Abstract
The Kv2 voltage-gated potassium channels, Kv2.1 and Kv2.2, are important regulators of neuronal excitability in mammalian brain. It has been shown that Kv2.1 channels are expressed in virtually all neurons in the brain. However, the cellular localization of Kv2.2 has not been fully elucidated. In this paper, we report that Kv2.2 is highly expressed in a subset of neurons in the magnocellular preoptic nucleus (MCPO) and the horizontal limb of the diagonal band of Broca (HDB) of the basal forebrain complex, which are areas highly implicated in the regulation of cortical activity and the sleep/wake-cycle. It has been shown that the MCPO and HDB contain distinct populations of neurons that differ in their neurochemicals, cholinergic, glutamatergic, and GABAergic neurons. Using specific immunolabeling and knock-in mice in which GFP is expressed in GABAergic neurons, we found that Kv2.2 is abundantly expressed in a large sub-population of the GABAergic neurons in the MCPO and HDB. These data offer Kv2.2 as a molecular target to study the role of the specific sub-population of basal forebrain GABAergic neurons.
Keywords: Magnocellular preoptic nucleus, potassium channel, sleep, corticopetal projection, antibody
Introduction
Voltage-gated potassium (Kv) channels play pivotal roles in regulating neuronal excitability, shaping action potentials, and modulating spiking patterns (Hille, 2001). Kv2 delayed rectified channels are particularly important in the regulation of somatodendritic excitability (Guan et al., 2007). Kv2.1 is widely expressed in most of brain areas in the mammalian brain, including cerebral cortex, cerebellum, hippocampus, striatum, thalamus, and hypothalamus (Trimmer, 1991; Hwang et al., 1993; Trimmer and Rhodes, 2004). In contrast, the information regarding the cellular localization of Kv2.2 is relatively limited. Previous studies reported that Kv2.2 is detected in the cerebral cortex, cerebellum, hippocampus, striatum, brain stem, and thalamus (Hwang et al., 1992; Hwang et al., 1993; Johnston et al., 2008). In this paper, we report a novel and abundant expression of Kv2.2 in the basal forebrain of the rat and mouse.
The basal forebrain (BF) complex, comprised of the substantia inominata, vertical and horizontal limbs of the diagonal band, the medial septum, and the magnocellular preoptic nucleus, is highly implicated in learning and memory (Bartus, 2000; Sarter and Bruno, 2004), attention (Everitt and Robbins, 1997), motivation (Whalen et al., 1994; Lin and Nicolelis, 2008), and the control of sleep-wake cycle (Szymusiak, 1995; Berntson et al., 2002; Jones, 2005). This area contains heterogeneous populations of neurons (Szymusiak and McGinty, 1986; Harkany et al., 2003; Nickerson Poulin et al., 2006)(Lin et al., 2003), with cholinergic and GABAergic neurons as their major components (Gritti et al., 1993; Gritti et al., 1997; Gritti et al., 2006). These neurons constitute the major cholinergic and GABAergic projections to the cerebral cortex (Divac, 1975; Kievit and Kuypers, 1975; Saper, 1984; Henny and Jones, 2008), where they are thought to regulate the activity of cortical neurons (Buzsaki et al., 1988). Although more than half of the basal forebrain neurons projecting to the cortex are GABAergic (Gritti et al., 1993), very little is known regarding the characteristics and functions of these GABAergic neurons (Sarter and Bruno, 2002; Lau and Salzman, 2008). This is mainly due to the lack of tools to target and study these neurons, as compared to those available for cholinergic neurons, such as IgG192-saporin (Wiley et al., 1991). Therefore, definitive molecular markers for BF GABAergic neurons have been demanded for better understanding of the physiological roles of these GABAergic neurons.
In the present study, we demonstrate that Kv2.2 is highly and selectively expressed in a subset of GABAergic neurons in the BF. Using highly specific antibodies, we found that Kv2.2 is abundantly expressed in neurons in the magnocellular preoptic nucleus (MCPO) and the horizontal limb of the diagonal band of Broca (HDB) and that these neurons are not cholinergic. Importantly, the protein expression levels of Kv2.2 in these nuclei were significantly greater than those in the cerebral cortex and striatum, suggesting the specific enrichment of Kv2.2 in the BF neurons. Furthermore, using the GFP knock-in technology, we identified that Kv2.2 is selectively expressed in a large subset of GABAergic neurons in the MCPO and HDB. This selective expression of Kv2.2 defines a novel sub-population of GABAergic neurons in the BF and also provides a novel molecular tool to target these neurons in studying their functions in animal behaviors.
Experimental Procedures
Animals
In this study, we used both rats and mice. Tissues from adult Sprague-Dawley rats (9 animals) were used in initial characterizations of Kv2.2 localization in the brain. In order to take advantage of knock-in mice technology (see below), we also used and characterized Kv2.2 localization in the brain of adult C57BL/6 mice (8 animals). Kv2.2-deficient mice were obtained from Texas A&M Institute for Genomic Medicine. In these animals, the second and the last exon of the Kv2.2 gene was replaced with a targeted vector containing β-geo. Heterozygous progeny was backcrossed to C57BL/6 mice. Two animals were used for this study. All animal use procedures were in strict accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institute of Health, and approved by the institutional animal use committee.
GAD67-GFP-knock-in mice
The generation of GAD67-GFP knock-in mice (Δneo) has been described previously (Tamamaki et al., 2003). Briefly, a cDNA-encoding enhanced GFP (EGFP; Clontech, Palo Alto, CA) was targeted to the locus encoding GAD67 using homologous recombination. Recombinant ES cells were used to generate chimeric male mice. GAD67-GFP knock-in mice were obtained by breeding the chimeric male mice with C57BL/6 or ICR females. Heterozygous progeny were backcrossed to C57BL/6 and referred to as GAD67-GFP mice. These GAD67-GFP mice retained the EGFP cDNA and the loxP-flanked PGK-neo cassette in the GAD67 locus. The loxP-flanked PGK-neo cassette was used as a positive selection marker for screening recombinant ES cells and was removed by mating the GAD67-GFP mice with the CAG-cre transgenic mice. The GAD-GFP mice without the PKG-neo cassette were referred to as GAD67-GFP (Δneo) mice. These mice are referred to as GAD67-GFP knock-in mice hereafter. Because the knock-out of both GAD67 alleles is lethal at birth, mice heterozygous for the altered GAD67 allele were used for all the experiments. GFP is expressed exclusively in GAD67-immunoreactive neurons in these mice (Tamamaki et al., 2003). Eight adult mice were used.
Antibodies Characterization
Please see Table 1 for a list of antibodies used.
Table 1.
Antibodies used in this study
| Antigen | Immunogen | Dilution | Manufactuere |
|---|---|---|---|
| Rat Kv2.1 | Synthetic peptide, aa 837-853 | 0.5 μg/ml | NeuroMab (Davis, CA), K89 |
| Rat Kv2.2 | Synthetic peptide, aa 859-873 | 0.1 μg/ml | Alomone (Israel), APC-120 |
| Rat Kv2.2 | Recombinant N-terminus 1-61 | 1 μg/ml | Neuromab, K37 |
| Mouse NeuN | Isolated nuclei from mouse brain | 1 μg/ml | Chemicon (Billerica, MA), MAB377 |
| Human choline acetyltransferase | Purified enzyme from human placenta | 1:5,000 | Chemicon, AB144 |
| GFP | Recombinant full-length GFP | 0.5 μg/ml | NeuroMab, N87 |
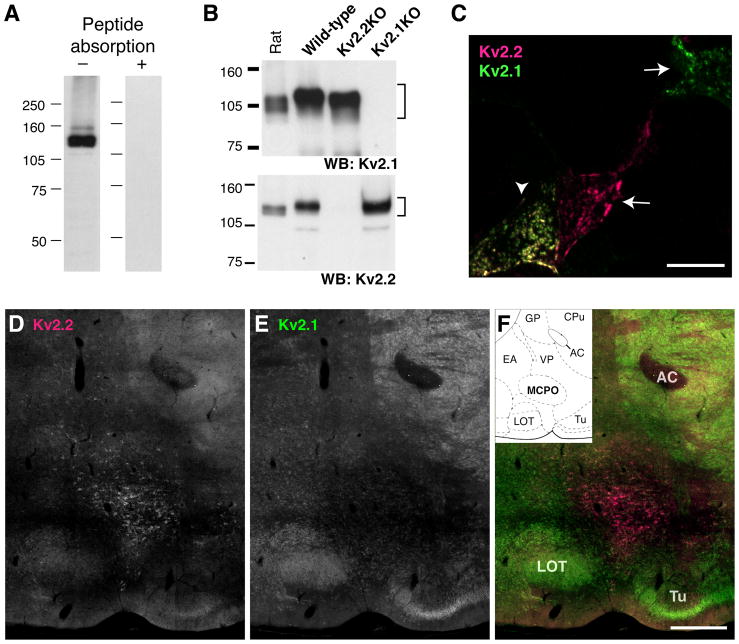
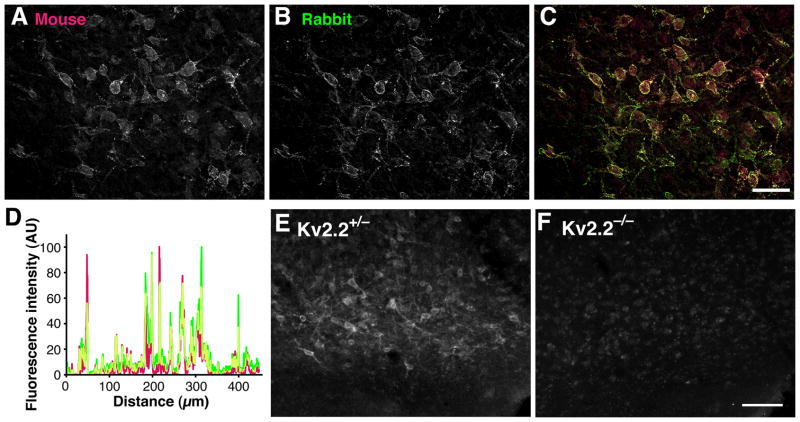
The rabbit Kv2.2 antibody recognized a major band of 140 kDa and a few minor bands in Western blotting. These bands were eliminated by preincubation of the diluted antibody (0.1 μg/ml) with 1 μg/ml of the antigen peptide. These bands were also undetectable in mice, in which the Kv2.2 gene had been deleted (see Fig. 1). Immunofluorescence staining was also eliminated by the preincubation procedure (see Fig. 2).
Fig. 1.
Specificity of the anti-Kv2.2 antibody. A: Rat brain membrane fraction was solubilized and subjected to Western blotting with (right lane) or without (left lane) the pre-absorption with the antigen peptide (1 μg/ml peptide to 0.1 μg/ml antibody). B: Brain lysates from wild-type, Kv2.1-deficient, and Kv2.2-deficient mice were subjected to Western blotting using K89 (top) or anti-Kv2.2 antibody (bottom). Numbers on the left denote the sizes of molecular weight markers in kDa. C: Immunostaining of HEK293 cells doubly transfected with Kv2.1 and Kv2.2. The arrows indicate cells solely stained either by K89 or Kv2.2 antibodies, and the arrowhead indicates a double-labeled cell. Non-transfected cells in the field were not labeled. The specificity was also verified in cells transfected with individual Kv2 subunit (not shown). D–F: Rat sagittal sections (lateral 2.62 mm) are stained with K89 anti-Kv2.1 and anti-Kv2.2 antibodies. The ventral surface of the brain between the nucleus of lateral olfactory tract (LOT) and the olfactory tubercle (Tu) is shown. AC, anterior commissure. Inset in F shows the rat brain atlas of the respective sagittal section. GP, globus pallidus; CPu, caudative putamen; EA, extended amygdala; VP, ventral pallidum. Scale bar, 1 mm.
Fig. 2.
Abundant expression of Kv2.2 in the BF of the rat. A–C: Overlapping patterns of immunolabeling with rabbit anti-Kv2.2C (Rabbit) and K37 anti-Kv2.2N (Mouse) antibodies. Scale bar, 100 μm. D: Line scanning analysis of a 450 μm segment in the fluorescence image in F. Green, rabbit Kv2.2 antibody; magenta, mouse Kv2.2 antibody. Overlap of signals is indicated in yellow. E–F: Kv2.2 immunostaining of heterozygous and homozygoous Kv2.2-deficient mice (Kv2.2+/− and Kv2.2−/−, respectively. Brain sections were stained using rabbit Kv2.2 antibody. Scale bar, 100 μm.
The K37 Kv2.2 antibody has been tested in heterologous cells expressing rat Kv2.2 gene in immunofluorescence staining (Maletic-Savatic et al., 1995). We have confirmed it in our hands as well (not shown). The antibody does not work in Western blotting.
The K89 Kv2.1 antibody has been extensively tested on heterologous cells and mice deficient of the Kv2.1 gene (Antonucci et al., 2001)(Misonou et al., 2006b). We have also confirmed that the antibody does not exhibit any detectable immunoreactivity in the Kv2.1 deficient mice in Western blotting (see Fig. 1). The Kv2.1-deficient mouse sample was a generous gift from Dr. Jeanne Nerbonne.
The N86 GFP antibody did not exhibit any detectable signals in naive HEK293 cells or wild-type mice, but showed strong immunoreactivity in HEK293 cells expressing EGFP (not shown) and in the GAD67-GFP knock-in mice (see Fig. 8).
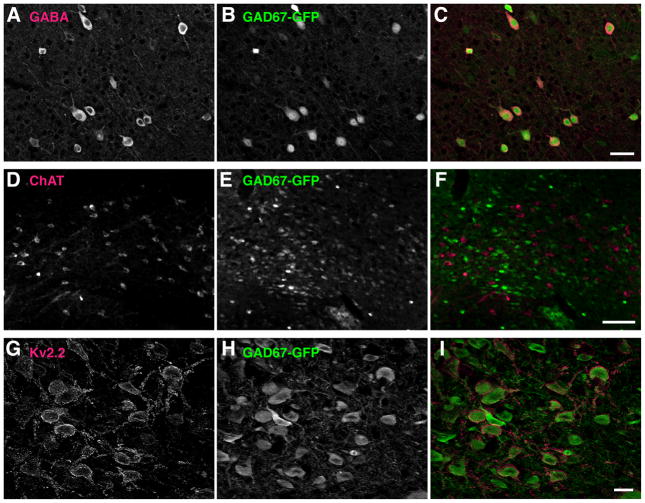
Fig. 8.
Selective expression of Kv2.2 in GABAergic neurons in the BF of mouse. A–C: Selective expression of GFP in GABAergic neurons in the cortrex of the GAD67-GFP knock-in mice. Coronal sections from GAD67-GFP knock-in mice were double immunostained with N86 anti-GFP and anti-GABA antibodies. Scale bar, 50 μm. D–F: GFP expressing neurons and ChAT positive neurons of the BF are distinct populations in the BF of the GAD67-GFP knock-in mice. Coronal sections from GAD67-GFP knock-in mice were double immunostained with N86 anti-GFP and anti-ChAT antibodies. Scale bar, 100 μm. G–I: Co-expression of Kv2.2 and GFP in the BF neurons of GAD67-GFP knock-in mice. Coronal sections from GAD67-GFP knock-in mice were double immunostained with anti-Kv2.2 and N86 anti-GFP antibodies. Scale bar, 20 μm.
The NeuN antibody recognized two bands around 50 kDa as previously reported (Mullen et al., 1992), and stained neuronal nuclei which is identical to previous reports (see Fig. 5).
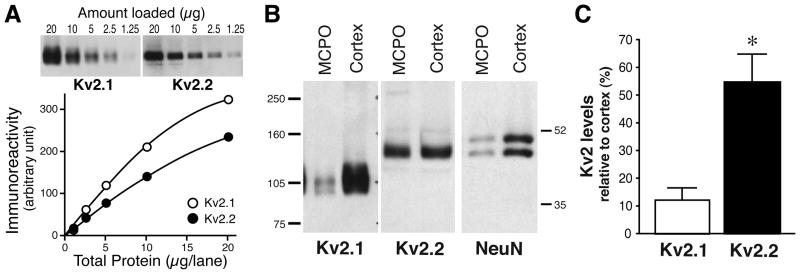
Fig. 5.
Reciprocal expression of Kv2.1 and Kv2.2 revealed by quantitative Western blotting. A: Micropunch tissue samples (0.5–1 mm) from the cortex were prepared and processed for western blot analysis using K89 anti-Kv2.1 and rabbit anti-Kv2.2 antibodies. Semi-linear relationships were obtained between immunoreactivity and the amount of samples for each of the antibodies. B: Micropunch tissues from the MCPO and the cortex was subjected to Western blotting using K89 anti-Kv2.1, anti-Kv2.2 and anti-NeuN antibodies. Numbers on the both ends denote the sizes of molecular weight markers in kDa. C: The relative levels of each Kv2 isotypes in the MCPO. Optical densities of immunoreactive bands in the MCPO samples were normalized to those of the cortex samples. Values are expressed as means ± SEM. *P< 0.05 (n= 7) by Student t-test.
The choline acetyltransferase antibody showed a staining pattern that is identical to published results obtained in the rat brain (Furuta et al., 2004) (see Fig. 6). The antibody did not work in Western blotting in our hands.
Fig. 6.
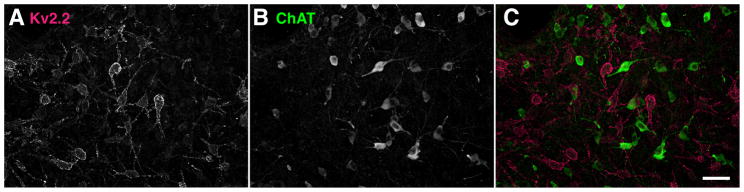
The expression of Kv2.2 in non-cholinergic neurons in the BF. Kv2.2-expressing neurons and cholinergic neurons are distinct sub-populations in the BF. Rat coronal sections were double immunostained with anti-Kv2.2 and anti-ChAT antibodies. Scale bar, 50 μm.
Monoclonal antibodies against Kv2.1, Kv2.2, and GFP are available from the UC Davis/NIH NeuroMab facility (Rhodes and Trimmer, 2008), supported by NIH grant U24NS050606 and maintained by the Department of Neurobiology, Physiology and Behavior, College of Biological Sciences, University of California Davis.
Preparation of tissue sections
Animals were anesthetized with pentobarbital (60 mg/Kg, ip) and briefly perfused with an ice-cold saline solution followed by a slow perfusion of 4% paraformaldehyde in PBS. Either sagittal or coronal brain sections (each 40 μm thick) were prepared as previously described (Misonou et al., 2006a).
Diaminobenzidine (DAB) and immunofluorescence staining of brain sections
Free-floating sections were blocked with 10% goat serum in a phosphate buffer containing 0.3% Triton X-100 for 1 h, then incubated overnight at 4°C with primary antibodies in the blocking buffer. For DAB staining, sections were incubated with rabbit anti-Kv2.2 antibody (0.3 μg/ml, Alomone Labs, Jerusalem, Israel) overnight, washed, and probed with a biotinylated anti-rabbit secondary antibody (1:250, Vector Labs, Burlingame, CA). Nickel-enhanced DAB reaction was performed using ABC kit (Vector Labs) according to the manufacturer’s instruction. For immunofluorescence staining, sections were incubated with combinations of primary antibodies that are different in species or IgG isotypes. Immunoreactivity was detected using Alexa dye-conjugated secondary antibodies (Invitrogen, Carlsbad, CA). For the detection of Kv2.2 and ChAT using K37 anti-Kv2.2 and anti-ChAT antibodies, antigen retrieval was necessary. Sections were incubated in 10 mM sodium citrate buffer (pH 8.5) for 30 min at 80°C prior to the immunostaining procedure described above (Jiao et al., 1999).
Imaging
Images were taken with a cooled CCD camera installed on an Axiovert-200M microscope, with 10×/0.5 NA, 20×/0.8 NA, 40×/1.3 NA, and 63×/1.4 NA lenses, using Axiovision software. Acquired images of sub-regions were manually tiled to create composite images of the entire basal forebrain. Optically-sectioned images were obtained using the Zeiss Apotome structured-illumination apparatus. Images were collapsed into single maximum projection images that are presented in the figures. Only minor corrections of brightness and contrast were performed.
Stereological analysis
Three consecutive coronal sections spanning 120 μm of the basal forebrain from three GAD67-GFP mice were used for stereological analysis. These sections were immunostained with anti-Kv2.2, N86 anti-GFP, and anti-NeuN antibodies. Cells that were positive for these antigens were counted within a region of 1 mm starting at the indentation of the ventral surface of the basal forebrain. The percentages of Kv2.2- and GFP-positive cells in NeuN-positive cells were averaged among three animals. The number of NeuN-positive cells used in the analysis is 885.
Cell culture and transfection
HEK293 cells were cultured in Dulbecco’s modified medium supplemented with 5 mM glutamine. Cells were transfected with DNA plasmids using Lipofectamine 2000 (Invitrogen). After 2 days, cells were either fixed in 4% paraformaldehyde for immunofluorescence staining or harvested for Western blotting. Immunofluorescence staining was performed as described for brain sections.
Micropunch tissues
Adult rat brains were quickly frozen in chilled isopentane at −70°C and then equilibrated to −10°C. Brains were then sectioned into 360 μm thick coronal sections. The sections were placed on a metal block on dry ice, and tissues from the MCPO and the anterior cingulate cortex were punched out using 0.5 – 1 mm diameter calipers. These tissues were solubilized in a lysis buffer (150 mM NaCl, 1% Triton X-100, protease inhibitors, 20 mM Tris-HCl, pH7.5) for 30 min on ice and then mixed with a SDS sample buffer.
Western blotting
Rat brain membrane fractions (RBM) were prepared as previously described (Misonou et al., 2004). Briefly, brains from adult rats were homogenized in 5 mM phosphate buffer (pH7.4) containing 320 mM sucrose and 100 mM NaF. The homogenate was centrifuged at 800 × g to remove nuclei, and the supernatant was centrifuged at 100,000 × g to obtain RBM. RBM was diluted to 1 mg/ml total protein with the SDS sample buffer. HEK293 cells were lysed in the lysis buffer for 30 min on ice. The lysate was diluted to 1 mg/ml total protein with the SDS sample buffer. Proteins were separated on 7.5% SDS gels and transferred to nitrocellulose membranes. Membranes were probed overnight with primary antibodies at the concentrations listed in Table I and incubated for 1 h with HRP-conjugated secondary antibodies (KPL, Gaithersburg, MD) followed by ECL detection (Perkin Elmer, Waltham, MA). Immunoreactive bands were visualized by exposing the membranes to X-ray films and quantified using ImageJ software.
Results
Validation of anti-Kv2.2 antibody
We used a rabbit polyclonal antibody of which the epitope is unique in the C-terminus of Kv2.2 (Johnston et al., 2008; Kihira et al., 2010). In order to validate the antibody, we first tested it in Western blotting with antigen preabsorption. Rat brain membrane fractions (RBM) were prepared and processed for Western blot analysis. The antibody labeled a major band around 140 kDa, which was not detected with the antibody preincubated with the antigen peptide (Fig. 1A), suggesting the specificity of the antibody to Kv2.2. The apparent size of the band was larger than the deduced molecular weight from the primary amino acid sequence of rat Kv2.2 (102.4 kDa). We have identified that this is attributable to, at least in part, phosphorylation of the protein in RBM (Kihira and Misonou, unpublished observations). Specificity was further validated using samples from Kv2.2 deficient mice. Brain lysates from wild-type, Kv2.2-deficient, and Kv2.1 deficient mice were probed with the anti-Kv2.2 antibody. As shown in Fig. 1B, the anti-Kv2.2 antibody exhibited immunoreactivity only in wild-type and Kv2.1-deficient mouse samples but not in Kv2.2-deficient samples. In contrast, the K89 monoclonal anti-Kv2.1 antibody exhibited immunoreactivity in wild-type and Kv2.2-deficient samples but not in Kv2.1-deficient samples, indicating their specificity and lack of cross-reactivity. The lack of cross-reactivity was also observed in immunoblotting (data not shown) and immunofluorescence staining of HEK293 cells expressing Kv2.1 and Kv2.2. Fig. 1C shows that antibodies against each Kv2 subunits has high specificity for their respective antigens expressed in HEK293 cells.
Abundant expression of Kv2.2 in the basal forebrain
Given the validation of the immuno-reagents, we have evaluated the cellular expression pattern of Kv2.2 in the rat brain. Unlike the ubiquitous expression of Kv2.1, the expression of Kv2.2 was relatively limited in certain brain areas. Detectable levels of Kv2.2 were observed in a fraction of cells in the cerebral cortex (Kihira et al., 2010), the superior colliculus, and the pons (not shown). However, we found that there was a cluster of cells expressing Kv2.2 at very high levels near the ventral surface of the forebrain (Fig. 1D–F). The area was identified as the basal forebrain (BF) based on the anatomical landmarks. The immunostaining was completely abolished by pre-incubating the antibody with the antigen peptide (data not shown). The expression of Kv2.2 in the BF was further confirmed by K37 mouse monoclonal antibody against the N-terminus of Kv2.2 (Maletic-Savatic et al., 1995). Double-staining of rat brain sections with the mouse and the rabbit Kv2.2 antibodies showed virtually complete colocalization of each signal in the BF (Fig. 2A–C), which are also represented by a substantial overlap of fluorescent signals in the line scanning analysis (Fig. 2D). We also observed similar localization of Kv2.2 staining in the BF of mouse (Fig. 2E). Importantly, the staining was completely absent in brain sections from Kv2.2-deficient mice (Fig. 2F).
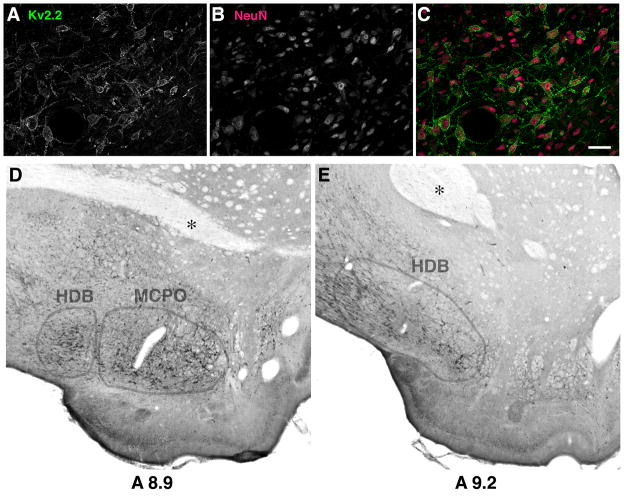
These cells expressing Kv2.2 were positive with NeuN, a pan-neuronal marker (Fig. 3A–C), indicating neuron-specific expression of Kv2.2 in the BF. To determine whether or not Kv2.2 is expressed in a specific nucleus of the BF, we immunostained serial brain sections using the nickel enhanced DAB staining, which provided us with better visualization of anatomical landmarks. We found that Kv2.2 positive neurons are located within the magnocellular preoptic nucleus (MCPO) and the horizontal limb of the diagonal band of Broca (HDB) of the BF (Fig. 3D and E).
Fig. 3.
Expression of Kv2.2 in the neurons of the magnocellular preoptic nucleus (MCPO) and the horizontal band of Broca (HDB). A–C: Expression of Kv2.2 in the NeuN-positive neuronal population in the basal forebrain. Scale bar, 50 μm. D–E: Nickel enhanced 3-3′diaminobenzidine (DAB) immunostaining was used to determine specific localization of Kv2.2-expressing neurons. Rat coronal sections (8.9 and 9.2mm anterior to the interaural line) were immunostained with the anti-Kv2.2 antibody. Anatomical landmarks such as the anterior commissure (indicated by asterisk) were used to locate the expression of Kv2.2 in the MCPO/HDB nuclei.
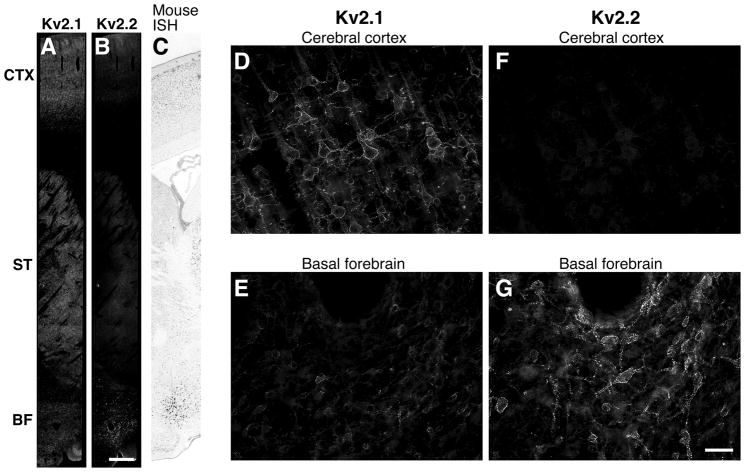
The expression level of Kv2.2 in MCPO/HDB neurons was incomparably higher than those in other brain areas. In low magnification images of rat sagittal sections, Kv2.2 immunoreactivity was readily detected in the BF but hardly detectable in the cortex and the striatum (Fig. 4A). In contrast, Kv2.1 exhibited high levels of expression in the cortex and the striatum but decreased levels in the BF (Fig. 4B). The enrichment of Kv2.2 immunostaining in the BF agrees with the published results of in situ hybridization in the mouse brain from the Allen Brain Institute (see the image obtained from their web site in Fig. 4C). Higher magnification images revealed that Kv2.2 is highly enriched in the MCPO/HDB not only in the number of positive neurons but also in individual neurons (Fig. 4D and E). Interestingly, the neurons expressing Kv2.2 in the MCPO/HDB exhibited low levels of Kv2.1 (Fig. 4F and G).
Fig. 4.
Reciprocal expression of Kv2.1 and Kv2.2 in the rat brain. A–C: Expression pattern of Kv2.1 (A) and Kv2.2 (B) in the rat brain. Dorsal to ventral view of rat sagittal brain sections immunostained with anti-Kv2.2 and K89 anti-Kv2.1 antibodies. The cortex (CTX), the striatum (STR) and the basal forebrain (BF) are shown. Mouse Kv2.2 in situ hybridiztion results from the Allen Brain Institute confirms Kv2.2 immunohistochemical data (C). Scale bar, 1 mm. D–G: Differential expression pattern of Kv2.1 and Kv2.2 in the cortex and the BF. Kv2.1 is highly expressed in neurons in the cortex and shows decreased expression levels in the BF. Kv2.2 is highly expressed in the BF and is only expressed in a subset of pyramidal neurons mainly in layers I/II and V at low expression levels. Scale bar, 50 μm.
To quantitatively demonstrate the enrichment of Kv2.2 in these nuclei, we employed a quantitative western blot analysis. Fig. 5A shows the quantifiability of our method in determining relative levels of Kv2.1 and Kv2.2 in brain tissues. Small tissue samples were collected from the MCPO/HDB and from the cingulate cortex, where the expression of Kv2.2 was highest in the cerebral cortex), using micropunch apparatuses. These tissues were subjected to the Western blot analysis for Kv2.1 and Kv2.2. The relative levels of these proteins in the MCPO/HDB to those in the cortex were determined by measuring the optical densities of Kv2.1 and Kv2.2 protein bands. We also used NeuN to estimate a difference in the number of neurons between these two areas, as the MCPO/HDB contained much less neurons than the cortex (about 10% of the number in the cingulate cortex). Consistent with the results of immunostaining, Kv2.1 protein is expressed at high levels in the cortex and at decreased levels in the MCPO/HDB (Fig. 5B). The relative abundance of Kv2.1 in the MCPO/HDB was 13.4 ± 3.2% (n= 6) of that in the cortex (Fig. 5C). In contrast, despite the lower neuronal density in the MCPO/HDB, evident by the decreased level of NeuN (Fig. 5B, 28.5 ± 4.7% of the cortical level), Kv2.2 protein was highly enriched in the MCPO/HDB tissues (55.3 ± 9.6% of the cortical level).
Selective expression of Kv2.2 in GABAergic neurons of the MCPO/HDB
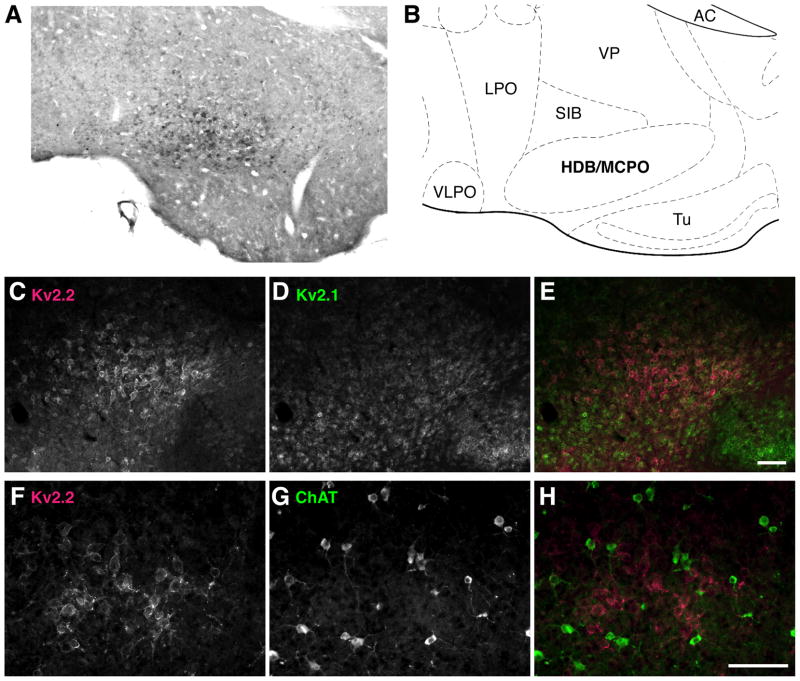
The BF contains multiple types of neurons that differ in their neurochemicals (Szymusiak, 1995; Henny and Jones, 2008). Therefore, we next investigated whether Kv2.2 is expressed in a specific population of neurons in the MCPO/HDB. Since Kv2.2-expressing neurons exhibited a multipolar morphology similar to that of cholinergic BF neurons, we tested whether Kv2.2 is expressed in these neurons. We immunostained rat coronal sections for Kv2.2 and choline acetyltransferase (ChAT), a specific marker for cholinergic neurons. As shown in Fig. 6, ChAT immunostaining identified a subset of neurons in the MCPO/HDB. However, we found that there was no overlap between Kv2.2- and ChAT-positive neurons in the MCPO of the BF, suggesting that Kv2.2-expressing neurons are a distinct population from the cholinergic neurons.
Therefore, we next tested whether GABAergic neurons express Kv2.2. We initially attempted double immunostaining for Kv2.2 and glutamate decarboxylase 67 (GAD67), a marker for GABAergic neurons, as well as for GABA itself. However, due to the dense distribution of GABAergic fibers and terminals in the BF, we were unable to identify GAD67-and GABA-positive cell bodies in this area (data not shown).
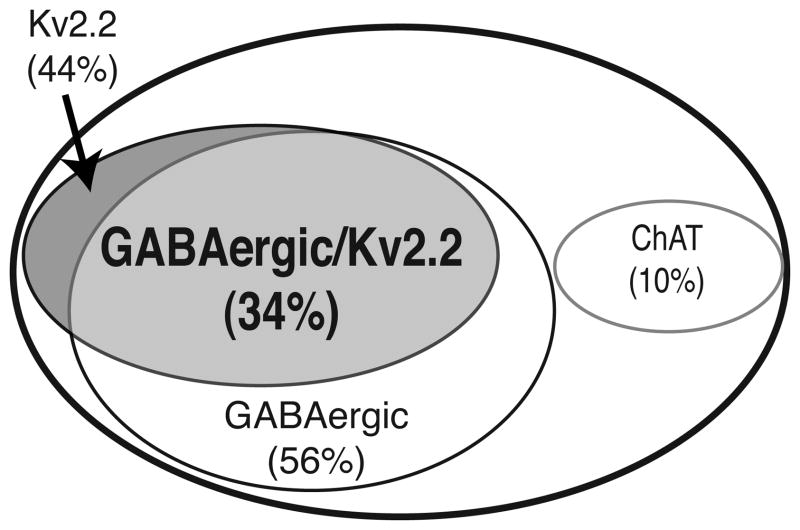
This led us to the use of transgenic mouse models in identifying the neuronal phenotype of Kv2.2-expressing neurons. First, we verified the abundant expression of Kv2.2 in the MCPO/HDB of the mouse brain. As shown in Fig. 7A–E, the enrichment of Kv2.2 and its reciprocal expression to Kv2.1 in the MCPO/HDB of the BF were similar to those observed in the rat brain. We also confirmed the lack of detectable Kv2.2 in ChAT-positive neurons in the mouse as well (Fig. 7F–H). We have utilized the GAD67-GFP knock-in mice, of which one allele of GAD67 is replaced with GFP, rendering GFP a reliable marker of GAD67-immunopositive GABAergic neurons (Tamamaki et al., 2003; Acuna-Goycolea et al., 2005; Ono et al., 2005; Brown et al., 2008; Kaneko et al., 2008). We validated that the expression of GFP overlaps with and is restricted to GABAergic neurons. Double immunostaining for GABA and GFP in the cortex, where GABA immunostaining provides reliable labeling of the somata of GABAergic neurons, showed a complete overlap between GABA-immunopositive neurons and GFP-positive neurons (Fig. 8A–C). We also confirmed non-overlapping distribution of ChAT- and GFP-positive neurons in the BF (Fig. 8D–F). Using this genetic tool, we tested whether or not Kv2.2 is expressed in GABAergic neurons in the MCPO/HDB. As shown in Fig. 8G–I, the immunostaining of Kv2.2 showed that Kv2.2 is highly expressed in GFP-positive GABAergic neurons in the MCPO/HDB of the GAD67 knock-in mice. In fact, our data using a stereological analysis showed that about 60% of GABAergic neurons in the MCPO/HDB express Kv2.2 (Fig. 9). Because GABAergic neurons make up 56% of the total NeuN-positive neuronal population in the MCPO/HDB Kv2.2-expressing neurons represent approximately 44% of the total population in the MCPO/HDB of the BF (Fig. 9).
Fig. 7.
Enriched expression of Kv2.2 in non-cholinergic neurons in the MCPO/HDB of the mouse brain. A: Nickel enhanced DAB immunostaining of Kv2.2-immunoreactive neurons in the mouse brain. A coronal section (3.94 mm anterior to the interaural line) was immunostained with the anti-Kv2.2 antibody. B: Corresponding mouse brain atlas to A. VLPO, ventrolateral preoptic nucleus; LPO, lateral preoptic nucleus; VP, ventral pallidum; AC, anterior commissure; SIB, substantia innominata basal; Tu, olfactory tubercle. C–E: Confirmation of the enrichment of Kv2.2 in the MCPO in immunofluorescence staining. Mouse coronal sections were double immunostained with K89 anti-Kv2.1 and anti-Kv2.2 antibodies. F–H: Reciprocal expression of Kv2.2 and ChAT in the MCPO of mouse. Mouse coronal sections were double immunostained with anti-Kv2.2 and anti-ChAT antibodies. Scale bars, 100 μm.
Fig. 9.
Kv2.2 expression defines a novel subpopulation of GABAergic neurons in the BF. Stereological analysis of three consecutive coronal brain sections spanning 120 μm of the MCPO/HDB (each 40 μm thick) from GAD67-GFP knock-in mice (n=3). These sections were immunostained with anti-Kv2.2, anti-GFP, and anti-NeuN antibodies. Labeled neurons were counted per section/per animal and averaged among animals.
Discussion
In the present paper, we report a novel finding that the Kv2.2 delayed rectifier channels are abundantly expressed in the BF, particularly in the MCPO/HDB. These findings are supported by immunofluorescence staining using two independent antibodies for Kv2.2 and also by quantitative Western blotting. We also found that the expression of Kv2.2 in the BF is not ubiquitous but rather restricted in a specific population of neurons. Using the GAD67-GFP knock-in mice (Tamamaki et al., 2003), we demonstrated that Kv2.2 is selectively expressed in GABAergic neurons in this area, whereas cholinergic neurons lack detectable levels of Kv2.2. Further analysis has shown that ~60% of GABAergic neurons in the MCPO/HDB express Kv2.2 at very high levels, whereas Kv2.2 was not detectable in the other ~40% of GABAergic neurons. This is consistent with the previous findings that the GABAergic population in the BF, like those in the hippocampus and cerebral cortex, is heterogeneous in gene expression (Gritti et al., 2003; Manns et al., 2003; Furuta et al., 2004) and their electrophysiological properties (Kirouac and Pittman, 1999; Duque et al., 2000; Manns et al., 2000; Borhegyi et al., 2004)(Hassani et al., 2009). Our study has identified a novel and major sub-population of GABAergic neurons in the MCPO/HDB, which differs in the expression of the somatic delayer rectifier Kv2.2 channels.
Although GABAergic neurons are the major neuronal component of the MCPO/HDB, this phenotypic heterogeneity has somewhat hampered the investigation of the physiological roles of these GABAergic neurons in sleep and in the regulation of cortical activity. In contrast, the significance of the BF cholinergic neurons in arousal, as well as in attention, has been well studied, due to the availability of IgG192-saporin (Wiley et al., 1991). This toxin-conjugated antibody targets the p75 NGF receptor, which is expressed specifically in these neurons, therefore enables specific lesion of the cholinergic population in the BF. Therefore, identification of a genetic marker that is specific to GABAergic neurons in the BF could also facilitate the investigation of their physiological roles in animal behaviors. Kv2.2 could be a particularly good marker because it is expressed in a specific subset of GABAergic neurons in the BF and because it is expressed on the cell surface. It could serve as an excellent target for specific lesioning of this subset of GABAergic neurons.
We currently do not know whether these Kv2.2/GABAergic neurons constitute the corticopetal GABAergic projection. It has been previously shown by others that many of GABAergic neurons in the basal forebrain including those in the MCPO and HDB send their projections to the cerebral cortex widely (Saper, 1984)(Freund and Meskenaite, 1992)(Gritti et al., 1997). Because Kv2.2 neurons represent about 60% of GABAergic neurons in the MCPO, it is likely that they also project to the cortical areas. Future efforts in determining their precise projection status is necessary to help better understanding of the physiological roles of these specific GABAergic neurons in the BF.
Kv2.1 potassium channels have been very well studied as compared to Kv2.2. The function of Kv2.1 is dynamically regulated by the activity of neurons through changes in the phosphorylation states of the channel subunits in cortical and hippocampal pyramidal neurons (Misonou et al., 2004; Misonou et al., 2006b; Misonou et al., 2008; Mulholland et al., 2008). It has been shown that this in turn modulates the excitability of these neurons and therefore has been considered as a form of homeostatic plasticity of neuronal excitability (Misonou, 2010). We have obtained preliminary data that Kv2.2 is also subjected to the activity-dependent regulation of phosphorylation in the brain (Kihira and Misonou, unpublished observations). Therefore, it is possible that the function of Kv2.2 in BF GABAergic neurons is also dynamically regulated by the activity of neurons, and thus provides a feedback to regulate the excitability of these inhibitory neurons in the BF.
Acknowledgments
This work was in part supported by the American Heart Association and the Epilepsy Foundation (H.M.), Grants-in-Aid from the MEXT, Japan, and Takeda Science Foundation (Y.Y.). We thank Dr. James S. Trimmer for the generous gift of the antibodies and Dr. Jeanne Nerbonne for Kv2.1 knockout samples. We also thank Dr. Adam Puche for providing us with the GAD67-GFP knock-in mice, and Drs. Jessica Mong, Gloria Hoffman, and Asaf Keller for critical discussion.
References
- Acuna-Goycolea C, Tamamaki N, Yanagawa Y, Obata K, van den Pol AN. Mechanisms of neuropeptide Y, peptide YY, and pancreatic polypeptide inhibition of identified green fluorescent protein-expressing GABA neurons in the hypothalamic neuroendocrine arcuate nucleus. J Neurosci. 2005;25:7406–7419. doi: 10.1523/JNEUROSCI.1008-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonucci DE, Lim ST, Vassanelli S, Trimmer JS. Dynamic localization and clustering of dendritic Kv2.1 voltage-dependent potassium channels in developing hippocampal neurons. Neuroscience. 2001;108:69–81. doi: 10.1016/s0306-4522(01)00476-6. [DOI] [PubMed] [Google Scholar]
- Bartus RT. On neurodegenerative diseases, models, and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp Neurol. 2000;163:495–529. doi: 10.1006/exnr.2000.7397. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Shafi R, Sarter M. Specific contributions of the basal forebrain corticopetal cholinergic system to electroencephalographic activity and sleep/waking behaviour. Eur J Neurosci. 2002;16:2453–2461. doi: 10.1046/j.1460-9568.2002.02310.x. [DOI] [PubMed] [Google Scholar]
- Borhegyi Z, Varga V, Szilagyi N, Fabo D, Freund TF. Phase segregation of medial septal GABAergic neurons during hippocampal theta activity. J Neurosci. 2004;24:8470–8479. doi: 10.1523/JNEUROSCI.1413-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, McKenna JT, Winston S, Basheer R, Yanagawa Y, Thakkar MM, McCarley RW. Characterization of GABAergic neurons in rapid-eye-movement sleep controlling regions of the brainstem reticular formation in GAD67-green fluorescent protein knock-in mice. Eur J Neurosci. 2008;27:352–363. doi: 10.1111/j.1460-9568.2008.06024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Bickford RG, Ponomareff G, Thal LJ, Mandel R, Gage FH. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci. 1988;8:4007–4026. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divac I. Magnocellular nuclei of the basal forebrain project to neocortex, brain stem, and olfactory bulb. Review of some functional correlates. Brain Res. 1975;93:385–398. doi: 10.1016/0006-8993(75)90178-x. [DOI] [PubMed] [Google Scholar]
- Duque A, Balatoni B, Detari L, Zaborszky L. EEG correlation of the discharge properties of identified neurons in the basal forebrain. J Neurophysiol. 2000;84:1627–1635. doi: 10.1152/jn.2000.84.3.1627. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Freund TF, Meskenaite V. gamma-Aminobutyric acid-containing basal forebrain neurons innervate inhibitory interneurons in the neocortex. Proc Natl Acad Sci U S A. 1992;89:738–742. doi: 10.1073/pnas.89.2.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta T, Koyano K, Tomioka R, Yanagawa Y, Kaneko T. GABAergic basal forebrain neurons that express receptor for neurokinin B and send axons to the cerebral cortex. J Comp Neurol. 2004;473:43–58. doi: 10.1002/cne.20087. [DOI] [PubMed] [Google Scholar]
- Gritti I, Henny P, Galloni F, Mainville L, Mariotti M, Jones BE. Stereological estimates of the basal forebrain cell population in the rat, including neurons containing choline acetyltransferase, glutamic acid decarboxylase or phosphate-activated glutaminase and colocalizing vesicular glutamate transporters. Neuroscience. 2006;143:1051–1064. doi: 10.1016/j.neuroscience.2006.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritti I, Mainville L, Jones BE. Codistribution of GABA- with acetylcholine-synthesizing neurons in the basal forebrain of the rat. J Comp Neurol. 1993;329:438–457. doi: 10.1002/cne.903290403. [DOI] [PubMed] [Google Scholar]
- Gritti I, Mainville L, Mancia M, Jones BE. GABAergic and other noncholinergic basal forebrain neurons, together with cholinergic neurons, project to the mesocortex and isocortex in the rat. J Comp Neurol. 1997;383:163–177. [PubMed] [Google Scholar]
- Gritti I, Manns ID, Mainville L, Jones BE. Parvalbumin, calbindin, or calretinin in cortically projecting and GABAergic, cholinergic, or glutamatergic basal forebrain neurons of the rat. J Comp Neurol. 2003;458:11–31. doi: 10.1002/cne.10505. [DOI] [PubMed] [Google Scholar]
- Guan D, Tkatch T, Surmeier DJ, Armstrong WE, Foehring RC. Kv2 Subunits Underlie Slowly Inactivating Potassium Current in Neocortical Pyramidal Neurons. J Physiol. 2007 doi: 10.1113/jphysiol.2007.128454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkany T, Hartig W, Berghuis P, Dobszay MB, Zilberter Y, Edwards RH, Mackie K, Ernfors P. Complementary distribution of type 1 cannabinoid receptors and vesicular glutamate transporter 3 in basal forebrain suggests input-specific retrograde signalling by cholinergic neurons. Eur J Neurosci. 2003;18:1979–1992. doi: 10.1046/j.1460-9568.2003.02898.x. [DOI] [PubMed] [Google Scholar]
- Hassani OK, Lee MG, Henny P, Jones BE. Discharge profiles of identified GABAergic in comparison to cholinergic and putative glutamatergic basal forebrain neurons across the sleep-wake cycle. J Neurosci. 2009;29:11828–11840. doi: 10.1523/JNEUROSCI.1259-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henny P, Jones BE. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur J Neurosci. 2008;27:654–670. doi: 10.1111/j.1460-9568.2008.06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic channels of excitable membranes. Sinauer; Sunderland, MA: 2001. [Google Scholar]
- Hwang PM, Fotuhi M, Bredt DS, Cunningham AM, Snyder SH. Contrasting immunohistochemical localizations in rat brain of two novel K+ channels of the Shab subfamily. J Neurosci. 1993;13:1569–1576. doi: 10.1523/JNEUROSCI.13-04-01569.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang PM, Glatt CE, Bredt DS, Yellen G, Snyder SH. A novel K+ channel with unique localizations in mammalian brain: molecular cloning and characterization. Neuron. 1992;8:473–481. doi: 10.1016/0896-6273(92)90275-i. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Sun Z, Lee T, Fusco FR, Kimble TD, Meade CA, Cuthbertson S, Reiner A. A simple and sensitive antigen retrieval method for free-floating and slide-mounted tissue sections. J Neurosci Methods. 1999;93:149–162. doi: 10.1016/s0165-0270(99)00142-9. [DOI] [PubMed] [Google Scholar]
- Johnston J, Griffin SJ, Baker C, Skrzypiec A, Chernova T, Forsythe ID. Initial segment Kv2.2 channels mediate a slow delayed rectifier and maintain high frequency action potential firing in medial nucleus of the trapezoid body neurons. J Physiol. 2008;586:3493–3509. doi: 10.1113/jphysiol.2008.153734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BE. From waking to sleeping: neuronal and chemical substrates. Trends Pharmacol Sci. 2005;26:578–586. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kaneko K, Tamamaki N, Owada H, Kakizaki T, Kume N, Totsuka M, Yamamoto T, Yawo H, Yagi T, Obata K, Yanagawa Y. Noradrenergic excitation of a subpopulation of GABAergic cells in the basolateral amygdala via both activation of nonselective cationic conductance and suppression of resting K+ conductance: a study using glutamate decarboxylase 67-green fluorescent protein knock-in mice. Neuroscience. 2008;157:781–797. doi: 10.1016/j.neuroscience.2008.09.029. [DOI] [PubMed] [Google Scholar]
- Kievit J, Kuypers HG. Basal forebrain and hypothalamic connection to frontal and parietal cortex in the Rhesus monkey. Science. 1975;187:660–662. doi: 10.1126/science.1114317. [DOI] [PubMed] [Google Scholar]
- Kihira Y, Hermanstyne TO, Misonou H. Formation of heteromeric Kv2 channels in mammalian brain neurons. J Biol Chem. 2010 doi: 10.1074/jbc.M109.074260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirouac GJ, Pittman QJ. Identification of barosensitive neurons in the mediobasal forebrain using juxtacellular labeling. Am J Physiol. 1999;276:R1766–71. doi: 10.1152/ajpregu.1999.276.6.R1766. [DOI] [PubMed] [Google Scholar]
- Lau B, Salzman CD. Noncholinergic neurons in the basal forebrain: often neglected but motivationally salient. Neuron. 2008;59:6–8. doi: 10.1016/j.neuron.2008.06.017. [DOI] [PubMed] [Google Scholar]
- Lin SC, Nicolelis MA. Neuronal ensemble bursting in the basal forebrain encodes salience irrespective of valence. Neuron. 2008;59:138–149. doi: 10.1016/j.neuron.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, McKinney K, Liu L, Lakhlani S, Jennes L. Distribution of vesicular glutamate transporter-2 messenger ribonucleic Acid and protein in the septum-hypothalamus of the rat. Endocrinology. 2003;144:662–670. doi: 10.1210/en.2002-220908. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M, Lenn NJ, Trimmer JS. Differential spatiotemporal expression of K+ channel polypeptides in rat hippocampal neurons developing in situ and in vitro. J Neurosci. 1995;15:3840–3851. doi: 10.1523/JNEUROSCI.15-05-03840.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns ID, Alonso A, Jones BE. Discharge properties of juxtacellularly labeled and immunohistochemically identified cholinergic basal forebrain neurons recorded in association with the electroencephalogram in anesthetized rats. J Neurosci. 2000;20:1505–1518. doi: 10.1523/JNEUROSCI.20-04-01505.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns ID, Lee MG, Modirrousta M, Hou YP, Jones BE. Alpha 2 adrenergic receptors on GABAergic, putative sleep-promoting basal forebrain neurons. Eur J Neurosci. 2003;18:723–727. doi: 10.1046/j.1460-9568.2003.02788.x. [DOI] [PubMed] [Google Scholar]
- Misonou H. Homeostatic regulation of neuronal excitability by K(+) channels in normal and diseased brains. Neuroscientist. 2010;16:51–64. doi: 10.1177/1073858409341085. [DOI] [PubMed] [Google Scholar]
- Misonou H, Menegola M, Buchwalder L, Park EW, Meredith A, Rhodes KJ, Aldrich RW, Trimmer JS. Immunolocalization of the Ca(2+)-activated K(+) channel Slo1 in axons and nerve terminals of mammalian brain and cultured neurons. J Comp Neurol. 2006a;496:289–302. doi: 10.1002/cne.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misonou H, Menegola M, Mohapatra DP, Guy LK, Park KS, Trimmer JS. Bidirectional activity-dependent regulation of neuronal ion channel phosphorylation. J Neurosci. 2006b;26:13505–13514. doi: 10.1523/JNEUROSCI.3970-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misonou H, Mohapatra DP, Park EW, Leung V, Zhen D, Misonou K, Anderson AE, Trimmer JS. Regulation of ion channel localization and phosphorylation by neuronal activity. Nat Neurosci. 2004;7:711–718. doi: 10.1038/nn1260. [DOI] [PubMed] [Google Scholar]
- Misonou H, Thompson SM, Cai X. Dynamic regulation of the Kv2.1 voltage-gated potassium channel during brain ischemia through neuroglial interaction. J Neurosci. 2008;28:8529–8538. doi: 10.1523/JNEUROSCI.1417-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland PJ, Carpenter-Hyland EP, Hearing MC, Becker HC, Woodward JJ, Chandler LJ. Glutamate transporters regulate extrasynaptic NMDA receptor modulation of Kv2.1 potassium channels. J Neurosci. 2008;28:8801–8809. doi: 10.1523/JNEUROSCI.2405-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Nickerson Poulin A, Guerci A, El Mestikawy S, Semba K. Vesicular glutamate transporter 3 immunoreactivity is present in cholinergic basal forebrain neurons projecting to the basolateral amygdala in rat. J Comp Neurol. 2006;498:690–711. doi: 10.1002/cne.21081. [DOI] [PubMed] [Google Scholar]
- Ono M, Yanagawa Y, Koyano K. GABAergic neurons in inferior colliculus of the GAD67-GFP knock-in mouse: electrophysiological and morphological properties. Neurosci Res. 2005;51:475–492. doi: 10.1016/j.neures.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Rhodes KJ, Trimmer JS. Antibody-based validation of CNS ion channel drug targets. J Gen Physiol. 2008;131:407–413. doi: 10.1085/jgp.200709926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB. Organization of cerebral cortical afferent systems in the rat. II. Magnocellular basal nucleus. J Comp Neurol. 1984;222:313–342. doi: 10.1002/cne.902220302. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP. The neglected constituent of the basal forebrain corticopetal projection system: GABAergic projections. Eur J Neurosci. 2002;15:1867–1873. doi: 10.1046/j.1460-9568.2002.02004.x. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP. Developmental origins of the age-related decline in cortical cholinergic function and associated cognitive abilities. Neurobiol Aging. 2004;25:1127–1139. doi: 10.1016/j.neurobiolaging.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Szymusiak R, McGinty D. Sleep-related neuronal discharge in the basal forebrain of cats. Brain Res. 1986;370:82–92. doi: 10.1016/0006-8993(86)91107-8. [DOI] [PubMed] [Google Scholar]
- Szymusiak R. Magnocellular nuclei of the basal forebrain: substrates of sleep and arousal regulation. Sleep. 1995;18:478–500. doi: 10.1093/sleep/18.6.478. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Trimmer JS. Immunological identification and characterization of a delayed rectifier K+ channel polypeptide in rat brain. Proc Natl Acad Sci U S A. 1991;88:10764–10768. doi: 10.1073/pnas.88.23.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimmer JS, Rhodes KJ. Localization of voltage-gated ion channels in mammalian brain. Annu Rev Physiol. 2004;66:477–519. doi: 10.1146/annurev.physiol.66.032102.113328. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Kapp BS, Pascoe JP. Neuronal activity within the nucleus basalis and conditioned neocortical electroencephalographic activation. J Neurosci. 1994;14:1623–1633. doi: 10.1523/JNEUROSCI.14-03-01623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley RG, Oeltmann TN, Lappi DA. Immunolesioning: selective destruction of neurons using immunotoxin to rat NGF receptor. Brain Res. 1991;562:149–153. doi: 10.1016/0006-8993(91)91199-b. [DOI] [PubMed] [Google Scholar]