Abstract
Possible functional roles for glutamate that is detectable at low concentrations in the extracellular space of intact brain and brain slices have not been explored. To determine whether this endogenous glutamate acts on metabotropic glutamate receptors (mGluRs), we obtained whole cell recordings from layer V pyramidal neurons of rat sensorimotor cortical slices. Blockade of mGluRs with (+)-α-amino-4-carboxy-α-methyl-benzeacetic acid (MCPG, a general mGluR antagonist) increased the mean amplitude of spontaneous excitatory postsynaptic currents (sEPSCs), an effect attributable to a selective increase in the occurrence of large amplitude sEPSCs. 2S-2-amino-2-(1S,2S-2-carboxycyclopropyl-1-yl)-3-(xanth-9-yl)propanoic acid (LY341495, a group II antagonist) increased, but R(−)-1-amino-2,3-dihydro-1H-indene-1,5-dicarboxylic acid (AIDA) and (RS)-hexyl-HIBO (group I antagonists) decreased sEPSC amplitude, and (R, S)-α-cyclopropyl-4-phosphonophenylglycine (CPPG, a group III antagonist) did not change it. The change in sEPSCs elicited by MCPG, AIDA, and LY341495 was absent in tetrodotoxin, suggesting that it was action potential-dependent. The increase in sEPSCs persisted in GABA receptor antagonists, indicating that it was not due to effects on inhibitory interneurons. AIDA and (S)-3,5-dihy-droxyphenylglycine (DHPG, a group I agonist) elicited positive and negative shifts in holding current, respectively. LY341495 and (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine (DCG-IV, a group II agonist) elicited negative and positive shifts in holding current, respectively. The AIDA and LY341495 elicited currents persisted in TTX. Finally, in current clamp, LY341495 depolarized cells by ~2 mV and increased the number of action potentials to a given depolarizing current pulse. Thus ambient levels of glutamate tonically activate mGluRs and regulate cortical excitability.
INTRODUCTION
Glutamate is rapidly released from presynaptic terminals and exerts its action on both ionotropic and metabotropic glutamate receptors, whose function has been carefully defined over many years (Anwyl 1999; Collingridge and Lester 1989; Conn and Pin 1997). In addition to this clearly demonstrated fast, precise, and well-controlled release, there is abundant evidence for a persistent low-level baseline concentration of glutamate (0.5–2 μM/L) in the brain in vivo (Meldrum 2000) and in perfusate from unstimulated brain slices (Bianchi et al. 1999; Kapetanovic et al. 1994). Although much is known about glutamate released during synaptic transmission, the function of steady-state low, but detectable, levels of glutamate in brain remains relatively unexplored. Of particular interest in this regard are metabotropic glutamate receptors (mGluRs), which are coupled to G-proteins and have the ability to respond to low concentrations of glutamate and modulate neuronal excitability. These mGluRs, which are present in neocortex, are divided into three major groups (I–III) on the basis of sequence homology and agonist affinity, and they have a wide variety of effects on neuronal communication and cellular excitability (Anwyl 1999; Conn and Pin 1997; Meldrum 2000).
The present set of experiments was undertaken to determine whether the baseline extracellular levels of glutamate in unstimulated neocortical slices, maintained under standard conditions, might tonically activate mGluRs, and modulate spontaneous synaptic transmission. Portions of this work have appeared in abstract form (Bandrowski et al. 2002).
METHODS
Brain slices were prepared from 13- to 20-day-old male and female Sprague-Dawley rats. Animals were anesthetized with pentobarbital (50 mg/kg), and their brains rapidly removed and placed in cold sucrose-artificial cerebrospinal fluid (ACSF) containing (in mM) 230 sucrose, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 0.5 CaCl2, 10 MgSO4, and 10 glucose. This “cutting solution” was gassed with 95% O2-5% CO2 and had a pH of ~7.4. Coronal slices, 300 μm in thickness, were obtained from sensorimotor cortex (Zilles et al. 1980) between the rostrocaudal landmarks of the anterior commissure and anterior hippocampus, using a TPI vibratome (St. Louis, MO). Slices were sequentially transferred into half sucrose-ACSF and then a standard ACSF solution containing (in mM) 126 NaCl, 3 KCl, 1.25 NaH2PO4, 26 NaHCO3, 2 CaCl2, 2 MgSO4, and 10 glucose, that had an osmolarity of 298 –302 mosmol and a pH of ~7.4 when gassed with 95% O2-5% CO2. After ~1 h of equilibration at 32°C in an interface holding chamber, temperature was allowed to return to ~24°C, and slices were transferred into a recording chamber where they were minimally submerged, perfused with ACSF at a rate of 1.5–2 ml/min, and maintained at 32°C. Typically, a light band ~550 – 650 μm from the pial surface, corresponding to superficial layer V, was identified using the ×2.5 objective of a Zeiss Axioskop (Carl Zeiss, Thornwood, NY; Fig. 1A), and unless otherwise stated, recordings were made at that location. Pyramidal cells were visualized with a ×60 water-immersion objective and identified as neurons with a single emerging apical dendrite extending toward the pial surface (Fig. 1A). A few such neurons were filled with biocytin (5% in the pipette solution), and the processed sections contained labeled typical layer V pyramidal neurons (see processing methods in Horikawa and Armstrong 1988; Tseng et al. 1991).
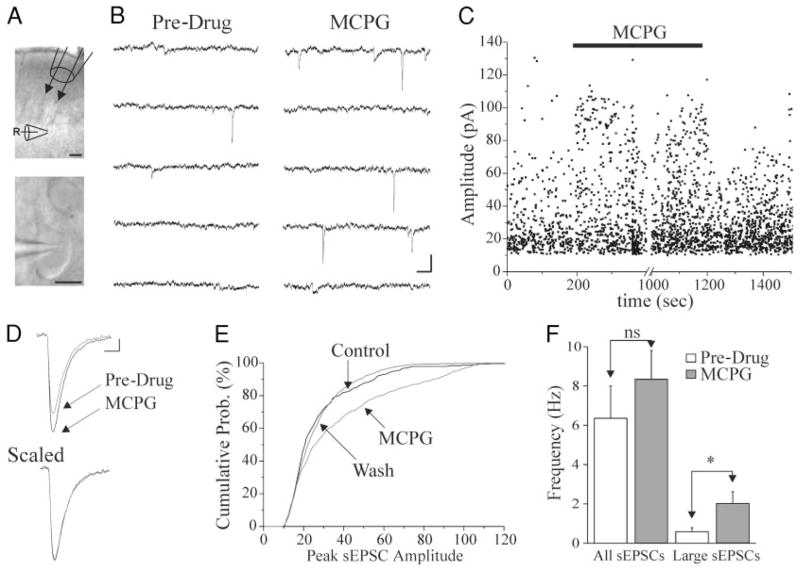
FIG. 1.
A: images obtained from a 300- μm-thick slice of sensorimotor cortex in the recording chamber (pial surface up). Top: light band lies in upper layer V where recording electrodes (R) were placed. The local perfusion pipette (top right) was positioned between layers I and III, in most experiments, so that flow of perfusate would encompass the area of the recording pipette. Calibration: 100 μm. Bottom: image shows a typical superficial layer V pyramidal cell soma and the proximal portion of the apical dendrite extending toward the pial surface. Patch pipette from which recordings were made extends from left side. Calibration: 10 μm. B: 6 s of continuous recording taken before drug application (pre-drug), at the 100-s marker in C, and in (+)-α-amino-4-carboxy-α-methyl-benzeacetic acid (MCPG, 0.2 mM), at the 300-s marker in C. Calibration: 40 pA, 50 ms. C: the peak amplitudes of sEPSCs are plotted vs. time for the cell of B. Each point represents a spontaneous excitatory postsynaptic current (sEPSC). After a 200-s pre-drug period, MCPG (0.2 mM) was superfused through the local perfusion system (■). MCPG perfusion elicits a reversible increase in large amplitude sEPSCs. D, top: MCPG perfusion (0.2 mM) increases mean sEPSC amplitude. Pre-Drug and MCPG traces are averages of 48 and 54 type 1 (see METHODS) sEPSCs, respectively, obtained during 100-s periods. Calibration: 5 pA, 3 ms. Bottom: averaged sEPSCs of A are scaled to the same amplitude to show that events Pre-Drug and in MCPG do not differ in rise times or decay time constants. E: a cumulative probability plot of sEPSC amplitudes from same cell. Median sEPSC amplitude was larger in MCPG than control (K–S: P < 0.01). F: graph of the mean frequency of sEPSCs in control (Pre) and MCPG-containing solutions (MCPG) in 15 cells. All sEPSCs, all detectible events (see METHODS). Large sEPSCs: events with peak amplitude >25 pA. *: P < 0.05. ns: P > 0.05.
Micropipettes for whole cell recording were pulled from borosilicate glass capillary tubes (ID: 0.84 mm, OD: 1.5 mm; WPI, Sarasota, FL). Pipettes had tip diameters of ~3 μm and were filled with a solution containing (in mM) 120 K+ gluconate, 10 KCl, 1 MgCl2, 1 CaCl2, 10 ethylene glycol-bis(beta-aminoethylether)-N, N, N′, N′-tetraacetic acid (EGTA), 10 N-2 hydroxyethylpiperazine-N′-2 ethane-sulfonic acid (HEPES), 3 ATP, and 0.2 GTP, pH adjusted to pH 7.3 with KOH (1.0 M). Final osmolarity was 278–292 mosM, and DC resistances were 1.5–3 MΩ. Series resistance (Ra) was monitored every 1–5 min by applying brief voltage steps, and data from a given cell were discarded if changes of >15% were detected during the recording, or if values exceeded 17 MΩ. Membrane currents and potentials were monitored and stored on a PC using Clampex8 software that interfaced with an Axopatch 200 amplifier via the Digidata 1322A (Axon Instruments, Union City, CA). Clampex8 software was programmed for stimulus delivery and data collection. The Axopatch 200 low-pass filter setting was 2 kHz, and the digitization rate was 5 kHz.
The following pharmacological agents were used: from Sigma/RBI (St Louis, MO): R(−)-1-amino-2,3-dihydro-1H-indene-1,5-dicar-boxylic acid (AIDA), 2-amino-5-phosphonovaleric acid (APV), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), (+)-α-amino-4-car-boxy-α-methyl-benzeacetic acid (MCPG), 6-imino-3-(4-methoxy-phenyl)-1(6H)-pyridazinebutanoic acid (SR 95531 hydrobromide or gabazine); from Tocris (Ellisville, MO): 2S-2-amino-2-(1S,2S-2-carboxycyclopropyl-1-yl)-3-(xanth-9-yl)propanoic acid (LY341495), (R, S)-α-cyclopropyl-4-phosphonophenylglycine (CPPG), (S)-3,5-di-hydroxyphenylglycine (DHPG), (2S,2′R,3′R)-2-(2′,3′-dicarboxycy-clopropyl)glycine (DCG IV); from Almone Labs: tetrodotoxin (TTX); from CGP: CGP55845. (RS)-hexyl-HIBO (HIBO) was generously donated by Dr. Ulf Madsen (compound 9 in Madsen et al. 2001). These drugs were prepared as a concentrated stock solution in distilled water, 0.1 M NaOH, solution (for MCPG, AIDA, HIBO, and LY341495) or a 50% water-50% DMSO solution (for CNQX) and diluted to final concentration in ACSF. Drugs were stored in aliquots, each of which was used only once to avoid loss of potency by the freeze-thaw process. Drugs were either added to the bathing medium (TTX, CNQX, APV, and gabazine) or applied locally to the slice using a perfusion pipette placed ~500 μm from the recording pipette (see also Sun et al. 2001). For local perfusion experiments, typically two barrels of the pipette were filled, one with ACSF and the other with the desired drug dissolved in ACSF. The area of the slice containing the recorded cell was perfused with a stream of ACSF from one barrel in the control and wash conditions, and with a stream of drug-containing ACSF from the second barrel for drug conditions. The time to “wash on” the drug was typically 1– 4 s, with some variation due to placement of perfusion pipette.
Spontaneous excitatory postsynaptic currents (sEPSCs) were analyzed using detector software (Ulrich and Huguenard 1996). They had average amplitudes of 9–27 pA, or about two to four times the baseline noise level of 2–8 pA, and were divided by the software into three types of events. Rise times, decay constants, amplitudes, and time of occurrence were recorded for each event. Type 1 sEPSCs were those that were not preceded or followed by another sEPSC within 20 ms, whereas types 2 and 3 sEPSCs were components of compound sEPSCs in which one event was closely preceded or followed by another. Holding potential (Vh), and membrane potential (Vm) measurements were made using Clampfit Software (Axon Instruments) by sampling a point every 10 s for the duration of data collection. Data were pooled, and averages were determined using Microsoft Excel. Graphs were generated using Microcal Origin software. Data are presented as means ± SE. Differences between means were evaluated using the t-test for unpaired samples, or where appropriate, t-test for paired samples. Statistical significance was set at P < 0.05.
RESULTS
We recorded from 104 visually identified superficial layer V pyramidal cells. Neurons included in the present data set had an Ra < 17 MΩs (mean: 11.7 ± 0.4 MΩ), input resistance of 58.6 ± 2.6 MΩ, and resting membrane potential of −60.4 ± 0.6 mV.
mGluR antagonism increases the amplitude of sEPSCs
To determine whether mGluRs were activated in unstimulated slices of neocortex, we applied MCPG, a general antagonist of mGluRs, and monitored ongoing spontaneous synaptic activity. sEPSCs had 10–90% rise times of 0.85 ± 0.09 ms, weighted decay time constants (τD, W) of 4.6 ± 0.4 ms, and were blocked by perfusion of APV/CNQX. Application of MCPG to 15 cells gave rise to a significant increase in sEPSC amplitude of 18.0 ± 5.6% (P < 0.01) that lasted for the duration of MCPG application (Fig. 1, B–E). This effect was reversible, but variable, ranging from a 0 to 56% increase. These data suggest that mGluRs are tonically activated in unstimulated neocortical slices, where they reduce sEPSC amplitudes in a significant proportion of neurons. Analysis of rise times and weighted decay time constants (τD, W) in responsive cells during MCPG application revealed no detectable change from control. Figure 1D shows an average of all type 1 events (see METHODS) obtained from one neuron during 100-s periods before (48 events) and during MCPG perfusion (54 events). Although sEPSCs increased in amplitude, no difference in rise or decay times was present when mean sEPSCs were scaled to the same amplitude (Fig. 1D). To determine whether all events were larger or whether larger amplitude events were more numerous, cumulative probability plots were generated. These cumulative probability distributions revealed that MCPG mainly increased the largest ~40% of events (Fig. 1E). Analysis of the frequency of sEPSCs revealed no significant change between pre-drug and MCPG conditions (Fig. 1F, all sEPSCs). However, when only events >25 pA were examined, a significant increase in sEPSC frequency was present in MCPG (P < 0.01; Fig. 1F, large sEPSCs). Thus MCPG selectively increases the frequency of larger-amplitude events. To test for nonspecific pH or osmolarity effects, we locally perfused ACSF containing 1.5 mM NaOH and found that this concentration NaOH, equivalent to that in local MCPG-containing perfusate, had no effect on the amplitude of sEPSCs (96.3 ± 4.6%, P > 0.05, n = 3, not shown).
Group II, but not I or III, mGluRs increase sEPSC amplitude
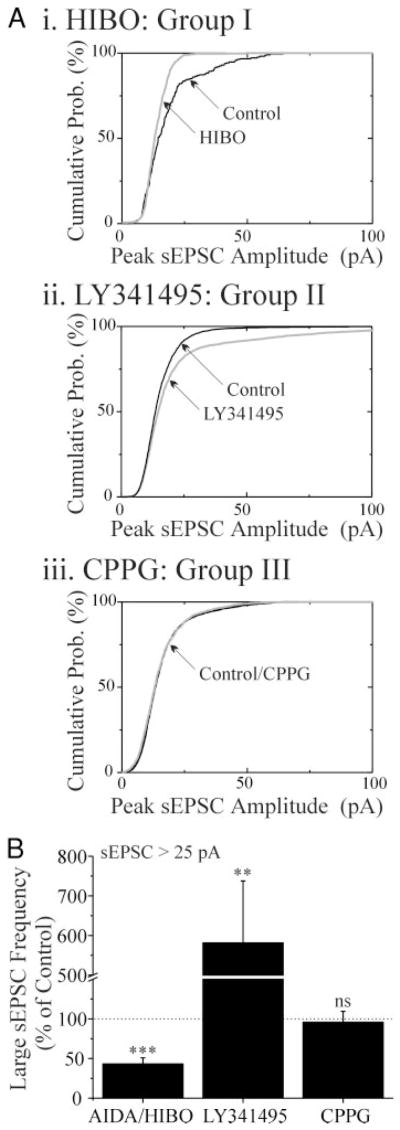
To determine which of the subtypes of mGluRs are responsible for the modulation of sEPSCs, we tested several mGluR group-specific antagonists. The effect of MCPG was mimicked by LY341495 (a group II antagonist), but HIBO and AIDA (group I antagonists) decreased sEPSC amplitude and CPPG (a group III antagonist) did not affect sEPSCs (Fig. 2). The effect of the specific group II mGluR antagonist, LY341495 (2 μM) was larger and less variable than that of MCPG, in that it increased sEPSC amplitude by an average of 42.0 ± 10.3% (P < 0.05, n = 11), as opposed to the mean 18% increase seen in MCPG. Individual cumulative probability plots show that, like MCPG, the change in amplitude in LY341495 was predominantly due to an increase in the number of large events with very little effect on the small sEPSCs (Fig. 3Aii). The effects of group I mGluR antagonists were examined in 14 neurons. AIDA (1mM) decreased sEPSC amplitude by 27.5 ± 6.0% (P < 0.05, n = 9; representative cell in Fig. 2A, top) and HIBO (200 μM) reduced sEPSC amplitude by 28.7 ± 11.8% (P < 0.05, n = 5; P > 0.05 AIDA vs. HIBO). The pooled data of Fig. 2B show the effects of AIDA and HIBO on sEPSC amplitude. Individual cumulative probability plots showed that the change in sEPSC amplitude in AIDA and HIBO was predominantly due to a decrease in the number of large events (Fig. 3Ai). Effects of MCPG, AIDA, and LY341495 were reversible during the washout period (amplitude: MCPG 95.4 ± 3.0%; AIDA 100.9 ± 20.0%; and LY341495 103.2 ± 4.6% of control levels following wash, P > 0.05 for all). These data suggest that the more modest effect of MCPG described in the preceding text may have resulted from a simultaneous increase of sEPSC amplitude due to antagonism of group II mGluRs, together with a decrease via blockade of group I mGluRs. The group III mGluR antagonist, CPPG (100 μM), was ineffective in changing amplitude or frequency of sEPSCs (n = 7, Figs. 2 and 3). These data indicate that mGluRs of groups I and II are activated in unstimulated slices and have a role in modulating sEPSCs.
FIG. 2.
Blockade of group II, but not group I or III mGluRs increases sEPSC amplitude. A: graphs of sEPSC amplitude vs. time for 3 cells, each exposed to an antagonist of a different metabotropic glutamate receptor (mGluR) group. After a 400-s pre-drug period, R(−)-1-amino-2,3-dihydro-1H-indene-1,5-dicarboxylic acid (AIDA, group I; 1 mM), 2S-2-amino-2-(1S,2S-2-carboxycyclopropyl-1-yl)-3-(xanth-9-yl)propanoic acid (LY341495, group II; 2 μM), and (R, S)-α-cyclopropyl-4-phosphonophenylglycine (CPPG, group III; 0.1 mM) were perfused for 400–500 s (□), followed by washout. ●, a single sEPSC. B: normalized mean sEPSC amplitude for groups of neurons exposed to mGluR antagonists. MCPG increased amplitude of sEPSCs (n = 15, P < 0.01); AIDA and (RS)-hexyl-HIBO (HIBO) decreased sEPSC amplitude (n = 14, results of 9 cells in AIDA and 5 cells in HIBO pooled, P < 0.001); LY341495 increased sEPSC amplitude (n = 11, P <0.01), while CPPG had no effect (−1.4 ± 4.4%, n = 7, P > 0.05). For wash values, see text.
FIG. 3.
Antagonism of group I or II mGluRs changes the frequency of large-amplitude sEPSCs. A: cumulative probability plot of sEPSC amplitudes from 3 neurons. Compared with the control conditions, there were fewer large (>25 pA) sEPSCs in the presence of HIBO (i), more large sEPSCs in the presence of LY341495 (ii), and no significant change in the number of large sEPSCs in the presence of CPPG (iii). Light lines: drug application; dark lines: control. B: graph showing the change in frequency of sEPSCs >25 pA in AIDA/HIBO (pooled data), LY341495, and CPPG. Probability values: **, P < 0.01; ***, P < 0.001; ns, P > 0.05.
Increase in sEPSCs is not due to disinhibition of pyramidal neurons
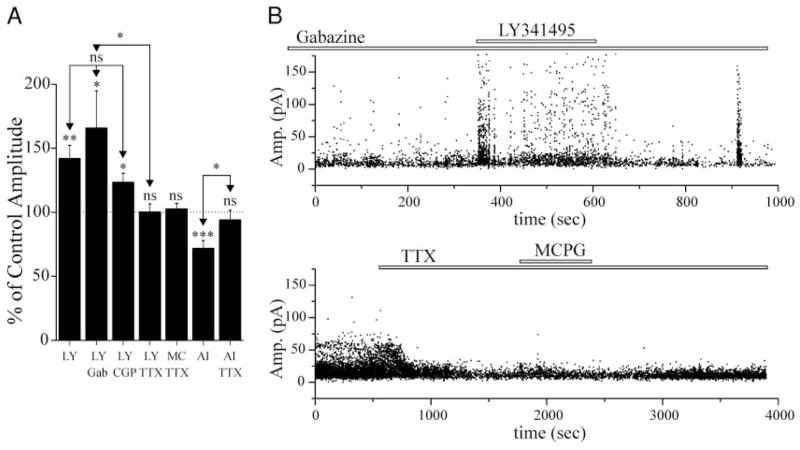
It has been shown that fast-spiking (GABAergic) cells can be depolarized and generate rhythmic activity when exposed to mGluR agonists (Boddeke et al. 1997; McBain et al. 1994; Whittington et al. 1995). Thus inactivation of mGluRs might result in hyperpolarization of these neurons, a reduction in their activity, and a secondary increase in the activity of downstream pyramidal cells. If the effects of mGluR antagonists on sEPSCs in pyramidal cells are indirect, i.e., due to disinhibition, the actions of these agents should be occluded by GABAA and GABAB antagonists. In the presence of the GABAA receptor antagonist gabazine (8 μM), which should block both phasic and tonic GABAA receptor-mediated inhibition (Stell and Mody 2002), LY341495 (2 μM) produced a significant (P < 0.05) increase in sEPSC amplitude in eight of eight cells (Fig. 4). This response was generally similar to that seen with perfusion of LY341495 alone (cf. Figs. 2A and 4B) with the exception that spontaneous bursts of EPSCs occasionally occurred, probably representing spontaneous epileptiform events (e.g., Fig. 4B, ~900 s). A similar significant effect of LY341495 was seen in seven of eight cells exposed to the GABAB receptor antagonist CGP 55845 (0.2–1 μM) (Blake et al. 1993). Therefore it is unlikely that mGluR actions on GABAergic cells are necessary for the observed increase in sEPSC amplitude.
FIG. 4.
Changes in sEPSC amplitude elicited by mGluR antagonists require action potential generation, but not involvement of GABAergic cells. A: graph showing the effect of LY341495 (2 μM), MCPG (100–200 μM), or AIDA (1 mM) on sEPSC amplitude under several conditions. The enhancement of sEPSC amplitude by LY341495 was not affected by concomitant exposure of slices to antagonists for either GABAA (gabazine, 8 μM, n = 8), or GABAB receptors (CGP55845, 0.2–1 μM, n = 8). LY341495, MCPG, or AIDA perfusion had no significant effect on sEPSCs in the presence of 1–2 μM TTX (LY341495: n = 6; MCPG: n = 7; AIDA: n = 6). *, a significant increase in sEPSC amplitude compared with control (P < 0.05). The effects of LY341495 alone on sEPSC amplitude were not significantly different from those obtained in gabazine or CGP55845. LY, LY341495; MC, MCPG; AI, AIDA; Gab, gabazine; CGP, CGP55845. B: graphs of sEPSC amplitude vs. time for 2 cells included in the bar graph of A. Top: cell was exposed to gabazine (8 μM) for >15 min before application of LY341495 (2 μM). Note, the burst of EPSCs at ~900 s, is likely due to a spontaneous epileptiform event. Bottom: cell exposed to TTX (2 μM), followed by MCPG (0.2 mM) ~1,500s later. ●, a single sEPSC.
Changes in sEPSC amplitude do not occur in TTX-containing solutions
Because mean action potential-dependent EPSCs may be larger in amplitude than miniature EPSCs (Berretta and Jones 1996), a change in presynaptic firing could account for the above effects of LY341495 and AIDA. When TTX (1–3 μM) was included in the bathing medium to block action potentials, neither MCPG (n = 7), AIDA (n = 6), nor LY341495 (n = 6) induced a change in sEPSC amplitudes (P > 0.05, Fig. 4A). Furthermore, in four cells where MCPG elicited an average increase in sEPSC amplitude of 31.1 ± 16.4%, TTX treatment during the MCPG exposure reduced the sEPSCs by 25.8 ± 8.8% (P < 0.05). Therefore the changes in the number of large events seen after block of group I or II mGluRs likely result from action-potential-evoked EPSCs. These data suggest that spike firing in at least some spontaneously active cells is apparently modified under resting conditions by activation of group I and II mGluRs.
Holding current and membrane potential are changed by mGluRs
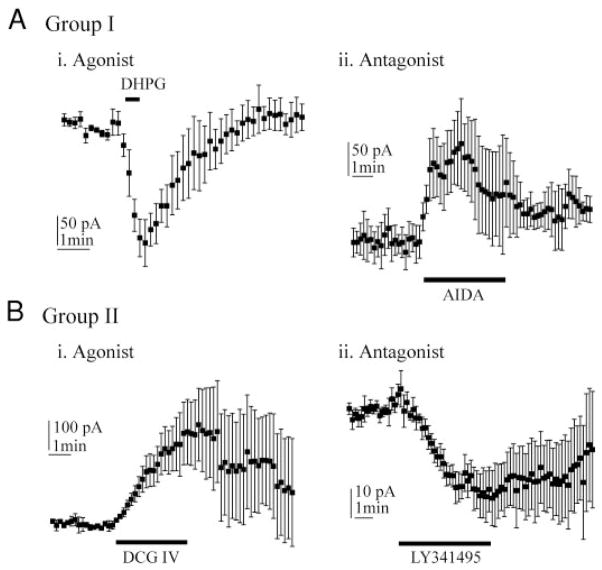
Application of group I and II mGluR agonists and antagonists elicited reversible shifts in holding currents in layer V pyramidal cells. AIDA, a group I antagonist, shifted holding current by an average 108 pA in the positive direction (Fig. 5Aii), indicating a negative or hyperpolarizing shift in Vm. The reversal potential for the shift, obtained from injection of voltage ramps, was −71 ± 6 mV (n = 4). A group I agonist, DHPG (100 μM), elicited an average 126-pA negative shift in holding current (Fig. 5Ai). In contrast to AIDA, the group II antagonist, LY341495, caused an average 28-pA negative shift of holding current (Fig. 5Bii). The group II agonist, DCG IV (1 μM) shifted the holding current by an average 209 pA in the positive direction (Fig. 5Bi). To determine whether the shifts in holding current elicited by LY341495 or by AIDA were dependent on impulse-related spontaneous synaptic activity, each antagonist was applied to six cells from slices bathed in TTX-containing perfusate. Under these conditions, AIDA elicited a positive shift (56 ± 24 pA, n = 6) and LY341495 elicited a negative shift in holding current (110 ± 33 pA, n = 6). These results suggest that blockade of group I and II mGluRs directly changes membrane potential (Vm), independent of an effect on impulse-related transmitter release. The reversal potential of the LY341495-induced current was −76 ± 4 mV (n = 4).
FIG. 5.
Group I and II mGluRs have opposite effects on holding current. A: group I drugs. Ai: agonist (S)-3,5-dihydroxyphenylglycine (DHPG, 100 μM, n = 5) shifts holding current in the negative direction. Aii: antagonist AIDA (1 mM, n = 5) shifts holding current in the positive direction. B: group II drugs. Bi: agonist DCG IV (1 μM, n = 7) shifts holding current in the positive direction. Bii: antagonist LY341495 (2 μM, n = 8) shifts holding current in the negative direction. All holding current measurements in A and B were made relative to pre-drug holding current.
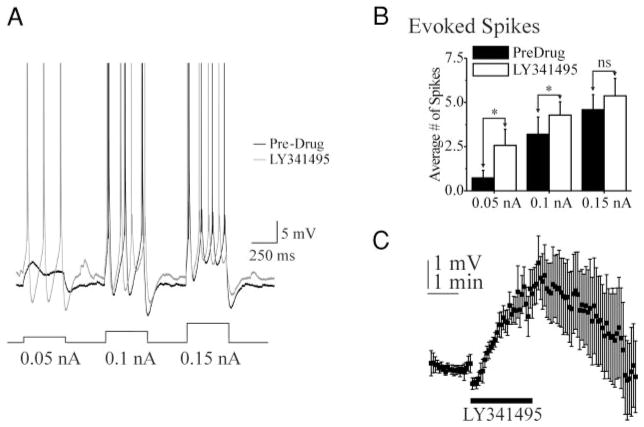
To further test the hypothesis that endogenous activation of group II mGluRs directly affects cellular excitability, current-clamp recordings were obtained from six cells with resting Vms between −57 and −61 mV, during LY341495 application. LY341495 (2 μM) reversibly depolarized Vm by 1– 4 mV (6 of 6 cells; average 2.3 ± 1.2 mV, P < 0.01; Fig. 6, A and C). In each cell, depolarizing current pulses evoked more spikes during LY341495 application than in control solution (Fig. 6, A and B). The occurrence of action potentials in 8-s intervals between current pulses was analyzed. LY341495 application induced action potential firing in two of six cells. The TTX data of Fig. 4 suggest that these changes in excitability likely resulted in alterations in the frequency of spontaneous action potentials.
FIG. 6.
A group II mGluR antagonist increases excitability of layer V pyramidal cells. A: LY341495 increases numbers of action potentials evoked by depolarizing current pulses. Current-clamp recording of responses to increasingly intense depolarizing current pulses before (darker trace, control) and after local perfusion of LY341495 (lighter trace). Vm: −61 mV in control and −59 mV after LY341495. Spikes are truncated. Spike height: 64 mV. B: graph of the average number of spikes evoked by current pulses with amplitudes shown in A. Data show means for 6 cells under control (Pre-Drug) and LY341495 conditions. Fifteen to 20 consecutive responses were used to generate the average for each cell, in each condition. *, P < 0.05, Pre-Drug vs. LY341495. C: graph of the membrane potential before, during, and after local perfusion of LY341495 (2 μM; n = 6; horizontal bar).
DISCUSSION
Our results show that antagonists of group I and II, but not group III, mGluRs alter cellular excitability and impulse-related EPSCs in layer V pyramidal neurons of neocortical slices. These findings suggest that the low concentrations of ambient glutamate known to be present in brain slices (see references in INTRODUCTION) are sufficient to activate these receptors. Judging from effects of antagonists, activation of group II mGluRs reduces excitability, whereas activation of group I mGluRs increases excitability. The effects of mGluR antagonists persist in GABAA or GABAB receptor blockers and are therefore not due to indirect actions resulting from disinhibition. These findings support the conclusion that spontaneous firing of layer V neurons is enhanced and suppressed by activated group I and II mGluRs, respectively.
The finding that group I mGluRs increase and group II mGluRs decrease excitability, when activated, is not entirely consistent with previous results obtained with application of exogenous mGluR agonists (for review see Anwyl 1999; Bockaert and Pin 1999; Schoepp 2001). For example, it has been previously reported that the group II agonist DCG IV does not affect membrane potential in layer V cells (Cho et al. 2000; Otani et al. 2002). Yet in our experiments, DCG IV and LY341495 had opposite effects on holding current, indicating that activation of group II mGluRs decreases cellular excitability. However, our data do agree with those of previous studies showing that DHPG produces a negative-going shift in holding current (for review see Wisniewski and Carr 2002), and we show that opposite effects are produced by antagonists of group I mGluRs, suggesting that group I mGluRs are activated in brain slices in the absence of overt stimulation. Our data support the conclusion that group I and II, but not III, mGluRs can directly alter membrane excitability and also affect release of glutamate in unstimulated slices through modulation of pyramidal cell spike frequency.
The reduction of sEPSCs is most likely to be a group-I-mediated effect. The reduction can be seen with both AIDA (1 mM) and HIBO (0.2 mM), molecules with different chemical structures that both antagonize group I mGluRs, ruling out some of the arguments for nonspecific effects of either drug. Furthermore, the effects of a group I mGluR agonist (DHPG), are opposite to the effects of AIDA, at least on the holding current, adding credibility to the claim that AIDA is acting as a group I antagonist. Additionally, the decrease does not appear to be an artifact of the drug application process, as sEPSCs are increased by group II antagonists and unchanged by NaOH application. Finally, the concentration of AIDA used, 1 mM, should affect both subtypes of group I mGluRs as this was reported to be the EC50 for mGluR5 (EC50 for mGluR1 is 0.2 mM) (see Pellicciari et al. 1995).
LY341495 is not a selective group II mGluR antagonist when used at higher concentrations (see Fitzjohn et al. 1998; Kingston et al. 1998; Ornstein et al. 1998; Schaffhauser et al. 1998); however, the concentration used in this study (2 μM) likely only affects group II mGluRs. Several lines of evidence suggest a specific role for group II mGluRs in the LY341495-mediated response. First, the increase in sEPSCs was also found for MCPG, a compound most active at group II and I mGluRs and almost inactive at group III sites (Jane et al. 1993; Pellicciari et al. 2001), indicating that the increase is not an effect of group III mGluRs. Second, group I mGluR antagonists AIDA (Moroni et al. 1997) and HIBO (Madsen et al. 2001) reduce, rather than enhance, sEPSC amplitudes, suggesting that the increase is not group I mediated. Third, the group III antagonist, CPPG (Jane et al. 1996), had no effect on sEPSCs, again suggesting that the increase is not group III mediated. Therefore the increase in sEPSCs is mediated via group II mGluRs, and the decrease is group I mediated. Because group I and II, but not group III, mGluRs have been found in extrasynaptic locations, it has been concluded that group I and II mGluRs are activated under conditions of lateral diffusion of glutamate or “spillover” (for review, see Anwyl 1999). It is possible that these receptors are activated in the presence of high-frequency neuronal activity that increases the ambient level of glutamate; however, the present results suggest that these receptors are also activated under resting conditions in brain slices.
The nature of the increase in sEPSCs is likely to be related to changes in spike frequency of cells within the neocortex. This conclusion is based on data showing that mGluR-mediated changes in the frequency of large-amplitude sEPSCs are absent in TTX. Previous reports show that TTX-insensitive miniature EPSCs (mEPSCs) are decreased in frequency by agonists of group II mGluRs in several preparations (Scanziani et al. 1995; Schoppa and Westbrook 1997; Tyler and Lovinger 1995), suggesting that group II mGluR antagonists should affect mEPSCs. However, mEPSCs are not significantly affected by LY341495 in our experiments, making it unlikely that mGluRs on the axon terminal are activated by endogenous glutamate in slices. Another possible interpretation of this result is that TTX reduces the concentration of ambient glutamate present in slices, resulting in a lower occupation of receptors and a decrease in the effects of both AIDA and LY341495. However, our data also show that AIDA and LY341495 continue to have an effect on the holding current of cells in the presence of TTX even though the effect of these drugs on sEPSCs is eliminated. Therefore it is unlikely that reduced ambient glutamate concentration in TTX accounts for the effect of these antagonists on large sEPSCs. The source of the excitatory synaptic events that are affected by mGluR antagonists is not clear from our results, although current-clamp recordings reveal that layer V cells, known to innervate neighboring pyramidal neurons (Gilbert 1985; Kisvarday et al. 1986; Lubke et al. 1996; Markram et al. 1997; Schwark and Jones 1989; for review see Markram 1997) did fire more action potentials in the presence of LY341495. It has not been determined if cells in other layers of the cortex or other brain regions are similarly affected by group I and II mGluR antagonists.
The nature of the conductance underlying the shift in membrane potential remains unexplored. The reversal potential of this current, about −70 mV, is not near EK or ECl, calculated to be −95 and −57 mV, respectively, in our experiments. This indicates that more than one conductance is activated by ambient glutamate as has been proposed for mGluR1 actions in CA1 cells (Crepel et al. 1994). The conductance activated by ambient glutamate would increase the electrotonic length of the recorded pyramidal neurons, and blockade of the resting conductance would be associated with a decreased electrotonic length and an improved space clamp. The resultant improved voltage-clamp fidelity would be expected to increase the amplitude of all distal synaptic events. However, we observed a specific increase in the frequency of large events, suggesting that altered voltage-clamp conditions cannot explain our results.
In a recent review, it was suggested that group II presynaptic mGluRs may be partially occupied (activated) at rest because these receptors are sensitive to glutamate in the micromolar range, and extracellular levels of glutamate readily reach these concentrations (Schoepp 2001). The present observations tend to support this conclusion because neither MCPG nor LY341495 should have any effect without some level of activation of mGluRs by an endogenous agonist in unstimulated slices. Furthermore, the findings that HIBO and AIDA decrease sEPSC amplitude and shift holding current suggest that group I mGluRs are also at least partially occupied at rest, whereas group III are not. Another possibility, at least for group I mGluRs, is that they may be constitutively active in the absence of glutamate, as was suggested by Ango and colleagues (2000). This constitutive activity may account for a portion of the present data obtained with antagonists; however, it is unlikely that constitutive activity accounts for a large portion of the effect as there is very little activity in wild-type cells containing Homer1a protein (Ango et al. 2000). The above-described effects of mGluRs should be greatly exaggerated in the intact brain as the levels of spontaneous activity, and therefore the extracellular concentration of glutamate, should be much higher (e.g., Pare et al. 1998; Steriade 2001). Therefore we believe that our results may underestimate the importance of mGluRs in the tonic control of excitability within cortical circuits.
Ambient levels of glutamate (0.5–2 μM) can also affect NMDA receptors that are sensitive to glutamate concentrations in the range of 2.5–3 μM (Meldrum 2000). Indeed, an APV-sensitive steady-state inward current can be observed in cortical neurons if Mg2+ is left out of the bathing medium (see Blanton et al. 1990; LoTurco et al. 1990), suggesting that extracellular levels of glutamate in brain slices are high enough to activate NMDA receptors under some circumstances. Low levels of glutamate may be critical in early stages of cortical development for activation of NMDA receptors present in embryonic cortical cells that have migrated away from the ventricular zone into the cortical plate (Blanton et al. 1990; LoTurco et al. 1991). These receptors are present before the emergence of synapses, suggesting that they may guide development of the immature neuron and be dependent on ambient levels of glutamate for their activation. mGluRs may also have an important developmental role during synaptogenesis as evidenced by changes in mGluR expression that accompany differentiation of cortical laminae (Furuta and Martin 1999; Muñoz et al. 1999). Also, mGluR expression patterns in visual cortex are altered during development and can be delayed by dark rearing (Reid et al. 1997). Many behavioral manifestations of brain function, such as alertness, sleep, and affective states, are best described as tonic in their time course. A variety of data from studies of glutamatergic (Blanton et al. 1990; LoTurco et al. 1990; present results) GABAergic (Hamann et al. 2002; Salin and Prince 1996) and perhaps other types of neurotransmission in the brain (Bunin and Wightman 1999) suggest that ongoing tonic extrasynaptic receptor activation is ubiquitous, raising the interesting hypothesis that ongoing tonic receptor activation has important functional behavioral consequences.
Acknowledgments
We thank Dr. Ulf Madsen for the generous donation of hexyl-HIBO, Drs. Alberto Bacci and Viktor Kharazia for helpful comments during the preparation of this manuscript, and Dr. QQ Sun for helpful discussions of the data.
This research was supported by National Institute of Neurological Sciences and Stroke Grant NS-12151 and by the Pimley Research and Training Funds.
References
- Ango F, Pin JP, Tu JC, Xiao B, Worley PF, Bockaert J, Fagni L. Dendritic and axonal targeting of type 5 metabotropic glutamate receptor is regulated by homer1 proteins and neuronal excitation. J Neurosci. 2000;20:8710–9716. doi: 10.1523/JNEUROSCI.20-23-08710.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev. 1999;29:83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- Bandrowski AE, Huguenard JR, Prince DA. Endogenous activation of group II mGluRs decreases the frequency of action potential-dependent excitatory synaptic currents in layer V pyramidal neurons of sensorimotor cortex. Soc Neurosci Abstr. 2002;28:339.19. [Google Scholar]
- Berretta N, Jones RS. A comparison of spontaneous EPSCs in layer II and layer IV–V neurons of the rat entorhinal cortex in vitro. J Neurophysiol. 1996;76:1089–1100. doi: 10.1152/jn.1996.76.2.1089. [DOI] [PubMed] [Google Scholar]
- Bianchi L, Della Corte L, Tipton KF. Simultaneous determination of basal and evoked output levels of aspartate, glutamate, taurine, and 4-aminobutyric acid during microdialysis and from superfused brain slices. J Chromatogr B Biomed Sci Appl. 1999;723:47–59. doi: 10.1016/s0378-4347(98)00519-2. [DOI] [PubMed] [Google Scholar]
- Blake JF, Cao CQ, Headley PM, Collingridge GL, Brugger F, Evans RH. Antagonism of baclofen-induced depression of whole-cell synaptic currents in spinal dorsal horn neurons by the potent GABAB antagonist CGP55845. Neuropharmacology. 1993;32:1437–1440. doi: 10.1016/0028-3908(93)90042-2. [DOI] [PubMed] [Google Scholar]
- Blanton MG, Lo Turco JJ, Kriegstein AR. Endogenous neurotransmitter activates N-methyl-D-aspartate receptors on differentiating neurons in embryonic cortex. Proc Natl Acad Sci USA. 1990;87:8027–8030. doi: 10.1073/pnas.87.20.8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockaert J, Pin JP. Molecular tinkering of G-protein-coupled receptors: an evolutionary success. EMBO J. 1999;18:1723–1729. doi: 10.1093/emboj/18.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddeke HW, Best R, Boeijinga PH. Synchronous 20 Hz rhythmic activity in hippocampal networks induced by activation of metabotropic glutamate receptors in vitro. Neuroscience. 1997;76:653–658. [PubMed] [Google Scholar]
- Bunin MA, Wightman RM. Paracrine neurotransmission in the CNS: involvement of 5-HT. Trends Neurosci. 1999;22:377–382. doi: 10.1016/s0166-2236(99)01410-1. [DOI] [PubMed] [Google Scholar]
- Cho K, Kemp N, Noel J, Aggleton JP, Brown MW, Bashir ZI. A new form of long-term depression in the perirhinal cortex. Nat Neurosci. 2000;3:150–156. doi: 10.1038/72093. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Lester RA. Excitatory amino acid receptors in the vertebrate CNS. Pharmacol Rev. 1989;41:143–210. [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Crepel V, Aniksztejn L, BenAri Y, Hammond C. Glutamate metabotropic receptors increase a Ca(2+)-activated nonspecific cationic current in CA1 hippocampal neurons. J Neurophysiol. 1994;72:1561–1569. doi: 10.1152/jn.1994.72.4.1561. [DOI] [PubMed] [Google Scholar]
- Fitzjohn SM, Bortolotto ZA, Palmer MJ, Doherty AJ, Ornstein PL, Schoepp DD, Kingston AE, Lodge D, Collingridge GL. The potent mGlu receptor antagonist LY341495 identifies roles for both cloned and novel mGlu receptors in hippocampal synaptic plasticity. Neuropharmacology. 1998;37:1445–1458. doi: 10.1016/s0028-3908(98)00145-2. [DOI] [PubMed] [Google Scholar]
- Furuta A, Martin LJ. Laminar segregation of the cortical plate during corticogenesis is accompanied by changes in glutamate receptor expression. J Neurobiol. 1999;39:67–80. [PubMed] [Google Scholar]
- Gilbert CD. Horizontal integration in the neocortex. Trends Neurosci. 1985;8:160–165. [Google Scholar]
- Hamann M, Rossi DJ, Attwell D. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron. 2002;33:625–633. doi: 10.1016/s0896-6273(02)00593-7. [DOI] [PubMed] [Google Scholar]
- Horikawa K, Armstrong WE. A versatile means of intracellular labeling: injection of biocytin and its detection with avidin conjugates. J Neurosci Methods. 1988;25:1–11. doi: 10.1016/0165-0270(88)90114-8. [DOI] [PubMed] [Google Scholar]
- Jane DE, Jones PL, Pook PC, Salt TE, Sunter DC, Watkins JC. Stereospecific antagonism by (+)-alpha-methyl-4-carboxyphenylglycine (MCPG) of (1S,3R)-ACPD-induced effects in neonatal rat motoneurones and rat thalamic neurons. Neuropharmacology. 1993;32:725–727. doi: 10.1016/0028-3908(93)90088-k. [DOI] [PubMed] [Google Scholar]
- Jane DE, Thomas NK, Tse HW, Watkins JC. Potent antagonists at the L-AP4- and (1S,3S)-ACPD-sensitive presynaptic metabotropic glutamate receptors in the neonatal rat spinal cord. Neuropharmacology. 1996;35:1029–1035. doi: 10.1016/s0028-3908(96)00048-2. [DOI] [PubMed] [Google Scholar]
- Kapetanovic V, Milovanovic L, Vladimirov S. Differential pulse polarographic determination of nizatidine in pharmaceutical formulations. Farmaco. 1994;49:377–379. [PubMed] [Google Scholar]
- Kingston AE, Ornstein PL, Wright RA, Johnson BG, Mayne NG, Burnett JP, Belagaje R, Wu S, Schoepp DD. LY341495 is a nanomolar potent and selective antagonist of group II metabotropic glutamate receptors. Neuropharmacology. 1998;37:1–12. doi: 10.1016/s0028-3908(97)00191-3. [DOI] [PubMed] [Google Scholar]
- Kisvarday ZF, Martin KA, Freund TF, Magloczky Z, Whitteridge D, Somogyi P. Synaptic targets of HRP-filled layer III pyramidal cells in the cat striate cortex. Exp Brain Res. 1986;64:541–552. doi: 10.1007/BF00340492. [DOI] [PubMed] [Google Scholar]
- LoTurco JJ, Blanton MG, Kriegstein AR. Initial expression and endogenous activation of NMDA channels in early neocortical development. J Neurosci. 1991;11:792–799. doi: 10.1523/JNEUROSCI.11-03-00792.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoTurco JJ, Mody I, Kriegstein AR. Differential activation of glutamate receptors by spontaneously released transmitter in slices of neocortex. Neurosci Lett. 1990;114:265–271. doi: 10.1016/0304-3940(90)90574-s. [DOI] [PubMed] [Google Scholar]
- Lubke J, Markram H, Frotscher M, Sakmann B. Frequency and dendritic distribution of autapses established by layer 5 pyramidal neurons in the developing rat neocortex: comparison with synaptic innervation of adjacent neurons of the same class. J Neurosci. 1996;16:3209–3218. doi: 10.1523/JNEUROSCI.16-10-03209.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen U, Brauner-Osborne H, Frydenvang K, Hvene L, Johansen TN, Nielsen B, Sanchez C, Stensbol TB, Bischoff F, Krogsgaard-Larsen P. Synthesis and pharmacology of 3-isoxazolol amino acids as selective antagonists at group I metabotropic glutamic acid receptors. J Med Chem. 2001;44:1051–1059. doi: 10.1021/jm000441t. [DOI] [PubMed] [Google Scholar]
- Markram H. A network of tufted layer 5 pyramidal neurons. Cereb Cortex. 1997;7:523–533. doi: 10.1093/cercor/7.6.523. [DOI] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Roth A, Sakmann B. Physiology and anatomy of synaptic connections between thick tufted pyramidal neurons in the developing rat neocortex. J Physiol. 1997;500:409–440. doi: 10.1113/jphysiol.1997.sp022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain CJ, DiChiara TJ, Kauer JA. Activation of metabotropic glutamate receptors differentially affects two classes of hippocampal interneurons and potentiates excitatory synaptic transmission. J Neurosci. 1994;14:4433–4445. doi: 10.1523/JNEUROSCI.14-07-04433.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130:1007S–1015S. doi: 10.1093/jn/130.4.1007S. [DOI] [PubMed] [Google Scholar]
- Moroni F, Lombardi G, Thomsen C, Leonardi P, Attucci S, Peruginelli F, Torregrossa SA, Pellegrini-Giampietro DE, Luneia R, Pellicciari R. Pharmacological characterization of 1-aminoindan-1,5-dicarboxylic acid, a potent mGluR1 antagonist. J Pharmacol Exp Ther. 1997;281:721–729. [PubMed] [Google Scholar]
- Munoz A, Liu XB, Jones EG. Development of metabotropic glutamate receptors from trigeminal nuclei to barrel cortex in postnatal mouse. J Comp Neurol. 1999;409:549–566. [PubMed] [Google Scholar]
- Ornstein PL, Arnold MB, Bleisch TJ, Wright RA, Wheeler WJ, Schoepp DD. [3H] LY341495, a highly potent, selective and novel radioligand for labeling Group II metabotropic glutamate receptors. Bioorg Med Chem Lett. 1998;8:1919–1922. doi: 10.1016/s0960-894x(98)00329-1. [DOI] [PubMed] [Google Scholar]
- Otani S, Daniel H, Takita M, Crepel F. Long-term depression induced by postsynaptic group II metabotropic glutamate receptors linked to phospholipase C and intracellular calcium rises in rat prefrontal cortex. J Neurosci. 2002;22:3434–3444. doi: 10.1523/JNEUROSCI.22-09-03434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D, Shink E, Gaudreau H, Destexhe A, Lang EJ. Impact of spontaneous synaptic activity on the resting properties of cat neocortical pyramidal neurons In vivo. J Neurophysiol. 1998;79:1450–1460. doi: 10.1152/jn.1998.79.3.1450. [DOI] [PubMed] [Google Scholar]
- Pellicciari R, Costantino G, Marinozzi M, Macchiarulo A, Camaioni E, Natalini B. Metabotropic glutamate receptors: structure and new subtype-selective ligands. Farmaco. 2001;56:91–94. doi: 10.1016/s0014-827x(01)01006-0. [DOI] [PubMed] [Google Scholar]
- Pellicciari R, Luneia R, Costantino G, Marinozzi M, Natalini B, Jakobsen P, Kanstrup A, Lombardi G, Moroni F, Thomsen C. 1-Aminoindan-1,5-dicarboxylic acid: a novel antagonist at phospholipase C-linked metabotropic glutamate receptors. J Med Chem. 1995;38:3717–3719. doi: 10.1021/jm00019a002. [DOI] [PubMed] [Google Scholar]
- Reid SN, Romano C, Hughes T, Daw NW. Developmental and sensory-dependent changes of phosphoinositide-linked metabotropic glutamate receptors. J Comp Neurol. 1997;389:577–583. doi: 10.1002/(sici)1096-9861(19971229)389:4<577::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Salin PA, Prince DA. Spontaneous GABAA receptor-mediated inhibitory currents in adult rat somatosensory cortex. J Neurophysiol. 1996;75:1573–1588. doi: 10.1152/jn.1996.75.4.1573. [DOI] [PubMed] [Google Scholar]
- Scanziani M, Gahwiler BH, Thompson SM. Presynaptic inhibition of excitatory synaptic transmission by muscarinic and metabotropic glutamate receptor activation in the hippocampus: are Ca2+channels involved? Neuropharmacology. 1995;34:1549–1557. doi: 10.1016/0028-3908(95)00119-q. [DOI] [PubMed] [Google Scholar]
- Schaffhauser H, Richards JG, Cartmell J, Chaboz S, Kemp JA, Klingelschmidt A, Messer J, Stadler H, Woltering T, Mutel V. In vitro binding characteristics of a new selective group II metabotropic glutamate receptor radioligand, [3H] LY354740, in rat brain. Mol Pharmacol. 1998;53:228–233. [PubMed] [Google Scholar]
- Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the CNS. J Pharmacol Exp Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- Schoppa NE, Westbrook GL. Modulation of mEPSCs in olfactory bulb mitral cells by metabotropic glutamate receptors. J Neurophysiol. 1997;78:1468–1475. doi: 10.1152/jn.1997.78.3.1468. [DOI] [PubMed] [Google Scholar]
- Schwark HD, Jones EG. The distribution of intrinsic cortical axons in area 3b of cat primary somatosensory cortex. Exp Brain Res. 1989;78:501–513. doi: 10.1007/BF00230238. [DOI] [PubMed] [Google Scholar]
- Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABA(A) conductances in hippocampal neurons. J Neurosci. 2002;22:RC223. doi: 10.1523/JNEUROSCI.22-10-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M. Impact of network activities on neuronal properties in corticothalamic systems. J Neurophysiol. 2001;86:1–39. doi: 10.1152/jn.2001.86.1.1. [DOI] [PubMed] [Google Scholar]
- Sun QQ, Huguenard JR, Prince DA. Neuropeptide Y receptors differentially modulate G protein-activated inwardly rectifying K+ channels and high-voltage-activated Ca2+ channels in rat thalamic neurons. J Physiol. 2001;531:67–79. doi: 10.1111/j.1469-7793.2001.0067j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng GF, Parada I, Prince DA. Double-labeling with rhodamine beads and biocytin: a technique for studying corticospinal and other projection neurons in vitro. J Neurosci Methods. 1991;37:121–131. doi: 10.1016/0165-0270(91)90122-g. [DOI] [PubMed] [Google Scholar]
- Tyler EC, Lovinger DM. Metabotropic glutamate receptor modulation of synaptic transmission in corticostriatal co-cultures: role of calcium influx. Neuropharmacology. 1995;34:939–952. doi: 10.1016/0028-3908(95)00066-f. [DOI] [PubMed] [Google Scholar]
- Ulrich D, Huguenard JR. GABAB receptor-mediated responses in GABAergic projection neurons of rat nucleus reticularis thalami in vitro. J Physiol. 1996;493:845–854. doi: 10.1113/jphysiol.1996.sp021427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Wisniewski K, Carr H. (S)-3,5-DHPG: A Review. CNS Drug Rev. 2002;8:101–116. doi: 10.1111/j.1527-3458.2002.tb00218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Zilles B, Schleicher A. A quantitative approach to cytoarchitectonics. VI. The areal pattern of the cortex of the albino rat. Anat Embryol (Berl) 1980;159:335–360. doi: 10.1007/BF00317655. [DOI] [PubMed] [Google Scholar]