Introduction
During the last decade, evidence has been collected on the central role played by growth stimulating factors (GsF) [1–9] and growth inhibiting factors (GiF) [10–12] in the mechanism of tissue growth regulation, using different experimental models [13]. The model of partial hepatectomy (PH) in rats has provided numerous findings on the existence of this regulatory process. In healthy mammals there is a well-defined relationship between body weight and liver weight [14]. When this relationship is altered either by surgical resection or by viral and toxic noxae, the liver quickly restores its volume [15]. A rapid growth of the residual liver mass is observed during the first few days after PH, indicating the prevalence of GsFs (Fig. 1). Later, a gradual decrease in liver growth is observed after the appearance of GiFs, which stop the regenerative process when the initial volume of the liver is restored (Fig. 1).
Fig. 1.
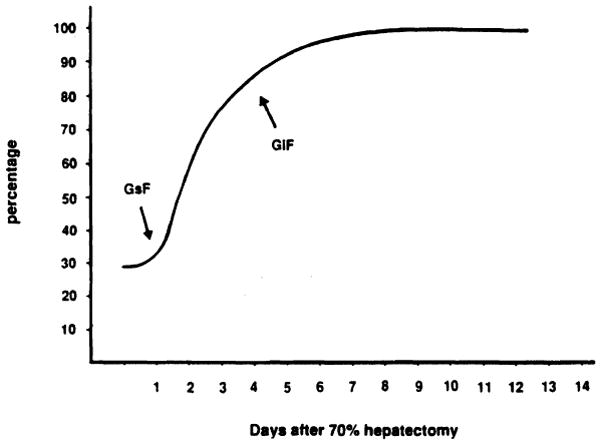
Liver mass recovery in rats after 70% hepatectomy. Liver resection was performed as described by Higgins and Anderson [15]. Liver mass is expressed as percentage of the liver weight in the intact animal. GsF, growth stimulatory factor; GiF, growth inhibitory factor.
Different liver growth factors (GFs) have been identified and classified, on the basis of their nature, as hormonal GF, GsF, and GiF (Table I). Recently, these factors have also been called initiators, progressors, and augmentors [16], on the basis of the time of their intervention in the cell cycle (Fig. 2).
TABLE I. Hormones and Growth Factors Involved in the Regulation of Liver Regeneration*.
| Hormones | Growth stimulating factors (GsF) | Growth inhibiting factors (GiF) |
|---|---|---|
| Prolactin | IGF II | TGF-β |
| Angiotensin | EGF | IL-1 |
| Vasopressin | TGF-α | IL-6 |
| Norepinephrine | HSS | RPM |
| Estradiol | HGF | |
| T3 | ||
| Insulin | ||
| Glucagon |
T3, triiodo-thyronine; IGF-II, insulin-like growth factor II; EGF, epidermal growth factor; TGF-α, transforming growth factor alpha; HSS, hepatic stimulatory substance; HGF, hepatocyte growth factor; TGF-β, transforming growth factor beta; IL-1, interleukin 1; IL-2, interleukin 2; RPM, rapamycin.
Fig. 2.
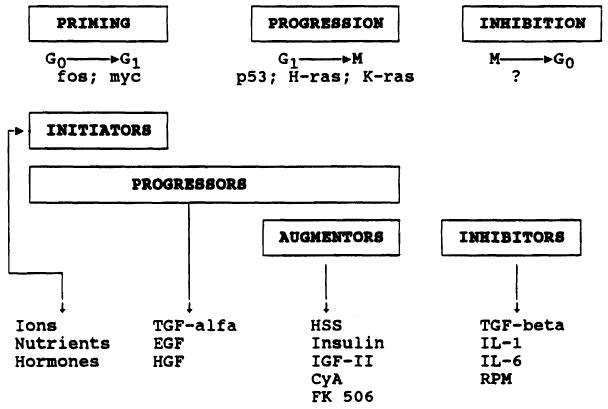
General pattern of the factors modulating liver regeneration and their time of intervention in the cell cycle. CyA, cyclosporin A; EGF, epidermal growth factor; HGF, hepatocyte growth factor; HSS, hepatic stimulatory substance; IGF, insulin-like growth factor; IL, interleukin; RPM, rapamycin; TGF, transforming growth factor.
Initiators are ions, nutrients, and hormones that are able to induce the G0–G1 transition in quiescent (G0) hepatocytes. The rapid increase of the expression of the protooncogenes myc and fos characterizes this phase [11]. Transforming growth factor alpha, hepatocyte (H)GF, and epidermal (E)GF, which are defined as progressors, induce the G0–G1 transition and allow hepatocytes to progress throughout the cell cycle. These GFs act both in vivo and in vitro [5,7,9]. H-ras, K-ras, and p53 are the biological markers of G1–S progression [11].
Hepatic stimulatory substance (HSS), cyclosporin A (CyA), FK 506, insulin, and insulin-like growth factor (IGF) II have been defined as augmentors [16–20]. They are not active in vitro, while in vivo they stimulate the proliferation of hepatocytes that have already completed the G0–G1 transition. The principal characteristics of some of these augmentors, as recently identified by our group, are reported here.
Hepatic Stimulatory Substance
HSS is a protein with a molecular weight of 33 kDa. It is found in cytosolic extracts of rat [4,8,21,22], rabbit [23], and dog [24] livers undergoing a proliferative response. The different steps of HSS preparation and purification are described in our previous papers [4,8], reporting, for the purest fraction (Acr-F4), a degree of purification of 380,000 times. This fraction is active only when administered in vivo and has been shown to be active both in the 40% hepatectomized rat model and in the portacaval shunt model [8]. In this latter model, it induces a proliferative response that exceeds that induced by other well-recognized GFs, at a dose of 20 ng/kg/day. This highly purified fraction does not stimulate the proliferation of hepatocytes in vitro in the presence or absence of either EGF or heparin [4]. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of Acr-F4 (Fig. 3) shows the presence of four different bands. However, western blot analysis demonstrates specific immunoreactivity with only one band, having an MW of 33 kDa (Fig. 3). Table II describes the physicochemical characteristics of HSS as currently recognized [8].
Fig. 3.
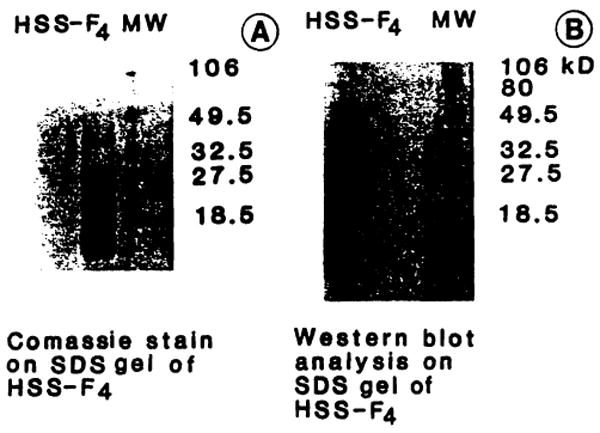
SDS-PAGE and Western blot analysis of acrylamide F4 (Ac-F4). For SDS-PAGE (A) final acrylamide concentration was 16%, in Tris 0.377 M, pH 8.8. Fifteen micrograms of protein underwent electrophoresis for 50 minutes at room temperature using 150 V, constant voltage, with Tris-HCI 0.125 M and 0.192 M glycine, pH 8.3, as reservoir buffer. Western blot analysis (B) was performed using an Immobilon membrane (Millipore). Protein transfer was conducted using 3-(cyclohexylamino)-1-propano-sulfonic acid (CAPS) 10 mM with 10% methanol, pH 11, as transfer buffer (90 minutes at 4°C with 70 V constant voltage). The membrane was blocked for 30 minutes with 3% gelatin in TTBS (Tris-buffered saline with 0.2% Tween 20) and incubated, for 60 minutes, with a specific monoclonal murine antibody against hepatic stimulatory substance (HSS) [8]. The membrane was then washed with TTBS and incubated with a second antibody in 1 % gelatin, against mouse immunoglobulins, carrying alkaline-phosphate substrate. The membrane was washed with TTBS and the immunoreactive band was identified.
TABLE II. Physicochemical Characteristics of HSS.
| Parameter | |
|---|---|
| Heat resistance | + |
| pH stability | 4.5–7.5 |
| Alcohol stability | + |
| Resistance to: | |
| Trypsin | − |
| Chymotrypsin | − |
| Neuroaminidase | + |
Cyclosporin A and Fk 506
In our recent work [17–20,25,26] we have shown that CyA and FK 506, two powerful immunosuppressors, stimulate hepatocyte proliferation in partially hepatectomized rats (Fig. 4). The effect of these drugs is mediated by their binding with a new family of cytokines called immunophilins [27], which are ubiquitous and are specific for each immunosuppressive agent [28].
Fig. 4.
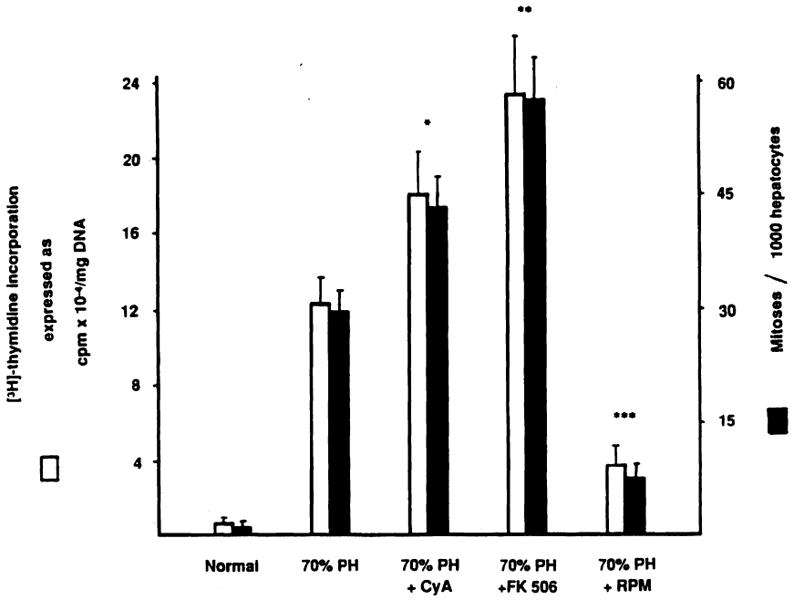
Effect of immunosuppressive agents on liver regeneration after 70% hepatectomy. [3H]thymidine incorporation and percentage of mitoses in normal and 70% hepatectomized rats treated or not treated with cyclosporin A (CyA), FK 506, and rapamycin (RPM). The animals were given oral CyA and intramuscular FK 506 or RPM at 10 mg, 1 mg, and 0.3 mg/kg body weight, respectively, for 3 days before surgery and again just after completing hepatic resection [15]. Values are the means from at least 15 rats ± SD. *, significantly different from 70% hepatectomized rats (P < .05). **, significantly different from 70% hepatectomized rats (P < .01) and CyA-treated 70% hepatectomized rats (P < .05). ***, significantly different from 70% hepatectomized rats (P < .01). Vehicle injections did not influence [3H]thymidine incorporation and percentage of mitoses in normal and 70% hepatectomized rats. PH, partial hepatectomy.
The role of immunosuppressive agents in liver regeneration has been further confirmed by another series of experiments using rapamycin (RPM) [26,29]. This drug, structurally similar to FK 506, has a negative effect on hepatocyte proliferation when administered to 70% hepatectomized rats (Fig. 4). These results indicate that immunophilins influence hepatic regeneration in both stimulatory or inhibitory ways. In addition, they indicate the presence in the cells of still unknown endogenous substances equivalent to FK 506, CyA, and RPM.
Recently two theories on the mechanism(s) regulating hepatic regeneration have been proposed to answer the central question of what starts liver regeneration. In the first theory, proposed by Michalopoulos et al. [30], the reduction of the liver mass generates in extrahepatic sites “signals” that act as complete mitogenic stimuli (Fig. 5). Two candidates have shown strong cumulative evidence as primary factors in the genesis of this stimulus: norepinephrine [31–33] and HGF [30]. In the second theory, proposed by Fausto et al. [11], the plasmatic changes induced by PH cause hepatocytes to produce their own mitogenic stimuli [transforming growth factor-alpha (TGF-α)]. In this case autocrine and paracrine loops involving nonparenchymal cells would control liver regeneration, keeping stimulatory and inhibitor “forces” stable or unbalancing them (Fig. 5). In both cases augmentors, which act on the G1–S transition, remain an important step in the chain of events involved in the control of regeneration.
Fig. 5.
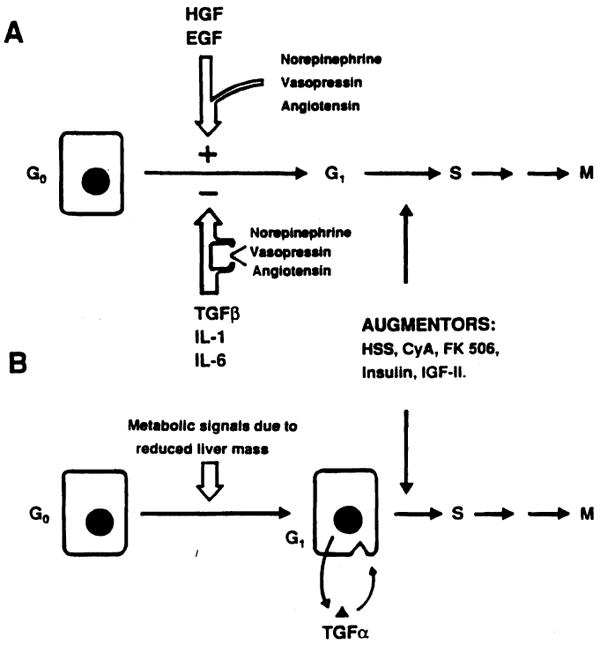
Possible mechanisms for the control of hepatic regeneration. The factors controlling hepatocyte proliferation are given, as described by Michalopoulos [1] (A) and Fausto [7] (B). Norepinephrine, vasopressin, and angiotensin potentiate GsF action and reduce GiF influence. For abbreviations, see Figure 2 legend.
The findings regarding the stimulatory activity of FK 506 and CyA have led investigators to explore another important aspect of the regenerative process, i.e., intracellular signal transduction. Actually, mitogenic stimuli are known to produce two types of intracellular signal transduction (Fig. 6). Peptides such as vasopressin, bombesin, and bradikinin bind to a guanine nucleotide protein, which activates a specific phospholipase C with formation of inositol trisphosphate [34]. On the other hand, growth factors such as EGF, platelet-derived growth factor (PDGF), and TGF-α bind to receptors having as internal domain a tyrosine kinase protein that can produce inositol trisphosphate either directly or through the same specific phospholipase C activated by the growth factors of the previous group [34]. In both cases inositol trisphosphate, which acts as transducer, binds to specific receptors of the endoplasmatic reticulum, determining a release of calcium and an increase of intracellular free-calcium concentration (Fig. 6). The calcium increase and the related pH change seem to be important steps leading to DNA replication.
Fig. 6.
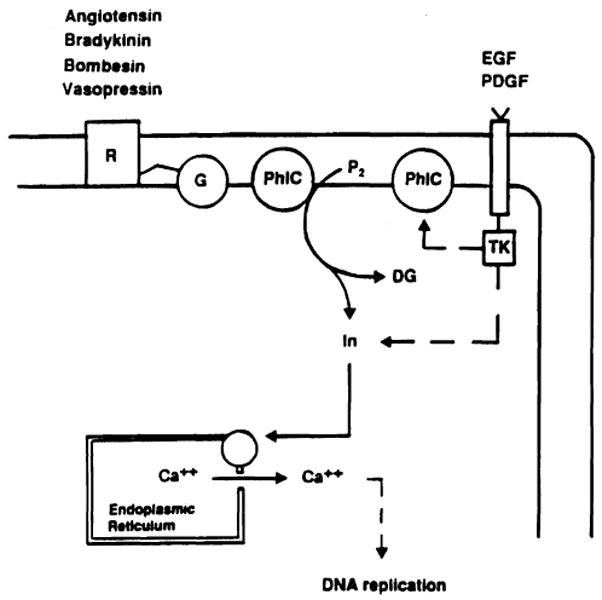
Signalling pathways of growth factors. R, cell surface receptor; G, guanine nucleotide binding protein; PhlC, phospholipase C; P2, phosphatidylinositol biphosphate; TK, tyrosine kinase; In, inositol; DG, diacylglycerol; PDGF, platelet-derived growth factor.
Recently Schreiber et al. [28] have demonstrated that when CyA and FK 506 bind to their specific immunophilins they constitute a drug-immunophilin complex (Fig. 7) that, in turn, binds with high affinity to the intracellular proteins calcineurin and calmodulin, forming a pentameric complex (Fig. 7). This pentameric complex has a high affinity for calcium channels and determines, like inositol triphosphate, a calcium release from the endoplasmic reticulum, followed by DNA replication (Fig. 8).
Fig. 7.
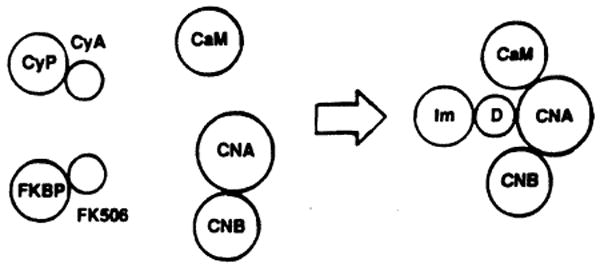
Interaction of CyA and FK 506 immunophilin complex with specific intracellular proteins. CyP, cyclophilin; FKBP, FK binding protein; CaM, calmodulin; CNA-CNB, calcineurin; Im, immunophilin; D, drug.
Fig. 8.
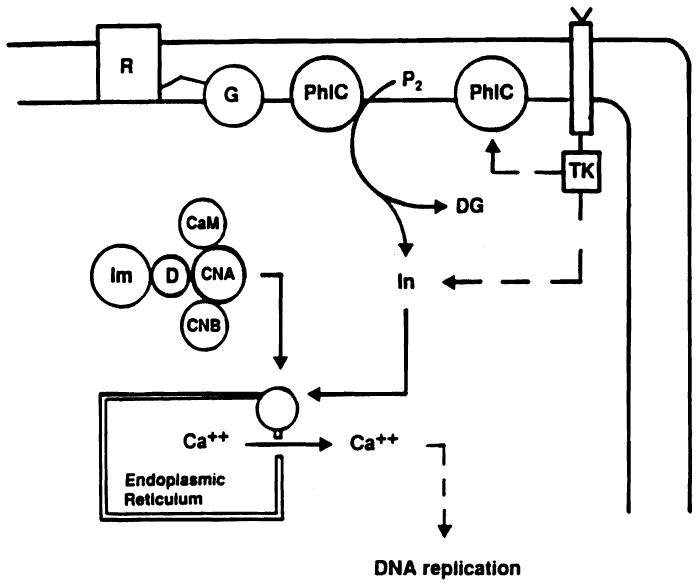
Intracellular signalling pathway of FK 506 and CyA. R, cell surface receptor; G, guanine nucleotide binding protein; PhlC, phospholipase C; P2, phosphatidylinositol biphosphate; TK, tyrosine kinase; In, inositol; DG, diacylglycerol; CaM, calmodulin; CNA-CNB, calcineurin; Im, immunophilin; D, drug.
The data obtained on liver regeneration using RPM suggest that this immunosuppressor has an opposite effect on calcium transport and therefore inhibits hepatocyte proliferation.
Conclusions
In the last decade important new data have enriched our knowledge of the mechanisms regulating liver regeneration. These findings, essentially concerning growth stimulating factors, have led to the identification of a new class of GFs defined as “augmentors.” On the other hand, very little is known about growth inhibiting factors, which undoubtedly play an important role in the control of cell proliferation.
Studies on the effect of immunosuppressive agents on liver regeneration have recently demonstrated that these agents can act either as growth inhibitors or growth stimulators. Moreover, such findings give indirect evidence of the existence of endogenous analogues of CyA, FK 506, and RPM that may constitute a connection between the immune system and the growth control system. It is likely that a deeper knowledge of all these factors will also help to define, in the near future, the mechanisms responsible for carcinogenesis.
Acknowledgments
This study was supported by Consiglio Nazionale delle Richerche, ACPR Program, grant 92.02187.PF39.
References
- 1.Michalopoulos G, Houck KA, Dolan ML, Luetteke NC. Control of hepatocvte replication by two serum factors. Cancer Res. 1984;44:4414–4419. [PubMed] [Google Scholar]
- 2.Goldberg M. Purification and partial characterization of a liver cell proliferation factor called hepatopoietin. J Cell Biochem. 1985;27:291–302. doi: 10.1002/jcb.240270310. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura T, Teramoto H, Ichihara A. Purification and characterization of a growth factor from rat platelets for mature parenchymal hepatocytes in primary cultures. Proc Natl Acad Sci USA. 1986;83:6489–6493. doi: 10.1073/pnas.83.17.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francavilla A, Ove P, Polimeno L, et al. Extraction and partial purification of a hepatic stimulatory substance in rats, mice, and dogs. Cancer Res. 1987;47:5600–5605. [PMC free article] [PubMed] [Google Scholar]
- 5.Olsen PS, Boesby S, Kirkegaard P, et al. Influence of epidermal growth factor on liver regeneration after partial hepatectomy in rats. Hepatology. 1988;8:992–996. doi: 10.1002/hep.1840080503. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura T, Nishizawa T, Hagiya M, et al. Molecular cloning and expression of human hepatocvte growth factor. Nature. 1989;342:440–443. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- 7.Mead JE, Fausto N. Transforming growth factor alpha may be a physiological regulator of liver regeneration by means of an autocrine mechanism. Proc Natl Acad Sci USA. 1989;86:1558–1562. doi: 10.1073/pnas.86.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francavilla A, Barone M, Van Thiei DH, et al. Further steps of HSS (hepatic stimulatory substance) purification. Dig Dis Sci. 1991;36:674–680. doi: 10.1007/BF01297037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michalopoulos GK, Zamegar R. Hepatocvte growth factor. Hepatology. 1992;15:149–154. doi: 10.1002/hep.1840150125. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura T, Arakaki R, Ichihara A. Interleukin-1 is a potent growth inhibitor of adult rat hepatocytes in primary culture. Exp Cell Res. 1988;179:488–497. doi: 10.1016/0014-4827(88)90286-8. [DOI] [PubMed] [Google Scholar]
- 11.Fausto N, Mead JE. Regulation of liver growth: protooncogenes and transforming growth factors. Lab Invest. 1989;60:4–13. [PubMed] [Google Scholar]
- 12.Mizel SB. The interleukines. FASEB J. 1989;3:2379–2388. doi: 10.1096/fasebj.3.12.2676681. [DOI] [PubMed] [Google Scholar]
- 13.Francavilla A, Starzl TE, Van Thiel DH, et al. Hepatic regeneration. In: LeBouton AV, editor. Molecular and Cell Biology of the Liver. Caldwell NJ: The Telford Press; 1993. in press. [Google Scholar]
- 14.Kam I, Lynch S, Svanas G, et al. Evidence that host size determines liver size: studies in dogs receiving orthotopic liver transplants. Hepatology. 1987;7:362–366. doi: 10.1002/hep.1840070225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins GM, Anderson RM. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186–202. [Google Scholar]
- 16.Francavilla A, Starzl TE, Porter K, et al. Screening for candidate hepatic growth factors by selective portal infusion after canine Eck's fistula. Hepatology. 1991;14:665–670. doi: 10.1016/0270-9139(91)90055-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francavilla A, Barone MB, Starzil TE, et al. FK 506 as a growth control factor. Transplant Proc. 1990;23:90–92. [PMC free article] [PubMed] [Google Scholar]
- 18.Francavilla A, Barone M, Todo S, et al. Augmentation of rat liver regeneration by FK 506 compared with cyclosporin. Lancet. 1989;25:1248–1249. doi: 10.1016/s0140-6736(89)91853-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francavilla A, Starzl TE, Barone M, et al. Studies on mechanisms of augmentation of liver regeneration by cyclosporin and FK506. Hepatology. 1991;14:140–143. doi: 10.1002/hep.1840140123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Francavilla A, Starzl TE, Barone M, et al. Studies on mechanisms of augmentation of liver regeneration by cyclosporine and FK 506. Hepatology. 1991;14:140–143. doi: 10.1002/hep.1840140123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Labrecque DR, Pesch LA. Preparation and partial characterization of hepatic regenerative stimulator substance (SS) from rat liver. J Physiol. 1975;14:273–284. doi: 10.1113/jphysiol.1975.sp010973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labrecque D, Steele G, Fogerty S, et al. Purification and physical-chemical characterization of hepatic stimulatory substance. Hepatology. 1987;7:100–105. doi: 10.1002/hep.1840070121. [DOI] [PubMed] [Google Scholar]
- 23.Fleig WE, Lehmann H, Wagner H, et al. Hepatic regenerative stimulator substance in the rabbit: relation to liver regeneration after partial hepatectomy. J Hepatol. 1986;3:19–25. doi: 10.1016/s0168-8278(86)80141-6. [DOI] [PubMed] [Google Scholar]
- 24.Starzl TE, Terblanche J, Porter KA, et al. Growth stimulating factor in regenerating canine liver. Lancet. 1979;2:127–130. doi: 10.1016/s0140-6736(79)90519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Starzl TE, Porter KA, Mazzaferro V, et al. Hepatotrophic effects of FK 506 in dogs. Transplantation. 1991;51:67–70. doi: 10.1097/00007890-199101000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francavilla A, Starzl TE, Carr B, et al. The effects of FK506, cyclosporin and rapamycin on liver growth in vitro and in vivo. Transplant Proc. 1991;23:2817–2820. [PMC free article] [PubMed] [Google Scholar]
- 27.Starzl TE, Schreiber SL, Albers MW, et al. Hepatotrophic properties in dogs of human FKBP, the binding protein for FK 506 and rapamycin. Transplantation. 1991;52:751–753. doi: 10.1097/00007890-199110000-00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schreiber SL, Liu J, Albers MW, et al. Immunophilin-ligand complexes as probes of intracellular signaling pathways. Transplant Proc. 1991;23:2839–2844. [PubMed] [Google Scholar]
- 29.Francavilla A, Carr BI, Starzl TE, et al. Effects of rapamycin on cultured hepatocyte proliferation and gene expression. Hepatoiogy. 1992;15:871–877. doi: 10.1002/hep.1840150520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michalopoulos GK. Liver regeneration: molecular mechanisms of growth control. FASEB. 1990;4:176–187. [PubMed] [Google Scholar]
- 31.Cruise JL, Knechtle SJ, Bollinger RR, et al. Alpha 1-adrenergic effects and liver regeneration. Hepatology. 1987;7:1189–1194. doi: 10.1002/hep.1840070604. [DOI] [PubMed] [Google Scholar]
- 32.Cruise JL, Houck KA, Michalopoulos G. Induction of DNA synthesis in cultured rat hepatocytes through stimulation of alpha-1 adrenoreceptor by norepinephrine. Science. 1985;227:749–751. doi: 10.1126/science.2982212. [DOI] [PubMed] [Google Scholar]
- 33.Houch KA, Cruise JL, Michalopoulos GK. Norinephrine modulates the growth-inhibitory effect of transforming growth factor-beta in primary hepatocyte cultures. J Cell Physiol. 1988;125:551–555. doi: 10.1002/jcp.1041350327. [DOI] [PubMed] [Google Scholar]
- 34.Powis G, Kozikowski A. Growth factor and oncogene signalling pathways as targets for rational anticancer drug development. Clin Biochem. 1991;24:385–397. doi: 10.1016/s0009-9120(05)80014-1. [DOI] [PubMed] [Google Scholar]


