Abstract
The clinical syndrome of aspirin-exacerbated respiratory disease (AERD) is a condition where inhibition of cyclooxygenase-1 (COX-1) induces attacks of upper and lower airway reactions, including rhinorrhea and varying degrees of bronchospasm and laryngospasm. Although the reaction is not IgE-mediated, patients can also present with anaphylactic hypersensitivity reactions, including hypotension, after exposure to COX-1 inhibiting drugs. All patients with AERD have underlying nasal polyps and intractable sinus disease which may be difficult to treat with standard medical and surgical interventions. This review article focuses on the management of AERD patients with a particular emphasis on aspirin desensitization and continuous treatment with aspirin.
Keywords: Aspirin-exacerbated respiratory disease, aspirin desensitization, aspirin sensitivity, chronic sinusitis, asthma, nasal polyps, Samter's triad
INTRODUCTION
What is aspirin-exacerbated respiratory disease?
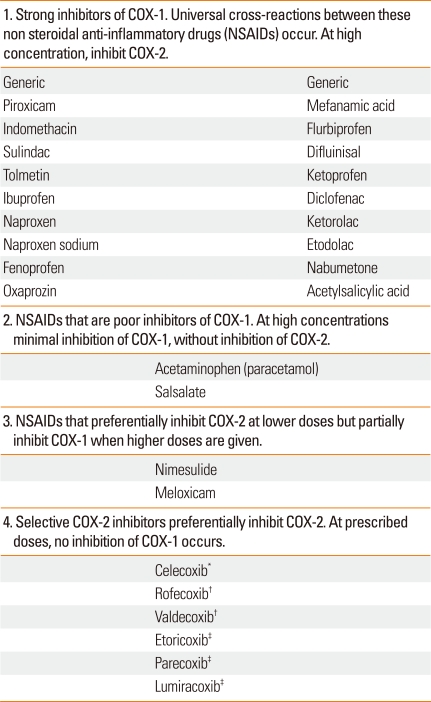
Aspirin-exacerbated respiratory disease (AERD) is a clinical tetrad of nasal polyps, chronic hypertrophic eosinophilic sinusitis, asthma and sensitivity to any medication that inhibits cyclooxygenase-1 (COX-1) enzymes, namely aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) (Table 1).1,2 Ingestion of aspirin, and most NSAIDs, results in a spectrum of upper and/or lower respiratory reactions, to include rhinitis, conjunctivitis, laryngospasm and bronchospasm.1,2 AERD affects 0.3-0.9% of the general population, but the prevalence rises to 10-20% of asthmatics and up to 30-40% in those asthmatics with nasal polyposis.3-7 The average age of onset is 34 years in a US study and is thought to be acquired between teenage to middle adulthood years with no ethnic predilection and rare familial associations.3-7 AERD is more commonly reported in females (57% vs. 43%).7,8
Table 1.
Four classes of NSAIDs based upon their pharmacologic function
*Available worldwide; †removed from the world market 2004 and 2005; ‡available outside the USA.
Clinical features of AERD
AERD has been described as "Samter's triad, aspirin induced asthma, aspirin sensitive asthma and aspirin hypersensitivity".1,9 Despite the fact that it was first described in 1922 by Widal et al.10, the pathophysiology of AERD is only partially understood and is not the focus of this review; however two reviews on this topic were recently published by Stevenson.2,11
Clinical features of AERD include onset of nasal congestion with anosmia and progression to chronic pansinusitis and nasal polyps which re-grow rapidly after surgery.2,7 Nocturnal nasal obstruction with sleep deprivation fatigue occurs routinely in these patients. Asthma may precede the upper airway disease or develop later. CT or plain radiographs of the sinuses reveal complete opacification in nearly all AERD patients.2,12 Normal imaging of the sinuses essentially rules out the diagnosis of AERD.
Diagnosis of AERD
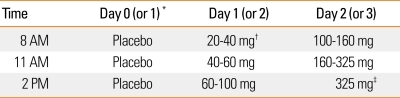
Aspirin challenge is the gold standard for diagnosing AERD.1,2,13 There are four routes of provocation challenges: oral, bronchial inhalation, nasal inhalation and intravenous.13-17 In the United States oral aspirin challenges are performed. The protocol used at the Scripps Clinic is found in Table 2.
Table 2.
Oral aspirin challenges in patients with suspected aspirin exacerbated respiratory disease
*A placebo challenge can be conducted the week before. Alternatively, if the patient's baseline FEV1 is the same as their prior best value and they have not used their albuterol rescue inhaler in the past week, you can skip the one day placebo challenge. †Using a pill cutter, 81 mg ASA tablet can be cut into a half or a fourth. ‡If patient has not reacted to 325 mg of ASA, they will not react to 650 mg. Therefore, if no reaction occurs in 3 hours after ASA 325 mg call it a negative challenge.
(1) Measure FEV1 every hour and wait three hours between doses.
(2) FEV1 should be at least 1.5 L and > 60% of predicted.
(3) Reactions can be:
- Naso-ocular alone
- Naso-ocular and a 15% or > decline in FEV1 (Classic reaction)
- Lower respiratory reaction only (FEV1 declines by >20%)
- Laryngospasm with or without a, b, c (flat or notched inspiratory curve)
- Systemic reaction: hives, flush, gastric pain, hypotension
(4) Aspirin desensitization
- After a reaction has been treated and resolved go to b.
- Repeat the ASA provoking dose.
- If no reaction, continue to escalate the doses as above.
- At 325 mg of ASA, desensitization is always completed.
- Give 650 mg as first dose and then treat with 650 mg bid.
History of an asthma attack following ingestion of aspirin or other NSAIDs is suggestive and sometimes diagnostic. However, 16% of patients who believed they had AERD, based upon a historical asthma attack after ingesting aspirin/NSAID, underwent negative oral aspirin challenges and therefore, did not meet criteria for AERD.18 On the other hand, in that same study, of patients who had nasal polyps, chronic sinusitis, asthma and were avoiding aspirin and NSAIDs, only 43% had positive oral aspirin challenges.18 Thus, AERD is both over diagnosed (coincidental history) or under diagnosed (no prior exposure to aspirin or COX-1 inhibitor during the time of the inflammatory respiratory disease).
TREATMENT OPTIONS FOR AERD
Avoidance or desensitization
Once patients are diagnosed, management options are limited to either complete avoidance of COX-1 inhibiting drugs or aspirin desensitization and continuous aspirin therapy.2,13 In order to completely avoid COX-1 inhibitors, it is essential that clinicians are familiar with cross-reacting NSAIDS that inhibit COX-1 (Table 1) and that they educate patients on avoidance of all the various cross-reacting NSAIDS. On the other hand, it is important to note that there are other NSAIDs that either partially inhibit COX-1 or are selective for COX-2, such as celecoxib, which lacks cross-reactivity with COX-1 inhibitors (Table 1). Well designed studies have shown that therapeutic doses of selective COX-2 inhibitors do not cross react with aspirin or other NSAIDs and therefore, can safely be given to patients with AERD.19-21 Due to the rare possibility of other types of reaction to COX-2 inhibitors, we recommend giving the first full dose in the physician's office.
Avoidance of aspirin is not always possible due to need for aspirin in the management cardiovascular diseases. In addition, even with avoidance of aspirin and NSAIDs, AERD patients usually experience progressive airways disease, despite aggressive surgical intervention and topical or systemic anti-inflammatory treatment with corticosteroids and leukotriene modifiers.1,2,7,8 Therefore, the purpose of this review will be to discuss the specific issues related to aspirin desensitization, the clinical benefits, patient selection, aspirin dosing, potential adverse reactions and newer methods that may improve on current protocols for aspirin desensitization.
CLINICAL EFFECTIVENESS AND THE BENEFITS OF ASPIRIN DESENSITIZATION IN AERD
Why recommend aspirin desensitization and therapy?
Multiple studies have shown that desensitization and daily treatment with aspirin can significantly improve overall symptoms and quality of life, decrease formation of nasal polyps and sinus infections, reduce the need for oral corticosteroids and sinus surgery and improve nasal and asthma scores in patient with AERD at both 6 months and after one year of therapy (P<0.0001).22-25 In a study from an otolaryngology clinic comparing outcomes of AERD patients who had surgery with aspirin desensitization versus surgery alone, none of the aspirin desensitized patients needed revision of their surgery, while 80% of the patients who did not undergo desensitization required additional surgery over a 2 year period (P=0.003).26
How soon can you expect clinical improvement?
Significant clinical improvement is seen in as little as 4 weeks after treatment with nasal scores, sense of smell and asthma scores improving significantly (P<0.0001) and for those taking systemic corticosteroids, prednisone doses decrease from an average of 10.7 mg to 5.9 mg daily (P=0.0003).27 From personal experience, there is a marked flattening of nasal membranes immediately after aspirin desensitization and many patients often note improvement in congestion and sense of smell within 24-48 hours after completing aspirin desensitization. Improvement in smell is more complicated, since the mechanism of compression of olfactory nerves by inflamed nasal tissues leads to nerve damage. Even when mucosal tissues return to normal, olfactory nerves may not regenerate.
Aspirin desensitization is cost effective
In addition to the clinical effectiveness of aspirin therapy, Shaker et al.28 performed a healthcare cost analysis to model the costs of aspirin desensitization for therapeutic and prophylactic use and found it to be cost-effective, even when factoring in the up front cost of aspirin desensitization. From an economic perspective, there is a substantial reduction of medical and surgical costs in the years following aspirin desensitization and daily aspirin treatment. Aspirin is also much less expensive for both treatment of primary and secondary prophylaxis in cardiovascular disease compared to other anti-platelet agents.28
PATIENT SELECTION AND OPTIMIZING DESENSITIZATION
Who should be desensitized?
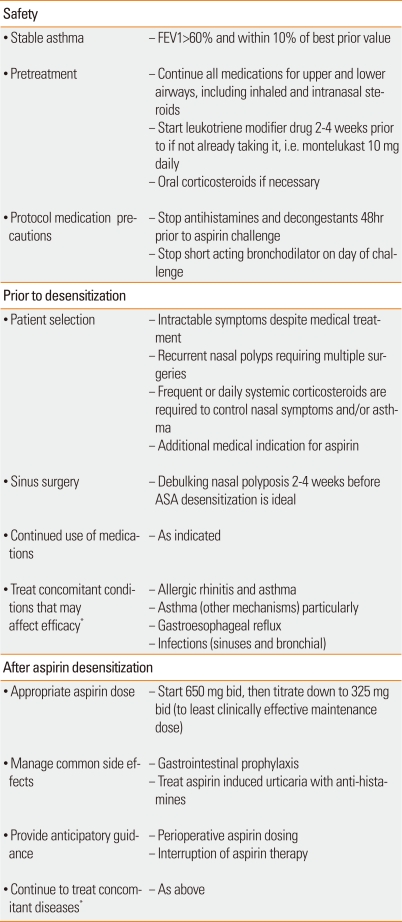
Most patients with AERD will benefit clinically from desensitization as described earlier, however this treatment is particularly helpful in patients who have suboptimal control with currently available pharmacotherapy or if they require multiple operations due to re-growth of nasal polyps or intractable sinus disease.29,30 Also, this procedure is indicated in AERD patients who require aspirin or NSAIDs for concomitant cardiovascular diseases, arthritis, or other medical indications (Table 3).29,30
Table 3.
Factors to consider in optimizing safe and effective aspirin desensitization in aspirin exacerbated respiratory disease
*Allergic rhinitis (nasal steroids, antihistamines, leukotriene modifiers, decongestants, immunotherapy, saline irrigation), asthma (inhaled steroids, long acting beta-agonists, leukotriene modifiers, anti-IgE therapy, immunotherapy for allergic asthma, systemic corticosteroids), gastroesophageal reflux (proton pump inhibitors, H2-antihistamines, if severe - consider fundoplication), infections (antibiotics).
Clinical factors to consider in optimizing ASA desensitization
It is important to note that AERD patients often have co-existing and provoking factors for upper and lower airway inflammation, including allergic rhinosinusitis, infections, gastroesophageal reflux disease, exercise-induced asthma, etc.2 Therefore, clinicians must recognize these additional provoking factors and aggressively treat them in order to optimize management. Patients with concomitant allergic rhinitis and asthma should be maintained and stable on antihistamines, topical steroids, leukotriene modifiers and immunotherapy. Sometimes omalizumab needs to be added to control this important co-morbid condition. Consider aggressive treatment of underlying gastroesophageal reflux disease with proton pump inhibitors or even Nissen fundoplication in severe cases recalcitrant to medical management. In addition, if patients have significant polyp or sinus disease, we routinely recommend surgical debulking 2-4 weeks prior to aspirin challenge and desensitization; we find that aspirin treatment works best in preventing new polyp formation. Although some patients experience marked regression of previously formed nasal polyps (Table 3), it is not possible to predict prospectively which patients might enjoy that benefit and to what extent that benefit might occur.
Premedication for aspirin challenge and desensitization
Most AERD patients synthesize excessive leukotrienes and severity of respiratory disease corresponds with the baseline production of leukotrienes.31,32 In addition, AERD patients express more cysteinyl LT1 receptors on their inflammatory cells.33 Aspirin and inhibitors of COX-1 divert arachidonic acid toward the lipoxygenase pathway, hence causing additional overproduction of inflammatory mediators.34 Interestingly, despite the role of leukotrienes in AERD, medications that modify this pathway, such as zileuton, a 5-lipoxygenase inhibitor, or inhibit cysteinyl leukotriene receptor (CysLT1), such as montelukast or zafirlukast, do not prevent upper airway reactions during aspirin challenges.35 Rather, CysLT1 inhibitors primarily prevent or attenuate bronchospasm.35 Studies have demonstrated that leukotriene modifier drugs positively impact treatment of AERD and, as we will discuss, also improve safety of aspirin challenges.36-38
We recommend that all patients have baseline spirometry showing FEV1 values to be more than 60% of predicted and at least 1.5 L. If they are not already taking a leukotriene modifier, we start either montelukast, zileuton or both prior to aspirin challenge. Patients are instructed to continue oral and topical corticosteroids and long-acting bronchodilators. We do ask that patients discontinue their antihistamines, decongestants and short-acting inhaled beta-agonists prior to aspirin challenge. These medications may mask a potential response and thus prevent diagnosis (Table 2). As an aspirin challenge and provocative reaction is the gold standard for diagnosis, it is necessary to measure a naso-ocular or bronchial reaction and thus confirm a diagnosis of AERD.
Inpatient or outpatient oral aspirin desensitization
Over 1,400 patients have successfully undergone aspirin challenge and desensitizations at the Scripps Clinic without any deaths or serious complications. In the past, the assumption was that the historical reactions to aspirin/NSAIDs should dictate the location of the aspirin desensitization procedure. The rational was that a bad reaction to aspirin 650 mg (or equivalent doses of NSAID) that required hospital intervention predicted a severe asthma attack during oral aspirin challenges and therefore should be conducted in a hospital ICU. However, Williams et al.39 at our institution compared the severity of prior aspirin/NSAID reactions and the degree of asthma induced during oral aspirin challenges and found that there was no correlation. A small number of severe reactions occurred during oral ASA challenges but they were just as likely to occur in patients with mild historical reactions. Furthermore, none of the "severe" reactions (FEV1 dropping >30% from baseline) required any more treatment than is available in all allergy outpatient offices.39 None required intubation or transfer to an ICU. Our explanation for the above is linked to dose response reactions. Historical reactions were induced by full therapeutic doses of aspirin or NSAIDs, compared to the average provoking dose of aspirin of 60 mg during oral aspirin challenges. Furthermore, the historical reactions may have occurred many years previously, usually without anti-leukotriene coverage. Also, the inflammatory respiratory disease may have changed over the years. In conclusion, historical reaction severity is not predictive of the degree of bronchospasm which might occur during controlled oral aspirin challenges.39
With experienced providers and staff, outpatient oral challenges are as safe as inpatient challenges and much more cost effective. At Scripps Clinic, beginning in 2003, we switched from inpatient (General Clinical Research Center) to outpatient Allergy Division offices and have conducted all oral aspirin challenges in our outpatient with one exception - inpatient desensitization should be strongly considered in patients receiving beta-blockers, recent myocardial infarction, and/or with severe or uncontrolled asthma. The only setting where we have encountered this need for ICU aspirin desensitization is with asthmatic patients admitted for an acute coronary syndrome urgently requiring aspirin therapy for either coronary stent or bypass surgery.
Although aspirin desensitization is beneficial in most patients with AERD, contraindications to aspirin desensitization include pregnancy, unstable asthma, gastric ulcers or bleeding disorders. We screen out patients with the above conditions and require that all patients sign informed consents prior to challenge and desensitization.
Oral aspirin challenge and desensitization protocol
Many variations of the oral aspirin challenge have been published.2,30 Hope et al.40 recently published a rational approach to aspirin challenge dosing which reviewed 420 aspirin desensitizations at the Scripps Clinic and used historical data to enhance the efficiency of the desensitization protocol. We found that most reactions occurred at 45 to 100 mg of aspirin and no patients reacted after the 650 mg dose.40 The average time to reaction was 102 minutes after last aspirin dose.40 Based on this rationale, we start with 20 to 40 mg of aspirin and advance every three hours (Table 2), and often complete the protocol in 2.5 days, 3 hours after the 325 mg asprin dose.40 If at any time a reaction occurs in the larynx or the bronchi, reactions are treated before continuation and the provocation dose is then repeated. If the reaction is limited to the nasal membranes, oxymetazoline nasal spray is used and the challenge is then continued with the next dose of aspirin.
ASPIRIN DOSAGES AND MAINTENANCE THERAPY AFTER SUCCESSFUL DESENSITIZATION
Patient specific optimal doses of aspirin cannot be predictable. This topic was evaluated by Lee et al.41 We found that no clinical distinguishing factor determined whether a patient needs 650 mg twice daily or 325 mg twice daily.41 Therefore, we recommend aspirin 1,300 mg daily (usually 650 mg bid) for one month. If there is significant improvement in upper and lower airway symptoms, we decrease by one tablet (325 mg) monthly to either 650 mg AM and 325 mg at night or ideally 325 mg bid (Table 3).41 A dose as low as 81 mg of aspirin can maintain the desensitized state, which may be sufficient for patients who need only cardiovascular prophylaxis, but is usually suboptimal with respect to blocking inflammatory tissues in the respiratory tract.30 In a small study by Rozsasi et al.42, AERD patients were desensitized with aspirin and then randomized to receive either aspirin 100 mg daily or 300 mg daily. One year later, careful re-examination revealed no polyp reformation in the any of the 300 mg daily group but nasal polyp reformation in all 7 of the patients treated with aspirin 100 mg daily.42 The same authors in an open study of 39 additional AERD patients, all of whom were treated with aspirin 300 mg daily, and none of these patients reformed nasal polyps at the end of one year of treatment.42 Maintenance treatment with aspirin should be at least 325 mg daily and ideally twice a day.
Since the introduction of leukotriene modifiers in the late 1990s, there have been several patients who were previously aspirin challenge positive, who subsequently stopped their aspirin therapy and were rechallenged while taking montelukast. They did not have a measureable reaction. However after taking aspirin 325 mg, they showed clinical flattening of the nasal turbinates and improvement of their nasal and asthma scores at one month after daily treatment with aspirin 650 mg twice daily (unpublished reports). Due to the potential of "silent desensitization" (under the blocking influence of montelukast), we recommend that all patients with history consistent with AERD continue aspirin for one month to evaluate clinical improvement. We are not aware of any studies that show that aspirin tolerant asthmatics receive any therapeutic benefit from aspirin treatment. Although we have proven silent desensitization in a few patients and thus have anecdotal evidence that silent desensitization can occur, concomitantly with or even without montelukast, further systematic studies are needed in order to better characterize this phenomena.
RISKS OF ORAL ASPIRIN CHALLENGES
Aspirin desensitization is highly effective, but underutilized. The main barrier to more widespread use of aspirin desensitization is the potential for aspirin induced severe bronchospasm, laryngospasm, and/or extra-respiratory side effects (cutaneous and gastric).39
A recent study analyzing 210 patients with suspected AERD found that naso-ocular reactions occurred in 90% and lower airway reactions (bronchial/laryngeal) occurred in 43%.39 Extra-pulmonary reactions were noted; gastrointestinal in 23% and cutaneous in 10%.39 Reactions during the challenges and desensitization are treated aggressively as indicated below. Bronchial reactions may be treated with inhaled beta-agonists. Laryngeal reactions respond to nebulized racemic epinephrine or intramuscular epinephrine. Nasal and ocular reactions are successfully treated with oral and topical antihistamines, mast cell stabilizers and decongestants. Aspirin induced flushing, urticaria, angioedema and pruritis may be treated with oral or intravenous antihistamines. Gastric symptoms, which include nausea, vomiting and abdominal cramping pain respond to oral or intravenous H2-antihistamines. For systemic reactions, intravenous fluid resuscitation and intramuscular epinephrine are available but rarely needed. In the 1,400 patients in whom we have conducted oral aspirin challenges, 3 (0.002%) experienced systemic reactions, which included hypotension. All three, responded quickly to one intramuscular injection of epinephrine.
Long term safety profile
In a group of 172 patients, who had undergone aspirin desensitization and treated with daily aspirin 650 mg twice daily for a year, 24 (14%) discontinued ASA treatment due to known side effects (i.e. gastric pain or bleeding, non-gastrointestinal bleeding, such as epistaxis, and urticaria).22 Eleven percent of patients stopped due to poor response and other reasons (i.e. planned surgeries, rash, death from other causes, etc).22 Of the remaining 126 patients who were continued on aspirin for a year, 110 (87%) benefitted from aspirin therapy.22
In the group of 16 "non responders", 12 had concomitant severe allergic respiratory disease to dust mutes and pets in the house. None were on immunotherapy and the study preceded the availability of omalizumab. The study design did not anticipate or account for this variable which may have negated the therapeutic effect of aspirin desensitization treatment.
There is a refractory period of about 48 to 72 hours following aspirin desensitization; this is maintained by continued aspirin therapy. Therefore, if there are brief interruptions to therapy (<48 hours) due to surgery or illness, aspirin may be restarted. However, if there is greater than a 48 hour disruption from treatment, we recommend repeat desensitization. The authors are aware of one patient, from another center, who lost the desensitized state after 24 hours.
NEW METHODS AND DOSING RATIONALE
In addition to the immediate risks of the procedure, another barrier to more widespread use is that it is time consuming, often lasting up to 3 days and longer if there are reactions requiring treatment and repeat doses. As mentioned earlier, Hope et al.40 published a rationale approach to dosing during oral challenges and desensitization which shortened the protocol from 3 to 2.5 days.
We recently presented a novel method of enhancing the challenge and desensitization process which was recently accepted for publication.43 The conception for this research evolved when White et al.44 demonstrated the use of intranasal ketorolac, in place of lysine-aspirin, as a nasal challenge provoking drug.
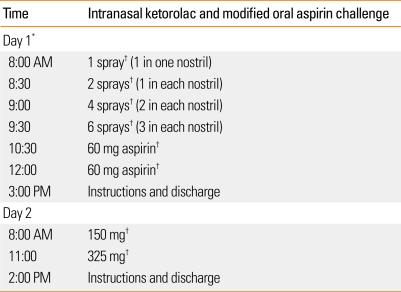
In Asia and Europe, intranasal lysine-aspirin is used as a diagnostic and therapeutic agent for AERD.45,46 Lysine-aspirin is not commercially available in the United States. Therefore, we were previously limited to only oral aspirin for challenge and treatment. However, we showed that intranasal ketorolac (Toradol®) is a safe and effective alternative for diagnosing AERD.44 In our study by White et al.44, there were several patients who had no subsequent reactions to oral aspirin challenges immediately following positive ketorolac challenges. We hypothesized that this procedure could potentially improve existing protocols for aspirin challenges and desensitization. In our study, we found that patients had significantly less laryngospasm and gastric side effects than a control group undergoing standard oral aspirin challenges.43 Perhaps as important, compared to an aspirin oral challenge control group, this new protocol significantly decreased the duration of the procedure as well (1.5 vs. 2.5 days of challenges and desensitization).43 This significantly decreases the cost of the procedure by one day or 40%. Currently at the Scripps Clinic, unless there is a contraindication to the procedure, such as complete nasal obstruction from polyps (which rarely occurs since pre procedure protocol calls for surgical debulking), AERD patients undergo this newer protocol using intranasal ketorolac and modified oral aspirin. See Figure for detailed instructions on how to prepare the nasal ketorolac and proceed with this protocol.
Figure.
Intranasal ketorolac protocol and directions for ketorolac solution preparation
*To prepare nasal ketorolac solution:
- Take ketorolac tromethamine (60 mg/2 mL) and preservative free normal saline (2.75 mL).
- Mix in an emptied Nasocort AQ® spray bottle.
- Prime with 5 sprays before use, then each spray actuates 1.26 mg of solution.
- Instruct patient and medical personnel to tilt head down while spraying and sniff gently to avoid swallowing solution.
†Clinical and objective evaluation with spirometry performed before each dose and as needed.
If there is no reaction 3 hours after the 325 mg dose of aspirin, this is a negative challenge.
Reactions can be:
- - Naso-ocular alone
- - Naso-ocular and a 15% or more decline in FEV1 (Classic reaction)
- - Lower respiratory reaction only (FEV1 declines by >20%)
- - Laryngospasm with or without a, b, c (flat or notched inspiratory curve)
- - Systemic reaction: hives, flush, gastric pain, hypotension
Aspirin desensitization:
- After a reaction has been treated and resolved, repeat provoking dose.
- If no reaction, continue to escalate the doses as above.
- At 325 mg of aspirin, desensitization is always completed.
CONCLUSIONS
Strong evidence supports the safety and clinical effectiveness of aspirin desensitization in the treatment of AERD. Aspirin desensitization reduces nasal congestion and polyp formation, improves respiratory symptoms and reduces the need for surgery and requirements for on-going medications. As awareness of AERD increases, greater availability of aspirin desensitization is required to successfully manage this condition. In addition, continued research efforts in understanding the pathophysiology, the phenomenon of "silent desensitization", treatment options, and possibly prevention are needed to better manage this condition.
Footnotes
The views expressed in this article are those of the author(s) and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense or the United States Government.
I am a military service member and employee of the U.S. Government. This work was prepared as part of my official duties. Title 17 U.S.C. 105 provides that 'Copyright protection under this title is not available for any work of the United States Government.' Title 17 U.S.C. 101 defines a United States Government work as a work prepared by a military service member or employee of the United States Government as part of that person's official duties.
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Szczeklik A, Nizankowska-Mogilnicka E, Sanak M. Hypersensitivity to aspirin and non-steroidal anti-inflammatory drugs. In: Adkinson NF Jr, Bochner BS, Busse WW, Holgate ST, Lemanske RF Jr, Simons FER, editors. Middleton's allergy: principles and practice. 7th ed. New York: Mosby; 2009. pp. 1227–1240. [Google Scholar]
- 2.Stevenson DD. Aspirin sensitivity and desensitization for asthma and sinusitis. Curr Allergy Asthma Rep. 2009;9:155–163. doi: 10.1007/s11882-009-0023-4. [DOI] [PubMed] [Google Scholar]
- 3.Jenkins C, Costello J, Hodge L. Systematic review of prevalence of aspirin induced asthma and its implications for clinical practice. BMJ. 2004;328:434. doi: 10.1136/bmj.328.7437.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonald JR, Mathison DA, Stevenson DD. Aspirin intolerance in asthma. Detection by oral challenge. J Allergy Clin Immunol. 1972;50:198–207. doi: 10.1016/0091-6749(72)90014-0. [DOI] [PubMed] [Google Scholar]
- 5.Weber RW, Hoffman M, Raine DA, Jr, Nelson HS. Incidence of bronchoconstriction due to aspirin, azo dyes, non-azo dyes, and preservatives in a population of perennial asthmatics. J Allergy Clin Immunol. 1979;64:32–37. doi: 10.1016/0091-6749(79)90080-0. [DOI] [PubMed] [Google Scholar]
- 6.Delaney JC. The diagnosis of aspirin idiosyncrasy by analgesic challenge. Clin Allergy. 1976;6:177–181. doi: 10.1111/j.1365-2222.1976.tb01896.x. [DOI] [PubMed] [Google Scholar]
- 7.Berges-Gimeno MP, Simon RA, Stevenson DD. The natural history and clinical characteristics of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2002;89:474–478. doi: 10.1016/S1081-1206(10)62084-4. [DOI] [PubMed] [Google Scholar]
- 8.Szczeklik A, Nizankowska E, Duplaga M AIANE Investigators. European Network on Aspirin-Induced Asthma. Natural history of aspirin-induced asthma. Eur Respir J. 2000;16:432–436. doi: 10.1034/j.1399-3003.2000.016003432.x. [DOI] [PubMed] [Google Scholar]
- 9.Samter M, Beers RF., Jr Intolerance to aspirin. Clinical studies and consideration of its pathogenesis. Ann Intern Med. 1968;68:975–983. doi: 10.7326/0003-4819-68-5-975. [DOI] [PubMed] [Google Scholar]
- 10.Widal MF, Abrami P, Lermeyez J. Idiosyncratic anaphylaxis. Presse Med. 1922;30:189–192. [Google Scholar]
- 11.Stevenson DD, Szczeklik A. Clinical and pathologic perspectives on aspirin sensitivity and asthma. J Allergy Clin Immunol. 2006;118:773–786. doi: 10.1016/j.jaci.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 12.Mascia K, Borish L, Patrie J, Hunt J, Phillips CD, Steinke JW. Chronic hyperplastic eosinophilic sinusitis as a predictor of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2005;94:652–657. doi: 10.1016/S1081-1206(10)61323-3. [DOI] [PubMed] [Google Scholar]
- 13.Stevenson DD. Aspirin desensitization in patients with AERD. Clin Rev Allergy Immunol. 2003;24:159–168. doi: 10.1385/CRIAI:24:2:159. [DOI] [PubMed] [Google Scholar]
- 14.Pfaar O, Klimek L. Eicosanoids, aspirin-intolerance and the upper airways--current standards and recent improvements of the desensitization therapy. J Physiol Pharmacol. 2006;57(Suppl 12):5–13. [PubMed] [Google Scholar]
- 15.Milewski M, Mastalerz L, Nizankowska E, Szczeklik A. Nasal provocation test with lysine-aspirin for diagnosis of aspirin-sensitive asthma. J Allergy Clin Immunol. 1998;101:581–586. doi: 10.1016/S0091-6749(98)70163-0. [DOI] [PubMed] [Google Scholar]
- 16.Nizankowska E, Bestynska-Krypel A, Cmiel A, Szczeklik A. Oral and bronchial provocation tests with aspirin for diagnosis of aspirin-induced asthma. Eur Respir J. 2000;15:863–869. doi: 10.1034/j.1399-3003.2000.15e09.x. [DOI] [PubMed] [Google Scholar]
- 17.Parikh AA, Scadding GK. Intranasal lysine-aspirin in aspirin-sensitive nasal polyposis: a controlled trial. Laryngoscope. 2005;115:1385–1390. doi: 10.1097/01.MLG.0000166702.38850.1B. [DOI] [PubMed] [Google Scholar]
- 18.Dursun AB, Woessner KA, Simon RA, Karasoy D, Stevenson DD. Predicting outcomes of oral aspirin challenges in patients with asthma, nasal polyps, and chronic sinusitis. Ann Allergy Asthma Immunol. 2008;100:420–425. doi: 10.1016/S1081-1206(10)60465-6. [DOI] [PubMed] [Google Scholar]
- 19.Gyllfors P, Bochenek G, Overholt J, Drupka D, Kumlin M, Sheller J, Nizankowska E, Isakson PC, Mejza F, Lefkowith JB, Dahlén SE, Szczeklik A, Murray JJ, Dahlen B. Biochemical and clinical evidence that aspirin-intolerant asthmatic subjects tolerate the cyclooxygenase 2-selective analgetic drug celecoxib. J Allergy Clin Immunol. 2003;111:1116–1121. doi: 10.1067/mai.2003.1450. [DOI] [PubMed] [Google Scholar]
- 20.Woessner KM, Simon RA, Stevenson DD. The safety of celecoxib in patients with aspirin-sensitive asthma. Arthritis Rheum. 2002;46:2201–2206. doi: 10.1002/art.10426. [DOI] [PubMed] [Google Scholar]
- 21.Stevenson DD, Simon RA. Lack of cross-reactivity between rofecoxib and aspirin in aspirin-sensitive patients with asthma. J Allergy Clin Immunol. 2001;108:47–51. doi: 10.1067/mai.2001.116290. [DOI] [PubMed] [Google Scholar]
- 22.Berges-Gimeno MP, Simon RA, Stevenson DD. Long-term treatment with aspirin desensitization in asthmatic patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2003;111:180–186. doi: 10.1067/mai.2003.7. [DOI] [PubMed] [Google Scholar]
- 23.Stevenson DD, Hankammer MA, Mathison DA, Christiansen SC, Simon RA. Aspirin desensitization treatment of aspirin-sensitive patients with rhinosinusitis-asthma: long-term outcomes. J Allergy Clin Immunol. 1996;98:751–758. doi: 10.1016/s0091-6749(96)70123-9. [DOI] [PubMed] [Google Scholar]
- 24.Kowalski ML, Grzelewska-Rzymowska I, Szmidt M, Rozniecki J. Clinical efficacy of aspirin in "desensitised" aspirin-sensitive asthmatics. Eur J Respir Dis. 1986;69:219–225. [PubMed] [Google Scholar]
- 25.Klimek L, Pfaar O. Aspirin intolerance: does desensitization alter the course of the disease? Immunol Allergy Clin North Am. 2009;29:669–675. doi: 10.1016/j.iac.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 26.McMains KC, Kountakis SE. Medical and surgical considerations in patients with Samter's triad. Am J Rhinol. 2006;20:573–576. doi: 10.2500/ajr.2006.20.2913. [DOI] [PubMed] [Google Scholar]
- 27.Berges-Gimeno MP, Simon RA, Stevenson DD. Early effects of aspirin desensitization treatment in asthmatic patients with aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2003;90:338–341. doi: 10.1016/S1081-1206(10)61803-0. [DOI] [PubMed] [Google Scholar]
- 28.Shaker M, Lobb A, Jenkins P, O'Rourke D, Takemoto SK, Sheth S, Burroughs T, Dykewicz MS. An economic analysis of aspirin desensitization in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2008;121:81–87. doi: 10.1016/j.jaci.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 29.Stevenson DD, Simon RA. Selection of patients for aspirin desensitization treatment. J Allergy Clin Immunol. 2006;118:801–804. doi: 10.1016/j.jaci.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 30.Macy E, Bernstein JA, Castells MC, Gawchik SM, Lee TH, Settipane RA, Simon RA, Wald J, Woessner KM. Aspirin challenge and desensitization for aspirin-exacerbated respiratory disease: a practice paper. Ann Allergy Asthma Immunol. 2007;98:172–174. doi: 10.1016/S1081-1206(10)60692-8. [DOI] [PubMed] [Google Scholar]
- 31.Daffern PJ, Muilenburg D, Hugli TE, Stevenson DD. Association of urinary leukotriene E4 excretion during aspirin challenges with severity of respiratory responses. J Allergy Clin Immunol. 1999;104:559–564. doi: 10.1016/s0091-6749(99)70324-6. [DOI] [PubMed] [Google Scholar]
- 32.Israel E, Fischer AR, Rosenberg MA, Lilly CM, Callery JC, Shapiro J, Cohn J, Rubin P, Drazen JM. The pivotal role of 5-lipoxygenase products in the reaction of aspirin-sensitive asthmatics to aspirin. Am Rev Respir Dis. 1993;148:1447–1451. doi: 10.1164/ajrccm/148.6_Pt_1.1447. [DOI] [PubMed] [Google Scholar]
- 33.Sousa AR, Parikh A, Scadding G, Corrigan CJ, Lee TH. Leukotriene-receptor expression on nasal mucosal inflammatory cells in aspirin-sensitive rhinosinusitis. N Engl J Med. 2002;347:1493–1499. doi: 10.1056/NEJMoa013508. [DOI] [PubMed] [Google Scholar]
- 34.Christie PE, Tagari P, Ford-Hutchinson AW, Charlesson S, Chee P, Arm JP, Lee TH. Urinary leukotriene E4 concentrations increase after aspirin challenge in aspirin-sensitive asthmatic subjects. Am Rev Respir Dis. 1991;143:1025–1029. doi: 10.1164/ajrccm/143.5_Pt_1.1025. [DOI] [PubMed] [Google Scholar]
- 35.Berges-Gimeno MP, Simon RA, Stevenson DD. The effect of leukotriene-modifier drugs on aspirin-induced asthma and rhinitis reactions. Clin Exp Allergy. 2002;32:1491–1496. doi: 10.1046/j.1365-2745.2002.01501.x. [DOI] [PubMed] [Google Scholar]
- 36.Dahlén SE, Malmström K, Nizankowska E, Dahlén B, Kuna P, Kowalski M, Lumry WR, Picado C, Stevenson DD, Bousquet J, Pauwels R, Holgate ST, Shahane A, Zhang J, Reiss TF, Szczeklik A. Improvement of aspirin-intolerant asthma by montelukast, a leukotriene antagonist: a randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165:9–14. doi: 10.1164/ajrccm.165.1.2010080. [DOI] [PubMed] [Google Scholar]
- 37.White A, Ludington E, Mehra P, Stevenson DD, Simon RA. Effect of leukotriene modifier drugs on the safety of oral aspirin challenges. Ann Allergy Asthma Immunol. 2006;97:688–693. doi: 10.1016/S1081-1206(10)61101-5. [DOI] [PubMed] [Google Scholar]
- 38.White AA, Stevenson DD, Simon RA. The blocking effect of essential controller medications during aspirin challenges in patients with aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2005;95:330–335. doi: 10.1016/S1081-1206(10)61150-7. [DOI] [PubMed] [Google Scholar]
- 39.Williams AN, Simon RA, Woessner KM, Stevenson DD. The relationship between historical aspirin-induced asthma and severity of asthma induced during oral aspirin challenges. J Allergy Clin Immunol. 2007;120:273–277. doi: 10.1016/j.jaci.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 40.Hope AP, Woessner KA, Simon RA, Stevenson DD. Rational approach to aspirin dosing during oral challenges and desensitization of patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2009;123:406–410. doi: 10.1016/j.jaci.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 41.Lee JY, Simon RA, Stevenson DD. Selection of aspirin dosages for aspirin desensitization treatment in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2007;119:157–164. doi: 10.1016/j.jaci.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 42.Rozsasi A, Polzehl D, Deutschle T, Smith E, Wiesmiller K, Riechelmann H, Keck T. Long-term treatment with aspirin desensitization: a prospective clinical trial comparing 100 and 300 mg aspirin daily. Allergy. 2008;63:1228–1234. doi: 10.1111/j.1398-9995.2008.01658.x. [DOI] [PubMed] [Google Scholar]
- 43.Lee RU, White D, Ding D, Durson AB, Woessner KM, Simon RA, Stevenson DD. Intranasal ketorolac and modified oral aspirin challenge enhances desensitization in aspirin exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2010;105:130–135. doi: 10.1016/j.anai.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 44.White A, Bigby T, Stevenson D. Intranasal ketorolac challenge for the diagnosis of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2006;97:190–195. doi: 10.1016/S1081-1206(10)60012-9. [DOI] [PubMed] [Google Scholar]
- 45.Casadevall J, Ventura PJ, Mullol J, Picado C. Intranasal challenge with aspirin in the diagnosis of aspirin intolerant asthma: evaluation of nasal response by acoustic rhinometry. Thorax. 2000;55:921–924. doi: 10.1136/thorax.55.11.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patriarca G, Bellioni P, Nucera E, Schiavino D, Papa G, Schinco G, Fais G, Pirotta LR. Intranasal treatment with lysine acetylsalicylate in patients with nasal polyposis. Ann Allergy. 1991;67:588–592. [PubMed] [Google Scholar]