Abstract
Aims
The apical membrane anion exchanger Pat-1 is expressed at significant levels in the lower villus epithelium of murine duodenum. However, previous studies of Cl−/HCO3− exchange in the lower villus have failed to demonstrate Pat-1 function. Those studies routinely included luminal glucose which induces Na+-coupled glucose transport and acidifies the villus epithelium. Since Pat-1 has been proposed to be an electrogenic 1Cl−/2HCO3− exchanger, membrane depolarization or cell acidification during glucose transport may obscure Pat-1 activity. Therefore, we investigated the effects of luminal glucose on Cl−IN/HCO3−OUT exchange activity in the lower villus epithelium.
Methods
Cl−IN/HCO3−OUT exchange of villus epithelium in duodenal mucosa from Pat-1 knockout (KO), Slc26a3 (Dra) KO, cystic fibrosis transmembrane conductance regulator (Cftr) KO and wild-type (WT) littermate mice was measured using the pH-sensitive dye BCECF. Short-circuit current (Isc) was measured in Ussing chambers.
Results
During glucose absorption, Cl−IN/HCO3−OUT exchange in the lower villus epithelium was abolished in the Dra KO and unaffected in the Pat-1 KO relative to WT. However, during electroneutral mannose absorption or electrogenic α-D-methyl glucoside absorption, Cl−IN/HCO3−OUT exchange was reduced in both Pat-1 KO and Dra KO villi. Exposure to high [K+] abolished Cl−IN/HCO3−OUT exchange in the Dra KO but not the Dra/Cftr double KO epithelium, suggesting that Pat-1 activity is little affected by membrane depolarization except in the presence of Cftr.
Conclusions
The metabolic and electrogenic activity of glucose transport obscures Cl−IN/HCO3−OUT exchange activity of Pat-1 in the lower villus. The inhibitory effects of membrane depolarization on Pat-1 Cl−IN/HCO3−OUT exchange may require concurrent membrane association with Cftr.
Keywords: anion exchange, Cftr, down regulated in adenoma, Dra, facilitated diffusion
Introduction
The physiology of the alkaline mucus barrier that protects the duodenal mucosa from gastric effluent was elucidated by the pioneering work of Gunnar Flemstrom and colleagues (Flemstrom 1994). Their work was instrumental in developing a model for active HCO3− secretion by the epithelium which postulated that the process involved both activities of Cl−/HCO3− exchanger(s) and an anion channel(Allen et al. 1993). It is now recognized that the cystic fibrosis transmembrane conductance regulator (Cftr), i.e., the anion channel that is mutated in cystic fibrosis, is conductive to both Cl− and HCO3− ions (ratio of ~4:1, respectively) and serves as the principal anion channel involved in basal and cyclic nucleotide-stimulated HCO3− secretion across the duodenum (Poulsen et al. 1994;Hogan et al. 1997;Seidler et al. 1997;Clarke and Harline 1998). Further, recent studies show that the HCO3− permeability of Cftr can be significantly increased by low intracellular [Cl−] or cell shrinkage through activation of a with-no-lysine (WNK) kinase cascade (Park et al. 2010). Discovery of the multifunctional anion exchanger family Slc26a identified two proteins capable of Cl−/HCO3− exchange that are expressed on the apical membrane of duodenum: Slc26a3, also known as down-regulated in adenoma (Dra) and Slc26a6, also known as putative anion transporter-1 (Pat-1)(Jacob et al. 2002;Wang et al. 2002). Based on pH stat studies of knockout mouse models, it has been shown that Pat-1 is responsible for < 30% of basal HCO3− secretion and does not participate in cAMP- stimulated secretion, although Pat-1 activity may be activated by PGE2 via a Ca2+-dependent pathway(Wang et al. 2005;Tuo et al. 2006). The Dra Cl−/HCO3− exchange contributes ~60% of basal HCO3− secretion and, during cAMP stimulation, on-going activity provides ~50% of net HCO3− secretion with remaining secretion apparently due to the Cftr HCO3− conductance (Walker et al. 2009).
Studies of recombinant Pat-1 and Dra have elucidated their regulation, relevant inhibitors and anion specificity but controversy has arisen with regard to exchange stoichiometry and electrogenicity. Species divergence in the amino acid identity between orthologous mouse and human Slc26a transporters and differing experimental conditions contribute to the confusion (Chernova et al. 2005). For Dra, most studies agree that human and mouse Dra Cl−/HCO3− exchange is electroneutral (ostensibly 1Cl−/1HCO3− exchange) (Melvin et al. 1999;Lamprecht et al. 2005;Horack et al. 2008), although one laboratory has proposed that mouse Dra demonstrates electrogenic 2Cl−/1HCO3− exchange (Shcheynikov et al. 2006). With regard to Pat-1, three studies using Xenopus oocyte expression of murine Pat-1 have shown evidence consistent with electrogenic exchange, with quantitative estimates from one laboratory of a 1Cl−/2HCO3− exchange stoichiometry (Jiang et al. 2002;Xie et al. 2002;Shcheynikov et al. 2006). In contrast, another laboratory using oocyte expression of murine Pat-1 did not find evidence of electrogenic Cl−/HCO3− exchange but confirmed previous observations of electrogenic Cl−/oxalate2− exchange by murine Pat-1 (Chernova et al. 2005). The question of electrogenicity is not trivial in that it alters the prediction of intestinal electrolyte and fluid responses during nutrient or drug absorption.
Studies using intact intestine, in particular from Pat-1 and Dra KO mice, have enabled localization of the exchangers’ activity along the villus axis in the duodenum (Walker et al. 2009). The level of Pat-1 and Dra expression reciprocates along the villus axis with Dra greatest in the lower villus/crypt and Pat-1 greatest in the upper villus, but both exchangers are well-represented throughout the villus length (Jacob et al. 2002;Walker et al. 2009). Microfluorimetry studies of the upper villus epithelium find that Pat-1 contributes 70% and Dra 30% of the total Cl−/HCO3− exchange(Simpson et al. 2007). In contrast, similar studies of the lower villus epithelium (10–15 enterocytes above crypt openings) indicate that Dra provides ~100% of the apical membrane Cl−/HCO3− exchange (Simpson et al. 2007). Thus, in the lower villus, an apparent discrepancy exists between functional studies and expression regarding Pat-1 activity. The complement of acid-base transporters at the apical membrane in the lower villus, including Cftr, the Na+/H+ exchanger Nhe3, Dra and Pat-1, may obscure the HCO3− transport function of a single anion exchanger. The complexity is increased by higher levels of Cftr expression relative to the upper villus (Ameen et al. 2000) which dominates the membrane potential, significantly affects electrochemical anion gradients and plays a major role in cell volume regulation (Simpson et al. 2007). Therefore, we re-examined the question of Pat-1 functional activity in the lower villus by altering conditions that may have obscured the activity of this anion exchanger in previous studies. In particular we focused on two aspects: 1) exposure to luminal glucose, which was used in previous studies to reduce pHi of the villus epithelium to a pH range suitable for the pH-sensitive dye BCECF. However, luminal glucose produces a strong inward Na+ current via the Na+-coupled glucose transporter Sglt1 which is predicted to affect Na+-dependent and electrogenic anion transport (Wright et al. 1994); and 2) measurement of Cl−IN/HCO3−OUT exchange rate, i.e., the “forward” mode of transport for anion exchangers. Previous studies have shown that luminal Cl− removal to measure Cl−OUT/HCO3−IN exchange in the lower villus also induces abrupt cell shrinkage and inhibits Nhe3 activity which counters cell alkalinization. Therefore, to ensure a steady-state condition, the villus epithelium was incubated for 20 min in a Cl− free solution prior to the initiation of Cl−IN/HCO3−OUT exchange.
Materials and Methods
Animals
The experiments were performed using mice with the gene-targeted disruptions of the murine homologs of Slc26a3 (Dra) (Schweinfest et al. 2006), Slc26a6 (Pat-1) (Wang et al. 2005) or Abcc7 (Cftr) (Snouwaert et al. 1992) on a mixed genetic background. All comparisons of homozygous knockout (−/−) mice (KO) were made with gender- and age-matched (+/+) siblings (WT). The mutant mice were identified by using a PCR-based analysis of tail snip DNA, as previously described (Clarke and Harline 1996). All mice were maintained ad libitum on standard laboratory chow (Formulab 5008 Rodent Chow; Ralston Purina) and tap water. The drinking water of the Dra and WT littermate mice contained 50% strength Pedialyte® to prevent dehydration (Walker et al. 2009) whereas the drinking water of the Cftr and WT littermate mice contained Colyte® laxative to prevent intestinal obstruction (Clarke et al. 1996). Mice (age 2–4 months) were fasted overnight prior to experimentation but were provided with water ad libitum. The mice were housed in the AAALAC-accredited Dalton Cardiovascular Research Center animal facility. All experiments involving animals were approved by the University of Missouri Animal Care and Use Committee.
Short-circuit current measurement
Freshly-excised proximal intestine was stripped of underlying muscle layers and mounted on voltage-clamped Ussing chambers for the measurement of short-circuit current (Isc) as previously described (Clarke and Harline 1998). The luminal and basolateral surface of the mucosa was bathed with Krebs bicarbonate Ringers (KBR) containing in mM: 140.0 Na+, 5.2 K+, 2.8 PO42−, 119.8 Cl−, 25.0 HCO3−, 1.2 Ca2+, 1.2 Mg2+, 4.8 gluconate−, 1 μM indomethacin and gassed with 95% O2: 5% CO2 at 37°C (pH 7.4). The luminal solution contained 10 mM mannitol and the basolateral solution contained 10 mM glucose and 0.1 μM tetrodotoxin (to inhibit neural activity). Following a 20 min equilibration period, 10 mM glucose, 10 mM α-D-methyl glucoside or 10 mM mannose was added to the luminal solution, balanced osmotically with 10 mM glucose addition to the basolateral solution and the change in Isc was recorded at five minute intervals.
Fluorescence Measurement of Intracellular pH and Image Analysis
The method used for imaging villus epithelial cells in intact murine intestine has been previously described (Gawenis et al. 2003;Simpson et al. 2005). Briefly, freshly-excised duodenum was stripped of the underlying muscle layers and mounted luminal side up in a horizontal Ussing-type perfusion chamber where luminal and serosal surfaces of the tissue were independently bathed. To gain access and enable rapid solution changes at the lower villus, the mucosa was gently stretched over a short 1.2 mm glass capillary piece on the underlying mesh using two 3–0 silk threads running parallel to the capillary. All tissues were treated with indomethacin (1 μM) bilaterally and tetrodotoxin (TTX, 0.1 μM) serosally to minimize the effect of endogenous prostaglandins and neural tone, respectively. The mucosal preparation was bathed on the luminal side with a Cl−-free isethionate− bicarbonate Ringer (IBR) solution containing (in mM) 140.0 Na+, 110.0 isethionate−, 25.0 HCO3−, 5.2 K+, 5.0 MES, 4.8 gluconate−, 2.8 PO42−, 1.2 Ca2+, 1.2 Mg2+, 10.0 glucose or mannose, and 6.8 mannitol that was gassed with 95% O2: 5% CO2 at 37°C (pH 7.4). The basolateral superfusate was IBR containing 10 mM glucose, 1 μM 5-(N-ethyl-n-isopropyl)-amiloride (EIPA) to block the basolateral membrane Na+/H+ exchanger Nhe1. Both solutions were gassed with 95% O2: 5% CO2 to pH 7.4 and warmed to 37°C. In some experiments, 80 mM K+ was substituted for Na+ on an equimolar basis.
After mounting in the tissue chamber, the preparations were incubated for 5 min with 100 μM DL-dithiothreitol to remove mucus followed by incubation on the luminal side with 16 μM of 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester (BCECF-AM) for 10 minutes. Using a 40x water immersion objective (Olympus, Melville, NY), 10 epithelial cells from the lower region of a single villus (approximately 10 – 15 cells vertical to a crypt mouth) were selected for ratiometric analysis. The mucosa was superfused for an additional 10 min before pHi acidification was induced by equimolar replacement of 55 mM isethionate− with Cl− in the luminal IBR. Changes in intracellular pH (pHi) were measured by the dual excitation wavelength technique (440 and 495 nm), and imaged at 535 nm emission. Ratiometric images were obtained at 20 second intervals with a Sensi-Cam digital camera (Cooke, Auburn Heights, MI) and processed using Slidebook 5.0 (Intelligent Imaging Innovations, Inc., Denver, CO). The 495:440 nm ratios were converted to pHi using a standard curve generated by the K+/nigericin technique (Thomas et al. 1979;Boyarsky et al. 1988). Intrinsic buffering capacity (βi) of duodenal villus cells was estimated by the ammonium prepulse technique and the total buffering capacity (βtotal) was calculated from the equation βtotal = βi + βHCO3− = βi + 2.3 × [HCO3−]i, where βHCO3− is the buffering capacity of the CO2/HCO3− system and [HCO3−]i is the intracellular concentration of HCO3− (Weintraub and Machen 1989). The rate of pHi change during the initial 90s period of linear ΔpH/Δt change was converted to transmembrane flux (J) of HCO3 measured in mM/min using the equation J = ΔpH/Δt × βtotal. The flux rate of HCO3− equivalents out of the cell are denoted as negative. Directionality of the Cl−/HCO3− exchange process is designated as Cl−IN/HCO3−OUT when referring the movement of Cl− into the cell across the luminal membrane in exchange for HCO3− moving out of the cell, and Cl−OUT/HCO3−IN when referring to the movement of Cl− out of the cell across the luminal membrane in exchange for HCO3− moving into the cell. To calculate the Nernst equilibrium potential for HCO3− across the luminal membrane, Veq HCO3−, the intracellular HCO3− was calculated from the pHi using the Henderson-Hasselbalch equation with a nominal extracellular HCO3− concentration of 25 mM.
Materials
The fluorescent dye BCECF acetoxymethyl ester was obtained from Invitrogen (Carlsbad, CA). Tetrodotoxin and forskolin were obtained from Biomol International L.P. (Plymouth Meeting, PA). All other materials were obtained from either Sigma Aldrich (St. Louis, MO) or Fisher Scientific (Springfield, NJ).
Statistics
All values are reported as mean ± SEM. Data between two treatment groups were compared using a 2-tailed unpaired Student t-test assuming equal variances between groups. Data from multiple treatment groups were compared using a one-way analysis of variance with a post hoc Holm-Sidak pairwise comparisons test. A probability value of p < 0.05 was considered statistically significant.
Results
Measurement of Cl−IN/HCO3−OUT exchange in the duodenal lower villus epithelium
Preliminary studies investigated the validity of the experimental design for the measurement of Cl−IN/HCO3−OUT exchange. After ~20 min incubation with luminal Cl− -free IBR, Cl−IN/HCO3−OUT exchange was initiated by the introduction of 55 mM Cl− into the luminal solution of the lower villus epithelium. To first determine whether Cl−/HCO3− exchange as opposed to Cl−/OH− exchange was measured, the duodenal mucosa was incubated in CO2/HCO3−-free IBR. As shown in Fig. 1(a), the pHi of the lower villus epithelium was unaffected by the introduction of luminal Cl− in the absence of extracellular CO2/HCO3−. In contrast, as shown in Fig. 1(b), the lower villus epithelium acidifies after introduction of luminal Cl− in the presence of extracellular CO2/HCO3−, consistent with Cl−IN/HCO3−OUT exchange (see inset).
Figure 1.
(a) Representative trace of pHi in WT duodenal lower villus epithelium before and after luminal Cl− addition in the absence of CO2/HCO3− containing solutions. (b) Representative trace of pHi in WT duodenal lower villus epithelium before and after luminal Cl− addition in the presence of CO2/HCO3− containing solutions. The dashed line indicates the slope of the initial 90 sec pHi change used to calculate the rate of Cl−IN/HCO3−OUT exchange. The luminal solution contained 10 mM mannose in both studies.
Lower villus Cl−IN/HCO3−OUT exchange in the presence of luminal glucose (10 mM)
Previous studies investigating Cl−/HCO3− exchange activity in the lower villus epithelium revealed domination of Dra activity with little contribution from Pat-1 (Walker et al. 2009). In these studies, luminal glucose (10 mM) was provided as a metabolic substrate in the luminal and basolateral baths to maintain pHi in the linear range for pH-sensitive BCECF fluorescence. However, given evidence of an electrogenic 1Cl−:2HCO3− stoichiometry for Pat-1 (Shcheynikov et al. 2006), inclusion of luminal glucose may affect Pat-1 function by activity of the Na+- coupled glucose transporter. Using the Cl−IN/HCO3−OUT exchange technique, the contribution of Pat-1 and Dra to Cl−/HCO3− exchange in the presence of luminal glucose was re-examined in the lower villus epithelium of duodena from Pat-1 KO and Dra KO mice and corresponding WT littermates. Since these experiments are initiated in the absence of extracellular [Cl−], we also calculated the equilibrium potential for HCO3− (Veq HCO3−) using pHi prior to introduction of luminal Cl− and initiation of Cl−IN/HCO3−OUT exchange. Although the electrochemical potential for HCO3− or Cl− cannot be calculated since membrane potential and [Cl−]i were not measured, the Veq HCO3− provides an evaluation of one aspect of the driving force for HCO3− movement. As shown in Fig. 2 (upper), the Veq HCO3− was near equilibrium in the absence of luminal Cl− for Dra KO, Dra WT and Pat-1 WT epithelium. However, Veq HCO3− was significantly inwardly directed in the Pat-1 KO due a more acidic pHi, suggesting that Pat-1 may provide HCO3− import to regulate pHi under luminal Cl− -free conditions. An inward Veq HCO3− is expected to reduce the availability of HCO3− for Cl−IN/HCO3−OUT exchange. Although there was a tendency for a lower Cl−IN/HCO3−OUT exchange rate in the Pat-1 KO epithelium, the rate of exchange was not significantly different from WT littermate epithelium (Fig. 2, lower). Thus, Cl−IN/HCO3−OUT exchange in the Pat-1 KO villus epithelium involves a strong contribution from Dra and possibly increased HCO3− conductance via Cftr as the membrane would be expected to hyperpolarize during luminal Cl− exposure. In contrast, Cl−IN/HCO3−OUT exchange was abolished in the Dra KO despite a more favorable Veq HCO3−, indicating a lack of contribution from Pat-1 and Cftr. Similar to previous findings (Walker et al. 2009), these studies indicate minimal contribution of Pat-1 to Cl−/HCO3− exchange in the lower villus epithelium during exposure to luminal glucose.
Figure 2.
Effect of luminal glucose (10 mM) on the HCO3− equilibrium potential across the apical membrane (Veq HCO3−, upper panel) and rates of Cl−IN/HCO3−OUT exchange (lower panel) in the lower villus epithelium of Pat-1 KO, Dra KO and WT littermate duodena. pHi before Cl− addition: Pat-1 WT 7.39 ± 0.05; Pat-1 KO 7.03 ± 0.08, Dra WT 7.28± 0.09, Dra KO 7.39 ± 0.06. *Significantly different from WT by unpaired t test, n = 6 – 8.
Lower villus Cl−/HCO3− exchange in the presence of luminal mannose (10 mM)
Mannose is a monosaccharide (C-2 epimer of glucose) that is often used as metabolic substrate in calibration studies of BCECF (Boyarsky et al. 1988). Mannose is transported across the apical membrane of intestinal epithelia primarily by electroneutral facilitated diffusion involving Glut2 (Slc2a2) (Kellet and Brot-Laroche 2005;Kellet et al. 2008), thus avoiding the effects of Na+ influx and membrane potential depolarization from Na+-coupled glucose transport. To determine the utility of mannose as a substitute for glucose, the effect of luminal mannose on pHi and electrogenic transport were evaluated. As compared to the absence of a luminal metabolic substrate (Control in Fig. 3(a)), the pHi of lower villus epithelia exposed to either luminal mannose or glucose (10 mM) was reduced to approximately the same extent and within the range of pH-sensitivity for the BCECF dye. To evaluate whether luminal mannose induces an ionic current, duodenal mucosal preparations were mounted in Ussing chambers for the measurement of short-circuit current (Isc) during acute addition of either glucose, α-D-methyl glucoside (a non-metabolizable substrate for Na+-coupled glucose cotransport(Turner and Black 2001)) or mannose (10 mM). As shown in Fig. 3(b), glucose and α-D-methyl glucoside treatments induced large inward currents as indexed by changes in the Isc (ΔIsc) whereas mannose addition had no appreciable effect on the Isc of the murine duodenum.
Figure 3.
(a) Effect of metabolic substrates glucose or mannose (10mM) in the luminal bath on pHi. Control luminal bath contained 10 mM of the non-metabolizable substrate mannitol. Note that the pHi of the Control is above the useable pH range for BCECF (dashed line; BCECF pH range = 6.5 – 8.0). a, bMeans with different letters are statistically different, n = 5 – 7. (b) Change in duodenal Isc (ΔIsc) after addition of 10 mM glucose, 10 mM α-D-methyl glucoside or 10 mM mannose to the luminal bath in Ussing chambers. ΔIsc was calculated by subtracting the pre-addition Isc from 15 min post-addition Isc. a, bMeans with different letters are statistically different by one-way ANOVA and Holm-Sidak pairwise comparisons, n = 5 – 7.
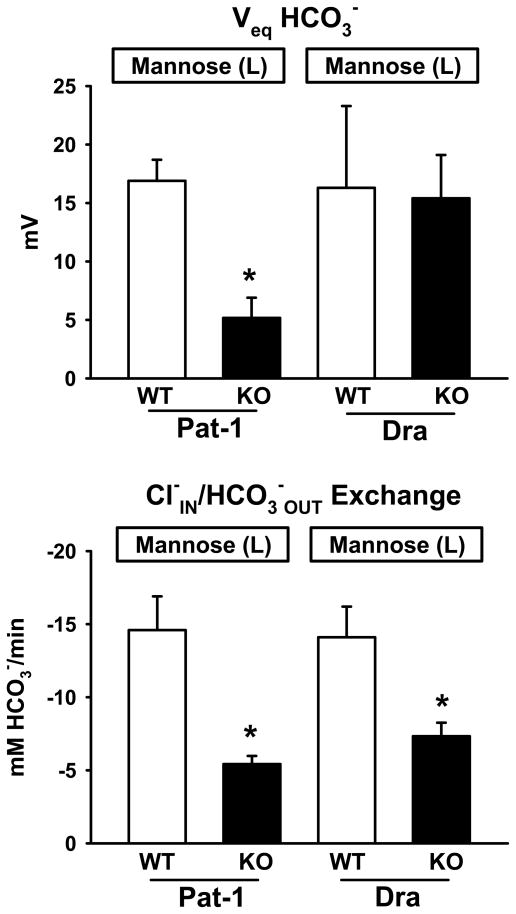
Using mannose as a luminal metabolic substrate, Veq HCO3− and rates of Cl−IN/HCO3−OUT exchange were determined in the lower villus epithelium of Pat-1 KO, Dra KO and WT littermate duodena. As shown in Fig. 4 (upper), the Veq HCO3− was outwardly directed in all duodena, suggesting that glucose uptake and metabolism significantly acidifies the intracellular milieu as compared to mannose. However, Veq HCO3− in the Pat-1 KO was again significantly less than in the WT littermate epithelium, again providing indirect evidence that Pat-1 functions to provide pHi regulation under these conditions. More direct evidence of Pat-1 activity in the lower villus is shown in Fig. 4 (lower) where the rate of Cl−IN/HCO3−OUT exchange in the Pat-1 KO is significantly reduced relative to that in the WT littermate epithelium. The rate of exchange in the Pat-1 KO exposed to luminal mannose was also significantly less than the Cl−IN/HCO3−OUT exchange rate measured during exposure to luminal glucose (p < 0.05). Since the Veq HCO3− likely contributes to the reduced Cl−IN/HCO3−OUT exchange rate in the Pat-1 KO, a better estimate of Pat-1 activity in the lower villus is shown in the Dra KO. Although the rate is reduced in the Dra KO relative to the WT, indicating Dra activity in the lower villus, the Dra KO shows a residual Cl−IN/HCO3−OUT exchange rate that is likely due to Pat-1 and a possible contribution of Cftr HCO3− conductance as the membrane hyperpolarizes during luminal Cl− exposure.
Figure 4.
Effect of luminal mannose (10 mM) on the HCO3− equilibrium potential across the apical membrane (Veq HCO3−, upper panel) and rates of Cl−IN/HCO3−OUT exchange (lower panel) in the lower villus epithelium of Pat-1 KO, Dra KO and WT littermate duodena. pHi before Cl− addition: Pat-1 WT 7.79 ± 0.07; Pat-1 KO 7.53 ± 0.03, Dra WT 7.72 ± 0.11, Dra KO 7.70 ± 0.06. *Significantly different from WT by unpaired t test, n = 6 – 8.
Lower villus Pat-1 activity in the presence of a non-metabolizable glucose substitute
To further examine the effect of luminal glucose on Veq HCO3− and Pat-1 Cl−/HCO3− exchange activity, the rates of Cl−IN/HCO3−OUT exchange were measured in Pat-1 KO and WT littermate lower villus during exposure to 10 mM α-D-methyl glucoside, a non-metabolizable substrate of Na+-coupled glucose transport(Turner and Black 2001). As shown in Fig 5 (left), the presence of 10 mM α-D-methyl glucoside together with 10 mM mannose in the luminal solution unexpectedly acidified the epithelium and induced an inward Veq HCO3−. The Veq HCO3− of WT and Pat-1 KO villus epithelium was significantly less in the presence of α-D-methyl glucoside as compared to luminal mannose alone (p < 0.05). Although the Veq HCO3− in the Pat-1 KO epithelium tended to be less than the WT during α-D-methyl glucoside incubation, it did not attain statistical significance. Nonetheless, as shown in Fig 5 (right), Pat-1 KO demonstrated a significant reduction in the rate of Cl−IN/HCO3−OUT exchange as compared to WT. The rate of Cl−IN/HCO3−OUT exchange in the Pat-1 KO exposed to luminal α-D-methyl glucoside was significantly less than that measured during exposure to luminal glucose (p < 0.05) but not different than the Cl−IN/HCO3−OUT exchange rate measured with luminal mannose alone.
Figure 5.
Effect of luminal α-D-methyl glucoside (10 mM) + mannose (10 mM) on the HCO3− equilibrium potential across the apical membrane (Veq HCO3−, left panel) and rates of Cl−IN/HCO3−OUT exchange (right panel) in the lower villus epithelium of Pat-1 KO and WT littermate duodena. pHi before Cl− addition: Pat-1 WT 7.34 ± 0.03; Pat-1 KO 7.27 ± 0.01. *Significantly different from WT by unpaired t test, n = 3.
Lower villus Pat-1 activity in the presence of high extracellular K+ concentration
The demonstrating loss of Cl−IN/HCO3−OUT exchange in the Pat-1 KO during exposure to luminal α-D-methyl glucoside suggest Pat-1 activity is unaffected by membrane depolarization, but this conclusion is complicated by an unfavorable Veq HCO3− at the initiation of Cl−IN/HCO3−OUT exchange and, possibly, changes in Dra and Cftr activities. In the Dra KO where Pat-1 activity is more isolated, exposure to luminal glucose abolished Cl−IN/HCO3−OUT exchange activity which may indicate that Cl−IN/HCO3−OUT exchange by Pat-1 is disadvantaged by offsetting effects on membrane potential by Na+-coupled glucose uptake (depolarization) and Cl− entry via Cftr (hyperpolarization), or the establishment of an unfavorable cell-to-lumen [Cl−] gradient from sustained Cl− entry. To help differentiate these factors, we measured Cl−IN/HCO3−OUT exchange in the lower villus of Dra KO mice (to isolate Pat-1) during membrane depolarization induced by exposure to 80 mM K+ in the luminal and basolateral baths. As shown in Fig 6, exposure of Dra KO villus epithelium to high extracellular [K+] in the presence of luminal mannose reduced the Veq HCO3− to near zero and abolished Cl−IN/HCO3−OUT exchange as compared to Dra KO epithelium exposed only to mannose. These changes were almost identical to the differences induced by luminal glucose (see Dra KO in Fig. 2). If Pat-1 has 1Cl−/2HCO3− exchange stoichiometry, membrane depolarization would favor Cl−OUT/HCO3−IN exchange activity and maintain Veq HCO3− near zero (compare with Pat-1 KO in Fig. 2) and disfavor Cl−IN/HCO3−OUT exchange during return of luminal Cl−. Next, the experimental design was applied to Dra/Cftr double knockout villus epithelium which should effectively isolate Pat-1 activity. Unexpectedly, an outwardly directed Veq HCO3− became apparent and Cl−IN/HCO3−OUT exchange activity was re-established, suggesting that Pat-1 in the absence of Cftr is unaffected by membrane depolarization.
Figure 6.
Effect of high extracellular [K+] (80 mM) + luminal mannose (10 mM) on the HCO3− equilibrium potential across the apical membrane (Veq HCO3−, upper panel) and rates of Cl−IN/HCO3−OUT exchange (lower panel) in the lower villus epithelium of Dra KO and Dra/Cftr double KO duodena. pHi before Cl− addition: Dra KO (5 mM K+) = 7.71 ± 0.07; Dra KO (80 mM K+) = 7.38 ± 0.06; Dra/Cftr double KO Dra KO (80 mM K+) = 7.60 ± 0.06. a, bMeans with different letters are statistically different by one-way ANOVA and Holm-Sidak pairwise comparisons, n = 3.
Discussion
The present study demonstrates functional Cl−/HCO3− exchange activity of Pat-1 in the lower villus epithelium of murine duodenum. In addition to the evidence for expression of Pat-1 in the lower villus of the duodenum (Wang et al. 2002;Walker et al. 2009), this conclusion is based on the following observations. First, in all instances where Pat-1 KO villus epithelium was examined, removal of luminal Cl− resulted in a more acidic pHi and an inwardly-directed Veq HCO3−. Reverse activity, i.e., Cl−OUT/HCO3−IN exchange, of Pat-1 would provide HCO3− loading to sustain pHi. Previous studies of Pat-1 KO mice have shown that basal pHi is acidic relative to WT littermates in the upper villus and renal proximal tubule (Simpson et al. 2007;Petrovic et al. 2008). More recently, it was shown that Pat-1 Cl−OUT/HCO3−IN exchange activity provides a HCO3− import pathway in upper villus epithelium to sustain pHi homeostasis during acid challenge by H+-dipeptide absorption (Simpson et al. 2010). These findings are consistent with a Pat-1-dependent HCO3− importing function. Second, under conditions which Veq HCO3− was near zero or outwardly directed, i.e., exposure to luminal mannose or luminal mannose plus α-D-methyl glucoside, the maximal rate of Cl−IN/HCO3−OUT exchange was reduced in the Pat-1 KO villus epithelium. Third, in the Dra KO villus, a significant residual Cl−IN/HCO3−OUT exchange was present during exposure to luminal mannose, which likely includes a contribution from Pat-1 activity. These findings are consistent with functional activity of Pat-1 in the lower villus which was apparently obscured in previous studies by the inclusion of luminal glucose for fluorescence imaging studies.
Several observations of the present study suggest that Pat-1 activity in the villus epithelium is electroneutral and therefore does not demonstrate 1Cl−/2HCO3− stoichiometry. Most studies of recombinant Slc26a6 have shown an associated electrogenic activity with anion transport which has led to the hypothesis that Pat-1 has a 1Cl−/2HCO3− stoichiometry (Xie et al. 2002). However, Chernova et al., in a comparison of murine and human Pat-1 expressed in Xenopus oocytes, did not find electrogenic Cl−/HCO3− exchange but recognized electrical activity that could be dissociated from exchange transport (Chernova et al. 2005). Moreover, transepithelial studies of intestine from Pat-1 KO mouse have not indicated significant changes in basal Isc as compared to WT littermates (Wang et al. 2005;Simpson et al. 2010). In the present study, two observations support the possibility that Pat-1 activity is electroneutral. First, as indicated by loss of Cl−/HCO3− exchange function in the Pat-1 KO, Pat-1 is active in the WT villus epithelium during luminal exposure to the non-metabolizable glucose epimer, α-D-methyl glucoside. However, α-D-methyl glucoside produces a strong inward Isc similar to luminal glucose and resultant membrane depolarization would be predicted to inhibit 1Cl−IN/2HCO3−OUT exchange in the Pat-1 WT. Secondly, and more definitively, was the novel observation of appreciable Cl−IN/HCO3−OUT exchange in the Dra/Cftr double KO epithelium during bilateral exposure to high extracellular [K+]. Pat-1 activity should be effectively isolated in the absence of Dra and Cftr in the lower villus and demonstration of an outward Veq HCO3− and appreciable rates of Cl−IN/HCO3−OUT exchange suggests that Pat-1 in isolation is not affected by membrane depolarization. This observation raises the question of why Pat-1 activity is apparently abolished in the presence of Cftr during membrane depolarization. One possibility is that Pat-1 exhibits either unidirectional or electrogenic transport in association with Cftr in the lower villus, unlike the upper villus where Cftr expression is low. Previous studies have shown that the C-terminal STAS domain of murine Pat-1 binds to and activates gating of Cftr (Ko et al. 2004), whereas studies of Pat-1 knockout mice suggest that Pat-1 may exert an inhibitory effect on Cftr in pancreatic duct epithelium (Wang et al. 2006). Thus, the interactions between Pat-1 and Cftr are likely complex but may involve conformational changes in Pat-1 that facilitate 1Cl−/2HCO3− exchange or prevent forward mode transport during membrane depolarization. Alternatively, a close association with Cftr may disfavor Cl−IN/HCO3−OUT exchange by localizing Cl− entry near Pat-1 during acute exposure to luminal Cl−, which would be enhanced by sustained membrane depolarization due to concurrent Na+-coupled glucose absorption. Future studies measuring membrane potential and intracellular [Cl−] in the villus epithelium will be necessary differentiate these possibilities.
The Cl−/HCO3− exchange capability of Pat-1 may play a physiological role in fluid absorption during non-electrogenic monosaccharide absorption. In the present study, Cl−IN/HCO3−OUT exchange by Pat-1 was operational in the villus epithelium during exposure to mannose which is primarily absorbed by electroneutral facilitated diffusion (Kellet and Brot-Laroche 2005). Recent studies have shown that Pat-1 also plays a significant role in salt and water absorption during fructose absorption by the electroneutral facilitated diffusion carrier Glut5 (Singh et al. 2008). In those studies, Pat-1-dependent fluid absorption was approximately 25% under basal conditions and approximately 67% during fructose-simulated fluid absorption in the small intestine. Although the exact physiology of this function is speculative, Pat-1 mediated fluid absorption may support efficient flow of monosaccharides absorbed by facilitated diffusion into the portal circulation. Since Pat-1 is involved in both intestinal and renal fluid absorption, high levels of dietary fructose have been shown to induce salt retention and hypertension in control but not Pat-1 KO mice (Singh et al. 2008).
In summary, the present study demonstrates functional Pat-1 Cl−/HCO3− exchange activity in the lower villus epithelium of the murine duodenum which, in previous studies, had been obscured by the inclusion of luminal glucose as a metabolic substrate. Substitution of luminal glucose with mannose provided a metabolic substrate that did not demonstrate electrogenic absorption and enabled the detection of Pat-1 Cl−IN/HCO3−OUT exchange by comparisons between Pat-1 KO and WT intestine. Since Pat-1 has been proposed to have an electrogenic 1Cl−/2HCO3− stoichiometry (Shcheynikov et al. 2006), Na+-coupled glucose absorption would be expected to depolarize the apical membrane potential and counter Pat-1 Cl−IN/HCO3−OUT exchange. However, when Pat-1 activity was isolated using Dra KO villus epithelium, membrane depolarization by exposure to high extracellular [K+] abolished Cl−IN/HCO3−OUT exchange in the presence of Cftr but not in the Dra/Cftr double KO epithelium. Thus, Pat-1 activity endogenously expressed but isolated in villus epithelium exhibited Cl−IN/HCO3−OUT exchange despite ostensibly strong membrane depolarization. In the presence of Cftr, exposure to high extracellular [K+] produced similar change in Pat-1 transport activity as demonstrated during luminal glucose exposure. These findings suggest a unique regulatory relationship between Pat-1 and Cftr in the lower villus epithelium which affects the Cl−/HCO3− exchange function of Pat-1. Of physiological significance, the present study provided additional evidence that Pat-1 contributes to pHi regulation in the villus epithelium by providing a HCO3− import pathway. Under several conditions, the absence of Pat-1 was associated with a more acidic pHi and an inwardly directed equilibrium potential for HCO3− as compared to either WT or Dra KO villus epithelia. Thus, conditions favoring enterocyte acidification such as H+-coupled peptide and amino acid absorption, or challenges from luminal acidity may be effectively buffered by the propensity for Pat-1 to operate in Cl−OUT/HCO3−IN exchange mode. A physiological role for Pat-1 Cl−/HCO3− was also suggested by its activity during monosaccharide absorption by facilitated diffusion. Similar to previous studies demonstrating a role of Pat-1 in fluid uptake during electroneutral fructose absorption via Glut5 (Singh et al. 2008), the Cl−/HCO3− exchange activity of Pat-1 was also apparent during electroneutral mannose absorption. Thus, Cl− absorption during electrogenic transport such as Na+-couple glucose absorption may be minimal but potentially has a central role during electroneutral nutrient absorption.
Acknowledgments
This study was supported by grants from the National Institutes of Health DK 48816 (to L.L.C.), T32-RR 07004 (to J.E.S.), CA-95172 (to C.W.S), DK 62809 (to M.S.) and the Cystic Fibrosis Foundation CLARKE05P0 (to L.L.C.)
Footnotes
Conflict of interest
The authors have no conflicts of interest to disclose.
References
- 1.Allen A, Flemstrom G, Garner A, Kivilaakso E. Gastroduodenal mucosal protection. Physiol Rev. 1993;73:823–857. doi: 10.1152/physrev.1993.73.4.823. [DOI] [PubMed] [Google Scholar]
- 2.Ameen NA, Alexis J, Salas P. Cellular localization of the cystic fibrosis transmembrane conductance regulator in mouse intestinal tract. Histochem Cell Biol. 2000;114:69–75. doi: 10.1007/s004180000164. [DOI] [PubMed] [Google Scholar]
- 3.Boyarsky G, Ganz MB, Sterzel RB, Boron WF. pH regulation in single glomerular mesangial cells I. Acid extrusion in absence and presence of HCO3−. Am J Physiol. 1988;255:C844–C856. doi: 10.1152/ajpcell.1988.255.6.C844. [DOI] [PubMed] [Google Scholar]
- 4.Chernova MN, Jiang L, Friedman DJ, Darman RB, Lohi H, Kere J, Vandorpe DH, Alper SL. Functional comparison of mouse slc26a6 anion exchanger with human SLC26A6 polypeptide variants: differences in anion selectivity, regulation, and electrogenicity. J Bio Chem. 2005;280:8564–8580. doi: 10.1074/jbc.M411703200. [DOI] [PubMed] [Google Scholar]
- 5.Clarke LL, Gawenis LR, Franklin CL, Harline MC. Increased survival of CFTR knockout mice using an oral osmotic laxative. Lab Animal Sci. 1996;46:612–618. [PubMed] [Google Scholar]
- 6.Clarke LL, Harline MC. CFTR is required for cAMP inhibition of intestinal Na+ absorption in a cystic fibrosis mouse model. Am J Physiol. 1996;270:G259–G267. doi: 10.1152/ajpgi.1996.270.2.G259. [DOI] [PubMed] [Google Scholar]
- 7.Clarke LL, Harline MC. Dual role of CFTR in cAMP-stimulated HCO3− secretion across murine duodenum. Am J Physiol. 1998;274:G718–G726. doi: 10.1152/ajpgi.1998.274.4.G718. [DOI] [PubMed] [Google Scholar]
- 8.Flemstrom G. Gastric and duodenal mucosal secretion of bicarbonate. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1994. pp. 1285–1309. [Google Scholar]
- 9.Gawenis LR, Franklin CL, Simpson JE, Palmer BA, Walker NM, Wiggins TM, Clarke LL. cAMP inhibition of murine intestinal Na+/H+ exchange requires CFTR-mediated cell shrinkage of villus epithelium. Gastroenterology. 2003;125:1148–1163. doi: 10.1016/s0016-5085(03)01212-5. [DOI] [PubMed] [Google Scholar]
- 10.Hogan DL, Crombie DL, Isenberg JI, Svendsen P, Schaffalitzky de Muckadell OB, Ainsworth MA. CFTR mediates cAMP- and Ca2+-activated duodenal epithelial HCO3− secretion. Am J Physiol. 1997;272:G872–G878. doi: 10.1152/ajpgi.1997.272.4.G872. [DOI] [PubMed] [Google Scholar]
- 11.Horack RZ, Simpson JE, Walker NM, Clarke LL. Evidence against electrogenicity of Slc26a3 (down-regulated in adenoma, Dra) Cl−/HCO3− exchange in murine large intestine. Gastroenterology. 2008;134:A108. [Google Scholar]
- 12.Jacob P, Rossmann H, Lamprecht G, Kretz A, Neff C, Lin-Wu E, Gregor M, Groneberg DA, Kere J, Seidler U. Down-regulated in adenoma mediates apical Cl−/HCO3− exchange in rabbit, rat, and human duodenum. Gastroenterology. 2002;122:709–724. doi: 10.1053/gast.2002.31875. [DOI] [PubMed] [Google Scholar]
- 13.Jiang Z, Grichtchenko II, Boron WF, Aronson PS. Specificity of anion exchange mediated by mouse Slc26a6. J Biol Chem. 2002;277:33963–33967. doi: 10.1074/jbc.M202660200. [DOI] [PubMed] [Google Scholar]
- 14.Kellet G, Brot-Laroche E. Apical GLUT2: a major pathway of intestinal sugar absorption. Diabetes. 2005;54:3056–3062. doi: 10.2337/diabetes.54.10.3056. [DOI] [PubMed] [Google Scholar]
- 15.Kellet G, Brot-Laroche E, Mace O, Leturque A. Sugar absorption in the intestine: the role of GLUT2. Annu Rev Nutr. 2008;28:35–54. doi: 10.1146/annurev.nutr.28.061807.155518. [DOI] [PubMed] [Google Scholar]
- 16.Ko SBH, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, Muallem S. Gating of CFTR by the STAS domain of SLC26 transporters. Nature Cell Biol. 2004;6:343–350. doi: 10.1038/ncb1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamprecht G, Baisch S, Schoenleber E, Gregor M. Transport properties of the human intestinal anion exchanger DRA (down-regulated in adenoma) in transfected HEK293 cells. Pfluegers Arch. 2005;449:479–490. doi: 10.1007/s00424-004-1342-x. [DOI] [PubMed] [Google Scholar]
- 18.Melvin JE, Park K, Richardson L, Schultheis PJ, Shull GE. Mouse down-regulated in adenoma (DRA) is an intestinal Cl−/HCO3− exchanger and is up-regulated in colon of mice lacking the NHE3 Na+/H+ exchanger. J Biol Chem. 1999;274:22855–22861. doi: 10.1074/jbc.274.32.22855. [DOI] [PubMed] [Google Scholar]
- 19.Park HW, Nam JH, Kim JY, Namkung W, Yoon JS, Lee JS, Kim KS, Venglovecz V, Gray MA, Kim KH, Lee MG. Dynamic regulation of CFTR bicarbonate permeability by [Cl−]i and its role in pancreatic bicarbonate secretion. Gastroenterology. 2010;139:620–631. doi: 10.1053/j.gastro.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Petrovic S, Barone S, Wang Z, McDonough AA, Amlal H, Soleimani M. Slc26a6 (PAT1) deletion downregulates the apical Na+/H+ exchanger in the straight segment of the proximal tubule. Am J Nephrol. 2008;28:330–338. doi: 10.1159/000111826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poulsen JH, Fischer H, Illek B, Machen TE. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci USA. 1994;91:5340–5344. doi: 10.1073/pnas.91.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schweinfest CW, Spyropoulos DD, Henderson KW, Kim JH, Chapman JM, Barone S, Worrell RT, Wang Z, Soleimani M. slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J Biol Chem. 2006;281:37962–37971. doi: 10.1074/jbc.M607527200. [DOI] [PubMed] [Google Scholar]
- 23.Seidler U, Blumenstein I, Kretz A, Viellard-Baron D, Rossmann H, Colledge WH, Evans M, Ratcliff R, Gregor M. A functional CFTR protein is required for mouse intestinal cAMP-,cGMP- and Ca2+-dependent HCO3− secretion. J Physiol (Lond ) 1997;505:411–423. doi: 10.1111/j.1469-7793.1997.411bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shcheynikov N, Wang Y, Park M, KO SBH, Dorwart M, Naruse S, Thomas PJ, Muallem S. Coupling modes and stoichiometry of Cl−/HCO3− exchange by slc26a3 and slc26a6. J Gen Physiol. 2006;127:511–524. doi: 10.1085/jgp.200509392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simpson JE, Gawenis LR, Walker NM, Boyle KT, Clarke LL. Chloride conductance of CFTR facilitates Cl−/HCO3− exchange in the villous epithelium of intact murine duodenum. Am J Physiol. 2005;288:1241–1251. doi: 10.1152/ajpgi.00493.2004. [DOI] [PubMed] [Google Scholar]
- 26.Simpson JE, Schweinfest CW, Shull GE, Gawenis LR, Walker NM, Boyle KT, Soleimani M, Clarke LL. PAT-1 (Slc26a6) is the predominant apical membrane Cl−/HCO3− exchanger in the upper villous epithelium of the murine duodenum. Am J Physiol. 2007;292:G1079–G1088. doi: 10.1152/ajpgi.00354.2006. [DOI] [PubMed] [Google Scholar]
- 27.Simpson JE, Walker NM, Supuran CT, Soleimani M, Clarke LL. Putative anion transporter-1 (Pat-1, Slc26a6) contributes to intracellular pH regulation during H+-dipeptide transport in the duodenal villous epithelium. Am J Physiol. 2010;298:G683–G691. doi: 10.1152/ajpgi.00293.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh AK, Amlal H, Haas PJ, Dringenberg U, Fussell S, Barone SL, Engelhardt R, Zuo J, Seidler U, Soleimani M. Fructose-induced hypertension: essential role of chloride and fructose absorbing transporters PAT1 and Glut5. Kidney Int. 2008;74:438–447. doi: 10.1038/ki.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, Koller BH. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- 30.Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979;18:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- 31.Tuo B, Riederer B, Wang Z, Colledge WH, Soleimani M, Seidler U. Involvement of the anion exchanger Slc26a6 in PGE2- but not forskolin-stimulated murine duodenal HCO3 secretion. Gastroenterology. 2006;130:349–358. doi: 10.1053/j.gastro.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 32.Turner JR, Black ED. NHE3-dependent cytoplasmic alkalinization is triggered by Na+-glucose cotransport in intestinal epithelia. Am J Physiol. 2001;281:C1533–C1541. doi: 10.1152/ajpcell.2001.281.5.C1533. [DOI] [PubMed] [Google Scholar]
- 33.Walker NM, Simpson JE, Brazill JM, Gill RK, Dudeja PK, Schweinfest CW, Clarke LL. Role of down-regulated in adenoma anion exchanger in HCO3− secretion across murine duodenum. Gastroenterology. 2009;136:893–901. doi: 10.1053/j.gastro.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Soyombo AA, Shcheynikov N, Zeng W, Dorwart M, Marino CR, Thomas PJ, Muallem S. Slc26a6 regulates CFTR activity in vivo to determine pancreatic duct HCO3− secretion: relevance to cystic fibrosis. Embo J. 2006;25:5049–5057. doi: 10.1038/sj.emboj.7601387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z, Wang T, Petrovic S, Tuo B, Riederer B, Barone S, Lorenz JN, Seidler U, Aronson PS, Soleimani M. Renal and intestine transport defects in Slc26a6-null mice. Am J Physiol. 2005;288:C957–C965. doi: 10.1152/ajpcell.00505.2004. [DOI] [PubMed] [Google Scholar]
- 36.Wang ZH, Petrovic S, Mann E, Soleimani M. Identification of an apical Cl−/HCO3− exchanger in the small intestine. Am J Physiol. 2002;282:G573–G579. doi: 10.1152/ajpgi.00338.2001. [DOI] [PubMed] [Google Scholar]
- 37.Weintraub WH, Machen TE. pH regulation in hepatoma cells: roles for Na-H exchange, Cl-HCO3 exchange, and Na-HCO3 cotransport. Am J Physiol. 1989;257:G317–G327. doi: 10.1152/ajpgi.1989.257.3.G317. [DOI] [PubMed] [Google Scholar]
- 38.Wright EM, Hirayama BA, Loo DD, Turk E, Hager K. Intestinal Sugar Transport. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1994. pp. 1751–1772. [Google Scholar]
- 39.Xie Q, Welch R, Mercado A, Romero MF, Mount DB. Molecular characterization of the murine Slc26a6 anion exchanger: functional comparison with Slc26a1. Am J Physiol. 2002;283:F826–F838. doi: 10.1152/ajprenal.00079.2002. [DOI] [PubMed] [Google Scholar]