Abstract
Following spinal cord injury (SCI) or peripheral neuropathy, increased levels of the p75NTR death receptor initiate the signal transduction cascade leading to cell death. Investigations of compounds that may ameliorate neuronal cell death have largely used rodent models, which are time consuming, expensive, and cumbersome to perform. Previous studies had demonstrated that steroids, particularly dexamethasone and its analog methylprednisolone sodium succinate, exhibit limited neuroprotective effects against neuronal injury. Significantly, many naturally occurring nonsteroidal plant compounds exhibit structural overlap with steroids. In this report, we present an in vitro cellular screen model to practically examine the efficacy of various phytoestrogens in modulating the ibuprofen-induced expression of p75NTR and reduced cell survival of CCFSTTG1 and U87MG cells in a rescue (postinjury) or prevention (preinjury) regimen. We show that the phytoestrogen, biochanin A, and, to a lesser extent, genistein are more effective than dexamethasone at reducing p75NTR expression and improving the viability of U87MG and CCFSTTG1 before and after p75NTR induction. Furthermore, these studies implicate biochanin A's inactivation of p38-MAPK as a possible contributor to reducing p75NTR with associated increased cell survival. This new in vitro assay facilitates a more time-efficient screening of compounds to suppress p75NTR expression and increase neuronal cell viability prior to their evaluation in animal models of neurological diseases.
Introduction
Neuronal cell death during development and injury is associated with upregulation of the p75 neurotrophin receptor (p75NTR).1 Several approaches have been suggested for amelioration of neuronal injury. Early research on glucocorticoids suggested that high-dose treatment of contused spinal cords could promote limited neurological recovery. Such animal studies demonstrated a neuroprotective effect for methylprednisolone sodium succinate (summarized in ref. 2). High doses of this compound appear to improve neurological recovery from acute spinal cord injury (SCI), but remain controversial.3 Treatment with methylprednisolone sodium succinate appears to improve motor scores marginally in patients with incomplete, but not complete, paralysis.4 Similarly, high-dose dexamethasone, a synthetic analog of methylprednisolone, has been shown to mitigate delayed SCI in a rat model by downregulating p75NTR expression, and concomitantly to decrease apoptotic cell number, ultimately accelerating functional recovery.5 Nevertheless, the neuroprotective effects of high-dose glucocorticoids appear to be marginal and confounded by undesirable side effects on the patient.6,7 Other steroids, including progesterone, androgens, and estrogens, have been suggested to provide neuroprotection after SCI8–14; however, their effects were variable and marred by side effects on other target organs. Hence, it appears that various steroid classes may exhibit limited neuroprotective effects to varying degrees of efficacy confounded by possible side effects and with undefined mechanisms of action, with the exception of dexamethasone suppression of the p75NTR.5
In common with other members of the tumor necrosis factor receptor super-family, p75NTR encodes an intracellular death domain responsible for apoptosis induction. In many instances, ligand-independent p75NTR expression initiates apoptosis. Indeed, a robust cause-and-effect relationship exists between increased p75NTR levels and cell death.15 Elevated p75NTR expression by genetic transfection or ibuprofen-induced mRNA stabilization induces cell death.16,17 Conversely, ligation of the cognate neurotrophins (e.g., nerve growth factor [NGF]) prevents p75NTR-dependent cell death.18 Many tumor cell types escape p75NTR-dependent cell death through loss of p75NTR mRNA stability.19 The observation that neuronal injury promotes a change in the ratio of p75NTR to ligand favoring p75NTR-mediated apoptosis suggests that p75NTR suppression could potentially reduce the severity of cell death.20 Indeed, small interfering RNA (siRNA) knockdown of p75NTR has been shown to reduce the level of degeneration in axotomized motor neurons.21
Significantly, many naturally occurring nonsteroid plant constituents exhibit structural overlap with steroids.22 Isoflavones and coumestans have been identified as the most common estrogenic compounds in plants and hence are named phytoestrogens.23 Several phytoestrogens are readily available or consumed as dietary supplements; for instance, soy is the major dietary source of phytoestrogens (genistein and daidzein), but it contains a smaller number of estrogenically active substances compared to red clover, which contains genistein, daidzein, biochanin A, and formononetin.24 Importantly, high-dose intake studies of these compounds suggest that they are well tolerated by humans and have no reported serious side effects.25 The testing of such available compounds highlights the need for a fast and reproducible cellular screening method that would pave the way to more elaborate, but narrowed-down, screens validating these compounds as potential alleviators of neuronal cell injury in rodent models of SCI.
In this work, we describe an in vitro assay for testing compounds that could modulate ibuprofen-induced p75NTR upregulation and decrease in cell survival. These studies confirm the limited protective effects of dexamethasone and identify the superior efficacy of biochanin A and to a lesser extent genistein, in reducing drug-induced p75NTR expression, potentially resulting in neuroprotection.
Materials and Methods
Cell lines, culture conditions, and reagents
U87MG glioblastoma and CCFSTTG1 astrocytoma cell lines were purchased from the tissue culture facility of Georgetown University Lombardi Comprehensive Cancer Center and maintained in 4.5 g/L glucose and L-glutamine-supplemented Dulbecco-modified Eagle medium (DMEM) and RPMI-1640 (Mediatech Inc, Manassas, VA), respectively, with 10% fetal bovine serum (FBS) and antibiotics (100 units/mL penicillin G, 100 μg/mL streptomycin), in the presence of 5% CO2 at 37°C. Ibuprofen, biochanin A, genistein, daidzein, formononetin, dexamethasone, prunetin, β-estradiol, and progesterone were purchased from Sigma (St. Louis, MO), and glycitein was from LC Laboratories (Woburn, MA).
Drug preparation and treatment
Stock and working solutions were dissolved in dimethylsulfoxide (DMSO; Sigma). For rescue regimens, cells were seeded overnight at 70% confluency in Phenol Red-free medium supplemented with 10% charcoal-stripped FBS, treated with ±2 mM ibuprofen for 24 h, followed by treatment with dexamethasone, β-estradiol, or progesterone (0, 10, 20, 40, 60, 80, and 100 nM) or phytoestrogens (0, 10, 20, 40, 60, 80, and 100 μM) + 2 mM ibuprofen for an additional 24 h. For prevention regimens, cells were seeded similarly, treated with dexamethasone, β-estradiol, or progesterone (0, 10, 20, 40, 60, 80, and 100 nM) or phytoestrogens (0, 10, 20, 40, 60, 80, and 100 μM) for 24 h followed by replenishment with same concentrations of hormones/phytoestrogens +2 mM ibuprofen for an additional 24 h.
Immunoblot analysis
Cell lysates for p75NTR or phospho-p38-MAPK detection were prepared and quantified as described previously.26 Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analysis were done as described previously.17 Primary antibodies used were: Mouse monoclonal anti-p75NTR (Millipore; 1:2,000); rabbit polyclonal anti-phospho-p38-MAPK (Thr180/Tyr182) and p38-MAPK (Cell Signaling Technology; 1:1,000); and mouse monoclonal anti–β-actin (Sigma; 1:5,000). Membranes used for detection of phospho- p38-MAPK and p75NTR were stripped and reprobed for total p38-MAPK and β-actin, respectively, to ensure equal loading. A875 whole cell lysates were used as a positive control for p75NTR.17
MTT survival assay
Cells from the above-described treatment regimens were incubated with 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide reagent (final concentration, 0.5 mg/mL; Sigma) for 4 h at 37°C. Subsequently, cells were mixed with 100 μL/well acidified isopropyl alcohol and quantified at 570 nm with a reference filter of 655 nm using a plate reader (Bio-Rad Laboratories). Controls used included: All compounds used were tested for possible interference by incubation alone (media + MTT labeling reagent only), and no change in the assay color has been observed with the addition of these compounds. Blank wells (media + MTT labeling reagent only) were included, and their values were subtracted from all readings to eliminate background noise. [(Ibu + cpds) − blank] values were normalized to (Ibu only − blank) values in wells. Control wells (cells + vehicle) were always included to verify the ibu effect on cell viability.
siRNA transfection
U87MG and CCFSTTG1 cells were transfected for 24 h with nontargeting or p75NTR-specific siRNA (Duplex D-009340-03, Dharmacon RNA Technologies, Lafayette, CO) at a final concentration of 100 nM according to the manufacturer's protocol using Oligofectamine (Invitrogen). After transfection, cells were treated with ibuprofen or biochanin A (BCA) for 24 h, then with ibuprofen + BCA for an additional 24 h, followed by the MTT assay.
Statistical analyses
Immunoblot band intensities were scanned from X-ray films and quantified by the ImageJ software (NIH, Bethesda, MD) and presented as means ± standard error of the mean (SEM). One-way analysis of variance (ANOVA) was used followed by a Dunnett test to determine significant differences between treatment groups, with a confidence interval of 95%. MTT experiments were conducted in triplicate and repeated at least three times.
Results
Ibuprofen induces p75NTR expression and reduces the survival of U87MG and CCFSTTG1 cells in a time-dependent manner
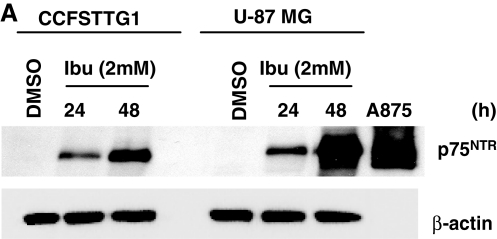
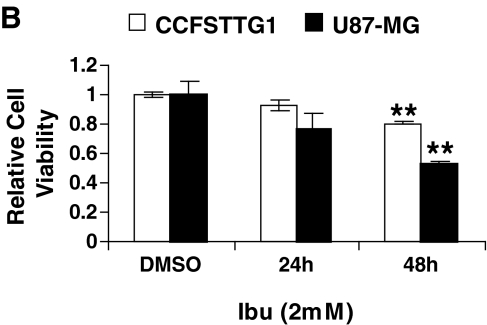
Previous work demonstrated that the nonsteroidal antiinflammatory drug ibuprofen induces p75NTR expression, but no other death receptors, and decreased the viability of bladder and prostate cancer cells.16,17 Similarly, we examined ibuprofen's effect on the U87MG and CCFSTTG1 cells lines. Ibuprofen treatment (2 mM) induced p75NTR expression in a time-dependent manner in both cell lines with a more pronounced increase in U87MG cells (Fig. 1A). Ibuprofen-induced expression of the p75NTR protein was accompanied by a corresponding reduction in cell survival, which was most pronounced (50% reduction with p < 0.01) in U87MG cells after 48 h (Fig. 1B). Because ibuprofen treatment resulted in p75NTR induction and a concomitant decrease in U87MG and CCFSTTG1 cell survival, it was subsequently used as a surrogate to mimic p75NTR induction and subsequent cell death, both of which are observed in the context of neuronal injury.
FIG. 1.
Ibuprofen treatment induces p75NTR expression and reduces the survival of U87MG and CCFSTTG1 cells in a time-dependent manner. (A) Representative Western blot of p75NTR levels in CCFSTTG1 and U87MG cells treated with 0 or 2 mM ibuprofen for 24 and 48 h. Cells were lysed, and equal amounts of protein (40 μg) were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Immunoblots were stripped and reprobed for β-actin to ensure equal loading, and the A875 melanoma lysates were used as a positive control for p75NTR. (B) Relative survival of CCFSTTG1 and U87MG cells after ibuprofen treatment for 24 or 48 h; data are presented as mean cell survival ± standard error of the mean (SEM), normalized to levels in vehicle-treated (dimethylsulfoxide [DMSO]) cells with ** indicating p < 0.01, as compared to vehicle-treated cells.
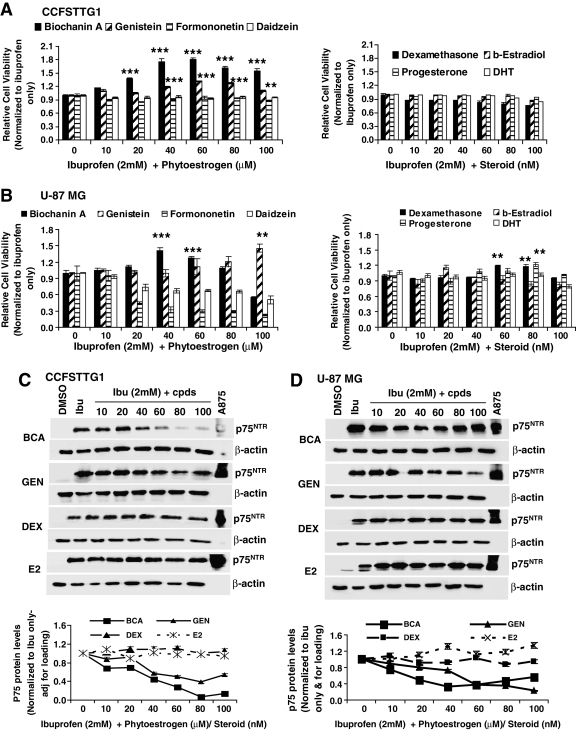
Biochanin A and genistein are more efficient than dexamethasone in decreasing ibuprofen-induced p75NTR levels and rescuing the viability of U87MG and CCFSTTG1 cells
To examine the effects of phytoestrogens on cell survival, U87MG or CCFSTTG1 cells were treated with 2 mM ibuprofen for 24 h (mimicking p75NTR upregulation after neuronal injury) followed by treatment with ibuprofen and the phytoestrogen to be tested for an additional 24 h and subjected to the MTT assay. In CCFSTTG1 cells, BCA treatment resulted in a significant increase in cell viability at 20 μM, reaching maximal levels at 60 μM (≈1.8-fold increase in relative cell number as compared to ibuprofen-treated cells, p < 0.001); genistein treatment also increased the number of viable CCFSTTG1 cells as compared to ibuprofen-treated cells. However, its effect was modest, not apparent before 40 μM, and reached 1.4-fold increase in relative cell viability at 60 μM as compared to control ibuprofen-treated cells (Fig. 2A, left). Conversely, formononetin and daidzein had no rescuing abilities suggesting a BCA- and genistein-selective effect (Fig. 2A, left). CCFSTTG1 cell viability was also unchanged by treatment with dexamethasone (DEX), β-estradiol, progesterone, or dihydroxytestosterone (DHT) (Fig. 2A, right). In U87MG cells, BCA treatment resulted in an ≈1.4 and 1.3-fold increase in cell viability as compared to ibuprofen-treated cells at 40 and 60 μM, respectively, whereas a 100 μM dose of genistein was needed to significantly increase the viability of U87MG cells as compared to ibuprofen-treated cells (Fig. 2B, left). Additional phytoestrogens, glycitein, and prunetin exhibited no positive effect on cell viability in the presence of ibuprofen (data not shown). Conversely, DEX and progesterone treatment recapitulated their documented abilities to rescue cell viability by increasing relative cell viability as compared to ibuprofen-treated cells at doses of 60–80 nM and 80 nM, respectively (Fig. 2B, right), which was very modest compared to BCA at <1.2-fold increase (p < 0.01). Having established the cell viability-rescuing abilities of BCA, genistein, and, to a lesser extent, DEX and progesterone, we subsequently determined p75NTR expression levels, when both cell lines were treated with the more efficacious compounds and using β-estradiol as a negative control because it showed no cell viability rescuing abilities in either cell line.
FIG. 2.
Biochanin A (BCA) is more effective than other phytoestrogens and steroids at increasing the survival of CCFSTTG1 and U87MG cells and reducing p75NTR levels in the presence of ibuprofen. MTT assay and p75NTR Western blot analysis of CCFSTTG1 (A and C) or U87MG (B and D) cells treated with ibuprofen (2 mM) for 24 h followed by ibuprofen (2 mM) + 0, 10, 20, 40, 60, 80, or 100 μM Biochanin A, genistein, formononetin, or daidzein for an additional 24 h (left panels of A and B), or by ibuprofen (2 mM) + 0, 10, 20, 40, 60, 80, or 100 nM dexamethasone, β-estradiol, progesterone, or DHT for an additional 24 h (right panels of A and B). Data are presented as mean cell survival ± standard error of the mean (SEM), normalized to ibuprofen-treated cells only, with ** and *** indicating p < 0.01 and 0.001, respectively, as compared to ibuprofen only-treated cells. For Western blots (C and D), cell lysates (40 μg) were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Immunoblots were stripped and reprobed for β-actin to ensure equal loading, and the A875 melanoma cell lysates were used as a positive control for p75NTR. Respective quantifications (from two independent experiments) of p75NTR expression are shown below for every cell type and normalized to levels in ibuprofen-treated cells.
In CCFSTTG1 cells, BCA treatment post ibuprofen treatment resulted in a significant reduction in p75NTR protein levels at 10 μM, with 30% reduction and reaching a more pronounced 75% reduction at 60 μM. Genistein treatment also slightly decreased ibuprofen-induced p75NTR expression as early as 40 μM, with the most significant reduction at 60–80 μM. Dexamethasone and β-estradiol did not affect p75NTR induction by ibuprofen in CCFSTTG1 cells (Fig. 2C).
In U87MG cells, BCA and genistein treatment significantly decreased p75NTR induction levels by ibuprofen, reaching a two-fold reduction at 20 μM, with a maximal p75NTR reduction of 70% at 40 μM for BCA and 60 μM for genistein, as compared to control ibuprofen-treated cells. Dexamethasone and β-estradiol treatment, however, failed to reduce the ibuprofen-induced levels of p75NTR (Fig. 2D).
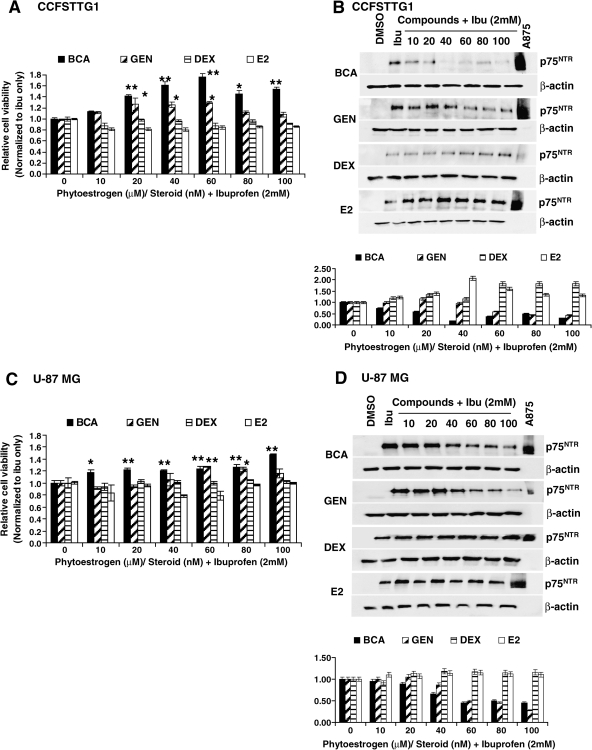
Biochanin A and, to a lesser extent, genistein mitigate p75NTR expression and the survival decrease by ibuprofen in a prevention regimen
We next examined whether any of the compounds tested would be beneficial in modulating p75NTR induction and subsequently cell viability when administered before ibuprofen treatment. Hence, CCFSTTG1 or U87MG cells were treated with BCA, genistein, DEX, or estradiol for 24 h, and then with fresh medium containing the same concentrations of phytoestrogens/steroids supplemented with ibuprofen was added for an additional 24 h, after which cells were assayed for MTT and p75NTR expression levels.
In this regimen, BCA increased the viability of CCFSTTG1 cells up to 1.4-fold and 1.6-fold (p < 0.01) by 20 and 40 μM, respectively. Genistein treatment also resulted in an increase in cell viability up to 1.2-fold as early as 20 μM (Fig. 3A). Dexamethasone and β-estradiol, however, had no significant positive effect on CCFSTTG1 viability when administered before ibuprofen (Fig. 3A). In U87MG cells, BCA increased cell viability with a dose as low as 10 μM and that remained significant throughout higher doses (Fig. 3C). However, a dose of 60 μM genistein was required to achieve a similar increase in cell viability as compared to control ibuprofen-treated cells, whereas DEX and estradiol again failed to positively affect cell survival in the presence of ibuprofen (Fig. 3C).
FIG. 3.
Effect of biochanin A (BCA) and other phytoestrogens/steroids on CCFSTTG1 and U87MG cells viability and p75NTR expression levels in a prevention regimen. MTT relative cell viability of CCFSTTG1 (A) and U87MG (C) cells treated with 0, 10, 20, 40, 60, 80, or 100 μM BCA or genistein (GEN), or 0, 10, 20, 40, 60, 80, or 100 nM dexamethsone (DEX) or estradiol (E2), for 24 h followed by treatment with ibuprofen (2 mM) + 0, 10, 20, 40, 60, 80, or 100 μM BCA or genistein, or 0, 10, 20, 40, 60, 80, or 100 nM DEX for an additional 24 h. Data are presented as mean cell survival ± standard error of the mean (SEM), normalized to ibuprofen-treated cells only, with * and ** indicating p < 0.05 and 0.01, respectively, as compared to ibuprofen only-treated cells. Representative Western blots of p75NTR levels in CCFSTTG1 (B) and U87MG (D) cells treated similarly as above, cells were lysed and similar amounts of protein (40 μg) were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Immunoblots were reprobed for β-actin to ensure for equal loading; A875 melanoma cell lysates were used as a positive control for p75NTR. Respective quantifications (from two independent experiments) of p75NTR expression are shown below as mean ± SEM for every cell type and normalized to levels in ibuprofen-treated cells.
We next examined the levels of p75NTR and their possible modulation by the addition of the above-mentioned compounds in a prevention regimen. BCA pretreatment resulted in a significant dose-dependent reduction in p75NTR levels as compared to ibuprofen alone in both CCFSTTG1 (≈80% reduction) and U87MG cells (≈60% reduction) (Fig. 3B,D). Genistein was also efficacious at modulating p75NTR levels in the prevention regimen, reaching a 60% reduction in p75NTR levels in both cells lines as compared to control ibuprofen-treated cells. DEX and estradiol not only failed to decrease p75NTR as compared to ibuprofen alone, but estradiol also resulted in a modest increase in p75NTR levels at doses higher than 40 nM, which coincided with a modest trend of decreasing survival in U87MG cells (Fig. 3C,D).
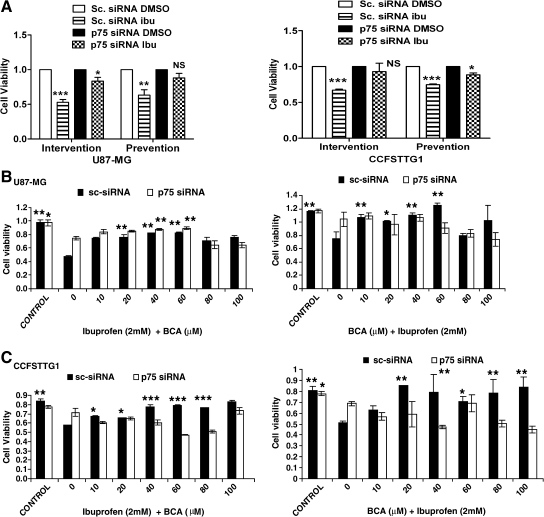
BCA rescuing abilities are dependent on p75NTR-induction by ibuprofen
Because the proposed in vitro assay is based on the induction of p75NTR and its modulation by BCA, we investigated whether BCA's effect on cell viability is mediated by its ability to decrease p75NTR levels, thereby eliminating a p75NTR-independent mechanism. Toward this aim, U87MG and CCFSTTG1 cells were transfected with scrambled or p75NTR-siRNA before ibuprofen or BCA treatment. In the presence of ibuprofen only, p75NTR knockdown in U87MG cells increased cell survival from 50% and 63% to 83% and 88% in intervention and prevention regimens, respectively (Fig. 4A, left). In CCFSTTG1, p75NTR knockdown resulted in an increase in survival from 67% and 74% to 93% and 88% in intervention and prevention regimens, respectively (Fig. 4A, right). This result validated the specificity of the p75NTR siRNA oligonucleotide, because the scrambled siRNA had no effect on cell viability (data not shown). Having demonstrated that p75NTR knockdown abolishes the ibuprofen-induced reduction in cell survival, we examined the ability of BCA to rescue U87MG and CCFSTTG1 cells in the presence of p75NTR siRNA. BCA-induced increase in U87MG and CCFSTTG1 cell survival in the presence of ibuprofen was recapitulated in the context of scrambled nontargeting siRNA in intervention and prevention settings (Fig. 4B,C). On the other hand, BCA's ability to rescue the viability of U87-MG cells was significantly mitigated in the presence of p75NTR siRNA in an intervention regimen (Fig. 4B, left) and completely abolished in the prevention regimen (Fig. 4B, right). In CCFSTTG1 cells, BCA's ability to rescue ibuprofen-induced reduction in cell survival was completely abolished in both prevention and intervention regimens (Fig. 4C), demonstrating the p75NTR-specific effect of BCA in our model.
FIG. 4.
Evaluation of biochanin A (BCA) effect in the presence of p75NTR siRNA. MTT analysis of U87 (A, left) and CCFSTTG1 (A, right) cells transfected with 100 nM scrambled (sc) or p75NTR siRNA, followed by treatment with ibuprofen (0 or 2 mM) after 24 h for 48 h (intervention regimen) or vehicle (dimethylsulfoxide [DMSO]) for 24 h and then ibuprofen for 24 h (prevention regimen). (B) U87 cells treated with scrambled (sc) or p75NTR siRNA for 24 h followed by ibuprofen (0 or 2 mM) for 24 h and then ibuprofen + BCA (0, 10, 20, 40, 60, 80, 100 μM) for an additional 24 h (left) or BCA (0, 10, 20, 40, 60, 80, 100 μM) for 24 h followed then by ibuprofen + BCA (0, 10, 20, 40, 60, 80, 100 μM) for an additional 24 h (right). (C) CCFSTTG1 cells treated with scrambled (sc) or p75NTR siRNA for 24 h followed by ibuprofen (2 mM) for 24 h and then ibuprofen + BCA (0, 10, 20, 40, 60, 80, 100 μM) for an additional 24 h (left) or BCA (0, 10, 20, 40, 60, 80, 100 μM) for 24 h followed by then ibuprofen + BCA (0, 10, 20, 40, 60, 80, 100 μM) for an additional 24 h (right). Data are presented as mean cell survival ± SEM, normalized to vehicle-treated cells only, with NS, *, **, and *** indicating nonsignificant, p < 0.05, 0.01, and 0.001, respectively, as compared to ibuprofen only-treated cells.
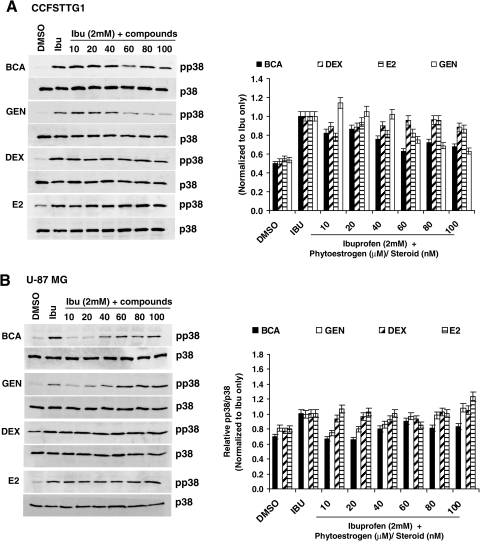
Evaluation of the effects of BCA and genistein's on ibuprofen-induced p38-MAPK activation
Previous work has demonstrated that p75NTR levels are stabilized at the mRNA level by p38-MAPK pathway activation, and p38-MAPK inhibition significantly reduces p75NTR in the presence of ibuprofen.27 Furthermore, p38-MAPK activation has also been reported upon SCI in astrocytes, microglia, and dorsal horn neurons in the spinal cord rostral to injury sites.28 Therefore, we examined whether p75NTR reduction by BCA and, to a lesser extent, genistein involved modulations in p38-MAPK activation. In the rescue regimen, when ibuprofen is administered before the phytoestrogens/steroids, BCA and genistein resulted in a 40% reduction in phospho-p38-MAPK levels at 60 and 80 μM, respectively, which closely coincided with the most pronounced downregulation of p75NTR levels in CCFSTTG1 cells (Fig. 5A). DEX and estradiol slightly decreased p38-MAPK activation levels by ≈10 and 20%, respectively (Fig. 5A). In U87MG cells, BCA (10 μM) induced a drastic 40% reduction in phopsho-p38-MAPK levels. Genistein also decreased the activation of p38-MAPK significantly at 10–20 μM (Fig. 5B).
FIG. 5.
p38-MAPK activation levels in CCFSSTG1 and U87MG cells treated with biochanin A (BCA) and other phytoestrogens/steroids in a rescue regimen. Representative Western blots of phospho-p38-MAPK (Thr180/Tyr182) and total p38-MAPK levels in CCFSTTG1 (A) and U87MG (B) cells treated with ibuprofen (2 mM) for 24 h, followed by treatment with ibuprofen (2 mM) for 24 h, and then followed by treatment with ibuprofen (2 mM) + 0, 10, 20, 40, 60, 80, and 100 μM BCA, genistein (GEN), or 0, 10, 20, 40, 60, 80, and 100 nM dexamethasone (DEX) or β-estradiol (E2) for an additional 24 h. Cells were lysed after treatment completion and equal amounts of protein (40 μg) were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Immunoblots were stripped and reprobed for total p38-MAPK levels. Respective quantification of mean of phospho-p38-MAPK/p38-MAPK ratio levels from two independent experiments is on the respective rights. Values are normalized to levels in ibuprofen-treated cells only.
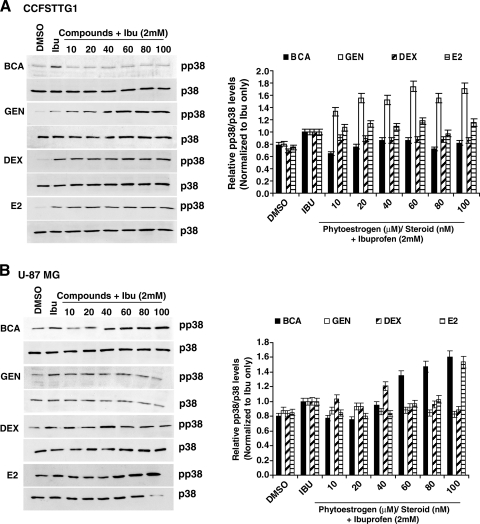
We also examined whether the prevention regimen could affect p38-MAPK activation by ibuprofen. BCA pretreatment resulted in the most drastic reduction in phospho-p38-MAPK levels in both cells lines (Fig. 6A,B), which was more pronounced in CCFSTTG1 cells concomitant with the most pronounced reduction in p75NTR in these cells (Fig. 3B), whereas the other compounds did not have a significant effect of the levels of phospho-p38-MAPK with the exception of estradiol, which exhibited a slight 10% reduction in phospho-p38-MAPK levels at 20–40 nM in U87MG cells only (Fig. 6B).
FIG. 6.
p38-MAPK activation levels in CCFSSTG1 and U87MG cells treated with biochanin A (BCA) and other phytoestrogens/steroids in a prevention regimen. Representative Western blots of phospho-p38-MAPK (Thr180/Tyr182) and total p38-MAPK levels in CCFSTTG1 (A) and U87MG (B) cells treated with 0, 10, 20, 40, 60, 80, and 100 μM BCA or genistein (GEN), or 0, 10, 20, 40, 60, 80, and 100 nM dexamethasone (DEX) or β-estradiol (E2) for 24 h, followed by treatment with ibuprofen (2 mM) for 24 h followed by treatment with ibuprofen (2 mM) + 0, 10, 20, 40, 60, 80, and 100 μM BCA, genistein or 0, 10, 20, 40, 60, 80, and 100 nM dexamethasone (DEX) or β-estradiol for an additional 24 h. Cells were lysed after treatment completion and equal amounts of protein (40 μg) were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Immunoblots were stripped and reprobed for total p38-MAPK levels. Quantification of the mean of phospho-p38-MAPK/p38-MAPK ratio levels from two independent experiments, respectively, is shown on the right. Values are normalized to levels in ibuprofen-treated cells only.
Discussion
We have made use of the p75NTR-inducing ability of ibuprofen as an in vitro model for screening compounds that could reduce p75NTR expression and would be of potential use in the treatment of ailments arising from p75NTR-dependent neuronal death such as SCI and peripheral neuropathy, following subsequent validation in primary neuronal cells and animal models. Using this model, we have demonstrated that BCA is more potent than traditionally used steroids such as dexamethasone, as well as other phytoestrogens, for increasing the survival of the cell lines tested when administered after ibuprofen treatment. BCA's rescuing ability was largely correlated with its ability to decrease ibuprofen-induced p75NTR expression levels and was largely mitigated or abolished when p75NTR induction was not present, as evidenced by siRNA experiments. The efficacy of BCA and other phytoestrogens/steroids was also evaluated in a prevention regimen where treatment was initiated before ibuprofen treatment and subsequent p75NTR induction. In this context, BCA also exhibited superior efficacy to other steroids or phytoestrogens tested, which translated into a more pronounced increase in survival and more drastic downregulation of p75NTR levels.
Growing evidence suggests that the pathophysiology of SCI involves primary and secondary mechanisms of injury, with the latter resulting in spreading of tissue damage that expands from the injury “epicenter” resulting in an increase in lesion size.29 Several processes that underlie the secondary injury have been identified including vascular abnormalities and edema,30 free radical formation and lipid peroxidation,31 excitoxicity,32 and inflammation,33 as well as necrotic and apoptotic cell death.34 Because these processes exacerbate injury, they represent attractive targets for potential therapies. It is now established that signal transduction from the neurotrophin receptor p75NTR causes cell death in the nervous system in vivo and in vitro.35 P75NTR levels are upregulated in various pathological conditions following mechanical damage and focal ischemia,36 and P75NTR expression is closely correlated to neuronal cell death.37 SCI seems to increase the ratio of p75NTR to trkA, favoring a downstream increase in ceramide38 and NF-κB,39 the respective mediators of cell death and inflammatory responses that represent hallmarks of the secondary injury in SCI models as well as peripheral neuropathy.
To mimic the induction of p75NTR after neuronal injury, we have made use of the ability of ibuprofen to induce p75NTR in the cell lines tested. Previously, it has been shown that ibuprofen-mediated decrease in cell survival is dependent on p75NTR induction because transfection of cancer cell lines with dominant negative p75NTR rescues, to a large extent, the viability of ibuprofen-treated cells,17 which was also observed in this study via p75NTR siRNA. This cause-and-effect relationship between p75NTR reduction and increased survival in both cell lines by BCA validated the MTT assay and p75NTR expression levels as end points of this in vitro assay. It is noteworthy that a modest cell survival increase was observed at doses where BCA was not shown to reduce p75NTR protein levels. This could be explained by a slight p75NTR independent effect on the survival of cells at low concentrations of BCA, which is yet unclear.
In this screening model, ibuprofen's ability to induce p75NTR expression in a time-dependent manner allows a better modeling of the various in vivo situations associated with p75NTR increase in the context of SCI. In fact, p75NTR expression has been reported as early as 1 day post-SCI.40 Furthermore, ibuprofen's ability to induce p75NTR as early as 24 h allowed for examining the effects of various phytoestrogens and steroids in a prevention regimen, while staying within an acceptable time range for the assay, especially for siRNA experiments. Interestingly, SCI occurrences as a result of surgical repair of thoracic/thoracoabdominal aortic disease41 remain a devastating complication of surgery and are ranked fouth as an underlying cause of SCI in females. Therefore, the efficacy of phytoestrogens/steroids in modulating p75NTR levels can also be evaluated when treatment is started prior to exposure of cells to ibuprofen, paving the way for in vivo experiments studying the chemopreventive effects of BCA prior to experimental SCI.
Because cell death following SCI has been documented in populations of neurons, oligodendrocytes, microglia, and astrocytes,34 we evaluated the efficacy of various steroids and phytoestrogens in modulating p75NTR expression in both the U87MG and CCFSTTG1 cell lines to determine any broad or cell-selective effects. We observed that BCA was the most efficacious compound in both cell lines in enhancing cell survival and decreasing p75NTR levels in both regimens. BCA's effects were more pronounced in CCFSTTG1 cells, which could be due to reduced ibuprofen sensitivity, as evidenced by a lower p75NTR induction as compared to U87MG cells. However, the possibility of a cell-selective efficacy cannot be completely eliminated. It is noteworthy that a caveat of the assay exists in the use of cancer cell lines. In fact, BCA has been demonstrated to inhibit the growth of various cancer cell lines,42–44 suggesting that in our assay extended exposure to BCA can have growth-arresting effects that would mask the increased survival effects mediated by the reduction in p75NTR levels. However this effect has not been reported in normal cells and is therefore not anticipated when testing BCA's efficacy in vivo. BCA was able to reduce p75NTR levels in CCFSTTG1 and U87MG cells and increase their viability with doses in the range of 10–40 μM with an half-maximal inhibitory concentration (IC50) of ≈20 μM in both cell lines. This concentration is physiologically achievable, because pharmacokinetic analyses of BCA have reported concentrations ≈25 μM in the serum of rats receiving BCA intraperitoneally with no adverse effects.45
To gain a better understanding of the mechanism by which BCA and, to a lesser extent, genistein may mediate the decrease in p75NTR levels, we examined p38-MAPK activation and its modulation by these compounds. It has been already established that p75NTR induction in prostate cancer cells is largely dependent on mRNA-stabilizing mechanisms downstream of p38-MAPK activation.27 The mitogen-activated protein kinases (MAPKs) are a family of signaling molecules comprising p38-MAPK and c-Jun N-terminal kinases (JNK), which transduce extracellular stress stimuli, thereby participating in injury responses and cell death.46 Interestingly, p38-MAPK activation in spinal microglia has been reported after SCI,47 as well as in other models of neuropathic pain.48 We observed a reduction in p38-MAPK activation as evidenced by decreased phosphorylation of Thr180/Tyr182 residues upon BCA treatment in both cell lines, concomitant with the most significant reduction of p75NTR expression, especially in the intervention regimen. However, some p75NTR reduction was also observed when p38-MAPK activity was unaltered, suggesting that other mechanisms could contribute to p75NTR downregulation. Furthermore, the ability of BCA but not other compounds to inhibit p38-MAPK activation at physiologically achievable doses (10–20 μM) in the U87MG cell line indicates that BCA is worth investigating in animal models of conditions associated with p38-MAPK activation in the nervous system and elsewhere, independent of its ability to modulate p75NTR.
In conclusion, this work has presented a new in vitro assay for the inexpensive and timely testing of compounds that may eliminate a screening step from animal model studies aimed at testing compounds that may alleviate neurological ailments resulting from the upregulation of p75NTR and or the activation of the p38-MAPK pathway. This study identifies BCA as the most efficacious of these compounds. This new in vitro assay may provide for a less expensive and less cumbersome method for compound screening, as well as providing insight into mechanism of action, prior to their evaluation in animal models of neurological diseases.
Acknowledgments
This work was funded by U.S. Army grant W81XWH-07-1-0366 and administered by the Telemedicine and Advanced Technology Research Center (TATRC), Fort Detrick, Maryland, MD. The content of this paper does not necessarily reflect the position or policy of the U.S. Government.
References
- 1.Kalb R. The protean actions of neurotrophins and their receptors on the life and death of neurons. Trends Neurosci. 2005;28:5–11. doi: 10.1016/j.tins.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Hall ED. Braughler JM. Non-surgical management of spinal cord injuries: A review of studies with the glucocorticoid steroid methylprednisolone. Acta Anaesthesiol Belg. 1987;38:405–409. [PubMed] [Google Scholar]
- 3.Bracken MB. Shepard MJ. Holford TR. Leo-Summers L. Aldrich EF. Fazl M. Fehlings M. Herr DL. Hitchon PW. Marshall LF. Nockels RP. Pascale V. Perot PL Jr. Piepmeier J. Sonntag VK. Wagner F. Wilberger JE. Winn HR. Young W. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277:1597–1604. [PubMed] [Google Scholar]
- 4.Tsutsumi S. Ueta T. Shiba K. Yamamoto S. Takagishi K. Effects of the Second National Acute Spinal Cord Injury Study of high-dose methylprednisolone therapy on acute cervical spinal cord injury-results in spinal injuries center. Spine. 2006;31:2992–2996. doi: 10.1097/01.brs.0000250273.28483.5c. [DOI] [PubMed] [Google Scholar]
- 5.Brandoli C. Shi B. Pflug B. Andrews P. Wrathall JR. Mocchetti I. Dexamethasone reduces the expression of p75 neurotrophin receptor and apoptosis in contused spinal cord. Brain Res Mol Brain Res. 2001;87:61–70. doi: 10.1016/s0169-328x(00)00284-9. [DOI] [PubMed] [Google Scholar]
- 6.Gorio A. Madaschi L. Di Stefano B. Carelli S. Di Giulio AM. De Biasi S. Coleman T. Cerami A. Brines M. Methylprednisolone neutralizes the beneficial effects of erythropoietin in experimental spinal cord injury. Proc Natl Acad Sci USA. 2005;102:16379–16384. doi: 10.1073/pnas.0508479102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumoto T. Tamaki T. Kawakami M. Yoshida M. Ando M. Yamada H. Early complications of high-dose methylprednisolone sodium succinate treatment in the follow-up of acute cervical spinal cord injury. Spine. 2001;26:426–430. doi: 10.1097/00007632-200102150-00020. [DOI] [PubMed] [Google Scholar]
- 8.Schumacher M. Guennoun R. Stein DG. De Nicola AF. Progesterone: Therapeutic opportunities for neuroprotection and myelin repair. Pharmacol Ther. 2007;116:77–106. doi: 10.1016/j.pharmthera.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Fee DB. Swartz KR. Joy KM. Roberts KN. Scheff NN. Scheff SW. Effects of progesterone on experimental spinal cord injury. Brain Res. 2007;1137:146–152. doi: 10.1016/j.brainres.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 10.Jones KJ. Brown TJ. Damaser M. Neuroprotective effects of gonadal steroids on regenerating peripheral motoneurons. Brain Res Brain Res Rev. 2001;37:372–382. doi: 10.1016/s0165-0173(01)00107-2. [DOI] [PubMed] [Google Scholar]
- 11.Schopp LH. Clark M. Mazurek MO. Hagglund KJ. Acuff ME. Sherman AK. Childers MK. Testosterone levels among men with spinal cord injury admitted to inpatient rehabilitation. Am J Phys Med Rehabil. 2006;85:678–684. doi: 10.1097/01.phm.0000228617.94079.4a. [DOI] [PubMed] [Google Scholar]
- 12.Chaovipoch P. Jelks KA. Gerhold LM. West EJ. Chongthammakun S. Floyd CL. 17beta-estradiol is protective in spinal cord injury in post- and pre-menopausal rats. J Neurotrauma. 2006;23:830–852. doi: 10.1089/neu.2006.23.830. [DOI] [PubMed] [Google Scholar]
- 13.Cordey M. Gundimeda U. Gopalakrishna R. Pike CJ. Estrogen activates protein kinase C in neurons: Role in neuroprotection. J Neurochem. 2003;84:1340–1348. doi: 10.1046/j.1471-4159.2003.01631.x. [DOI] [PubMed] [Google Scholar]
- 14.Smith SS. Woolley CS. Cellular and molecular effects of steroid hormones on CNS excitability. Cleveland Clin J Med. 2004;71:S4–S10. doi: 10.3949/ccjm.71.suppl_2.s4. [DOI] [PubMed] [Google Scholar]
- 15.Tabassum A. Khwaja F. Djakiew D. The p75(NTR) tumor suppressor induces caspase-mediated apoptosis in bladder tumor cells. Int J Cancer. 2003;105:47–52. doi: 10.1002/ijc.11038. [DOI] [PubMed] [Google Scholar]
- 16.Khwaja F. Allen J. Lynch J. Andrews P. Djakiew D. Ibuprofen inhibits survival of bladder cancer cells by induced expression of the p75NTR tumor suppressor protein. Cancer Res. 2004;64:6207–6213. doi: 10.1158/0008-5472.CAN-03-3814. [DOI] [PubMed] [Google Scholar]
- 17.Quann EJ. Khwaja F. Zavitz KH. Djakiew D. The aryl propionic acid R-flurbiprofen selectively induces p75NTR-dependent decreased survival of prostate tumor cells. Cancer Res. 2007;67:3254–3262. doi: 10.1158/0008-5472.CAN-06-3657. [DOI] [PubMed] [Google Scholar]
- 18.Khwaja F. Tabassum A. Allen J. Djakiew D. The p75(NTR) tumor suppressor induces cell cycle arrest facilitating caspase mediated apoptosis in prostate tumor cells. Biochem Biophys Res Commun. 2006;341:1184–1192. doi: 10.1016/j.bbrc.2006.01.073. [DOI] [PubMed] [Google Scholar]
- 19.Krygier S. Djakiew D. Molecular characterization of the loss of p75(NTR) expression in human prostate tumor cells. Mol Carcinog. 2001;31:46–55. doi: 10.1002/mc.1038. [DOI] [PubMed] [Google Scholar]
- 20.Yan P. Liu N. Kim GM. Xu J. Xu J. Li Q. Hsu CY. Xu XM. Expression of the type 1 and type 2 receptors for tumor necrosis factor after traumatic spinal cord injury in adult rats. Exp Neurol. 2003;183:286–297. doi: 10.1016/s0014-4886(03)00135-3. [DOI] [PubMed] [Google Scholar]
- 21.Lowry KS. Murray SS. Coulson EJ. Epa R. Bartlett PF. Barrett G. Cheema SS. Systemic administration of antisense p75(NTR) oligodeoxynucleotides rescues axotomised spinal motor neurons. J Neurosci Res. 2001;64:11–17. doi: 10.1002/jnr.1048. [DOI] [PubMed] [Google Scholar]
- 22.Kuiper GG. Carlsson B. Grandien K. Enmark E. Häggblad J. Nilsson S. Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 23.Price KR. Fenwick GR. Naturally occurring oestrogens in foods-a review. Food Addit Contam. 1985;2:73–106. doi: 10.1080/02652038509373531. [DOI] [PubMed] [Google Scholar]
- 24.Wang SW. Chen Y. Joseph T. Hu M. Variable isoflavone content of red clover products affects intestinal disposition of biochanin A, formononetin, genistein, and daidzein. J Altern Complement Med. 2008;14:287–297. doi: 10.1089/acm.2007.0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munro IC. Harwood M. Hlywka JJ. Stephen AM. Doull J. Flamm WG. Adlercreutz H. Soy isoflavones: A safety review. Nutr Rev. 2003;61:1–33. doi: 10.1301/nr.2003.janr.1-33. [DOI] [PubMed] [Google Scholar]
- 26.Allen J. Khwaja F. Byers S. Djakiew D. The p75NTR mediates a bifurcated signal transduction cascade through the NF kappa B and JNK pathways to inhibit cell survival. Exp Cell Res. 2005;304:69–80. doi: 10.1016/j.yexcr.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 27.Quann EJ. Khwaja F. Djakiew D. The p38 MAPK pathway mediates aryl propionic acid induced messenger RNA stability of p75 NTR in prostate cancer cells. Cancer Res. 2007;67:11402–11410. doi: 10.1158/0008-5472.CAN-07-1792. [DOI] [PubMed] [Google Scholar]
- 28.Crown ED. Gwak YS. Ye Z. Johnson KM. Hulsebosch CE. Activation of p38 MAP kinase is involved in central neuropathic pain following spinal cord injury. Exp Neurol. 2008;213:257–267. doi: 10.1016/j.expneurol.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwon BK. Tetzlaff W. Grauer JN. Beiner J. Vaccaro AR. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004;4:451–464. doi: 10.1016/j.spinee.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Tator CH. Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75:15–26. doi: 10.3171/jns.1991.75.1.0015. [DOI] [PubMed] [Google Scholar]
- 31.Cuzzocrea S. Riley DP. Caputi AP. Salvemini D. Antioxidant therapy: A new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol Rev. 2001;53:135–159. [PubMed] [Google Scholar]
- 32.Wrathall JR. Teng YD. Choiniere D. Amelioration of functional deficits from spinal cord trauma with systemically administered NBQX, an antagonist of non-N-methyl-D-aspartate receptors. Exp Neurol. 1996;137:119–126. doi: 10.1006/exnr.1996.0012. [DOI] [PubMed] [Google Scholar]
- 33.Dusart I. Schwab ME. Secondary cell death and the inflammatory reaction after dorsal hemisection of the rat spinal cord. Eur J Neurosci. 1994;6:712–724. doi: 10.1111/j.1460-9568.1994.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 34.Beattie MS. Farooqui AA. Bresnahan JC. Review of current evidence for apoptosis after spinal cord injury. J Neurotrauma. 2000;17:915–925. doi: 10.1089/neu.2000.17.915. [DOI] [PubMed] [Google Scholar]
- 35.Dechant G. Barde YA. The neurotrophin receptor p75(NTR): Novel functions and implications for diseases of the nervous system. Nat Neurosci. 2002;5:1131–1136. doi: 10.1038/nn1102-1131. [DOI] [PubMed] [Google Scholar]
- 36.Rende M. Provenzano C. Tonali P. Modulation of low-affinity nerve growth factor receptor in injured adult rat spinal cord motoneurons. J Comp Neurol. 1993;338:560–574. doi: 10.1002/cne.903380406. [DOI] [PubMed] [Google Scholar]
- 37.Giehl KM. Röhrig S. Bonatz H. Gutjahr M. Leiner B. Bartke I. Yan Q. Reichardt LF. Backus C. Welcher AA. Dethleffsen K. Mestres P. Meyer M. Endogenous brain-derived neurotrophic factor and neurotrophin-3 antagonistically regulate survival of axotomized corticospinal neurons in vivo. J Neurosci. 2001;21:3492–3502. doi: 10.1523/JNEUROSCI.21-10-03492.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dobrowsky RT. Werner MH. Castellino AM. Chao MV. Hannun YA. Activation of the sphingomyelin cycle through the low-affinity neurotrophin receptor. Science. 1994;265:1596–1599. doi: 10.1126/science.8079174. [DOI] [PubMed] [Google Scholar]
- 39.Carter BD. Kaltschmidt C. Kaltschmidt B. Offenhäuser N. Böhm-Matthaei R. Baeuerle PA. Barde YA. Selective activation of NF-kappa B by nerve growth factor through the neurotrophin receptor p75. Science. 1996;272:542–545. doi: 10.1126/science.272.5261.542. [DOI] [PubMed] [Google Scholar]
- 40.Yune TY. Lee JY. Jung GY. Kim SJ. Jiang MH. Kim YC. Oh YJ. Markelonis GJ. Oh TH. Minocycline alleviates death of oligodendrocytes by inhibiting pro-nerve growth factor production in microglia after spinal cord injury. J Neurosci. 2007;27:7751–7761. doi: 10.1523/JNEUROSCI.1661-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shamji MF. Maziak DE. Shamji FM. Ginsberg RJ. Pon R. Circulation of the spinal cord: an important consideration for thoracic surgeons. Ann Thorac Surg. 2003;76:315–321. doi: 10.1016/s0003-4975(03)00139-5. [DOI] [PubMed] [Google Scholar]
- 42.Moon YJ. Shin BS. An G. Morris ME. Biochanin A inhibits breast cancer tumor growth in a murine xenograft model. Pharm Res. 2008;25:2158–2163. doi: 10.1007/s11095-008-9583-6. [DOI] [PubMed] [Google Scholar]
- 43.Su SJ. Yeh TM. Lei HY. Chow NH. The potential of soybean foods as a chemoprevention approach for human urinary tract cancer. Clin Cancer Res. 2000;6:230–236. [PubMed] [Google Scholar]
- 44.Wang S. DeGroff VL. Clinton SK. Tomato and soy polyphenols reduce insulin-like growth factor-I-stimulated rat prostate cancer cell proliferation and apoptotic resistance in vitro via inhibition of intracellular signaling pathways involving tyrosine kinase. J Nutr. 2003;133:2367–2376. doi: 10.1093/jn/133.7.2367. [DOI] [PubMed] [Google Scholar]
- 45.Moon YJ. Sagawa K. Frederick K. Zhang S. Morris ME. Pharmacokinetics and bioavailability of the isoflavone biochanin A in rats. AAPS J. 2006;8:E433–442. doi: 10.1208/aapsj080351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Widmann C. Gibson S. Jarpe MB. Johnson GL. Mitogen-activated protein kinase: Conservation of a three-kinase module from yeast to human. Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 47.Hains BC. Waxman SG. Activated microglia contributes to the maintenance of chronic pain after spinal cord injury. J Neurosci. 2006;26:4308–4317. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ji RR. Suter MR. p38MAPK, microglial signaling, and neuropathic pain. Mol Pain. 2007;3:33–41. doi: 10.1186/1744-8069-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]