Abstract
Objectives
This study examined differences in cessation success based on smokers' self-initiated pre-quit reductions in cigarettes per day (cpd).
Methods
The study utilized data from a nicotine replacement + behavioral therapy smoking cessation intervention conducted in a female prison facility with 179 participants who were wait-listed for 6 months prior to intervention. We compared two groups of smokers based on whether they self-selected to reduce smoking prior to their cessation attempt (n = 77) or whether they increased smoking or did not reduce (n= 102). General Estimating Equations (GEE) were used to model smoking cessation through 12-month follow-up.
Results
Examination of pre-cessation cpd showed that those who reduced were heavier smokers at baseline, relative to those who did not reduce (p < .001). By the week prior to the quit attempt (week 3) heavier smokers at baseline smoked significantly fewer cigarettes (p < .001) and had lower CO levels (p < .05) compared to baseline lighter smokers. GEE analyses showed that individuals who reduced prior to their quit attempt had significantly higher quit rates during early treatment but these gains were not sustained by follow-up points.
Conclusions
Participant-initiated pre-cessation smoking reduction may be initially helpful in preparing to quit smoking, or may serve as a marker for participant motivation to quit smoking, but these differences do not sustain over time. More intensive interventions are still needed for successful cessation.
1. Introduction
Smoking remains the leading cause of preventable death in the United States and novel interventions are needed to assist smokers in quitting (MMWR, 2007). While smoking prevalence in the general population has declined in recent years, smoking remains highly prevalent in prison populations with approximately 70-80% of prisoners identified as current smokers (Cropsey, Eldridge, Weaver, Villalobos, & Stitzer, 2006; Sieminska, Jassem, & Konopa, 2006; Belcher, Butler, Richmond, Wodak, & Wilhelm, 2006; Cropsey & Kristeller, 2003; 2005; Conklin, Lincoln, Tuthill, 2000; Colsher et al., 1992; Cropsey, Eldridge, and Ladner, 2004). These high rates of smoking are not gender specific (Cropsey & Kristeller, 2003; 2005; Cropsey et al., 2006; Cropsey et al., 2008) and have a grave impact on the health of prisoners. The most common medical problems in prisoners are smoking-related illnesses such as heart disease, respiratory problems, and liver and kidney disease (Maruschak & Beck, 2001). Despite the fact that smoking cessation treatment is clearly needed for incarcerated populations and the incarceration period presents an ideal opportunity to intervene, few studies of cessation interventions have been conducted in correctional facilities.
There are many contributing factors for the lack of cessation treatment in prisons. One key barrier is the cost to develop and implement evidence-based tobacco cessation treatment. Despite evidence suggesting that treating tobacco use would actually save costs incurred from the treatment of smoking-related health conditions, there has not been enough fiscal support to offer treatment in many institutions. Smoking bans in correctional facilities have been a more recent phenomenon and over half of states have implemented smoking bans within their facilities (Kauffman, Ferketich, & Wewers, 2008). While smoking bans can be an important public health policy to reduce smoking, implementing these bans in prisons without cessation treatment creates problems with an underground economy and may not lead to sustained cessation (Cropsey et al., 2003; Kauffman, Ferketich, & Wewers, 2008). A review of smoking policies in prisons found that while most institutions with indoor tobacco bans (86%) reported having some form of tobacco treatment, few (<40%) continued such programs after the initial period of introducing the ban (Kauffman, Ferketich, & Wewers, 2008). With new inmates entering prison daily, treatment to address abrupt cessation due to smoking bans will continue to be needed.
One strategy that has recently been investigated is smoking reduction as a precursor to cessation; i.e., gradual cessation. Reduction interventions generally consist of either structured reductions (e.g., having participants reduce their cpd by a certain percentage each week until they are close to smoking no cigarettes and can quit) or an unstructured reduction (allowing the participant to decide the rate to reduce their smoking). Reduction techniques may include behavioral treatment, pharmacotherapy, or both. Reduction in advance of a planned quit attempt may enhance likelihood of eventual success (Rose & Behm, 2004), particularly if the individual gains self-efficacy and/or lessens nicotine dependence through reduction (Carpenter, Hughes, & Keely, 2003; Carpenter, Hughes, Solomon, & Callas, 2004).
Smoking reduction has generally been examined as a potential marker or precursor of future quitting behavior among otherwise unmotivated smokers or as a directed intervention prior to a quit attempt. Such was the nature of the studies reviewed by Hughes and Carpenter (2006) where outcomes were examined in smokers that were not motivated to quit prior to the reduction initiation. They concluded that smoking reduction promotes eventual cessation and does not hinder overall motivation to quit (Hughes & Carpenter, 2006). However, a more recent review by Lindson, Aveyard, and Hughes (2010) found no appreciable differences between standard, abrupt quit attempts and reduction methods.
Two studies by Cinciripini and colleagues have looked at gradual reduction in smokers who were motivated to quit. The first looked at scheduled reduction versus minimal contact and found that reduction significantly improved cessation outcomes through one year follow-up (Cinciripini et al., 1994). The second study tested scheduled gradual reduction versus nonscheduled reduction (reducing cpd by eliminating whichever cigarettes participants chose), scheduled non-reduction (continue smoking the same amount at fixed intervals) or nonscheduled non-reduction (smoke the same amount as usual). They found that the scheduled reduction group had the best abstinence rates and the non-scheduled reduction had the worst, suggesting that the structure provided by scheduling may play a key role in success (Cinciripini, et al., 1995). However, neither study looked at self-initiated reduction.
There are reasons to believe gradual cessation techniques may be useful for promoting cessation in recalcitrant prison populations. One reason is that reduction can serve as the first step in the cessation process by increasing the participant's self-efficacy (Bandura & Adams, 1977; Hughes & Carpenter, 2006). This could be particularly useful in a prison population with markedly less access to positive reinforcement and limited treatment options. A second reason gradual cessation may be a useful approach for prisoners is that it slowly reduces nicotine dependence, which is a well-established barrier to cessation success (Colby et. al. 2000; Shadel et al., 2000; Hughes & Carpenter, 2006).
The optimal test of a reduction intervention on eventual cessation would be to randomize prisoners to either a reduction versus no reduction intervention prior to cessation. While no such test has been conducted among prisoners, the present study, based on a secondary analysis of a recently completed cessation trial (Cropsey et al., 2008), offers an indirect test of this. We compared female prisoners who self-selected to reduce during a 6-month interval prior to their enrollment in a smoking cessation program to those who did not. We hypothesized that self-initiated reduction would be positively correlated with subsequent quit success.
2. Method
2.1 Participants
Inclusion criteria for the original study included being female, at least 18 years of age, smoking at least 5 cpd, maintenance in the general prison population (e.g., not held in segregation), and a desire to quit smoking. We set the inclusion cutoff of smoking at least 5 cpd as an indication of daily smokers (and not chippers or occasional smokers) who would be able to use the nicotine replacement therapy (NRT) provided. Exclusion criteria included allergies to nicotine patches, having less than one year remaining in their prison sentence, non-English speaking, or active serious mental illness (e.g., suicidal ideation or psychosis), mental retardation, or any cognitive impairment that would limit their ability to provide informed consent. Participants with other disabilities such as illiteracy or legal blindness were admitted into the study and were assisted in completing their survey instruments.
Procedures
Detailed study procedures have been described previously (Cropsey et al., 2008). Between 2004 and 2006 participants were recruited though announcements and study fliers in prison housing units at a medium-maximum security female prison. The prison allowed unrestricted smoking within an individual's cell as well as all outdoor areas. The prison did not allow smoking within the medical areas, vocational or educational areas, food preparation areas, or chapel. Prisoners could purchase loose tobacco and rolling papers as well as name brand cigarettes on commissary. The initial study was a randomized prospective (12 month) controlled trial testing a behavioral intervention (described below) to promote cessation versus a wait-list control group (6-month wait-list). This study received Institutional Review Board (IRB) approval from Virginia Commonwealth University, the Virginia Department of Corrections, and the University of Alabama at Birmingham. A Certificate of Confidentiality was obtained from the federal government to further protect confidentiality of research data.
Upon enrolling in the study, participants completed a baseline assessment of smoking history and demographic information. Participants were then placed on a wait-list control for six months and were not specifically instructed to change their smoking. Thus, individuals who made reductions during the six month wait-list period reduced spontaneously on their own. After six months participants entered the 10 week group intervention, and completed weekly measures of daily smoking, concentration of expired carbon monoxide (CO) (Vitalograph BreathCO®), type of cigarette smoked, and height and weight were collected. An expired carbon monoxide reading of 3 parts per million (ppm) or higher was used to indicate current smoking, which was the optimal cutoff indicated in a previous investigation with smoking and non-smoking female prisoners (Cropsey et al, 2006).
The behavioral intervention used for this study was Mood Management (MM) Training to Prevent Smoking Relapse (Hall et al., 1994), which was modified for use in a correctional setting. It included examples of smoking triggers encountered in prison and acceptable coping strategies that could be used in the prison environment. A full description of how this 10-week intervention was modified was reported previously (Cropsey et al., 2008). In addition to the group intervention, all participants received NRT (NicoDerm CQ®) following the manufacturer's suggested dosing regimen as follows: for those smoking 20 cpd or less, 6 weeks at 14 mg followed by 2 weeks at 7 mg and for those smoking 21 cpd or more, 6 weeks at 21 mg, 2 weeks at 14 mg, and 2 weeks at 7 mg. Participants started NRT and were asked to make a quit attempt between week 3 and week 4 of the 10-week intervention. Participants completed assessments at End-of-Treatment (EOT or week 10) and at 3, 6, and 12 month follow-ups. Participants who started on 21 mg nicotine patches had an additional medication check-in the week after the EOT assessment to refill medication and assess for side effects. All study outcomes through the 12-month follow-up are presented below.
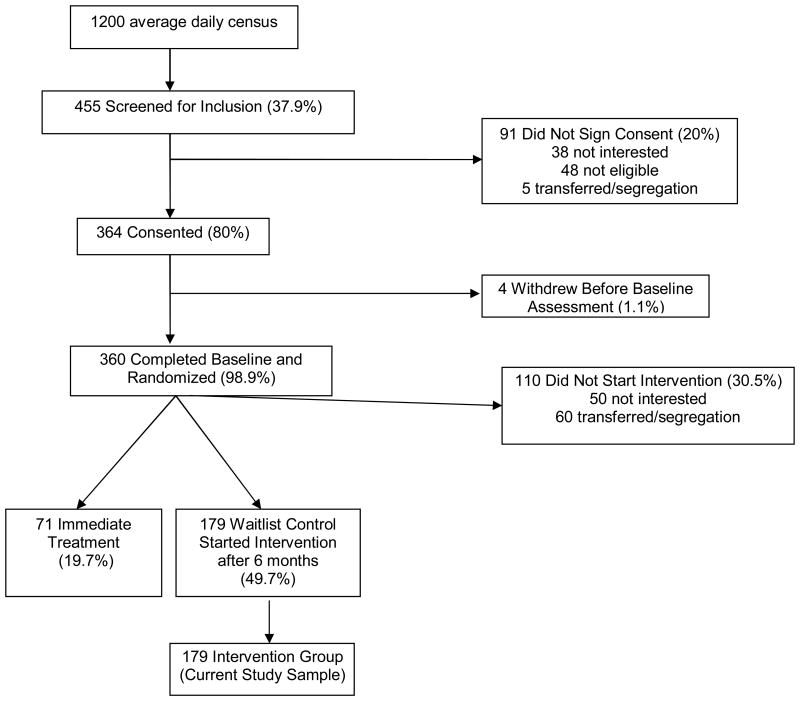
The flow of study participants is depicted in Figure 1 and was previously reported in Cropsey et al. (2008). A total of 364 participants signed informed consent, 360 participants completed the baseline assessment, and 250 participants started the intervention either immediately after the baseline assessment (N = 71) or after a 6 month wait list period (N = 179). For the current study, reduction information and cessation outcomes were analyzed for participants who started the intervention after being assigned to the 6 month wait-list control group (N= 179). Smoking data were collected as self-reported cpd verified by a breath CO reading at each of the 10 weekly visits and at 3-months and 6-month follow-ups.
Figure 1.
Study Flow (N= 179).
Data Analysis
Self-reported pre-quit reductions were used for group assignment versus expired CO levels. This decision was made due to the influence of time since last cigarette on CO concentrations. Out of the 179 participants in the initial wait-list control group, two groups were formed based upon the participant's self-reported change in cpd prior to their instructed quit attempt. Reduction was calculated by dividing their cpd during the week prior to the quit date by their baseline cpd obtained approximately six months earlier. Those participants with any reduction in cpd were classified into one group (N= 77) and had an average reduction of 54.7%. Participants with no reduction in cpd or an increase in cpd between baseline and week 3 were in the second group (N=102) and had an average of a 33.7% increase during this same period.
Comparison of baseline characteristics between reduction groups was done using chi-square and ANOVA procedures where appropriate. The outcome variable, smoking abstinence, was obtained by asking participants if they smoked in the past seven days and was confirmed by expired CO, with participants coded as abstinent if they denied any smoking in the past week and had a CO level of 2 ppm or less. This more stringent CO criterion was found to be the most sensitive and specific to detect smoking among female prisoners in a previous study (see Cropsey et al., 2006 for a full description of these methods). Abstinence at each time point was defined by a 7-day interval of no reported smoking, which was biochemically confirmed with CO monitoring.
Group comparisons on initial smoking behavior for weeks 1- 3 of the treatment period (prior to Week 4, where participants were instructed to make a quit attempt) were conducted through ANOVA analyses. Groups were compared on the number of cigarettes smoked on each day prior to their Baseline, week 1, week2, and week 3 assessments.
To examine the long-term impact of pre-quit reduction of smoking rates on quit success, a generalized estimating equation (GEE) method was used. GEE is used with longitudinal, dichotomous outcome data to provide the best estimation of the relationships across time of the variables of interest. The model for this analysis included the two reduction groups (reduction or no reduction); time (15 time points: baseline, weeks 1-10 of group treatment, a medication check one week after group termination, 3, 6, and 12-month follow-up); and a time by reduction group interaction term. The outcome variable of interest was 7-day smoking cessation at each time point (smoking vs. quit). A p-value <0.05 was used for all analyses to indicate significance. As a final step in the analysis, cessation rates were compared between groups at each time-point using a chi-square analysis. This provided point comparisons in addition to the overall effect of the model which was examined through the GEE analysis.
3. Results
Demographics
As seen in Table 1, there were no significant between-group differences in demographic or historical smoking characteristics during the pre-treatment waiting period. However, baseline smoking rate was higher among those who reduced prior to the quit day than those who did not (17.5 cpd vs 11.9 cpd, respectively; p < .001).
Table 1. Demographic and Smoking Characteristics (N=179).
| No Reduction (n=102) |
Reduction (n=77) |
p- value | |
|---|---|---|---|
| % or Mean (SD) |
% or Mean (SD) |
||
| Race | 0.81 | ||
| White | 47.9 | 43.2 | |
| Black | 50.0 | 45.9 | |
| Other | 2.1 | 2.7 | |
| Education | 0.69 | ||
| Less than High School | 28.4 | 33.8 | |
| High School/GED | 41.1 | 40.5 | |
| Some College or Higher | 30.5 | 25.7 | |
| Marital Status | 0.98 | ||
| Never Married | 49.5 | 50.0 | |
| Married | 15.5 | 16.2 | |
| Separated, Divorced, Widowed | 35.1 | 33.8 | |
| Age | 33.2 (9.4) |
3.1 (9.0) |
.90 |
| Age 1st Smoke | 13.31 (4.92) |
13.95 (4.40) |
0.38 |
| Age Regular Smoke | 17.39 (4.90) |
15.91 (4.18) |
0.43 |
| Average CPD | 14.91 (7.58) |
18.53 (11.06) |
0.01 |
| Yesterday CPD | 11.92 (7.10) |
17.51 (9.67) |
0.001 |
| Highest CPD | 24.20 (15.62) |
23.60 (21.91) |
0.85 |
| Baseline Carbon Monoxide (CO) in ppm | 14.19 (8.83) |
15.26 (8.00) |
0.43 |
| Total # Years of smoking | 18.05 (10.02) |
17.86 (9.85) |
0.90 |
| Stage of Change | 0.29 | ||
| Precontemplation | 5.4 | 2.1 | |
| Contemplation | 67.6 | 77.1 | |
| Preparation | 27.0 | 20.8 | |
| FTND | 0.10 | ||
| 5.82 (2.28) |
6.39 (1.91) |
||
Changes in smoking behavior
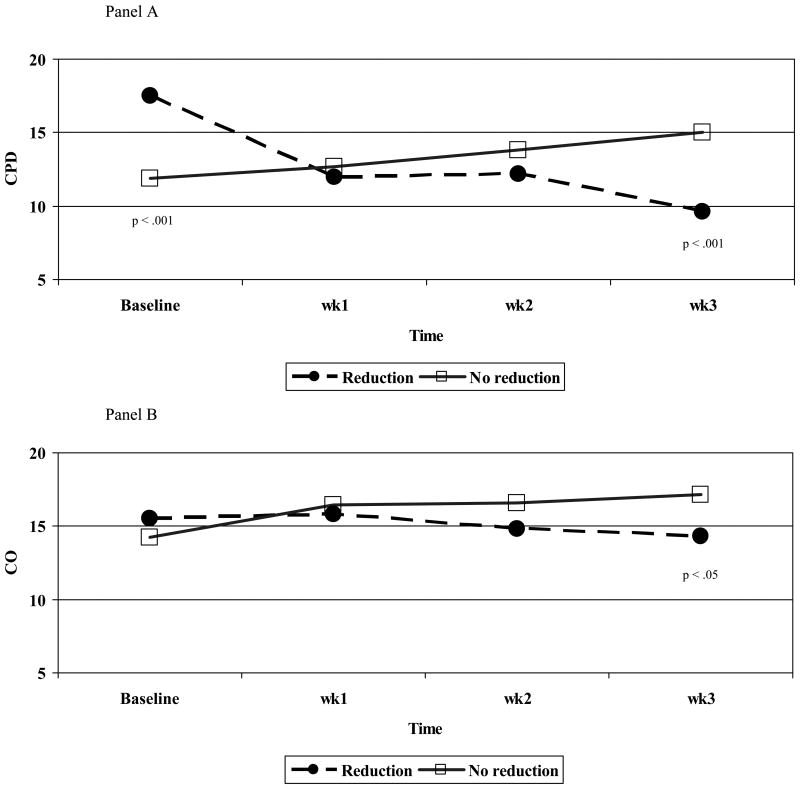
Changes in smoking and CO levels from baseline to pre-quit are shown in Fig. 2, Panel A. Group differences found at baseline and Week 3 are denoted by their significance level. During the week prior to the scheduled quit attempt, reducers decreased their smoking on average by almost 8 cpd (from 17.5±9.7 to 9.6±6.2 cpd) while non-reducers increased smoking on average by 3 cpd (from 12.0±7.3 to 15.0±7.9 cpd). At this pre-quit time point (week 3), the reduction group reported smoking significantly fewer cpd than the no reduction group, (M= 9.6 vs. 15.0 cpd, respectively; p < .001. Similarly, CO levels for these groups changed such that by the week prior to quitting (week 3), reducers had significantly lower CO levels compared to the non-reducers (14.4±8.2 vs. 17.2±9.2 ppm, p < 0.05; see Fig.2, Panel B).
Figure 2.
Initial smoking levels (Yesterday CPD) and CO by reduction group (N= 179).
Note: Significant p-values listed from Chi-square time-point comparisons
Reduction and smoking cessation outcomes
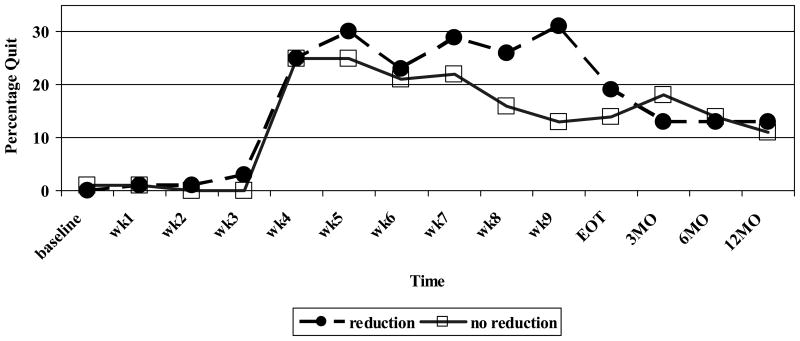
The results of the GEE analysis comparing reduction groups on smoking cessation found significant interaction effects for the reduction group across time. Smokers who showed no pre-quit reduction had significantly lower quit rates early in cessation treatment compared to those who had reduced prior to quitting, although the differences between the groups were not maintained either at the end of treatment or during the follow-up points. See Table 2 and Figure 3 for details.
Table 2.
GEE test of model effects with smoking cessation as outcome variable and reduction group, time, and reduction*time interaction as explanatory variables (N = 179).
| Variable | Wald Chi-Square | df | p-value |
|---|---|---|---|
| Intercept | 502.28 | 1 | <0.001 |
| Reduction Group | 34.22 | 1 | <0.001 |
| Time | 841.20 | 14 | <0.001 |
| Reduction*Time | 28.29 | 11 | 0.003 |
Figure 3.
CO-Verified Quit Rates by Pre-quit Reduction Behavior (N=179).
4. Discussion
Our study showed that the smokers who reduced prior to their quit attempt showed better cessation rates during initial post-quit weeks of the smoking cessation program than did those who did not reduce. However, these effects disappeared by the end of treatment and at follow-up points. The finding of a short-term improvement of cessation outcomes for those participants who evidenced pre-quit smoking reduction is inconsistent with null findings from previous studies (Hughes et al., 1999). The fact that these differences in cessation outcomes did not sustain through follow-up was consistent with most previous findings (Pisinger et al., 2005), with the one notable exception being the study by Falba and colleagues (2004) which showed long-term (two year) benefits associated with moderate-large reductions versus no reduction at all. In our study we found no long-term quit benefit for self-initiated reduction versus no reduction. It is important to note, however, the extent of reduction in our sample may have been smaller than that observed in previous studies given that fact that we compared any reduction versus none at all. Finally, the findings of this study are similar to those of Hughes and Carpenter (2006) in refuting the idea that pre-quit reduction hinders cessation. While we found no sustainable improvement in cessation for those that were able to reduce smoking, we also found no evidence of a negative impact of reduction.
This study proposes an interesting theory regarding which smokers attempt to reduce smoking prior to their established quit date. We found that the heaviest smokers were more inclined to reduce, while lighter smokers did not reduce their cpd prior to the quit date. A possible explanation for this is that lighter smokers may feel less dependent upon nicotine and anticipate less difficulty in quitting, while heavy, chronic smokers may have attempted to quit in the past and anticipate more difficulty during cessation. Partial support for this is found with the fact that individuals who reduced had slightly higher scores on the FTND, although these differences were not statistically significant. Alternatively, since non-reducers were similar to reducers in the highest number of cigarettes ever smoked in their lifetime, they may represent a group that had cut down gradually prior to entering the study, but had been unable to completely quit on their own and were relying on the intervention to help them quit or reduce further. No significant differences were found between the reduction groups on baseline Stage of Change which suggests that both groups were at a similar point of motivation to quit.
Our study had several limitations. The first is the lack of a scheduled reduction condition to allow us to test whether structured intervals would improve outcomes as seen in a previous study (Cinciripini et al., 1995). Group assignments were based on participant-reported reductions in cpd. While CO expirations served as a biochemical indication of smoking abstinence, we did not use it as the main indicator of reduction given the impact of time lapse on CO concentration, which could inadvertently miss smoker's who may have reduced their cpd, but smoked their last cigarette immediately prior to the collection of the sample. Further, given that this study involved a female prisoner population, it is not clear how these results would apply to non-prisoner populations, although they likely generalize to other correctional settings since male and female prisoners have similar smoking rates (Cropsey et al., 2004). Given that these smokers were not instructed to reduce on their own or given any strategies for doing so, this study is not comparable to other studies which have specifically instructed or assisted participants to reduce smoking. Also, we do not know if cessation outcomes would extend beyond prisoner release, i.e., long term. There is a reasonable expectation that some successful quitters will relapse once they return to the community. While the scope of this study was to compare cessation outcomes between smokers who made any self-directed change in their smoking behavior versus those that did not, it may be useful in future studies to examine outcomes based on the amount of reduction.
Despite these limitations, this study contributes to existing cessation literature in that it is the first to examine the impact of participant-initiated reduction on smoking cessation in a prison population. As previously noted, studies on scheduled reduction have shown more promising results; our data suggest that reduction might be promising as a pre-quit strategy with this population, although a subsequent study with this population should directly test this hypothesis. Self- initiated smoking reduction may serve as a marker for participant motivation to quit smoking although this was not apparent on baseline Stage of Change measure. Nevertheless, the limited and short-lived nature of the improved outcomes noted suggest that more intensive interventions are still needed for cessation even among those who may spontaneously cut down prior to a quit attempt. Future studies of novel smoking cessation interventions may wish to consider investigating scheduled reductions combined with pharmacotherapy as a means of promoting cessation with correctional populations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Karen L. Cropsey, University of Alabama at Birmingham
Dorothy O. Jackson, University of Alabama at Birmingham
Galen J. Hale, University of Alabama at Birmingham
Matthew J. Carpenter, Medical University of South Carolina
Maxine L. Stitzer, Johns Hopkins University
References
- Bandura A, Adams NE. Analysis of self-efficacy theory of behavioral change. Cognitive Therapy and Research. 1977;1:287–310. [Google Scholar]
- Belcher J, Butler TG, Richmond R, Wodak A, Wilhelm K. Smoking and its correlates in an Australian prisoner population. Drug and Alcohol Review. 2006;25:343–348. doi: 10.1080/09595230600741198. [DOI] [PubMed] [Google Scholar]
- Carpenter MJ, Hughes JR, Keely JP. Effect of smoking reduction on later cessation: a pilot experimental study. Nicotine and Tobacco Research. 2003;5:155–62. doi: 10.1080/146222003100007385. [DOI] [PubMed] [Google Scholar]
- Carpenter MJ, Hughes JR, Solomon LJ, Callas PW. Both smoking reduction with nicotine replacement therapy and motivational advice increase future cessation among smokers unmotivated to quit. Journal of Consulting and Clinical Psychology. 2004;72:371–381. doi: 10.1037/0022-006X.72.3.371. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Cigarette Smoking Among Adults—United States, 2006. Morbidity and Mortality Weekly Report. 2007;56(44):1157–1161. serial online. [cited 2007 Nov 8 ]. Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5644a2.htm. [PubMed]
- Cinciripini PM, Lapitsky LG, Wallfisch A, Mace R, Nezami E, Van Vunakis H. An evaluation of a multicomponent treatment program involving scheduled smoking and relapse prevention procedures: Initial findings. Addictive Behavior. 1994;19:13–22. doi: 10.1016/0306-4603(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Cinciripini PM, Lapitsky L, Seay S, Wallfisch A, Kitchens K. The effects of smoking schedules on cessation outcome: Can we improve on common methods of gradual and abrupt nicotine withdrawal? Journal of Consulting and Clinical Psychology. 1995;63:388–399. doi: 10.1037//0022-006x.63.3.388. [DOI] [PubMed] [Google Scholar]
- Colby SM, Tiffany ST, Shiffman S, Niaura RS. Measuring nicotine dependence among youth: a review of available approaches and instruments. Drug Alcohol Depend. 2000;59(Suppl. 1):S23–S39. doi: 10.1016/s0376-8716(99)00163-5. [DOI] [PubMed] [Google Scholar]
- Colsher PL, Wallace RB, Loeffelholz PL, Sales M. Health status of older male prisoners: A comprehensive review. American Journal of Public Health. 1992;82:881–884. doi: 10.2105/ajph.82.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin TJ, Lincoln T, Tuthill RW. Self-reported health and prior health behaviors of newly admitted correctional inmates. American Journal of Public Health. 2000;90:1939–1941. doi: 10.2105/ajph.90.12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropsey KL, Eldridge GD, Weaver MF, Villalobos GC, Stitzer ML, Best AM. Smoking cessation intervention for female prisoners: addressing an urgent public health need. American Journal of Public Health. 2008;98:1894–901. doi: 10.2105/AJPH.2007.128207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropsey KL, Eldridge GD, Ladner T. Smoking among female prisoners: an ignored public health epidemic. Addictive Behaviors. 2004;29:425–431. doi: 10.1016/j.addbeh.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Cropsey KL, Eldridge GD, Weaver MF, Villalobos GC, Stitzer ML. Expired carbon monoxide levels in self-reported smokers and non-smokers in prison. Nicotine & Tobacco Research. 2006;8:653–659. doi: 10.1080/14622200600789684. [DOI] [PubMed] [Google Scholar]
- Cropsey KL, Kristeller JL. Motivational factors related to quitting smoking among prisoners during a smoking ban. Addictive Behaviors. 2003;28:1081–1093. doi: 10.1016/s0306-4603(02)00230-7. [DOI] [PubMed] [Google Scholar]
- Cropsey KL, Kristeller JL. The effects of a prison smoking ban on smoking behavior and withdrawal symptoms. Addictive Behaviors. 2005;30:589–594. doi: 10.1016/j.addbeh.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Falba T, Jofre-Bonet M, Busch S, Duchovny N, Sindelar J. Reduction of quantity smoked predicts future cessation among older smokers. Addiction. 2004;99:93–102. doi: 10.1111/j.1360-0443.2004.00574.x. [DOI] [PubMed] [Google Scholar]
- Farkas AJ. When does cigarette fading increase the likelihood of future cessation? Annals of Behavioral Medicine. 1999;21:1–6. doi: 10.1007/BF02895036. [DOI] [PubMed] [Google Scholar]
- Hall SM, Muñoz RF, Reus VI. Cognitive-behavioral intervention increases abstinence rates for depressive-history smokers. Journal of Consulting and Clinical Psychology. 1994;62:141–146. doi: 10.1037//0022-006x.62.1.141. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Carpenter MJ. Does smoking reduction increase future cessation and decrease disease risk? A qualitative review. Nicotine and Tobacco Research. 2006;8:739–49. doi: 10.1080/14622200600789726. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Cummings KM, Hyland A. Ability of smokers to reduce their smoking and its association with future smoking cessation. Addiction. 1999;94:109–114. doi: 10.1046/j.1360-0443.1999.9411097.x. [DOI] [PubMed] [Google Scholar]
- Kauffman RM, Ferketich AK, Wewers ME. Tobacco policy in American prisons, 2007. Tobacco Control. 2008;17:357–360. doi: 10.1136/tc.2007.024448. [DOI] [PubMed] [Google Scholar]
- Lindson N, Aveyard P, Hughes JR. Reduction versus abrupt cessation in smokers who want to quit. Cochrane Database of Systematic Reviews. 2010;(3) doi: 10.1002/14651858.CD008033.pub2. Art. No.: CD008033. [DOI] [PubMed] [Google Scholar]
- Maruschak LM, Beck AL. Medical problems of inmates, 1997. U.S. Department of Justice, Bureau of Justice Statistics; 2001. NCJ 181644. [Google Scholar]
- Pisinger C, Vestbo J, Borch-Johnsen K, Jørgensen T. Smoking reduction intervention in a large population-based study. The Inter99 study. Preventive Medicine. 2005;40:112–118. doi: 10.1016/j.ypmed.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM. Extinguishing the rewarding value of smoke cues: pharmacological and behavioral treatments. Nicotine and Tobacco Research. 2004;6:523–32. doi: 10.1080/14622200410001696501. [DOI] [PubMed] [Google Scholar]
- Shadel WG, Shiffman S, Niaura R, Nichter M, Abrams DB. Current models of nicotine dependence: What is known and what is needed to advance understanding of tobacco etiology among youth. Drug Alcohol Depend. 2000b;59:9–22. doi: 10.1016/s0376-8716(99)00162-3. [DOI] [PubMed] [Google Scholar]
- Sieminska A, Jassem E, Konopa K. Prisoners' attitudes towards cigarette smoking and smoking cessation: a questionnaire study in Poland. BMC Public Health. 2006;6:181, 1–9. doi: 10.1186/1471-2458-6-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MD, Weinstock MC, Herman DS, Anderson BJ. Respiratory symptom relief related to reduction in cigarette use. Journal of General Internal Medicine. 2005;20:889–894. doi: 10.1111/j.1525-1497.2005.0190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]