon.
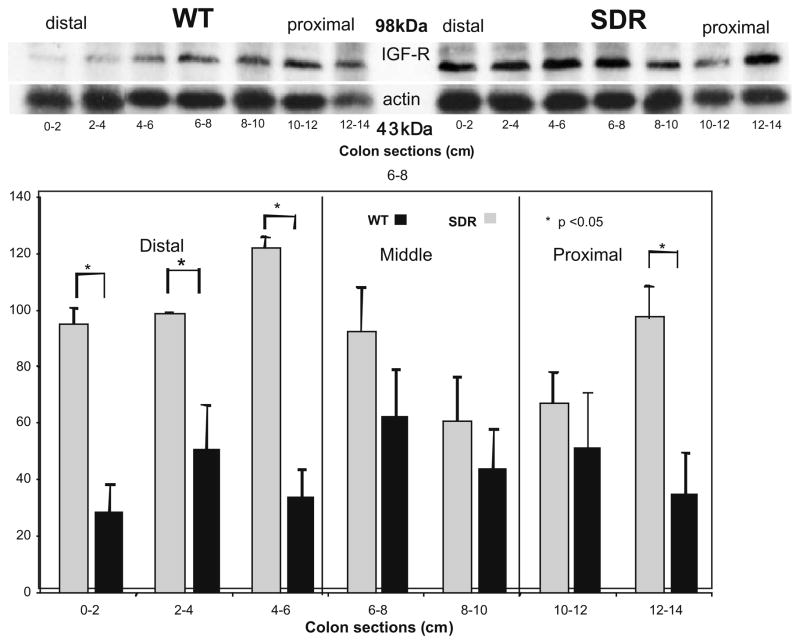
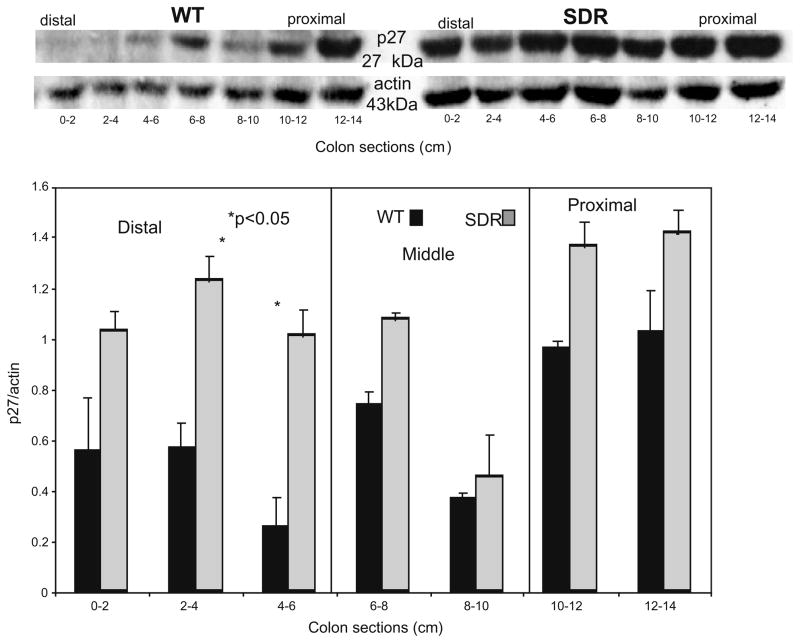
As shown in Fig 6, the expression of IGF-I receptor protein (IGF-IR) was increased in the distal 6 cm of the colon in SDR compared to WT rats, while levels in the middle and proximal segments of the colon do not differ significantly though were relatively higher in the SDR rat. Finally, Western blotting revealed that the expression of p27Kip/Cip decreases along the length of the WT colon (Fig. 7). In contrast, p27Kip/Cip is expressed uniformly along the length of the colon in SDR rats, and its expression is increased in the distal colon of SDR rats compared to WT (Fig. 7).
Fig. 6.
A representative Western blot for IGF-receptor and actin prepared from whole cell lysates of sequentially sectioned colons of control WT and SDR rats. Digital photographs of the relevant bands were obtained using an Eagle Eye imager, quantitated with Adobe Photoshop software, and normalized relative to actin intensity. Bar graphs calculated from 3 blots prepared from six control animals compare protein expression in distal, middle and proximal colon (mean ± SEM).
Fig. 7.
A representative Western blot for p27 and actin prepared from whole cell lysates of sequentially sectioned colons of control WT and SDR rats. Digital photographs of the relevant bands were obtained using an Eagle Eye imager, quantitated with Adobe Photoshop software, and normalized relative to actin intensity. Bar graphs calculated from 3 blots prepared from six control animals compare protein expression in distal, middle and proximal colon (mean ± SEM).
4. Discussion
Current knowledge regarding specific mechanisms by which GH promotes malignant transformation of the colon is limited and contradictory. A body of published literature suggests that IGF ligands and the IGF-I and insulin receptors are overexpressed in malignant tissue (reviewed in [4]). These individual receptors or malignant “hybrid” receptors are capable of downstream signaling both through the AKT → mTOR pathway and ras → MAPK signaling pathways resulting in proliferation and inhibition of apoptosis [38]. Furthermore, by enhancing angiogenesis, tumor invasion, and drug resistance these same signaling pathways may promote tumor metastasis [4]. Growth hormone effects are likely to be more indirect and mediated by IGF-I production by the liver and/or insulin production by the pancreas. Furthermore, attempt to block the IGF-I receptor can lead to increased GH levels and increase insulin and insulin receptor signaling [38]. Since GH deficiency in the SDR rat is due to a point mutation within the GH gene itself [39], the SDR rat provides a model whereby the effects of GH deficiency on the development of colon cancer can be examined under conditions where other pituitary functions are normal.
A major finding of this study is that there is dramatic reduction in the ability of AOM to induce the formation of ACFs, an early neoplastic lesion, in the colon of SDR rats compared to wild type litter-mates while reduction in tumor formation was more modest. A single report, in mice, has also shown ACF reduction in an AOM model with murine females expressing reduced serum IGF levels (LID mice) but not in their male counterparts. Tumor size but not multiplicity or incidence was also reduced in these animals though proliferative and/or apoptotic pathways were not explored [40]. ACF formation is reduced both by agents that promote apoptosis [41] or suppress crypt proliferation [42]. Previous studies have shown that the formation of DNA adducts induced by AOM is similar in susceptible and resistant mouse strains [43], while the proliferative response to this damage correlates strongly with strain susceptibility [36]. Previous studies also have shown that adduct formation induced by AOM is higher in distal vs. proximal colon [44]. Although we did not study the ability of AOM to induce DNA adducts in the colonic epithelium of SDR rats, we have previously shown that the ability of N-methyl-N-nitrosourea (MNU), an alkylating agent, to induce DNA adduct formation is similar in SDR and WT animals, while the formation of MNU-induced breast tumors is significantly reduced, indicating that other steps in tumor development are altered in GH-deficient animals [45].
In the present study, we evaluated both proliferation and apoptosis as early steps thought to be involved in AOM-induced ACF and tumor formation in the colon. AOM treatment induces apoptosis 6–8 h post exposure and this occurs predominately in the distal colon [18]. The DNA damage induced by these agents is associated with activation and cleavage of PARP [46] and caspase proteins [47] in response to DNA strand breaks. We have previously shown in p21-deficient mice that reduced apoptosis and cleaved caspase-3 expression in response to AOM resulted in significantly increased ACF formation [48], indicating that an inverse relationship between apoptotic rate and ACF formation has been demonstrated.
IGF-I, a major mediator of GH action, has been thought to have an anti-apoptotic role in prostate [49] and lung cancer [50] cells. Although circulating levels of IGF-I are markedly reduced in the SDR, TUNEL assay (which tests for DNA cleavage), PARP activation and cleavage (which reflects a response to DNA damage) and caspase-3 cleavage (the earliest committed step in apoptosis) indicate that the colonic epithelium of SDR rats is not more sensitive to AOM-induced apoptosis compared to WT rats. These findings indicate apoptosis is not increased (and likely to be reduced) in SDRs, and that differences in apoptotic events are not responsible for the reduction in ACF and tumor formation observed in SDRs.
In contrast, we find that the ability of AOM to stimulate proliferation is blunted in the colonic epithelium of SDRs compared to WT rats. This difference in proliferative response in the SDR is evident 72 h after AOM exposure and persists 25 weeks after treatment. Also, this effect is confined predominately to the distal colon where ACFs and tumor occur. Additional studies will be required to determine whether this reduction in the proliferative response to AOM treatment is due to differences in circulating levels of IGF-I, which are reduced in SDR rats, or to local effects of GH, including effects on other signaling pathways [51,52].
We found that the expression of IGF-I receptor and p27 proteins are increased in the distal colon of SDR rats compared to WT. The expression of the IGF-I receptor has been shown to be increased in other tissues in GH deficiency, and are suppressed by GH treatment [53], presumably due to effects of IGF-I on the expression of its own receptor. This suggests that GH and circulating or locally produced IGFs may contribute to the regulation of IGF-I receptor expression in the colon. Since IGF-I can both suppress and enhance the expression of the p27 [54], it is not unexpected that p27 expression is increased in the colon of the SDR rat. The role of p27 as a regulator of cdk2 and an inhibitor of proliferation within the crypt proliferative zone and its ability to promote both spontaneous and carcinogen-induced neoplasia when genetically abrogated [55] reflect the central role of this cell cycle protein in regulating response to DNA damage. Additionally, we have recently shown that NO–NSAID compounds which reduce DNA damage and ACF formation are associated with a corresponding increase in p27 expression within the distal colon [56]. In conflict with this finding is that baseline epithelial cell proliferation is increased despite the relatively abundant p27 expression in SDR rats. Yet, others have shown nuclear export of p27 results in increased crypt proliferation through up regulation of cdk2 activity [57]. It will be important in future studies to more closely examine intra-cellular localization and function of p27 proteins and other cell cycle regulatory proteins in the colon of SDR and WT animals.
We also found that the formation and size of tumors at 28 weeks is reduced in male SDRs compared to WT rats. However, this effect on tumor formation is less pronounced than that seen for ACF formation. This result contrasts with the bulk of published studies in the AOM-murine model in which ACF and tumor formation closely correspond [58]. In other animal tumor models, inhibition of the GH/IGF axis markedly reduced breast cancer development [59] but had little effect on prostate cancer formation [60] suggesting sex or organ differences in response to these endocrine signaling pathways. Given that the initial effect of AOM on apoptosis is not enhanced in SDR rats, and that GH deficiency does not appear to reduce the ability of carcinogens to induce potentially mutagenic chemical adducts in other settings, it is interesting to speculate that GH deficiency may delay the expansion of premalignant clones, and therefore subsequently limit the multiplicity and size of colon tumors in SDR vs. WT rats. It will be of interest to examine the effect of GH deficiency on the development of other early neoplastic lesions recently associated with the development of colon tumors, including the formation of ACFs associated with aberrant β-catenin signaling [61] and mucin depleted foci and dysplasia [62].
Unexpectedly, we found that baseline cellular proliferation is increased in the colon of SDRs compared to WT rats, based on both metaphase analysis and BrdU labeling studies. The mechanism(s) responsible for this effect are not clear. Others have found that proliferative activity increases in the aging rat colon [63], and we also found that baseline cellular proliferation increases with age in WT Sprague-Dawley rats. It has been shown that aging is accompanied by a reduction in GH secretion [64] and IGF-I production [65]. Additionally, loss of SOCS-2 expression, a negative regulator of GH action and indirectly EGF-receptor action, may allow increased receptor signaling and increased crypt proliferation in the aging rat [66]. This aging effect in the SDR rat is not seen. Further studies to examine the signaling pathways modulated by the GH/IGF axis in the colon over time will be of great interest.
Caloric restriction in previous studies reduces the occurrence of colon tumors in response to AOM [67] and other carcinogens [68]. Caloric restriction is associated with reduced levels of IGF-I, and IGF infusion partially restores the occurrence of carcinogen-induced tumors. Similarly, in a two-stage skin cancer model, caloric restriction reduces tumor promotion by reducing dermal proliferation [69] while dermal over-expression of IGF-I promotes tumor development and IGF-I reduction reduces tumor formation and proliferation in response to carcinogen [70]. We believe the reduced circulating levels of IGF-I and GH, in the SDR rat, are responsible for the reduction in ACF and tumor formation in this SDR animal model.
GH supplementation and/or over-expression has been show to protect against low dose radiation injury in rats [71] and in a DSS colitis model [72]. Thus, GH has demonstrated protective effect against colonic mucosal injury but perhaps at the cost of increased susceptibility to colon carcinogens. This may suggest that GH and IGF-I represent an example of antagonistic pleiotropy [14] in which these hormones promote mucosal repair in response to injury, and thus increasing relative fitness but at the expense of increased susceptibility to diseases of aging like colon cancer. Drugs that target GH/IGF signaling have recently entered clinical trials (reviewed in [38]. Understanding the role of the GH/IGF system in aging and colon carcinogenesis may be important in colon cancer treatment and/or chemoprevention.
Acknowledgments
The authors thank Jai Marchandani, Heliodoro Medina, Young Choi, Sean Lee, Tatiana Kouznetsova and Kevin Leung for contributing to the apoptosis and proliferation experiments. Financial support was provided by NIH grants CA-80360 (REC), DK41430 (TGU) and CA099904 (SMS) DK044525 (ALT) and the Department of Veterans Affairs Merit Review Program (R.E.C., R.B.R., and T.G.U.). This project was performed in part using compound(s) provided by the National Cancer Institute’s Chemical Carcinogen Reference Standards Repository operated under contract by Midwest Research Institute, No. N02-CB-07008.
References
- 1.Lann D, LeRoith D. The role of endocrine insulin-like growth factor-I and insulin in breast cancer. J Mammary Gland Biol Neoplasia. 2008;13:371–379. doi: 10.1007/s10911-008-9100-x. [DOI] [PubMed] [Google Scholar]
- 2.Nam RK, Trachtenberg J, Jewett MA, Toi A, Evans A, Emami M, Narod SA, Pollak M. Serum insulin-like growth factor-I levels and prostatic intraepithelial neoplasia: a clue to the relationship between IGF-I physiology and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14:1270–1273. doi: 10.1158/1055-9965.EPI-04-0430. [DOI] [PubMed] [Google Scholar]
- 3.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131:3109S–3120S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 4.Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins PJ, Frajese V, Jones AM, Camacho-Hubner C, Lowe DG, Fairclough PD, Chew SL, Grossman AB, Monson JP, Besser GM. Insulin-like growth factor I and the development of colorectal neoplasia in acromegaly. J Clin Endocrinol Metab. 2000;85:3218–3221. doi: 10.1210/jcem.85.9.6806. [DOI] [PubMed] [Google Scholar]
- 6.Matano Y, Okada T, Suzuki A, Yoneda T, Takeda Y, Mabuchi H. Risk of colorectal neoplasm in patients with acromegaly and its relationship with serum growth hormone levels. Am J Gastroenterol. 2005;100:1154–1160. doi: 10.1111/j.1572-0241.2005.40808.x. [DOI] [PubMed] [Google Scholar]
- 7.Le Marchand L, Donlon T, Seifried A, Kaaks R, Rinaldi S, Wilkens LR. Association of a common polymorphism in the human GH1 gene with colorectal neoplasia. J Natl Cancer Inst. 2002;94:454–460. doi: 10.1093/jnci/94.6.454. [DOI] [PubMed] [Google Scholar]
- 8.Schoen RE, Weissfeld JL, Kuller LH, Thaete FL, Evans RW, Hayes RB, Rosen CJ. Insulin-like growth factor-I and insulin are associated with the presence and advancement of adenomatous polyps. Gastroenterology. 2005;129:464–475. doi: 10.1016/j.gastro.2005.05.051. [DOI] [PubMed] [Google Scholar]
- 9.Saydah SH, Platz EA, Rifai N, Pollak MN, Brancati FL, Helzlsouer KJ. Association of markers of insulin and glucose control with subsequent colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2003;12:412–418. [PubMed] [Google Scholar]
- 10.Nomura AM, Stemmermann GN, Lee J, Pollak MN. Serum insulin-like growth factor I and subsequent risk of colorectal cancer among Japanese–American men. Am J Epidemiol. 2003;158:424–431. doi: 10.1093/aje/kwg176. [DOI] [PubMed] [Google Scholar]
- 11.Gargosky SE, Nanto-Salonen K, Tapanainen P, Rosenfeld RG. Pregnancy in growth hormone-deficient rats: assessment of insulin-like growth factors (IGFs), IGF-binding proteins (IGFBPs) and IGFBP protease activity. J Endocrinol. 1993;136:479–489. doi: 10.1677/joe.0.1360479. [DOI] [PubMed] [Google Scholar]
- 12.Okuma S. Study of growth hormone in spontaneous dwarf rat. Nippon Naibunpi Gakkai Zasshi. 1984;60:1005–1014. doi: 10.1507/endocrine1927.60.8_1005. [DOI] [PubMed] [Google Scholar]
- 13.Takeuchi T, Suzuki H, Sakurai S, Nogami H, Okuma S, Ishikawa H. Molecular mechanism of growth hormone (GH) deficiency in the spontaneous dwarf rat: detection of abnormal splicing of GH messenger ribonucleic acid by the polymerase chain reaction. Endocrinology. 1990;126:31–38. doi: 10.1210/endo-126-1-31. [DOI] [PubMed] [Google Scholar]
- 14.Sonntag WE, Carter CS, Ikeno Y, Ekenstedt K, Carlson CS, Loeser RF, Chakrabarty S, Lee S, Bennett C, Ingram R, Moore T, Ramsey M. Adult-onset growth hormone and insulin-like growth factor I deficiency reduces neoplastic disease, modifies age-related pathology, and increases life span. Endocrinology. 2005;146:2920–2932. doi: 10.1210/en.2005-0058. [DOI] [PubMed] [Google Scholar]
- 15.Dipple A. DNA adducts of chemical carcinogens. Carcinogenesis. 1995;16:437–441. doi: 10.1093/carcin/16.3.437. [DOI] [PubMed] [Google Scholar]
- 16.Bird RP. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett. 1987;37:147–151. doi: 10.1016/0304-3835(87)90157-1. [DOI] [PubMed] [Google Scholar]
- 17.Hirose Y, Yoshimi N, Makita H, Hara A, Tanaka T, Mori H. Early alterations of apoptosis and cell proliferation in azoxymethane-initiated rat colonic epithelium. Jpn J Cancer Res. 1996;87:575–582. doi: 10.1111/j.1349-7006.1996.tb00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ponder BA, Wilkinson MM. Direct examination of the clonality of carcinogen-induced colonic epithelial dysplasia in chimeric mice. J Natl Cancer Inst. 1986;77:967–976. [PubMed] [Google Scholar]
- 19.Siu IM, Robinson DR, Schwartz S, Kung HJ, Pretlow TG, Petersen RB, Pretlow TP. The identification of monoclonality in human aberrant crypt foci. Cancer Res. 1999;59:63–66. [PubMed] [Google Scholar]
- 20.Park HS, Goodlad RA, Wright NA. Crypt fission in the small intestine and colon. A mechanism for the emergence of G6PD locus-mutated crypts after treatment with mutagens. Am J Pathol. 1995;147:1416–1427. [PMC free article] [PubMed] [Google Scholar]
- 21.Kristt D, Bryan K, Gal R. Colonic aberrant crypts may originate from impaired fissioning: relevance to increased risk of neoplasia. Hum Pathol. 1999;30:1449–1458. doi: 10.1016/s0046-8177(99)90167-3. [DOI] [PubMed] [Google Scholar]
- 22.Nogami H, Tachibana T, Ishikawa H. Intrauterine growth retardation due to growth hormone deficiency in rats. Biol Neonate. 1995;68:412–418. doi: 10.1159/000244266. [DOI] [PubMed] [Google Scholar]
- 23.McLellan EA, Bird RP. Aberrant crypts: potential preneoplastic lesions in the murine colon. Cancer Res. 1988;48:6187–6192. [PubMed] [Google Scholar]
- 24.Prophet EB. American Registry of Pathology. Washington, DC: 1994. Armed Forces Institute of Pathology (US) and American Registry of Pathology, Laboratory Methods in Histotechnology. [Google Scholar]
- 25.Alferez D, Goodlad RA. To best measure cell proliferation in samples from the intestine. Cell Prolif. 2007;40:231–240. doi: 10.1111/j.1365-2184.2007.00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weidner N, Moore DH, 2nd, Ljung BM, Waldman FM, Goodson WH, 3rd, Mayall B, Chew K, Smith HS. Correlation of bromodeoxyuridine (BRDU) labeling of breast carcinoma cells with mitotic figure content and tumor grade. Am J Surg Pathol. 1993;17:987–994. doi: 10.1097/00000478-199310000-00003. [DOI] [PubMed] [Google Scholar]
- 27.McGinley JN, Knott KK, Thompson HJ. Effect of fixation and epitope retrieval on BrdU indices in mammary carcinomas. J Histochem Cytochem. 2000;48:355–362. doi: 10.1177/002215540004800305. [DOI] [PubMed] [Google Scholar]
- 28.Carroll RE, Matkowskyj KA, Chakrabarti S, McDonald TJ, Benya RV. Aberrant expression of gastrin-releasing peptide and its receptor by well-differentiated colon cancers in humans. Am J Physiol. 1999;276:G655–G665. doi: 10.1152/ajpgi.1999.276.3.G655. [DOI] [PubMed] [Google Scholar]
- 29.Taneja N, Coy PE, Lee I, Bryson JM, Robey RB. Proinflammatory interleukin-1 cytokines increase mesangial cell hexokinase activity and hexokinase II isoform abundance. Am J Physiol Cell Physiol. 2004;287:C548–C557. doi: 10.1152/ajpcell.00126.2003. [DOI] [PubMed] [Google Scholar]
- 30.Nogami H, Watanabe T, Kobayashi S. IGF-I and IGF-binding protein gene expressions in spontaneous dwarf rat. Am J Physiol. 1994;267:E396–E401. doi: 10.1152/ajpendo.1994.267.3.E396. [DOI] [PubMed] [Google Scholar]
- 31.Tei TM, Kissmeyer-Nielsen P, Christensen H, Flyvbjerg A. Growth hormone treatment increases transmural colonic growth in GH-deficient dwarf rats. Growth Horm IGF Res. 2000;10:85–92. doi: 10.1054/ghir.2000.0144. [DOI] [PubMed] [Google Scholar]
- 32.Papanikolaou A, Wang QS, Delker DA, Rosenberg DW. Azoxymethane-induced colon tumors and aberrant crypt foci in mice of different genetic susceptibility. Cancer Lett. 1998;130:29–34. doi: 10.1016/s0304-3835(98)00101-3. [DOI] [PubMed] [Google Scholar]
- 33.Papanikolaou A, Wang Q, Delker DA, Rosenberg DW. Azoxymethane-induced colon tumors and aberrant crypt foci in mice of different genetic susceptibility. Cancer Lett. 1998;130:29–34. doi: 10.1016/s0304-3835(98)00101-3. [DOI] [PubMed] [Google Scholar]
- 34.Cameron IL, Garza J, Hardman WE. Distribution of lymphoid nodules, aberrant crypt foci and tumours in the colon of carcinogen-treated rats. Br J Cancer. 1996;73:893–898. doi: 10.1038/bjc.1996.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deschner EE, Maskens AP. Significance of the labeling index and labeling distribution as kinetic parameters in colorectal mucosa of cancer patients and DMH treated animals. Cancer. 1982;50:1136–1141. doi: 10.1002/1097-0142(19820915)50:6<1136::aid-cncr2820500617>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 36.Robey RB, Hay N. Mitochondrial hexokinases: guardians of the mitochondria. Cell Cycle. 2005;4:654–658. doi: 10.4161/cc.4.5.1678. [DOI] [PubMed] [Google Scholar]
- 37.van Diest PJ, Brugal G, Baak JP. Proliferation markers in tumours: interpretation and clinical value. J Clin Pathol. 1998;51:716–724. doi: 10.1136/jcp.51.10.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 39.Charlton HM, Clark RG, Robinson IC, Goff AE, Cox BS, Bugnon C, Bloch BA. Growth hormone-deficient dwarfism in the rat: a new mutation. J Endocrinol. 1988;119:51–58. doi: 10.1677/joe.0.1190051. [DOI] [PubMed] [Google Scholar]
- 40.Ealey KN, Xuan W, Lu S, Archer MC. Colon carcinogenesis in liver-specific IGF-I-deficient (LID) mice. Int J Cancer. 2008;122:472–476. doi: 10.1002/ijc.23102. [DOI] [PubMed] [Google Scholar]
- 41.Volate SR, Davenport DM, Muga SJ, Wargovich MJ. Modulation of aberrant crypt foci and apoptosis by dietary herbal supplements (quercetin, curcumin, silymarin, ginseng and rutin) Carcinogenesis. 2005;26:1450–1456. doi: 10.1093/carcin/bgi089. [DOI] [PubMed] [Google Scholar]
- 42.Reddy BS. Prevention of colon cancer by pre- and probiotics: evidence from laboratory studies. Br J Nutr. 1998;80:S219–S223. [PubMed] [Google Scholar]
- 43.Papanikolaou A, Shank RC, Delker DA, Povey A, Cooper DP, Rosenberg DW. Initial levels of azoxymethane-induced DNA methyl adducts are not predictive of tumor susceptibility in inbred mice. Toxicol Appl Pharmacol. 1998;150:196–203. doi: 10.1006/taap.1998.8393. [DOI] [PubMed] [Google Scholar]
- 44.Jackson PE, O’Connor PJ, Cooper DP, Margison GP, Povey AC. Associations between tissue-specific DNA alkylation, DNA repair and cell proliferation in the colon and colon tumour yield in mice treated with 1, 2-dimethylhydrazine. Carcinogenesis. 2003;24:527–533. doi: 10.1093/carcin/24.3.527. [DOI] [PubMed] [Google Scholar]
- 45.Swanson SM, Unterman TG. The growth hormone-deficient Spontaneous Dwarf rat is resistant to chemically induced mammary carcinogenesis. Carcinogenesis. 2002;23:977–982. doi: 10.1093/carcin/23.6.977. [DOI] [PubMed] [Google Scholar]
- 46.Kupper JH, Muller M, Wolf I. NAD(+) consumption in carcinogen-treated hamster cells overexpressing a dominant negative mutant of poly(ADP-ribose) polymerase. Biochem Biophys Res Commun. 1999;265:525–529. doi: 10.1006/bbrc.1999.1690. [DOI] [PubMed] [Google Scholar]
- 47.Nordling MM, Nygren J, Bergman J, Sundberg K, Rafter JJ. Toxicological characterization of a novel in vivo benzo[a]pyrene metabolite, 7-oxo-benz[d]anthracene-3,4-dicarboxylic acid anhydride. Chem Res Toxicol. 2002;15:1274–1280. doi: 10.1021/tx025549l. [DOI] [PubMed] [Google Scholar]
- 48.Poole AJ, Heap D, Carroll RE, Tyner AL. Tumor suppressor functions for the Cdk inhibitor p21 in the mouse colon. Oncogene. 2004;23:8128–8134. doi: 10.1038/sj.onc.1207994. [DOI] [PubMed] [Google Scholar]
- 49.Ngo TH, Barnard RJ, Leung PS, Cohen P, Aronson WJ. Insulin-like growth factor I (IGF-I) and IGF binding protein-1 modulate prostate cancer cell growth and apoptosis: possible mediators for the effects of diet and exercise on cancer cell survival. Endocrinology. 2003;144:2319–2324. doi: 10.1210/en.2003-221028. [DOI] [PubMed] [Google Scholar]
- 50.Hurbin A, Coll JL, Dubrez-Daloz L, Mari B, Auberger P, Brambilla C, Favrot MC. Cooperation of amphiregulin and insulin-like growth factor-1 inhibits Bax- and Bad-mediated apoptosis via a protein kinase C-dependent pathway in non-small cell lung cancer cells. J Biol Chem. 2005;280:19757–19767. doi: 10.1074/jbc.M413516200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Costoya JA, Finidori J, Moutoussamy S, Searis R, Devesa J, Arce VM. Activation of growth hormone receptor delivers an antiapoptotic signal: evidence for a role of Akt in this pathway. Endocrinology. 1999;140:5937–5943. doi: 10.1210/endo.140.12.7209. [DOI] [PubMed] [Google Scholar]
- 52.Yamauchi T, Ueki K, Tobe K, Tamemoto H, Sekine N, Wada M, Honjo M, Takahashi M, Takahashi T, Hirai H, Tsushima T, Akanuma Y, Fujita T, Komuro I, Yazaki Y, Kadowaki T. Growth hormone-induced tyrosine phosphorylation of EGF receptor as an essential element leading to MAP kinase activation and gene expression. Endocr J. 1998;45(Suppl):S27–S31. doi: 10.1507/endocrj.45.suppl_s27. [DOI] [PubMed] [Google Scholar]
- 53.Eshet R, Werner H, Klinger B, Silbergeld A, Laron Z, LeRoith D, Roberts CT., Jr Up-regulation of insulin-like growth factor-I (IGF-I) receptor gene expression in patients with reduced serum IGF-I levels. J Mol Endocrinol. 1993;10:115–120. doi: 10.1677/jme.0.0100115. [DOI] [PubMed] [Google Scholar]
- 54.Ewton DZ, Kansra S, Lim S, Friedman E. Insulin-like growth factor-I has a biphasic effect on colon carcinoma cells through transient inactivation of forkhead1, initially mitogenic, then mediating growth arrest and differentiation. Int J Cancer. 2002;98:665–673. doi: 10.1002/ijc.10229. [DOI] [PubMed] [Google Scholar]
- 55.Yang W, Bancroft L, Nicholas C, Lozonschi I, Augenlicht LH. Targeted inactivation of p27kip1 is sufficient for large and small intestinal tumorigenesis in the mouse, which can be augmented by a Western-style high-risk diet. Cancer Res. 2003;63:4990–4996. [PubMed] [Google Scholar]
- 56.Hagos GK, Carroll RE, Kouznetsova T, Li Q, Toader V, Fernandez PA, Swanson SM, Thatcher GR. Colon cancer chemoprevention by a novel NO chimera that shows anti-inflammatory and antiproliferative activity in vitro and in vivo. Mol Cancer Ther. 2007;6:2230–2239. doi: 10.1158/1535-7163.MCT-07-0069. [DOI] [PubMed] [Google Scholar]
- 57.Besson A. p27Kip1: tumor suppressor and oncogene? Med Sci (Paris) 2007;23:1089–1091. doi: 10.1051/medsci/200723121089. [DOI] [PubMed] [Google Scholar]
- 58.Corpet DE, Tache S. Most effective colon cancer chemopreventive agents in rats: a systematic review of aberrant crypt foci and tumor data, ranked by potency. Nutr Cancer. 2002;43:1–21. doi: 10.1207/S15327914NC431_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pollak M, Blouin MJ, Zhang JC, Kopchick JJ. Reduced mammary gland carcinogenesis in transgenic mice expressing a growth hormone antagonist. Br J Cancer. 2001;85:428–430. doi: 10.1054/bjoc.2001.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anzo M, Cobb LJ, Hwang DL, Mehta H, Said JW, Yakar S, LeRoith D, Cohen P. Targeted deletion of hepatic Igf1 in TRAMP mice leads to dramatic alterations in the circulating insulin-like growth factor axis but does not reduce tumor progression. Cancer Res. 2008;68:3342–3349. doi: 10.1158/0008-5472.CAN-07-3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamada Y, Yoshimi N, Hirose Y, Kawabata K, Matsunaga K, Shimizu M, Hara A, Mori H. Frequent beta-catenin gene mutations and accumulations of the protein in the putative preneoplastic lesions lacking macroscopic aberrant crypt foci appearance, in rat colon carcinogenesis. Cancer Res. 2000;60:3323–3327. [PubMed] [Google Scholar]
- 62.Femia AP, Dolara P, Caderni G. Mucin-depleted foci (MDF) in the colon of rats treated with azoxymethane (AOM) are useful biomarkers for colon carcinogenesis. Carcinogenesis. 2004;25:277–281. doi: 10.1093/carcin/bgh005. [DOI] [PubMed] [Google Scholar]
- 63.Schmelz EM, Levi E, Du J, Xu H, Majumdar AP. Age-related loss of EGF-receptor related protein (ERRP) in the aging colon is a potential risk factor for colon cancer. Mech Ageing Dev. 2004;125:917–922. doi: 10.1016/j.mad.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 64.Martinoli MG, Ouellet J, Rheaume E, Pelletier G. Growth hormone and somatostatin gene expression in adult and aging rats as measured by quantitative in situ hybridization. Neuroendocrinology. 1991;54:607–615. doi: 10.1159/000125967. [DOI] [PubMed] [Google Scholar]
- 65.Velasco B, Cacicedo L, Melian E, Fernandez-Vazquez G, Sanchez-Franco F. Sensitivity to exogenous GH and reversibility of the reduced IGF-I gene expression in aging rats. Eur J Endocrinol. 2001;145:73–85. doi: 10.1530/eje.0.1450073. [DOI] [PubMed] [Google Scholar]
- 66.Michaylira CZ, Simmons JG, Ramocki NM, Scull BP, McNaughton KK, Fuller CR, Lund PK. Suppressor of cytokine signaling-2 limits intestinal growth and enterotrophic actions of IGF-I in vivo. Am J Physiol Gastrointest Liver Physiol. 2006;291:G472–G481. doi: 10.1152/ajpgi.00218.2005. [DOI] [PubMed] [Google Scholar]
- 67.Klurfeld DM, Weber MM, Kritchevsky D. Inhibition of chemically induced mammary and colon tumor promotion by caloric restriction in rats fed increased dietary fat. Cancer Res. 1987;47:2759–2762. [PubMed] [Google Scholar]
- 68.Dunn SE, Kari FW, French J, Leininger JR, Travlos G, Wilson R, Barrett JC. Dietary restriction reduces insulin-like growth factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53-deficient mice. Cancer Res. 1997;57:4667–4672. [PubMed] [Google Scholar]
- 69.Stewart JW, Koehler K, Jackson W, Hawley J, Wang W, Au A, Myers R, Birt DF. Prevention of mouse skin tumor promotion by dietary energy restriction requires an intact adrenal gland and glucocorticoid supplementation restores inhibition. Carcinogenesis. 2005;26:1077–1084. doi: 10.1093/carcin/bgi051. [DOI] [PubMed] [Google Scholar]
- 70.Moore T, Carbajal S, Beltran L, Perkins SN, Yakar S, Leroith D, Hursting SD, Digiovanni J. Reduced susceptibility to two-stage skin carcinogenesis in mice with low circulating insulin-like growth factor I levels. Cancer Res. 2008;68:3680–3688. doi: 10.1158/0008-5472.CAN-07-6271. [DOI] [PubMed] [Google Scholar]
- 71.Raguso CA, Leverve X, Pichard C. Protective effects of recombinant growth hormone on intestinal mucosa in rats receiving abdominal radiotherapy. Clin Nutr. 2002;21:487–490. doi: 10.1054/clnu.2002.0579. [DOI] [PubMed] [Google Scholar]
- 72.Williams KL, Fuller CR, Dieleman LA, DaCosta CM, Haldeman KM, Sartor RB, Lund PK. Enhanced survival and mucosal repair after dextran sodium sulfate-induced colitis in transgenic mice that overexpress growth hormone. Gastroenterology. 2001;120:925–937. doi: 10.1053/gast.2001.22470. [DOI] [PubMed] [Google Scholar]