Introduction
The technical problems and clinical course after transplantation of the homologous dog liver have been recently described (Moore et al., 1960; Starzl et al., 1960, 1961). The rejection pattern was similar to that seen with other vascularized homografts. Survival averaged six to ten days but was as long as twenty and a half days (Starzl et al., 1961). The present study characterizes the histopathologic changes in 80 animals in which homotransplantation of the liver was performed.
After homografting of various organs, pathologic changes have not been generally demonstrable in the host organs. Simonsen et al. (1953) found no consistent alterations in the spleen, liver, heart or adrenal glands after renal homotransplantation. In a study of liver homografts, Moore (Moore et al., 1960) did not mention a systemic response. In the present study, a marked systemic response in a variety of host organs will be described which, it is thought, was indicative of total reticuloendothelial participation in the rejection of the liver homograft.
Methods
The technique of liver transplantation was previously described (Starzl et al., 1960). The graft was placed within the liver fossa after removal of the recipient dog's liver. Splenectomy was performed.
Autopsies were performed on all animals and the specimens were fixed in formalin. Hematoxylin and eosin stains were used routinely. Periodic acid-Schiff, Masson, iron reticulum and oil red O or Sudan black B stains were also employed to aid in interpreting the sequence of events in parenchymal and mesenchymal tissues but data from these stains will not be presented at this time since they would appear to warrant a separate report.
Results
Alterations Related to Surgical Artefacts of the Transplantation Procedure
Well-defined outflow block (Fig. 1), patterned by constant hepatic vein and variable distal (central) venous congestion with marked perivenous lymphatic dilatation commonly characterized the livers of those animals surviving 48 hours or less. Focal areas of central vein endothelial necrosis and obstruction occurred. Residual evidence of outflow block was sometimes present in long-term survivors. Such changes are known to be due to hepatic ischemia rather than a specific effect of homotransplantation (Starzl et al., 1960).
Fig. 1.
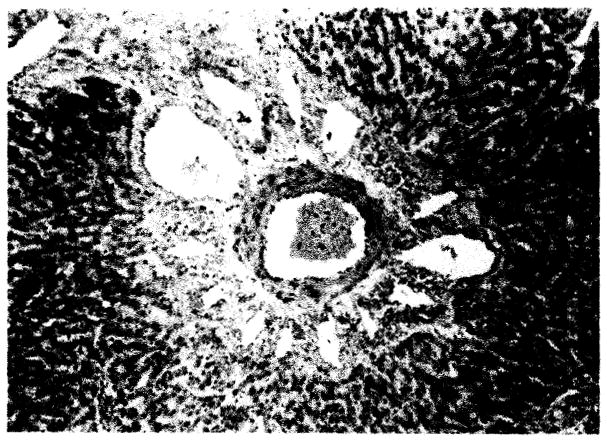
Liver from dog surviving 10 hours. Note portal venous and lymphatic engorgement characteristic of outflow block, × 43.
Two specific control procedures were carried out. In the first, cholecystojejunostomy with entero-enterostomy was performed after ligation of the common duct in four dogs, since this was the means of provision for internal biliary drainage in the transplants. The dogs were sacrificed at 5, 13, 14, and 15 days. Liver function studies were uniformly normal. Histologic studies of the liver were either normal or revealed a minimal periportal polymorphonuclear leukocytic infiltrate always with an undisturbed hepatic architecture. All other organs were histologically normal.
In the second, four dogs had autografts of the liver, employing the same technique as with homografts. These animals were sacrificed after 7 to 15 days. Liver architecture was completely preserved with no evidence of infiltrate. Focal myocardial infarcts were present, but there were no other changes in host organs.
Alterations Not Related to Surgical Artefacts of the Transplantation Procedure
The liver
Generalized Kupfer cell enlargement with increased nuclear basophilism was noted at 10 hours (Fig. 2). This change was constant throughout all subsequent survival intervals. Focal Kupfer cell proliferation did not occur. Endothelial activation was present in all sized vessels as evidenced by increased nuclear basophilism and increased size of the endothelial cells at 18 hours. Endothelial and adventitial proliferation was seen regularly after 5 days. Occasionally, arteriolar hyalin degeneration was manifest at this time (Fig. 3).
Fig. 2.

Liver from dog surviving 10 hours illustrating Kupfer cell activation, × 263.
Fig. 3.
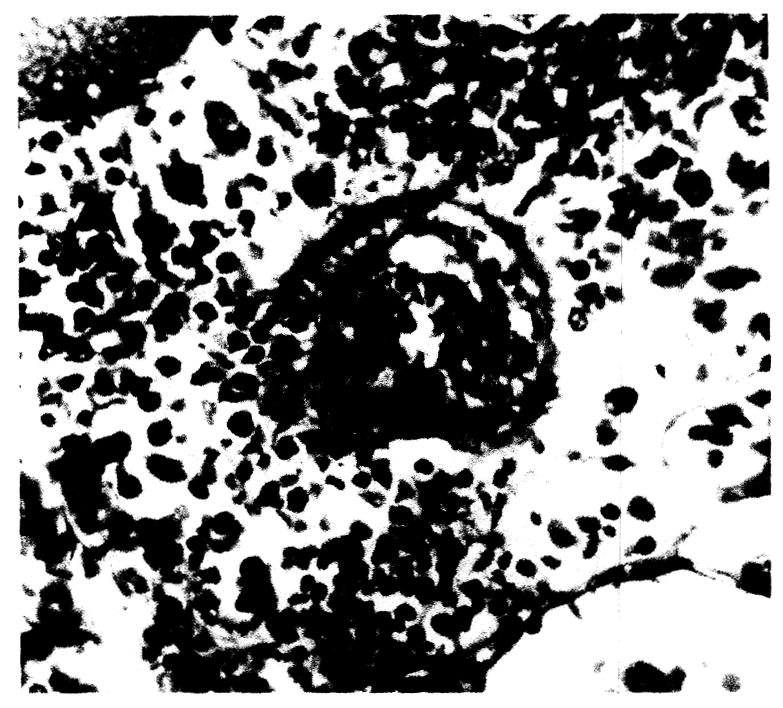
Arteriole of liver showing endothelial and perithelial proliferation with focal hyaline degeneration, × 263.
Mononuclear cell aggregates which were absent during the first 48 hours and scantily present at 4 days were very numerous after 5 days (Fig. 4). These cells were ovoid or irregularly shaped and resembled plasmacytes. They possessed an eccentric deeply basophilic ovoid nucleus and variable amounts of light basophilic cytoplasm. The aggregates were commonly intimately associated with fixed proliferating elongated cells in perivascular areas or portal tract supportive tissue. In addition, similar isolated cells were present diffusely within the hepatic parenchyma.
Fig. 4.

Liver: 10 day survival, × 263. Periportal and parenchymal infiltrate is primarily plasmacytic.
Parenchymal architecture was well-preserved during the first 4 or 5 days after surgery. Shortly after the above-described mononuclear infiltration, varying degrees of hepatic cell loss developed most prominently around the central vein. After 6 days, undisturbed architecture was noted in only one dog. The degree of parenchymal loss after 5 days was crudely related to the period of survival and in several cases was virtually complete (Figs. 5a and b). Intrahepatic ductal epithelium behaved in a similar manner, being extensively sloughed after the fifth day.
Fig. 5.
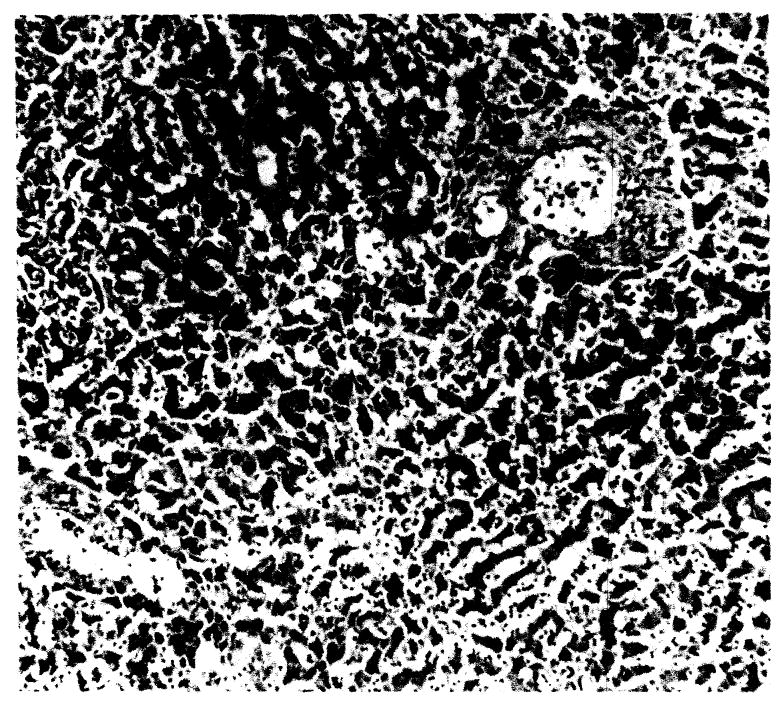

General hepatic architecture: (a) 9 day survival, × 42; (b) another dog, 8 day survival, × 42.
Despite very extensive parenchymal destruction, reticulum stains revealed the reticular matrix to be intact. Fatty alterations presented no consistent pattern.
Recipient tissues
Lymph nodes
Progressive changes occurred in the enlarged mediastinal, cervical and mesenteric lymph nodes. As early as the fifth day definite cortical thinning was present with decreased numbers of follicles. The follicles were discrete and composed primarily of small round deeply basophilic lymphocytes and occasional reticulum cells. The adjacent cortical area and medullary cords revealed a loose pattern of plasmacytes and larger similar but irregular plasmacytoid cells. In addition, significant numbers of small round lymphocytes and a small number of nonphagocytizing reticulum cells were present. The congested sinusoids contained many macrophagic reticulum cells, some of which contained hemosiderin. Definite endothelial and/or adventitial proliferation was present.
By the eighth day, recession of follicles had progressed, and occasionally these were completely absent (Fig. 6a). The thin cortex and medullary cords were composed of densely packed plasmacytes and larger plasmacytoid cells (Fig. 6b). The previously abundant lymphocytes were greatly decreased in number. The cells within sinusoids and lymphatic channels were almost exclusively large reticulum cells occasionally actively phagocytic and often with PAS positive cytoplasm.
Fig. 6.
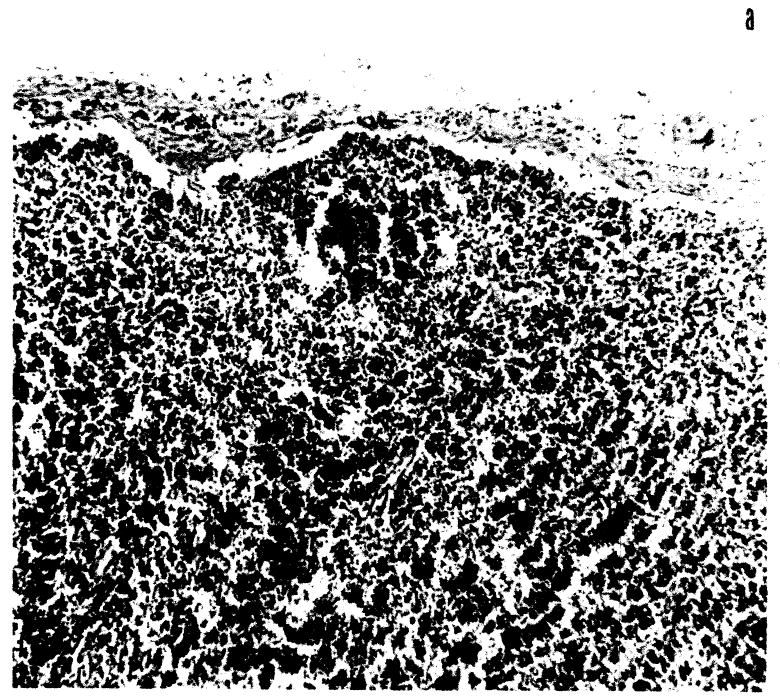

Lymph node from dog surviving 20 days: (a) cortical depletion, × 82; (b) cortical cell type primarily mononuclear and plasmacytic, × 232.
Kidney
Within 4 days small focal aggregates of plasmacytes and large plasmacytoid cells were seen in the periglomerular and perivascular areas. These were principally cortical. At this time occasional vessel wall edema was noted. Endothelial and adventitial proliferation occurred. The cellular aggregates developed slowly during the first 6 to 7 days after which cellular reaction was quite marked (Fig. 7a). At this time definite areas of thickening of tubular basement membrane and accentuation of nuclei in this area were noted (Fig. 7b). Concomitantly, perirenal and periadrenal supportive tissue demonstrated capillary engorgement and/or proliferation with perivascular aggregation of plasmacytes and lymphocytes (Fig. 8).
Fig. 7.
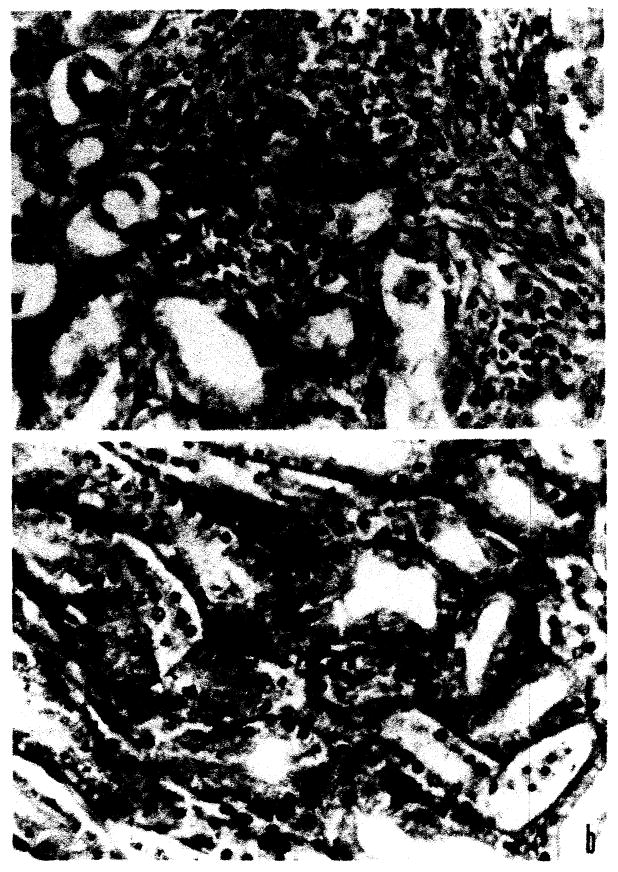
Kidney: (a) plasmacytic aggregation, × 97; (b) tubular basement membrane activation, × 97.
Fig. 8.
Perirenal plasmacytic and mononuclear infiltration, × 97.
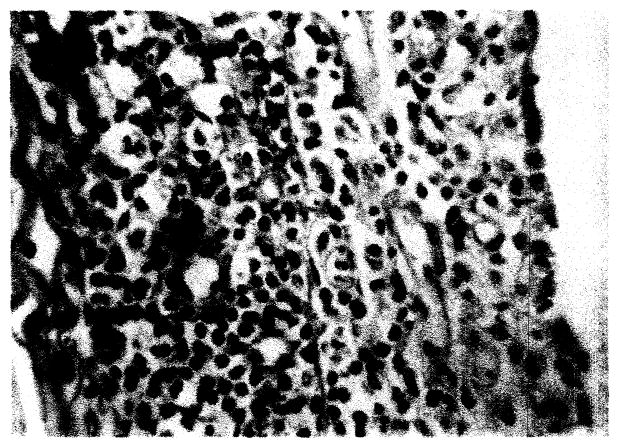
Lung
After 4 days, alveolar wall proliferation, diffusely or as nodules three to nine cells in thickness, occurred. These cells were ovoid or spindle-form frequently with a hyperchromatic nucleus. Mitoses were infrequently present. Multinucleated bizarre giant cells were irregularly formed in the alveolar wall (Fig. 9). Occasional areas of hyaline thickening of the alveolar wall were present.
Fig. 9.
Lung. Focal alveolar wall proliferation, mononuclear cell infiltrate and giant cell formation, × 225.
Mononuclear cell infiltrate, predominantly plasmacytic, involved the peribronchial supportive tissue and alveolar walls.
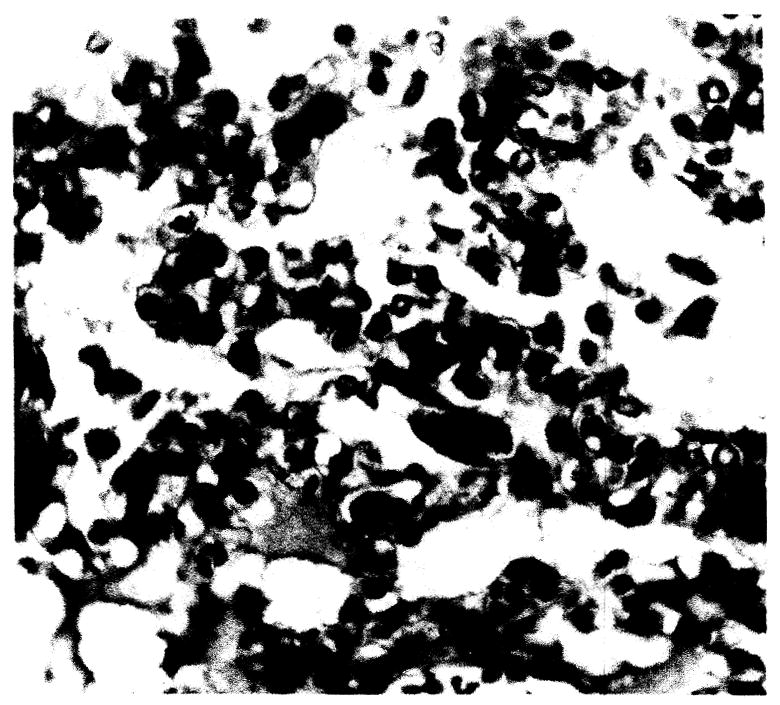
Bone marrow
Post-mortem bone marrow studies were performed routinely on animals surviving more than 4 days. All revealed increased plasmacytes (Fig. 10).
Fig. 10.
Bone marrow. Note increased plasmacytes, × 392.
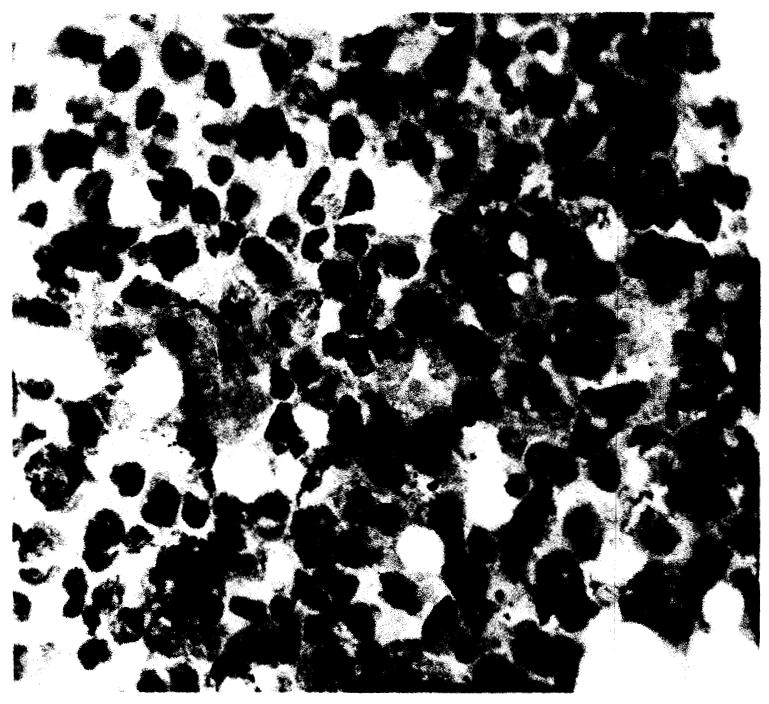
Gastrointestinal tract
Congestive changes with associated mucosal slough of the small intestine was a constant finding regardless of survival time. Mucosal infiltration of plasmacytes and less numbers of lymphocytes was present in all animals. The plasmacytic infiltrate increased to a maximum at the fourth to fifth day (Fig. 11). The submucosal and muscularis of the entire tract was markedly congested with areas of interstitial edema. Single or multiple discrete punched out duodenal ulcers were present in many animals surviving more than 4 days, and in some dogs perforation had occurred.
Fig. 11.
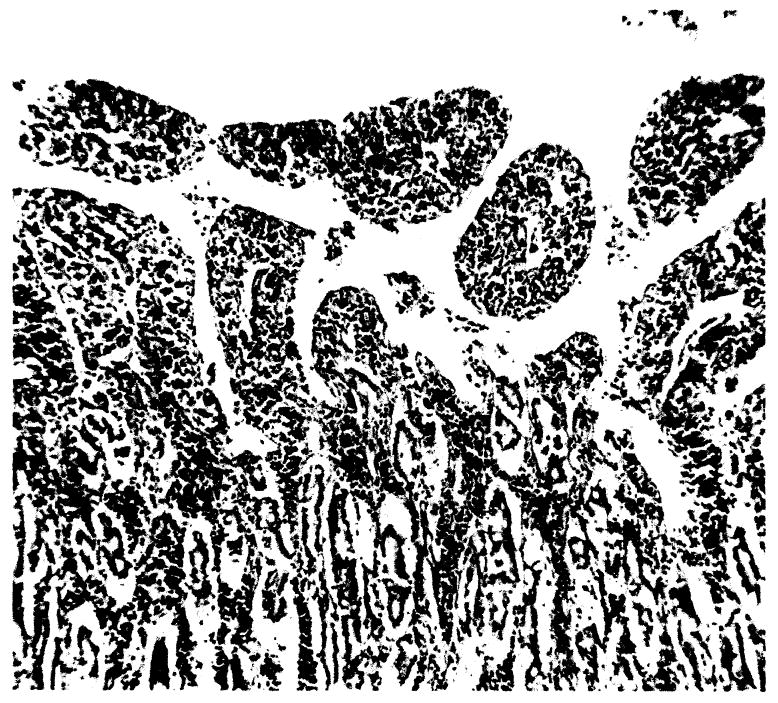
Small intestine. Mucosal slough and mononuclear infiltration, × 42.
Other organs and tissues
Adrenal gland parenchyma, skeletal muscle, large arteries and veins, and skin revealed no significant alterations. Numerous dogs revealed small myocardial infarctions which were often limited to the right ventricle and similar to those provoked in control animals. Initially in the 10- to 24-hour survivors the infarcts were characterized by focal necrosis. This typically was followed by minimal infiltrate with early organization evidenced in the longer survivors.
Discussion
The occurrence of mononuclear, lymphocytic and plasmacytic infiltrates in the donor organ is uniformly cited in early phases of whole organ [spleen (Moore et al., 1960), kidney (Dempster, 1953; Hume et al., 1960; Simonsen et al., 1953), liver (Moore et al., 1960; Starzl et al., 1961)] homotransplantation. A similar infiltrate is seen in freegrafts of skin (Medawar, 1944). After the third to fifth day, this cellular accumulation becomes accelerated. Various degrees of parenchymal and functional deterioration occur. Histologic evidence of rejection is well established as early as the fifth day.
The origin of the mononuclear, lymphocytic and plasmacytic accumulation in the homograft has been of considerable interest. Simonsen et al. (1950) and Dempster (1953), working with renal transplants, suggested that these cells originated in the donor and not in the host and represented an effort of the kidney transplant to reject the host. Hume et al. (1960) and Mannick et al. (1959) recently showed, however, that kidneys homotransplanted for long periods to irradiated hosts were devoid of infiltrate. The lack of donor organ infiltrate under these circumstances was thought by Hume et al. (1960) to be strong evidence that the infiltrate is of host origin and not indicative of a graft versus host reaction. Conversely, Hume et al. (1960) showed that massive irradiation of the donor kidney did not present the prompt appearance of a mononuclear infiltrate after homotransplantation to a normal host.
Contemporary experience in this laboratory (Starzl et al., 1962a) has also strengthened our belief that the cellular infiltrate in the rejecting liver homograft is primarily of host origin. Donor livers were subjected to 1000-1200 r irradiation 18 to 24 hours before transplantation to normal hosts. The tempo and character of mononuclear infiltration in these grafts was no different than when the donor tissues were untreated.
If the cellular infiltrate in the liver graft is of host origin, what is the explanation of the similar infiltrates to be found in a variety of host tissues, including lymph nodes, bone marrow, lung, and kidney? These host changes have not been described with most homografts, and have only been seen after whole organ hepatic (Starzl et al., 1961) or multiple organ (Starzl et al., 1962b) homotransplantation. Considerable evidence indicates that these changes are part of a host response to the massive antigenic stimulus of a bulky graft. The cellular aggregation is limited to those tissues known to have a reticuloendothelial potential. The involved tissues do not undergo functional deterioration as would be expected in a graft versus host reaction. Finally, immunologic paralysis of the donor liver by irradiation with 1000 to 1200 r does not abolish the host response (Starzl et al., 1962a).
The demonstration of generalized host reticuloendothelial participation in the rejection of large homografts provides a link between the rejection process and certain other hypersensitivity phenomena. For example, Bjornboe and Gormsen (1943) have shown that prolonged and intense stimulation with bacterial and protein antigens evokes a generalized reticuloendothelial response similar to that seen with liver homografts, and these observations have been extended by Kojima (1960) to include lipid antigens. In their experiments, Bjornboe and Gormsen (1943) showed a correlation between host reticuloendothelial activity and increases in gamma globulin, and concluded that the plasma cell aggregates were the chief source of this protein fraction. Working with liver transplants, Kukral et al. (1961) have shown a similar rise in gamma globulin during the rejection phase.
Summary
Homotransplanted livers in dogs developed mononuclear, lymphocytic and plasmacytic infiltration and hepatic cell degeneration roughly paralleling survival time.
Extensive histologic alterations of host reticuloendothelial structures occurred. Proliferation and infiltration of mononuclear cells, principally plasmacytes, were noted in lung, kidney, perirenal supportive tissue, bone marrow, and lymph nodes. Lymph nodes, in addition, were characterized by cortical and follicular depletion. These changes were considered to represent extensive host reticuloendothelial mobilization coincident to liver homotransplant rejection. The relation between these alterations and those found in other hypersensitivity states is discussed.
Footnotes
Aided by Grant A-3176 from the United States Public Health Service, National Institutes of Health, Bethesda, Maryland.
References
- Bjornboe M, Gormsen H. Experimental studies on the role of plasma cells as antibody producers. Acta Pathol Microbiol Scand. 1943;20:649–692. doi: 10.1111/j.1600-0463.2007.apm_681a.x. [DOI] [PubMed] [Google Scholar]
- Dempster WJ. Kidney homotransplantation. Brit J Surg. 1953;40:447–465. doi: 10.1002/bjs.18004016309. [DOI] [PubMed] [Google Scholar]
- Hume DM, Jackson BT, Zukoski CF, Lee HM, Kauffman HM, Egdahl RH. The homotransplantation of kidneys and of fetal liver and spleen after total body irradiation. Ann Surg. 1960;152:354–373. [PMC free article] [PubMed] [Google Scholar]
- Kojima M. Morphologic changes accompanying RES stimulation. Ann NY Acad Sci. 1960;88(1):196–202. doi: 10.1111/j.1749-6632.1960.tb20019.x. [DOI] [PubMed] [Google Scholar]
- Kukral JC, Littlejohn MH, Butz GW, Jr, Starzl TE. Biochemical studies of the homotransplanted canine liver. Surg Forum. 1961;12:112–113. [PMC free article] [PubMed] [Google Scholar]
- Mannick JA, Lochte HL, Jr, Ashley CA, Thomas ED, Ferrebee JW. A functioning kidney homotransplant in the dog. Surgery. 1959;46:821–828. [PubMed] [Google Scholar]
- Medawar PB. Behavior and fate of skin autografts and skin homografts in rabbits. J Anat. 1944;78:176–199. [PMC free article] [PubMed] [Google Scholar]
- Moore FD, Wheeler HB, Demissianos HV, Smith LL, Belankura O, Abel LK, Greenberg JB, Dammin GJ. Experimental whole organ transplantation of the liver and of the spleen. Ann Surg. 1960;152:374–387. [PMC free article] [PubMed] [Google Scholar]
- Simonsen M, Buemann J, Gammeltoft A, Jensen F, Jorgenson K. Biological incompatibility in kidney transplantation in dogs. I. Experimental and morphologic investigations. Acta Pathol Microbiol Scand. 1953;32:1–35. doi: 10.1111/j.1699-0463.1953.tb00229.x. [DOI] [PubMed] [Google Scholar]
- Starzl TE, Kaupp HA, Jr, Brock DR, Lazarus RE, Johnson RV. Reconstructive problems in canine liver homotransplantation with special reference to the postoperative role of hepatic venous flow. Surg Gynecol Obstet. 1960;111:733–742. [PMC free article] [PubMed] [Google Scholar]
- Starzl TE, Kaupp HA, Jr, Brock DR, Linman JW. Studies on the rejection of the transplanted homologous dog liver. Surg Gynecol Obstet. 1961;112:135–144. [PMC free article] [PubMed] [Google Scholar]
- Starzl TE, Butz GW, Jr, Brock DR, Linman JT, Moss WT. The effect of host and graft irradiation on canine liver homotransplants. 1962a doi: 10.1001/archsurg.1962.01310030108016. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzl TE, Kaupp HA, Jr, Brock DR, Butz GW, Linman JW. Homotransplantation of multiple visceral organs. Ant J Surg. 1962b;103:219–229. doi: 10.1016/0002-9610(62)90491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


