Abstract
Thioredoxin 2 (Trx2) is a mitochondrially localized antioxidant and anti-apoptotic protein, whose functions are mainly dependent on the conserved cysteines at its redox active center. In the current study, we showed by mass spectrometry that a thiol alkylating agent, N-ethylmaleimide (NEM), alkylated a single cysteine residue in the active center of Trx2. The interaction between NEM and Trx2 in intact cells was confirmed by redox Western analysis. Overexpression of Trx2 in cultured 143B osteosarcoma cells caused increased sensitivity to NEM. Covalent modification by NEM resulted in a dominant-negative effect and increased the interaction between Trx2 and peroxiredoxin 3 (Prx3). Our data suggest that the alkylation of the essential thiol(s) of Trx2 has profound impact on the mitochondrial redox circuitry and such effects are distinct from the responses to agents causing reversible disulfide bond formation between the vicinal dithiols in the active center.
Keywords: thioredoxin, alkylating agent, mitochondria, redox, peroxiredoxin
Introduction
The thiol/disulfide redox status is an important regulatory factor in controlling tissue and cell functions. Modifications of critical thiols of protein kinases and phosphatases can result in either loss or gain of function. For instance, exposure of endothelial cells to peroxynitrite caused glutathiolation of p21ras at Cys118, which subsequently led to its membrane translocation and increased binding with Raf-1 (1). Alkylation of Cys199 by NEM augmented dephosphorylation and inactivation of cAMP-dependent protein kinase A (2). NEM also inhibited platelet-derived growth factor-induced Akt phosphorylation (3); but increased the activity of protein phosphatase 2A (4).
There are a number of enzymatic systems that control the cellular redox status utilizing NAD(P)H as the ultimate electron donor (5). Among them, thioredoxins (Trxs) are a family of small pleiotropic proteins that are ubiquitously expressed and evolutionarily conserved from prokaryotes to mammals (6). Trxs contain an invariant active center, -Cys-Gly-Pro-Cys-. The two cysteine residues can undergo reversible oxidation/reduction in the presence of thioredoxin reductase and NADPH. In mammalian cells, the cytosolic thioredoxin 1 (Trx1) protein is implicated in stimulation of cell proliferation, regulation of transcription of cell survival genes and inhibition of apoptosis (7). The mitochondrially localized thioredoxin 2 (Trx2) is an indispensable component of the mitochondrial antioxidant system (8, 9) and shows anti-apoptotic functions against various stimuli (10, 11). Trx2 interacts with Prx3, which is a major detoxification enzyme responsible for the clearance of mitochondrially generated hydrogen peroxide, and keeps it in the reduced and active state (12).
In our previous studies, we showed that Trx2 was susceptible to oxidation caused by various inducers of oxidative stress, such as peroxides (13), thiol cross-linker (14), heavy metal (15) and tumor necrosis factor-α (16). Under those conditions, a disulfide bond was reversibly formed between Cys90 and Cys93 in the active center. The oxidized cysteines often returned to the reduced state within relative short time after the initial oxidation (13). How modification of a single cysteine affects the functions of this protein has not been well characterized. In the present study, we showed that NEM caused higher cytotoxicity in cells overexpressing Trx2. Direct modification of Trx2 was confirmed by mass spectrometry and redox Western analysis. NEM treatment increased the complex formation between Trx2 and Prx3. Taken together, our data suggest that covalent modification of a single cysteine of Trx2 resulted in dominant-negative effects and irreversibly inhibited its function.
Experimental Procedures
Materials and Cell culture
Tris(2-carboxyethyl)phosphine (TCEP) and 4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid (AMS) were purchased from Invitrogen (Carlsbad, CA). Other agents were purchased from Sigma (St. Louis, MO). Human 143 B osteosarcoma cells were obtained from American Type Culture Collection (ATCC, Manassas, VA), and were cultured in Dulbecco’s modified Eagle’s medium (Mediatech, Hemdon, VA) supplemented with 10% fetal bovine serum (Sigma) in a humidified CO2 incubator at 37°C. For all experiments, 80-90% confluent cells were used for treatment. Cells stably transfected with Trx2 were described previously (10). The C90S and C93S mutants were generated by in vitro site-directed mutagenesis (GeneEditor, Promega, Madison, WI). Oligos 5′-CAC GCA CAG TGG AGT GGA CCC TG-3′ and 5′-GTG TGG ACC CAG CAA GAT CC-3′ were used as mutagenesis primers to introduce the C90S and C93S mutation, respectively.
Measurement of Trx2 Modification by Redox Western analysis
To test whether NEM can directly modify Trx2, we applied a previously described redox Western method with modifications (14). Briefly, cellular proteins were precipitated with 10% trichloroacetic acid after different treatments. The acid-insoluble proteins were washed once with acetone and then incubated with TCEP or H2O2 at final concentration of 1 mM for 10 min to reduce or oxidize the protein contents, respectively. After reacting with AMS, samples were resolved on 20% non-reducing SDS-PAGE, transferred to nitrocellulose membranes and probed with antibodies of interest. Signals were detected and quantified on an infrared imaging system (Odyssey, LI-COR, Lincoln, NE).
Measurement of Trx2 Modification by Mass spectrometry
The in vitro interaction between Trx2 and NEM was analyzed by Matrix-Assisted Laser Desorption/Ionization Time-Of-Flight Mass Spectrometry (MALDI-TOF MS). Purified recombinant human Trx2 protein (14) and NEM-treated Trx2 were digested with trypsin (Promega) and desalted (Ziptip, Millipore, Billerica, MA). Positive-ion MALDI-TOF MS analysis was performed using a 4700 Proteomics Analyzer MALDI-TOF/TOF (Applied Biosystems, Foster City, CA); α-cyano-2-hydroxycinnamic acid (Agilent technology, Santa Clara, CA) was used as the matrix.
Preparation of Subcellular Fractions and Western Blot Analysis
Cells were harvested by trypsinization and subcellular fractions were prepared as previously described using digitonin to selectively permeabilize the plasma membrane (10). After measuring the protein concentrations, the Trx2 contents in both the mitochondria-enriched fraction and the cytosolic fraction were analyzed by SDS-PAGE and Western blot analysis using an antibody against Trx2 (14). The membranes were reprobed with anti-cytochrome c antibody to control for the loading.
Measurement of Interaction between Trx2 and Prx3 by Co-Immunoprecipitation (Co-IP)
Cells overexpressing Trx2 were treated with either NEM or hydrogen peroxide, and lysed with 1 ml of lysis buffer [50 mM Tris-Cl, pH 7.5, 150 mM NaCl, 1% Triton-100 supplemented with protease inhibitors (cocktail III, Calbiochem, San Diego, CA)]. Trx2 was immunoprecipitated with the specific antibody (10). After stringent washing, the immunoprecipitates were boiled in 2 × SDS loading buffer and subjected to Western blot analysis. Anti-Prx3 antibody was obtained from Affinity BioReagents (Golden, CO).
Cell viability assay
Cell viability was measured by flow cytometry (BD Immunocytometry systems, San Jose, CA) as described (LIVE/DEAD viability/cytotoxicity kit, Invitrogen) (10). The intracellular GSH content was measured by HPLC (17), after 1 h treatment with different concentrations of NEM.
Results
Sensitization to NEM in 143B cells overexpressing Trx2
The active center cysteines are essential for the functions of the Trx proteins and tend to be susceptible to modifications by electrophilic and thiol-reactive compounds. To determine whether Trx2 affected sensitivity to thiol alkylating agents, NEM-induced cytotoxicities were measured and compared between 143B human osteosarcoma cells transfected with either Trx2 or an empty vector. The same types of cells were used in our previous studies in which Trx2 protected against apoptosis induced by peroxides and diamide treatment (10, 14).
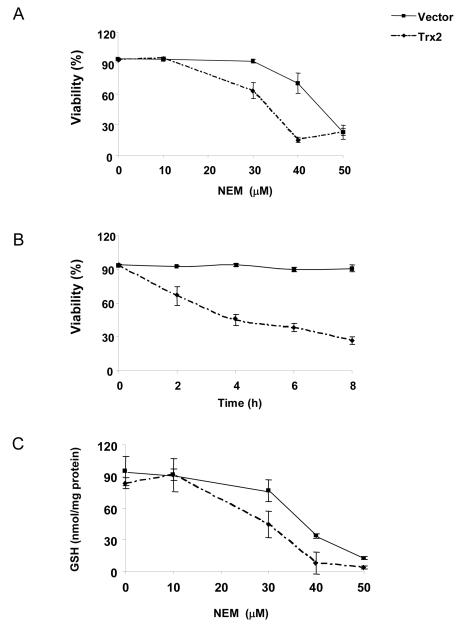
Overexpression of Trx2 caused increased sensitivity to NEM-induced cell death (Figure 1A). At 30 μM, NEM had minimal cytotoxicity in vector-transfected cells, but induced 37% cell death at 2 h in Trx2 overexpressing cells. The differences were even greater at 40 μM. When measured for the time course, 30 μM NEM induced cell death in Trx2 overexpressing cells at as early as 2 h, but not in vector-transfected cells (Fig. 1B). The percentage of cell death increased with time only in Trx2-transfected cells. Extending exposure time to 16 h did not change the pattern of responses (data not shown), indicating that the effects of Trx2 were not merely due to accelerating the cell death process. Consistent with the viability data, NEM caused more pronounced glutathione depletion in cells transfected with Trx2 (Fig. 1C).
Fig. 1. Cells with increased Trx2 level were more sensitive to NEM toxicity.
(A) 143 B cells transfected with empty vector or Trx2 were exposed to different concentrations of NEM for 2 h. Cells were then harvested and the viability was measured by flow cytometry. (B) Cells were treated with 30 μM of NEM and the viability was measured at the indicated time points. (C) Cells were treated with different concentrations of NEM for 1 h and intracellular GSH were measured by HPLC. Results are the average of three separate experiments (mean ± SEM).
NEM directly modified Trx2 in vitro
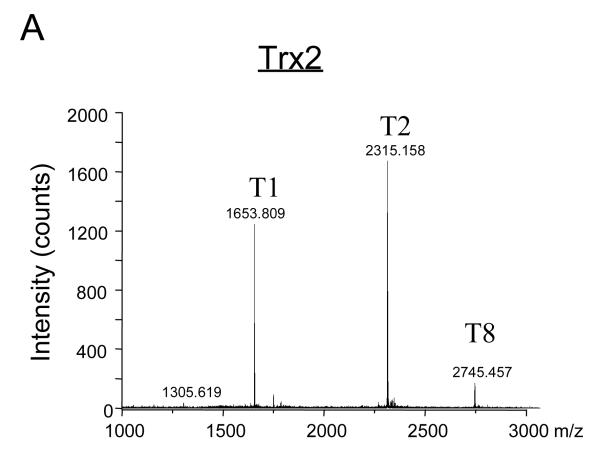
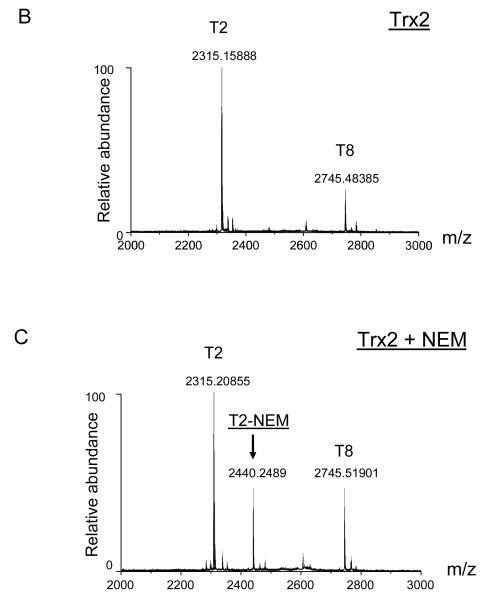
NEM alkylates accessible protein thiols and alters their activities. MS approach was used to map Trx2 peptides that had been directly modified by NEM. Purified recombinant human Trx2 protein was first reduced with TCEP and then incubated with NEM at various molar ratios of NEM to Trx2 for 1 h at 30°C. After the binding reaction, Trx2 was digested with trypsin and the peptides obtained after tryptic digestion were subjected to MS analysis. The predicted peptides are listed in Table 1 and are labeled as T1 through T12. The two cysteine residues in T2 are indicted as italic and underlined letters. The mass spectrum shown in Figure 2A displays the tryptic fragments (T1, T2, and T8) of the reduced Trx2 with mass/charge ratio (m/z) higher than 1000. Enlarged areas of the mass spectrum for Trx2 before and after reacting with NEM are shown in Figures 2B and 2C, respectively. A new peak at m/z of 2440 was detected in the mass spectrum, corresponding to a single addition of NEM to the T2 peptide (Fig. 2C, T2-NEM). At 1:5 molar ratio of Trx2 to NEM, no peak corresponding to two NEM additions was detected (Table 1 and Fig. 2C).
Table 1. Expected and observed monoisotopic masses (MH+) of tryptic fragments from NEM-reacted mitochondrial Trx 2.
Trx 2 (100 μM) was reduced with TCEP, treated with NEM (Trx 2 to NEM; 1:5), digested with trypsin, and analyzed by MALDI-TOF. The amino acid sequences of the tryptic fragments are indicated (T1 - T12). T2 is the Cys-containing fragment. NEM-modified fragments by one addition (−NEM) or two additions (− 2NEM) of NEM are indicated.
| Fragment | Expected mass (MH+) | Observed mass (MH+) |
|---|---|---|
| Cys-containing peptides | ||
| T2 dithiols (Cys 90, Cys93) | 2315.09 | 2315.15 |
| T2-1NEM | 2440.09 | 2440.2 |
| T2-2NEM | 2565.09 | ND |
|
| ||
| Other identified peptides | ||
| T1 | 1653.75 | 1653.80 |
| T8 | 2744.36 | 2745.45 |
|
| ||
| Amino acid sequences of the Trx2 tryptic fragments | ||
| 60 TTFNIQDGPDFQDR(T1), VVNSET PVVVDFHAQWCGPCK(T2), 95 ILGPR(T3), LEK(T4), MVAK(T5), QHG K(T6),VVMAK(T7), 116 VDIDDHTDLAIEYEVSAVPTVLAMK(T8), NGDVVDK(T9), 149 FVGIK(T10), DEDQLEAFLKK(T11), LIG(T12) | ||
| The mitochondrial localization signal from 1 to 59, which is removed upon mitochondrial import, is not shown. | ||
Fig. 2. MALDI-TOF mass spectrum of tryptic fragments of Trx2 with or without NEM modification.
(A) MALDI-TOF mass spectrum of tryptic fragments of purified Trx2. The identified fragments (T1, T2 and T8) were labeled as described in Table 1. (B) MALDI-TOF mass spectrum of m/z in the range of 2000-3000 was enlarged to demonstrate the T2 tryptic peptide which contained the active center cysteines Cys90 and Cys93. (C) After being incubated with NEM, a modified peptide (T2-NEM) at m/z 2440 emerged on the MALDI-TOF mass spectrum of Trx2. It corresponded to a single addition of NEM (125 Da).
NEM directly modified Trx2 in cells
We further applied a modified redox Western blot approach to investigate whether modification of Trx2 by NEM occurred in intact cells. Under normal conditions, the majority of Trx2 protein is in the reduced state (14) and the two sulfhydryl groups in the redox active center can react with the thiol alkylating agent AMS (14). When samples were resolved on a SDS-PAGE, the reduced and oxidized forms of Trx2 can be distinguished by the molecular weight increased due to additions of two molecules of AMS. If any of the two cysteine residues in the active center of Trx2 is modified by NEM, the subsequent addition of AMS will be blocked and a new band with different molecular weight will emerge on the SDS-PAGE.
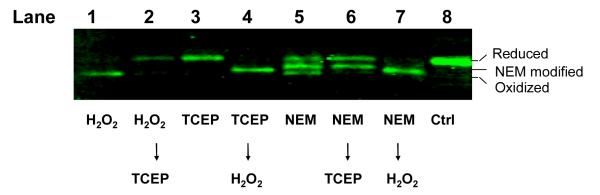
As shown in Fig. 3, reduced Trx2 was the prevalent form in untreated cells (lane 8) and in cells being exposed to the reducing agent TCEP (lane 3). Incubation of cells with H2O2 led to a rapid oxidation of Trx2 within 10 min (lane 1). Oxidation of Trx2 also occurred in cells treated with 50 μM NEM for 10 min (lane 5). In addition, a third band with an intermediate molecular weight between the oxidized and the reduced band emerged after exposure to NEM (lane 5). Applying TCEP or H2O2 to the cell lysates can efficiently reverse the oxidation or reduction of Trx2 in vitro (lanes 2 and 4). As expected from an alkylation reaction, TCEP could only reduce the oxidized band but not the new band generated from NEM treatment (lane 6). We also treated the lysate of NEM-treated cells with H2O2. Oxidation of the cysteines prevented addition of AMS and diminished both reduced and the new band (lane 7). The redox Western data suggested that, consistent with the findings from the MS analyses, NEM predominantly interacted with one of the two cysteine residues in the active center.
Fig. 3. NEM directly modified Trx2 in cells.
Cells were treated with 50 μM of NEM for 10 min and samples were harvested and prepared for redox Western analysis on 20% non-reducing SDS-PAGE. Data are representative of three separate experiments.
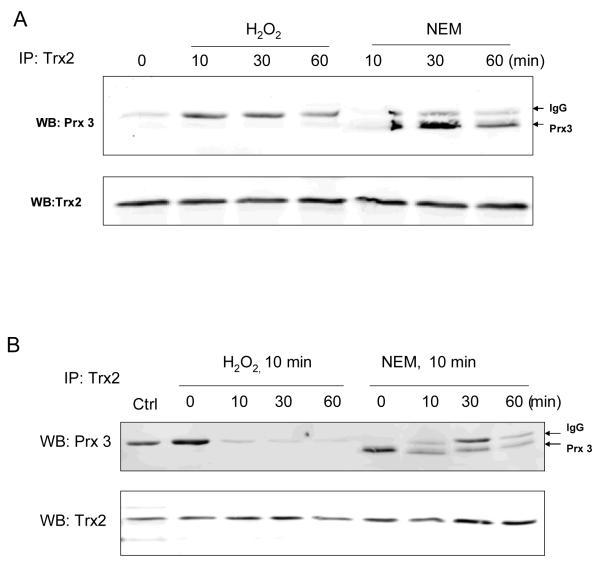
Both cysteines at the active center are required for the interaction of Trx2 with its substrate proteins including Prx3. To determine the effects of thiol alkylation at a single cysteine on protein-protein interactions, we performed Co-IP assay to measure the binding between Prx3 and Trx2 after cells had been exposed to NEM. Results (Fig. 4A) showed that under control conditions when Trx2 was largely reduced (Fig. 3), the majority of Trx2 did not bind to Prx3. Upon NEM treatment, the interaction was significantly increased. The effect of NEM was distinct from the response to hydrogen peroxide. Only a small fraction of Trx2 bound to Prx3 in cells exposed to 300 μM H2O2 for 60 min (Fig. 4A). Furthermore, even after NEM had been removed and cells were recovered for up to 60 min, binding between Prx3 and Trx2 was still detectable by Co-IP assay (Fig. 4B), indicating that the increased interaction by modification of NEM was not a transient effect.
Fig. 4. NEM inhibited the dissociation of Trx2 from Prx3.
(A) Cells were treated with either 300 μM hydrogen peroxide or 50 μM NEM for the indicated time. Trx2 was immunoprecipitated (IP) and the bound Prx3 was determined by Western blot analyses (WB). The position of the light chain of immunoglobulin (IgG) was indicated by arrows. (B) Hydrogen peroxide and NEM were removed after the initial 10 min exposure. Cells were then recovered for the indicated time. Interactions between Trx2 and Prx3 were determined by Co-IP.
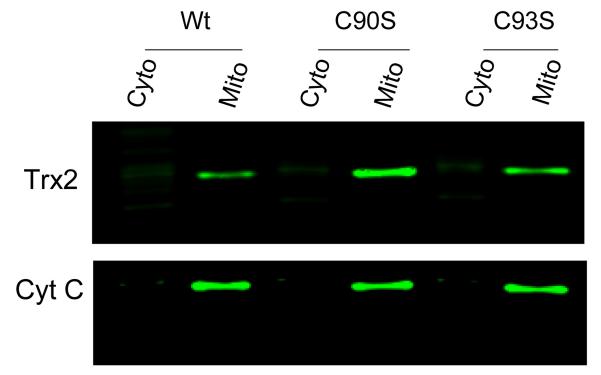
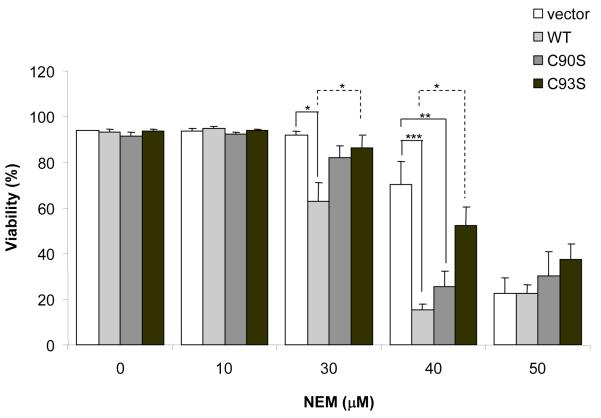
To further determine the functional consequences of binding of NEM to the redox active cysteine, we transfected cells with Trx2 engineered to carry a mutation at either Cys90 or Cys93 (C90S or C93S, respectively) in the active center. The expression and the mitochondrial localization of both mutants were confirmed by subcellular fractionation and Western blot analysis (Fig. 5). When cells were maintained under unstressed conditions, transfection of both mutants did not affect cell proliferation and viability. After being exposed to 40 μM NEM, cells transfected with C90S exhibited significantly higher percentage of cell death, which was similar to the response of cells overexpressing wild type Trx2 (Fig. 6). Such sensitization was not observed in cells overexpressing the C93S mutant. Mutant Trx2 proteins had one cysteine mutated to serine and the other cysteine likely occupied by NEM. Therefore, their effects on NEM-induced cytotoxicities might be redox independent.
Fig. 5. Western blot analysis of subcellular localization of wild-type and site-directed mutants of Trx2.
Cytosolic and mitochondria-enriched fractions from cells transfected with wild type, C90S or C93S mutants of Trx2 were run on 15% SDS-PAGE and blotted with anti-Trx2 antibody. Anti-cytochrome c was used as mitochondrial marker. Data presented are representative of three separate experiments.
Fig. 6. The C93S mutant was less sensitive than wild type Trx2 in response to NEM.
Cells transfected with wild-type, C90S Trx2 or C93S Trx2 were treated with different concentrations of NEM. Cell viability was measured at 6 h by flow cytometry. Results are the average of three separate experiments (mean ± SEM). * P<0.05; ** P<0.01, One-way ANOVA and Turkey’s Post hoc test.
Discussion
Trx and its reductase are essential components of the cellular antioxidant defensive system. They are also susceptible targets of oxidative damage. Various environmental toxicants and reactive metabolites, such as 4-hydroxy-2-nonenal (23, 27), acrolein (23, 28), heavy metals like mercury and gold compounds (15, 29), and metabolites of halogenated ethane (30) directly alkylate and inhibit the functions of Trx. Most of the earlier studies used in vitro binding and activity assays; however, recent findings with proteomic approaches, along with molecular biology techniques, have provided convincing experimental evidence of modifications of the cysteine(s) in intact cells by the electrophilic compounds (22, 23, 31).
After being imported into mitochondria and cleaved of its targeting sequence, the mature form of Trx2 has two cysteines, Cys90 and Cys93, at its active center. Previous studies using site-directed mutants showed that the interactions between Trx2 and apoptosis signaling kinase 1 (19) and Prx3 (20) require the cysteines. In the current study, we showed by MS (Fig. 2) and redox Western (Fig. 3) analyses that NEM preferentially modified one of the active center cysteines. Modification of both cysteines by NEM was not observed on the MS spectrum (Table 1 and Fig. 2C). This could due to steric hindrance or protein conformational change after the binding with the first NEM. Both Cys90 and Cys93 are close to the C-terminus of the T2 peptide (Table 1). The spectrum of the y-series ions from the Electrospray Ionisation MS/MS analyses did not provide unambiguous evidence for the site of modification (data not shown). For Trx family proteins, the cysteine responsible for the initial binding with the substrate proteins is often different from the site of modification by either endogenous or exogenous compounds. The Cys73 of cytosolic Trx1 protein is the preferential site for glutathionylation (21) and modification by thiol-reactive electrophiles (22, 23). S-nitrosylation occurs at Cys69 of Trx1 (24), which can form a second intramolecular disulfide bond with Cys62 (25). Preferential modification of the second cysteine (Cys35) has been reported for Trx1 exposed to 15-deoxy-Δ12,14-prostaglandin J2 (26). Future studies will be needed to determine how these agents modify Trx2 and regulate its functions both in vitro and in vivo.
Trx2 forms intermolecular mixed disulfide with other mitochondrial proteins such as Prx3. Results from the Co-IP experiments indicated that only marginal level of Prx3 bound to Trx2 under unstressed conditions (Fig. 4), indicating this enzymatic detoxification system is highly efficient and the protein components are mostly in the reduced state. When alkylated by NEM, however, Trx2 showed much increased binding with Prx3 (Fig. 4). The C93S mutant but not the C90S mutant had similar increased binding with Prx3 (20). The amino acid sequence of the active center is identical between Trx1 and Trx2. The redox chemistry of Trx1 has been studied extensively and it is generally accepted that the two cysteines have different kinetic properties (18). The first cysteine has lower pKa and is involved in the initial disulfide bond formation with the substrate proteins. Once the intermediate complex is formed between Trx and a substrate, the second cysteine reduces the intermolecular disulfide bond and releases Trx from the complex. Inactivation of Cys93, either site-directed mutagenesis or covalent modification, will inhibit the dissociation of Trx2 from its substrate proteins and result in dominant negative effects.
We have previously reported that Trx2 is particularly susceptible to oxidation and forms disulfide bond between Cys90 and Cys93 (13, 14). It is likely that alkylation and oxidation have distinct effects on the redox properties of Trx2. Oxidation induced by hydrogen peroxide was transient (14) and did not potentiate the formation of the Trx2/Prx3 complex (Fig. 4). Alkylation of Trx2 by NEM as short as 10 min resulted in persistently increased binding with Prx3, even at 60 min after the removal of NEM from the culture media (Fig. 4B). The data suggest that alkylation at a single Cys residue caused much more potent and persistent inhibition of the Trx2 function. The binding of NEM to Trx2 may also elicit redox-independent effects, as implied by the studies using site-directed mutants (Fig. 6). In cells treated with 30 μM NEM, neither C90S nor C93S potentiated the NEM toxicity. In both mutants, one cysteine had been mutated to serine and the other was likely occupied by NEM. Therefore, neither could function as a dominant negative factor like the wild type Trx2. However, in cells treated with 40 μM NEM, the C90S showed similar response as the wild type Trx2 (Fig. 6). The results suggested that the alkylation at Cys93 of Trx2 could have affected other mitochondrial proteins which function downstream of Trx2 but remain to be identified. The binding between such proteins with Trx2 may occur at a different site but is still affected by the modification of Cys93.
The findings from our current study have potential clinical implications in cancer therapy. It is well established that the protein and mRNA levels of Trx1, the cytosolic functional homologue of Trx2, are often increased in tumors (32-34). A series of alkyl 2-imidazolyl disulfide have been developed to target the Trx1/TrxR1 system (35-37) and they were shown to inhibit cell growth and cause cell death in a variety of cancer cell lines (38). Trx2 is an antioxidant and anti-apoptotic protein. Its expression is increased in Imexon-resistant myeloma cells (39). By its analogy to Trx1, the Trx2 system can be another target for chemotherapeutics. NEM does not have therapeutic value and was only used as a model drug for the current study. It is a global alkylating agent and reacts with accessible protein cysteines (22) and glutathione (Fig. 1C) with low specificity. Potential candidate compounds would include thiol alkylating agents that can penetrate into mitochondria and become concentrated in this negatively charged subcellular compartment.
In summary, we have measured the direct modification of the active center cysteine of Trx2 by NEM, both in vitro and in intact cells. Alkylation of the cysteine inhibited the dissociation of Trx2 from its substrate protein, Prx3, and interfered with the mitochondrial redox regulation which is essential in protecting against oxidative stress.
Acknowledgements
This work was supported by NIH grants ES009047, ES014668, Alston Callahan Postdoctoral Scholar Award (to YC) from the International Retinal Research Foundation, a departmental Challenge Grant and Sybil Harrington Award from Research to Prevent Blindness, Inc.
Abbreviations
- AMS
4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid
- IP
immunoprecipitation
- m/z
mass/charge ratio
- NEM
N-ethylmaleimide
- Prx3
peroxiredoxin 3
- TCEP
Tris(2-carboxyethyl)phosphine
- Trx
thioredoxin
References
- (1).Clavreul N, Adachi T, Pimental DR, Ido Y, Schoneich C, Cohen RA. S-glutathiolation by peroxynitrite of p21ras at cysteine-118 mediates its direct activation and downstream signaling in endothelial cells. Faseb J. 2006;20:518–520. doi: 10.1096/fj.05-4875fje. [DOI] [PubMed] [Google Scholar]
- (2).Humphries KM, Deal MS, Taylor SS. Enhanced dephosphorylation of cAMP-dependent protein kinase by oxidation and thiol modification. J Biol Chem. 2005;280:2750–2758. doi: 10.1074/jbc.M410242200. [DOI] [PubMed] [Google Scholar]
- (3).Bhanoori M, Yellaturu CR, Ghosh SK, Hassid A, Jennings LK, Rao GN. Thiol alkylation inhibits the mitogenic effects of platelet-derived growth factor and renders it proapoptotic via activation of STATs and p53 and induction of expression of caspase1 and p21(waf1/cip1) Oncogene. 2003;22:117–130. doi: 10.1038/sj.onc.1206065. [DOI] [PubMed] [Google Scholar]
- (4).Yellaturu CR, Bhanoori M, Neeli I, Rao GN. N-Ethylmaleimide inhibits platelet-derived growth factor BB-stimulated Akt phosphorylation via activation of protein phosphatase 2A. J Biol Chem. 2002;277:40148–40155. doi: 10.1074/jbc.M206376200. [DOI] [PubMed] [Google Scholar]
- (5).Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- (6).Arner ES, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- (7).Powis G, Montfort WR. Properties and biological activities of thioredoxins. Annu Rev Pharmacol Toxicol. 2001;41:261–295. doi: 10.1146/annurev.pharmtox.41.1.261. [DOI] [PubMed] [Google Scholar]
- (8).Nonn L, Williams RR, Erickson RP, Powis G. The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol Cell Biol. 2003;23:916–922. doi: 10.1128/MCB.23.3.916-922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Tanaka T, Hosoi F, Yamaguchi-Iwai Y, Nakamura H, Masutani H, Ueda S, Nishiyama A, Takeda S, Wada H, Spyrou G, Yodoi J. Thioredoxin-2 (TRX-2) is an essential gene regulating mitochondria-dependent apoptosis. Embo J. 2002;21:1695–1703. doi: 10.1093/emboj/21.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Chen Y, Cai J, Murphy TJ, Jones DP. Overexpressed human mitochondrial thioredoxin confers resistance to oxidant-induced apoptosis in human osteosarcoma cells. J Biol Chem. 2002;277:33242–33248. doi: 10.1074/jbc.M202026200. [DOI] [PubMed] [Google Scholar]
- (11).Damdimopoulos AE, Miranda-Vizuete A, Pelto-Huikko M, Gustafsson JA, Spyrou G. Human mitochondrial thioredoxin. Involvement in mitochondrial membrane potential and cell death. J Biol Chem. 2002;277:33249–33257. doi: 10.1074/jbc.M203036200. [DOI] [PubMed] [Google Scholar]
- (12).Nonn L, Berggren M, Powis G. Increased expression of mitochondrial peroxiredoxin-3 (thioredoxin peroxidase-2) protects cancer cells against hypoxia and drug-induced hydrogen peroxide-dependent apoptosis. Mol Cancer Res. 2003;1:682–689. [PubMed] [Google Scholar]
- (13).Chen Y, Yu M, Jones DP, Greenamyre JT, Cai J. Protection against oxidant-induced apoptosis by mitochondrial thioredoxin in SH-SY5Y neuroblastoma cells. Toxicol Appl Pharmacol. 2006;216:256–262. doi: 10.1016/j.taap.2006.05.006. [DOI] [PubMed] [Google Scholar]
- (14).Chen Y, Cai J, Jones DP. Mitochondrial thioredoxin in regulation of oxidant-induced cell death. FEBS Lett. 2006;580:6596–6602. doi: 10.1016/j.febslet.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Hansen JM, Zhang H, Jones DP. Differential oxidation of thioredoxin-1, thioredoxin-2, and glutathione by metal ions. Free Radic Biol Med. 2006;40:138–145. doi: 10.1016/j.freeradbiomed.2005.09.023. [DOI] [PubMed] [Google Scholar]
- (16).Hansen JM, Zhang H, Jones DP. Mitochondrial thioredoxin-2 has a key role in determining tumor necrosis factor-alpha-induced reactive oxygen species generation, NF-kappaB activation, and apoptosis. Toxicol Sci. 2006;91:643–650. doi: 10.1093/toxsci/kfj175. [DOI] [PubMed] [Google Scholar]
- (17).Ha KN, Chen Y, Cai J, Sternberg P., Jr. Increased glutathione synthesis through an ARE-Nrf2-dependent pathway by zinc in the RPE: implication for protection against oxidative stress. Invest Ophthalmol Vis Sci. 2006;47:2709–2715. doi: 10.1167/iovs.05-1322. [DOI] [PubMed] [Google Scholar]
- (18).Kallis GB, Holmgren A. Differential reactivity of the functional sulfhydryl groups of cysteine-32 and cysteine-35 present in the reduced form of thioredoxin from Escherichia coli. J Biol Chem. 1980;255:10261–10265. [PubMed] [Google Scholar]
- (19).Zhang R, Al-Lamki R, Bai L, Streb JW, Miano JM, Bradley J, Min W. Thioredoxin-2 inhibits mitochondria-located ASK1-mediated apoptosis in a JNK-independent manner. Circ Res. 2004;94:1483–1491. doi: 10.1161/01.RES.0000130525.37646.a7. [DOI] [PubMed] [Google Scholar]
- (20).Zhang H, Go YM, Jones DP. Mitochondrial thioredoxin-2/peroxiredoxin-3 system functions in parallel with mitochondrial GSH system in protection against oxidative stress. Arch Biochem Biophys. 2007;465:119–126. doi: 10.1016/j.abb.2007.05.001. [DOI] [PubMed] [Google Scholar]
- (21).Casagrande S, Bonetto V, Fratelli M, Gianazza E, Eberini I, Massignan T, Salmona M, Chang G, Holmgren A, Ghezzi P. Glutathionylation of human thioredoxin: a possible crosstalk between the glutathione and thioredoxin systems. Proc Natl Acad Sci U S A. 2002;99:9745–9749. doi: 10.1073/pnas.152168599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Dennehy MK, Richards KA, Wernke GR, Shyr Y, Liebler DC. Cytosolic and nuclear protein targets of thiol-reactive electrophiles. Chem Res Toxicol. 2006;19:20–29. doi: 10.1021/tx050312l. [DOI] [PubMed] [Google Scholar]
- (23).Go YM, Halvey PJ, Hansen JM, Reed M, Pohl J, Jones DP. Reactive aldehyde modification of thioredoxin-1 activates early steps of inflammation and cell adhesion. Am J Pathol. 2007;171:1670–1681. doi: 10.2353/ajpath.2007.070218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Haendeler J, Hoffmann J, Tischler V, Berk BC, Zeiher AM, Dimmeler S. Redox regulatory and anti-apoptotic functions of thioredoxin depend on S-nitrosylation at cysteine 69. Nat Cell Biol. 2002;4:743–749. doi: 10.1038/ncb851. [DOI] [PubMed] [Google Scholar]
- (25).Watson WH, Pohl J, Montfort WR, Stuchlik O, Reed MS, Powis G, Jones DP. Redox potential of human thioredoxin 1 and identification of a second dithiol/disulfide motif. J Biol Chem. 2003;278:33408–33415. doi: 10.1074/jbc.M211107200. [DOI] [PubMed] [Google Scholar]
- (26).Shibata T, Yamada T, Ishii T, Kumazawa S, Nakamura H, Masutani H, Yodoi J, Uchida K. Thioredoxin as a molecular target of cyclopentenone prostaglandins. J Biol Chem. 2003;278:26046–26054. doi: 10.1074/jbc.M303690200. [DOI] [PubMed] [Google Scholar]
- (27).Fang J, Holmgren A. Inhibition of thioredoxin and thioredoxin reductase by 4-hydroxy-2-nonenal in vitro and in vivo. J Am Chem Soc. 2006;128:1879–1885. doi: 10.1021/ja057358l. [DOI] [PubMed] [Google Scholar]
- (28).Yang X, Wu X, Choi YE, Kern JC, Kehrer JP. Effect of acrolein and glutathione depleting agents on thioredoxin. Toxicology. 2004;204:209–218. doi: 10.1016/j.tox.2004.06.056. [DOI] [PubMed] [Google Scholar]
- (29).Bragadin M, Scutari G, Folda A, Bindoli A, Rigobello MP. Effect of metal complexes on thioredoxin reductase and the regulation of mitochondrial permeability conditions. Ann N Y Acad Sci. 2004;1030:348–354. doi: 10.1196/annals.1329.043. [DOI] [PubMed] [Google Scholar]
- (30).Meyer M, Jensen ON, Barofsky E, Barofsky DF, Reed DJ. Thioredoxin alkylation by a dihaloethane-glutathione conjugate. Chem Res Toxicol. 1994;7:659–665. doi: 10.1021/tx00041a011. [DOI] [PubMed] [Google Scholar]
- (31).Liebler DC. Proteomic approaches to characterize protein modifications: new tools to study the effects of environmental exposures. Environ Health Perspect. 2002;110(Suppl 1):3–9. doi: 10.1289/ehp.02110s113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Shao L, Diccianni MB, Tanaka T, Gribi R, Yu AL, Pullen JD, Camitta BM, Yu J. Thioredoxin expression in primary T-cell acute lymphoblastic leukemia and its therapeutic implication. Cancer Res. 2001;61:7333–7338. [PubMed] [Google Scholar]
- (33).Raffel J, Bhattacharyya AK, Gallegos A, Cui H, Einspahr JG, Alberts DS, Powis G. Increased expression of thioredoxin-1 in human colorectal cancer is associated with decreased patient survival. J Lab Clin Med. 2003;142:46–51. doi: 10.1016/S0022-2143(03)00068-4. [DOI] [PubMed] [Google Scholar]
- (34).Grogan TM, Fenoglio-Prieser C, Zeheb R, Bellamy W, Frutiger Y, Vela E, Stemmerman G, Macdonald J, Richter L, Gallegos A, Powis G. Thioredoxin, a putative oncogene product, is overexpressed in gastric carcinoma and associated with increased proliferation and increased cell survival. Hum Pathol. 2000;31:475–481. doi: 10.1053/hp.2000.6546. [DOI] [PubMed] [Google Scholar]
- (35).Engman L, Al-Maharik N, McNaughton M, Birmingham A, Powis G. Thioredoxin reductase and cancer cell growth inhibition by organotellurium antioxidants. Anticancer Drugs. 2003;14:153–161. doi: 10.1097/00001813-200302000-00009. [DOI] [PubMed] [Google Scholar]
- (36).Ramanathan RK, Kirkpatrick DL, Belani CP, Friedland D, Green SB, Chow HH, Cordova CA, Stratton SP, Sharlow ER, Baker A, Dragovich T. A Phase I pharmacokinetic and pharmacodynamic study of PX-12, a novel inhibitor of thioredoxin-1, in patients with advanced solid tumors. Clin Cancer Res. 2007;13:2109–2114. doi: 10.1158/1078-0432.CCR-06-2250. [DOI] [PubMed] [Google Scholar]
- (37).Hashemy SI, Ungerstedt JS, Avval F. Zahedi, Holmgren A. Motexafin gadolinium, a tumor-selective drug targeting thioredoxin reductase and ribonucleotide reductase. J Biol Chem. 2006;281:10691–10697. doi: 10.1074/jbc.M511373200. [DOI] [PubMed] [Google Scholar]
- (38).Kirkpatrick DL, Kuperus M, Dowdeswell M, Potier N, Donald LJ, Kunkel M, Berggren M, Angulo M, Powis G. Mechanisms of inhibition of the thioredoxin growth factor system by antitumor 2-imidazolyl disulfides. Biochem Pharmacol. 1998;55:987–994. doi: 10.1016/s0006-2952(97)00597-2. [DOI] [PubMed] [Google Scholar]
- (39).Dvorakova K, Payne CM, Tome ME, Briehl MM, Vasquez MA, Waltmire CN, Coon A, Dorr RT. Molecular and cellular characterization of imexon-resistant RPMI8226/I myeloma cells. Mol Cancer Ther. 2002;1:185–195. [PubMed] [Google Scholar]