Abstract
Objective
To assess the effects of lisdexamfetamine dimesylate (LDX) on executive function (EF) behaviors in children with attention-deficit/hyperactivity disorder (ADHD).
Methods
This observational, open-label, 7-week, dose-optimization study of LDX (20–70 mg/day) in children with ADHD evaluated efficacy with the ADHD Rating Scale IV; safety measures included adverse events (AEs). EF was assessed with the Behavior Rating Inventory of Executive Function (BRIEF). Post hoc analyses examined BRIEF scores by sex, ADHD subtype, comorbid psychiatric symptoms, and common treatment-emergent AEs (TEAEs). ADHD Rating Scale IV scores were assessed in subjects categorized by baseline BRIEF global executive composite T scores with clinically significant (≥65) or not clinically significant (<65) impairment in EF.
Results
Mean (standard deviation) change from baseline to endpoint for BRIEF of −17.9 (12.5) for Global Executive Composite, −15.4 (12.6) for Behavioral Regulation Index, and −17.6 (12.3) for Metacognition Index demonstrated improvement with LDX (pooled doses; p < 0.0001 for all). Improvements in BRIEF scores were seen regardless of sex, ADHD subtype, comorbid psychiatric symptoms, common TEAEs, or baseline EF impairment category. TEAEs included decreased appetite, decreased weight, irritability, insomnia, headache, upper abdominal pain, and initial insomnia.
Conclusions
Improvements were demonstrated in EF behaviors and ADHD symptoms with LDX. LDX safety profile was consistent with long-acting stimulant use.
Introduction
Executive function (EF) is defined as a group of processes (e.g., inhibition, working memory, and the ability to plan and organize) (Gioia et al. 2000) that are dependent on and, in turn, influence more basic cognitive abilities such as attention, language, and perception. This top-down model of behavior control posits that the EFs are collectively essential for setting goals and solving problems (Miller and Cohen 2001). Similar to the functional organization of the frontal lobe (Badre 2008), EF is theorized to be hierarchically organized and may best be viewed as involving multiple levels of increasingly complex functioning, including new, more multifaceted abilities, skills, and goals and increasingly intricate nested sets of behavioral sequences needed to achieve those goals via management of lower levels (Changeux and Dehaene 2000).
Barkley (1997) proposed the hypothesis that attention-deficit/hyperactivity disorder (ADHD) symptoms may be due to EF deficits. In a meta-analysis of 83 studies, children and adolescents with ADHD exhibited significant deficits compared to those without ADHD in neuropsychological measures of EF; the EF domains that showed impairments included planning, spatial and verbal working memory, response inhibition, and vigilance (Willcutt et al. 2005). The primacy of EF deficits in ADHD has not been clearly established, with some experts (Scheres et al. 2004; Willcutt et al. 2005) accepting that some—but not all—patients with ADHD suffer from significant EF deficits; other experts assert that all patients with ADHD have EF deficits and that ADHD is essentially a developmental impairment of EF (Brown 2006).
Laboratory neuropsychological tests evaluate specific components of tasks thought to be associated with EF (Pennington and Ozonoff 1996). For those children with ADHD who had EF deficits identified by neuropsychological tests, outcomes may be worse than in those without identifiable EF impairments. Children with ADHD having EF impairments demonstrated in a laboratory setting also had worse task performance in a real-life setting than did control subjects without executive dysfunction (Lawrence et al. 2002, 2004). Moreover, children with both ADHD and executive dysfunction are at greater risk for poor academic outcomes (e.g., learning disabilities or repeating a grade) than are children diagnosed with ADHD without concurrent executive dysfunction (Biederman et al. 2004). Impairments in specific EFs identified with neuropsychological tests appear to be associated with worse outcome for subjects with ADHD. However, these tests have demonstrated considerable variability from patient to patient (Doyle 2006).
Significant group differences exist in neuropsychological testing between subjects with and without ADHD (Willcutt et al. 2005), but no more than half of tested subjects with ADHD exhibit impairment on any particular neuropsychological test. In fact, inhibitory control, the most common ADHD-associated EF impairment, has been found in only 40%–50% of subjects (Doyle 2006). Thus, impaired neuropsychological performance may be generally predictive of ADHD, but scores in the normal range do not rule out ADHD. This may be because EF neuropsychological impairment is not a universal feature of ADHD, because the subject developed adequate compensatory mechanisms to offset impairment on a specific test, or because the tests chosen were not the appropriate ones for the particular subject (Doyle 2006).
Most traditional tests of neuropsychological function do not correlate well with real-world or ecological functional outcomes (Gioia et al. 2000). Some researchers believe that EF impairments may be more pervasive and more apparent in observations or assessments of how patients with ADHD perform daily tasks over time. Such assessments provide a complementary perspective to laboratory tests. This has resulted in the development of parent-reported questionnaire assessments of EF behaviors, theoretically based on how specific traits apparent in neuropsychological tests may be observable in day-to-day behavior. The Behavior Rating Inventory of Executive Function (BRIEF) is one such tool designed to evaluate EF using eight domains of executive functioning: Inhibition, shifting (i.e., moving freely from one thing to another), emotional control, initiation (i.e., beginning a task), working memory, planning/organizing, organizing materials, and monitoring (i.e., checking work) (Gioia et al. 2000). The BRIEF has previously been used in children with and without ADHD to evaluate EF behaviors (Jarratt et al. 2005; Bodnar et al. 2007; Mahone and Hoffman 2007). The BRIEF was designed to assess real-world EF behaviors in the home and/or school as assessed by parents and teachers using a behavior rating scale (Gioia et al. 2000) and may capture deficits not evident on isolated laboratory performance tests. The BRIEF has demonstrated convergent and divergent validity with a number of measures of behavioral and emotional functioning, respectively (Gioia et al. 2000). Recent data suggest partial convergence between neuropsychological testing of EF and the behavioral ratings of EF as assessed by the parent- and teacher-rated BRIEF (Toplak et al. 2009).
Emotional regulation is thought to be an important aspect of EF and is one of the domains assessed by the BRIEF (Gioia et al. 2000). Alterations in emotional regulation due to problems related to behavioral inhibition have been hypothesized to be important components in the EF deficits associated with ADHD (Barkley 1997). Other experts posit that managing frustration and regulating emotion comprise one of several cognitive functions affected by deficits in executive functioning (Gioia et al. 2002; Brown 2006). Accompanying analysis of EF with concurrent analysis of emotional regulation may provide a more complete assessment of the impact and functional impairments associated with ADHD.
Stimulants have demonstrated efficacy in the management of ADHD symptoms in children (Brown et al. 2005). Moreover, stimulants may improve EF; in an open-label pilot study, adults diagnosed with ADHD who were administered a long-acting stimulant had significant improvement in EF (p ≤ 0.0085) (Fallu et al. 2006). Emotional regulation may also be impacted by the administration and subsequent wearing off of a stimulant. Thirty percent of children with ADHD who were given a stimulant experienced some rebound when the medication began to wear off before the next dose; symptoms included sadness, crying, insomnia, irritability, or euphoria, but this rebound was serious in only 8.7% of the cases (Carlson and Kelly 2003). Switching medications from a stimulant to either a different stimulant or a nonstimulant has also been shown to improve emotional expression in some children with ADHD (Kratochvil et al. 2007).
Lisdexamfetamine dimesylate (LDX; Vyvanse®; Shire US Inc.) is the first long-acting prodrug stimulant and is indicated for the treatment of ADHD in children 6–12 years of age and in adults. LDX is a therapeutically inactive molecule (Leroux et al. 2009). After oral ingestion, LDX is converted to l-lysine and active d-amphetamine, which is responsible for the therapeutic effect. Although a small amount of LDX is hydrolyzed to d-amphetamine in the gastrointestinal tract, the conversion of LDX into active d-amphetamine occurs primarily in the blood. The combination of l-lysine and d-amphetamine created a new chemical entity with a prodrug technology of delivery of d-amphetamine (Pennick 2010).
In a randomized, controlled trial in children with ADHD, LDX was effective throughout the day, up to 6 p.m., as measured by parent ratings. Efficacy was also demonstrated by clinician measures (Biederman et al. 2007b). The most common adverse events (AEs) associated with LDX included decreased appetite, insomnia, abdominal pain, and irritability (Biederman et al. 2007b). LDX was effective from 1.5 to 13 hours postdose in a randomized, controlled trial in children in a laboratory classroom study (Wigal et al. 2009). AEs were consistent with other pediatric studies of LDX.
A 7-week, open-label, dose-optimization study of LDX in children aged 6–12 years with ADHD provided an opportunity to further evaluate the effectiveness and safety of LDX and to evaluate the impact of LDX on EF (Findling et al. 2009). This analysis presents a priori and post hoc analyses of data related to EF and emotional expression with the goals of providing a broad assessment of EF deficits and LDX treatment-related improvements in EF in children with ADHD, and to examine whether factors such as sex, ADHD subtype, and comorbid psychiatric symptoms influence EF.
Methods
Study design
This prospective, open-label, multicenter (46 centers throughout the United States; 42 sites enrolled subjects), dose-optimization study was conducted between June 2007 and January 2008 to evaluate the efficacy and safety of LDX (20, 30, 40, 50, 60, and 70 mg/day) in children aged 6–12 years with a primary diagnosis of ADHD. The study protocol was approved by the institutional review board at each study center, and the study was performed in accordance with the principles of the International Conference on Harmonization Good Clinical Practice, 18th World Medical Assembly (Helsinki, 1964), and amendments of the 29th (Tokyo, 1975), the 35th (Venice, 1983), the 41st (Hong Kong, 1989), and the 48th (South Africa, 1996) World Medical Assemblies. All subjects' parents or legally authorized representatives read and signed an informed consent form.
The study consisted of three phases: (1) Screening and medication washout (2 weeks); (2) open-label dose optimization (5 weeks) and maintenance (2 weeks); and (3) 30-day safety follow-up.
Dose-optimization phase
The 5-week dose-optimization phase was initiated after the completion of the baseline visit (visit 0); the morning after the baseline visit, all subjects were administered LDX at a dosage of 20 mg/day for the entire week. An acceptable response was defined as a significant reduction in ADHD symptoms, defined by a ≥ 30% reduction in the ADHD Rating Scale IV (ADHD-RS-IV) score and a Clinical Global Impressions-Improvement scale score of 1 (very much improved) or 2 (much improved) with tolerable side effects. If a subject had an acceptable response, that dosage was maintained for the remainder of the study. If an acceptable response was not achieved, the dosage was increased in a stepwise manner by 10 mg/week (i.e., LDX dosages available: 20–70 mg/day). In the event of intolerable side effects during the dose-optimization phase, one downward titration was permitted, but if side effects persisted at the next lower dose, the subject was discontinued from the study. Week 5 was the last visit that a dose could be modified; subjects who did not achieve an acceptable response or had intolerable AEs were discontinued from the study. During the titration phase, if an acceptable dose was well tolerated but, in the opinion of the clinical investigator, a subject could potentially achieve greater symptom reduction, the dose could be increased to the next strength.
Maintenance and follow-up phase
The maintenance phase of the study was during weeks 6 and 7, at which visits efficacy and safety assessments were completed. For follow-up, subjects were contacted by telephone ∼30 days after the last dose of LDX to monitor for the presence of ongoing or new AEs, serious AEs, and concomitant medications. Follow-up continued until all AEs were resolved.
Subjects
Male and female children, aged 6–12 years, with a primary diagnosis of ADHD according to the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision (DSM-IV-TR) (American Psychiatric Association 2000), and a baseline ADHD-RS-IV score ≥28 were eligible for the study. Additional eligibility criteria included blood pressure measurements within the 95th percentile for age, sex, and height. Subjects diagnosed with conduct disorder, a comorbid psychiatric disorder with significant symptoms, and a history (within the past 6 months) of or suspected substance abuse or dependence disorder (excluding nicotine) were excluded from the study. Subjects were also excluded if they had a positive urine drug test, clinically significant (CS) electrocardiogram or laboratory abnormality, weight <50 lb, or body mass index >98th percentile. Subjects well controlled on their current ADHD therapy were ineligible for the study as well as were individuals taking other medications that have central nervous system effects or affect performance.
Efficacy and safety measures
The primary efficacy measure was the clinician-completed ADHD-RS-IV score (Faries et al. 2001), based on the parent and teacher ADHD-RS developed by DuPaul et al. (1998), at endpoint (the score collected on day 49 or the last score collected postbaseline) compared with the ADHD-RS-IV score at baseline. ADHD-RS-IV measurements were collected at baseline and weekly during the dose-optimization and maintenance phase. Three scores were derived from the ADHD-RS-IV: The total score and 2 subscales (inattention and hyperactivity-impulsivity), which evaluate the 18 Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (American Psychiatric Association 1994) symptom domains of ADHD. Items on the ADHD-RS-IV are scored from 0 (never or rarely exhibits symptom) to 3 (very often exhibits symptom); ADHD-RS-IV total score can range from 0 to 54, whereas each subscale score can range from 0 to 27 (DuPaul et al. 1998; Faries et al. 2001).
Other efficacy measures collected during this study included the Expression and Emotion Scale for Children (EESC) (Perwien et al. 2008) and the BRIEF. The parent-rated EESC was administered at baseline and the final study visit (visit 7 or early termination). The EESC includes 29 questions, with a 5-point rating scale ranging from 1 (not at all true) to 5 (very much true) and has three subscales: Emotional flatness, positive emotions, and emotional lability (Perwien et al. 2008). Questions are designed to allow parents to rate characteristics of their child's emotional expression: My child does not talk enough; my child's mood is flat; my child has sparkle in his/her personality; my child seems happy; my child gets upset easily; my child's personality seems “dampened,” etc.
The BRIEF was administered at baseline and the final study visit (visit 7 or early termination). This parent-rated measure contains 86 items, scored as 1 (never), 2 (sometimes), or 3 (often) (Gioia et al. 2002). These 86 items are categorized based on assessment of eight domains of EF behaviors (Gioia et al. 2000): Emotional control (e.g., overreacts to small problems); initiate (e.g., is not a self-starter); working memory (e.g., when given three things to do, only remembers the first or last); organization of materials (e.g., leaves playroom a mess); shift (e.g., tries the same approach to a problem over and over when it does not work); plan/organize (e.g., does not bring home homework, assignment sheets, materials, etc.); monitor (e.g., does not check work for mistakes); and inhibit (e.g., interrupts others). Although several factor models for the BRIEF have been proposed, the current study used the original model in which the BRIEF has three summation indices: The Behavioral Regulation Index (BRI), which comprises inhibit, shift, and emotional control domains; the Metacognition Index (MCI), which comprises working memory, initiate, plan/organize, organization of materials, and monitor domains; and the Global Executive Composite (GEC) index, which includes all eight of the previously described domains. Each subject's raw BRIEF index score, GEC, BRI, MCI, and individual BRIEF domains were used to derive standardized T scores from tables in the BRIEF Professional Manual (Gioia et al. 2000). BRIEF T scores can be categorized as CS (scores ≥65, 1½ standard deviation [SD] above the population norm) or not clinically significant (scores <65, <1½ SD above the population norm), with higher BRIEF T scores indicating poor executive functioning (Mares et al. 2007; McCandless and O'Laughlin 2007). The relationship between scores on the ADHD-RS-IV total and subscale scores and GEC composite score of the BRIEF were analyzed post hoc for correlations using Pearson correlation coefficients.
Post hoc analyses were also conducted for treatment outcomes by sex, by ADHD subtype, and by presence or absence of history of other psychiatric symptoms including psychiatric diagnosis or condition (e.g., oppositional defiant disorder, depression, insomnia, initial insomnia, and emotional lability) significant enough to be reported on the medical history intake form, but not enough to be exclusionary and in subjects with the presence or absence of common treatment-emergent AEs (TEAEs; i.e., reported by ≥5% of subjects). For some analyses, subjects were categorized by ADHD-RS-IV subtypes for assessment of change from baseline in BRIEF subscales scores.
TEAEs were ascertained by nonleading questions asked by the investigator and reported for this study. TEAEs were collected at screening, baseline, weekly during the dose-optimization and maintenance phase, and during the follow-up phase. TEAEs, defined as events that started on or after the first day of receiving LDX and no later than 3 days after the cessation of receiving LDX, were coded by using the Medical Dictionary for Regulatory Affairs (MedDRA, Version 10.0).
Statistics
Efficacy of LDX was evaluated in the intention-to-treat population, which was defined as all patients who received ≥1 dose of LDX and had ≥1 postbaseline ADHD-RS-IV measurement. The safety and tolerability of LDX was evaluated in the safety population, which was defined as all patients who received ≥1 dose of LDX.
The primary efficacy measure, or the change in ADHD-RS-IV total score from baseline to endpoint, was analyzed by a one-sample t-test. Endpoint was defined as the last total ADHD-RS-IV measure, which for most patients was during visit 7, or the last visit of the dose-optimization and maintenance phase.
Results
Demographics and disposition of subjects have been previously reported (Findling et al. 2009). Some pertinent applicable findings are reviewed here. Of enrolled subjects, 50 required and completed medication washout. The safety population (n = 317) had a mean (SD) age of 9.1 (1.9) years and was predominantly male (70.7%) and white (70.7%); 87.4% completed the study. Of the subjects who discontinued the study (n = 40), 4.1% of withdrawals were attributed to AEs and 0.6% were attributed to lack of efficacy. Combined ADHD subtype was diagnosed in 259 (81.7%), inattentive in 54 (17.0%), and hyperactive-impulsive in 4 (1.3%).
Primary and some secondary efficacy measures have also been previously reported (Findling et al. 2009). The mean (SD) change in ADHD-RS-IV total score from baseline to endpoint and at each weekly visit demonstrated significant improvement (p < 0.0001). The change in EESC score from baseline to endpoint demonstrated a mean (SD) change in the total score of −7.4 (18.3) (p < 0.0001).
At baseline, mean (SD) BRIEF T scores for the three indices for all doses were 74.0 (8.9) for the GEC, 71.1 (11.8) for the BRI, and 73.1 (8.4) for the MCI (Table 1). At endpoint, mean (SD) for each index T score had decreased to <65 for subjects by final dose and for all doses combined. At endpoint, mean (SD) index T score for all doses was 56.1 (12.0) for GEC, 55.7 (12.5) for BRI, and 55.5 (11.5) for MCI (Table 1).
Table 1.
Behavior Rating Inventory of Executive Function T Scores at Baseline and End of Study by Final Dose Level (Intention-to-Treat Population, n = 308)
| |
LDX, final dose level |
|
|||||
|---|---|---|---|---|---|---|---|
| 20 mg/day (n = 31) | 30 mg/day (n = 67) | 40 mg/day (n = 60) | 50 mg/day (n = 70) | 60 mg/day (n = 47) | 70 mg/day (n = 33) | All doses (n = 308) | |
| Global Executive Composite, mean (SD) | |||||||
| Baseline | 73.1 (8.0) | 70.9 (8.8) | 74.9 (8.3) | 73.5 (9.8) | 77.2 (8.2) | 76.1 (8.2) | 74.0 (8.9) |
| End of study | 55.0 (13.4) | 50.3 (9.8) | 56.2 (11.7) | 55.3 (11.0) | 61.2 (12.6) | 63.2 (10.3) | 56.1 (12.0) |
| Behavioral Regulation Index, mean (SD) | |||||||
| Baseline | 69.3 (11.0) | 66.8 (11.5) | 71.7 (12.3) | 70.6 (11.7) | 75.2 (10.5) | 75.8 (11.0) | 71.1 (11.8) |
| End of study | 53.9 (13.1) | 49.9 (9.6) | 56.3 (12.7) | 54.8 (11.5) | 60.7 (14.2) | 63.5 (10.6) | 55.7 (12.5) |
| Metacognition Index, mean (SD) | |||||||
| Baseline | 73.0 (8.1) | 71.1 (8.6) | 74.0 (7.1) | 72.6 (9.9) | 75.3 (7.8) | 73.5 (7.5) | 73.1 (8.4) |
| End of study | 54.8 (13.1) | 50.4 (10.1) | 55.6 (11.0) | 54.9 (10.9) | 60.1 (11.7) | 61.4 (9.9) | 55.5 (11.5) |
Behavior Rating Inventory of Executive Function, with T scores ≥65 indicative of clinically significant executive dysfunction and scores <65 as clinically insignificant.
Abbreviations: LDX = lisdexamfetamine dimesylate; SD = standard deviation.
Of the 308 subjects in the intention-to-treat population who had both baseline and endpoint (visit 7 or early termination) BRIEF measurements, there was a significant improvement in BRIEF indices, as demonstrated by the mean (SD) changes in the GEC (−17.9 [12.5], p < 0.0001), BRI (−15.4 [12.6], p < 0.0001), and MCI (−17.6 [12.3], p < 0.0001). Moreover, there was an improvement in all eight domains of the BRIEF; the mean change in T score from baseline to the last visit significantly improved for all eight domains (p < 0.0001) (Table 2).
Table 2.
Mean Behavior Rating Inventory of Executive Function T Score Changes from Baseline to End-of-Study Visit (Intention-to-Treat Population)
| BRIEF subscale | Mean (SD) changea |
|---|---|
| Inhibit | −16.3 (12.4) |
| Shift | −12.6 (12.9) |
| Emotional control | −10.9 (12.8) |
| Initiate | −13.8 (11.8) |
| Working memory | −17.9 (12.4) |
| Plan/organize | −16.6 (12.6) |
| Organization of materials | −10.6 (10.0) |
| Monitor | −14.7 (12.4) |
p < 0.0001 for all subscales based on one-sample t-test.
Abbreviation: BRIEF = Behavior Rating Inventory of Executive Function; SD = standard deviation.
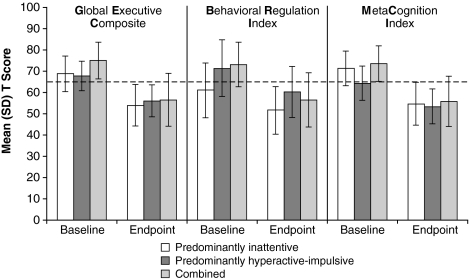
For the evaluation of treatment outcome by ADHD subtype, the mean (SD) GEC T scores for subjects with inattentive, hyperactive-impulsive, and combined ADHD subtypes were CS at baseline. Also at baseline, the mean (SD) MCI T score was CS in the inattentive and the combined subtype, but not the hyperactive-impulsive (T = 64.3 [8.1]), whereas the mean (SD) BRI T score was CS in the combined and hyperactive-impulsive subtypes but not the inattentive (T = 61.2 [12.8]). At endpoint, scores of none of the BRIEF indices remained CS (Fig. 1).
FIG. 1.
Mean (SD) BRIEF T scores at baseline and end of study by ADHD subtype (intention-to-treat population). At baseline and endpoint, n = 54 and 51 for the inattentive, n = 4 and 4 for the hyperactive-impulsive, n = 257 and 253 for the combined groups, respectively. Dashed line represents standardized T score of 65. T scores ≥65 have potential clinical significance. Error bars indicate SD. ADHD = attention-deficit/hyperactivity disorder; BRIEF = Behavior Rating Inventory of Executive Function; SD = standard deviation.
Analysis demonstrated that both sexes had CS baseline T scores and improvement on all indices. For female subjects with both baseline and endpoint BRIEF scores (n = 90), mean (SD) baseline GEC, BRI, and MCI scores were 76.1 (9.9), 72.1 (12.1), and 76.0 (9.4), respectively. For male subjects with both baseline and endpoint BRIEF scores (n = 218), mean (SD) baseline GEC, BRI, and MCI scores were 73.1 (8.3), 70.7 (11.6), and 71.9 (7.7), respectively. At endpoint, scores of none of the BRIEF indices remained CS, and all showed significant improvement (p < 0.0001) from baseline for both female and male subjects. Mean (SD) changes from baseline scores for female subjects were −19.4 (12.4), −15.9 (12.5), and −19.8 (12.3) for GEC, BRI, and MCI indices, respectively. Mean (SD) changes from baseline scores for male subjects were −17.3 (12.5), −15.2 (12.6), and −16.6 (12.3) for GEC, BRI, and MCI indices, respectively.
Post hoc assessment showed significant (p < 0.0001) correlation coefficients of 0.509, 0.396, and 0.346 between baseline BRIEF GEC index and ADHD-RS-IV total, hyperactivity/impulsivity, and inattentiveness subscale scores, respectively. At endpoint, correlation coefficients between BRIEF GEC index and ADHD-RS-IV total, hyperactivity/impulsivity, and inattentiveness subscale scores were 0.648, 0.554, and 0.636, respectively (p < 0.0001). Similarly, for change from baseline to endpoint scores, correlation coefficients between BRIEF GEC index and ADHD-RS-IV total, hyperactivity/impulsivity, and inattentiveness subscale scores were 0.605, 0.505, and 0.548, respectively (p < 0.0001).
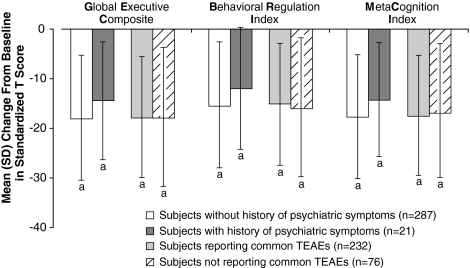
Similar findings were observed for subjects with and without a history of other psychiatric symptoms; LDX was effective for both groups: At baseline, the mean T scores for all three BRIEF indices were >65. At endpoint, the mean (SD) change in GEC, BRI, and MCI scores for subjects with and without a history of comorbid psychiatric symptoms, psychiatric diagnosis, or condition (including oppositional defiant disorder, depression, insomnia, initial insomnia, and emotional lability) was improved compared with baseline scores (Fig. 2). Also, for subjects who reported common TEAEs (those with overall incidence ≥5%) and for those who did not report common TEAEs, LDX was effective; at baseline, the mean T scores for all three BRIEF indices were >65. At endpoint, the mean (SD) change in GEC, BRI, and MCI scores for subjects who did or did not report commonly occurring TEAEs improved compared with baseline scores (Fig. 2).
FIG. 2.
Mean BRIEF T score changes from baseline at end of study/early termination by history of psychiatric symptoms on medical history and by incidence of common TEAEs (intention-to-treat population). Common TEAEs were those reported by ≥5% of patients with both baseline and end-of-study BRIEF assessments (i.e., upper abdominal pain, affect lability, decreased appetite, headache, initial insomnia, insomnia, irritability, nausea, vomiting, and decreased weight). ap ≤ 0.0002 based on one-sample t-test versus baseline. Error bars indicate SD. TEAEs = treatment-emergent adverse events.
The safety profile of LDX was evaluated in the safety population (n = 317), which was defined as all subjects who received ≥1 dose of medication. Detailed safety data for this study are presented elsewhere (Findling et al. 2009). There were no deaths and the incidence of serious TEAEs was low (two subjects). Of the subjects who had serious TEAEs, one subject had syncope and the other had sinus pauses, but both of these subjects had a history of cardiovascular issues. The overall incidence of TEAEs was 84.9%. The most common TEAEs were decreased appetite, decreased weight, insomnia, irritability, headache, upper abdominal pain, and initial insomnia. These TEAEs were of mild to moderate severity in the majority of subjects (Findling et al. 2009).
Discussion
The results of this study indicate that LDX was effective in improving parent ratings of behaviors related to executive dysfunction and investigator ratings of ADHD symptoms in children with ADHD. Overall, emotional expression also improved slightly in children with LDX treatment. Significant improvement in BRIEF composite, index, and domain scores as well as improvement in ADHD-RS-IV scores from baseline to endpoint were seen. EF behaviors improved from baseline BRIEF index T scores that were indicative of CS executive dysfunction to endpoint scores that were no longer considered CS.
A number of studies using the BRIEF have characterized EF behavior deficits and assessed the extent of the functional impact of such EF deficits in children and adolescents with ADHD (Jarratt et al. 2005; Riccio et al. 2006; Mares et al. 2007; McCandless and O'Laughlin 2007; Qian et al. 2007; Qian and Wang 2007; Sullivan and Riccio 2007; Toplak et al. 2009). To date, few published studies have analyzed the effects of treatment on aspects of EF as measured by the BRIEF. Unlike the study by Qian et al. (2007) of children with ADHD, which showed improvement in EF behaviors with long-acting oral osmotic-release methylphenidate treatment in GEC, MCI, and some individual domain scores (working memory, monitor, and inhibition), there was consistent improvement in all BRIEF indices and subscales in this study with LDX.
Significant correlations between ADHD-RS-IV total scores and BRIEF GEC index were demonstrated. While r values for correlations of endpoint and change scores were generally in the range considered high, those at baseline were somewhat lower, in the medium range. There has been limited analysis of the correlation between ADHD symptoms and behavioral domains of EF. In a recent study of adolescents with ADHD, no significant associations were noted between ADHD symptoms and performance-based (e.g., neuropsychologic) measures of EF. Conversely, parent-rated inattention and hyperactivity/impulsivity on the Conners' Parent Rating Scales were significantly correlated (p = 0.001) with parent-rated BRIEF scores (Toplak et al. 2009). In a recent neuropsychiatric assessment of effortful control in children with varying levels of ADHD symptoms, children with higher scores for ADHD symptoms demonstrated poorer effortful control by several measures (Wiersema and Roeyers 2009). Although the findings of the current study indicate that EF behaviors may be associated with specific ADHD symptomatology and that improvements in EF behaviors track with ADHD symptom improvement, this correlation analysis cannot provide direct evidence that specific executive dysfunctions cause these symptoms. Additionally, the level of correlation between the BRIEF and ADHD-RS-IV seen in the current study raises interesting questions related to the degree of commonality between assessments of ADHD symptoms and of EF behaviors: How do these assessments overlap and how do they diverge? Addressing such questions with further analysis may provide useful information on the clinical utility of applying broadened assessment measures to obtain a more complete picture of the impact of ADHD and the benefits of treatment.
In a further analysis of ADHD symptom groups (e.g., combined, inattentive, and hyperactive-impulsive subtypes), the majority of baseline mean EF GEC, BRI, and MCI scores for subjects categorized with all three ADHD subtypes were >65 and considered CS. The exceptions at baseline are, again, consistent with what one might expect: Those diagnosed with predominantly inattentive subtype of ADHD did not reach the CS level on the BRIEF and those found to have predominantly hyperactive-impulsive ADHD subtype did not reach the CS level on the MCI. All mean BRIEF index scores, regardless of ADHD subtype, improved and were <65 at study endpoint. A difference in the EF of subjects by ADHD subtype has previously been observed, although these results differ between studies (Gioia et al. 2000; Geurts et al. 2005; Willcutt et al. 2005). A meta-analysis found differences in EF of subjects with combined or inattentive ADHD subtype, although subjects with hyperactive-impulsive ADHD subtype appeared to have fewer EF deficits (Willcutt et al. 2005). In another study, children with combined ADHD subtype had deficits in the inhibition domain of EF compared with normal controls, whereas no differences in EF were found between the children with combined ADHD subtype and children with inattentive ADHD subtype (Geurts et al. 2005). Sex differences in another study may have confounded the results in detecting differences between the EF of the different ADHD subtypes; although children with either inattentive or combined ADHD subtype did not exhibit differences in EF, when male subjects were evaluated separately, differences in EF between the two ADHD subtypes were demonstrated (Riccio et al. 2006).
For subjects with and without history of other psychiatric symptoms, EF behavior dysfunction was similar as demonstrated at baseline by T scores >65. At endpoint, significant improvement to scores <65 was seen regardless of history of comorbid psychiatric symptoms. EF deficits have been found in patients with other neuropsychiatric disorders such as depression (DeBattista 2005), bipolar disorder (Quraishi and Frangou 2002; Doyle et al. 2005), and high-functioning autism (Geurts et al. 2004; Verté et al. 2005). However, there is no clear interrelationship between comorbid neuropsychiatric disorders and the occurrence or severity of EF deficits (Geurts et al. 2004; Oosterlaan et al. 2005; Sarkis et al. 2005). The findings of this study suggest that improvement in EF behavioral deficits with LDX treatment are not significantly affected by the presence or absence of comorbid psychiatric symptoms. Similarly, when evaluated by sex, CS impairment in EF was seen at baseline in both male and female subjects, and treatment with LDX resulted in significant improvement in EF for subjects of both sexes. Although EF and, for that matter, ADHD have been more extensively studied in boys (Biederman et al. 2008), evidence confirms that girls are affected by EF deficits in multiple function domains and settings (Houghton et al. 1999; Biederman et al. 2008; Wodka et al. 2008).
The majority of TEAEs reported in this study were mild to moderate in severity. TEAEs of highest incidence included decreased appetite, decreased weight, irritability, and insomnia. The incidence, profile, and severity of TEAEs were similar to those reported in other recent studies of LDX in children with ADHD (Biederman et al. 2007a, 2007b; Findling et al. 2008; Wigal et al. 2009), as well as similar to those reported for other long-acting stimulants (Wolraich et al. 2001; McCracken et al. 2003; McGough et al. 2005; Steele et al. 2006). In general, LDX demonstrated a safety profile consistent with that of long-acting stimulant use.
There are limitations to the ability to draw conclusions from this study based on inherent design features. As an open-label study without a comparator arm, the ability to draw definitive conclusions regarding overall efficacy and safety of LDX is limited. In addition, studies that evaluate executive dysfunction frequently use normal controls to assess the characteristics and severity of deficits found in the subjects with ADHD. Although normative scores for the BRIEF composite and subscores have been proposed to allow evaluation of EF deficits in the absence of such normal controls, the numbers of subjects assessed to date, especially in clinical settings outside the auspices of a research protocol, are limited. Additionally, this study used parent-rated, but not teacher-rated BRIEF assessments. The differing perspectives of parents and teachers can be a valuable source for broader and more thorough assessment when logistical and scientific considerations allow for analysis of both forms simultaneously. This study used post hoc data analyses, which evaluated baseline and treatment-related changes in EF where some subgroups of subjects were small (e.g., four subjects in the hyperactivity/impulsivity ADHD subtype group). Also, inherent in post hoc analysis, the ability to use statistical measures of hypothesis testing is limited. Further statistical analyses and additional studies may shed light on the important interrelationship of core ADHD symptoms and EF deficits. Finally, the study was of relatively short duration; therefore, it was not possible to evaluate long-term safety and effectiveness of LDX and its impact on EF.
In conclusion, treatment with LDX was associated with improvement in EF behaviors regardless of ADHD subtype, the presence or absence of comorbid psychiatric symptoms, or the occurrence of common TEAEs. Significant improvements were seen in all identified domains of EF behavior. This study in a large sample of children with ADHD provides evidence that optimized treatment of ADHD with the long-acting prodrug stimulant LDX was effective in reducing parent-reported EF behavioral deficits from levels considered CS and may, thus, provide relief from the negative impact of ADHD-related EF deficits in real-world settings. These findings provide additional evidence of the presence and extent of EF behavioral deficits from a post hoc analysis by sex in a large group of boys and girls with ADHD, adding substantially to the overall numbers of subjects assessed by this method. Overall, these findings strengthen the notion that real-world behaviors, reflecting EF deficits, adversely affect many children with ADHD, and the negative impact on different domains of EF behavior may improve significantly with ADHD pharmacotherapy.
Disclosures
Dr. Turgay received research grant support or served as a consultant or speaker for AstraZeneca, Canadian Counseling Foundation, Canadian Independent Films Institute, Canadian Psychiatric Association, CellTech, Eli Lilly, GlaxoSmithKline, Hospital for Sick Children, Janssen Cilag, Janssen Ortho, Lundback, Nestle, Novartis, Ontario Ministry of Children's Services, Ontario Psychiatric Association, Purdue Pharma, Sanofi Aventis, Shire Canada, Shire UK, Shire US, The Scarborough Hospital, Turkish Medical Research Council, University of Ottawa, and Wellesley Foundation Canada.
Dr. Ginsberg receives or has received research grant support from Abbott Laboratories, Alkermes, AstraZeneca, Bristol-Myers Squibb, Cephalon, Cyberonics, Eli Lilly, Forest, GlaxoSmithKline, Janssen, McNeil, Neurocrine Biosciences, Neuropharm, New River, Novartis, Organon, Ortho-McNeil, Otsuka, Pam Labs, Pfizer, Sanofi-Aventis, Seaside Therapeutics, Sepracor, Shire, Takeda, UCB Pharma, Validus, and Wyeth; served on a speaker's bureau for Alkermes, AstraZeneca, Bristol-Myers Squibb, Cephalon, Cyberonics, Eli Lilly, Forest, GSK, Janssen, Jazz, JDS Pharmaceuticals, McNeil, Novartis, Noven, Organon, Pamlab, Pfizer, Sanofi-Aventis, Schwarz, Sepracor, Shire, Takeda, UCB Pharma, Validus, and Wyeth; and is a consultant for AstraZeneca, Bristol-Myers Squibb, Cephalon, Cyberonics, Eli Lilly, Forest, GlaxoSmithKline, Janssen, McNeil, Novartis, Ortho-McNeil, Pfizer, Sanofi-Aventis, Sepracor, Shire, Takeda, UCB Pharma, Validus, and Wyeth.
Dr. Sarkis receives or has received research grant support from Abbott Laboratories, Addrenex, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Cephalon, Eli Lilly, GlaxoSmithKline, Janssen, Jazz Pharmaceuticals, Labopharm, Lexicor, Novartis, Organon, Ortho-McNeil, Pfizer, PGxHealth, PediaMed, Pharmacia, Repligen, Sanofi-Aventis, Sepracor, Shire, Somerset, Supernus, Synosia, Takeda, Targacept, and Wyeth; served on an advisory board for Eli Lilly; and served on a Speaker's Bureau for Eli Lilly, Forest Pharmaceuticals, and Novartis.
Dr. Jain or Saundra Jain receives or has received grant research support from Abbott, Addrenex, Aspect, Forest, Lilly, and Pfizer; served as a consultant for Addrenex, Impax, Lilly, and Shire; served on a speaker's bureau for Cyberonics, GlaxoSmithKline, Jazz, Pamlab, Pfizer, Shire, and Takeda; and received honorarium from Cyberonics, Forest, Jazz, Lilly, Pfizer, Roche, Shire, and Takeda.
Mr. Adeyi is a Shire employee and has stocks and/or stock options from Shire.
Dr. Gao is a Shire employee and has stocks and/or stock options from Shire.
Dr. Dirks is a Shire employee and has stocks and/or stock options from Johnson & Johnson and Shire.
Dr. Babcock is a Shire employee and has stocks and/or stock options from Shire.
Dr. Scheckner is a Shire employee and has stocks and/or stock options from Shire.
Dr. Richards is a Shire employee and has stocks and/or stock options from Shire.
Dr. Lasser is a Shire employee and has stocks and/or stock options from Shire.
Dr. Findling receives or has received research support, acted as a consultant and/or served on a speaker's bureau for Abbott, Addrenex, AstraZeneca, Biovail, Bristol-Myers Squibb, Forest, GlaxoSmithKline, Johnson & Johnson, KemPharm Lilly, Lundbeck, Neuropharm, Novartis, Noven, Organon, Otsuka, Pfizer, Sanofi-Aventis, Sepracor, Shire, Solvay, Supernus Pharmaceuticals, Validus, and Wyeth.
Acknowledgments
Clinical research was funded by Shire Development Inc. Authors directed writing assistance from Susan Kralian, Ph.D., and Michael Pucci, Ph.D., former and current employees of Health Learning Systems, respectively. Editorial assistance in the form of proofreading, copy editing, and fact checking was also provided by Health Learning Systems. Health Learning Systems was funded by Shire Development Inc. for support in writing and editing this article. Although the sponsor was involved in the design, collection, analysis, interpretation, and fact checking of information, the content of this article the ultimate interpretation, and the decision to submit it for publication in Journal of Child and Adolescent Psychopharmacology was made by the authors independently.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Association; 1994. DSM-IV. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Association; 2000. Text Revision (DSM-IV-TR) [Google Scholar]
- Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends Cogn Sci. 2008;12:193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Biederman J. Boellner SW. Childress A. Lopez FA. Krishnan S. Zhang Y. Lisdexamfetamine dimesylate and mixed amphetamine salts extended-release in children with ADHD: A double-blind, placebo-controlled, crossover analog classroom study. Biol Psychiatry. 2007a;62:970–976. doi: 10.1016/j.biopsych.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Biederman J. Krishnan S. Zhang Y. McGough JJ. Findling RL. Efficacy and tolerability of lisdexamfetamine dimesylate (NRP-104) in children with attention-deficit/hyperactivity disorder: A phase III, multicenter, randomized, double-blind, forced-dose, parallel-group study. Clin Ther. 2007b;29:450–463. doi: 10.1016/s0149-2918(07)80083-x. [DOI] [PubMed] [Google Scholar]
- Biederman J. Monuteaux MC. Doyle AE. Seidman LJ. Wilens TE. Ferrero F, et al. Impact of executive function deficits and attention-deficit/hyperactivity disorder (ADHD) on academic outcomes in children. J Consult Clin Psychol. 2004;72:757–766. doi: 10.1037/0022-006X.72.5.757. [DOI] [PubMed] [Google Scholar]
- Biederman J. Petty CR. Doyle AE. Spencer T. Henderson CS. Marion B, et al. Stability of executive function deficits in girls with ADHD: A prospective longitudinal followup study into adolescence. Dev Neuropsychol. 2008;33:44–61. doi: 10.1080/87565640701729755. [DOI] [PubMed] [Google Scholar]
- Bodnar LE. Prahme MC. Cutting LE. Denckla MB. Mahone EM. Construct validity of parent ratings of inhibitory control. Child Neuropsychol. 2007;13:345–362. doi: 10.1080/09297040600899867. [DOI] [PubMed] [Google Scholar]
- Brown RT. Amler RW. Freeman WS. Perrin JM. Stein MT. Feldman HM, et al. American Academy of Pediatrics Committee on Quality Improvement; American Academy of Pediatrics Subcommittee on Attention-Deficit/Hyperactivity Disorder: Treatment of attention-deficit/hyperactivity disorder: Overview of the evidence. Pediatrics. 2005;115:e749–e757. doi: 10.1542/peds.2004-2560. [DOI] [PubMed] [Google Scholar]
- Brown TE. Executive functions and attention deficit hyperactivity disorder: Implications of two conflicting views. Int J Disabil Dev Educ. 2006;53:35–46. [Google Scholar]
- Carlson GA. Kelly KL. Stimulant rebound: How common is it and what does it mean? J Child Adolesc Psychopharmacol. 2003;13:137–142. doi: 10.1089/104454603322163853. [DOI] [PubMed] [Google Scholar]
- Changeux JP. Dehaene S. Hierarchical neuronal modeling of cognitive functions: From synaptic transmission to the Tower of London. Int J Psychophysiol. 2000;35:179–187. doi: 10.1016/s0167-8760(99)00052-5. [DOI] [PubMed] [Google Scholar]
- DeBattista C. Executive dysfunction in major depressive disorder. Expert Rev Neurother. 2005;5:79–83. doi: 10.1586/14737175.5.1.79. [DOI] [PubMed] [Google Scholar]
- Doyle AE. Executive functions in attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67(Suppl 8):21–26. [PubMed] [Google Scholar]
- Doyle AE. Wilens TE. Kwon A. Seidman LJ. Faraone SV. Fried R, et al. Neuropsychological functioning in youth with bipolar disorder. Biol Psychiatry. 2005;58:540–548. doi: 10.1016/j.biopsych.2005.07.019. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ. Power TJ. Anastopoulos AD. Reid R. Checklists, Norms, and Clinical Interpretation. New York, NY: Guilford Press; 1998. ADHD Rating Scale—IV. [Google Scholar]
- Fallu A. Richard C. Prinzo R. Binder C. Does OROS-methylphenidate improve core symptoms and deficits in executive function? Results of an open-label trial in adults with attention deficit hyperactivity disorder. Curr Med Res Opin. 2006;22:2557–2566. doi: 10.1185/030079906X154132. [DOI] [PubMed] [Google Scholar]
- Faries DE. Yalcin I. Harder D. Heiligenstein JH. Validation of the ADHD Rating Scale as a clinician administered and scored instrument. J Atten Disord. 2001;5:107–115. [Google Scholar]
- Findling RL. Childress AC. Krishnan S. McGough JJ. Long-term effectiveness and safety of lisdexamfetamine dimesylate in school-aged children with attention-deficit/hyperactivity disorder. CNS Spectrums. 2008;13:614–620. doi: 10.1017/s1092852900016898. [DOI] [PubMed] [Google Scholar]
- Findling RL. Ginsberg LD. Jain R. Gao J. Effectiveness, safety, and tolerability of lisdexamfetamine dimesylate in children with attention-deficit/hyperactivity disorder: An open-label, dose-optimization study. J Child Adolesc Psychopharmacol. 2009;19:649–662. doi: 10.1089/cap.2008.0165. [DOI] [PubMed] [Google Scholar]
- Geurts HM. Verté S. Oosterlaan J. Roeyers H. Sergeant JA. ADHD subtypes: Do they differ in their executive functioning profile? Arch Clin Neuropsychol. 2005;20:457–477. doi: 10.1016/j.acn.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Geurts HM. Verté S. Oosterlaan J. Roeyers H. Sergeant JA. How specific are executive functioning deficits in attention deficit hyperactivity disorder and autism? J Child Psychol Psychiatry. 2004;45:836–854. doi: 10.1111/j.1469-7610.2004.00276.x. [DOI] [PubMed] [Google Scholar]
- Gioia GA. Isquith PK. Guy SC. Kenworthy L. BRIEF: Behavior Rating Inventory of Executive Function: Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc.; 2000. [Google Scholar]
- Gioia GA. Isquith PK. Retzlaff PD. Espy KA. Confirmatory factor analysis of the Behavior Rating Inventory of Executive Function (BRIEF) in a clinical sample. Child Neuropsychol. 2002;8:249–257. doi: 10.1076/chin.8.4.249.13513. [DOI] [PubMed] [Google Scholar]
- Houghton S. Douglas G. West J. Whiting K. Wall M. Langsford S, et al. Differential patterns of executive function in children with attention-deficit hyperactivity disorder according to gender and subtype. J Child Neurol. 1999;14:801–805. doi: 10.1177/088307389901401206. [DOI] [PubMed] [Google Scholar]
- Jarratt KP. Riccio CA. Siekierski BM. Assessment of attention deficit hyperactivity disorder (ADHD) using the BASC and BRIEF. Appl Neuropsychol. 2005;12:83–93. doi: 10.1207/s15324826an1202_4. [DOI] [PubMed] [Google Scholar]
- Kratochvil CJ. Faries D. Vaughan B. Perwien A. Busner J. Saylor K, et al. Emotional expression during attention-deficit/hyperactivity disorders treatment: Initial assessment of treatment effects. J Child Adolesc Psychopharmacol. 2007;17:51–62. doi: 10.1089/cap.2006.0018. [DOI] [PubMed] [Google Scholar]
- Lawrence V. Houghton S. Douglas G. Durkin K. Whiting K. Tannock R. Executive function and ADHD: A comparison of children's performance during neuropsychological testing and real-world activities. J Atten Disord. 2004;7:137–149. doi: 10.1177/108705470400700302. [DOI] [PubMed] [Google Scholar]
- Lawrence V. Houghton S. Tannock R. Douglas G. Durkin K. Whiting K. ADHD outside the laboratory: Boys' executive function performance on tasks in videogame play and on a visit to the zoo. J Abnorm Child Psychol. 2002;30:447–462. doi: 10.1023/a:1019812829706. [DOI] [PubMed] [Google Scholar]
- Leroux JR. Turgay A. Quinn D. Advances in ADHD treatment. Can J Diagn. 2009;26:49–52. [Google Scholar]
- Mahone EM. Hoffman J. Behavior ratings of executive function among preschoolers with ADHD. Clin Neuropsychol. 2007;21:569–586. doi: 10.1080/13854040600762724. [DOI] [PubMed] [Google Scholar]
- Mares D. McLuckie A. Schwartz M. Saini M. Executive function impairments in children with attention-deficit hyperactivity disorder: Do they differ between school and home environments? Can J Psychiatry. 2007;52:527–534. doi: 10.1177/070674370705200811. [DOI] [PubMed] [Google Scholar]
- McCandless S. O'Laughlin L. The clinical utility of the Behavior Rating Inventory of executive function (BRIEF) in the diagnosis of ADHD. J Atten Disord. 2007;10:381–389. doi: 10.1177/1087054706292115. [DOI] [PubMed] [Google Scholar]
- McCracken JT. Biederman J. Greenhill LL. Swanson JM. McGough JJ. Spencer TJ, et al. Analog classroom assessment of a once-daily mixed amphetamine formulation, SLI381 (Adderall XR), in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2003;42:673–683. doi: 10.1097/01.CHI.0000046863.56865.FE. [DOI] [PubMed] [Google Scholar]
- McGough JJ. Biederman J. Wigal SB. Lopez FA. McCracken JT. Spencer T, et al. Long-term tolerability and effectiveness of once-daily mixed amphetamine salts (Adderall XR) in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2005;44:530–538. doi: 10.1097/01.chi.0000157550.94702.a2. [DOI] [PubMed] [Google Scholar]
- Miller EK. Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Oosterlaan J. Scheres A. Sergeant JA. Which executive functioning deficits are associated with AD/HD, ODD/CD and comorbid AD/HD+ODD/CD? J Abnorm Child Psychol. 2005;33:69–85. doi: 10.1007/s10802-005-0935-y. [DOI] [PubMed] [Google Scholar]
- Pennick M. Absorption of the prodrug lisdexamfetamine dimesylate and its subsequent enzymatic conversion to the active moiety d-amphetamine. Neuropsychiatr Dis Treat. 2010;6:317–327. doi: 10.2147/ndt.s9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington BF. Ozonoff S. Executive functions and developmental psychopathology. J Child Psychol Psychiatry. 1996;37:51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Perwien AR. Kratochvil CJ. Faries D. Vaughan B. Busner J. Saylor KE, et al. Emotional expression in children treated with ADHD medication: Development of a new measure. J Atten Disord. 2008;11:568–579. doi: 10.1177/1087054707306117. [DOI] [PubMed] [Google Scholar]
- Qian Y. Cao QJ. Wang YF. [Effect of extended-release methylphenidate on the ecological executive function for attention deficit hyperactivity disorder] Beijing Da Xue Xue Bao. 2007;39:299–303. [PubMed] [Google Scholar]
- Qian Y. Wang YF. [Reliability and validity of behavior rating scale of executive function parent form for school age children in China] Beijing Da Xue Xue Bao. 2007;39:277–283. [PubMed] [Google Scholar]
- Quraishi S. Frangou S. Neuropsychology of bipolar disorder: A review. J Affect Disord. 2002;72:209–226. doi: 10.1016/s0165-0327(02)00091-5. [DOI] [PubMed] [Google Scholar]
- Riccio CA. Homack S. Jarratt KP. Wolfe ME. Differences in academic and executive function domains among children with ADHD Predominantly Inattentive and Combined Types. Arch Clin Neuropsychol. 2006;21:657–667. doi: 10.1016/j.acn.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Sarkis SM. Sarkis EH. Marshall D. Archer J. Self-regulation and inhibition in comorbid ADHD children: An evaluation of executive functions. J Atten Disord. 2005;8:96–108. doi: 10.1177/1087054705277265. [DOI] [PubMed] [Google Scholar]
- Scheres A. Oosterlaan J. Geurts H. Morein-Zamir S. Meiran N. Schut H, et al. Executive functioning in boys with ADHD: Primarily an inhibition deficit? Arch Clin Neuropsychol. 2004;19:569–594. doi: 10.1016/j.acn.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Steele M. Weiss M. Swanson J. Wang J. Prinzo RS. Binder CE. A randomized, controlled effectiveness trial of OROS-methylphenidate compared to usual care with immediate-release methylphenidate in attention deficit-hyperactivity disorder. Can J Clin Pharmacol. 2006;13:e50–e62. [PubMed] [Google Scholar]
- Sullivan JR. Riccio CA. Diagnostic group differences in parent and teacher ratings on the BRIEF and Conners' Scales. J Atten Disord. 2007;11:398–406. doi: 10.1177/1087054707299399. [DOI] [PubMed] [Google Scholar]
- Toplak ME. Bucciarelli SM. Jain U. Tannock R. Executive functions: Performance-based measures and the Behavior Rating Inventory of Executive Function (BRIEF) in adolescents with attention deficit/hyperactivity disorder (ADHD) Child Neuropsychol. 2009;15:53–72. doi: 10.1080/09297040802070929. [DOI] [PubMed] [Google Scholar]
- Verté S. Geurts HM. Roeyers H. Oosterlaan J. Sergeant JA. Executive functioning in children with autism and Tourette syndrome. Dev Psychopathol. 2005;17:415–445. doi: 10.1017/s0954579405050200. [DOI] [PubMed] [Google Scholar]
- Wiersema JR. Roeyers H. ERP correlates of effortful control in children with varying levels of ADHD symptoms. J Abnorm Child Psychol. 2009;37:327–336. doi: 10.1007/s10802-008-9288-7. [DOI] [PubMed] [Google Scholar]
- Wigal SB. Kollins SH. Childress AC. Squires L. A 13-hour laboratory school study of lisdexamfetamine dimesylate in school-aged children with attention-deficit/hyperactivity disorder. Child Adolesc Psychiatry Ment Health. 2009;3:17. doi: 10.1186/1753-2000-3-17. for the 311 Study Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt EG. Doyle AE. Nigg JT. Faraone SV. Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biol Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Wodka EL. Mostofsky SH. Prahme C. Gidley Larson JC. Loftis C. Denckla MB, et al. Process examination of executive function in ADHD: Sex and subtype effects. Clin Neuropsychol. 2008;22:826–841. doi: 10.1080/13854040701563583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolraich ML. Greenhill LL. Pelham W. Swanson J. Wilens T. Palumbo D, et al. for the Concerta Study Group. Randomized, controlled trial of OROS methylphenidate once a day in children with attention-deficit/hyperactivity disorder. Pediatrics. 2001;108:883–892. doi: 10.1542/peds.108.4.883. [DOI] [PubMed] [Google Scholar]