Abstract
Pseudoxanthoma elasticum (PXE) is a heritable disorder characterized by ectopic mineralization of connective tissues primarily in the skin, eyes, and the cardiovascular system. PXE is caused by mutations in the ABCC6 gene. While PXE is associated with considerable morbidity and mortality, there is currently no effective or specific treatment. In this study, we tested oral phosphate binders for treatment of a mouse model of PXE which we have developed by targeted ablation of the corresponding mouse gene (Abcc6 −/−). This “knock‐out” (KO) mouse model recapitulates features of PXE and demonstrates mineralization of a number of tissues, including the connective tissue capsule surrounding vibrissae in the muzzle skin which serves as an early biomarker of the mineralization process. Treatment of these mice with a magnesium carbonate‐enriched diet (magnesium concentration being 5‐fold higher than in the control diet) completely prevented mineralization of the vibrissae up to 6 months of age, as demonstrated by computerized morphometric analysis of histopathology as well as by calcium and phosphate chemical assays. The magnesium carbonate‐enriched diet also prevented the progression of mineralization when the mice were placed on that experimental diet at 3 months of age and followed up to 6 months of age. Treatment with magnesium carbonate was associated with a slight increase in the serum concentration of magnesium, with no effect on serum calcium and phosphorus levels. In contrast, concentration of calcium in the urine was increased over 10‐fold while the concentration of phosphorus was markedly decreased, being essentially undetectable after long‐term (>4 month) treatment. No significant changes were noted in the serum parathyroid hormone levels. Computerized axial tomography scan of bones in mice placed on magnesium carbonate‐enriched diet showed no differences in the bone density compared to mice on the control diet, and chemical assays showed a small increase in the calcium and phosphate content of the femurs by chemical assay, in comparison to mice on control diet. Similar experiments with another experimental diet supplemented with lanthanum carbonate did not interfere with the mineralization process in Abcc6 −/− mice. These results suggest that magnesium carbonate may offer a potential treatment modality for PXE, a currently intractable disease, as well as for other conditions characterized by ectopic mineralization of connective tissues.
Keywords: pseudoxanthoma elasticum, heritable mineralization disorders, phosphate binders, Abcc6−/− mouse
Introduction
Pseudoxanthoma elasticum (PXE) is a heritable, multi‐system disorder characterized by ectopic mineralization of peripheral connective tissues. 1 The clinical manifestations derive primarily from the involvement of three organ systems: the skin, the eyes, and the cardiovascular system. In the skin, histopathology demonstrates accumulation of pleiomorphic elastic structures in the mid‐ and upper reticular dermis with profound, progressive mineralization. The eye manifestations characteristically consist of angioid streaks due to mineralization of an elastin‐rich Bruch's membrane behind the pigmented retina. 2 Mineralization of this membrane causes ruptures of blood vessels with subsequent neovascularization, associated with bleeding to the eye and leads to loss of visual acuity and occasionally to blindness. The cardiovascular manifestations result from mineralization of the arterial blood vessels, and clinically manifest with intermittent claudication, hemorrhage from the gastric arteries, and occasionally, early myocardial infarcts. 3 The mineral deposits in the affected tissues have been shown by specific histochemical staining methods (Alizarin Red and von Kossa) to consist of calcium and phosphate, and this composition has been confirmed by X‐ray diffraction analysis. 4
The pathomechanisms of ectopic mineralization in PXE are currently unknown. It has been established, however, that the classic forms of PXE are caused by mutations in the ABCC6 gene, which encodes a putative transmembrane transporter protein, ABCC6, expressed primarily in the baso‐lateral surface of hepatocytes. 5 ABCC6 has been shown by in vitro studies to serve as an eflux pump that transports anionic small molecular weight conjugates. 6 However, ABCC6 does not transport calcium or phosphate, and its physiologic ligands in vivo are currently unknown. PXE‐like cutaneous findings have also been seen in a select number of patients with mutations in the GGCX gene. 7 , 8 This gene encodes a γ‐glutamyl carboxylase enzyme necessary for activation of Gla‐proteins, including the vitamin K‐dependent coagulation factors within the hepatocytes. 9 , 10 In the peripheral tissues, matrix Gla‐protein (MGP) also needs to be activated by γ‐glutamyl carboxylation. Since MGP serves as a powerful anti‐mineralization protein, as demonstrated by extensive mineralization in MGP knock‐out mice, 11 and since the carboxylation reaction requires reduced vitamin K, a hypothesis has been put forward that a vitamin K conjugate might be a physiologic substrate of ABCC6, 12 thus tying the vitamin K transport and ABCC6 mutations to the development of PXE phenotypes.
PXE demonstrates considerable, both intra‐ and inter‐familial heterogeneity, and genetic modifying factors have been suggested to play a role in the severity of this disease. 1 , 13 , 14 , 15 , 16 At the same time, early retrospective studies on PXE patients suggested that variations in the diet may inf uence the age of onset and extent of mineralization in these patients. 4 , 17 Specifically, it was suggested that patients who had high consumption of dairy products, rich in calcium and phosphate, during childhood or early adolescence may have more severe PXE phenotype. In this context, it is important to note that serum concentrations of calcium and phosphate, as well as urinary output of these minerals are entirely within normal limits in PXE, and there is no evidence of perturbations in the parathyroid hormone and/or vitamin D metabolism. 4 The prevailing view is that PXE is a metabolic disease due to lack of critical anti‐mineralization factors in the circulation, and an imbalance between the mineralization/ anti‐mineralization factors allow development of late onset, and slow, yet progressive, mineralization of the peripheral connective tissues to take place. 1 , 18
There is currently no effective or specific treatment for PXE. However, since the clinical manifestations are clearly a sequela of aberrant mineralization of connective tissues, prevention of this process by pharmacologic means would be expected to ameliorate, and even perhaps cure, PXE. Phosphate binders, a group of drugs currently in use for treatment of hyperphosphatemia primarily in patients with chronic kidney disease, could offer a means of reducing the calcium/phosphate load in patients with PXE. 19 A recent study consisting of six patients treated with aluminum hydroxide‐containing oral phosphate binder suggested that three of these patients showed significant improvement of skin lesions and revealed histopathologic regression of the disease. 20 At the same time, no deterioration of the eye disease was seen in any of the six patients at 1‐year follow‐up. However, since aluminum‐containing oral phosphate binders are associated with aluminum toxicity, including cognitive disturbances, osteomalacia, and anemia, their long‐term use in general practice is not common. 19 In this study, we have explored two alternate oral phosphate binders, specifically containing magnesium carbonate or lanthanum carbonate, for counteracting mineralization in PXE. For this purpose, we treated Abcc6 −/− mice, which we have developed by targeted ablation of the corresponding mouse gene, resulting in mineralization which by histopathologic and ultrastructural means mimics those seen in patients with PXE. 21 Our results suggest that magnesium carbonate‐containing oral phosphate binder may provide an effective way to counteract the mineralization that causes considerable morbidity and even mortality in PXE.
Materials and methods
Mice and diets
A PXE mouse model was developed by targeted ablation of the Abcc6 gene. 21 Heterozygous mice (Abcc6 +/−) were backcrossed with C57BL/6J mice for five generations to generate Abcc6 knock‐out (Abcc6 −/−) and wild‐type (Ab c c 6 +/+) mice on C57BL/6J background. These mice were housed in the Animal Facility of Thomas Jefferson University where they were maintained in a climate‐controlled environment with free access to water and a 12‐hour light/dark cycle.
Wild‐type and Abcc6 −/− mice were placed on the control diet (Laboratory Autoclavable Meal Rodent Diet 5010; PMI Nutrition, Brentwood, MO) following weaning at 4 weeks of age and continued for an additional 2 or 5 months on the same diet. The Abcc6 −/− mice were divided into different groups and placed on the control diet supplemented with either magnesium carbonate or lanthanum carbonate, 5 to 9 mice per group. Three groups of mice received control diet supplemented with magnesium carbonate, with a 5‐fold increase in magnesium (from 0.22% to 1.10%). The first and second group of mice were fed the magnesium carbonate‐enriched diet following weaning at 4 weeks of age and maintained on that diet for an additional 2 months or 5 months (1m → 3m and 1m → 6m), respectively. The mice in the third group were given the control diet following weaning at 4 weeks of age and continued for 2 months, and then the mice were switched to the magnesium carbonate‐containing diet for 3 additional months (3m → 6m). Another three groups of mice were fed the control diet supplemented with lanthanum carbonate (Fosrenol), 1 g of lanthanum carbonate in 1 kg of control diet. The first and second group of mice were fed the lanthanum carbonate‐enriched diet following weaning at 4 weeks of age and continued for an additional 2 months or 5 months (1m → 3m and 1m → 6m, respectively). The mice in the third group were given the control diet following weaning at 4 weeks of age and continued for 2 months, and then these mice were switched to a lanthanum carbonate‐containing diet for another 3 months (3m + 6m).
This study was approved by the Institutional Animal Care and Use Committee of Thomas Jef erson University.
Histological analysis
For histopathological analysis of mineralization, biopsies from muzzle skin containing vibrissae were fixed in 10% phosphate‐buffered formalin and embedded in parafin. Parafin sections (5 μm) were stained with hematoxylin‐eosin, Alizarin Red, or von Kossa using standard methods.
Quantification of tissue mineralization by computerized morphometric analysis
Computerized morphometric analysis of hematoxylin and eosinstained sections of muzzle skin was performed as described elsewhere. 22 The sections were examined with a Nikon model Te2000 microscope furnished with an Auto Quant Imaging system (Watervliet, New York, NY). The number of vibrissae with and without evidence of mineralization was determined in all sections, and the extent of mineralization was expressed as the percentage of area of mineralization per total area of vibrissae examined.
Quantification of calcium and phosphate
To quantify the calcium/phosphate deposition in mouse vibrissae, the muzzle skin, which contains the vibrissae, was harvested and decalcified with 0.15 N HCl for 48 hours at room temperature. The calcium content was determined colorimetrically by the o‐cresolphthalein complexone method (Calcium (CPC) Liquicolor; Stanbio Laboratory, Boerne, TX). The phosphate content was determined with Malachite Green Phosphate Assay kit (BioAssay Systems, Hayward, CA). The values for calcium and phosphate were normalized to tissue weight. Calcium and phosphorus in the serum and urine samples were quantitatively assayed as above.
The left femurs of the mice were collected after euthanization and decalcified in 1 N HCl for 2 weeks at room temperature. Calcium and phosphorus content in the femur were then quantitatively assayed as above.
Quantification of magnesium
The magnesium concentrations in the mouse serum and urine were measured using the QuantiChrom Magnesium Assay Kit (BioAssay Systems).
Serum parathyroid hormone (PTH) assay
Mouse serum PTH concentrations were measured using Mouse Intact PTH Elisa Kit (Immutopics Inc., San Clemente, CA).
Computerized axial tomography (CAT) scan analysis
At the end of each experiment on different diets, wild‐type mice on control diet for 5 months, Abcc6 −/− mice on control diet for 5 months, and Abcc6 −/− mice on high‐magnesium diet for 5 months were anesthetized with a Xylazine‐Ketamine‐Acetopromazine cocktail (160 μL per 25 grams body weight of 10 mg/kg Xylazine, 200 mg/kg Ketamine, 2 mg/kg Acetopromazine). Following anesthesia the mice were restrained in a perforated 50 mL conical plastic tube, and scanned by Computerized Axial Tomography (CAT scan) in a MicroCAT II scanner (ImTek Inc, Oak Ridge, TN). Bone parameters of the femur were assessed using Inveon Research Workplace software (Siemens Corporation, New York, NY).
Statistical analysis
The results in different groups of mice receiving various diets were first analyzed for normal distribution using Hamilton's test. Comparisons of continuous measures across all groups were completed using two‐sided Kruskal–Wallis nonparametric tests. 23 The Kruskal–Wallis test is comparable to one‐way analysis of variance, but without the parametric assumptions. For each of the paired group comparisons, an exact two‐sided Wilcoxon's test was computed. For some comparisons, Student's two‐tailed t ‐test was also used. All statistical computations were completed using SPSS version 15.0 sofware.
Results
Magnesium carbonate prevents mineralization in PXE mice
The Abcc6 −/− mice show progressive mineralization of the connective tissue capsule of vibrissae, while their corresponding littermates (Abcc6 +/+) do not show such calcification up to 2 years of follow‐up. 21 , 24 These observations were confirmed in this study by demonstration of mineralization in the vibrissae of 3‐month and 6‐month old Abcc6 −/− mice ( Figure 1b and c ), while no mineralization was noted in the wild‐type littermates at 6 months of age ( Figure 1a ). This mouse model was then used to study the effect of magnesium carbonate‐containing diet. In one set of experiments, the mice were placed on this experimental diet at 4 weeks of age after weaning, and mineralization was determined at 3 or 6 months of age. In both groups, there was no evidence of the development of mineralization in the vibrissae of these KO mice ( Figure 1d , e). Thus, diet enriched with magnesium carbonate entirely prevents the development of mineralization in Abcc6 −/− mice, at least up to 6 months of age.
Figure 1.
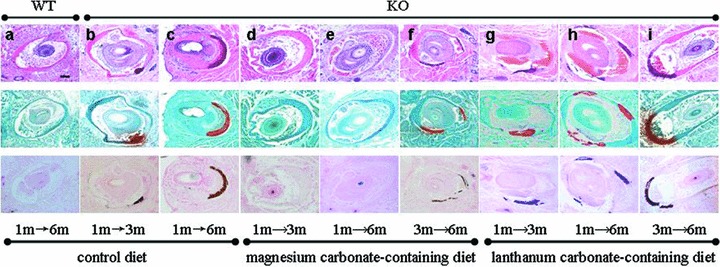
Histopathologic evaluation of mineralization of the vibrissae in Abcc6 −/− mice (KO) or their corresponding wild‐type (WT) counterparts kept on control, magnesium carbonate‐containing, or lanthanum carbonate‐containing diet. The mice were placed on the experimental diet either at 1 month or 3 months of age, and biopsies from the muzzle skin containing the vibrissae were taken at 3 or 6 months of age. The tissue specimens were processed and stained with hematoxylin and eosin, Alizarin Red or von Kossa stains (top, middle, and bottom panels, respectively). All figures have the same magnification: bar, 100 μm.
In another set of experiments, the Abcc6 −/− mice were allowed to develop mineralization up to 3 months of age and they were then placed on the experimental diet and followed for an additional 3 months. At this point, at 6 months of age, the muzzle skin was biopsied and mineralization was determined. The mice now showed relatively little mineralization, comparable to that of Abcc6 −/− mice at 3 months of age kept on control diet ( Figure 1f ). In fact, the degree of mineralization, as determined by computerized morphometric analysis in the mice ( Table 1 ), was lower than mineralization in age‐matched 6‐month‐old mice kept on control diet; however, this difference was not statistically significant by Wilcoxon's test, but was significant with Student's t‐test (p < 0.05). Thus, the magnesium carbonate‐containing diet arrests, and may even reverse, mineralization, in the Abcc6 −/− mice.
Table 1.
Quantitation of connective tissue mineralization of vibrissae*.
| Mouse | Diet | Diet started → diet end (months) | No. of mice | Mineralized vibrissae (per mouse) | Total vibrissae examined (per mouse) | Percent mineralized† | Area of mineralization per total area of vibrissae (%)† | Fold‡ | p‐value |
|---|---|---|---|---|---|---|---|---|---|
| Wild‐type | Control | 1 → 6 | 9 | 0 | 11.0 | 0 | 0 | ||
| Abcc6−/− | Control | 1 → 3 | 8 | 5.5 | 10.3 | 58.1 ± 5.5 | 0.69 ± 0.14 | 1.0 | 0.001§ |
| Abcc6−/− | Control | 1 → 6 | 9 | 6.4 | 8.8 | 75.2 ± 5.2 | 2.78 ± 0.76 | 4.0 | 0.001§ |
| Abcc6−/− | Magnesium carbonate | 1 → 3 | 6 | 0 | 7.7 | 0 | 0 | 0 | 0.001∥ |
| Abcc6−/− | Magnesium carbonate | 1 → 6 | 5 | 0.2 | 6.8 | 4.0 ± 4.0 | 0.15 ± 0.15 | 0.2 | 0.003∥ |
| Abcc6−/− | Magnesium carbonate | 3 → 6 | 8 | 4.6 | 8.4 | 49.3 ± 12.4 | 0.97 ± 0.33 | 1.4 | 0.074∥,¶ |
| Abcc6−/− | Lanthanum carbonate | 1 → 3 | 6 | 2.5 | 13.2 | 23.3 ± 15.9 | 0.72 ± 0.39 | 1.0 | 0.555∥ |
| Abcc6−/− | Lanthanum carbonate | 1 → 6 | 5 | 5.6 | 6.0 | 92.5 ± 5.0 | 1.92 ± 0.28 | 2.8 | 0.884∥ |
| Abcc6−/− | Lanthanum carbonate | 3 → 6 | 5 | 5.0 | 8.5 | 60.0 ± 5.7 | 2.32 ± 0.49 | 3.4 | 0.897∥ |
* Skin sections containing vibrissae were stained with H&E and examined by computerized morphometric analysis.
† Values presented as mean ± S.E.
‡ Calculated using the 3‐month‐old Abcc6 −/− mice as 1.0.
§ Compared with the wild type mice.
∥ Compared with age‐matched Abcc6 −/− mice on control diet.
¶ Statistical difference by Student's t‐test, p= 0.0497.
The degree of mineralization in the same mice analyzed by computerized morphometric analysis was also determined by chemical assays of calcium and phosphate in the biopsies taken from the muzzle skin containing the vibrissae. The results supported the observations made by computerized morphometric analyses, and demonstrated that the Ca × P content in the vibrissae of mice treated with magnesium carbonate‐containing diet for 2 or 5 months (1m → 3m and 1m → 6m) was very low, essentially the same as in wild‐type mice of 6 months of age ( Figure 2 ). Also, mineralization in the mice placed on magnesium carbonate‐containing diet at 3 months of age and biopsied 3 months later at 6 months of age showed statistically significant reduction in the Ca × P product, as compared to age‐matched (6m) mice kept on control diet ( Figure 2 ). Thus, collectively, our results derived either from computerized morphometric analyses or chemical calcium and phosphate assays independently indicate that magnesium carbonate in the diet is a powerful inhibitor of ectopic mineralization in the vibrissae of Abcc6 −/− mice.
Figure 2.
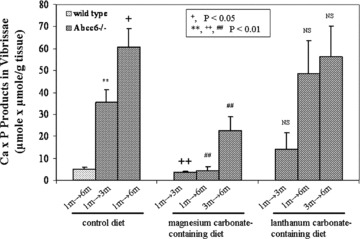
Quantitation of calcium and phosphate in the vibrissae of mice depicted in Figure 1 Biopsy specimens were processed as described in the Materials and methods section, and calcium and phosphate were quantitatively determined by chemical assays. The Ca x P product was correlated with the weight of the biopsied tissue. Values are expressed as mean ± S.E. and the statistical significance was determined by Wilcoxon's test as shown in the inset. Statistical significance: **= in comparison with wild‐type mice;+,++= in comparison with 3‐month‐old Abcc6 −/− mice on control diet; ##= in comparison with 6‐month‐old Abcc6 −/− mice on control diet; NS = the values are not statistically different from those in age‐matched mice on control diet.
Lanthanum carbonate‐enriched diet does not alter the mineralization
Lanthanum carbonate is another oral phosphate binder which is calcium and aluminum free and has few side effects. 19 It has been demonstrated to be effective in controlling serum phosphate levels in hyperphosphatemia. 25 , 26 To test its efficacy in our preclinical model of PXE, the Abcc6 −/− mice were similarly placed on lanthanum carbonate‐containing diet either at 1 or 3 months of age using a similar experimental design as was used for the magnesium carbonate diet. Computerized morphometric analysis of the vibrissae of lanthanum carbonate‐treated mice at 3 or 6 months of age showed extensive mineralization ( Figure 1g , h, i), similar to the age‐match Abcc6 −/− mice kept on control diet. In fact, the values were not statistically different in any of three groups of mice treated with lanthanum carbonate from the age‐matched mice kept on control diet ( Table 1 ). These observations were confirmed by chemical assays of calcium and phosphate in the muzzle skin containing the vibrissae, which showed no statistical difference from those in age‐matched Abcc6 −/− mice kept on control diet ( Figure 2 ).
Serum and urine metabolic analysis
To potentially gain insight into the mechanisms of the oral phosphate binders and to gauge potential side effects in our mouse model system, we determined calcium, phosphorus, and magnesium concentrations both in serum and urine. Assay of serum calcium and phosphorus in mice either on magnesium carbonate or lanthanum carbonate‐containing diet did not show values different from those in mice kept on control diet, and the calcium/phosphate ratio remained unaltered ( Table 2 ). Assay of magnesium in the serum of mice kept on magnesium carbonate‐enriched diet revealed slight, but statistically significant, increase in mice kept 5 months on this experimental diet, in comparison to the age‐matched (6m) mice kept on control diet ( Table 2 ). Mice kept on magnesium carbonate‐enriched diet for 2 or 3 months (1m → 3m and 3m → 6m) showed normal magnesium levels.
Table 2.
Calcium, phosphorus and magnesium concentrations in the serum of Abcc6‐/‐ mice placed on different diets*.
| Parameter | Concentration (mean ± S.E.) in group† | |||||||
|---|---|---|---|---|---|---|---|---|
| Control diet | Magnesium carbonate‐containing diet | Lanthanum carbonate‐containing diet | ||||||
| lm → 3m | lm → 6m | lm → 3m | lm → 6m | 3m → 6m | lm → 3m | lm → 6m | 3m → 6m | |
| Calcium (mg/dL) | 10.70 ± 0.69 | 11.54 ± 0.42 | 10.94 ± 0.30 | 12.72 ± 0.62 | 10.95 ± 0.28 | 11.25 ± 0.32 | 11.26 ± 0.22 | 11.27 ± 0.11 |
| Phosphorus (mg/dL) | 14.29 ± 1.87 | 15.55 ± 1.38 | 16.03 ± 0.50 | 16.28 ± 0.95 | 13.59 ± 1.89 | 15.72 ± 0.23 | 15.46 ± 0.91 | 13.98 ± 1.28 |
| Ca/P ratio | 0.75 ± 0.06 | 0.78 ± 0.08 | 0.68 ± 0.02 | 0.78 ± 0.02 | 0.91 ± 0.11 | 0.72 ± 0.02 | 0.74 ± 0.05 | 0.84 ± 0.06 |
| Magnesium (mg/dL) | 3.07 ± 0.31 | 3.11 ± 0.14 | 3.00 ± 0.12 | 3.94 ± 0.18‡ | 3.06 ± 0.21 | ND | ND | ND |
*Abcc6 −/− mice at 1 or 3 months of age were placed on different diets. Serum concentrations of calcium, phosphorus and magnesium were determined at either 3 or 6 months of age.
† Statistical significance in comparison to the Abcc6 −/− mice on control diet is indicated: ‡ p < 0.05 (n= 5–9).
ND = not determined.
Assay of urinary calcium and phosphorus in mice kept on magnesium carbonate‐enriched diet demonstrated dramatic changes ( Table 3 ). First, the urinary calcium concentration was elevated up to over 10‐fold in mice kept on magnesium carbonate‐containing diet when measured at 3, 4, 5, or 6 months of age, as compared to the mice kept on control diet ( Table 3 ). At the same time, the urinary phosphorus concentration was markedly reduced and in fact, mice kept 3 to 5 months on magnesium carbonate‐containing diet showed essentially undetectable levels of phosphorus. In this group of mice, very small (∼5%) increases in the urinary magnesium concentration were noted ( Table 3 ).
Table 3.
Calcium, phosphorus and magnesium concentrations in the urine of Abcc6 −/− mice placed on different diets*.
| Parameter | Concentration (mean ± S.E.) in group† | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control diet | Magnesium carbonate‐containing diet | Lanthanum carbonate‐containing diet | ||||||||||
| 1m → 3m | 1m → 6m | 1m → 3m | 1m → 4m | 1m → 5m | 1m → 6m | 3m → 6m | 1m → 3m | 1m → 4m | 1m → 5m | 1m → 6m | 3m → 6m | |
| Calcium (mg/dL) | 11.06 ± 2.73 | 9.16 ± 1.45 | 95.41 ± 19.3† | 90.77 ± 15.74† | 115.42 ± 16.48† | 132.89 ± 16.13† | 119.04 ± 15.35† | 19.06 ± 6.09 | 10.13 ± 0.89 | 21.53 ± 5.51 | 6.86 ± 2.93 | 10.22 ± 2.90 |
| Phosphorus (mg/dL) | 443.31 ± 118.42 | 334.78 ± 110.23 | 105.91 ± 24.13‡ | 19.77 ± 19.77† | 0.02 ± 0.02† | 1.26 ± 1.26† | 0 ± 0+ | 319.35 ± 136.3 | 370.94 ± 82.15 | 194.68 ± 56.75 | 165.93 ± 16.07 | 244.30 ± 86.43 |
| Magnesium (mg/dL) | 28.49 ± 1.10 | 28.78 ± 0.44 | 30.83 ± 0.46‡ | 29.63 ± 0.57 | 30.73 ± 0.12† | 30.90 ± 0.16† | 30.64 ± 0.53‡ | ND | ND | ND | ND | ND |
*Abcc6−/− mice at 1 or 3 months of age were placed on different diets. Urine concentrations of calcium, phosphorus and magnesium were determined at 3, 4, 5 and 6 months of age.
†Statistical significance in comparison to the Abcc6 −/− mice on control diet is indicated: ‡ p < 0.05; † p < 0.01 (n = 5–9).
ND = not determined.
Examination of calcium and phosphorus in mice kept on lanthanum carbonate‐containing diet showed considerable variability in the values, and the overall mean values were not statistically different from the mice kept on control diet ( Table 3 ).
Finally, we measured the parathyroid hormone (PTH) concentration in the sera of mice kept on either control diet for 5 months (WT and KO mice) or on magnesium carbonate‐enriched diet for 3 months (3m → 6m) or 5 months (1m → 6m) (KO mice). All mice were examined at 6 months of age. Assay of PTH by an ELISA revealed that values in WT and KO groups kept on control diet were not statistically different, 97.25 ± 11.03 versus 86.72 ± 7.01 pg/mL (mean ± S.E.; p > 0.05). Similarly, the PTH concentrations in the sera of Abcc6 −/− mice kept on magnesium‐rich diet either for 3 or 5 months were not statistically different from the wild‐type mice or those Abcc6 −/− mice kept on control diet (72.57 ± 6.62 and 95.14 ± 7.49 pg/mL, respectively).
Computerized axial tomography (CAT) scan and mineral content of bones in mice on magnesium carbonate‐enriched diet
Although the precise role of dietary magnesium on integrity and composition of bones is somewhat unclear (see Discussion), there are suggestions that long‐term excessive magnesium supplementation may be deleterious to the functional integrity of the bones. 27 Therefore, we determined the bone density and calcium and phosphorus content of bones in mice kept 5 months on magnesium carbonate‐enriched diet (1m → 6m). First, bone density in selected mice (2 to 3 per group) was determined by CAT scan including WT mice on control diet, KO mice on control diet, and KO mice on magnesium carbonate‐enriched diet for 5 months. As shown in Figure 3 , there were no gross differences in the morphology of femurs of these mice and the bone densities were statistically not different. We also determined the calcium and phosphorus content in the left femur of the same animals by quantitative chemical assays. As shown in Table 4 , KO mice kept on control diet for 5 months (and analyzed at the age of 6 months) demonstrated calcium and phosphorus values which were not different from WT mice kept on control diet for the same time period. However, KO mice kept on experimental magnesium carbonate‐enriched diet for 5 months showed a statistically significant increase in both calcium and phosphorus content as compared to their KO counterparts kept on control diet for the same time period ( Table 4 ). Thus, there is no apparent deleterious effect on the bone as a result of 5‐month treatment with magnesium carbonate‐enriched diet, and in fact, our findings support the view that magnesium intake may be beneficial for bone integrity.
Figure 3.
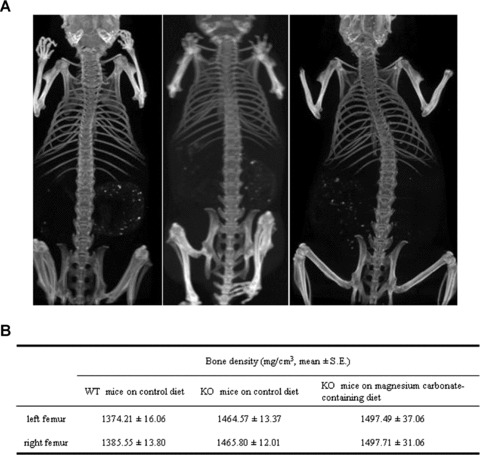
Evaluation of bone integrity in mice treated with magnesium carbonate‐containing diet. (A) 2‐Di‐mensional images of computerized axial tomography (CAT) scan from a WT mouse on control diet (left); KO mouse on control diet (center); and KO mouse on magnesium carbonate‐enriched diet (right) for 5 months; all mice are imaged at the age of 6 months. (B) Bone density assays of the groups of mice represented in A. The values in KO mice on control diet are not statistically different from the WT mice on control diet or from KO mice on magnesium carbonate‐containing diet.
Table 4.
Calcium and phosphorus content in the left femur of the mice*.
| Mice | No. of mice examined | Ca (mg/g)† | P (mg/g)‡ |
|---|---|---|---|
| WT on control diet | 6 | 87.82 ± 4.90 | 62.18 ± 3.90 |
| KO on control diet | 4 | 75.23 ± 5.47 | 57.15 ± 4.28 |
| KO on magnesium carbonate‐containing diet | 5 | 106.91 ± 7.61‡ | 73.93 ± 4.36‡ |
* Wild‐type (WT) and Abcc6 −/− mice (KO) at 1 month of age were placed on different diets for 5 months. Calcium and phosphorus content were determined at 6 months of age and expressed as mean ± S.E.
† Statistical significance in comparison to the KO mice on control diet is indicated: ‡ p < 0.05.
Discussion
The results of our study clearly demonstrate that magnesium carbonate, when added to the mouse diet in amounts that increase the magnesium concentration by 5‐fold, is able to prevent the ectopic mineralization noted in Abcc6 −/− mice, an animal model for PXE. The observations of our study suggest, therefore, that dietary magnesium might also be helpful for treatment of patients with PXE, a notion that could be tested in controlled clinical trials. The efficacy of dietary magnesium carbonate is most likely due to the magnesium ion because another oral phosphate binder tested, lanthanum carbonate, was ineffective in eliciting changes in the mineralization of the PXE mouse model. The control mouse diet contains 0.22% magnesium, and enrichment with magnesium carbonate increased the magnesium content of the mouse food to 1.10%, i.e., a 5‐fold increase. The mouse control diet does not contain any lanthanum, and the dose for enrichment of the food with lanthanum carbonate (Fosrenol) was based on recommended maximum human dose (1,500 mg per day), 25 but increased again by 5‐fold. The inclusion of the lanthanum carbonate in the mouse diet did not have an effect on the mineralization process and did not change serum or urinary calcium and phosphorus levels. It is unclear, therefore, why lanthanum carbonate is not effective for treatment of mice for ectopic mineralization but might elicit an effect in humans. Nevertheless, magnesium carbonate clearly had a major impact on the mineralization process and should have similar effects on patients with PXE. It should be noted that the daily dose of magnesium administered to the mice was higher than the recommended daily dose for humans (500 mg per day). Thus, the efficacy in humans should be tested in carefully controlled clinical trials.
The side effects of magnesium include gastrointestinal disturbances, and evaluation of long‐term effects on vascular calcif cation and bone integrity has been suggested. 19 In our study, no overt side effects were noted in the mice. We also examined the bone density by CAT scan as well as the mineral content (Ca and P) in the femurs of mice treated for 5 months (1m → 6m) with magnesium carbonate. No changes in the bone density by CAT scan and a slight increase in the calcium and phosphorus contents by chemical assays were noted. These observations in the mineral content do not exclude any changes in the functional quality of long bones, such as those related to collagen multimer composition. These observations are consistent with early suggestions that magnesium deficiency in the rat alters bone and mineral metabolism resulting in bone loss. 28 In addition, magnesium intake has been associated with greater bone mineral density at the hip for both men and women and in the forearm of men in a both cross‐section (baseline) and 4‐year longitudinal study of a cohort of the Framingham Heart Study individuals. 29 These beneficial events were tied to the hypothesis that alkaline producing dietary components, such as potassium, magnesium, and fruit and vegetables, contribute to the maintenance of bone mineral density.
It should be noted that control of mineralization by dietary magnesium may have broader implications beyond PXE. A study by investigators at the Centers for Disease Control and Prevention (CDC) in the United States, based on a National Health and Nutrition Examination Survey 1999–2000, concluded that substantial numbers of US adults failed to consume adequate amounts of magnesium in their diets. 30 These observations suggested that assessment of dietary magnesium, not only in patients with PXE, but also in other conditions with ectopic mineralization of connective tissues, such as arteriosclerosis, 31 may be warranted.
Acknowledgments
We thank Devakumar Devadhas for assistance in CAT scan, Reid Oldenburg in preparing histology sections, and GianPaolo Guercio for manuscript preparation. This study was supported by DHHS, NIH/NIAMS grants R01 AR28450, R01 AR55225, and R01 AR 52627.
References
- 1. Li Q, Jiang Q, Pfendner E, Váradi A, Uitto J. Pseudoxanthoma elasticum: clinical phenotypes, molecular genetics and putative pathomechanisms. Exp Dermatol. 2009; 18: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Georgalas I, Papaconstantinou D, Koutsandrea C, Kalantzis G, Karagiannis D, Georgopoulos G, Ladas I. Angioid streaks, clinical course, complications, and current therapeutic management. Ther Clin Risk Manag. 2009; 5: 81–89. [PMC free article] [PubMed] [Google Scholar]
- 3. Lebwohl M, Halperin J, Phelps RG. Occult pseudoxanthoma elasticum in patients with premature cardiovascular disease. N Engl J Med. 1993; 329: 1237–1239. [DOI] [PubMed] [Google Scholar]
- 4. Neldner KH. Pseudoxanthoma elasticum. Clin Dermatol. 1988; 6: 1–159. [DOI] [PubMed] [Google Scholar]
- 5. Pfendner EG, Vanakker O, Terry SF, Vourthis S, McAndrew P, McLain MR, Fratta S, Marais AS, Hariri S, Coucke PJ, Ramsey M, Viljoen D, Tery PF, Uitto J, Bercovitch LG. Mutation detection in the ABCC6 gene and genotype‐phenotype analysis in a large international case series affected by pseudoxanthoma elasticum. J Med Genet. 2007; 44: 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iliás A, Urban Z, Seidl TL, Le Saux O, Sinkó E, Boyd CD, Sarkadi B, Váradi A. Loss of ATP‐dependent transport activity in pseudoxanthoma elasticum‐associated mutants of human ABCC6 (MRP6). J Biol Chem. 2002; 277: 16860–16867. [DOI] [PubMed] [Google Scholar]
- 7. Vanakker OM, Martin L, Gheduzzi D, Leroy BP, Loeys BL, Guerci VI, Matthys D, Terry SF, Coucke PJ, Pasquali‐Ronchetti I, De Paepe A. Pseudoxanthoma elasticum‐like phenotype with cutis laxa and multiple coagulation factor deficiency represents a separate genetic entity. J Invest Dermatol. 2007; 27: 581–587. [DOI] [PubMed] [Google Scholar]
- 8. Li Q, Schurgers L, Smith A, Tsokos M, Uitto J, Cowen E. Co‐existent pseudoxanthoma elasticum and vitamin K‐dependent coagulation factor deficiency. Am J Pathol. 2009; 174: 534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berkner KL. The vitamin K‐dependent carboxylase. Ann Rev Nutr. 2005; 25: 127–149. [DOI] [PubMed] [Google Scholar]
- 10. Shearer MJ. Role of vitamin K and Gla proteins in the pathophysiology of osteoporosis and vascular calcification. Curr Opin Clin Nutr Mtab Care. 2000; 3: 433–438. [DOI] [PubMed] [Google Scholar]
- 11. Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997; 386: 78–81. [DOI] [PubMed] [Google Scholar]
- 12. Borst P, Van De Wetering K, Schlingemann R. Does the absence of ABCC6 (multidrug resistance protein 6) in patients with pseudoxanthoma elasticum prevent the liver from providing sufficient vitamin K to the periphery? Cell Cycle. 2008; 7: 1575–1579. [DOI] [PubMed] [Google Scholar]
- 13. Hendig D, Arndt M, Szliska C, Kleesiek K, Götting C. SPP1 promoter polymorphisms: identification of the first modifier gene for pseudoxanthoma elasticum. Clin Chem. 2007; 53: 829–836. [DOI] [PubMed] [Google Scholar]
- 14. Schöen S, Schulz V, Prante C, Hendig D, Szaliska C, Kuhn J, Kleesiek K, Götting C. Polymorphisms in the xylosyltransferase genes cause higher serum XT‐I activity in patients with pseudoxanthoma elasticum (PXE) and are involved in a severe disease course. J Med Genet. 2006; 43: 745–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zarbock R, Hendig D, Szliska C, Kleesiek K, Götting C. Pseudoxanthoma elasticum: genetic variations in antioxidant genes are risk factors for early disease onset. Clin Chem. 2007; 53: 1734–1740. [DOI] [PubMed] [Google Scholar]
- 16. Li Q, Grange D, Armstrong N, Whelan A, Hurley M, Rishavy M, Hallgren K, Berkner K, Schurgers L, Jiang Q, Uitto J. Mutations in the GGCX and ABCC6 genes in a family with pseudoxanthoma elasticum‐like phenotypes. J Invest Dermatol. 2007; 129: 553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Renie WA, Pyeritz RE, Combs J, Fine SL. Pseudoxanthoma elasticum: high calcium intake in early life correlates with severity. Am J Med Genet. 1984; 19: 235–244. [DOI] [PubMed] [Google Scholar]
- 18. Uitto J, Pulkkinen L, Ringpfeil F. Molecular genetics of pseudoxanthoma elasticum: a metabolic disorder at the environment‐genome interface? Trends Mol Med. 2001; 7: 13–17. [DOI] [PubMed] [Google Scholar]
- 19. Hutchinson AJ. Oral phosphate binders. Kidney Int. 2009; 75: 906–914. [DOI] [PubMed] [Google Scholar]
- 20. Sherer DW, Singer G, Uribarri J, Phelps RG, Sapadin AN, Freund KB, Yanuzzi L, Fuchs W, Lebwohl M. Oral phosphate binders in the treatment of pseudoxanthoma elasticum. J Am Acad Dermatol. 2005; 53: 610–615. [DOI] [PubMed] [Google Scholar]
- 21. Klement JF, Matsuzaki Y, Jiang Q‐J, Terlizzi J, Choi HY, Fujimoto N, Li K, Pulkkinen L, Birk DE, Sundberg JP, Uitto J. Targeted ablation of the ABCC6 gene results in ectopic mineralization of connective tissues. Mol Cell Biol. 2005; 25: 8299–8310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. LaRusso J, Jiang Q, Li Q, Uitto J. Ectopic mineralization of connective tissue in Abcc6 −/−mice: effects of dietary modifications and a phosphate binder—a preliminary study. Exp Dermatol. 2008; 17: 203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Siegel S, Castellan N. Nonparametric Statistics for the Behavioral Sciences. Singapore : McGraw‐Hill, 1988, 180–181. [Google Scholar]
- 24. Jiang Q, Li Q, Uitto J. Aberrant mineralization of connective tissues in a mouse model of pseudoxanthoma elasticum: systemic and local regulatory factors. J Invest Dermatol. 2007; 127: 1392–1402. [DOI] [PubMed] [Google Scholar]
- 25. Joy MS, Kshirsagar A, Candiani C, Brooks T, Hudson JQ. Lanthanum carbonate. Ann Pharma-cother. 2006; 40: 234–240. [DOI] [PubMed] [Google Scholar]
- 26. Hutchinson AJ, Barnett ME, Krause R, Kwan JTC, Siami GA. Long‐term efficacy and safety profile of lanthanum carbonate: results for up to 6 years of treatment. Nephron Clin Pract. 2008; 110: 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Riond JL, Hartmann P, Steiner P, Ursprung R, Wanner M, Forrer R, Spichiger UE, Thomsen JS, Mosekilde L. Long‐term excessive magnesium supplementation is deleterious whereas suboptimal supply is beneficial for bones in rats. Magnes Res. 2000; 13: 249–264. [PubMed] [Google Scholar]
- 28. Rude RK, Kirchen ME, Gruber HE, Stasky AA, Meyer MH. Magensium defiency induces bone loss in the rat. Miner Electrolyte Metab. 1998; 24: 314–320. [DOI] [PubMed] [Google Scholar]
- 29. Tucker KL, Hannan MT, Chen H, Cupples LA, Wilson PWF, Kiel DP. Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in eldery men and women. Am J Clin Nutr. 1999; 69: 727–736. [DOI] [PubMed] [Google Scholar]
- 30. Ford ES, Mokdad AH. Dietary magnesium intake in a national sample of US adults. J Nutr. 2003; 133: 2879–2882. [DOI] [PubMed] [Google Scholar]
- 31. Persy V, D’Haese P. Vascular calcification and bone disease: the calcification paradox. Tren Mol Med. 2009; 15: 405–416. [DOI] [PubMed] [Google Scholar]


