Abstract
A factor has been isolated from weanling rat liver which stimulates in vivo hepatic DNA synthesis in a dose dependent manner when injected into 40% hepatectomized rats. The factor has been partially purified by successive steps, involving ethanol precipitation, ultrafiltration through an Amicon PM 30 membrane, and finally fast protein liquid chromatography, resulting in a 38,000-fold increase in specific activity over that in the original cytosol. The factor contains a few bands in the molecular weight range of 14,000-50,000 on sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Active fractions from fast protein liquid chromatography (F158), when injected into 40% hepatectomized rats, increased hepatic DNA synthesis 3-fold over the background stimulation due to the hepatectomy. The response was dose dependent over a range from 1.76 μg to 6.8 μg per 200-g (body weight) rat. Mitotic and labeling indexes confirmed that F150 stimulates both replicative DNA synthesis and cell proliferation. The factor is heat and neuraminidase resistant, trypsin sensitive, organ specific, but not species specific.
Introduction
Since the demonstration by Higgins and Anderson (1) of the remarkable capacity of the liver to regenerate, following surgical removal of 70% of the tissue, many investigators have attempted to elucidate the mechanism(s) that triggers the regenerative response. The use of the parabiotic model in rats yielded evidence that the regenerative stimulus, which initiates and maintains DNA synthesis and cell division, is transmitted via the circulation (2). However, the nature of the humoral factors involved is still undefined, despite the impetus provided to their study by the use of hepatocytes in primary cultures (3–8).
Regenerating liver or weanling rat liver have been investigated as primary sources of hepatomitogens. In addition, a variety of hormones and defined growth factors have been shown to modulate the regenerative response in vivo or to stimulate DNA synthesis in hepatocytes in primary culture. Among these are insulin and glucagon (9), EGF3 (3, 9), proline (10, 11), norepinephrine (12–14), and platelet derived growth factors (15, 16).
A number of investigators have isolated factors from serum of PH rats capable of stimulating DNA synthesis of hepatocytes in primary culture (17–20). However, these preparations have not been shown, thus far, to be active in vivo. There are also a number of reports on the isolation and partial purification of substances from regenerating rat, dog, or rabbit livers or from weanling rat liver (19, 21–30). Notably the presence of a HSS in regenerating and weanling rat liver was first reported by LaBrecque and Pesch (21). We have previously reported that crude preparations from proliferating liver tissues stimulated DNA synthesis in vivo (27, 28). In this report we describe the isolation and purification of a factor from weanling rat liver which stimulates DNA synthesis in vivo in a dose dependent manner.
Materials and Methods
Animals
Male Fischer (F344) rats (180–200 g) and weanling rats (60–90 g), female CF-1 mice, and male mongrel dogs (15–20 kg) were purchased from Hilltop Lab Animals, Scottsdale, PA, and were kept in temperature and light controlled rooms. They received food and water ad libitum.
Surgical Procedures
Partial hepatectomies of either 40% or 70% were performed in all animals between 7:30 and 9 a.m. Hepatectomies were performed in rats and mice according to the method of Higgins and Anderson (1) and in dogs as described previously (23). Control animals underwent a sham operation consisting of laparotomy and manual manipulation of the liver.
Materials
Neuraminidase type V and proteins used as molecular weight markers were purchased from Sigma Chemical Company, St Louis, MO. [methyl3H]Thymidine (50–80 Ci/mmol), l25I-EGF (200 μCi/μg), and 125I-vasopressin (1820 μCi/μg) were purchased from New England Nuclear, Boston, MA. 125I-Insulin (100 μCi/μg) and 125I-glucagon (200 μCi/μg) were purchased from Amersham-Searle Corp., Arlington Heights, IL, and l-1-tosylamido-2-phenylethyl chloromethyl ketone-trypsin was purchased from Worthington Biochemical Corp., Bedford, MA. Amicon ultrafiltration membrane filters were purchased from Amicon Corporation, Danvers, MA.
Preparation of Hepatic Extracts
Cytosols
Cytosols were prepared from livers of normal rats, from regenerating liver remnants 24 h after 70% PH, and livers of weanling unoperated rats. The livers were excised, placed in 4 volumes (w/v) of ice-cold buffer (0.27 m sucrose-12 mm Tris-HCl-1 mm EDTA, pH 7.6), and homogenized, using a Potter-Elvenhjem tissue grinder. The homogenates were centrifuged for 10 min at 10,000 × g and 4°C, and the supernatant was again centrifuged for 1 h at 100,000 × g using a Spinco ultracentrifuge.
HSS Preparation and Purification
Ethanol Precipitation Fraction
This fraction (OH-F) was prepared essentially as described by LaBrecque et al. (21, 25, 26) with slight modification. The livers from sham-operated rats, weanling unoperated rats, 70% PH rats, or 70% PH dogs were homogenized in cold 100 mm sodium acetate, pH 4.65 (35% w/v), between 7 and 8 a.m. A pH of between 4 and 5 was determined to be necessary for the most efficient extraction of HSS. Homogenates were heated at 65°C for 15 min and centrifuged at 30,000 × g for 20 min at 4°C. Six columns of cold ethanol were added to the supernatants. After being stirred for 2 h at 4°C the supernatants were centrifuged at 30,000 × g for 30 min at 4°C. The resulting pellet was dissolved in 150 mm ammonium acetate, lyophilized, and stored at −70°C until use. The amount of HSS obtained in the ethanol precipitate was identical whether the homogenate was heated directly or the heating step was applied to the cytosol. For injection into animals, the lyophilized OH-F was dissolved in 5 mm phosphate buffer, pH 7.4.
Amicon Membrane Ultrafiltration
Lyophilized OH-F, dissolved in 150 mm ammonium acetate, or freshly prepared OH-F in 150 mm ammonium acetate was filtered through Amicon PM 30 ultrafiltration membranes. The PM 30 filtrate was then concentrated, using Amicon YC05 membranes with a molecular weight limit of 500. The retained concentrated solution was designated as the Mr 30,000 fraction. This fraction was lyophilized and stored at −70°C.
Fast Protein Liquid Chromatography
The lyophilized Mr 30,000 fraction (20 mg) was suspended in 5 mm phosphate buffer, pH 6, and chromatographed on a Mono Q HR 5/5 column, using ABS-751 FPLC apparatus (Pharmacia, Uppsala, Sweden). Elution of material was achieved with a linear 0–200 mm NaCl gradient in 5 mm phosphate buffer, pH 6, at a flow rate of 2 ml/min. UV absorbance peaks (280 nm) were collected and dialyzed against 150 mm ammonium acetate, lyophilized, and stored at −70°C until use. The fractions were dissolved in 5 mm phosphate buffer, pH 7.4, and, after the determination of protein, tested for stimulatory activity. Some additional absorbance material could be eluted with 500 mm NaCl, but no protein was detected in this fraction. Sixteen mg of total protein (80%) were recovered, including the void volume.
Analyses of Physical and Chemical Properties
Active fractions eluted from the Mono Q column were tested for trypsin sensitivity; aliquots (3 μg) were dissolved in distilled water, brought to pH 7.6, and incubated at 37°C with l-1-tosylamido-2-phenylethyl chloromethyl ketone-trypsin (100 μg/ml). After 2 h the reaction was stopped by addition of 2 μg of soybean trypsin inhibitor/μg of trypsin. A mixture of similar amounts of trypsin and inhibitor was preincubated for 30 min at 20°C before addition to the active fraction and was similarly treated as a control (31). To test for heat stability, aliquots (3 μg) were dissolved in PBS, pH 7.6, and heated at 95°C in boiling water for 10 min. Neuraminidase sensitivity (19) was tested on aliquots (3 μg) dissolved in water, adjusted to pH 5.5, and incubated at 37°C for 1 h in the presence of 0.5 unit of neuraminidase/ml. The reaction was terminated by heating at 95°C for 30 min; after centrifugation the supernatant was used for i.p. injections. As a control, 0.5 unit of neuraminidase, without any HSS present, was treated the same way and injected.
SDS-Polyacrylamide Gel Electrophoresis
SDS-polyacrylamide gradient slab gels, 7.5 to 20% with a 5% stacking gel, were prepared and developed according to the method of Laemmli (32). Protein bands were visualized by Coomassie Blue R 250 according to the method of Weber and Osborn (33).
Protein Determination
Protein was determined by the method of Lowry et al. (34) or by the method of McKnight (35) for the determination of submicrogram quantities.
Determination of the Activity of HSS and Its Fractions
Fraction activity was determined in vivo using rats and mice. Experiments were carried out according to the method of LaBrecque and Pesch (21). Briefly a heightened background of DNA synthetic activity in vivo was induced in host rats and mice by a 40% PH. The 40% hepatectomized animal model was chosen for its sensitivity to recognize either an inhibitor or a stimulatory factor. Six h after PH the rats were given i.p. injections of 2 ml of 5 mm phosphate buffer, pH 7.4 (PBS-control), cytosol, ethanol precipitation fraction (OH-F), Mr 30,000 fraction, and the active FPLC fraction (Fl50), at protein concentrations as indicated in the tables. Seventeen h later, 50 μCi [3H]thymidine were injected i.p., and the animals were sacrificed 1 h later.
Extracts (0.2-ml volume) were also administered i.p. to mice at 30 h after 40% PH and DNA synthesis was studied 18 h later. [3H]Thymidine (10 μCi/mouse) was injected i.p. 1 h before sacrifice (i.e., 47 h following PH). Nonhepatectomized rats received injections of extracts 24 and 18 h before determination of [3H]thymidine incorporation, and mice received injections at 48, 24, and 18 h. [3H]Thymidine incorporation, labeling, and mitotic indexes were determined as described previously (23). An augmentation of all 3 parameters, beyond the modest response that is usually present in 40% PH or in unoperated animals, was considered to be indicative of a proliferative inducing activity of the liver extracts.
DNA Synthesis Determination in Organs Other Than the liver
DNA synthesis in the small intestine, spleen, heart, and kidney was also determined in 40% PH rats, following injection of F150, and was compared to DNA synthesis observed in the same organs of 40% PH rats given injections of PBS.
Statistical Analysis
Statistical analysis of groups were made by one way analysis of variance using SPSS/PC statistical software (SPSS, Inc., Chicago, IL) on an IBM-AT microcomputer.
Results
Determination of HSS Activity in Cytosol
Five ml of cytosol (75 mg of total protein) of weanling rat liver or regenerating rat liver remnants 24 h after 70% PH stimulated hepatic DNA synthesis when injected into recipient rats with a 40% PH. The results shown in Fig. 1 represent at least 5 separate experiments. A 40% PH alone resulted in a 2- to 3-fold increase in hepatic thymidine incorporation when compared to control sham-operated nonhepatectomized rats. Injection of cytosol prepared from normal adult rat liver did not result in a further stimulation. However, cytosol prepared from regenerating rat liver or from weanling rat liver produced a further stimulation of 3-fold (P < 0.05). Since the same degree of stimulation was achieved with either source, weanling rat liver was used in all subsequent experiments for purification of HSS. Since there was no difference in liver DNA synthesis between rats given injections of 5 mm PBS and those injected with cytosol prepared from the livers of normal nonhepatectomized animals, phosphate buffer was used as controls in all subsequent experiments. Nonhepatectomized recipient rats did not respond with an increased hepatic DNA synthetic activity when given injections of cytosol, regardless of the source, i.e., regenerating or nonregenerating (results not shown). Thus, an augmentation of hepatic regeneration by cytosol prepared from proliferating liver cells could be elicited only in a heightened background of hepatic DNA synthesis produced by 40% PH.
Fig. 1.
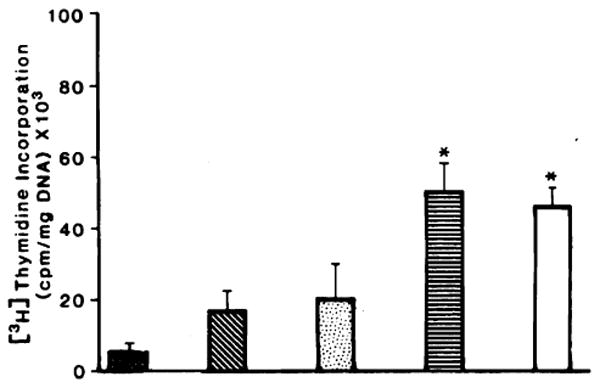
DNA synthesis in sham-operated and 40% hepatectomized rats after the i.p. injection of rat liver cytosol. DNA synthesis in the liver was determined as described under “Materials and Methods.” ■, sham-operated rats given injections of PBS; ■, 40% hepatectomy plus PBS; □, 40% hepatectomy plus cytosol from normal rat liver; ■, 40% hepatectomy plus cytosol from 24-h regenerating rat liver; □, 40% hepatectomy plus cytosol from weanling rat liver. Columms, means from 20 rats; bars, SD. *, significantly different from the control value (P <0.05).
Determination of HSS Activity in Ethanol Precipitate
The first step of cytosol purification by ethanol precipitation produced a 7.5-fold enrichment factor (Table 1). OH-F extracted from weanling rat liver or from regenerating rat liver 24 h after 70% PH stimulated hepatic incorporation of tritiated thymidine by 4-fold with respect to the animals given injections of OH-F prepared from sham-operated rats.
Table 1. Effect of OH-F prepared from different sources on DNA synthesis, percentage of labeled nuclei, and percentage of mitosis in 40% hepatectomized rats.
The preparation of ethanol precipitate fraction (OH-F), determination of [3H] thymidine incorporation, labeling index, and mitotic index have been described in “Materials and Methods.” Rats received 10 mg of OH-F i.p. in 2 ml 5 mm phosphate buffer.
| Source of OH-F | No. of rats | [3H]thymidine incorporation (cpm/mg DNA) | % of labeled nuclei | % of mitosis |
|---|---|---|---|---|
| Sham-operated rat liver (control) | 8 | 13,130 ± 690a | 12.8 ± 1.8 | 0.7 ± 0.1 |
| 70% PH rat liver | 14 | 63,520 ± 18,100b | ||
| Weanling rat liver | 16 | 60,750 ± 13,320b | 25.8 ± 5b | 1.8 ± 0.2b |
Mean ± SD.
Significantly different from sham-operated rat liver (control value); P< 0.05.
Determination of HSS Activity of Mr 30,000 Fraction
Further purification (4-fold) was achieved by ultrafiltration on Amicon membranes and yielded the Mr 30,000 fraction. One-fourth the amount of protein of this Mr fraction resulted in the same stimulation of DNA synthesis as did 10 mg of OH-F. These results are shown in Fig. 2. Also shown in Fig. 2 are the mitotic and the labeling indexes confirming that OH-F and the Mr 30,000 fraction stimulate both replicative DNA synthesis and cell proliferation.
Fig. 2.
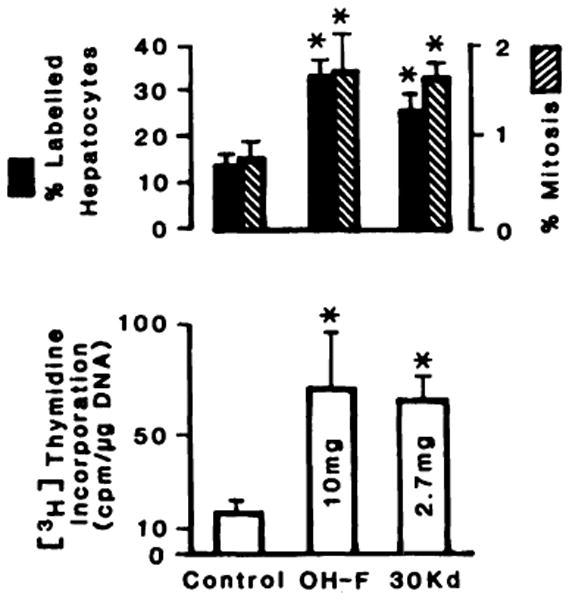
Effect of the ethanol precipitate fraction and Mr 30,000 fraction (30 Kd) on DNA synthesis and proliferation. Experimental conditions are described under “Materials and Methods.” Columns, average of 30 rats; bars, SD. *, significantly different (P < 0.05).
Determination of HSS Activity in FPLC Fraction
Application of the Mr 30,000 fraction to a FPLC column resulted in a further substantial purification and a 38,000-fold increase in specific activity. The elution profile in a linear 0–200 mm NaCl gradient in 5 mm phosphate buffer, pH 6, and the activity of the various fractions is shown in Fig. 3. The activity of these fractions was evaluated in 40% PH rats and expressed as cpm/μg DNA/μg of protein injected. Although several of the elution peaks demonstrated some activity, the material eluting at 150 mm NaCl produced the greatest stimulation. This fraction was designated F150.
Fig. 3.
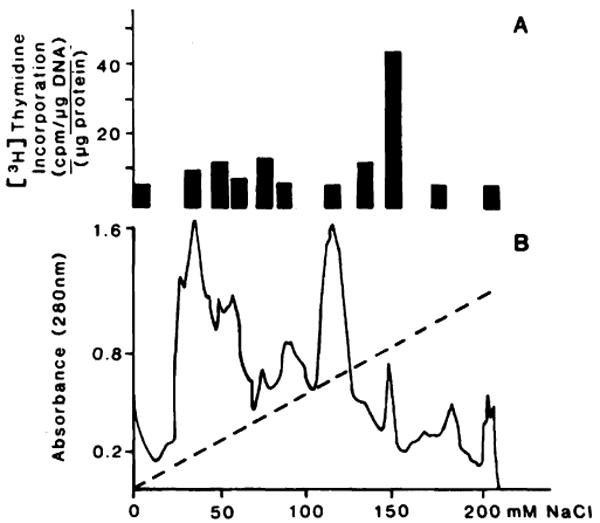
Elution and activity profile of HSS from FPLC. Stimulatory activity of FPLC fraction (A). Elution profile of 30 Kd (B). The amount of protein injected for each fraction was 3 μg except for F150 of which only 1.5 μg were used for this experiment [3H]Thymidine incorporation in rats given injections of 3 μg of bovine serum albumin was 5378 ± 690 cpm/μg DNA/μg protein. Each was an average from 6 different animals. The statistical analysis shows that only the value of F150 was significant (P < 0.0001).
The activity of this fraction was dose dependent over a range of 1.76 to 6.8 μg/rat with an average weight of 200 g. [3H] Thymidine incorporation results were confirmed by labeling and mitotic index results, as shown in Fig. 4.
Fig. 4.
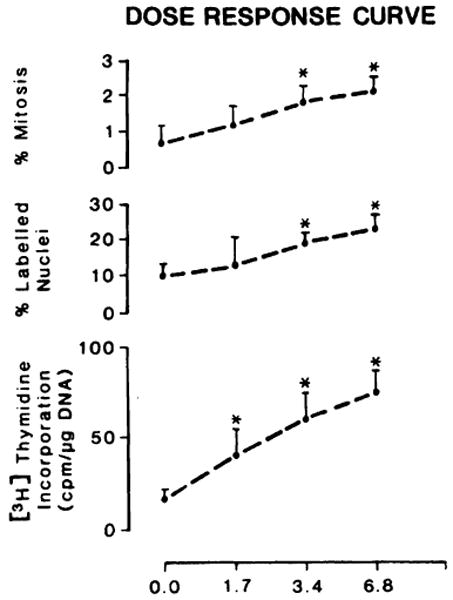
Dose-response curve in 40% hepatectomized rats given injections of F150. The experimental conditions are the same as those reported in Fig. 1. Control animals (10) were given injections of PBS containing 6.6 μg of serum albumin. DNA synthesis, percentage of labeled nuclei, and percentage of mitosis were done as reported in “Materials and Methods.” Points, averages of 10 determinations for each level of F150; bars, SD. *, significantly different from the control value (PBS) (P < 0.05).
The administration of OH-F, Mr 30,000 fraction, or F150 into sham-operated rats, as already demonstrated with cytosol, did not result in stimulation of DNA synthesis. Even multiple injections of these stimulatory fractions did not augment DNA synthesis activity in sham-operated rats (data not reported).
The degree of purification of the activity achieved thus far is reported in Table 2. In calculating the purification, the original cytosol was used as the starting material. Ethanol precipitation resulted in only modest purification as did ultrafiltration on an Amicon membrane filter with a molecular weight limit exclusion of 30,000. The purification was significantly improved by fractionation with FPLC, resulting in a 38,000-fold increase in the specific activity of F150 over that in the original cytosol. It must be noted that injection of these extracts into non-PH rats and mice under the conditions reported in “Materials and Methods” was ineffective (data not shown). The activity in F150 was resistant to neuraminidase and to heating at 95° for 10 min but was trypsin sensitive.
Table 2. Steps of purification of HSS and chemical and physical properties of fraction F150 obtained from weanling rat liver.
The purification scheme of HSS has been described in “Materials and Methods.” The determination of [3H]thymidine incorporation was as for Table 1. The [3H]thymidine incorporation in a 40% hepatectomized rat given an injection of PBS was 16,550 ± 3,000 cpm/mg DNA. The numbers are the averages from no less than 20 different rats ± SD.
| Material injected | Protein injected/rat (mg) | DNA synthesis (cpm/mg DNA) | Specific activity (units/mg protein) | Fold of purification |
|---|---|---|---|---|
| Cytosol | 75 | 43,350 ± 8,820 | 0.02 | |
| 65°C supernatant | 20 | 56,720 ± 10,240 | 0.12 | 6 |
| OH-F | 10.5 | 66,350 ± 11,350 | 0.30 | 15 |
| Mr 30,000 fraction | 2.7 | 63,520 ± 13,220 | 1.05 | 102 |
| FPLC F150 | 0.003 | 54,380 ± 10,200 | 762.0 | 38,100 |
To rule out the possibility that the stimulatory activity exhibited by the fractions in these studies could be due to the presence of hormones known to stimulate hepatic DNA synthesis in vivo or in vitro, 125I-labeled vasopressin, insulin, glucagon, and EGF were mixed with Mr 30,000 fraction and passed through the FPLC Mono Q column. All counts were eluted with less than 50 mm NaCl concentrations, well before the major active peak which eluted at 150 mm NaCl (data not shown). It has been shown previously that the in vitro activity of HSS is not due to contamination with these or other well known hormones (26).
The results of SDS-polyacrylamide gel electrophoresis are illustrated in Fig. 5; ethanol precipitate, Mr 30,000 fraction, and F150 were compared. F150 still contained several bands, with molecular weights ranging from 14,000 to 50,000.
Fig. 5.
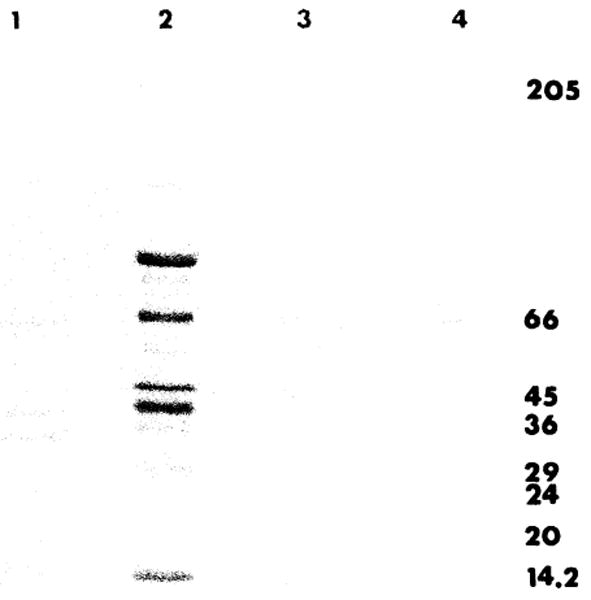
SDS-polyacrylamide gel electrophoresis of different purification steps of HSS. The gel electrophoresis preparation is reported in “Materials and Methods.” Slot 1, Mr 30,000 fraction; slot 2, fraction OH-F; slot 3, fraction F150; slot 4, a mixture of protein standards (Sigma) with molecular weights multiplied by 10−3.
Organ and Species Specific Activity Study
F150 was used to determine the organ specificity of HSS, as shown in Fig. 6. Injection of F150 into 40% PH recipient rats resulted in a significant stimulation of DNA synthesis only in the liver (P < 0.001), without affecting DNA synthesis of the heart, kidney, spleen, and small intestine.
Fig. 6.
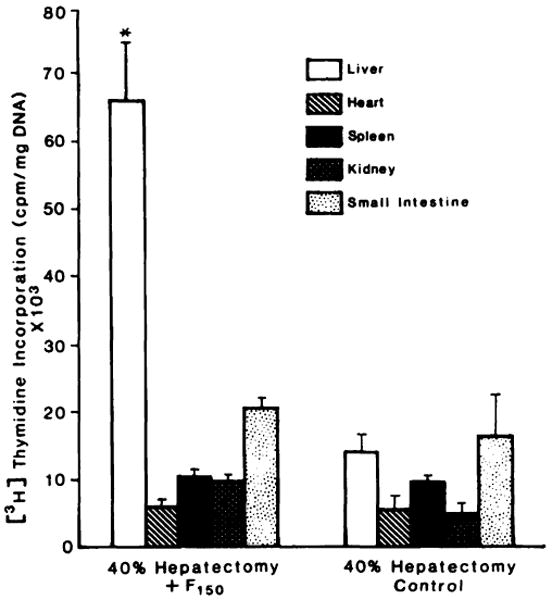
Specificity of F150 for hepatic DNA synthesis. Weanling rat liver F150 was injected into a 40% hepatectomized rat. DNA synthesis was determined in the liver, the heart, small intestine, kidney, and spleen, as described in “Materials and Methods.” Columns, averages from 5 rats; bars, SD. *, significantly different (P < 0.001) from the value observed in the liver of the control animals.
The results listed in Table 3 indicate that F150 activity is not species specific. Indeed, F150 prepared from weanling rats stimulated DNA synthesis in 40% PH female CF-1 mice, while F150 prepared from canine liver remnants at 48 h after 70% PH significantly stimulated DNA synthesis in 40% PH rats.
Table 3. Species specificity of F150 obtained from liver of weanling rats and 70% hepatectomized dogs and injected into mice or rats.
A 40% PH was performed on the recipient mice and rats. Injections and DNA determination with rats were done as described in Fig. 4. Mice were given injections of the indicated amounts of F150 in a volume of 0.2 ml 30 h after 40% PH. DNA synthesis was determined after a 1-h exposure at 48 h after the operation. Values are the averages from 6 animals ± SD.
| Source and amount of F150(μg) | [3H]Thymidine incorporation (cpm/mg DNA) in recipient animals | |
|---|---|---|
| Mice | Rats | |
| PBS | 7,250 ± 1,025 | 16,550 ± 3,000 |
| Rat 0.5 | 16,650 ± 2,055a | |
| Rat 1.0 | 25,750 ± 6,500a | |
| Dog 3 | 24,250 ± 4,875a | |
| Dog 6 | 32,175 ± 9,875a | |
| Dog 9 | 41,750 ± 10,300a | |
Significantly different from the control value (PBS) (P < 0.05).
Discussion
The purpose of this study was to purify and characterize HSS, a protein found in the liver which has been reported to stimulate hepatic DNA synthesis and hepatocyte replication and which was previously identified by LaBrecque et al. (21, 25, 26), by Francavilla et al. (27, 28) in rat liver, and by Starzl et al. (23, 24) in the canine liver. Despite the numerous attempts, a true liver specific mitogen has not been purified to any great extent. LaBrecque, who first used the term hepatocyte stimulatory substance, in recent abstracts (36, 37) has reported a significant purification of HSS. In our study the evaluation of proliferating activity of various fractions was carried out in an in vivo model using 40% PH rats. This model has been demonstrated by us (27, 28, 38) and by others (21, 25, 26) to be the most sensitive and reproducible assay system for detecting the presence of activity in such studies.
Physicochemical studies of the F150 fraction demonstrated that HSS seems to be a protein with a molecular weight between 50,000 and 14,000, which is resistant to neuraminidase, is destroyed by trypsin, and is resistant to heating at 95°C for 10 min. Each of the preparations obtained from FPLC chromatography was completely free of recognizable hormones such as insulin, glucagon, vasopressin, and EGF. Specifically, insulin and glucagon are soluble in alcohol, while HSS is precipitated in alcohol. Furthermore, these hormones, labeled with 125I, eluted from a Mono Q column in a position completely different from that of F150 (data not shown).
Table 4 reports all the attempts made since 1957 to isolate or prove the presence of a specific growth factor in the liver of different species. In all cases, the factors isolated are reported to be organ specific and species unspecific, all were prepared from rat liver homogenate, and in only one case (39) was it isolated from cellular membrane.
Table 4. Summary of the literature.
| Investigator | Time period | Biological source | Name of substance | Biological assay system | Physicochemical characteristics: (resistance) | Response organ/species | % of purification | Mrb | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Heat | Trypsin | Chymotrypsin | Neuraminidase | ||||||||
| Blomqvist | 1957 | NbReLa | in vivo: NR | ||||||||
| LaBrecque | 1975–1987 | WRL-ReRL | HSS | in vitro; HTC cells | Yes | No | No | Yes | Yes No | 110,000 | 14,000–15,000 |
| in vivo: 40% HeR | |||||||||||
| Hatase | 1979 | NL | in vitro: L-929 fibroblast | No | No | 30,000 | |||||
| in vivo: N and 34% HeR | |||||||||||
| Starzl | 1979 | ReL from 70% He dog | in vivo: dog with portacaval shunt | Yes | |||||||
| Goldberg | 1980–1985 | ReL from 70% He rat | Hepatopoietin | in vivo: NR | Yes | No | No | No | Yes No | 13,000 | 38,000 |
| Terblanche | 1980 | Re dog L | in vivo: NR | ||||||||
| Francavilla | 1984–1985 | WRL | HSS | in vivo: 40% HeR | Yes | No | No | Yes | Yes No | 38,000 | 15,000–50,000 |
| Schwarz | 1985 | ReL from 70% He R and Pig | in vivo: 34% He female R and WR | Yes | No | 14,000–25,000 | |||||
| in vitro: hepatocyte cells | |||||||||||
| Lieberman | 1984 | Mouse plasma membrane | in vitro: NR-6 line fibroblast | No | Yes | No | No | ||||
| Fleig | 1986 | ReL from 60% He rabbit | in vivo: NR | No | No | ||||||
L, liver; N, normal; Nb, newborn; R, rat; Re, regenerating; He, hepatectomized; W, weanling.
Determined by SDS-polyacrylamide gel electrophoresis
Concerning the physicochemical characteristics, all factors were sensitive to trypsin, but not all had heat stability, while the molecular weight ranged between 14,000 and 45,000. Among all these reports, only 3, including ours, address the problem of purification.
At present it is difficult to compare the HSS preparation of LaBrecque and our preparation. Although many chemical and physical characteristics are similar, there are substantial differences in the activity. For example, our HSS stimulates DNA synthesis in vivo only in animals with a 40% hepatectomy and the stimulation is dose dependent. LaBrecque and Bachur (25) have reported that a crude HSS preparation stimulates hepatic DNA synthesis in normal rats and mice but have not tested their newest preparation (40) in vivo or with hepatocytes in primary culture. Their primary assay system has been an HTC culture system. We have not been able to confirm that our HSS stimulates DNA synthesis in HTC cells. This might be due to differences in HTC lines maintained in different laboratories. Since neither our HSS nor the HSS of LaBrecque is completely pure, a final comparison is possible only after complete purification.
It is important to note that factors which have been able to stimulate hepatocyte regeneration have been found in the perfusate of isolated and partial hepatectomized livers of rats and dogs, confirming the possibility of the passage of this factor into the circulation. Serum factors, in fact, have recently been found by several authors (15–20, 29, 31, 41, 42).
Although the final identification and purification of the specific growth factor(s) derived from the liver remain to be defined, our data represent a significant advancement. A high degree of purification has been achieved and it has been clearly demonstrated that F150 is active only on hepatocytes exhibiting a dose-response phenomenon by both DNA synthesis and percentage of mitosis. The fraction, as it presently exists, still contains a few bands on SDS polyacrylamide gel electrophoresis and it therefore cannot be ruled out that some of them might contain inhibitory activity.
The potential of these contaminating proteins may also be the reason for the need to use 40% hepatectomized rats in our assay system. Once complete purification has been achieved it is conceivable that HSS can exhibit more potent activity which will allow a better definition of the role of HSS in the chain of the liver regeneration. Similarly a completely pure HSS might stimulate DNA synthesis of hepatocytes in primary culture.
Apart from the biological implications, the exact definition of HSS and other hepatic growth factors has important clinical implications. The use of growth factor therapy for acute liver failure in animals and in humans is, in fact, the main objective of this study. We have already shown that this type of therapy, using fractions obtained during the HSS purification, improves the survival rate of rats intoxicated with the selective hepatotoxin d-galactosamine (43).
Acknowledgments
We are grateful to John Prelich for technical assistance.
Footnotes
This study was supported by a research project grant from the Veterans Administration, Project Grant AM-29961 from the NIH, Bethesda, MD, and Grant 885/02 16544 from Consiglio) Nazionale delle Ricerche, Italy.
The abbreviations used are: EGF, epidermal growth factor; PH, partially hepatectomized; HSS, hepatocyte stimulating substance; FPLC, fast protein liquid chromatography; PBS, phosphate buffered saline; SDS, sodium dodecyl sulfate.
References
- 1.Higgins GM, Anderson RM. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186–202. [Google Scholar]
- 2.Moolten FL, Bucher NRL. Regeneration of rat liver. Transfer of humoral agent by crosscirculation. Science (Wash DC) 1967;158:272–274. doi: 10.1126/science.158.3798.272. [DOI] [PubMed] [Google Scholar]
- 3.Richman RA, Claus TH, Pilkes SJ, Friedman DL. Hormonal stimulation of DNA synthesis in primary cultures of adult rat hepatocytes. Proc Natl Acad Sci USA. 1976;73:3589–3592. doi: 10.1073/pnas.73.10.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams GM, Gunn JM. Long-term cell culture of adult rat liver epithelial cells. Exp Cell Res. 1974;89:139–142. doi: 10.1016/0014-4827(74)90196-7. [DOI] [PubMed] [Google Scholar]
- 5.Michalopoulos G, Pitot HC. Primary culture of parenchymal liver cells on collagen membranes. Exp Cell Res. 1975;94:70–78. doi: 10.1016/0014-4827(75)90532-7. [DOI] [PubMed] [Google Scholar]
- 6.Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 7.Leffert H, Moran T, Sell S, Skelly H, Ibsen K, Muellers M, Arias I. Growth state-dependent phenotypes of adult hepatocytes in primary monolayer cultures. Proc Natl Acad Sci USA. 1978;75:1834–1838. doi: 10.1073/pnas.75.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonney RJ, Becker JE, Walker PR, Potter VR. Primary monolayer cultures of adult rat liver parenchymal cells suitable for study of the regulation of enzyme synthesis. In Vitro (Rockville) 1974;99:399–413. doi: 10.1007/BF02615992. [DOI] [PubMed] [Google Scholar]
- 9.McGowan JA, Strain AJ, Bucher NRL. DNA synthesis in primary cultures of adult rat hepatocytes in a defined medium: effects of epidermal growth factor, insulin, glucagon, and cyclic-AMP. J Cell Physiol. 1987;108:353–363. doi: 10.1002/jcp.1041080309. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura T, Teramoto H, Tomita Y, Ichihara A. l-Proline is an essential amino acid for hepatocyte growth in culture. Biochem Biophys Res Commun. 1984;122:884–891. doi: 10.1016/0006-291x(84)91173-2. [DOI] [PubMed] [Google Scholar]
- 11.Houck KA, Michalopoulos G. Proline is required for the stimulation of DNA synthesis in hepatocyte cultures by EGF. In vitro cell Dev Biol. 1985;21:121–124. doi: 10.1007/BF02620953. [DOI] [PubMed] [Google Scholar]
- 12.Cruise JL, Houck KA, Michalopoulos GK. Induction of DNA synthesis in cultured rat hepatocytes through stimulation of α1-adrenoreceptor by norepinephrine. Science (Wash DC) 1985;227:749–751. doi: 10.1126/science.2982212. [DOI] [PubMed] [Google Scholar]
- 13.Cruise JL, Michalopoulos GK. Norepinephrine and epidermal growth factor dynamics of their interaction in the stimulation of hepatocyte DNA synthesis. J Cell Physiol. 1985;125:45–50. doi: 10.1002/jcp.1041250107. [DOI] [PubMed] [Google Scholar]
- 14.Cruise JL, Cotecchia S, Michalopoulos GK. Norepinephrine decreases EGF binding in primary rat hepatocyte cultures. J Cell Physiol. 1986;127:39–44. doi: 10.1002/jcp.1041270106. [DOI] [PubMed] [Google Scholar]
- 15.Russell WE, McGowan JA, Bucher NRL. Partial characterization of a hepatocyte growth factor from rat platelets. J Cell Physiol. 1984;119:183–192. doi: 10.1002/jcp.1041190207. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura N, Teramoto H, Ichihara A. Purification and characterization of a growth factor from rat platelets for mature parenchymal hepatocytes in primary cultures. Proc Natl Acad Sci USA. 1986;83:6489–6493. doi: 10.1073/pnas.83.17.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michalopoulos G, Houck KA, Dolan ML, Luetteke NC. Control of hepatocyte replication by two serum factors. Cancer Res. 1984;44:4414–4419. [PubMed] [Google Scholar]
- 18.Nakamura T, Nawa K, Ichihara A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun. 1984;122:1450–1459. doi: 10.1016/0006-291x(84)91253-1. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg M. Purification and partial characterization of a liver cell proliferation factor called hepatopoietin. J Cell Biochem. 1985;27:291–302. doi: 10.1002/jcb.240270310. [DOI] [PubMed] [Google Scholar]
- 20.Diaz-Gil JJ, Escartin P, Garcia-Canero R, Trilla C, Veloso JJ, Sanchez G, Moreno-Capparros A, Enrigue de Salamanca C, Lozano R, Gavilanes JG, Garcia-Segura JM. Purification of a liver DNA synthesis promoter from plasma of partially hepatectomized rats. Biochem J. 1986;235:49–55. doi: 10.1042/bj2350049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LaBrecque DR, Pesch LA. Preparation and partial characterization of hepatic regenerative stimulator substance (SS) from rat liver. J Physiol. 1975;248:273–284. doi: 10.1113/jphysiol.1975.sp010973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatase O, Fujii T, Kuramitsu M, Itano T, Takahashi F, Murakami T, Nisida I. Co-existence of inhibitory and stimulatory factors modulating cell proliferation in rat liver cytoplasm. Acta Med Okayama. 1979;33:73–80. [PubMed] [Google Scholar]
- 23.Starzl TE, Terblanche J, Porter KA, Jones AF, Usiu S, Mazzoni G. Growth-stimulating factor in regenerating canine liver. Lancet. 1979 Jan 20;2:127–130. doi: 10.1016/s0140-6736(79)90519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terblanche J, Porter KA, Starzl TE, Moore J, Patzelt L, Hayashida N. Stimulation of hepatic regeneration after partial hepatectomy by infusion of a cytosol extract from regenerating dog liver. Surg Gynecol Obstet. 1980;151:538–544. [PMC free article] [PubMed] [Google Scholar]
- 25.LaBrecque DR, Bachur NR. Hepatic stimulator substance physicochemical characteristics and specificity. Am J Physiol. 1982;242:G281–G288. doi: 10.1152/ajpgi.1982.242.3.G281. [DOI] [PubMed] [Google Scholar]
- 26.LaBrecque DR, Wilson M, Fogerty S. Stimulation of HTC hepatoma cell growth in vitro by hepatic stimulator substance (HSS) Exp Cell Res. 1984;150:419–429. doi: 10.1016/0014-4827(84)90585-8. [DOI] [PubMed] [Google Scholar]
- 27.Francavilla A, Ove P, Van Thiel DH, Coetzee ML, Wu SZ, DiLeo A, Starzl TE. Induction of hepatocyte stimulating activity by T3 and appearance of the activity despite inhibition of DNA synthesis by Adriamycin. Horrm Metab Res. 1984;16:237–242. doi: 10.1055/s-2007-1014755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Francavilla A, Ove P, Polimeno L, Coetzee M, Van Thiel DH, Starzl TE. Extraction and partial purification of hepatic stimulatory activity (HSA) which stimulates hepatocyte proliferation in vivo and in vitro. Hepatology. 1985;5:922. [Google Scholar]
- 29.Fleig WE, Lehmann H, Wagner H, Hoss G, Ditschuneit H. Hepatic regenerative stimulator substance in the rabbit: relation to liver regeneration after partial hepatectomy. J Hepatol. 1986;3:19–26. doi: 10.1016/s0168-8278(86)80141-6. [DOI] [PubMed] [Google Scholar]
- 30.Schwarz LC, Makowka L, Falk JA, Falk R. The characterization and partial purification of hepatocyte proliferation factor. Ann Surg. 1985;202:296–302. doi: 10.1097/00000658-198509000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thaler FJ, Michalopoulos GK. Hepatopoietin A: partial characterization and trypsin activation of a hepatocyte growth factor. Cancer Res. 1985;45:2545–2549. [PubMed] [Google Scholar]
- 32.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (Lond) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 33.Weber K, Osborn M. The reliability of molecular weight determinations by dodecyl sulfate polyacrylamide gel electrophoresis. J Biol Chem. 1969;244:4406–4412. [PubMed] [Google Scholar]
- 34.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 35.McKnight GS. A colorimetric method for the determination of submicrogram quantities of protein. Anal Biochem. 1979;78:86–92. doi: 10.1016/0003-2697(77)90011-2. [DOI] [PubMed] [Google Scholar]
- 36.LaBrecque DR, Steele G, Fogerty S. Further purification of hepatic stimulator substance (HSS)—a liver specific growth factor. Fed Proc. 1983;42:438. [Google Scholar]
- 37.LaBrecque DR, Wilson M, Rinderknecht C, Barton J. Purification of hepatic stimulator substance (HSS) and characterization of the early steps in its initiation of HTC hepatoma cell DNA synthesis. Hepatology. 1986;6:505. [Google Scholar]
- 38.Francavilla A, Porter KA, Benichou J, Jones AF, Starzl TE. Liver regeneration in dogs: morphologic and chemical changes. J Surg Res. 1979;25:409–419. doi: 10.1016/s0022-4804(78)80005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lieberman MA. The presence of both growth inhibitory and growth stimulatory factors on membranes prepared from mouse liver. Biochem Biophys Res Commun. 1984;120:891–897. doi: 10.1016/s0006-291x(84)80191-6. [DOI] [PubMed] [Google Scholar]
- 40.LaBrecque DR, Steele G, Fogerty S, Wilson M, Barton J. Purification and physical-chemical characterization of hepatic stimulator substance. Hepatology. 1987;7:100–106. doi: 10.1002/hep.1840070121. [DOI] [PubMed] [Google Scholar]
- 41.Morley CGD, Kingdon HS. The regulation of cell growth. I. Identification and partial characterization of a DNA synthesis stimulating factor from serum of partially hepatectomized rats. Biochim Biophys Acta. 1973;308:260–274. doi: 10.1016/0005-2787(73)90156-1. [DOI] [PubMed] [Google Scholar]
- 42.Thorgeirsson SS, Song MKH, Cone JL, Roller PP, Huggett A. Characterization of a serum growth factor which stimulates hepatocyte proliferation. Fed Proc. 1985;44:1653. [Google Scholar]
- 43.Francavilla A, DiLeo A, Polimeno L, Gavaler J, Pellicci R, Todo S, Kam I, Prelich J, Makowka L, Starzl TE. The effect of hepatic stimulatory substance (HSS) isolated from regenerating hepatic cytosol and 50,000 and 300,000 subtractions in enhancing survival in experimental acute hepatic failure in rats treated with d-galactosamine. Hepatology. 1986;6:1346–1351. doi: 10.1002/hep.1840060621. [DOI] [PMC free article] [PubMed] [Google Scholar]


