Abstract
Objective
To determine whether video-based coping skills (VCS) training with telephone coaching reduces psychosocial and biological markers of distress in primary caregivers of a relative with Alzheimer’s Disease or related dementia (ADRD)
Methods
A controlled clinical trial was conducted with 116 ADRD caregivers who were assigned, alternately as they qualified for the study, to a Wait List control condition or the VCS training arm in which they viewed two modules/week of a version of the Williams LifeSkills Video adapted for ADRD family care contexts, did the exercises and homework for each module presented in an accompanying Workbook, and received one telephone coaching call per week for five weeks on each week’s two modules. Questionnaire-assessed depressive symptoms, state and trait anger and anxiety, perceived stress, hostility, caregiver self-efficacy, salivary cortisol across the day and before and after a stress protocol, and blood pressure and heart rate during a stress protocol were assessed prior to VCS training, seven weeks after training was completed and at three and six months follow-up.
Results
Compared to controls, participants who received VCS training plus telephone coaching showed significantly greater improvements in depressive symptoms, trait anxiety, perceived stress, and average systolic and diastolic blood pressure that were maintained over the six-month follow-up period.
Conclusions
VCS training augmented by telephone coaching reduced psychosocial and biological indicators of distress in ADRD caregivers. Future studies should determine the long-term benefits to mental and physical health from this intervention.
Keywords: Alzheimer’s Disease, coping skills training, caregiving stress
INTRODUCTION
It is estimated that more than 5.3 million Americans aged sixty-five and older suffer from Alzheimer’s disease or a related dementia (ADRD). That number is expected to rise to 7.7 million by the year 2030 as medical advances enable people to live longer (1) thus increasing the strain on society both socially and financially. (2) Care for individuals with ADRD often falls to informal caregivers, such as family members, friends and and/or neighbors, who can suffer significant stress from shouldering this responsibility.
An extensive body of research documents the adverse effects of prolonged stress associated with caring for a family member with ADRD. These effects include a greater risk for depression (3–9), anxiety (3, 10), physical morbidity (3), mortality (11, 12), and obesity (13). Caregiver status is also associated with poorer sleep quality (14), increased levels of stress hormones (15–18), reduced immune function (15, 17, 19), slower wound healing (20), new cases of hypertension (21), and new cases of coronary heart disease (22–24). Recent research has additionally linked the depression often experienced by ADRD caregivers with an increased incidence of emergency department visits (25). These findings are consistent with caregivers’ self-reports of poor health and well being.1
Despite these potential adverse effects, some family caregivers view certain aspects of caregiving in a positive light (26) and handle the stress of caring for a family member with ADRD with little difficulty (8, 27). Specifically, research has shown that higher levels of perceived mastery of the caregiver role (28–30), higher levels of social support (31), and the use of coping strategies that seek to regulate emotions experienced during stressful events (26) serve as protective factors that buffer the negative health-damaging and emotional effects of caring for a family member with ADRD. These findings suggest that behavioral interventions that improve coping strategies and competencies among caregivers may ameliorate the health-damaging effects of caregiver stress.
Research does suggest that interventions that emphasize skill building, education, treatment of depression, and family support are most successful in ameliorating the negative health-damaging effects of caregiver stress (32, 33). To date, the most effective interventions are thought to be those that provide a skills-based approach and active educational engagement (i.e., educating about the disease, giving practical advice, teaching problem-specific skills and decision making). (34) Such interventions have been successful in reducing depression, improving sleep quality, reducing burden and increasing social support in caregivers and delaying nursing home placement of the care recipient. (7, 35–38) In addition, successful interventions are structured (38) and provide prolonged, continuous support for caregivers. (34) While such interventions have proven efficacious in some studies, reviews of interventions that focus uniquely on caregiving have revealed them to be generally ineffective in reducing the burden of caregiving. (34, 39)
Three limitations may have impeded the development of successful interventions. One issue in caregiver research thus far has been that studies have often excluded ADRD caregivers who were not able to travel to a training site (e.g. 7), although these individuals may well have been among the most highly burdened members of the population. Research has shown that the most burdened caregivers (e.g. those with the lowest self-efficacy scores) benefit the most from interventions (28). By excluding caregivers for such logistical reasons, studies may have underestimated the potential benefits of the interventions. A second issue is that interventions have often focused on the benefits of counseling and support groups (40) that would not likely be available to caregivers or would be too costly. Although research suggests that “the most successful interventions are based on prolonged continuous support of the caregiver – i.e., for years,” (34), such programs would be time-demanding and costly to implement. Finally research to date has also been limited by a focus solely on psychosocial markers of stress. None of the studies cited above included stress biomarkers, such as measures of hypothalamic-pituitary adrenocortical, sympathoadrenal medullary, or cardiovascular reactivity that are cited (15–21) as the likely mediators of the health-damaging effects of caregiver stress.
Research to date suggests that a successful intervention needs to train caregivers to use a wide variety of coping skills and it must do so in an easy-to-deliver and cost-effective manner. Ideally, the intervention should improve both psychological and biological markers of caregiver stress with both immediate and long-term benefits. The Williams LifeSkills Video (WLV) may meet these requirements. The WLV is based on the Williams LifeSkills Workshop (41) that has been shown in randomized controlled trials to reduce hostility and blood pressure in post-MI patients (42) and to reduce both psychosocial (depression, trait anger, and perceived stress) and biological (blood pressure and heart rate at rest and during stress) risk factors in patients following coronary bypass surgery (43). . The WLV presents 10 standardized modules that provide training in skills to identify and manage stressful situations and to improve the quality of interpersonal relationships. When tested in a group of community volunteers who experienced psychosocial distress, the WLV reduced trait anxiety and perceived stress compared to a wait-list condition, with persistent benefits maintained during a 6-month follow-up.(44) In a randomized controlled trial of the Williams LifeSkills Workshop, reductions in resting SBP and DBP were significantly larger in hypertensive patients randomized to the treatment arm compared to usual care, with even larger reductions in patients reporting high levels of job demand. (45) Another randomized controlled trial testing a version of the Williams LifeSkills Workshop adapted for high school students found a significantly larger reduction in daytime average ambulatory SBP in those randomized to the Workshop arm compared to no intervention. (46) With respect to other biomarkers of stress, a 10-week cognitive behavioral stress management program that contains many elements in common with the WLV was found to produce a significant reduction in afternoon serum cortisol compared to a 1-day psychoeducational seminar in women with nonmetastatic breast cancer. (47)
Given these prior findings, we hypothesized that a version of the WLV that was adapted to address the stressful situations frequently encountered by Alzheimer’s caregivers and complemented by telephone coaching to enhance skills acquisition would accomplish a reduction in caregiver stress. Thus, the present controlled trial was designed to evaluate whether the Video-Based Coping Skills (VCS) program produces immediate and persistent improvements in the same psychosocial and biological stress markers that showed improvements with LifeSkills training in prior studies, both at the end of training and during up to six months follow-up.
METHODS
Participants
Participants were recruited over a two year interval through local advertisements, support groups, personal referrals, and referrals from the Bryan Alzheimer’s Disease Research Center’s Memory Disorders Clinic. Potential participants underwent an initial screening to exclude individuals with severe medical problems, such as cancer or severe heart disease and those who were not the primary caregiver for a relative with ADRD. We based our estimate of the sample size required to detect differences in outcome variables between VCS and Wait List groups on the findings for CES-D scores for depressive symptoms in the Bishop et al. (43) randomized controlled trial of post-CABG patients. In this trial the Williams Lifeskills workshop reduced CES-D scores at 3-month follow-up by 7 points, with a reported effect size d = .71, indicating that the SD of the change is 10 points. In contrast, the control intervention was associated with an increase of 4 points (d = −.48, SD change = 8). The difference between groups in CES-D change at 3 months follow-up was 11 points. We propose that in the current trial a treatment effect that is 50% of this difference or greater would be meaningful. For our comparison of VCS and Wait List groups, therefore, we selected a sample size that would enable us to detect a difference in CES-D change of 5 points. After calculating the associated effect size for a two-group t-test (d = (M1–M2) / SD = 5 / 9 = .56) and consulting standard tables for a 2-tailed test, we determine that N=50 subjects per group would be required to insure detection of an effect of this size with 80% likelihood.
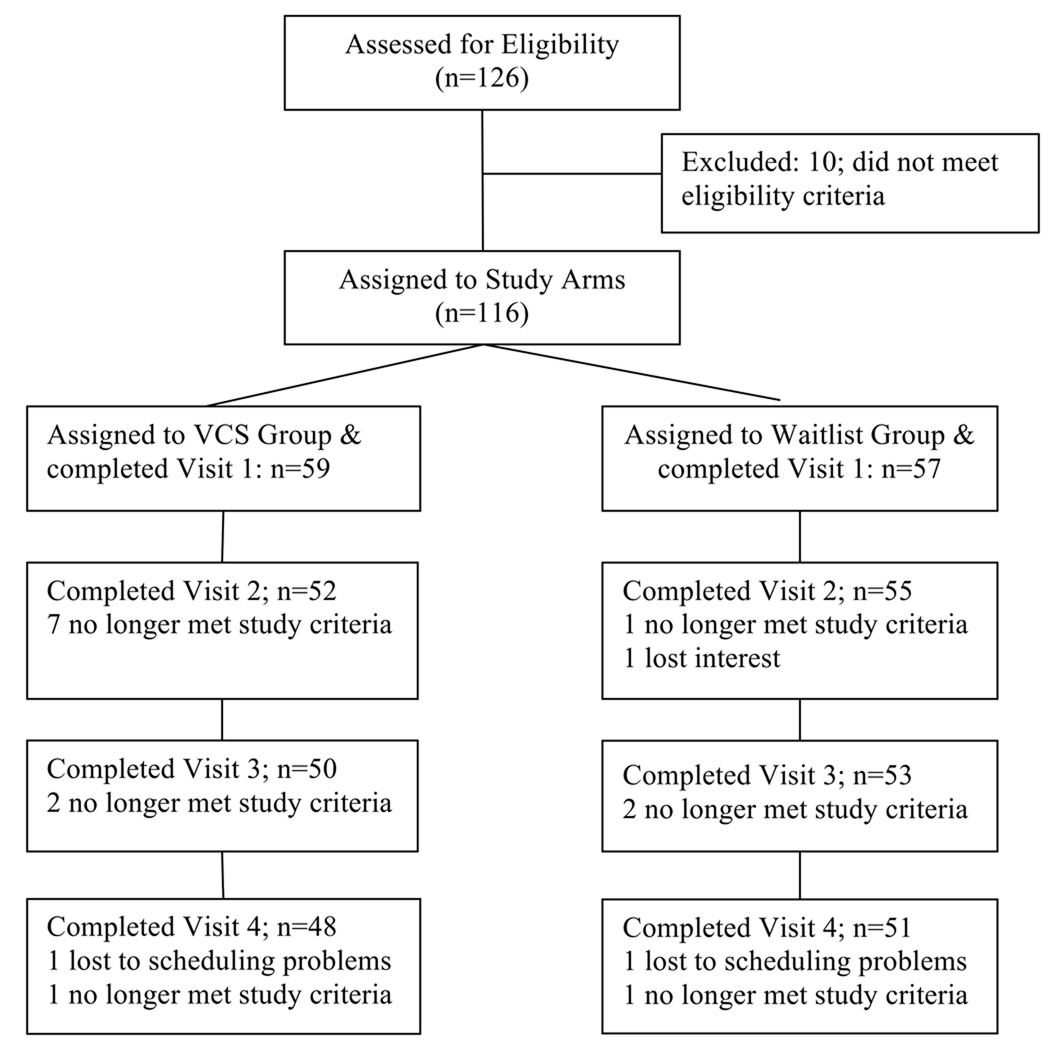
As shown in the Study Flow Chart (Figure 1) the final study sample consisted of 116 primary caregivers of relatives or friends with ADRD who agreed to participate in a controlled trial to test the efficacy of the VCS, a multicomponent, skills-based, psychoeducational intervention, in improving physiological and psychological markers of stress and well-being. Subjects were automatically assigned to the two study arms on an alternating basis based solely on the time they contacted the study coordinator and were assigned a subject number. This alternating assignment, while not fully random in the strictest sense, did ensure that each study participant had an equal chance of assignment to treatment or control groups, based solely on the time of contact and assignment of subject number. Of the 116 caregivers enrolled, 59 were assigned to the VCS group and 57 to the Wait List group. Demographic and other baseline characteristics of the two groups are shown in Table 1. Overall, the average age (± S.D.) of participants was 60.5 ± 13.4 years. Most participants were African American or Caucasian and varied in education and socioeconomic status. Most of the participants cared for a spouse (N=47) or a parent (N=58), but the sample also contained individuals caring for parents-in-law (N=3), aunts/uncles (N=2), grandparents (N=2), godparents (N=1) and others (N=3). Participant reports indicated that the care recipient’s symptoms first appeared an average of 69.3 ± 4.7 months prior to study enrollment. All participants lived within approximately a 150 mile radius of Durham, NC. Ninety-six percent of the spousal caregivers lived with the care recipient, versus only 60% of non-spouse caregivers. Data collection began in January, 2007 and concluded in April, 2009. The study was approved by the Duke University Health Systems Institutional Review Board and informed consent was obtained prior to participation.
Figure 1.
Flow chart identifying the number of participants in each arm who completed each stage of the study and reasons for participants lost to follow-up.
Table 1.
Participant Characteristics at Baseline (Visit 1)
| Variable | VCS Group (n= 59) | Waitlist Group (n= 57) | P Value |
|---|---|---|---|
| Age, mean (SD), yr 56,57 | 62.1 (13.6) | 59.0 (12.8) | 0.21 |
| Race/ethnicity, No. (%) 58,56 | 0.61 | ||
| Caucasian | 37 (64) | 36 (64) | |
| African American | 20 (35) | 20 (35) | |
| Other | 1 (2) | 0 (0) | |
| Female, No. (%) 59,57 | 44 (75) | 46 (81) | 0.43 |
| Education Level, No. (%) 59,57 | 0.72 | ||
| <High School | 1 (2) | 0 (0) | |
| High School | 10 (17) | 8 (14) | |
| Some College | 17 (29) | 17 (30) | |
| College | 18 (31) | 14 (25) | |
| Some Post-Grad | 5 (8) | 5 (9) | |
| Post-Grad | 8 (14) | 13 (23) | |
| Income, No. (%) | 0.52 | ||
| $0–$20,000 | 16 (27) | 11 (19) | |
| >$20,000–$40,000 | 20 (34) | 16 (28) | |
| >$40,000–$60,000 | 11 (19) | 12 (21) | |
| >$60,000 | 11 (19) | 16 (28) | |
| No answer | 1 (2) | 2 (4) | |
| Relationship to care recipient, No. (%) | 0.03* | ||
| Spouse | 30 (51) | 17 (30) | |
| Child | 22 (37) | 36 (63) | |
| Other | 7 (12) | 4 (7) | |
| (coding responses of “Parent” in the Child category as a confused response) | |||
| Resides with patient, No. (%) | 50 (85) | 37 (65) | 0.01 |
| Depression (CES-D), mean (SD) 58,55 | 18.7 (10.6) | 14.4 (9.6) | 0.03 |
| Self Efficacy (CGSE), mean (SD) | |||
| 1. Obtaining respite 54,45 | 53.8 (29.9) | 61.9 (30.1) | 0.19 |
| 2. Responding 51,49 | 67.4 (24.4) | 69.8 (27.4) | 0.65 |
| 3. Controlling thoughts 55,52 | 59.2 (22.9) | 63.1 (23.1) | 0.38 |
| Anxiety, (STAI) mean (SD) | |||
| State 58,54 | 41.9 (11.1) | 37.3 (12.5) | 0.04 |
| Trait 58,55 | 41.6 (10.3) | 38.4 (11.1) | 0.10 |
| Anger, (STAXI) mean (SD) | |||
| State 58,55 | 21.7 (8.5) | 21.0 (8.8) | 0.65 |
| Trait 58,55 | 27.2 (7.3) | 26.0 (8.0) | 0.43 |
| Hostility (Ho), mean (SD) 58,55 | 9.1 (4.7) | 8.3 (5.0) | 0.35 |
| Perceived Stress (PSS), mean (SD) 58,54 | 21.5 (6.7) | 19.1 (7.2) | 0.08 |
| Sleep Disturbance (PSQI), mean (SD) 50,51 | 8.2 (3.0) | 7.4 (3.9) | 0.27 |
| Resting Blood Pressure, mean (SD) 57,57 | |||
| SBP | 124.0 (20.0) | 117.1 (16.9) | 0.05 |
| DBP | 71.3 (9.5) | 67.8 (8.5) | 0.05 |
| Resting Heart Rate, mean (SD) | 67.1 (11.1) | 69.5 (11.0) | 0.25 |
| Cortisol, mean (SEM) | 3.0 (0.2) | 3.3 (0.2) | 0.33 |
Note: CES-D: Center for Epidemiologic Studies Depression Scale; CGSE: Revised Scale for Caregiver Self-efficacy; STAI: Spielberger State-Trait Anxiety Inventory; STAXI: Spielberger State-Trait Anger Expression Inventory; Ho: Cook-Medley Hostility Scale; PSS: Perceived Stress Scale; PSQI: Pittsburgh Sleep Quality Index; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure.
Comparing Spouse vs. Child/Other
Procedure
Participants were seen for data collection either at the Williams LifeSkills office in Durham, NC, or at their own homes in the surrounding area. The location was determined by the choice of the participant, based on personal convenience. This allowed for inclusion of individuals who could not leave the home due to their caregiver responsibilities. Proportions of participants seen at the Williams LifeSkills office or at home did not differ between treatment arms.
All participants were assessed for psychological and biological stress markers (see Measures, below) on each of four visits. Following completion of data collection during Visit 1, participants were informed which group – VCS or Waitlist – they had been assigned to. Those in the VCS treatment arm received the VCS training materials and were informed about the provisions for telephone coaching (see below) that they would be receiving. Following Visit 1 the psychological and biological stress markers were re-assessed at three additional visits – seven weeks (Visit 2), three months (Visit 3) and six months (Visit 4) after Visit 1. Subjects enrolled in the study received $30 compensation for completion of each of the first three study visits and $60 compensation for the fourth study visit
VCS Training Protocol
The VCS program consists of ten video modules each of 7 to 10 minutes in length that provided training in the ten coping skills presented by the WLV. (44) These coping skills are listed in Table 2. To adapt the VCS to the specific needs of caregivers, the broad range of generically stressful situations used to illustrate use of each skill in the WLV in each VCS module was replaced by a dramatization of a caregiving situation that calls for the use of that module’s skill. In the opening scene of each module, failure to employ that skill leads to increased distress in patient and caregiver. Then the video instructor calls attention to how the caregiver’s behavior contributed to the distress. The instructor then describes that module’s skill and how it might have been used. A second dramatization of the situation shows the caregiver using that module’s skill effectively, resulting in a less distressing and more positive outcome. The VCS video program was accompanied by a workbook that provided additional information about each skill, including tips on how to use it in caregiving situations and homework exercises in which that skill is applied first to typical caregiving situations and then to examples drawn from the caregiver’s own life. Participants assigned to the VCS training arm were instructed to view two modules per week – in sequence, and to complete the exercises and homework presented for each module in the workbook.
Table 2.
The ten LifeSkills modules presented in the VCS
| 1. Increasing awareness of and objectivity in distressing situations; |
| 2. Evaluating one’s reactions to those situations to decide whether to try to change one’s reactions or to take actions to try to change the situations |
| 3. Changing one’s reaction to distressing situations |
| 4. Using assertion to get others to change their behavior |
| 5. Problem solving to change distressing situations |
| 6. Saying No to reduce exposure to distressing situations; |
| 7. Speaking clearly so others really listen |
| 8, Listening skills to make sure you hear what others are saying |
| 9. Empathizing to increase understanding of others’ behavior |
| 10. Increasing the positives in your interactions with others |
Telephone Coaching
Telephone coaches (LPG, ELB) trained to facilitate the LifeSkills modules called participants once a week for five weeks to teach each week’s two stress reducing skills designed to increase the effectiveness of their caregiving and to reduce biological and psycho-behavioral indices of stress. The calls and the ten skills that were covered in sequential pairs of modules is shown in Table 2, with a focus on the goals outlined for each coaching session. The coaching followed a standardized format focusing on situations presented by the caregiver, as described in the VCS Coaching Manual developed for this study.
Each skill set presented in the Caregiver Video portrayed caregiving scenarios with typical stress producing circumstances or events. Viewing the scenarios often served to “normalize” caregiver experiences or behaviors that were difficult for some caregivers to admit, particularly those involving anger at the ill individual. Some caregivers reported that just being able to admit their anger as normal lessened the pain. Caregivers were encouraged to practice the skills they learned to deal successfully with normal but stressful situations faced by caregivers.
Measures
Demographics
Age, race/ethnicity, gender, education level, family income, relation to care recipient and living arrangement with care recipient were recorded at Visit 1 (Table 1), with checks for any changes at subsequent visits.
Psychological Measures
Perceived Stress
The Perceived Stress Scale (48) consists of 10 items which are scored on a 5-point Likert scale. This scale assesses the degree to which individuals feel that the events in their lives are unpredictable or uncontrollable. Higher scores indicate higher levels of perceived stress, i.e., feelings that one’s life is unpredictable or uncontrollable.
Anxiety
State and trait anxiety were measured using the Spielberger State-Trait Anxiety Inventory (STAI) (49). The state and trait forms of the STAI each consisted of 20 self-report items which are scored on a 4-point Likert scale (1: not at all, 2: somewhat, 3: moderately so and 4: very much so).
Anger
State and trait anger were measured using the Spielberger State-Trait Anger Inventory (STAXI). (50) For continuity of instructions within the questionnaire battery, the STAXI was divided into individual inventories for state and trait anger. Both inventories consist of 15 self-report items which are scored on a 4-point Likert scale (1: almost never, 2: sometimes, 3: often and 4: almost always).
Depressive Symptoms
Depressive symptoms were measured using The Center for Epidemiologic Studies Depression Scale (CES-D), (51) a widely used 20-item self-report scale designed to measure depressive symptoms (i.e., depressive affect, well-being, somatic complaints and interpersonal concerns) in a general population. The CES-D has been frequently used in studies of caregiver psychiatric morbidity and well-being. (3–9) The questions target symptoms experienced during the past week and capture affective, somatic, well-being and interpersonal domains. The CES-D is scored on a 4-point Likert scale, with higher scores indicating stronger symptoms.
Hostility
Hostility was measured using the MMPI-based Cook-Medley Hostility Scale. (52) For the purpose of this study, the number of questions was pared down from 50 to 27 items as previous research has shown the 27-item version of the Cook-Medley Hostility Scale, comprised of three subscales – cynicism, hostile affect and aggressive responding – to be a stronger predictor of increased risk of mortality due to Coronary Heart Disease than other items on the 50-item scale. (49)
Personal Mastery
Perceived personal mastery for caregiving tasks was assessed using the Revised Scale for Caregiving Self-Efficacy (CGSE). (54) The scale consists of 51 items which assess various aspects of perceived personal mastery of the caregiving role (e.g., asking for help, responding well to aggravating patient behaviors and controlling negative thoughts). The items on the scale form subscales for three domains of caregiving self-efficacy: obtaining respite, responding to disruptive patient behaviors and controlling upsetting thoughts. Higher subscale scores and summed subscale scores generally reflect higher levels of perceived personal mastery. We chose to use this scale due to the body of research which suggests that higher levels of perceived personal mastery can act as buffers against the negative effects of caregiving on psychiatric morbidity. (28–30, 55)
Sleep
Sleep quality was measured using the Pittsburgh Sleep Quality Index (PSQI). (56) The PSQI consists of 18 items that assess the quality of sleep (e.g., time to fall asleep and sleep disrupting events) and manifestations of poor sleep quality (e.g., medications taken for sleep and tiredness or sluggishness during the day) over the last month. The questions on the PSQI make up seven subscales: overall sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, problems with daytime functioning and medications taken for sleep. Two additional questions – 1. Do you nap during the day? and 2. Do you share the same bedroom with your spouse? – were added to the PSQI for the present study.
Biomarkers
Stress testing
Blood pressure (BP) and heart rate (HR) were recorded during stress testing using an automatic blood pressure monitor (DINAMAP PRO 100, Critikon, Tampa, Florida or Accutorr Plus, Datascope Corp., Mahwah, New Jersey). Stress testing began with a 10-minute baseline period of quiet rest during which BP and HR were measured at 1-minute intervals. Participants were then asked to recall a recent caregiving experience that they found difficult to manage. They were instructed to speak continuously for 4–5 minutes about this experience. Five BP and HR measurements were taken during the stressor period at 1-minute intervals. After recalling the experience, the caregivers were asked to stop talking and remain silent. Five additional measurements were collected in a 5-minute recovery period. Approximately 53% of the study participants chose to have their blood pressure monitored during stress testing at home and the remainder chose to come to the Williams LifeSkills offices for testing
Salivary Cortisol
Five salivary cortisol samples were collected at each study visit using Salivettes® (SARSTEDT, Newton, NC). A salivary cortisol sample was collected at the beginning of each study visit and at the conclusion of the stress protocol. Study participants were also asked to collect three additional samples on the day after each visit: upon waking; 30 minutes after waking; and the last sample after 6:00pm. Assays for salivary free cortisol were conducted by the Pharmacology and Cancer Biology Laboratory within the Duke University Health System.
Statistical Analysis
Treatment effects were evaluated with intention to treat analyses that used all of the data available for each participant. The number available at each time point is shown in the flow chart of Figure 1. Based on prior findings of benefits in trials testing the Williams LifeSkills Workshop and Video (42–46), depressive symptoms (CES-D), Trait Anxiety (STAI), Trait Anger (STAXI), and perceived stress (PSS), hostility (Ho) were chosen as the primary psychological outcome variables. Average SBP and DBP were selected as primary biological outcome variables. Repeated measures analysis of covariance (ANCOVA) tested differences between the VCS and Wait List groups across the three follow-up visits, with adjustment for baseline values (Visit 1). ANCOVA provided statistical control for the observed differences at baseline in some variables (Table 1). We predicted that participants in the VCS arm would show significantly larger improvements in these psychological and biological outcomes than those in the Wait List arm.
With less prior evidence for LifeSkills efficacy, we designated self-efficacy (CGSE), sleep disturbance (PSQI), BP reactivity to mental stress, HR and cortisol measures as secondary outcomes, which could benefit from VCS training. Because prior research has not shown benefits of LifeSkills training for the more transitory State Anger and Anxiety variables, we did not predict benefits for them. For each outcome variable, a two-factor statistical model was tested that included the Visit 1 level as a covariate, with factors for treatment group and the repeated assessments over time. Visit 2 (seven weeks after Visit 1, when the VCS group had just finished the training) provided data on short-term effects, while Visit 3 (three months) and Visit 4 (six months) provided data on the persistence of treatment effects. The Group × Visit interaction was calculated for all outcome variables. Analyses of the biomarkers included an additional time factor and Group × Visit × Time interaction. The third factor designated time of collection, which represented time of day for salivary cortisol or phase of the stress test for BP, HR, and salivary cortisol. When an interaction was found to be significant, simple effects were examined to determine where the group comparisons reached significance. Analyses were conducted using Proc MIXED (SAS ver. 9.0, SAS Institute, Inc., Cary, North Carolina) and the significance criterion was set at P < .05.
RESULTS
The number of participants in each group over time and reasons for attrition are shown in Figure 1. The overall dropout rate over the course of the study was 18.1%. Over the four visits, the percentage of patients dropping out in the two study arms did not differ by Fisher’s exact test. The majority of the VCS group dropouts occurred following the first visit. The reasons for dropout were death of the care recipient (N=6), major illness developing in the caregiver (N=2), not being able to make the time commitment necessary for participation (N=2), moving away from NC (1), insufficient English fluency (1) and ceasing to be the caregiver (N=1), with four unknown. One hundred and sixteen patients were randomly assigned to the VCS (N=59) or Waitlist (N=57) group. Baseline characteristics of the VCS and Waitlist groups are shown in Table 1. In general, participants’ demographic backgrounds in terms of age, race, gender and SES (education and income) were similar across the two study arms. However, the groups significantly differed in terms of the relationship of the participant to the care recipient and whether the caregiver resided with the patient. The VCS group included a greater percentage of spouse caregivers (P=0.03) as well as caregivers who resided with the care recipient (P=0.01), suggesting that this group may experience a greater burden of care. The VCS group also had significantly higher Baseline CES-D and State Anxiety scores and SBP and DBP levels, with trends (P≤0.10) toward higher Trait Anxiety and Perceived Stress scores -- possibly a reflection of this greater burden. When “residing with” and “relationship to care recipient” were covaried, the baseline differences in State Anxiety and SBP became non-significant, providing some support for this interpretation. None of the other psychological or biological stress markers differed significantly between the two study arms at Baseline. There were no differences between the VCS and Wait List groups in the time since the care recipient was diagnosed with ADRD (42+/−35 vs 45+/−32 months) or since dementia symptoms were first noticed (68+/−52 vs 73+/−51 months), indicating there was no difference in length of exposure to caregiving responsibilities.
Psychological Improvements
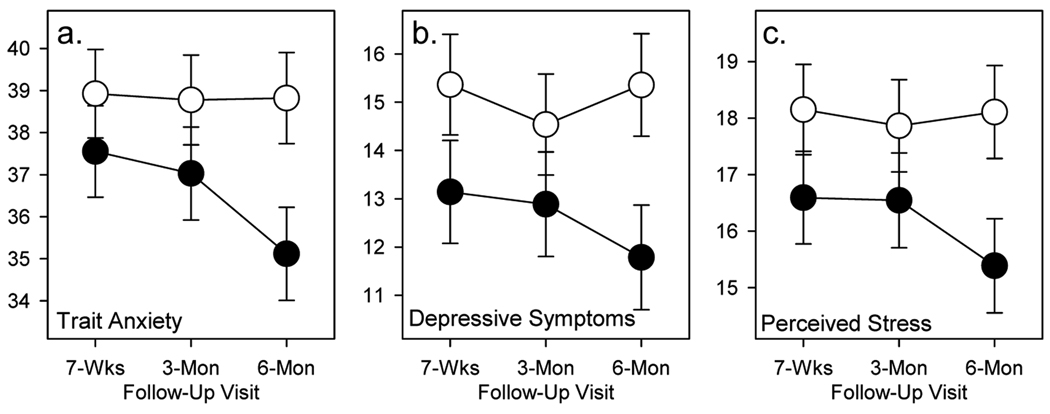
Among the five primary psychological outcome variables, three showed effects in the predicted direction. The group main effect was significant for depressive symptoms [CES-D; F(1,101)=4.19, P=0.04] and perceived stress [PSS; F(1,101)=4.08, P<0.05], and there was a trend for the group effect for trait anxiety [F(1,101)=3.17, P=0.08]. None of the Group × Visit interactions were significant. As shown in Figure 2, depressive symptoms, perceived stress and trait anxiety levels were all lower across Visits 2, 3 and 4 in the VCS group than the Wait List group. This pattern indicates that when the higher baseline levels of these variables in the VCS group are controlled, those caregivers who received the VCS training reported lower levels of depressive symptoms, perceived stress, and trait anxiety than those in the Wait List group, and that these benefits were maintained across the entire six-month follow-up period. Effect sizes (Cohen’s d; 57) for the degree of improvement at the six months follow-up assessment were 0.63 for depressive symptoms, 0.90 for perceived stress and 0.64 for trait anxiety. No significant Group effects or Group × Visit interactions were found for state anxiety, state and trait anger, hostility, disruptive sleep and the subscales of the Revised Scale for Caregiver Self-efficacy (all ps >.0.10).
Figure 2.
Follow-up differences in mood scales for VCS treatment (filled circles) and Wait List control (open circles) groups. Mean values (SEM error bars) post-treatment for STAI Trait scale (a), CESD depressive symptoms scale (b), and Perceived Stress Scale (c), with statistical adjustment for baseline values.
Physiological Improvements
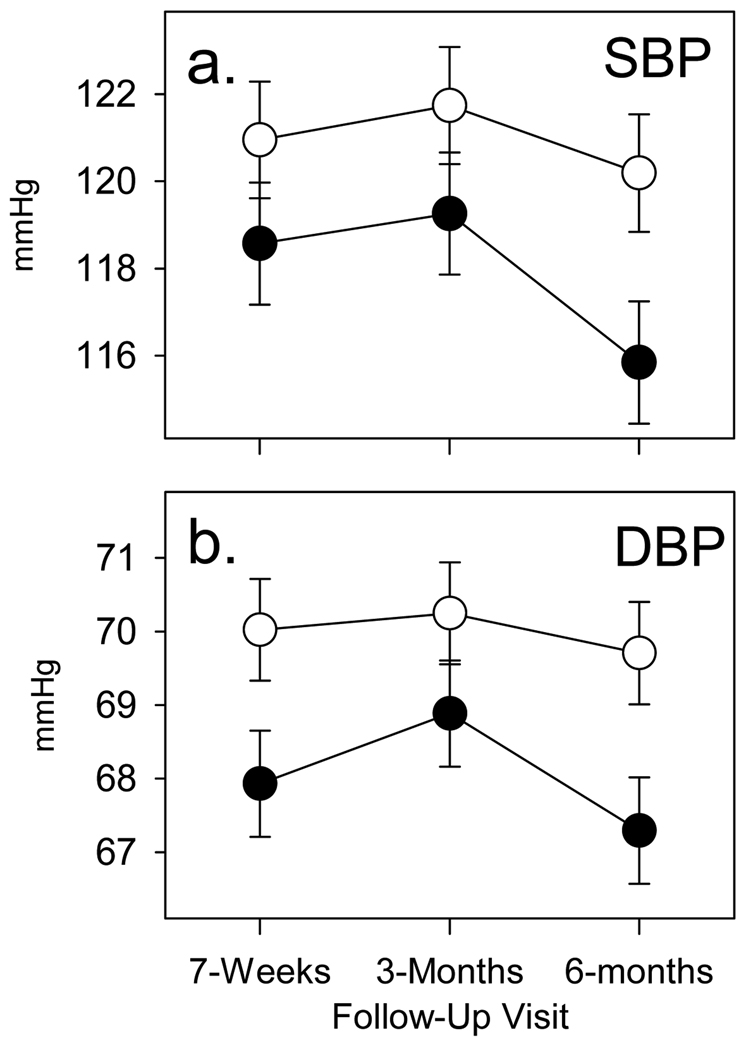
Both primary biological outcome variables showed effects in the predicted direction. A Group × Visit interaction was found for both mean (i.e., averaged across rest, stress and recovery periods for each Visit) systolic [F(2,192)=5.37, P=0.005] and mean diastolic [F(2,192)=3.38, P=0.04] blood pressure, with a significant group main effect for mean diastolic blood pressure as well [F(1,101)=3.96, P<0.05]. As shown in Figure 3, mean systolic blood pressure and mean diastolic blood pressure were lower in the VCS group across Visits 2–4 and decreased significantly more at Visit 4 in the VCS group than the Waitlist group. Effect sizes (d) for the degree of improvement at the six months follow-up assessment were 0.40 for SBP and 0.42 for DBP.
Figure 3.
Follow-up blood pressure averages for VCS treatment (filled circle) and Wait List control (open circle) groups. Mean values (SEM error bars) for systolic blood pressure (a) and diastolic blood pressure (b) with statistical adjustment for baseline values.
There were no Group or Group × Visit effects with respect to blood pressure or heart rate reactivity to stress. Mean heart rate demonstrated a significant Group × Time interaction [F(2,192)=16.9, P<.0001], with different patterns of change in the two groups The VCS and Wait list groups had similar heart rate levels across Visits 2 and 3 but the VCS group exhibited an increase in mean heart rate from Visit 3 to Visit 4 while the Waitlist group showed a decrease during this same interval.
Cortisol measurement included daily fluctuation (at awakening, 30 min post awakening and after 6pm) as well as stress response (pre and post stress). Neither mean levels of cortisol across visits nor the changes across the day or pre/post-stress differed significantly between the VCS and Waitlist groups (all ps >.05).
DISCUSSION
The results of this study suggest that the VCS program reduced stress in ADRD caregivers. Three of five primary psychological outcome measures and both primary biological stress markers were lower (depressive symptoms, trait anxiety, perceived stress, DBP) or showed a larger fall (SBP and DBP) across a six-month follow-up period in caregivers assigned to the VCS arm of the trial than levels observed in the Wait List control group. In contrast to prior successful interventions in caregivers that were continuously delivered, in some cases, over periods of years (34) these improvements were observed immediately at the conclusion of the 5-week training period and were sustained, with no further reinforcement, over the 5-month follow-up period after the five weeks of training,. This suggests that caregivers in the VCS arm continued to use the skills they had learned.
The potential clinical importance of these improvements is highlighted (Figure 2) by the decrease in CES-D scores from a mean of 18.7 at Visit 1 that was above the clinical threshold level of 16 (58, 59) at Visit 1 to a level of 12 at the six months follow-up assessment. In addition to these improvements in psychosocial factors, VCS training was also associated with decreases of 8 mmHg in SBP and 4 mmHg in DBP by the six months follow-up. The 8 mmHg in SBP compares favorably with other studies of non-pharmacologic approaches to BP reduction. (60) To our knowledge, this is the first demonstration of a physiological improvement in association with a behavioral intervention in caregivers. Effect sizes for both psychosocial and biological stress indices that were significantly improved by VCS training were in the moderate to large range and similar to those found in a previous randomized controlled trial of Williams LifeSkills training in coronary bypass patients (43).
Prior reviews of the literature (32, 37) suggested that the improvements produced by behavioral interventions in earlier trials of caregivers were often found with interventions that excluded caregivers who cannot travel to test sites – i.e., those who are likely to be the most burdened and who could be either more or less likely to show benefits. This study had no such limitation because the VCS, Workbook and telephone coaching were delivered in the home to caregivers whose mobility and hence ability to attend group or other counseling sessions, might have been greatly restricted. We found that those who could not travel to a testing center also benefited from the intervention. Because the VCS program tested in this study constitutes a behavioral intervention that can be delivered on a mass basis, it has the potential to reach the large numbers of caregivers who are unable to attend counseling and support groups.
Telephone coaching has the added effect of being personalized to the specific concerns of the caregiver. This is not always possible in a group setting, such as the caregiver support group, one of the more common resources offering support, education and consumer information to the caregiver. Telephone coaching is totally person-focused for the time set aside for the call—a luxury seldom available in a support group setting. The need to be “heard” is critical for many caregivers. When this need is met, the person is more open to consider and accept skills that help with preventing or coping with stress producing situations. The combination of VCS, workbook and telephone coaching is particularly useful where time, cost and other constraints determine that caregivers cannot travel to a treatment facility, thereby showing it can be delivered on a mass basis to caregivers whose mobility is limited.
Several limitations must be acknowledged. First, rather than some form of attention placebo control, we used a no-treatment wait-list condition as a control for the VCS treatment. We believe this is a valid control, because that is what the vast majority of caregivers in the population are getting – no active intervention to allay their distress. The home visits (or visits to WLS offices) for assessments could be construed as a kind of attention, but we agree that in future, larger studies it will be appropriate to include a more credible attention-placebo control. Second, the alternating assignment to study arms did not achieve balance of the treatment and control groups on all of the demographic factors. The group assigned to the VCS treatment arm contained larger proportions of spouse caregivers and caregivers who resided with the care recipient compared to the control group. This imbalance likely contributed to the higher psychosocial risk factor and blood pressure levels in the VCS group at baseline. Because the levels of psychosocial and biological stress markers were significantly lower in the VCS group compared to the Wait List group across a six month follow-up period even after statistical control for these baseline differences, it is unlikely that this imbalance accounted for the observed treatment benefits in the VCS group. Our confidence in the validity of improvements in the VCS arm of this study is further increased by findings of similar improvements in these same measures in an earlier randomized controlled trial of the LifeSkills Workshop in coronary bypass surgery patients,(43) in which randomization did achieve similar levels in these psychosocial and biological measures in both study arms at baseline. An additional limitation is that our pre-specified primary outcomes in this efficacy study included two biological and five psychological indicators of stress, of which four were statistically significant at conventional levels with a fifth at the trend level. A conservative approach would require a Bonferroni correction, which changes the statistical outcome. Nonetheless, the signal is clearly in the predicted direction of benefit, and future research employing a fully randomized controlled design in a larger sample will enable us to confirm that the benefits of LifeSkills VCS training for caregivers found in this study are real.
In conclusion, the current study provides encouraging evidence that a program of video-based coping skills with telephone coaching has the potential to produce improvements in both psychosocial stress indicators and average blood pressure levels in caregivers of a family member with ADRD. These improvements suggest that such VCS training may result in improved mental and physical health outcomes as well as reduced medical care costs among caregivers. Further research, in adequately powered randomized controlled trials, will be required to confirm that the benefits found in the current study are replicable and to determine whether clinical and cost benefits can be achieved with this technology
Acknowledgments
Supported by an SBIR grant from the National Institute on Aging (2 R44 AG025593) to Williams LifeSkills, Inc. Lisa Gwyther and Edna Ballard gratefully acknowledge support from the National Institute on Aging to the Duke Bryan Alzheimer’s Disease Research Center at Duke Medicine (P30 AG028377). Redford and Virginia Williams are founders and major stockholders in Williams LifeSkills, Inc. Lauren Bishop-Fitzpatrick, Analise Vendittelli and Tiffany Hutchens are present or former employees of Williams LifeSkills, Inc., and James D. Lane has been a consultant. Virginia Williams had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Glossary
- ADRD
Alzheimer’s Disease or related dementia
- VCS
video-based coping skills
- WLV
Williams LifeSkills Video
- CES-D
Center for Epidemiological Studies Depression Scale
- STAI
Spielberger State-Trait Anxiety Inventory
- STAXI
Spielberger State-Trait Anger Inventory
- CGSE
Revised Scale for Caregiving Self-Efficacy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration: http://www.clinicaltrials.gov; #NCT00396285.
REFERENCES
- 1.Mebane-Sims I. 2009 Alzheimer's Disease facts and figures. Alzheimer's & Dementia. 2009;5(3):234–270. doi: 10.1016/j.jalz.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Cotter VT. The burden of dementia. The American Journal of Managed Care. 2007;13(8):S193–S197. [PubMed] [Google Scholar]
- 3.Schulz R, Obrien AT, Bookwala J, Fleissner K. Psychiatric and physical morbidity effects of dementia caregiving - prevalence, correlates, and causes. Gerontologist. 1995 Dec;35(6):771–791. doi: 10.1093/geront/35.6.771. [DOI] [PubMed] [Google Scholar]
- 4.Covinsky KE, Newcomer R, Fox P, Wood J, Sands L, Dane K, Yaffe K. Patient and caregiver characteristics associated with depression in caregivers of patients with dementia. Journal of General Internal Medicine. 2003;18(12):1006–1014. doi: 10.1111/j.1525-1497.2003.30103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neundorfer MM, McClendon MJ, Smyth KA, Strauss ME, McCallum TJ. Does depression prior to caregiving increase vulnerability to depressive symptoms among caregivers of persons with Alzheimer's disease? Aging & Mental Health. 2006;10(6):606–615. doi: 10.1080/13607860600641036. [DOI] [PubMed] [Google Scholar]
- 6.Russo J, Vitaliano PP, Brewer DD, Katon W, Becker J. Psychiatric disorders in spouse caregivers of care recipience with Alzheimer's Disease and matched controls: A diathesis-stress model of psychopathology. Journal of Abnormal Psychology. 1995;104(1):197–204. doi: 10.1037//0021-843x.104.1.197. [DOI] [PubMed] [Google Scholar]
- 7.Schulz R, Belle SH, Czaja SJ, McGinnis KA, Stevens A, Zhang S. Long-term care placement of dementia patients and caregiver health and well-being. Journal of the American Medical Association. 2004;292(8):961–967. doi: 10.1001/jama.292.8.961. [DOI] [PubMed] [Google Scholar]
- 8.Yaffe K, Fox P, Newcomer R, Sands L, Lindquist K, Dane K, Covinsky KE. Patient and Caregiver characteristics and nursing home placement in patients with dementia. Journal of the American Medical Association. 2002;287(16):2090–2097. doi: 10.1001/jama.287.16.2090. [DOI] [PubMed] [Google Scholar]
- 9.Schulz R, Mendelsohn AB, Haley WE, Mahoney D, Allen RS, Zhang S, Thompson L, Belle SH. End-of-life care and the effects of bereavement on family caregivers of persons with dementia. New England Journal of Medicine. 2003 Nov;349(20):1936–1942. doi: 10.1056/NEJMsa035373. [DOI] [PubMed] [Google Scholar]
- 10.Cooper C, Balamurali TBS, Livingston G. A systematic review of the prevalence and covariates of anxiety in caregivers of people with dementia. International Psychogeriatrics. 2007;19(2):175–195. doi: 10.1017/S1041610206004297. [DOI] [PubMed] [Google Scholar]
- 11.Christakis NA, Allison PD. Mortality after the hospitalization of a spouse. New England Journal of Medicine. 2006;354(7):719–730. doi: 10.1056/NEJMsa050196. [DOI] [PubMed] [Google Scholar]
- 12.Schulz R, Beach SR. Caregivers as a risk factor for mortality: The Caregiver Health Effects Study. Journal of the American Medical Association. 1999;282(23):2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 13.Vitaliano PP, Russo J, Scanlan JM, Greeno CG. Weight changes in caregivers of Alzheimer's care recipients: Psychobehavioral predictors. Psychology & Aging. 1996;11(1):155–163. doi: 10.1037//0882-7974.11.1.155. [DOI] [PubMed] [Google Scholar]
- 14.Brummett BH, Krystal AD, Ashley-Koch A, Kuhn C, Züchner S, Siegler IC, Barefoot JC, Ballard EL, Gwyther LP, Williams RB. Sleep quality varies as a function of 5-HTTLPR genotype and stress. Psychosomatic Medicine. 2007;69:621–624. doi: 10.1097/PSY.0b013e31814b8de6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiecolt-Glaser JK, Dura JR, Speicher CE, Trask OJ, Glaser R. Spousal caregivers of dementia victims: longitudinal changes in immunity and health. Psychosomatic Medicine. 1991;53(4):345–362. doi: 10.1097/00006842-199107000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Lutgendorf SK, Garland L, Buckwalter KC, Reimer TT, Hong SY, Lubaroff DM. Life stress, mood disturbance, and elevated interleukin-6 in healthy older women. Journals of Gerontology Series A-Biological Sciences and Medical Sciences. 1999;54(9):M434–M439. doi: 10.1093/gerona/54.9.m434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vitaliano PP, Zhang JP, Scanlan JM. Is caregiving hazardous to one's physical health? A meta-analysis. Psychological Bulletion. 2003;129(6):946–972. doi: 10.1037/0033-2909.129.6.946. [DOI] [PubMed] [Google Scholar]
- 18.Von Kanel R, Dimsdale JE, Ancoli-Israel S, Mills PJ, Patterson TL, McKibbin CL, Archuleta C, Grant I. Poor sleep is associated with higher plasma proinflammatory cytokine interleukin-6 and procoagulant marker fibrin D-dimer in older caregivers of people with Alzheimer's disease. Journal of the American Geriatrics Society. 2006;54(3):431–437. doi: 10.1111/j.1532-5415.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- 19.Kiecolt-Glaser JK, Glaser R, Gravenstein S, Malarkey WB, Sheridan J. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc. Natl. Acad. Sci. U. S. A. 1996 Apr;93(7):3043–3047. doi: 10.1073/pnas.93.7.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiecolt-Glaser JK, Marucha PT, Malarkey WB, Mercado AM, Glaser R. Slowing of would-healing by psychological stress. The Lancet. 1995;346(8984):1194–1196. doi: 10.1016/s0140-6736(95)92899-5. [DOI] [PubMed] [Google Scholar]
- 21.Shaw WS, Patterson TL, Ziegler MG, Dimsdale JE, Semple SJ, Grant I. Accelerated risk of hypertensive blood pressure recordings among Alzheimer caregivers. Journal of Psychosomatic Research. 1999;46(3):215–227. doi: 10.1016/s0022-3999(98)00084-1. [DOI] [PubMed] [Google Scholar]
- 22.Vitaliano PP, Scanlan JM, Zhang JP, Savage MV, Hirsch IB, Siegler IC. A path model of chronic stress, the metabolic syndrome, and coronary heart disease. Psychosomatic Medicine. 2002 May–Jun;64(3):418–435. doi: 10.1097/00006842-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Von Kanel R, Mausbach BT, Patterson TL, Dimsdale JE, Aschbacher K, Mills PJ, Ziegler MG, Ancoli-Israel S, Grant I. Increased Framingham coronary heart disease risk score in dementia caregivers relative to non-caregiving controls. Gerontology. 2008;54(3):131–137. doi: 10.1159/000113649. [DOI] [PubMed] [Google Scholar]
- 24.Aschbacher K, Mills PJ, von Kanel R, Hong S, Mausbach BT, Roepke SK, Dimsdale JE, Patterson TL, Ziegler MG, Ancoli-Israel S, Grant I. Effects of depressive and anxious symptoms on norepinephrine and platelet P-selectin responses to acute psychological stress among elderly caregivers. Brain Behav. Immun. 2008 May;22(4):493–502. doi: 10.1016/j.bbi.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schubert CC, Boustani M, Callahan CM, Perkins AJ, Hui S, Hendrie HC. Acute Care Utilization by Dementia Caregivers Within Urban Primary Care Practices. Journal of General Internal Medicine. 2008 Nov;23(11):1736–1740. doi: 10.1007/s11606-008-0711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen CA, Colantonio A, Vernich L. Positive aspects of caregiving: rounding out the caregiver experience. Int. J. Geriatr. Psychiatr. 2002 Feb;17(2):184–188. doi: 10.1002/gps.561. [DOI] [PubMed] [Google Scholar]
- 27.Farran CJ, Miller BH, Kaufman JE, Donner E, Fogg L. Finding meaning through caregiving: Development of an instrument for family caregivers of persons with Alzheimer's disease. J. Clin. Psychol. 1999 Sep;55(9):1107–1125. doi: 10.1002/(sici)1097-4679(199909)55:9<1107::aid-jclp8>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 28.Devor M, Renvall M. An educational intervention to support caregivers of elders with dementia. Am. J. Alzheimers Dis. Other Dement. 2008 Jun–Jul;23(3):233–241. doi: 10.1177/1533317508315336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roepke SK, Mausbach BT, Aschbacher K, Ziegler MG, Dimsdale JE, Mills PJ, von Känel R, Ancoli-Israel S, Patterson TL, Grant I. Personal mastery is associated with reduced sympathetic arousal in stressed Alzheimer caregivers. Am. J. Geriatr. Psychiatr. 2008 Apr;16(4):310–317. doi: 10.1097/JGP.0b013e3181662a80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mausbach BT, Patterson TL, Von Kanel R, Mills PJ, Dimsdale JE, Ancoli-Israel S, Grant I. The attenuating effect of personal mastery on the relations between stress and Alzheimer caregiver health: A five-year longitudinal analysis. Aging & Mental Health. 2007 Nov;11(6):637–644. doi: 10.1080/13607860701787043. [DOI] [PubMed] [Google Scholar]
- 31.Wilks SE, Croom B. Perceived stress and resilience in Alzheimer's disease caregivers: Testing moderation and mediation models of social support. Aging & Mental Health. 2008;12(3):357–365. doi: 10.1080/13607860801933323. [DOI] [PubMed] [Google Scholar]
- 32.Gallagher-Thompson D, Coon DW. Evidence-based psychological treatments for distress in family caregivers of older adults. Psychol. Aging. 2007 Mar;22(1):37–51. doi: 10.1037/0882-7974.22.1.37. [DOI] [PubMed] [Google Scholar]
- 33.Logsdon RG. Dementia: psychosocial interventions for family caregivers. Lancet. 2008 Jul;372(9634):182–183. doi: 10.1016/S0140-6736(08)61048-X. [DOI] [PubMed] [Google Scholar]
- 34.Martin-Carrasco M, Martin MF, Valero CP, Millán PR, García CI, Montalbán SR, Vázquez AL, Piris SP, Vilanova MB. Effectiveness of a psychoeducational intervention program in the reduction of caregiver burden in alzheimer's disease patients' caregivers. Int. J. Geriatr. Psychiatr. 2009 May;24(5):489–499. doi: 10.1002/gps.2142. [DOI] [PubMed] [Google Scholar]
- 35.Belle SH, Burgio L, Burns R, Coon D, Czaja SJ, Gallagher-Thompson D, Gitlin LN, Klinger J, Koepke KM, Lee CC, Martindale-Adams J, Nichols L, Schulz R, Stahl S, Stevens A, Winter L, Zhang S. Resources for Enhancing Alzheimer's Caregiver Health (REACH) II Investigators.. Enhancing the quality of life of dementia caregivers from different ethnic or racial groups - A randomized, controlled trial. Ann. Intern. Med. 2006 Nov;145(10):727–738. doi: 10.7326/0003-4819-145-10-200611210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCurry SM, Logsdon RG, Vitiello MV, Teri L. Successful behavioral treatment for reported sleep problems in elderly caregivers of dementia patients: A controlled study. J. Gerontol. Ser. B-Psychol. Sci. Soc. Sci. 1998 Mar;53(2):P122–P129. doi: 10.1093/geronb/53b.2.p122. [DOI] [PubMed] [Google Scholar]
- 37.Schulz R, Martire LM, Klinger JN. Evidence-based caregiver interventions in geriatric psychiatry. Psychiatr. Clin. North Amer. 2005 Dec;28(4):1007–1038. doi: 10.1016/j.psc.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Brodaty H, Green A, Koschera A. Meta-analysis of psychosocial interventions for caregivers of people with dementia. Journal of the American Geriatrics Society. 2003 May;51(5):657–664. doi: 10.1034/j.1600-0579.2003.00210.x. [DOI] [PubMed] [Google Scholar]
- 39.Torti FM, Gwyther LP, Reed SD, Friedman JY, Schulman KA. A multinational review of recent trends and reports in dementia caregiver burden. Alzheimer Dis. Assoc. Dis. 2004 Apr–Jun;18(2):99–109. doi: 10.1097/01.wad.0000126902.37908.b2. [DOI] [PubMed] [Google Scholar]
- 40.Mittelman MS, Roth DL, Coon DW, Haley WE. Sustained benefit of supportive intervention for depressive symptoms in caregivers of patients with Alzheimer's disease. Am. J. Psychiat. 2004 May;161(5):850–856. doi: 10.1176/appi.ajp.161.5.850. [DOI] [PubMed] [Google Scholar]
- 41.Williams VP, Williams RB. LifeSkills: 8 simple ways to build stronger relationships, communicate more clearly, improve your health and even the health of those around you. New York: Times Books/Random House; 1998. [Google Scholar]
- 42.Gidron Y, Davidson K, Bata I. The short-term effects of a hostility-reduction intervention on male coronary heart disease patients. Health Psychology. 1999 Jul;18(4):416–420. doi: 10.1037//0278-6133.18.4.416. [DOI] [PubMed] [Google Scholar]
- 43.Bishop GD, Kaur D, Tan VLM, Chua YL, Liew SM, Mak KH. Effects of a psychosocial skills training workshop on psychophysiological and psychosocial risk in patients undergoing coronary artery bypass grafting. American Heart Journal. 2005;150(3):602–609. doi: 10.1016/j.ahj.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 44.Kirby ED, Williams VP, Hocking MC, Lane JD, Williams RB. Psychosocial benefits of three formats of a standardized behavioral stress management program. Psychosomatic Medicine. 2006 Nov–Dec;68(6):816–823. doi: 10.1097/01.psy.0000238452.81926.d3. [DOI] [PubMed] [Google Scholar]
- 45.Clemow LP, Pickering TG, Davidson KW, Liriano C. Multi-component Stress Management for Hypertensives: For whom does it work best?. Paper presented at the American Psychosomatic Society Annual Meeting; March 4–7, 2009; Chicago, IL. [Google Scholar]
- 46.Barnes VA, Williams VP, Williams RB, Johnson MH, Murrell AS, Shenbagarajan VP. Williams Lifeskills® training lowers school-time ambulatory blood pressure in adolescents. Paper presented at the Society of Behavioral Medicine Annual Meeting; April 22–25, 2009; Montreal, Canada. [Google Scholar]
- 47.Phillips KM, Antoni MH, Lechner SC, Blomberg BB, Llabre MM, Avisar E, Gluck S, DerHagopian R, Carver CS. Stress management intervention reduces serum cortisol and increases relaxation during treatment for nonmetastatic breast cancer. Psychosomatic Medicine. 2008;70:1044–1049. doi: 10.1097/PSY.0b013e318186fb27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen S, Williamson GM. Perceived stress in a probability sample of the United States. In: S S, S O, editors. The Social Psychology of Health. Newbury Park: Sage Publications; 1988. pp. 31–67. [Google Scholar]
- 49.Spielberger CD. Manual for the State-Trait anxiety inventory (Form Y) "Self-Evaluation Questionnaire". Palo Alto, CA: Consultion Psychologists Press, Inc.; 1983. [Google Scholar]
- 50.Spielberger CD. Manual for the State-Trait Anger Expression Inventory (STAXI) Orlando, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- 51.Radloff L. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 52.Cook W, Medley D. Proposed hostility and pharasaic-virtue scales for the MMPI. Journal of Applied Psychology. 1954;38:414–418. [Google Scholar]
- 53.Barefoot JC, Dodge KA, Peterson BL, Dahlstrom WG, Williams RB. The Cook-Medley Hostility Scale - Item content and ability to predict survival. Psychosomatic Medicine. 1989 Jan–Feb;51(1):46–57. doi: 10.1097/00006842-198901000-00005. [DOI] [PubMed] [Google Scholar]
- 54.Steffen AM, McKibbin C, Zeiss AM, Gallagher-Thompson D, Bandura A. The revised scale for caregiving self-efficacy: Reliability and validity studies. J. Gerontol. Ser. B-Psychol. Sci. Soc. Sci. 2002 Jan;57(1):P74–P86. doi: 10.1093/geronb/57.1.p74. [DOI] [PubMed] [Google Scholar]
- 55.Mausbach BT, Patterson TL, von Kanel R, Mills PJ, Ancoli-Israel S, Dimsdale JE, Grant I. Personal mastery attenuates the effect of caregiving stress on psychiatric morbidity. J. Nerv. Ment. Dis. 2006 Feb;194(2):132–134. doi: 10.1097/01.nmd.0000198198.21928.e7. [DOI] [PubMed] [Google Scholar]
- 56.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index - A new instrument for psychiatric practice and research. Psychiatry Res. 1989 May;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 57.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale (NJ): Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 58.Ensel W. Measuring depression: The CES-D scale. In: Lin N, Dean A, Ensel W, editors. Social Support, Life Events and Depression. New York, NY: Academic Press; 1986. pp. 51–70. [Google Scholar]
- 59.Zich JM, Attkisson CC, Greenfield TK. Screening for depression in primary care clinics: The CED-D and the BDI. International Journal of Psychiatry in Medicine. 1990;20(3):259–277. doi: 10.2190/LYKR-7VHP-YJEM-MKM2. [DOI] [PubMed] [Google Scholar]
- 60.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The jnc 7 report. JAMA. 2003;289:2560–2571. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]