Summary
Peanuts are a frequent cause of food allergy and the most common cause of fatal food-induced anaphylaxis in the U.S. Advances during the past two years have promoted our understanding of peanut allergens and peanut allergy prevalence, etiology, diagnosis and therapy. The advances highlighted in this review include evidence that the peanut allergens most important in disease differ in different parts of the world, that early oral exposure to peanuts may decrease the frequency of peanut allergy, while early non-oral exposure may have the opposite effect, that complement activation by peanut constituents appears to promote peanut-induced anaphylaxis and that oral immunotherapy, anti-IgE antibody and an herbal formulation are promising approaches for treatment of this disorder.
Introduction
In allergic disorders, the normal protective role of the immune system is perverted by responses designed to protect against pathogens that are made against generally harmless foreign molecules. These responses, which include increased production of Th2 cytokines (IL-4, IL-5, IL-9, IL-13) and inflammatory cytokines (particularly IL-3, TNF and, in some cases, IL-17) induce inflammation characterized by the presence and activation of eosinophils, basophils, mast cells, alternatively activated macrophages and, to a variable extent, neutrophils. Allergy-associated cytokines additionally induce important changes in the function of cells that are not conventionally thought of as elements of the immune system, including epithelial cells (increased permeability and mucus and chemokines production), smooth muscle cells (increased contractility and chemokines production) and vascular endothelial cells (increased permeability and expression of molecules involved in inflammatory cell migration)[1].
The characteristics of an allergic response depend predominantly on the tissues affected and the cytokines produced, which, in turn, determine inflammatory cell and non-immune cell involvement. Of all of the allergic disorders, anaphylaxis, which affects approximately 150,000 Americans a year [2], is probably the most dramatic. In this disease, rapid production of large quantities of vasoactive mediators, including histamine, platelet-activating factor (PAF), leukotrienes and serotonin, as well as Th2 and inflammatory cytokines and proteolytic enzymes by mast cells, basophils and macrophages causes increases in vascular permeability and smooth muscle contraction that can cause urticaria (hives), swelling of the face, mouth, tongue and pharynx (angioedema), diarrhea, asthma, decreased myocardial function, hypotension and, in approximately 1,500 Americans a year, death [3]. Although anaphylaxis can be induced by a variety of antigens delivered through several different routes, the most common causes are insect venoms, drugs and foods [4].
This review is limited to food allergy, which are thought to cause >50,000 cases of anaphylaxis and approximately 100 deaths a year in the U.S. and focuses on allergy to peanuts (Arachis hypogaea), which affects approximately 1% of children and 0.6% of adults in the U.S. [5]. Foods associated with allergy can be broadly divided into 2 groups: those, such as milk and eggs, which are the most common causes of food allergy in infants and young children, but rarely cause food allergy in adults (and rarely cause death) and those, including peanuts, tree nuts, fish and shellfish, that cause food allergy in both children and adults and are more likely to cause severe shock and death. Of the allergens in the second group, peanuts, which are inexpensive and frequently eaten in unmodified form and as components of many different prepared foods, cause the largest number of cases of severe anaphylaxis and death in the U.S. [5]. This and evidence that the incidence of peanut allergy is increasing has led many students of allergy to focus their research efforts on peanut allergens and their clinical effects. This review, an update on these efforts, concentrates on papers published in the past 2 years and comments on observations that have extended our knowledge of peanut allergens and peanut allergy prevalence, etiology, diagnosis, prognosis and treatment.
Peanut allergens
Allergens are typically identified by their ability to bind IgE in the serum of allergic individuals and to activate mast cells and/or basophils that have been sensitized with IgE-containing serum. Based on these criteria, 11 peanut allergens have been identified (Table I). Of these, Ara h 2 and the related allergen, Ara h 6, have been determined to be considerably more potent than Ara h 1 and Ara h 3, the other peanut allergens that bind IgE from a large percentage of peanut allergic individuals [6]. Consequently, some studies aimed at modifying peanuts to reduce their allergenicity have focused on decreasing Ara h 2 and Ara h 6 concentration. Although these studies suggest that this approach is feasible [7], they have not yet tested the basic hypothesis that Ara h 2/h 6-depleted peanuts will be less allergenic than conventional peanuts. One concern about this approach is that different geographically- or ethnically-defined populations are predominantly sensitive to different peanut allergens. Two groups, for example, have demonstrated that Ara h 9, a peanut protein that is relatively unimportant as an allergen in the U.S., is a more important allergen than Ara h 1, Ara h 2, or Ara h 3 for peanut-allergic patients from the Mediterranean area [8,9]. A second concern is that even though the 11 peanut allergens, particularly Ara h2 and Ara h 6, account for the binding of the great majority of the peanut-specific IgE in peanut-allergic individuals (and thus, for the effector phase of peanut allergy), other components may be more critical for the sensitization phase. In this regard, crude peanut extract is considerably more immunogenic in animal studies than any of the purified major allergens tested [10].
Table 1.
Characteristics of Peanut Allergens
| Allergen | Molecular Mass | Characteristics |
|---|---|---|
| Ara h 1 | 63 k-Da | Member of vicilin family of seed storage proteins, a 7S globulin |
| Ara h 2 | 17–19 k-Da | Member of conglutin family of seed storage proteins, a 2S albumin |
| Ara h 3 | 14–45 k-Da, processed from 64 k-Da protein | Member of glycinin family of seed storage proteins; heteromultimeric protein formed from differently proteoltically processed products of the same gene, an 11S globulin |
| Ara h 4 | 37 k-Da | Isoform of Ara h 3 |
| Ara h 5 | 15 k-Da | Member of profilin family of G-actin-binding proteins |
| Ara h 6 | 15 k-Da | Member of conglutin family of seed storage proteins, a 2S albumin |
| Ara h 7 | 17 k-Da | Member of conglutin family of seed storage proteins, a 2S albumin |
| Ara h 8 | 16 k-Da | Homologous to major birch pollen allergen, Bet v 1 and other pathogenesis-related proteins |
| Ara h 9 | 9.8 k-Da | Lipid transfer protein |
| Ara h 10 | 16 k-Da | Oleosin seed storage protein |
| Ara h 11 | 14 k-Da | Oleosin seed storage protein |
Studies aimed at defining the epitopes of Ara h 2 that are bound by IgE from peanut-allergic individuals have demonstrated that most IgE binds to conformational epitopes (i.e.; 3-dimensional epitopes generated by the native folding of the Ara h 2 polypeptide) rather than by linear epitopes (i.e.; epitopes generated by consecutive amino acids that does not depend on polypeptide folding). It is not known how this relates to previous evidence that allergic symptoms are usually worst in individuals who have relatively high concentrations of IgE specific for linear epitopes, especially multiple linear epitopes [5].
Prevalence
Several studies have evaluated the prevalence of peanut allergy in the U.S. and Canada and investigated whether prevalence is changing. In one study, 1.4% of subjects in the US reported peanut or tree nut allergy in a random telephone survey [11]. This percentage has not increased for adults, but increased for children less than 18 years old from 0.6% in 1997 to 1.2% in 2002 and 2.1% in 2007. 1% of individuals reported peanut allergy in a similar survey in Canada [12]. Consistent with an increase in peanut allergy prevalence in children, an analysis of hospitalization rates for peanut-related anaphylaxis in New York state showed a >4-fold increase from 1990 to 2006 for individuals <20 years old [12]. Another study, however, indicated that not all “peanut-allergic” individuals actually develop allergic symptoms following peanut ingestion. Only 22% of 8 year old children considered to be peanut-sensitized on the basis of skin prick testing or IgE measurements had a positive response to oral peanut challenge. IgE and skin prick responses to Ara h 2 were the best predictor of a positive response to oral challenge in this study [13]. Other studies evaluated clinical implications of peanut allergy. For example, a retrospective chart review study found that children with asthma who had peanut allergy had 2.32-fold more frequent hospitalizations for asthma and a 1.59-fold greater rate of systemic steroid use than asthmatic children without peanut allergy [14]. Additionally, a survey of parents indicated a 6–7 fold increased incidence (8.5%) of peanut allergy in siblings of peanut-allergic patients compared to siblings of children not allergic to peanuts [15]. The authors of this study suggested this frequency was sufficiently high to justify testing of siblings of peanut-allergic individuals before they are allowed to eat peanut-containing foods.
Etiology
Two important British studies evaluated the relationship of peanut exposure to the development of peanut allergy. The first of these demonstrated that the prevalence of peanut allergy in Israeli Jewish children was <10% than of Jewish children in the UK [16]. It was hypothesized that this difference may be related to the early consumption of a peanut product (bamba, made from peanut butter and puffed corn) by Israeli infants, while infants in the UK have avoided peanuts. In contrast, increased non-oral exposure to peanuts, especially peanut butter, during infancy was associated with increased risk of sensitization to peanuts, although maternal peanut consumption during pregnancy or lactation did not have a detectable effect [17]. Because these studies suggest, but do not prove that ingestion of peanuts or peanut products early in life protects against the development of peanut allergy [18, 19], a prospective trial has been initiated to test this hypothesis with a cohort of infants who are at high risk for food allergy.
Mouse model studies published during the past 2 years provide mechanistic insight into peanut allergy. IgE, mast cells and IL-13 were shown to be required for peanut allergy development [20]. Although the requirements for IgE and mast cells are similar to those demonstrated in previous murine food allergy models, the requirement for IL-13 had not previously been shown and suggests that IL-13 effects on vascular and epithelial cell permeability and smooth muscle contractility may be critical factors in food allergy pathophysiology. If so, IL-13 antagonists (along with IgE and mast cell antagonists) may be useful food allergy therapeutics. A separate study revealed that aqueous extracts of peanuts and tree nuts, but neither milk nor eggs, have the ability to activate complement in both mouse and human plasma [21]. This led to the production of C3a, which induces basophils and macrophages to produce PAF and mast cells, to a lesser extent, to produce histamine in in vivo mouse experiments (Figure 1). Although the vasoactive mediators produced by this mechanism were insufficient to cause shock in otherwise untreated mice, they caused shock in mice made more sensitive to vasoactive mediators by pretreatment with IL-4 and a β-adrenergic antagonist. More importantly, peanut extract acted through a complement-dependent mechanism to considerably exacerbate the severity of IgE-mediated anaphylaxis in mice. Taken together, these observations suggest that complement activation by peanut components (and by a number of other allergens, such as some insect venoms [22]), contributes importantly to their ability to induce anaphylaxis. This, along with other peanut characteristics, such as peanut allergen resistance to digestion [23,24], increased availability of peanut allergens caused by the roasting process [25] and dendritic cell activation by Ara h 1 [26] may explain why peanuts are so much more likely to cause severe anaphylaxis than most other foods. In addition, because activated complement can act as an adjuvant [27], complement activation by peanut components may contribute to the sensitization phase, as well as the elicitation phase of peanut allergy.
Figure 1. Peanut activation of complement exacerbates anaphylaxis.
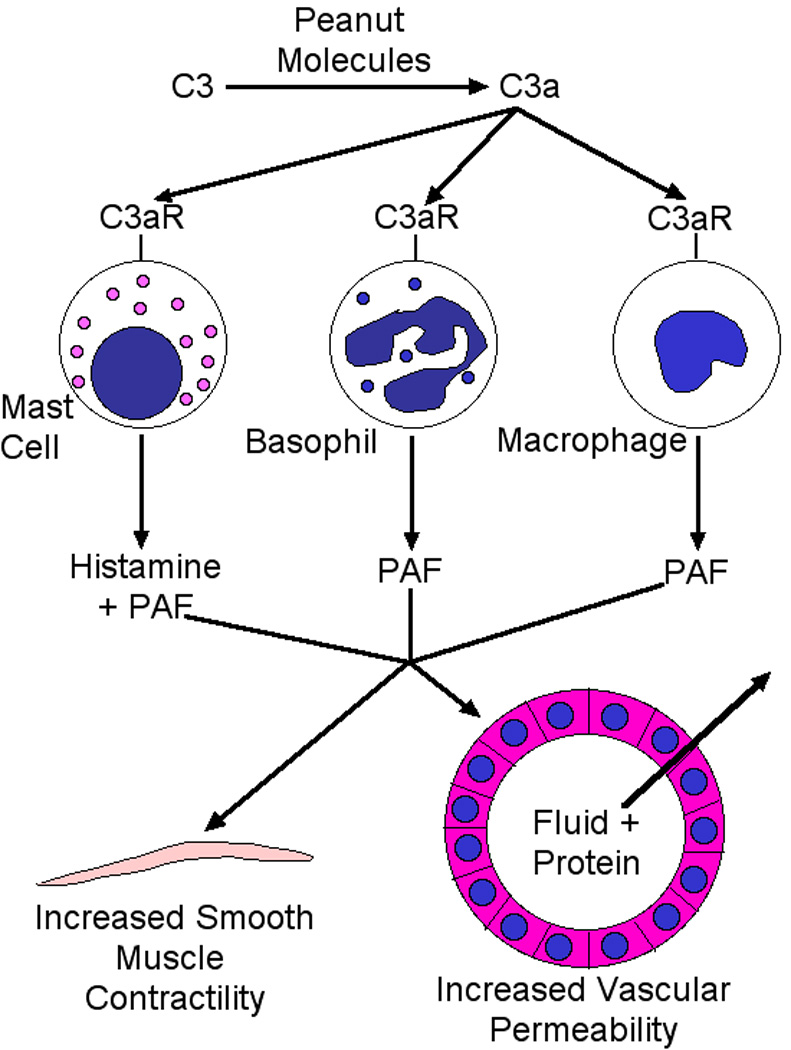
Peanut molecules activate complement, causing production of C3a, which acts through C3a receptors on mast cells, basophils and macrophages to induce production of PAF and histamine, which directly and indirectly increase vascular permeability and smooth muscle contractility.
An unrelated line of investigation has shed light on the etiology of a specific allergic disorder, eosinophilic esophagitis. Patients with this disorder, which is characterized histologically by an increased number of eosinophils in the esophagus, typically have severe heartburn that does not respond well to acid neutralization [28]. These patients usually are allergic to multiple foods, including peanuts [29]. Unlike patients with more typical peanut allergy, whose T cells produce considerably more IL-4 than IL-5 in response to peanut allergen stimulation, T cells from peanut-allergic patients with eosinophilic eosphagitis also produce large amounts of IL-5 [30]. The ability of IL-5 to promote eosinophilopoiesis, survival, migration and activation and the ability of anti-IL-5 monoclonal antibody to ameliorate eosinophilic esophagitis in clinical trials [31] provides evidence that this increased T cell IL-5 production leads to the development of eosinophilic eosphagitis rather than more typical food allergy.
Diagnosis and prognosis
Although the gold standard for diagnosing peanut allergy is a double-blinded oral food challenge, an Australian study demonstrated that for children, peanut skin prick test wheal size and specific IgE level, but not history of peanut-induced anaphylaxis, were predictive of the severity of allergic response to oral challenge [32]. This observation should be useful for determining which patients should be given an oral challenge and what precautions should be taken.
Treatment
Advances were reported in the use of oral immunotherapy, anti-IgE monoclonal antibody treatment, and treatment with an herbal formulation based on traditional Chinese medicine. Oral immunotherapy over 7 months, in one study, was able to increase tolerance for peanuts an average of 5-fold in 18/22 allergic children age 3 – 14. Mild to moderate side effects accompanied 2.6% of doses and symptoms of pulmonary obstruction developed in 1.3%. Oral immunotherapy was associated with an increase in peanut-specific IgG4 levels and decreased peanut specific IL-2, IL-4 and IL-5 by peripheral blood mononuclear cells. Oral immunotherapy was discontinued in 4 of 22 patients because of adverse effects [33]. In a second study, an oral immunotherapy protocol for children that featured initial day escalation, buildup and maintenance phases was well tolerated and successful for >90% of patients. Decreases in responses to skin prick testing, basophil activation and peanut-specific IgE were found along with increases in peanut-specific IgG4, IL-4, IL-10, IFN-γ and TNF secretion. Foxp3+ T cells first increased, then decreased [34]. 20 of 22 patients completed a third, similar study that included home dosing. Upper respiratory tract and abdominal symptoms were experienced by most patients during the initial escalation day. Symptoms were less common during the buildup phase and occurred with only 3.5% of home doses. Only 0.7% of home doses caused symptoms that required treatment, which included 1 dose of epinephrine each for 2 subjects [35]. One additional caution to the use of oral immunotherapy is that peanut allergy has been observed to recur even during regular ingestion of significant amounts of peanut protein [36]. Because the relative risks of oral immunotherapy vs. avoidance, optimal dosing regimen, optimal patient selection, and optimal patient selection post-desensitization remain uncertain, experts in this area have suggested that oral immunotherapy for peanut allergy is not ready for clinical use [37].
One study of asthmatic patients treated with omalizumab, an IgE-specific monoclonal antibody that prevents IgE binding to FcεRI and is FDA-approved for treatment of asthma, but not food allergy, reported increased tolerance for food allergens, including peanuts, after 12–24 weeks of treatment in patients who had food allergy as well as asthma [38]. This seems to confirm the results of a previous study with a different anti-IgE monoclonal antibody [39]. Unfortunately, a separate report described decreased efficacy of omalizumab treatment in patients in whom a high percentage of total serum IgE was specific for the offending antigen and noted that 33% of peanut-allergic patients fell in this category [40]. In addition, omalizumab itself has been reported to induce an unusual form of slow-developing anaphylaxis [41].
Two studies of an herbal formulation, Food Allergy Herbal Formula-2 (FAHF-2) provided promising results. The first, a mouse model study, demonstrated that FAHF-2 treatment of peanut-allergic mice protected them from anaphylaxis for >36 weeks after discontinuing treatment. Effects of treatment included decreased peanut-specific IgE and Th2 cytokine production and increased IgG2a and IFN-γ production by CD8+ T cells. Efficacy was CD8+ T cell and IFN-γ-dependent, suggesting that FAHF-2 induces CD8+ T cell production of IFN-γ, which is known to suppress IgE and Th2 cytokine production and effects [42]. The second study, a phase I, randomized double-blinded, placebo-controlled dose-escalation trial of FAHF-2 showed no unacceptable toxicity as well as decreased IL-5 and increased IFN-γ and IL-10 production by PBMCs [43]. Thus, there is reason to hope that this apparently safe therapy may be useful for treatment of human food allergy and enable identification of specific constituents that can be used therapeutically.
A final report showed that earlier observations that PAF antagonists suppress food allergy symptoms in mouse models [44] are applicable in a mouse model of peanut allergy. Additionally, although histamine or leukotriene antagonists, by themselves, had no therapeutic effect, the combination of histamine and PAF antagonists was more effective than PAF anagonists alone [45]. Combined with other animal model studies [46] and a recent human study that shows increased serum PAF during human anaphylaxis and more severe anaphylaxis in individuals who slowly catabolize this mediator [47], this mouse study creates a rationale to develop PAF antagonists (which were previously tested and found inefficacious as asthma therapeutics [48]) for use as prophylactics in suppression of food allergy.
Conclusion
Work performed during the past 10 years, including the recent studies highlighted in this review, have improved our understanding of the epidemiology, etiology and pathophysiology of peanut allergy and anaphylaxis, but have not yet led to a novel, accepted therapy for this disease. Future studies are likely to apply these recent advances to the development of prophylactic and therapeutic approaches that are practical and safer and more convenient than peanut avoidance.
Acknowledgements
Our own work in the field of anaphylaxis has been supported by a Merit Award from the United States Department of Veterans Affairs, National Institutes of Health Grant R21 AI079947, and a grant from the Food Allergy and Anaphylaxis Network. I thank my colleagues, Marat Khodoun, Marc Rothenberg, Simon Hogan, and Eric Brandt for contributions to our research and my views on the subjects discussed in this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The author is an Associate Editor of the Journal of Allergy and Clinical Immunology, but has no financial or other relationships that could constitute a conflict of interest in relation to this paper.
References
- 1.Finkelman FD, Hogan SP, Hershey GK, Rothenberg ME, Wills-Karp M. Importance of cytokines in murine allergic airway disease and human asthma. J Immunol. 2010;184:1663–1674. doi: 10.4049/jimmunol.0902185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark S, Camargo CA., Jr. Epidemiology of anaphylaxis. Immunol Allergy Clin North Am. 2007;27:145–163. doi: 10.1016/j.iac.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Matasar MJ, Neugut AI. Epidemiology of anaphylaxis in the United States. Curr Allergy Asthma Rep. 2003;3:30–35. doi: 10.1007/s11882-003-0007-8. [DOI] [PubMed] [Google Scholar]
- 4. Simons FE. Anaphylaxis. J Allergy Clin Immunol. 2010;125:S161–S181. doi: 10.1016/j.jaci.2009.12.981.. An excellent broad review of the field of anaphylaxis.
- 5. Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2010;125:S116–S125. doi: 10.1016/j.jaci.2009.08.028.. An up-to-date review of food allergy by two experts in this area.
- 6. Blanc F, Adel-Patient K, Drumare MF, Paty E, Wal JM, Bernard H. Capacity of purified peanut allergens to induce degranulation in a functional in vitro assay: Ara h 2 and Ara h 6 are the most efficient elicitors. Clin Exp Allergy. 2009;39:1277–1285. doi: 10.1111/j.1365-2222.2009.03294.x.. Provides evidence that Ara h 2 and the related allergen, Ara h 6, are the two most potent and immunogenic peanut allergens.
- 7.Dodo HW, Konan KN, Chen FC, Egnin M, Viquez OM. Alleviating peanut allergy using genetic engineering: the silencing of the immunodominant allergen Ara h 2 leads to its significant reduction and a decrease in peanut allergenicity. Plant Biotechnol J. 2008;6:135–145. doi: 10.1111/j.1467-7652.2007.00292.x. [DOI] [PubMed] [Google Scholar]
- 8. Krause S, Reese G, Randow S, Zennaro D, Quaratino D, Palazzo P, Ciardiello MA, Petersen A, Becker WM, Mari A. Lipid transfer protein (Ara h 9) as a new peanut allergen relevant for a Mediterranean allergic population. J Allergy Clin Immunol. 2009;124:771–778. doi: 10.1016/j.jaci.2009.06.008.. Provides evidence that Ara h 9 is the most important peanut allergen in the Mediterranean area.
- 9. Lauer I, Dueringer N, Pokoj S, Rehm S, Zoccatelli G, Reese G, Miguel-Moncin MS, Cistero-Bahima A, Enrique E, Lidholm J, et al. The non-specific lipid transfer protein, Ara h 9, is an important allergen in peanut. Clin Exp Allergy. 2009;39:1427–1437. doi: 10.1111/j.1365-2222.2009.03312.x.. Provides evidence that Ara h 9 is the most important peanut allergen in the Mediterranean area.
- 10.van Wijk F, Nierkens S, Hassing I, Feijen M, Koppelman SJ, de Jong GA, Pieters R, Knippels LM. The effect of the food matrix on in vivo immune responses to purified peanut allergens. Toxicol Sci. 2005;86:333–341. doi: 10.1093/toxsci/kfi187. [DOI] [PubMed] [Google Scholar]
- 11.Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125:1322–1326. doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Shoshan M, Harrington DW, Soller L, Fragapane J, Joseph L, St Pierre Y, Godefroy SB, Elliot SJ, Clarke AE. A population-based study on peanut, tree nut, fish, shellfish, and sesame allergy prevalence in Canada. J Allergy Clin Immunol. 2010;125:1327–1335. doi: 10.1016/j.jaci.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Nicolaou N, Poorafshar M, Murray C, Simpson A, Winell H, Kerry G, Harlin A, Woodcock A, Ahlstedt S, Custovic A. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol. 2010;125:191–197. doi: 10.1016/j.jaci.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Simpson AB, Yousef E, Hossain J. Association between peanut allergy and asthma morbidity. J Pediatr. 2010;156:777–781. doi: 10.1016/j.jpeds.2009.11.080. [DOI] [PubMed] [Google Scholar]
- 15.Liem JJ, Huq S, Kozyrskyj AL, Becker AB. Should younger siblings of peanut-allergic children be assessed by an allergist before being fed peanut? Allergy Asthma Clin Immunol. 2008;4:144–149. doi: 10.1186/1710-1492-4-4-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Du Toit G, Katz Y, Sasieni P, Mesher D, Maleki SJ, Fisher HR, Fox AT, Turcanu V, Amir T, Zadik-Mnuhin G, et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol. 2008;122:984–991. doi: 10.1016/j.jaci.2008.08.039.. A comparison of the prevalence of peanut allergy among Israeli Jews, who eat peanut-containing food in infancy with its prevalence among British Jews, who generally refrain from eating peanuts until they are older, suggests that early peanut ingestion may be protective.
- 17. Fox AT, Sasieni P, du Toit G, Syed H, Lack G. Household peanut consumption as a risk factor for the development of peanut allergy. J Allergy Clin Immunol. 2009;123:417–423. doi: 10.1016/j.jaci.2008.12.014.. Suggests that non-oral exposure to peanuts and peanut products early in life increases the risk of developing peanut allergy.
- 18.Thompson RL, Miles LM, Lunn J, Devereux G, Dearman RJ, Strid J, Buttriss JL. Peanut sensitization and allergy: influence of early life exposure to peanuts. Br J Nutr. 2010;103:1278–1286. doi: 10.1017/S000711450999376X. [DOI] [PubMed] [Google Scholar]
- 19. Burks AW. Early peanut consumption: postpone or promote? J Allergy Clin Immunol. 2009;123:424–425. doi: 10.1016/j.jaci.2008.12.015.. A well thought-out editorial about this important issue.
- 20. Wang M, Takeda K, Shiraishi Y, Okamoto M, Dakhama A, Joetham A, Gelfand EW. Peanut-induced intestinal allergy is mediated through a mast cell-IgEFc RI-IL-13 pathway. J Allergy Clin Immunol. 2010 doi: 10.1016/j.jaci.2010.05.017. epub ahead of print.. Indicates a role for IL-13, which does not directly influence IgE production or mast cells in mice, in peanut-induced intestinal allergy.
- 21. Khodoun M, Strait R, Orekov T, Hogan S, Karasuyama H, Herbert DR, Kohl J, Finkelman FD. Peanuts can contribute to anaphylactic shock by activating complement. J Allergy Clin Immunol. 2009;123:342–351. doi: 10.1016/j.jaci.2008.11.004.. Demonstrates that complement activation by peanut components contributes to development of anaphylaxis through a C3a- and PAF-dependent mechanism.
- 22.van der Linden PW, Hack CE, Kerckhaert JA, Struyvenberg A, van der Zwan JC. Preliminary report: complement activation in wasp-sting anaphylaxis. Lancet. 1990;336:904–906. doi: 10.1016/0140-6736(90)92272-j. [DOI] [PubMed] [Google Scholar]
- 23.Sen M, Kopper R, Pons L, Abraham EC, Burks AW, Bannon GA. Protein structure plays a critical role in peanut allergen stability and may determine immunodominant IgE-binding epitopes. J Immunol. 2002;169:882–887. doi: 10.4049/jimmunol.169.2.882. [DOI] [PubMed] [Google Scholar]
- 24.Maleki SJ, Kopper RA, Shin DS, Park CW, Compadre CM, Sampson H, Burks AW, Bannon GA. Structure of the major peanut allergen Ara h 1 may protect IgE-binding epitopes from degradation. J Immunol. 2000;164:5844–5849. doi: 10.4049/jimmunol.164.11.5844. [DOI] [PubMed] [Google Scholar]
- 25.Kopper RA, Odum NJ, Sen M, Helm RM, Stanley JS, Burks AW. Peanut protein allergens: the effect of roasting on solubility and allergenicity. Int Arch Allergy Immunol. 2005;136:16–22. doi: 10.1159/000082580. [DOI] [PubMed] [Google Scholar]
- 26.Shreffler WG, Castro RR, Kucuk ZY, Charlop-Powers Z, Grishina G, Yoo S, Burks AW, Sampson HA. The major glycoprotein allergen from Arachis hypogaea, Ara h 1, is a ligand of dendritic cell-specific ICAM-grabbing nonintegrin and acts as a Th2 adjuvant in vitro. J Immunol. 2006;177:3677–3685. doi: 10.4049/jimmunol.177.6.3677. [DOI] [PubMed] [Google Scholar]
- 27.Van den Berg CW, Hazenberg MA, Hofhuis FM, Van Rooyen SM, Van Dijk H. C3-and T-cell-dependent adjuvant activity of in vivo formed immune complexes. Immunology. 1991;73:264–270. [PMC free article] [PubMed] [Google Scholar]
- 28.DeBrosse CW, Collins MH, Buckmeier Butz BK, Allen CL, King EC, Assa'ad AH, Abonia JP, Putnam PE, Rothenberg ME, Franciosi JP. Identification, epidemiology, and chronicity of pediatric esophageal eosinophilia, 1982–1999. J Allergy Clin Immunol. 2010;126:112–119. doi: 10.1016/j.jaci.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erwin EA, James HR, Gutekunst HM, Russo JM, Kelleher KJ, Platts-Mills TA. Serum IgE measurement and detection of food allergy in pediatric patients with eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2010;104:496–502. doi: 10.1016/j.anai.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prussin C, Lee J, Foster F. Eosinophilic gastrointestinal disease and peanut allergy are alternatively associated with IL-5+ and IL-5− Th2 responses. J Allergy Clin Immunol. 2009;124:1326–1332. doi: 10.1016/j.jaci.2009.09.048.. Demonstrates that differential production of IL-5 may be responsible for the alternative development of conventional peanut allergy vs. eosinophilic esophagitis.
- 31.Stein ML, Collins MH, Villanueva JM, Kushner JP, Putnam PE, Buckmeier BK, Filipovich AH, Assa'ad AH, Rothenberg ME. Anti-IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J Allergy Clin Immunol. 2006;18:1312–1319. doi: 10.1016/j.jaci.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Wainstein BK, Studdert J, Ziegler M, Ziegler JB. Prediction of anaphylaxis during peanut food challenge: usefulness of the peanut skin prick test (SPT) and specific IgE level. Pediatr Allergy Immunol. 2010;21:603–611. doi: 10.1111/j.1399-3038.2010.01063.x. [DOI] [PubMed] [Google Scholar]
- 33. Blumchen K, Ulbricht H, Staden U, Dobberstein K, Beschorner J, de Oliveira LC, Shreffler WG, Sampson HA, Niggemann B, Wahn U, et al. Oral peanut immunotherapy in children with peanut anaphylaxis. J Allergy Clin Immunol. 2010;126:83–91. doi: 10.1016/j.jaci.2010.04.030.. One of three excellent papers cited that demonstrate the efficacy and risks of oral immunotherapy for peanut allergy.
- 34. Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, Shreffler WG, Steele P, Henry KA, Adair M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124:292–300. doi: 10.1016/j.jaci.2009.05.022.. One of three excellent papers cited that demonstrate the efficacy and risks of oral immunotherapy for peanut allergy.
- 35. Hofmann AM, Scurlock AM, Jones SM, Palmer KP, Lokhnygina Y, Steele PH, Kamilaris J, Burks AW. Safety of a peanut oral immunotherapy protocol in children with peanut allergy. J Allergy Clin Immunol. 2009;124:286–291. doi: 10.1016/j.jaci.2009.03.045.. One of three excellent papers cited that demonstrate the efficacy and risks of oral immunotherapy for peanut allergy.
- 36.Boyle RJ, Tang ML. Recurrent peanut allergy may not be prevented by continued peanut ingestion. Int Arch Allergy Immunol. 2008;147:260–262. doi: 10.1159/000142051. [DOI] [PubMed] [Google Scholar]
- 37. Thyagarajan A, Varshney P, Jones SM, Sicherer S, Wood R, Vickery BP, Sampson H, Burks AW. Peanut oral immunotherapy is not ready for clinical use. J Allergy Clin Immunol. 2010;126:31–32. doi: 10.1016/j.jaci.2010.05.012.. A well-balanced editorial that aims to provide a consensus about this subject.
- 38.Rafi A, Do LT, Katz R, Sheinkopf LE, Simons CW, Klaustermeyer W. Effects of omalizumab in patients with food allergy. Allergy Asthma Proc. 2010;31:76–83. doi: 10.2500/aap.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 39.Leung DY, Sampson HA, Yunginger JW, Burks AW, Jr., Schneider LC, Wortel CH, Davis FM, Hyun JD, Shanahan WR., Jr. Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med. 2003;348:986–993. doi: 10.1056/NEJMoa022613. [DOI] [PubMed] [Google Scholar]
- 40.Hamilton RG, MacGlashan DW, Jr., Saini SS. IgE antibody-specific activity in human allergic disease. Immunol Res. 2010;47:273–284. doi: 10.1007/s12026-009-8160-3. [DOI] [PubMed] [Google Scholar]
- 41.Cox L, Platts-Mills TA, Finegold I, Schwartz LB, Simons FE, Wallace DV. American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma and Immunology Joint Task Force Report on omalizumabassociated anaphylaxis. J Allergy Clin Immunol. 2007;120:1373–1377. doi: 10.1016/j.jaci.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 42. Srivastava KD, Qu C, Zhang T, Goldfarb J, Sampson HA, Li XM. Food Allergy Herbal Formula-2 silences peanut-induced anaphylaxis for a prolonged posttreatment period via IFN-γ-producing CD8+ T cells. J Allergy Clin Immunol. 2009;123:443–451. doi: 10.1016/j.jaci.2008.12.1107.. A mouse model study that provides evidence for efficacy and mechanism of action of this herbal formulation for the treatment of peanut-induced anaphylaxis.
- 43.Wang J, Patil SP, Yang N, Ko J, Lee J, Noone S, Sampson HA, Li XM. Safety, tolerability, and immunologic effects of a food allergy herbal formula in food allergic individuals: a randomized, double-blinded, placebo-controlled, dose escalation, phase 1 study. Ann Allergy Asthma Immunol. 2010;105:75–84. doi: 10.1016/j.anai.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, Zimmermann N, Finkelman FD, Rothenberg ME. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest. 2003;112:1666–1677. doi: 10.1172/JCI19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Arias K, Baig M, Colangelo M, Chu D, Walker T, Goncharova S, Coyle A, Vadas P, Waserman S, Jordana M. Concurrent blockade of platelet-activating factor and histamine prevents life-threatening peanut-induced anaphylactic reactions. J Allergy Clin Immunol. 2009;124:307–314. doi: 10.1016/j.jaci.2009.03.012.. Mouse model study that provides additional impetus for development of PAF anatagonists as prophylactics against food allergy.
- 46.Strait RT, Morris SC, Yang M, Qu XW, Finkelman FD. Pathways of anaphylaxis in the mouse. J Allergy Clin Immunol. 2002;109:658–668. doi: 10.1067/mai.2002.123302. [DOI] [PubMed] [Google Scholar]
- 47. Vadas P, Gold M, Perelman B, Liss GM, Lack G, Blyth T, Simons FE, Simons KJ, Cass D, Yeung J. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med. 2008;358:28–35. doi: 10.1056/NEJMoa070030.. Provides strong direct and indirect evidence that PAF has an important role in human anaphylaxis.
- 48.Gomez FP, Rodriguez-Roisin R. Platelet-activating factor antagonists: current status in asthma. BioDrugs. 2000;14:21–30. doi: 10.2165/00063030-200014010-00003. [DOI] [PubMed] [Google Scholar]


