Introduction
By 2015, nearly 15% of the U.S. population will be over the age of 65. In 2030, there will be over 70 million older Americans1. This increase in the elderly population has prompted interest in recent years toward the study of “frail” older adults. Clinicians use the term “frail” to describe a person over 65 years old who is vulnerable to any kind of change in health status, such as infection or physical injury2. These individuals are at high risk of complications during a medical illness and have prolonged recovery times3. An international consensus report in 2006 characterized frail elders as having impairments in mobility, balance, strength, motor processing, cognition, nutrition, endurance (fatigue) and physical activity4.
In 1992, Buchner first proposed a formal definition of frailty as a syndrome of weakness, impaired mobility, balance, and minimal reserve5. Using epidemiological data from the Cardiovascular Health Study (CHS), Fried further characterized frailty as individuals who had unintentional weight loss (ten or more pounds in the past year), fatigue or exercise intolerance, weakness, slowed motor performance, and low physical activity. A person was considered frail if they demonstrated at least three of these attributes (see Figure 1), and pre-frail if they had one or two of these characteristics. Fried found those who were frail had an increased risk of falls, ADL disability, hospitalization, and death over a three year period2. In the Women’s Health and Aging I (WHAS-I) study, the risk of ADL dependence increased with the number of frailty criteria fulfilled6. Mortality in the frail cohort of the Cardiovascular Health Study was approximately three times higher when compared to the non-frail cohort (43% versus 12%) at seven years. Interestingly, 25% of frail subjects in the Cardiovascular Health Study had only one chronic disease, defined as osteoarthritis, diabetes, hypertension, angina, congestive heart failure, cancer or pulmonary disease2, suggesting that frailty was not always associated with multiple co-morbidities. The underlying mechanisms causing the phenotype of frailty remain to be fully elucidated. Buchner and Wagner initially proposed that declines in neurological processes, musculoskeletal functioning, and energy metabolism were the cause of frailty5. Lipsitz later suggested that frailty might be caused by the loss of the ability of the cardiovascular and nervous systems to respond appropriately to stressors due to age-related changes7. Using these concepts, frailty is now thought to be due altered function in multiple physiological systems (including inflammatory, skeletal muscle, endocrine, clotting, and hematological) and dysregulation of mechanisms between these systems to maintain homeostasis8.
Figure 1. Components of frailty.
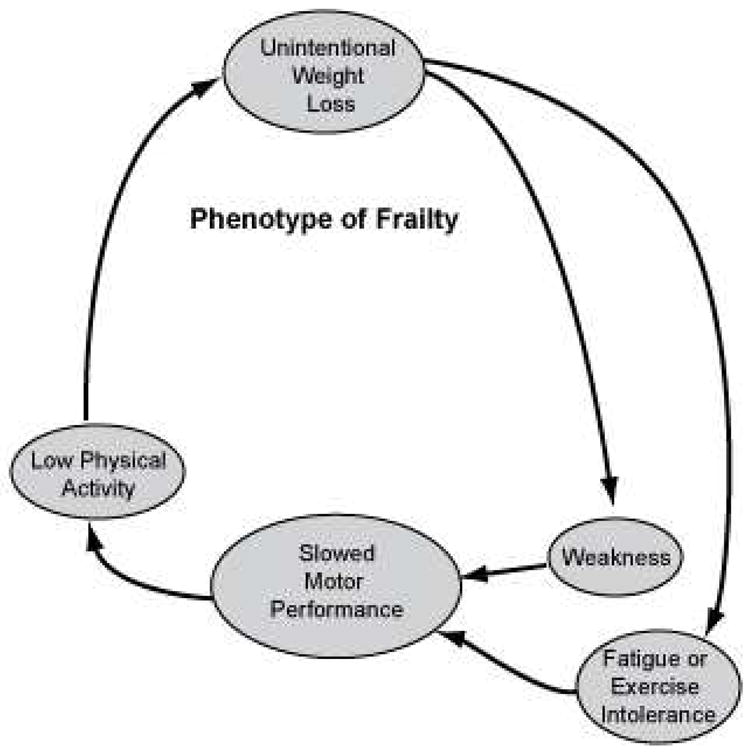
Adapted from Fried LP, Xue QL, Cappola AR, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. Oct 2009;64(10): 1050.
With increasing age, there is a well-described decline in voluntary physical activity which is associated with decreases in several measures of exercise tolerance including maximal aerobic capacity, muscle strength, and fatigueability9, leading to an increase risk of frailty. In recent years, increased physical activity or regular exercise training has been proposed as preventive strategies for frailty and its adverse outcomes, as it can target four of the frailty criteria: weakness, low physical activity, slowed motor performance, and exercise intolerance. Epidemiological studies suggest that regular physical activity is associated with a decreased risk of ADL disability in older adults, which is an adverse outcome of frailty. In a large scale cohort study done in Taiwan, Wu et al found older adults who were physically active, defined as participation in dancing, hiking, jogging or walking at least twice a week, were less likely to have ADL disabilities at the end of a three year period when compared to their sedentary counterparts10. These findings were confirmed by the Longitudinal Study of Aging, which found elders who were physically active, defined a walking at least a mile a week, were less likely to develop impairments in their ADL/IADLs over a six year period, after adjusting for age, gender, co-morbidities, and baseline disability11.
The major goal of this chapter is to review the literature investigating the utility of aerobic and resistance exercise training as an intervention for frailty in older adults. In addition, areas of future research will be addressed, including concerns related to the dissemination of exercise interventions on a widespread scale. Finally, guidelines for an “exercise prescription” for frail older adults will be briefly outlined.
Aerobic/endurance exercise training
There are two mechanisms by which aerobic exercise is thought to alter the frailty phenotype: improvement in the maximal oxygen uptake (VO2 peak) and increased muscle mass. VO2 peak is defined as the maximum rate of oxygen consumption measured during vigorous exercise and is closely related to sub-maximal endurance exercise capacity and exercise tolerance.
In an intervention study of 64 frail men and women, a nine month program of strength training and walking aimed at reaching 78% of peak heart rate was found to increase endurance by improving VO2 peak by 14%12. A small study of healthy elderly sedentary women also found a 12 week regimen of cycle ergometer training improved maximal aerobic capacity by 30% from baseline. This study demonstrated that the endurance training increased quadriceps muscle mass by 12%13. Although previous studies have shown that aerobic exercise did not alter muscle size in older adults 14–16, a recent cross-sectional study by Sugawara17 demonstrated that those who undergo aerobic training have a higher percentage of muscle mass in their extremities when compared to their sedentary counterparts.
Resistance exercise training
There is well-documented evidence that muscle strength decreases with advancing age. Muscle strength decreases approximately 12% to 15% per decade after the age of 50 years18 in both males and females19. Additionally, muscle mass also decreases with increased age. In a cross-sectional study of healthy men of equal mass, muscle mass comprised 24% of total mass in those 20 to 29 years old, but decreased to 13% in subjects aged 70 to 79 years20. Several studies have found that the decline in strength in the older adult is due to this age-related loss of muscle mass21–23. While resistance exercise training has been shown to increase muscle mass and therefore muscle strength, this response is attenuated in older adults with mobility limitations or other co-morbidities. In healthy older men and women, four months of resistance training resulted in a 16 to 23% increase in muscle mass, compared to a 2.5–9% increase in frail or institutionalized older adults24–26.
Despite these age-related effects on muscle, resistance exercise training still has been found to increase strength in older adults. Multiple studies have demonstrated that these changes can occur even into the ninth decade of life24, 27–31. In a systemic review of 41 articles by Latham, resistance exercise training in older adults was associated with gains in strength32, and a Cochrane review of 74 studies found similar results33. Both reviews used studies that examined both healthy older adults and frail adults. Of note, Fiatarone showed nursing home residents were able to increase their strength on average 97% after 10 weeks of resistance exercise training25. As nursing home residents represent the frailest segment of older adults, this demonstrates the intervention is beneficial for even those most severely affected.
Motor performance in older adults has also been shown to improve after resistance training. In her systemic review, Latham found resistance exercise training in healthy and frail elders improved gait speed in 14 studies and increased distances in the six minute walk test in six trials32. In their respective studies of frail elders living in the nursing home and in the community, Fiatarone and Chandler each showed that 10 weeks of resistance training resulted in improved gait speed25, 34. There may be a dose response relationship between resistance exercise and motor performance, as Galvao and Taaffe found subjects who did more repetitions of resistance exercise had almost twice the improvement in their 400 meter walk time compared to those who did fewer repetitions35.
Combined aerobic and resistance exercise
Given the beneficial results seen with aerobic or resistance exercise alone and that both types of exercise target specific distinct features of frailty, there has been recent interest in whether an intervention with both components is beneficial for frail older adults. In a randomized controlled trial of elderly subjects who had undergone surgical repair of a femur fracture, a six month intervention of aerobic activity and progressive resistance exercise was associated with a mean improvement of 19 meters per minute in walking speed36. Similar changes in motor function were demonstrated in the Lifestyles Interventions and Independence for Elders (LIFE) study, which found that a 12 month program of walking, resistance exercise, and flexibility training improved scores on the Short Physical Performance Battery (SPPB) and prevented a decline in 400 meter walk speed in older adults at high risk for disability one year after follow up37.
Effect of exercise interventions on the adverse outcomes of frailty
Several studies have examined the effect of exercise on falls, a common adverse outcome of frailty. After a single fall, the risk of skilled nursing facility placement in older adults increases three-fold, after adjustment for cognitive, psychological, social, functional and medical factors38. In a studying examining women undergoing resistance training for 25 weeks, risk of falls was reduced by 57% from baseline39. A meta-analysis of six studies by Baker showed a combined regimen of aerobic, resistance, balance and flexibility exercises was found to decrease fall risk40. A Cochrane review of 111 trials found that a combination of aerobic and resistance exercise reduced the risk of falls by 17% in community dwelling elders41.
In addition to falls, ADL disability is of major concern in frail individuals as it is associated with higher rates of mortality42. In the systemic review of 41 studies conducted by Latham32, resistance exercise training did not decrease the risk of ADL disability in an elderly population. In contrast, a Cochrane review of 121 trials found an association between resistance training and reduced ADL disability33. Neither review stratified their results by frailty severity. In a randomized controlled trial of a six month home-based program that combined resistance exercise training with balance training and home safety and assistive device evaluations, rates of ADL disability decreased only in those with moderate frailty, but not in those with not in those with severe frailty. Moderate frailty was defined as either the inability to perform a rapid gait test (requiring more than 10 seconds to walk a 3 meter course) or stand up from a chair with arms folded, and severe frailty was defined as having both characteristics43, 44. In contrast, Binder did not find an improvement in the Older American Resources and Services (OARS) ADL score in a group of mild to moderately frail subjects45 after a regimen of resistance, balance, and flexibility training. Currently, final results of the FRASI (FRAilty, Screening and Intervention) are pending, and will look at the effect of an eight week exercise regimen on the on the time of onset of ADL disability in community dwelling frail elders46. Table 1 lists key randomized controlled trials that studied frail older adults (Table 1).
Table 1.
Randomized controlled trials in frail older adults
| Study | Population | Intervention | Outcome |
|---|---|---|---|
| Fiatarone et al 199425 | Nursing home residents 70 years or older and able to walk 6 meters | 3 sessions per week of resistance training of lower extremities for 10 weeks versus regular recreational activities | Mean improvement in muscle strength of lower extremities was 97% for intervention group compared to 12% for control group |
| Chandler et al 199834 | Community dwelling older adults 65 years and older and unable to climb stairs without a handrail | 3 sessions per week of progressive resistance training of lower extremities with stair stepping and chair rises for 10 weeks versus normal activities | Mean improvement in muscle strength of lower extremities was 10 to 13% for intervention group compared to 1% improvement to 3% decline in control group |
| Binder et al 200245 | Community dwelling older adults 78 years and older who had 2 of the following criteria: modified PPT score 18 to 32, VO2 peak 10 to 18 mL/kg/min, or difficulty with either 1 ADL or 2 IADLs | 3 sessions a week of resistance training of upper and lower extremities, aerobic training with walking, cycling or rowing, and flexibility/balance training for 9 months versus flexibility exercises | Mean improvement in lower extremity strength was 19% to 23% for intervention group compared to 5% improvement to 5% decline in control group Mean VO2 peak improved 13% for intervention group compared to 2.6% decline in control group |
| Gill 200244 | Community dwelling older adults 75 years and older who required more than 10 seconds to walk 3 meters and/or unable to stand up from a chair with arms folded | Average of 16 sessions in the home of resistance training of upper and lower extremities, flexibility and balance exercises, and home safety and assistive device evaluations for 6 months versus education program | 66% improvement in disability scores for those with moderate frailty but no significant improvement for those with severe frailty in intervention group, using control group as baseline |
Effect on the phenotype of frailty
Although there have been multiple trials studying the effects of exercise on the various characteristics of frailty and the adverse outcomes of frailty, there have been relatively few studies to see whether exercise can alter or even reverse frailty status in older adults. In a study conducted with subjects who were frail or at high risk for frailty, a telephone intervention encouraging exercise decreased the proportion of frail elders by 18% at 6 months follow-up47. Currently, the Frailty Intervention Trial (FIT) study is examining whether a 12 month intervention of aerobic and resistance training can change frailty status in a cohort of already frail older adults48.
Adverse outcomes of exercise
Reports of adverse outcomes with both aerobic and resistance training while not uncommon, are rarely life threatening. In a study that examined resistance training in elderly women, most of the adverse outcomes were musculoskeletal complaints39. Latham found that the risk ratio for adverse events increased to 3.6 in those who underwent of ten weeks of resistance training49. However, no reports of death or cardiovascular events were found in a systemic review of 62 trials of resistance exercise32. In a randomized controlled trial studying the effect of a 12 month intervention of walking, resistance exercise, and flexibility, similar rates of serious and non-serious adverse events were found for both the intervention and control subjects37.
Future Directions
Clearly, exercise and physical activity are promising interventions for frailty, and several studies are currently underway to examine their impact. However, there are several related areas that need further investigation this intervention can be disseminated to frail older adults on a widespread basis. First, adherence to an exercise regimen is key to its beneficial effects, and strategies to overcome this barrier need to be developed before exercise as treatment modality is implemented on a wide scale. Schneider found that subjects were interested in exercise for its medical and psychological benefits, but had concerns about the time required and about their abilities to perform adequate exercise50. In addition, cognition is a factor that should be considered. A significant proportion of older adults are cognitively impaired, which may impact their ability to properly adhere to a regular exercise regimen. However, if caregivers are involved, this may not be such a barrier. In a randomized clinical trial, subjects with Alzheimer’s dementia participated in a home-based exercise program of aerobic and resistance exercise under the supervision of their caregivers. At three months, the dementia subjects were more active and had better motor functioning compared to the controls51. Almost all the trials reviewed in this chapter were clinic or facility based. Home-based programs are more accessible, and would eliminate the barrier of transportation for many elders. In a study that examined a six month home-based program that combined resistance and balance training with home safety and assistive device evaluations, there was no improvement in motor performance. However, a later Cochrane review did find that home-based exercise programs reduced the risk of falls in older adults by 23%41. Finally, whether these exercise interventions would require supervision by a rehabilitation professional or could be conducted in the community, is still unclear. If supervision is necessary, this adds to the cost of the intervention. Future and ongoing trials should include an analysis of the costs and benefits of a physical activity intervention.
Conclusion: An exercise “prescription”
While more investigation is still needed, the majority of studies suggest that clinicians should recommend regular physical activity or exercise training to frail older adults. The current guidelines from the U.S. Department of Health and Human Services state that all adults over 65 years should participate in 150 minutes (2 hours and 30 minutes) of moderate aerobic exercise per week52. Although most trials studied resistance exercise training, we would encourage frail older adults to start with an aerobic activity such as walking, as it is more accessible. If possible, resistance exercise training should be added. Depending on the degree of frailty, supervision may or may not be required. For individuals with severe frailty, evaluation by a rehabilitation profession is recommended.
The majority of evidence shows that regular physical activity or exercise is beneficial for older adults who are frail or at high risk of frailty. Studies have shown the number adverse events are minimal, and the gains of regular exercise clearly outweigh the risks. Although there are still several areas related to the intervention that require further investigation, for older adults regular physical activity or exercise is highly recommended as a means to modify frailty and its adverse outcomes.
Acknowledgments
Dr. Liu was supported by a Health Resources and Services Administration grant from the Bureau of Health Professions (# D01 HP08796) and by Boston Medical Center, Boston, MA 02118. Dr. Fielding was supported by the Boston Claude D. Pepper Older Americans Independence Center (1P30AG031679). This material is based upon work supported by the U.S. Department of Agriculture, under agreement No. 58-1950-7-707.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.www.americangeriatrics.org.
- 2.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 Mar;56(3):M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 3.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004 Mar;59(3):255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 4.Ferrucci L, Guralnik JM, Studenski S, Fried LP, Cutler GB, Jr, Walston JD. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc. 2004 Apr;52(4):625–634. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- 5.Buchner DM, Wagner EH. Preventing frail health. Clin Geriatr Med. 1992 Feb;8(1):1–17. [PubMed] [Google Scholar]
- 6.Boyd CM, Xue QL, Simpson CF, Guralnik JM, Fried LP. Frailty, hospitalization, and progression of disability in a cohort of disabled older women. Am J Med. 2005 Nov;118(11):1225–1231. doi: 10.1016/j.amjmed.2005.01.062. [DOI] [PubMed] [Google Scholar]
- 7.Lipsitz LA. Dynamics of stability: the physiologic basis of functional health and frailty. J Gerontol A Biol Sci Med Sci. 2002 Mar;57(3):B115–125. doi: 10.1093/gerona/57.3.b115. [DOI] [PubMed] [Google Scholar]
- 8.Fried LP, Xue QL, Cappola AR, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009 Oct;64(10):1049–1057. doi: 10.1093/gerona/glp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006 Jun;54(6):991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 10.Wu SC, Leu SY, Li CY. Incidence of and predictors for chronic disability in activities of daily living among older people in Taiwan. J Am Geriatr Soc. 1999 Sep;47(9):1082–1086. doi: 10.1111/j.1532-5415.1999.tb05231.x. [DOI] [PubMed] [Google Scholar]
- 11.Miller ME, Rejeski WJ, Reboussin BA, Ten Have TR, Ettinger WH. Physical activity, functional limitations, and disability in older adults. J Am Geriatr Soc. 2000 Oct;48(10):1264–1272. doi: 10.1111/j.1532-5415.2000.tb02600.x. [DOI] [PubMed] [Google Scholar]
- 12.Ehsani AA, Spina RJ, Peterson LR, et al. Attenuation of cardiovascular adaptations to exercise in frail octogenarians. J Appl Physiol. 2003 Nov;95(5):1781–1788. doi: 10.1152/japplphysiol.00194.2003. [DOI] [PubMed] [Google Scholar]
- 13.Harber MP, Konopka AR, Douglass MD, et al. Aerobic exercise training improves whole muscle and single myofiber size and function in older women. Am J Physiol Regul Integr Comp Physiol. 2009 Nov;297(5):R1452–1459. doi: 10.1152/ajpregu.00354.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verney J, Kadi F, Saafi MA, Piehl-Aulin K, Denis C. Combined lower body endurance and upper body resistance training improves performance and health parameters in healthy active elderly. Eur J Appl Physiol. 2006 Jun;97(3):288–297. doi: 10.1007/s00421-006-0175-z. [DOI] [PubMed] [Google Scholar]
- 15.Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab. 2004 Jan;286(1):E92–101. doi: 10.1152/ajpendo.00366.2003. [DOI] [PubMed] [Google Scholar]
- 16.Ferrara CM, Goldberg AP, Ortmeyer HK, Ryan AS. Effects of aerobic and resistive exercise training on glucose disposal and skeletal muscle metabolism in older men. J Gerontol A Biol Sci Med Sci. 2006 May;61(5):480–487. doi: 10.1093/gerona/61.5.480. [DOI] [PubMed] [Google Scholar]
- 17.Sugawara J, Miyachi M, Moreau KL, Dinenno FA, DeSouza CA, Tanaka H. Age-related reductions in appendicular skeletal muscle mass: association with habitual aerobic exercise status. Clin Physiol Funct Imaging. 2002 May;22(3):169–172. doi: 10.1046/j.1475-097x.2002.00413.x. [DOI] [PubMed] [Google Scholar]
- 18.Larsson L. Histochemical characteristics of human skeletal muscle during aging. Acta Physiol Scand. 1983 Mar;117(3):469–471. doi: 10.1111/j.1748-1716.1983.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 19.Lindle RS, Metter EJ, Lynch NA, et al. Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. J Appl Physiol. 1997 Nov;83(5):1581–1587. doi: 10.1152/jappl.1997.83.5.1581. [DOI] [PubMed] [Google Scholar]
- 20.Munro HN. Aging. In: Kinney JMJK, Hill GL, Owen OE, editors. Nutrition and metabolism in patient care. Philadelphia, PA: WB Saunders Company; 1988. pp. 145–166. [Google Scholar]
- 21.Pearson MB, Bassey EJ, Bendall MJ. The effects of age on muscle strength and anthropometric indices within a group of elderly men and women. Age Ageing. 1985 Jul;14(4):230–234. doi: 10.1093/ageing/14.4.230. [DOI] [PubMed] [Google Scholar]
- 22.Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J Appl Physiol. 1991 Aug;71(2):644–650. doi: 10.1152/jappl.1991.71.2.644. [DOI] [PubMed] [Google Scholar]
- 23.Newman AB, Haggerty CL, Goodpaster B, et al. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003 Mar;51(3):323–330. doi: 10.1046/j.1532-5415.2003.51105.x. [DOI] [PubMed] [Google Scholar]
- 24.Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA. 1990 Jun 13;263(22):3029–3034. [PubMed] [Google Scholar]
- 25.Fiatarone MA, O'Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994 Jun 23;330(25):1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 26.Binder EF, Yarasheski KE, Steger-May K, et al. Effects of progressive resistance training on body composition in frail older adults: results of a randomized, controlled trial. J Gerontol A Biol Sci Med Sci. 2005 Nov;60(11):1425–1431. doi: 10.1093/gerona/60.11.1425. [DOI] [PubMed] [Google Scholar]
- 27.McCartney N, Hicks AL, Martin J, Webber CE. Long-term resistance training in the elderly: effects on dynamic strength, exercise capacity, muscle, and bone. J Gerontol A Biol Sci Med Sci. 1995 Mar;50(2):B97–104. doi: 10.1093/gerona/50a.2.b97. [DOI] [PubMed] [Google Scholar]
- 28.McCartney N, Hicks AL, Martin J, Webber CE. A longitudinal trial of weight training in the elderly: continued improvements in year 2. J Gerontol A Biol Sci Med Sci. 1996 Nov;51(6):B425–433. doi: 10.1093/gerona/51a.6.b425. [DOI] [PubMed] [Google Scholar]
- 29.Valkeinen H, Hakkinen K, Pakarinen A, et al. Muscle hypertrophy, strength development, and serum hormones during strength training in elderly women with fibromyalgia. Scand J Rheumatol. 2005 Jul–Aug;34(4):309–314. doi: 10.1080/03009740510018697. [DOI] [PubMed] [Google Scholar]
- 30.Ferri A, Scaglioni G, Pousson M, Capodaglio P, Van Hoecke J, Narici MV. Strength and power changes of the human plantar flexors and knee extensors in response to resistance training in old age. Acta Physiol Scand. 2003 Jan;177(1):69–78. doi: 10.1046/j.1365-201X.2003.01050.x. [DOI] [PubMed] [Google Scholar]
- 31.Adams KJ, Swank AM, Berning JM, Sevene-Adams PG, Barnard KL, Shimp-Bowerman J. Progressive strength training in sedentary, older African American women. Med Sci Sports Exerc. 2001 Sep;33(9):1567–1576. doi: 10.1097/00005768-200109000-00021. [DOI] [PubMed] [Google Scholar]
- 32.Latham NK, Bennett DA, Stretton CM, Anderson CS. Systematic review of progressive resistance strength training in older adults. J Gerontol A Biol Sci Med Sci. 2004 Jan;59(1):48–61. doi: 10.1093/gerona/59.1.m48. [DOI] [PubMed] [Google Scholar]
- 33.Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev. 2009;(3):CD002759. doi: 10.1002/14651858.CD002759.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chandler JM, Duncan PW, Kochersberger G, Studenski S. Is lower extremity strength gain associated with improvement in physical performance and disability in frail, community-dwelling elders? Arch Phys Med Rehabil. 1998 Jan;79(1):24–30. doi: 10.1016/s0003-9993(98)90202-7. [DOI] [PubMed] [Google Scholar]
- 35.Galvao DA, Taaffe DR. Resistance exercise dosage in older adults: single-versus multiset effects on physical performance and body composition. J Am Geriatr Soc. 2005 Dec;53(12):2090–2097. doi: 10.1111/j.1532-5415.2005.00494.x. [DOI] [PubMed] [Google Scholar]
- 36.Binder EF, Brown M, Sinacore DR, Steger-May K, Yarasheski KE, Schechtman KB. Effects of extended outpatient rehabilitation after hip fracture: a randomized controlled trial. JAMA. 2004 Aug 18;292(7):837–846. doi: 10.1001/jama.292.7.837. [DOI] [PubMed] [Google Scholar]
- 37.Pahor M, Blair SN, Espeland M, et al. Effects of a physical activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006 Nov;61(11):1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 38.Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. N Engl J Med. 1997 Oct 30;337(18):1279–1284. doi: 10.1056/NEJM199710303371806. [DOI] [PubMed] [Google Scholar]
- 39.Liu-Ambrose T, Khan KM, Eng JJ, Janssen PA, Lord SR, McKay HA. Resistance and agility training reduce fall risk in women aged 75 to 85 with low bone mass: a 6-month randomized, controlled trial. J Am Geriatr Soc. 2004 May;52(5):657–665. doi: 10.1111/j.1532-5415.2004.52200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baker MK, Atlantis E, Fiatarone Singh MA. Multi-modal exercise programs for older adults. Age Ageing. 2007 Jul;36(4):375–381. doi: 10.1093/ageing/afm054. [DOI] [PubMed] [Google Scholar]
- 41.Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2009;(2):CD007146. doi: 10.1002/14651858.CD007146.pub2. [DOI] [PubMed] [Google Scholar]
- 42.Fried LP, Kronmal RA, Newman AB, et al. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. Jama. 1998 Feb 25;279(8):585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 43.Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Van Ness PH. A prehabilitation program for the prevention of functional decline: effect on higher-level physical function. Arch Phys Med Rehabil. 2004 Jul;85(7):1043–1049. doi: 10.1016/j.apmr.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 44.Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Byers A. A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med. 2002 Oct 3;347(14):1068–1074. doi: 10.1056/NEJMoa020423. [DOI] [PubMed] [Google Scholar]
- 45.Binder EF, Schechtman KB, Ehsani AA, et al. Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc. 2002 Dec;50(12):1921–1928. doi: 10.1046/j.1532-5415.2002.50601.x. [DOI] [PubMed] [Google Scholar]
- 46.Bandinelli S, Lauretani F, Boscherini V, et al. A randomized, controlled trial of disability prevention in frail older patients screened in primary care: the FRASI study. Design and baseline evaluation. Aging Clin Exp Res. 2006 Oct;18(5):359–366. doi: 10.1007/bf03324831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peterson MJ, Sloane R, Cohen HJ, Crowley GM, Pieper CF, Morey MC. Effect of telephone exercise counseling on frailty in older veterans: project LIFE. Am J Mens Health. 2007 Dec;1(4):326–334. doi: 10.1177/1557988307306153. [DOI] [PubMed] [Google Scholar]
- 48.Fairhall N, Aggar C, Kurrle SE, et al. Frailty Intervention Trial (FIT) BMC Geriatr. 2008;8:27. doi: 10.1186/1471-2318-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Latham NK, Anderson CS, Lee A, Bennett DA, Moseley A, Cameron ID. A randomized, controlled trial of quadriceps resistance exercise and vitamin D in frail older people: the Frailty Interventions Trial in Elderly Subjects (FITNESS) J Am Geriatr Soc. 2003 Mar;51(3):291–299. doi: 10.1046/j.1532-5415.2003.51101.x. [DOI] [PubMed] [Google Scholar]
- 50.Schneider JK, Eveker A, Bronder DR, Meiner SE, Binder EF. Exercise training program for older adults. Incentives and disincentives for participation. J Gerontol Nurs. 2003 Sep;29(9):21–31. doi: 10.3928/0098-9134-20030901-06. [DOI] [PubMed] [Google Scholar]
- 51.Teri L, Gibbons LE, McCurry SM, et al. Exercise plus behavioral management in patients with Alzheimer disease: a randomized controlled trial. JAMA. 2003 Oct 15;290(15):2015–2022. doi: 10.1001/jama.290.15.2015. [DOI] [PubMed] [Google Scholar]
- 52.www.health.gov/paguidelines/guidelines/default.aspx.


