SUMMARY
A technique for the isolation of human intrahepatic bile ductular epithelium, and the establishment of primary cultures using a serum- and growth-factor-supplemented medium combined with a connective tissue substrata is described. Initial cell isolates and monolayer cultures display phenotypic characteristics of biliary epithelial cells (low molecular weight prekeratin positive; albumin, alphafetoprotein, and Factor VIII-related antigen negative). Ultrastructural features of the cultured cells show cell polarization with surface microvilli, numerous interepithelial junctional complexes and cytoplasmic intermediate prekeratin filaments.
Keywords: biliary epithelium, bile duct, cell culture, human
INTRODUCTION
The biliary epithelium is a relatively select target of lymphocytic attack in liver allograft rejection and primary biliary cirrhosis (1–3). However, the interactions between these epithelial cells and T-lymphocytes, which putatively mediate the epithelial cell damage in these conditions, have yet to be defined. An ideal approach to study these interactions would obviously be the use of an in vitro system, utilizing bile duct and lymphocyte cell cultures. Bile duct cell (4,5) and nonparenchymal epithelial (NPE) cell cultures from normal rat livers, with the establishment of serially passable cell lines, has been successful to some extent (6–8). However, the exact origin of these rat NPE cells that are serially passable is uncertain (9–13). We were unable to find any reference to human intrahepatic bile ductular epithelium (BDE) or NPE cell cultures in the literature. The objective of these studies was to devise a method for the isolation of bile ductular cells from normal human livers and the establishment of primary cultures. Initial media selection was based on reported experience with establishment of human hepatocyte and breast ductular epithelial cell cultures (14,15). Successful establishment of primary cultures of BDE was achieved using a serum-supplemented media in combination with a connective tissue substrate.
MATERIALS AND METHODS
Source of Tissue
The majority of the study tissues were either intact human livers (unused donor grafts) or large segments of normal liver obtained from partial hepatectomies performed before transplantation. Unused normal donor livers became available in situations where the recipient was unable to undergo orthotopic liver transplantation, such as those with inoperable hepatic malignancies.
Biliary epithelial cell isolation
The entire organ or segment of liver was placed on a sterile drape under a laminar flow hood with all the subsequent steps performed using sterile techniques. The hepatic bile ducts were identified by their yellow luminal color and by milking the organ to produce a back-flow of bile. A silastic cannula (2 mm × 12 in.) was advanced into the duct and sutured in place. A small segment (1 to 2 cm) of the most lateral portion of the respective lobe was resected and the biliary tract was then flushed with Hanks’ balanced salt solution (HBSS) (calcium and magnesium-free HBSS, GIBCO Lab., Life Technologies Inc., Grand Island, NY) until the effluent streams emerging from the cut liver surface were bile free. The biliary tree was then filled with a 0.25% collagenase IV (Worthington Diagnostics System Inc., Freehold, NJ) solution in HBSS, kept at 37° C, and left for 30 min to free up the cells.
After collagenase incubation, the specific bile duct tree was again flushed with HBSS and the digestion effluent was collected. The fluids were then transferred to 50-ml plastic centrifuge tubes and spun at 1200 rpm for 10 min. The pellet was resuspended in a minimal volume of culture medium and plated on Falcon tissue culture plates previously coated with Matrigel (Collaborative Res. Inc., Lexington, MA).
Tissue Culture Medium and Substrate
Basal media used for culturing the epithelial cells (Table 1), included CEM 2000 (16) (Scott Lab., West Warwick, RI), Williams E with l-glutamine or a 50/50 (vol/vol) mixture of Ham’s F12 and Dulbecco’s modified Eagle’s medium (DMEM). Serum supplementation was with 10% heat inactivated fetal bovine serum. Supplements (Tables 1 and 2) consisted of SGF-7 (1 ml/100 ml medium), SGF-9 (1 ml/100 ml/medium, Scott Lab.), 100 µg/ml endothelial cell growth factor (Collaborative Res. Inc.), and 250 µg/ml Fungizone, 56 µg/ml Gentamycin (GIBCO).
TABLE 1.
CONSTITUENTS OF SERUM-SUPPLEMENTED BASAL MEDIA
| Constituent | Amount |
|---|---|
| Basal media | 90% |
| Fetal bovine serum | 10% |
| SGF-7 | 1 ml/100 mla |
| SGF-9 | 1 ml/100 mla |
| Endothelial cell growth factor | 100 µg/ml |
| Fungizone | 250 µg/ml |
| Gentamycin | 56 µ/ml |
See Table 2 for a listing of the constituents and their final concentrations.
TABLE 2.
CONSTITUENTS AND FINAL CONCENTRATION OF GROWTH SUPPLEMENTS (SGF-7 AND SGF-9) GROWTH-FACTOR-SUPPLEMENTED BASAL MEDIA
| Constituent | Concentration |
|---|---|
| Epidermal growth factor | 17.5 ng/ml |
| Transferrin (iron saturated) | 5.0 µg/ml |
| Insulin (bovine) | 1.1 µg/ml |
| Fetuin | 500 µg/ml |
| Hydrocortisone | 750 ng/ml |
| Selenous acid | 5.6 ng/ml |
| T3 | 1.5 ng/ml |
| Progesterone | 1.5 ng/ml |
| Oleic-BSA and linoleic acid-BSA | 500 ng/ml |
| 2-Aminoethanol | 920 ng/ml |
| O-Phosphorylethanolamine | 2.1 µg/ml |
Follow up of primary NPE cultures
The plates were washed free of nonadherent organoids or cells or both after 2 d. Half of the BDE culture medium was removed every 2nd d and replaced with fresh complete medium in the above described form. Once the cells were firmly attached (4 to 5 d) the medium was totally replaced by fresh medium every 2 to 3 d.
Characterization and Identification
Cells obtained from the digestion procedure shortly after harvesting from liver segments were fixed with cold acetone on gelatin-coated microscopic slides using a cytospin for 5 min at 1000 rpm for light or immunostaining microscopy or in 2% glutaraldehyde for transmission electron microscopy. Cells were harvested from confluent monolayers with 0.1% collagenase solution in HBSS, using a 30-min incubation at 37° C with gentle scratching of the plates. Cells were fixed as described and stained for gammaglutamyl transpeptidase or for prekeratin, AE-1 (Behringer Biochemical), albumin, and Factor VIII-related antigen (Dako Corp. Santa Barbara, CA) using an indirect immunofluorescent or immunoperoxidase technique with biotinylated primary antibodies. The AE-1 antibody reacts against acidic (type I) keratins of 40, 48, 50, and 56.5 kilodaltons (kD) molecular weight found in simple epithelia such as bile ducts but not in hepatocytes. Color development was achieved with diaminobenzidine.
Transmission electron microscopy was performed on both the initial cell isolates and on intact monolayers grown in Lab-Tek (Miles Scientific, Naperville, IL) tissue culture chamber-slides. Cell monolayers were fixed in situ with 2% glutaraldehyde (1 h) and 1% osmium tetroxide (30 min). The monolayers were dehydrated in graded ethyl alcohol and propylene oxide, then infiltrated with Epon-araldite embedding resin. After infiltration (2 h), the slides with the monolayers were inverted over an embedding capsule which had been filled with resin, and then polymerized overnight at 60° C. The following day the cooled block was removed from the slide with a sharp twist. The monolayer was now on the surface of the block. Thin sections were cut and stained with uranyl acetate and lead citrate.
RESULTS
Bile Duct Cell Isolates
Identification, purity, and viability
Microscopic examination of tissue sections of livers from which biliary cells were removed using the above techniques revealed an intact lobular architecture with undisturbed hepatocytes. The biliary epithelium of the larger intrahepatic septal ducts and some smaller (100 to 200 µm i.d.) ducts were either devoid of epithelial cells, had only a partial circumference of the duct lined by epithelial cells, or had epithelial cells lying free in the ductal lumena (Fig. 1 a, b). Therefore, the cells that we were attempting to isolate were indeed dislodged from the underlying connective tissue. Immunoperoxidase staining for prekeratin using a polyclonal screening antibody to detect 52, 56, and 58 kD subunits and monoclonal AE-1 (acidic low molecular weight keratins) and gammaglutamyl transpeptidase in tissue sections from the liver either before or after BDE harvesting revealed staining only in the biliary epithelial cell.
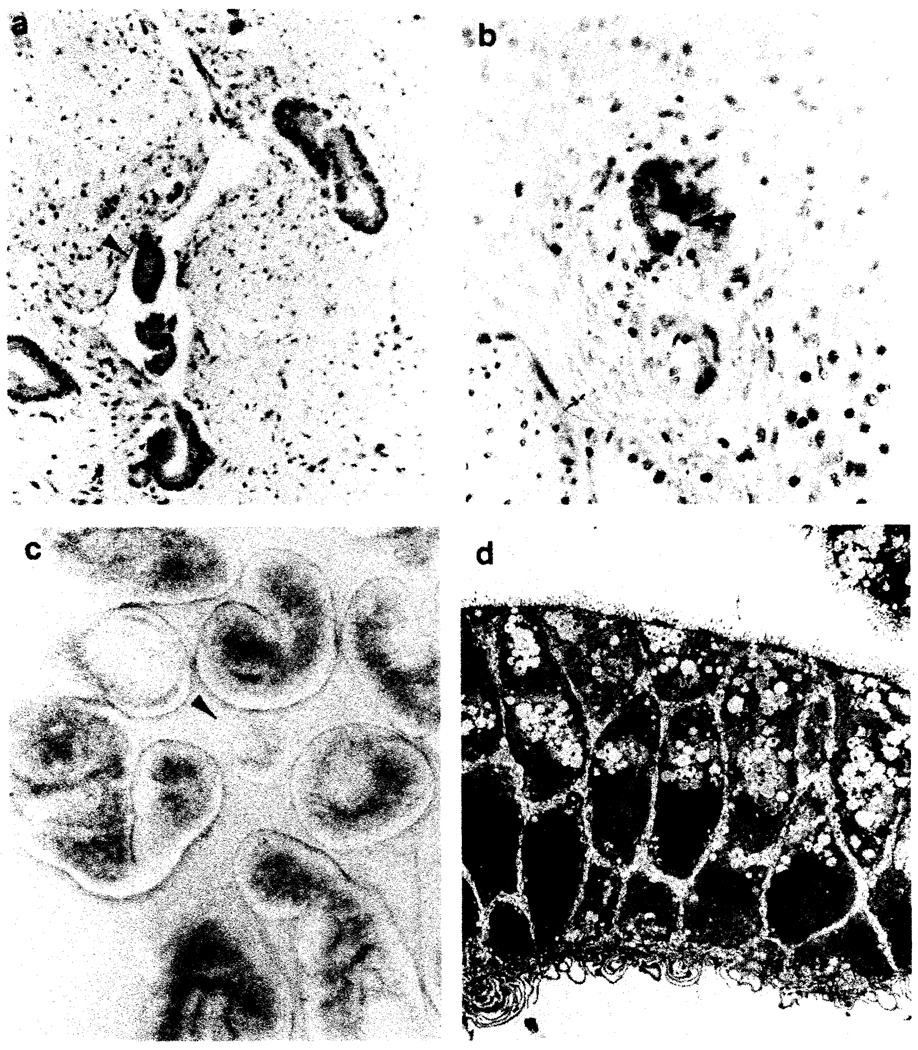
FIG. 1.
Appearance of the liver after digestion and morphologic characteristics of initial cell isolates. Cross section of large septal bile duct (a. H&E, X100) and smaller ductule (b, prekeratin immunoperoxidase, X400). Phase contrast microscopy of organbid clusters of epithelial cells (c, X40), postdigestion eluate. Note the similar appearance of the organoids in a and c (arrows). Ultrastructural characteristics of initial isolates (d, X1800). Note the polarization of the cells, surface microvilli, lipid droplets, and few cytoplasmic organelles.
Phase contrast microscopy of eluates obtained immediately after tissue digestion revealed organoid clusters of cells, intermixed with many fewer single small round cells ranging in size from 10 to 15 µm in diameter. Resuspension of the eluates in 50-ml plastic centrifuge tubes, followed by gravity sedimentation and removal of all but 2 to 3 ml of the supernatant, revealed the sediment fraction to be enriched in organoid cell clusters (Fig. 1 c).
Immunoperoxidase or histochemical staining of the organoid clusters, using the same prekeratin antibodies or for gammaglutamyl transpeptidase was positive, whereas Factor VIII-related antigen (endothelial) and albumin (hepatocytes) staining were negative. Staining of the supernatant that contained the greater percentage of single cells revealed that 70% of the cells had staining characteristics similar to the organoid clusters. The organoid clusters and the single cell suspensions exhibited from 70 to 90% viability based on trypan blue exclusion.
Electron microscopic examination of the inital eluates revealed clusters and sheets of cells with surface microvilli connected by tight junctional complexes. Numerous interdigitating cytoplasmic processes were seen in the intercellular spaces. Scattered small mitochondria, sparse rough endoplasmic reticulum, glycogen granules, and intracytoplasmic lipid droplets were seen in the cytoplasm (Fig. 1 d).
Effects of substrata
Attempts were made to culture the epithelial cells with complete media on three different substrates: Falcon tissue culture plastic alone and tissue culture plastic coated with human fibronectin or Matrigel.
Plastic alone resulted in rather poor plating efficiency in serum-free media (< 5% attachment of the organoid clusters at 24 h). The attachment efficiency increased in serum-supplemented media to near 25% of the organoids. The cells that did attach would spread rapidly over the plates, but sustained cell growth was not observed. More typically, the attached cells would become large and squamoid in appearance at about the end of the 1st wk, and lift from the plate.
Human fibronectin-coated plates increased the percentage of attached organoids to 10% in serum-free media. Cell spreading and early cell growth to near confluence were seen during the 1st wk to 10 d, but thereafter the appearance of the cultures was similar to that seen when the cells were plated on plastic alone. This outcome persisted despite continued addition of fibronectin to fresh culture media every 3rd d.
The Matrigel-coated plates resulted in 30% organoid attachment using serum-supplemented media, at 24 h, a clearly superior attachment when compared to the other methods. Cell spreading could be seen within 24 h, and the cells grew to confluence in 7 to 10 d. Other connective tissue substrata such as type IV collagen and laminin were not tested for their effect on cell attachment efficiency and culture maintenance.
Serum-supplemented media increased the efficiency of cell attachment regardless of the substrata. The Matrigel substrata, however, continued to prove superior to the others with regard to cell attachment and culture maintenance.
Effects of culture medium
Attempts to establish cell cultures on a Matrigel substrate were performed with the following: a) basal medium alone, b) basal medium plus SGF-7 and SGF-9, c) basal medium supplemented with 10% fetal bovine serum, and d) basal media with serum and growth-factor supplements as described in Table 1.
Initial cell attachment was limited to less than 5% of the organoid clusters in serum-free media despite fetuin supplementation in all substrata. Serum supplementation was essential therefore for initiation of the cultures.
Nonvacuolated monolayers could be achieved by using any of the basal media (see Materials and Methods) but required supplementation with fetal bovine serum. Serum-free medium, on the other hand, despite the addition of fetuin and growth-factor supplements, was unable to support the initiation of cultures. However, once established, cultures could be maintained with progressively lesser amounts (less than 2%) of serum supplementation.
Morphologic and phenotypic characteristic of cultures
Bile ductular epithelium cultures were examined routinely each day using an inverted phase contrast microscope. The cells would begin to spread from the organoid clusters within 24 h and growth to confluence occurred in most instances by 8 to 12 d. Confluent wells of BDE presented as a monolayer with a characteristic cobblestone appearance. The individual cells were mostly small and polygonal with smooth intercellular borders and slightly granular cytoplasm; however, as the cultures aged, some cells became larger and more irregularly shaped. At times ridges of piled-up cells could be seen at the spreading edges.
Cells plated on the Matrigel, maintained a cobblestone appearance for several weeks with a small, slightly granular cytoplasm. Those plated on fibronectin became quite large and squamoid in appearance with a clear cytoplasm and detached from the plate in 7 to 10 d.
After 2 wk in culture, two different cellular populations could be recognized by phase contrast microscopy. One population of cells was small, polygonal, and formed cobblestone sheets with smooth, intercellular borders. Aggregates of these cells were intermixed with larger angular cells and slightly spindled cells with clear cytoplasm that on occasion would become multinucleated. On daily observation it seemed as though the smaller cells would transform into larger and more irregularly shaped ones. In turn, these larger cells would become multinucleate, senescent, and peel from the plate. In addition, tubularlike structures with hollow centers could be seen with back-and-forth focusing of the phase contrast microscope (Fig. 2).
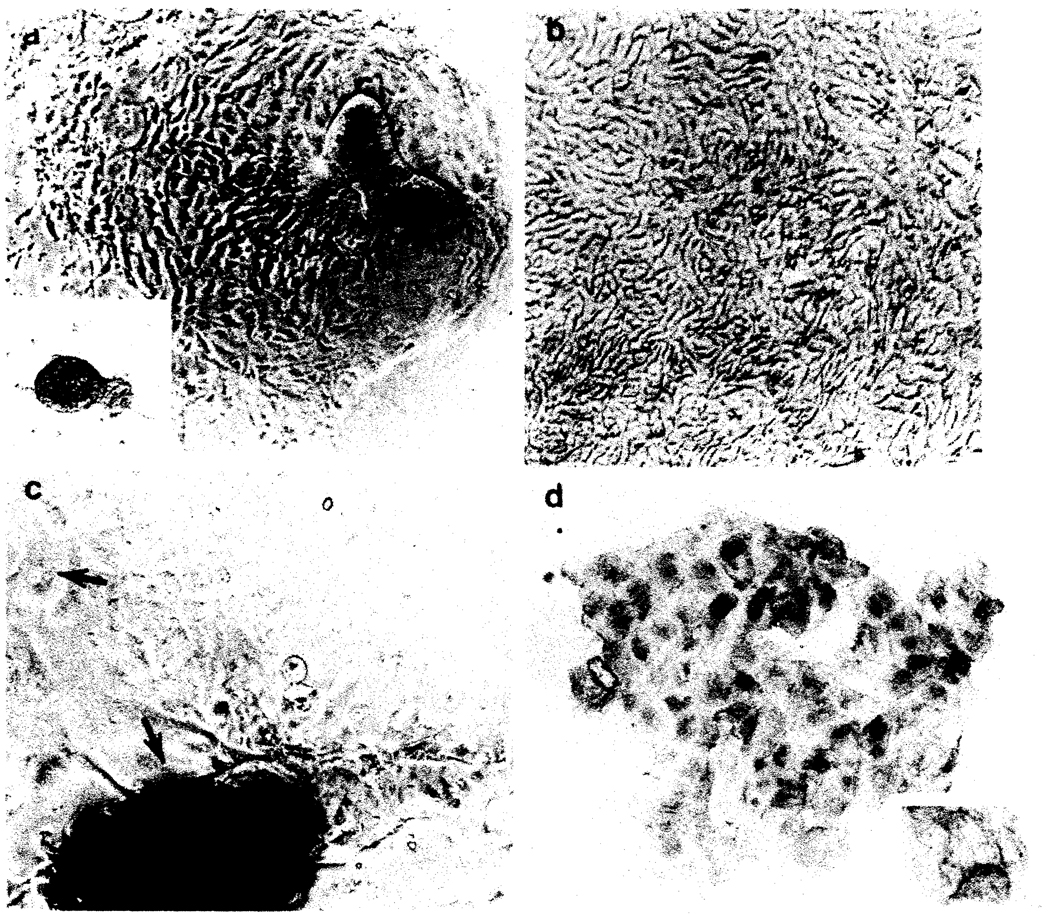
FIG. 2.
Phase contrast microscopy of the cell cultures. a, Initial isolates consist largely of “organoid” clusters of cells (inset) which after 24 h begin to spread over the plate. b, After 8 to 12 d the plates become confluent with a cobblestone appearance. c, The cells begin to form spheres containing debris after 3 to 4 wk (bottom arrow). Note the smaller polygonal cells in the top center of c and the nearby multinucleated cell (top arrow). Immunoperoxidase staining for prekeratin reveals that virtually all cells are positive (d) showing crisscrossing fibrils in the cytoplasm (inset).
The monolayers, including all cellular populations recognized by phase contrast microscopy were uniformly and nearly 100% prekeratin positive. Staining for albumin to detect hepatocytic differentiation, alphafetoprotein, and Factor VIII-related antigen to detect endothelial cell differentiation were negative. Staining for gammaglutamyl transpeptidase seemed weakly positive, but the staining intensity was too weak to ascertain that the cells demonstrated this characteristic.
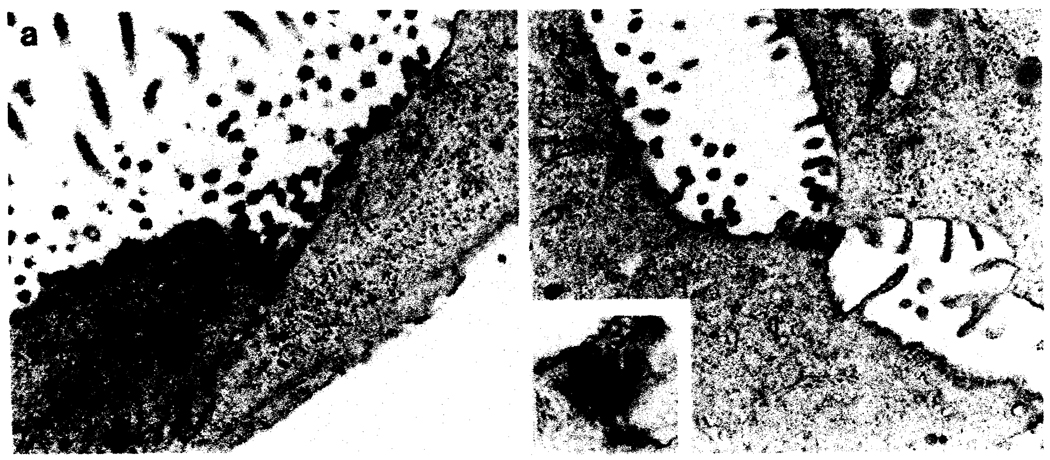
Ultrastructural examination of the monolayers (Fig. 3) revealed numerous intercellular epithelial-type junctional complexes (tight and desmosomal), surface microvilli, and lumenal-like intercellular spaces. The cells contained a few cytoplasmic organelles such as small mitochondria, focal rough endoplasmic reticulum, sparse glycogen granules, and cytoplasmic intermediate filaments that were recognized as prekeratin by light microscopy immunoperoxidase. No cells with endothelial characteristics (i.e. Wiebel-Palade bodies) were seen.
FIG. 3.
Ultrastructural characteristics of the cell cultures revealing surface microvilli, intermediate cytoplasmic filaments (arrow in a), and interepithelial junctional complexes.
Attempts at Passage
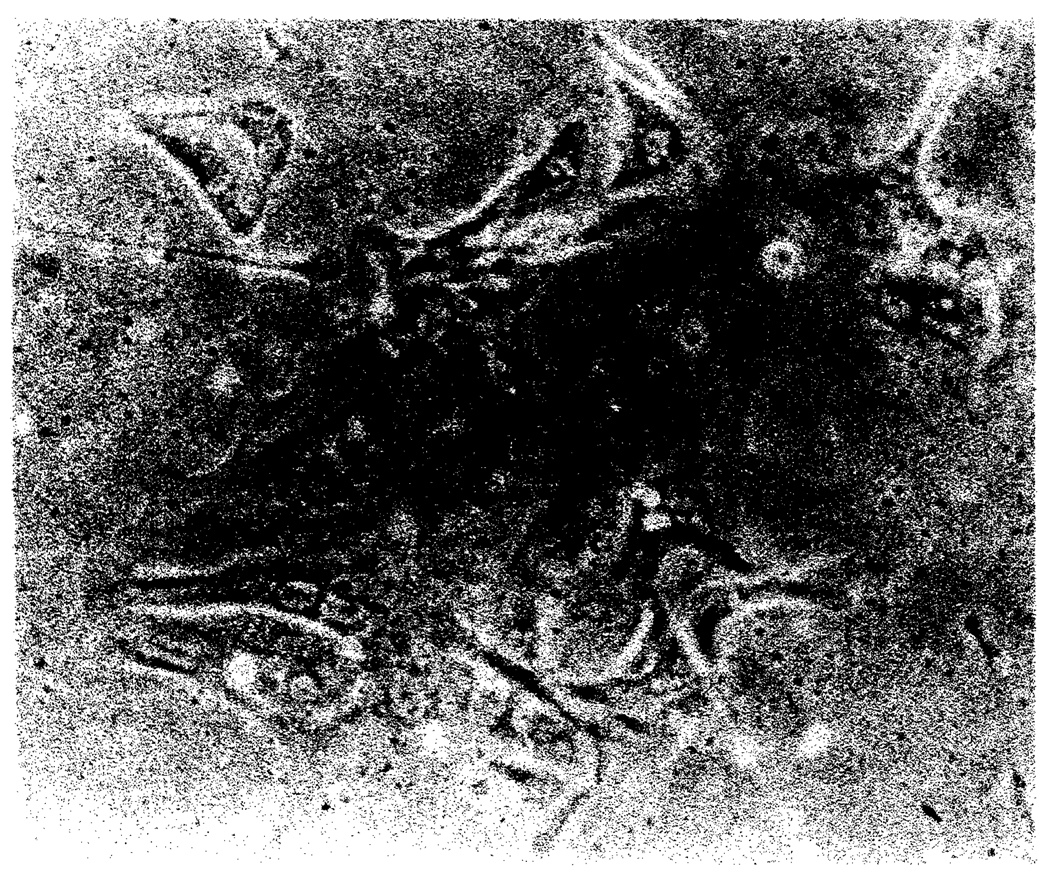
After growth to confluence, the monolayers were subjected to trypsinization, which in most instances resulted in detached sheets of cells rather than single cell suspensions. The cells were then replated on Matrigel-coated plates. In two instances, a 1:2 split resulted in reconfluent plates within 2 wk. However, after the 2nd passage, and in some instances on the 1st passage, many cells would become large, multinucleated, and cease to grow (Fig. 4). This was particularly true when vigorous digestion was used to produce single cell suspensions. These changes were followed by peeling from the plate, pynkosis, and loss of cell cultures.
FIG. 4.
Phase contrast appearance of biliary epithelial cells after 2 passages. Note the large cell size and multinucleation.
DISCUSSION
The primary goal of this study was to isolate liver epithelial cells derived from the human biliary tract, because in vivo they display immunologic properties different from hepatocytes (17). We therefore chose not to attempt isolation of biliary epithelium from whole organ homogenates using Pronase digestion and centrifugal elutriation or other techniques as employed in the isolation and culture of rat duct cells (4,5,8), because of the greater potential of hepatocyte or endothelial cell contamination.
Cells isolated by our technique were in all probability derived from septal biliary ducts. This contention is based on the appearance of histologic sections of the liver after dislodging and harvesting the cells, the phase contrast, electron microscopic, and immunohistochemical staining characteristics of the isolates immediately after harvesting, and the routine, immunohistologic, and ultrastructural characteristics of monolayer cultures. Using this harvesting technique, the most likely cell contaminants seem to be fibroblasts and connective tissue dendritic cells which often lie near the ducts; endothelial cell and hepatocyte contamination is much less likely and could be excluded on the basis of the phenotypic characteristics of the isolated cells and their immunohistologic and ultrastructural properties. The hydrocortisone in the media, progressively decreasing supplementation with serum, and the keratin positivity of greater than 90% of the cells in monolayers, suggest that the level of contamination, if present, was negligible.
Basal media selection with serum supplementation was based on reported experience with rat and human Liver parenchymal cell and breast ductal epithelial cell cultures (4,6,8,11,15). Attempts made to sustain the cultures using a serum-free, growth-factor-supplemented medium were based on reports that serum supplementation may be inhibitory to epithelial cell growth (15). The growth supplements selected were those reported to be stimulatory for epithelial cell cultures (15). Addition of endothelial cell growth factor (ECGF) to the media was based on the finding that crude central nervous system extracts similar to the ECGF used in these studies were found to stimulate epithelial cell growth (15). Unfortunately, our work to date does not give a clear indication as to which media or combinations of growth factors or both was optimal, other than the requirement of serum for initial cell attachment.
The need for and the interactions between differentiated epithelial cells and connective tissue stroma in cell culture systems have been previously emphasized (14,17). The use of the Matrigel substrate was clearly superior in enhancement of cell attachment and culture maintenance when compared to plastic alone or plastic coated with human fibronectin.
Sustained growth of the BDE beyond 2 passages was not achieved. This may be due to the differentiated state of the isolated cells, suboptimal growth conditions, or the use of serum-supplemented media.
Epithelial cells that serve as “stem” cells in vivo, such as those derived from the basal layers of the epidermis, continue to display these characteristics in vitro as long as they retain contact with the appropriate mesenchyme (14,16,18). The concept of a “liver epithelial stem cell” seems to be supported by the establishment of lines of NPE harvested from rat livers and maintained in serum-supplemented media (6,9). These cells display, or could be induced to dislay, phenotypic characteristics not only of bile ducts, (GGTP, Alkaline phosphatase positive) but also of hepatocytes (albumin positive). In addition to the dualistic phenotypic characteristics displayed by such cells, alpha fetoprotein (AFP) may also be detected (9), indicative of a “fetal” state. The cells isolated in this study, under the conditions employed however, displayed characterisice more consistent with differentiated biliary epithelium (prekeratin positive; AFP and albumin negative). In that regard, our findings are quite similar to those of Sirica et al. (4) in that the cells cultured expressed characteristics of true bile ductular epithelium, in contrast to “oval” cells derived from carcinogen-treated rats. The relationship between these two cell types however remains a point of controversy.
Further experimentation is necessary to more precisely define the optimal growth conditions of these cells in culture, and to further characterize the cell type phenotypically, immunologically, and biochemically. However, we feel that this methodology is a useful starting point for the in vitro study of certain hepatic disorders, such as primary biliary cirrhosis, biliary atresia, and liver allograft rejection in which bile duct cells play a major role, and could be used as targets in immunologic studies.
Acknowledgments
We express our thanks to Dr. B. Lombardi, for encouragement and helpful discussions during performance of this work.
REFERENCES
- 1.Demetris AJ, Lasky S, Van Thiel DH, et al. Pathology of hepatic transplantation: a review of 62 adult allograft recipients immunosuppressed with a cyclosporine/steroid regimen. Am. J. Pathol. 1985;118:151–161. [PMC free article] [PubMed] [Google Scholar]
- 2.Snover DC, Sibley RK, Freese DK, et al. Orthotopic liver transplantation: a pathologic study of 63 serial liver biopsies from 17 patients with special reference to the diagnostic features and natural history of rejection. Hepatology. 1984;4:1212–1222. doi: 10.1002/hep.1840040620. [DOI] [PubMed] [Google Scholar]
- 3.MacSween RNM, Burt AD. The cellular pathology of primary biliary cirrhosis. Molecular aspects Med. 1985;8:269–291. doi: 10.1016/0098-2997(85)90010-x. [DOI] [PubMed] [Google Scholar]
- 4.Sirica AE, Sattler CA, Cihla HP. Characterization of a primary bide ductular cell culture from the livers of rats during extrahepatic cholestasis. Am. J. Pathol. 1985;120:67–78. [PMC free article] [PubMed] [Google Scholar]
- 5.Grant AG, Billing BH. The isolation and characterization of a bile ductule cell population from normal and bile-duct ligated rat livers. Br. J. Exp. Pathol. 1977;58:301–310. [PMC free article] [PubMed] [Google Scholar]
- 6.Ming-Sound T, Smith JD, Nelson KG, et al. A diploid epithelial cell line from normal adult rat liver with phenotypic properties of “oval” cells. Exp. Cell Res. 1984;154:38–52. doi: 10.1016/0014-4827(84)90666-9. [DOI] [PubMed] [Google Scholar]
- 7.Yoshimura H, Harris R, Yokoyama S, et al. Anaplastic carcinomas in nude mice and in original donor strain rats inoculated with cultured oval cells. Am. J. Pathol. 1983;110:322–332. [PMC free article] [PubMed] [Google Scholar]
- 8.Fausto N, Thompson NL, Braum L. Purification and culture of oval cells from rat liver. In: Pretlow TG, Pretlow TP, editors. Cell separation: methods and selected applications. New York: Academic Press; 1987. pp. 45–78. [Google Scholar]
- 9.Grisham JW, Hartroft WS. Morphologic identification by electron microscopy of “oval” cells in experimented hepatic regeneration. Lab. Invest. 1961;10:317–332. [PubMed] [Google Scholar]
- 10.Grisham JW, Porta EA. Origin and fate of proliferated hepatic ductal cells in the rat: electron microscopic and auto-radiographic studies. Exp. Mol. Pathol. 1964;3:242–261. doi: 10.1016/0014-4800(64)90057-7. [DOI] [PubMed] [Google Scholar]
- 11.Grisham JW. Cell types in long-term passable cultures of rat liver. Ann. NY Acad. Sci. 1980;349:128–137. doi: 10.1111/j.1749-6632.1980.tb29521.x. [DOI] [PubMed] [Google Scholar]
- 12.Hayner NT, Braun L, Yaswen P, et al. Isoenzyme profiles of oval cells, parenchymal cells, and biliary cells isolated by centrifugal elutriation from normal and preneoplastic livers. Cancer Res. 1984;44:332–338. [PubMed] [Google Scholar]
- 13.Petropoulos C, Yaswen P, Panzcia M, et al. Cell lineage in liver carcinogenesis: possible clues from studies of the distribution of alphofeto-protein RNA sequences in all populations isolated from normal, regenerating and preneoplastic livers. Cancer Res. 1985;45:5762–5768. [PubMed] [Google Scholar]
- 14.Ried LM, Jefferson DM. Culturing hepatocytes and other differentiated cells. Hepatology. 1984;4:548–559. doi: 10.1002/hep.1840040332. [DOI] [PubMed] [Google Scholar]
- 15.Hammond SL, Ham RG, Stampfer MR. Serum-free growth of human mammary epithelial cells: Rapid clonal growth in defined medium and extended serial passage with pituitary extract. Proc. Natl. Acad. Sci. USA. 1984;81:5435–5439. doi: 10.1073/pnas.81.17.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jauregni HO, McMillan PN, Driscoll J, et al. Attachment and long-term survival of adult rat hepatocytes in primary monolayer cultures: comparison of different substrata and tissue culture media formulations. In Vitro. 1986;22:13–22. doi: 10.1007/BF02623436. [DOI] [PubMed] [Google Scholar]
- 17.Demetris AJ, Lasky S, Van Thiel DH, et al. Induction of DR/Ia antigens in human liver allografts. Transplantation. 1984;40:504–509. doi: 10.1097/00007890-198511000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green H, Rheinwald JG, Sun TT. Properties of an epithelial cell type in culture: the epidermal keratinocyte and it’s dependence on products of the fibroblast. In: Pollack R, editor. Reading in mamalian cell culture. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; pp. 149–156. [Google Scholar]