Abstract
Cholecystokinin (CCK), a peptide originally discovered in the gastrointestinal tract, is one of the most the abundant and widely distributed neuropeptides in the brain. In spite of its abundance, recent data indicate that that CCK modulates intrinsic neuronal excitability and synaptic transmission in a surprisingly cell-type specific manner, acting as a key molecular switch to regulate the functional output of neuronal circuits. The central importance of CCK in neuronal networks is also reflected in its involvement in a variety of neuropsychiatric and neurological disorders including panic attacks and epilepsy.
Keywords: neuropeptide, GABA, interneuron, cannabinoid, anxiety
INTRODUCTION
Chole-cysto-kinin (literary “bile-sack-move”, from Greek) was originally identified as pancreozymin in the early 20th century as a gastrin-like molecule of the gastrointestinal tract, where it plays an important role in the release of pancreatic enzymes, gall bladder contraction, and gastric motility. The first report of a peptide in the central nervous system (CNS) reacting with anti-gastrin antibodies was published in 1975, and this peptide was then identified as CCK (Vanderhaeghen et al., 1975). As an initial indication of its functional importance, CCK has been shown to be one of the most abundant neuropeptides, present in microgram quantities in the brain, expressed in especially high levels in the hippocampus, amygdala, septum, olfactory tubercles, caudate nucleus and the hypothalamus (Beinfeld et al., 1981; Crawley, 1985). Furthermore, CCK colocalizes with several key neurotransmitter and neuromodulatory systems, most notably γ-aminobutyric acid (GABA), as well as endocannabinoids, dopamine, serotonin and vasoactive intestinal peptide (Hokfelt et al., 1980; van der Kooy et al., 1981; Hendry et al., 1983; Somogyi et al., 1984; Kosaka et al., 1985; Nunzi et al., 1985; Katona et al., 1999), providing an additional clue to its wide-ranging role in physiological functions in neuronal networks. Indeed, it is now clear that CCK is intimately involved in a diversity of normal behaviors such as learning and memory, feeding, nociception, satiety, and it is also strongly linked to several CNS disorders including anxiety, panic attacks, deficits in fear extinction, schizophrenia and epilepsy (reviewed in Noble and Roques, 2006).
What are the neurobiological bases of the normal and abnormal actions of CCK in the brain? As highlighted in this review, recent research has provided fundamentally new insights into the cellular-synaptic mechanisms underlying the surprisingly selective effects of CCK on particular cell types and at specific synapses in neuronal circuits.
SETTING THE STAGE: SYNTHESIS AND PROCESSING OF NEURONAL CCK
The cell-type specific nature of CCK begins with the synthesis and complex processing of the precursor molecule preprocholecystokinin, a sequence of events that has the potential to result in the generation of a family of CCK-related neuroactive molecules in a spatially and temporarily highly selective manner. CCK is first synthesized as preprocholecystokinin (Deschenes et al., 1984), which is then subject to extensive tissue-specific post-translational processing (Figure 1). Several prohormone convertases have been identified that are thought to take part in the tissue- and perhaps even cell type-specific cleaving of the 115-amino acid form into several CCK molecules of various sizes (Cain et al., 2003). As a result, a combination of various CCK molecules (mostly larger forms such as CCK-22 and 33) exist particularly in the gut and in the plasma, while the sulfated, 8-amino acid C-terminal fragment (CCK-8s) exists as the predominant form of CCK in neurons (Dockray, 1980). CCK8 has been shown to be cleaved by aminopeptidase A in vivo, and by tripeptidyl peptidase II (Migaud et al., 1996; Rose et al., 1996). But as the breakdown of CCK8 is relatively slower in rat brain membranes as compared to other neuropeptides (Roques, 2000), other clearing mechanisms may also play a role in its metabolism (Migaud et al., 1995), bringing a further specialization into the processing of neuronal CCK.
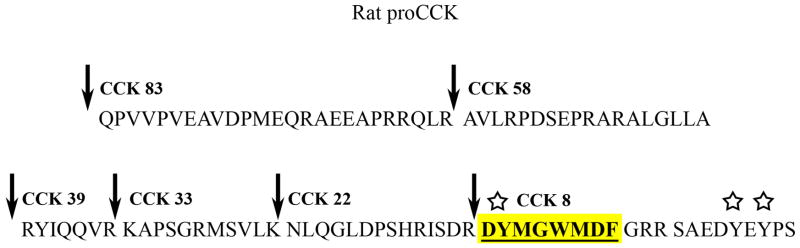
Figure 1. Synthesis and Processing of Neuronal CCK.
The rat proCCK amino acid sequence is illustrated with the CCK peptides numbered backwards from the carboxyl terminal of CCK-8. Prohormone convertases cleave the initial proCCK into several different active forms (the amino terminal end of the major forms of amidated CCK peptides is indicated by the arrows). Endocrine cells contain a mixture of the small and larger peptides, while the predominant form of CCK in neurons is CCK-8s, highlighted in yellow. Stars mark the location of the tyrosine sulfation sites. [Modified with permission of Elsevier from Beinfeld (2003)].
Single-cell RT-PCR and immunocytochemical studies revealed the expression patterns of CCK in great detail. In the neocortex, for example, CCK mRNA has been shown to be present in certain types of GABAergic interneurons and also in pyramidal cells (Gallopin et al., 2006). However, the CCK protein itself is frequently more difficult to unequivocally demonstrate in pyramidal cells, most likely due to lower levels of the protein, or perhaps owing to differences in post-translational processing (Toledo-Rodriguez et al., 2005). On the other hand, the CCK protein is strongly expressed at especially high levels in specific sub-classes of GABAergic interneurons that are collectively referred to as “CCK interneurons”, and, as described below, CCK is now recognized as a key molecular agent that plays critical roles in the regulation of inhibitory networks in the brain.
CELL-TYPE SPECIFIC ACTIONS OF CCK IN HIPPOCAMPAL MICROCIRCUITS
A particularly striking example of the cell-type specific regulation of CCK has been recently identified to control the perisomatically-targeting inhibitory interneurons of the hippocampus, where CCK is expressed in especially high levels. Perisomatic GABAergic inhibition plays a crucial role in controlling the output and synchrony of principal cell populations (Freund 2003; Freund and Katona, 2007). The main source of perisomatic inhibition is provided by the basket cells, which, through their selective innervation of the somata and the proximal dendrites of hundreds of postsynaptic neurons, are in a strategic position to powerfully control the input-output function of large neuronal ensembles. Basket cells can be divided into two non-overlapping classes, namely, the parvalbumin (PV)-expressing and the CCK-expressing cells. These two classes of basket cells play very distinct roles to exert their influence over the network. The parvalbumin-expressing cells, with their ability to fire fast, non-accommodating action potentials, fast membrane time constants and prominent glutamatergic inputs, are thought to primarily serve to generate precisely timed synchronized membrane potential oscillations in the circuit. In contrast, the CCK-expressing basket cells, with their accommodating spike discharge patterns, slower membrane time constants and inputs from several modulatory subcortical sources, are thought of as a “fine-tuning”, highly plastic system that is more prone to both short- and long-term modulatory influences (Freund, 2003; Glickfeld and Scanziani, 2006; Soltesz, 2006; Freund and Katona, 2007). Thus, basket cells are currently recognized to perform distinct, cell-type specific roles related to timing and plasticity of network activity (Figure 2a). But what is the role of CCK itself in the regulation of basket cell functions, beyond serving as a convenient marker for one of the two basic types of basket cells? The first clue to the latter question came from observations that showed that application of CCK-8s to acute rat hippocampal slices significantly increased the frequency of spontaneous, action potential dependent postsynaptic inhibitory events (sIPSCs) in CA1 pyramidal cells and dentate gyrus granule cells (Miller et al., 1997; Deng and Lei, 2006; Foldy et al., 2007; Karson et al., 2008). Subsequently, the increase in sIPSCs was unequivocally demonstrated to be due to the selective, direct, CCK mediated depolarization and robust firing of PV-expressing basket cells (Figure 2b) through the activation of CCK2 receptors (see below). This increased excitation was observed with low nanomolar concentrations of CCK, indicating that the parvalbumin-expressing cells are extremely sensitive to CCK levels, acting essentially as high-affinity biosensors for the neuropeptide in the circuit (Foldy et al., 2007).
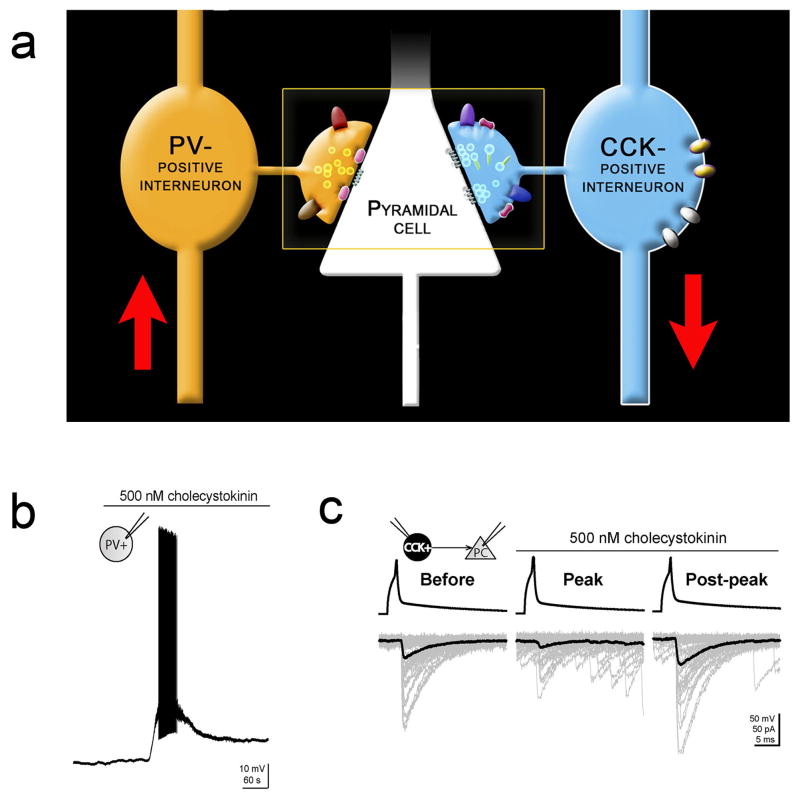
Figure 2. Cell-type specific actions of CCK in Hippocampal Microcircuits.
(a) CCK-expressing and PV-expressing basket cells provide the main source of perisomatic inhibition to the pyramidal cells. CCK strongly depolarizes the PV-expressing basket cells, but suppresses GABAergic transmission from the CCK-expressing basket cells (CCK effects are indicated by the direction of the red arrows) [Modified with permission of Neuron from Freund and Katona (2007)] (b) Sample trace from a PV-expressing basket cell shows the CCK-induced increase in firing. (c) CCK significantly inhibited the unitary IPSCs in pyramidal cells that were evoked by presynaptic CCK-expressing basket cells [Modified with permission of Nature Publishing Group from Földy et al. (2007)].
Even more strikingly, not only that CCK selectively excites PV-expressing basket cells without significantly depolarizing neighboring pyramidal cells or CCK expressing basket cells, but CCK also has the ability to depress the synaptic output from the CCK basket cells themselves through an indirect pathway involving endocannabinoid-mediated retrograde signaling (but see Karson et al., 2008). Specifically, paired recordings between synaptically coupled CCK expressing basket cells and postsynaptic CA1 pyramidal cells showed that CCK significantly suppressed the unitary GABAergic IPSCs recorded from the pyramidal cell in response to presynaptic action potentials (Figure 2c). This CCK-evoked suppression of presynaptic GABA release is due to a cannabinoid type-1 (CB1) receptor-mediated mechanism, as CCK-containing interneurons express the CB1 receptors on their axon terminals and preterminal segments (Katona et al., 1999; Nyiri et al., 2005). Activation of CB1 receptors reduces neurotransmitter release, and indeed, CB1 receptor antagonists were able to block the CCK induced suppression of GABA release. The source of the cannabinoids is most likely from the postsynaptic pyramidal cells themselves, as blocking G protein coupled receptor (GPCR) function in the postsynaptic cell was also able to block the CCK induced suppression of GABA release (Foldy et al., 2007). Thus, CCK reduces GABA release from the CCK-expressing basket cells through an indirect effect involving CCK receptors the postsynaptic pyramidal cells as the primary sites of action and the subsequent downstream, Gq/11-protein dependent synthesis and release of lipid derived retrograde synaptic messengers which, in turn, bind to presynaptic CB1 receptors to suppress transmitter release through a Gi-protein coupled second messenger pathway that depresses N-type calcium channel activity.
Therefore, these data demonstrate that CCK serves dual, opposing, direct (excitation of PV basket cells) and indirect (suppression of GABA release from CCK basket cells) roles in neuronal microcircuits, acting as a molecular switch to gate the different sources of perisomatic inhibition for hippocampal principal cells. The CCK mediated enhancement of PV-expressing basket cell output, in conjunction with the concomitant reduction of the CCK-expressing basket cell output, likely promotes tasks requiring precise timing and integration over narrow time windows (Hefft and Jonas, 2005; Glickfeld and Scanziani, 2006). Interestingly, both CCK2 receptors and CB1 receptors have been implicated in mood-related disorders, and behavioral studies also support the hypothesis that the direct interactions between the CCK and endocannabinoid systems play a critical role in modulating anxiety, stress and fear extinction (Kurrikoff et al., 2008; Chhatwal et al., 2009); see also below).
It should also be noted that, in addition to the actions of CCK on GABAergic interneurons, CCK can also modulate the glutamatergic excitatory system. Specifically, CCK was found to result in increases in glutamate concentration in hippocampal slice perfusates and synaptosomes (Migaud et al., 1994; Breukel et al., 1997), and CCK has been recently demonstrated to facilitate glutamate release from most, but not all, major hippocampal excitatory pathways through a mechanism involving phospholipase C, intracellular Ca2+ release and protein kinase Cγ that ultimately increases the number of readily releasable vesicles and release probability (Deng et al., 2010). Taken together, these observations indicate that CCK, while being widely expressed at high quantities in the brain, is nevertheless able to exert a variety of highly selective effects at specific points in neuronal circuits to coordinate network function and particular aspects of behavior.
CCK AS A NEURONAL KALEIDOSCOPE: INSIGHTS INTO THE GENERATION OF MOLECULAR AND CELLULAR SOURCES OF DIVERSITY OF NEUROPEPTIDE ACTIONS
In addition to the cell-type specific actions discussed so far, the CCK system exhibits additional mechanistically and functionally distinct actions in the CNS. A prominent source of variation is the CCK receptor. Although CCK2 receptors predominate in the brain (indeed, the receptor was originally named “CCK-B” for “brain”), the CCK1 receptor (originally named “CCK-A” for “alimentary”) is also expressed in discrete areas, including the interpeduncular nucleus, area prostrema, nucleus tractus solitarius, and hypothalamus (Innis and Snyder, 1980; Sankaran et al., 1980; Van Dijk et al., 1984; Hill et al., 1987; Honda et al., 1993). The area- and perhaps cell-type specific expression of the two receptor classes is likely to be functionally relevant, as they exhibit unique pharmacological affinities for various forms of CCK, including sulfated and non-sulfated forms (Saito et al., 1980). Furthermore, although both classes of CCK receptors are GPCRs generally thought to couple to the Gq/PLC pathway (reviewed in Wank, 1995; Noble and Roques, 2006), it is increasingly being recognized that CCK2 receptors may also couple to a pertussis toxin sensitive (and thus non-Gq) G-protein in certain neuronal types (reviewed in Noble et al., 1999; Pommier et al., 1999; Pommier et al., 2003; Noble and Roques, 2006; Oz et al., 2007), raising the possibility that CCK2 receptors couple to diverse signaling pathways in different neuronal systems. Indeed, our recent studies indicate that the CCK induced robust depolarization of PV expressing basket cells is also due to a non-canonical, pertussis toxin sensitive pathway (S.Y.L. and I.S., unpublished observations).
Although less well understood, it is likely that an additional source of diversity of action may be related to the rules that govern the activity-dependent release of endogenous CCK. CCK can be isolated from purified synaptosome preparations (isolated nerve terminals), and like other neuropeptides and neurohormones, is thought to be stored in dense core vesicles. These large vesicles appear as dense granules under the electron microscope and are located further away from the active zone, conceivably accounting for different release properties and kinetics as compared to classical neurotransmitters (Zhu et al., 1986; Verhage et al., 1991). Early studies on the release of CCK have been performed in synaptosomes, acute brain slices, and by in vivo microdialysis, mostly by using chemical methods of stimulating nerve terminals. Depolarization of terminals using elevated levels of extracellular potassium or 4-aminopyridine can trigger CCK release in these preparations through a Ca2+-dependent manner (Dodd et al., 1980; Emson et al., 1980; Verhage et al., 1991; You et al., 1994). However, direct CCK release under more physiologically relevant electrical stimulations has been difficult to measure, potentially due to degradation of CCK in the tissue, or because the amount of CCK released by local stimulation in slices or in vivo may not be large enough to detect by currently available methods (Ghijsen et al., 2001). Thus, although there are reports of electrophysiologically detectable synaptic effects of endogenously released of CCK in acute hippocampal slices (Karson et al., 2008; Deng et al., 2010), the precise conditions and molecular mechanisms regulating the endogenous release of CCK under physiological conditions in neuronal circuits remain a critically important, currently still largely unresolved question.
While the precise rules underlying the release of CCK remain to be studied further, there is now a clear recognition that CCK-expressing interneurons form synapses, and perhaps release CCK, not only on the perisomatic regions but also on the proximal and distal dendrites of principal cells, in addition to innervating other GABAergic interneurons (Acsady et al., 1998; Karson et al., 2009). For example, in the hippocampus where the organization of the CCK interneuronal microcircuits is particularly well understood, there are, in addition to the CCK expressing basket cells that target the soma and proximal dendrites, the so-called Schaffer-collateral associated cells targeting the CA1 pyramidal cell dendrites that receive excitatory inputs from CA3 pyramidal cells, the so-called apical dendrite-innervating cells that preferentially innervate the main apical dendrites, and the perforant path-associated cells that innervate the apical tuft of principal cells (reviewed in Klausberger et al., 2005; Klausberger, 2009). The domain-specificity of these CCK-containing inputs ensures a finely-tuned spatiotemporal regulation of the hippocampal network by CCK-containing cells, represented by the timing of discharges at different time periods during hippocampal oscillations (Klausberger et al., 2005). Another layer of variation in the synaptic-cellular effects of CCK expressing cells arises from the fact that these neurons can release GABA in a highly asynchronous manner (Hefft and Jonas, 2005; Daw et al., 2009), due to the coupling of the N-type Ca2+ channels to the Ca2+ sensor for exocytosis. Interestingly, the asynchronous release may also involve a longer presynaptic Ca2+ transient in the CCK-positive terminals, which is consistent with the idea that a long-lasting Ca2+ transient is needed for peptide release from dense-core vesicles (Verhage et al., 1991; Hefft and Jonas, 2005). Furthermore, the release of GABA, and presumably also CCK, is subject to regulation by a variety of presynaptic receptors expressed by the CCK-containing interneurons, including, in addition to the above-mentioned CB1 receptors, GABAB, 5-HT3, and nicotinic α7 (or α4) receptors, which allow for selective modulation of these cells from various cortical sources as a function of behavioral states (reviewed in Freund, 2003; Freund and Katona, 2007). In addition, the distinct classes of CCK-expressing interneurons mentioned in the previous paragraph appear to exhibit significantly different sensitivity to presynaptic modulators, as illustrated recently by the demonstration that all three forms of endocannabinoid mediated regulation of GABA release are expressed more prominently at perisomatic versus dendritic synapses originating from distinct types of CCK-containing interneurons (Lee et al., 2010).
FROM CELLULAR ACTIONS OF CCK TO ROLES IN NORMAL BEHAVIORS AND PATHOLOGICAL STATES
Given the widespread distribution and multiple cellular and synaptic actions of CCK, it is not surprising that CCK plays active roles in several forms of normal behavior, as well as in pathological states. Importantly, we are beginning to bridge the gap between the molecular and cellular actions of CCK and whole animal behavior. For example, in the mouse basolateral amygdala, a region known to be involved in generating anxiety and fear, projection neurons have been shown to respond to application of CCK with a depolarizing current mediated by transient receptor potential (TRP)-like channels, through actions of the CCK2 receptors and Gq/11 proteins (Chung and Moore, 2007; Meis et al., 2007). CCK-8s also directly activates orexin neurons of the hypothalamus through the CCK1 receptor and non-selective cation channels, implicating a role of CCK in sleep and arousal states (Tsujino et al., 2005). In addition, CCK has been demonstrated to depolarize rat thalamic reticular neurons, but not cells in the ventrobasal thalamus, by suppressing a potassium conductance, revealing a potentially significant role of CCK in the modulation of thalamic rhythms (Cox et al., 1995; Cox et al., 1997). CCK may also play a role in normal development. In the neonatal spinal cord, CCK depolarizes motorneurons and an unidentified group of ventral horn neurons by reducing a potassium conductance through CCK2 receptors and most likely Gi/o-proteins, modulating the occurrence of developmentally important spontaneous depolarizations and discharges from lumbosacral ventral roots (Oz et al., 2007). Finally, it has recently been shown that CCK-containing cells contain clusters of synaptic vesicles which are immunoreactive for estrogen receptor α, and that estrogen is able to mobilize these vesicle clusters closer to synapses (Hart et al., 2007). CCK levels fluctuate during the various stages of the estrous cycle (Micevych and Sinchak, 2001; Hilke et al., 2007), and estrogen may have significant influence in controlling the levels of CCK in vivo.
The multiple neuronal actions of CCK are reflected by its active role in several neuropsychiatric states. CCK is a known anxiogenic agent, which was first discovered when it was found that injections of CCK could reliably induce panic attacks in healthy volunteers. Patients already predisposed to panic attacks became even more sensitive after CCK injections (de Montigny, 1989; Bradwejn et al., 1991; Harro, 2006). In addition, the interactions between the CCK and endocannabinoid systems discussed earlier have been further demonstrated in behavioral studies as well. Stress-induced analgesia (SIA) serves as an important coping mechanism in response to stressful stimuli, and is thought to involve the opioid and endcannabinoid systems. CCK2 receptor knockout mice showed a significantly delayed development of SIA as compared to the wild-type, and CB1 receptor antagonism was able to block SIA in wild-type, but not the knockout mice, indicating that eliminating CCK2 receptors also eliminated the endocannabinoid-mediated aspects of SIA (Kurrikoff et al., 2008). The CCK-CB1 system interaction was also reported to play a role in fear extinction learning, which is known to induce endocannabinoid production (Marsicano et al., 2002). Administration of a CCK2 receptor agonist, or administration of the CB1 receptor antagonist SR141716, significantly impaired fear extinction learning. However, the SR141716-induced impairment in fear extinction learning could be reversed if a CCK2R antagonist was co-administered with SR141716, showing that CCK and its interactions with the endocannabinoid system plays a critical role in fear extinction learning (Figure 3a; Chhatwal et al., 2009).
Figure 3. CCK in behavioral and pathological states.
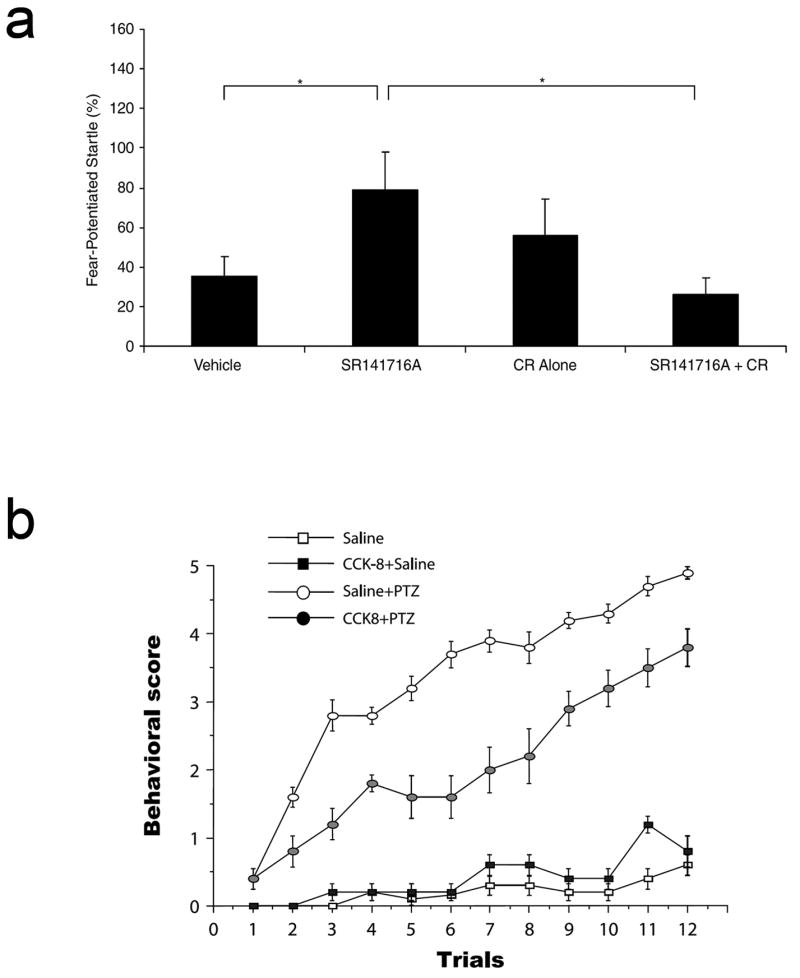
(a) Administration of a CB1 receptor antagonist (SR14176A, 5mg/kg, i.p.) in rats potently inhibited fear extinction learning, showing higher levels of fear-potentiated startle compared to vehicle treated controls when tested 96 hours after extinction. Notably, rats treated with a CCK2 receptor antagonist CR2945 (CR, 3mg/kg, i.p.) in combination with the CB1 receptor antagonist SR14176A showed significantly less fear-potentiated than those receiving SR14176A alone [Modified with permission of Nature Publishing Group from Chhatwal et al. (2009)]. (b) Pretreatment with CCK-8 delays the manifestation of behavioral changes during the course of PTZ-kindling in rats. Average score of the rat population monitored for 30 min after PTZ or Saline injection at each time point [Modified with permission of Elsevier from Tirassa et al. (2005)].
Alterations in the CCK system have also been correlated with additional major neuropsychiatric and neurological disorders. In particular, post-mortem studies of schizophrenic patients have shown a decrease in the levels of CCK in the brain (Ferrier et al., 1983; Kerwin et al., 1992). The CCK receptors are also affected as well, as polymorphisms in CCK receptors have associated with both schizophrenia and panic attacks (Kennedy et al., 1999; Wei and Hemmings, 1999; Sanjuan et al., 2004). In epilepsy, the CCK-containing cells themselves can be lost. For example, in the kindling model, in which repeated, brief, high-frequency stimulation progressively increase seizure susceptibility and eventually may evoke spontaneous seizures, there was a loss of paired-pulse inhibition and reduction of GABAergic evoked IPSCs, and this loss corresponded with a reduction of CCK-labeled interneurons and punctate staining in the hilus and granule cell layer of the dentate gyrus (Sayin et al., 2003). Importantly, the CCK-CB1 receptor interaction appears to be a significant component in epilepsy as well, as CB1 receptors, which are located on the CCK-expressing cells, are upregulated during febrile seizures in rats. The subsequent alterations in GABA release from these cells may be a contributing factor to limbic hyperexcitability observed in developmental seizure models (Chen et al., 2003; Chen et al., 2007).
CONCLUSIONS
In summary, rapidly accumulating molecular, cellular and behavioral data indicate that CCK acts as a highly versatile yet spatially and temporally specific regulatory element in a variety of neuronal circuits. The sources of diversity of the CCK system are manifold, from the CCK receptor gene polymorphisms to variations in CCK synthesis and release and diversity of receptors and second messenger pathways, providing a rich repertoire of alternative molecular steps and pathways that can be combined in a large number of ways to result in the emergence of its highly cell-type specific actions. Through interactions with several other key neurotransmitter and neuromodulatory systems, CCK is able to modify a surprising variety of normal behaviors and contribute to a number of significant disorders. It is anticipated that future research efforts will continue to provide further new insights into this multi-faceted neuropeptide system.
Acknowledgments
This work was supported by the US National Institutes of Health grant NS38580 (to I.S.) and the American Epilepsy Society (to S.Y.L.).
LIST OF REFERENCES
- Acsady L, Kamondi A, Sik A, Freund T, Buzsaki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J Neurosci. 1998;18:3386–3403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinfeld MC, Meyer DK, Eskay RL, Jensen RT, Brownstein MJ. The distribution of cholecystokinin immunoreactivity in the central nervous system of the rat as determined by radioimmunoassay. Brain Res. 1981;212:51–57. doi: 10.1016/0006-8993(81)90031-7. [DOI] [PubMed] [Google Scholar]
- Bradwejn J, Koszycki D, Shriqui C. Enhanced sensitivity to cholecystokinin tetrapeptide in panic disorder. Clinical and behavioral findings. Arch Gen Psychiatry. 1991;48:603–610. doi: 10.1001/archpsyc.1991.01810310021005. [DOI] [PubMed] [Google Scholar]
- Breukel AI, Lopes da Silva FH, Ghijsen WE. Cholecystokinin (CCK-8) modulates vesicular release of excitatory amino acids in rat hippocampal nerve endings. Neurosci Lett. 1997;234:67–70. doi: 10.1016/s0304-3940(97)00678-2. [DOI] [PubMed] [Google Scholar]
- Cain BM, Connolly K, Blum A, Vishnuvardhan D, Marchand JE, Beinfeld MC. Distribution and colocalization of cholecystokinin with the prohormone convertase enzymes PC1, PC2, and PC5 in rat brain. J Comp Neurol. 2003;467:307–325. doi: 10.1002/cne.10924. [DOI] [PubMed] [Google Scholar]
- Chen K, Neu A, Howard AL, Foldy C, Echegoyen J, Hilgenberg L, Smith M, Mackie K, Soltesz I. Prevention of plasticity of endocannabinoid signaling inhibits persistent limbic hyperexcitability caused by developmental seizures. J Neurosci. 2007;27:46–58. doi: 10.1523/JNEUROSCI.3966-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Ratzliff A, Hilgenberg L, Gulyas A, Freund TF, Smith M, Dinh TP, Piomelli D, Mackie K, Soltesz I. Long-term plasticity of endocannabinoid signaling induced by developmental febrile seizures. Neuron. 2003;39:599–611. doi: 10.1016/s0896-6273(03)00499-9. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Gutman AR, Maguschak KA, Bowser ME, Yang Y, Davis M, Ressler KJ. Functional interactions between endocannabinoid and CCK neurotransmitter systems may be critical for extinction learning. Neuropsychopharmacology. 2009;34:509–521. doi: 10.1038/npp.2008.97. [DOI] [PubMed] [Google Scholar]
- Chung L, Moore SD. Cholecystokinin enhances GABAergic inhibitory transmission in basolateral amygdala. Neuropeptides. 2007;41:453–463. doi: 10.1016/j.npep.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Cox CL, Huguenard JR, Prince DA. Cholecystokinin depolarizes rat thalamic reticular neurons by suppressing a K+ conductance. J Neurophysiol. 1995;74:990–1000. doi: 10.1152/jn.1995.74.3.990. [DOI] [PubMed] [Google Scholar]
- Cox CL, Huguenard JR, Prince DA. Peptidergic modulation of intrathalamic circuit activity in vitro: actions of cholecystokinin. J Neurosci. 1997;17:70–82. doi: 10.1523/JNEUROSCI.17-01-00070.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Comparative distribution of cholecystokinin and other neuropeptides. Why is this peptide different from all other peptides? Ann N Y Acad Sci. 1985;448:1–8. doi: 10.1111/j.1749-6632.1985.tb29900.x. [DOI] [PubMed] [Google Scholar]
- Daw MI, Tricoire L, Erdelyi F, Szabo G, McBain CJ. Asynchronous transmitter release from cholecystokinin-containing inhibitory interneurons is widespread and target-cell independent. J Neurosci. 2009;29:11112–11122. doi: 10.1523/JNEUROSCI.5760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Montigny C. Cholecystokinin tetrapeptide induces panic-like attacks in healthy volunteers. Preliminary findings. Arch Gen Psychiatry. 1989;46:511–517. doi: 10.1001/archpsyc.1989.01810060031006. [DOI] [PubMed] [Google Scholar]
- Deng PY, Lei S. Bidirectional modulation of GABAergic transmission by cholecystokinin in hippocampal dentate gyrus granule cells of juvenile rats. J Physiol. 2006;572:425–442. doi: 10.1113/jphysiol.2005.104463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Xiao Z, Jha A, Ramonet D, Matsui T, Leitges M, Shin HS, Porter JE, Geiger JD, Lei S. Cholecystokinin facilitates glutamate release by increasing the number of readily releasable vesicles and releasing probability. J Neurosci. 2010;30:5136–5148. doi: 10.1523/JNEUROSCI.5711-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes RJ, Lorenz LJ, Haun RS, Roos BA, Collier KJ, Dixon JE. Cloning and sequence analysis of a cDNA encoding rat preprocholecystokinin. Proc Natl Acad Sci U S A. 1984;81:726–730. doi: 10.1073/pnas.81.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray GJ. Cholecystokinins in rat cerebral cortex: identification, purification and characterization by immunochemical methods. Brain Res. 1980;188:155–165. doi: 10.1016/0006-8993(80)90564-8. [DOI] [PubMed] [Google Scholar]
- Dodd PR, Edwardson JA, Dockray GJ. The depolarization-induced release of cholecystokinin C-terminal octapeptide (CCK-8) from rat synaptosomes and brain slices. Regul Pept. 1980:17–29. [Google Scholar]
- Emson PC, Lee CM, Rehfeld JF. Cholecystokinin octapeptide: vesicular localization and calcium dependent release from rat brain in vitro. Life Sci. 1980;26:2157–2163. doi: 10.1016/0024-3205(80)90603-7. [DOI] [PubMed] [Google Scholar]
- Ferrier IN, Roberts GW, Crow TJ, Johnstone EC, Owens DG, Lee YC, O’Shaughnessy D, Adrian TE, Polak JM, Bloom SR. Reduced cholecystokinin-like and somatostatin-like immunoreactivity in limbic lobe is associated with negative symptoms in schizophrenia. Life Sci. 1983;33:475–482. doi: 10.1016/0024-3205(83)90797-x. [DOI] [PubMed] [Google Scholar]
- Foldy C, Lee SY, Szabadics J, Neu A, Soltesz I. Cell type-specific gating of perisomatic inhibition by cholecystokinin. Nat Neurosci. 2007;10:1128–1130. doi: 10.1038/nn1952. [DOI] [PubMed] [Google Scholar]
- Freund TF. Interneuron Diversity series: Rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26:489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56:33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Gallopin T, Geoffroy H, Rossier J, Lambolez B. Cortical sources of CRF, NKB, and CCK and their effects on pyramidal cells in the neocortex. Cereb Cortex. 2006;16:1440–1452. doi: 10.1093/cercor/bhj081. [DOI] [PubMed] [Google Scholar]
- Ghijsen WE, Leenders AG, Wiegant VM. Regulation of cholecystokinin release from central nerve terminals. Peptides. 2001;22:1213–1221. doi: 10.1016/s0196-9781(01)00444-2. [DOI] [PubMed] [Google Scholar]
- Glickfeld LL, Scanziani M. Distinct timing in the activity of cannabinoid-sensitive and cannabinoid-insensitive basket cells. Nat Neurosci. 2006;9:807–815. doi: 10.1038/nn1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harro J. CCK and NPY as anti-anxiety treatment targets: promises, pitfalls, and strategies. Amino Acids. 2006;31:215–230. doi: 10.1007/s00726-006-0334-x. [DOI] [PubMed] [Google Scholar]
- Hart SA, Snyder MA, Smejkalova T, Woolley CS. Estrogen mobilizes a subset of estrogen receptor-alpha-immunoreactive vesicles in inhibitory presynaptic boutons in hippocampal CA1. J Neurosci. 2007;27:2102–2111. doi: 10.1523/JNEUROSCI.5436-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefft S, Jonas P. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat Neurosci. 2005;8:1319–1328. doi: 10.1038/nn1542. [DOI] [PubMed] [Google Scholar]
- Hendry SH, Jones EG, Beinfeld MC. Cholecystokinin-immunoreactive neurons in rat and monkey cerebral cortex make symmetric synapses and have intimate associations with blood vessels. Proc Natl Acad Sci U S A. 1983;80:2400–2404. doi: 10.1073/pnas.80.8.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilke S, Hokfelt T, Darwish M, Theodorsson E. Cholecystokinin levels in the rat brain during the estrous cycle. Brain Res. 2007;1144:70–73. doi: 10.1016/j.brainres.2007.01.107. [DOI] [PubMed] [Google Scholar]
- Hill DR, Campbell NJ, Shaw TM, Woodruff GN. Autoradiographic localization and biochemical characterization of peripheral type CCK receptors in rat CNS using highly selective nonpeptide CCK antagonists. J Neurosci. 1987;7:2967–2976. doi: 10.1523/JNEUROSCI.07-09-02967.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokfelt T, Rehfeld JF, Skirboll L, Ivemark B, Goldstein M, Markey K. Evidence for coexistence of dopamine and CCK in meso-limbic neurones. Nature. 1980;285:476–478. doi: 10.1038/285476a0. [DOI] [PubMed] [Google Scholar]
- Honda T, Wada E, Battey JF, Wank SA. Differential Gene Expression of CCK(A) and CCK(B) Receptors in the Rat Brain. Mol Cell Neurosci. 1993;4:143–154. doi: 10.1006/mcne.1993.1018. [DOI] [PubMed] [Google Scholar]
- Innis RB, Snyder SH. Distinct cholecystokinin receptors in brain and pancreas. Proc Natl Acad Sci U S A. 1980;77:6917–6921. doi: 10.1073/pnas.77.11.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karson MA, Tang AH, Milner TA, Alger BE. Synaptic cross talk between perisomatic-targeting interneuron classes expressing cholecystokinin and parvalbumin in hippocampus. J Neurosci. 2009;29:4140–4154. doi: 10.1523/JNEUROSCI.5264-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karson MA, Whittington KC, Alger BE. Cholecystokinin inhibits endocannabinoid-sensitive hippocampal IPSPs and stimulates others. Neuropharmacology. 2008;54:117–128. doi: 10.1016/j.neuropharm.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy JL, Bradwejn J, Koszycki D, King N, Crowe R, Vincent J, Fourie O. Investigation of cholecystokinin system genes in panic disorder. Mol Psychiatry. 1999;4:284–285. doi: 10.1038/sj.mp.4000507. [DOI] [PubMed] [Google Scholar]
- Kerwin R, Robinson P, Stephenson J. Distribution of CCK binding sites in the human hippocampal formation and their alteration in schizophrenia: a post-mortem autoradiographic study. Psychol Med. 1992;22:37–43. doi: 10.1017/s0033291700032700. [DOI] [PubMed] [Google Scholar]
- Klausberger T. GABAergic interneurons targeting dendrites of pyramidal cells in the CA1 area of the hippocampus. Eur J Neurosci. 2009;30:947–957. doi: 10.1111/j.1460-9568.2009.06913.x. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Marton LF, O’Neill J, Huck JH, Dalezios Y, Fuentealba P, Suen WY, Papp E, Kaneko T, Watanabe M, Csicsvari J, Somogyi P. Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J Neurosci. 2005;25:9782–9793. doi: 10.1523/JNEUROSCI.3269-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka T, Kosaka K, Tateishi K, Hamaoka Y, Yanaihara N, Wu JY, Hama K. GABAergic neurons containing CCK-8-like and/or VIP-like immunoreactivities in the rat hippocampus and dentate gyrus. J Comp Neurol. 1985;239:420–430. doi: 10.1002/cne.902390408. [DOI] [PubMed] [Google Scholar]
- Kurrikoff K, Inno J, Matsui T, Vasar E. Stress-induced analgesia in mice: evidence for interaction between endocannabinoids and cholecystokinin. Eur J Neurosci. 2008;27:2147–2155. doi: 10.1111/j.1460-9568.2008.06160.x. [DOI] [PubMed] [Google Scholar]
- Lee SH, Foldy F, Soltesz I. Distinct endocannabinoid control of GABA release at perisomatic and dendritic synapses in the hippocampus. Journal of Neuroscience. 2010 doi: 10.1523/JNEUROSCI.6238-09.2010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Meis S, Munsch T, Sosulina L, Pape HC. Postsynaptic mechanisms underlying responsiveness of amygdaloid neurons to cholecystokinin are mediated by a transient receptor potential-like current. Mol Cell Neurosci. 2007;35:356–367. doi: 10.1016/j.mcn.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Micevych P, Sinchak K. Estrogen and endogenous opioids regulate CCK in reproductive circuits. Peptides. 2001;22:1235–1244. doi: 10.1016/s0196-9781(01)00447-8. [DOI] [PubMed] [Google Scholar]
- Migaud M, Durieux C, Viereck J, Soroca-Lucas E, Fournie-Zaluski MC, Roques BP. The in vivo metabolism of cholecystokinin (CCK-8) is essentially ensured by aminopeptidase A. Peptides. 1996;17:601–607. doi: 10.1016/0196-9781(96)00036-8. [DOI] [PubMed] [Google Scholar]
- Migaud M, Roques BP, Durieux C. Effects of cholecystokinin octapeptide and BC 264, a potent and selective CCK-B agonist on aspartate and glutamate release from rat hippocampal slices. Neuropharmacology. 1994;33:737–743. doi: 10.1016/0028-3908(94)90113-9. [DOI] [PubMed] [Google Scholar]
- Migaud M, Roques BP, Durieux C. Evidence for a high-affinity uptake system for cholecystokinin octapeptide (CCK8) in rat cortical synaptosomes. Eur J Neurosci. 1995;7:1074–1079. doi: 10.1111/j.1460-9568.1995.tb01094.x. [DOI] [PubMed] [Google Scholar]
- Miller KK, Hoffer A, Svoboda KR, Lupica CR. Cholecystokinin increases GABA release by inhibiting a resting K+ conductance in hippocampal interneurons. J Neurosci. 1997;17:4994–5003. doi: 10.1523/JNEUROSCI.17-13-04994.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble F, Roques B. Cholecystokinin Peptides in Brain Function. Handbook of Neurochemistry and Molecular Neurobiology. 2006:545–571. [Google Scholar]
- Noble F, Wank SA, Crawley JN, Bradwejn J, Seroogy KB, Hamon M, Roques BP. International Union of Pharmacology. XXI. Structure, distribution, and functions of cholecystokinin receptors. Pharmacol Rev. 1999;51:745–781. [PubMed] [Google Scholar]
- Nunzi MG, Gorio A, Milan F, Freund TF, Somogyi P, Smith AD. Cholecystokinin-immunoreactive cells form symmetrical synaptic contacts with pyramidal and nonpyramidal neurons in the hippocampus. J Comp Neurol. 1985;237:485–505. doi: 10.1002/cne.902370406. [DOI] [PubMed] [Google Scholar]
- Nyiri G, Cserep C, Szabadits E, Mackie K, Freund TF. CB1 cannabinoid receptors are enriched in the perisynaptic annulus and on preterminal segments of hippocampal GABAergic axons. Neuroscience. 2005;136:811–822. doi: 10.1016/j.neuroscience.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Oz M, Yang KH, Shippenberg TS, Renaud LP, O’Donovan MJ. Cholecystokinin B-type receptors mediate a G-protein-dependent depolarizing action of sulphated cholecystokinin ocatapeptide (CCK-8s) on rodent neonatal spinal ventral horn neurons. J Neurophysiol. 2007;98:1108–1114. doi: 10.1152/jn.00148.2007. [DOI] [PubMed] [Google Scholar]
- Pommier B, Da Nascimento S, Dumont S, Bellier B, Million E, Garbay C, Roques BP, Noble F. The cholecystokininB receptor is coupled to two effector pathways through pertussis toxin-sensitive and -insensitive G proteins. J Neurochem. 1999;73:281–288. doi: 10.1046/j.1471-4159.1999.0730281.x. [DOI] [PubMed] [Google Scholar]
- Pommier B, Marie-Claire C, Da Nascimento S, Wang HL, Roques BP, Noble F. Further evidence that the CCK2 receptor is coupled to two transduction pathways using site-directed mutagenesis. J Neurochem. 2003;85:454–461. doi: 10.1046/j.1471-4159.2003.01690.x. [DOI] [PubMed] [Google Scholar]
- Roques BP. Novel approaches to targeting neuropeptide systems. Trends Pharmacol Sci. 2000;21:475–483. doi: 10.1016/s0165-6147(00)01571-6. [DOI] [PubMed] [Google Scholar]
- Rose C, Vargas F, Facchinetti P, Bourgeat P, Bambal RB, Bishop PB, Chan SM, Moore AN, Ganellin CR, Schwartz JC. Characterization and inhibition of a cholecystokinin-inactivating serine peptidase. Nature. 1996;380:403–409. doi: 10.1038/380403a0. [DOI] [PubMed] [Google Scholar]
- Saito A, Sankaran H, Goldfine ID, Williams JA. Cholecystokinin receptors in the brain: characterization and distribution. Science. 1980;208:1155–1156. doi: 10.1126/science.6246582. [DOI] [PubMed] [Google Scholar]
- Sanjuan J, Toirac I, Gonzalez JC, Leal C, Molto MD, Najera C, De Frutos R. A possible association between the CCK-AR gene and persistent auditory hallucinations in schizophrenia. Eur Psychiatry. 2004;19:349–353. doi: 10.1016/j.eurpsy.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Sankaran H, Goldfine ID, Deveney CW, Wong KY, Williams JA. Binding of cholecystokinin to high affinity receptors on isolated rat pancreatic acini. J Biol Chem. 1980;255:1849–1853. [PubMed] [Google Scholar]
- Sayin U, Osting S, Hagen J, Rutecki P, Sutula T. Spontaneous seizures and loss of axo-axonic and axo-somatic inhibition induced by repeated brief seizures in kindled rats. J Neurosci. 2003;23:2759–2768. doi: 10.1523/JNEUROSCI.23-07-02759.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltesz I. Diversity in the Neuronal Machine: Order and Variability in Interneuronal Microcircuits. New York: Oxford University Press; 2006. [Google Scholar]
- Somogyi P, Hodgson AJ, Smith AD, Nunzi MG, Gorio A, Wu JY. Different populations of GABAergic neurons in the visual cortex and hippocampus of cat contain somatostatin-or cholecystokinin-immunoreactive material. J Neurosci. 1984;4:2590–2603. doi: 10.1523/JNEUROSCI.04-10-02590.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Rodriguez M, Goodman P, Illic M, Wu C, Markram H. Neuropeptide and calcium-binding protein gene expression profiles predict neuronal anatomical type in the juvenile rat. J Physiol. 2005;567:401–413. doi: 10.1113/jphysiol.2005.089250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujino N, Yamanaka A, Ichiki K, Muraki Y, Kilduff TS, Yagami K, Takahashi S, Goto K, Sakurai T. Cholecystokinin activates orexin/hypocretin neurons through the cholecystokinin A receptor. J Neurosci. 2005;25:7459–7469. doi: 10.1523/JNEUROSCI.1193-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooy D, Hunt SP, Steinbusch HW, Verhofstad AA. Separate populations of cholecystokinin and 5-hydroxytryptamine-containing neuronal cells in the rat dorsal raphe, and their contribution to the ascending raphe projections. Neurosci Lett. 1981;26:25–30. doi: 10.1016/0304-3940(81)90420-1. [DOI] [PubMed] [Google Scholar]
- Van Dijk A, Richards JG, Trzeciak A, Gillessen D, Mohler H. Cholecystokinin receptors: biochemical demonstration and autoradiographical localization in rat brain and pancreas using [3H] cholecystokinin8 as radioligand. J Neurosci. 1984;4:1021–1033. doi: 10.1523/JNEUROSCI.04-04-01021.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhaeghen JJ, Signeau JC, Gepts W. New peptide in the vertebrate CNS reacting with antigastrin antibodies. Nature. 1975;257:604–605. doi: 10.1038/257604a0. [DOI] [PubMed] [Google Scholar]
- Verhage M, Ghijsen WE, Nicholls DG, Wiegant VM. Characterization of the release of cholecystokinin-8 from isolated nerve terminals and comparison with exocytosis of classical transmitters. J Neurochem. 1991;56:1394–1400. doi: 10.1111/j.1471-4159.1991.tb11437.x. [DOI] [PubMed] [Google Scholar]
- Verhage M, McMahon HT, Ghijsen WE, Boomsma F, Scholten G, Wiegant VM, Nicholls DG. Differential release of amino acids, neuropeptides, and catecholamines from isolated nerve terminals. Neuron. 1991;6:517–524. doi: 10.1016/0896-6273(91)90054-4. [DOI] [PubMed] [Google Scholar]
- Wank SA. Cholecystokinin receptors. Am J Physiol. 1995;269:G628–646. doi: 10.1152/ajpgi.1995.269.5.G628. [DOI] [PubMed] [Google Scholar]
- Wei J, Hemmings GP. The CCK-A receptor gene possibly associated with auditory hallucinations in schizophrenia. Eur Psychiatry. 1999;14:67–70. doi: 10.1016/s0924-9338(99)80719-6. [DOI] [PubMed] [Google Scholar]
- You ZB, Herrera-Marschitz M, Brodin E, Meana JJ, Morino P, Hokfelt T, Silveira R, Goiny M, Ungerstedt U. On the origin of striatal cholecystokinin release: studies with in vivo microdialysis. J Neurochem. 1994;62:76–85. doi: 10.1046/j.1471-4159.1994.62010076.x. [DOI] [PubMed] [Google Scholar]
- Zhu PC, Thureson-Klein A, Klein RL. Exocytosis from large dense cored vesicles outside the active synaptic zones of terminals within the trigeminal subnucleus caudalis: a possible mechanism for neuropeptide release. Neuroscience. 1986;19:43–54. doi: 10.1016/0306-4522(86)90004-7. [DOI] [PubMed] [Google Scholar]