Abstract
Interleukin (IL)-17 (also known as IL-17A) is produced by activated T cells. It is a marker cytokine of the TH17 lineage. IL-17 production is induced in infections, autoimmune diseases and other inflammatory events. IL-17 is involved in host defense, but also inflammatory tissue destruction. Vascular disease, mostly in the chronic form of atherosclerosis, is a leading cause of death. While normal vessels harbor only few leukocytes, large numbers of both innate and adaptive immune cells accumulate during vascular inflammation, both in chronic forms such as atherosclerosis and in acute vasculitis. IL-17 has a role in chronic vascular inflammation of atherosclerosis and possibly hypertensive vascular changes. In acute inflammation, IL-17 is elevated and may be causally involved in the autoimmune vasculitides including vasculitis in systemic lupus erythematodes. Blood vessels are important targets in alloimmune graft rejection and a number of studies provide data on a role of IL-17 in this context. This brief review summarizes the currently available evidence for and putative mechanisms of action of IL-17 in mouse models of and human vascular disease.
Keywords: Interleukin 17, T cells, atherosclerosis, vasculitis, vasculopathy
Introduction
During vascular inflammation, in addition to changes in vascular endothelial and smooth muscle cells, both innate and adaptive immune cells invade the vessel wall. Among them are T cells, some of them producing interleukin (IL)-17. Vascular inflammation is a pathogenic mechanism in atherosclerosis that accounts for a large proportion of global mortality [1]. Acute vascular inflammation is less common but acutely life-threatening in a number of autoimmune conditions [2]. Allo-immune chronic and acute vascular inflammation is pathogenically important in solid organ graft rejection [3]. It also occurs secondary to intravascular events such as thrombosis or embolism.
Leukocytes in the vascular wall
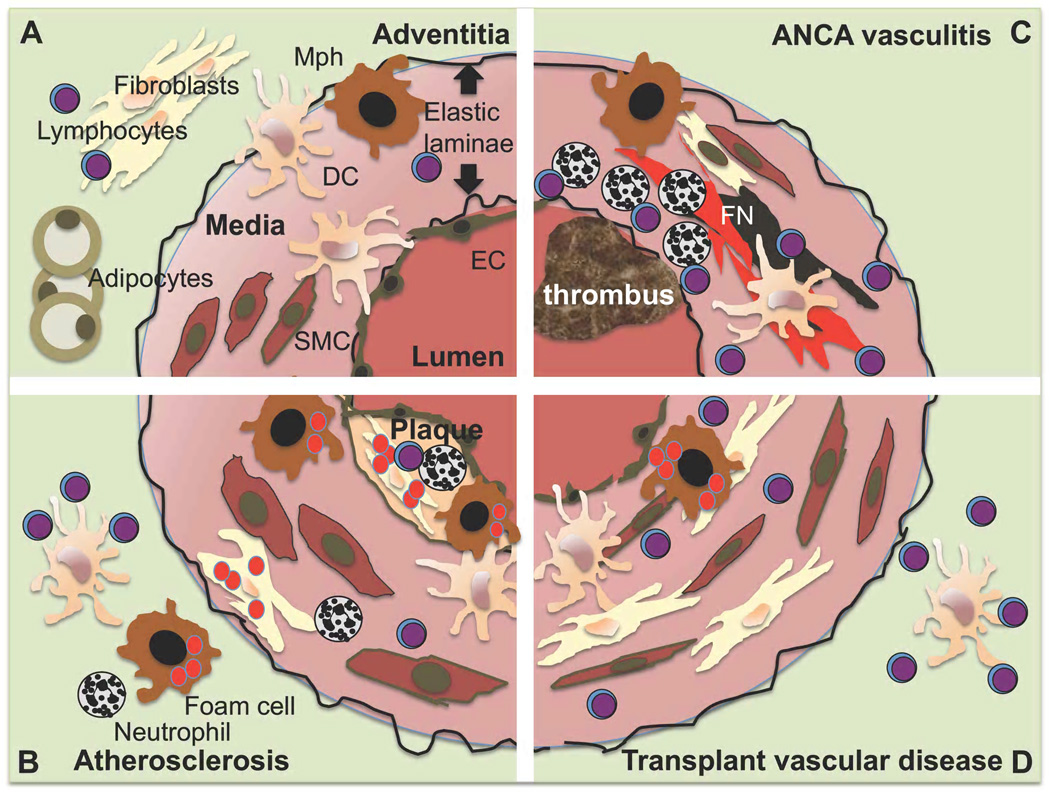
The normal vessel wall in arteries, where vascular inflammatory events are concentrated, is a concentric structure with distinct layers that are separated by elastic laminae [3]. The intima mainly consists of endothelial cells, smooth muscle cells are the predominant cell type in the arterial tunica media and the adventitial layers contain large numbers of fibroblasts and adipocytes (figure 1A). However, both innate and adaptive leukocytes populate the vessel wall even under normal conditions (figure 1A). Normal human arteries were analyzed for leukocyte contents in a series of carotid arteries from children killed in accidents [4]. Dendritic cells as defined by CD1a, macrophages staining for CD68, mast cells positive for tryptase and CD3+ T cells were found in the intima of the carotid bifurcation. In normal wild-type C57Bl/6 mice, macrophages and T cells, as defined by CD68 and CD3 expression, respectively, were found by confocal microscopy in the intima of the ascending aorta [5]. They were concentrated in the lesser curvature, an area of non-laminar flow that is prone to atherosclerosis development. Flow cytometry analysis of aortas from normal C57Bl/6 mice also revealed the presence of leukocytes, predominantly B and T lymphocytes [6]. These two methods are complementary in vessel wall analysis as immunostaining of histologic sections localizes the cells and flow cytometry of enzymatically digested vessels allows to analyze multiple surface markers at the same time and assess total cell numbers. Both methods suggest that leukocytes are an integral part of the normal vessel wall.
Figure 1. Leukocytes in the arterial wall in health and disease.
The arterial wall is organized in intimal, medial and adventitial layers divided by elastic laminae. Endothelial cells (EC) line the vascular lumen, smooth muscle cells (SC) are most abundant in the medial, muscular layer and the adventitial layer contains large numbers of fibroblasts and adipose tissue. Lymphocytes (Ly), macrophages (Mph) and dendritic cells (DC) have been demonstrated in normal mouse and human arteries (A)[4–6]. The most striking find in atherosclerosis is lipid uptake and foam cell formation in the plaque area of the neo-intima but also other regions of the vessel (B). ANCA associated small vessel vasculitis is characterized by fibrinoid necrosis (FN), intravascular thrombus formation and both myeloid and lymphoid cell invasion (C). Transplant vasculopathy invovlves multiple changes, the chronic form most prominently involves concentric stenosis and vascular muscular hypertrophy and fibrosis as well as lipid deposition (D).
During inflammation, leukocytes migrate into the vascular wall and their numbers greatly increase [6, 7]. In addition, proliferation of vascular leukocytes has been documented, in atherosclerosis most prominently among vascular macrophages [8, 9]. This occurs together with alterations in smooth muscle, fibrous tissue and intimal layers that respond to mechanical stress [10, 11] and in chronic inflammation such as atherosclerotic lesion formation [9, 12]. The most detailed work on leukocyte populations in vascular inflammation addresses atherosclerosis development [6, 7, 13]. In this condition, absolute numbers of all leukocyte subsets increase, but the amount of macrophages grows most prominently and they are the most abundant leukocyte in the atherosclerotic vessel [6]. This increase in macrophage numbers in atheroslcerosis is due to both immigration and local proliferation [8, 9]. Aortic T cell numbers also increase during atherosclerosis development. In both mice [14, 15] and humans [16], T cells have been detected in the adventitia and in all regions of the atherosclerotic plaque in intima and media.
The T cell cytokine IL-17, also known as IL-17A, has recently emerged as an important mediator of host defense and autoimmunity [17, 18]. While αβTCR+CD4+ T cells (TH17 cells) are the best-investigated source of IL-17A and prominent in settings of autoimmunity, other cell-types such as γδTCR+ cells, NK and NKT cells also produce IL-17 [17, 18]. In vitro, IL-17 production is induced by a combination of IL-6, IL-1, TGF-β and its production stabilized by IL-23 in mice and humans [19, 20]. IL-17 elicits an array of effector mechanisms, among them cytokine and chemokine production from epithelial and myeloid cells, attracting innate immune effector cells such as neutrophils and monocytes [21].
The role of IL-17 in atherosclerosis
Development of atherosclerotic plaques is closely associated with immune cell invasion of the vessel wall (figure 1B) [22]. T cells promote atherosclerotic lesion formation in a number of models [13, 23]. Apolipoprotein E- deficient (Apoe−/−) mice, a strain that spontaneously develops atherosclerosis and is commonly used for atherosclerosis studies, with severe combined immunodeficiency (SCID) or on recombinase activating gene (RAG) deficient backgrounds developed smaller atherosclerotic lesions [24–26]. αβTCR-deficient mice also developed smaller lesions [27] and injection of αβTCR+CD4+ T cells into SCID mice increased atherosclerotic lesion formation [26]. There is strong evidence supporting a pro-atherogenic role of TH1 cells, the T helper subtype characterized by the transcription factor T-bet and secretion of interferon (IFN)-γ. Mice were protected from atherosclerotic lesion formation when IFN-γ or T-bet were absent [28–31] and exogenous IFN-γ enhanced atherosclerotic lesion formation [32]. The role for TH2 cells characterized by IL-4 and IL-5 production in atherosclerotic lesion formation is equivocal [13, 23], but a protective role for antiinflammatory regulatory T cells (Treg) is well established [33–35].
Recent publications have begun to address the role of IL-17 and IL-17-producing T cells in atherosclerosis. Eid and colleagues studied circulating IL-17 in matched healthy controls and patients with coronary artery disease and in coronary arteries from explanted hearts or cadaveric donor organs [36]. Among coronary artery infiltrating T cells, IL-17-producing T cells were observed, although tenfold less anbundant than IFN-γ producers. Circulating IL-17 levels spanned a wide range and were non-detectable in a large proportion of patients and controls (detection limit of the sandwich ELISA used was 7.8 pg/ml). The proportion of individuals with detectable serum IL-17 was significantly elevated in coronary artery disease patients compared to healthy young laboratory workers, but not age-matched control patients without coronary artery disease. IL-17 levels did not correlate with disease extent. Taleb et al. [37] found IL-17 positive cells in the media of wild-type mice and around the media-adventitial junction of human arteries with early atherosclerotic lesions. Relatively more IL-17 was found in regions with few macrophages and high contents of smooth muscle cells and fibrous tissue.
In aged Apoe−/− mice (40–55 week old), IL-17 serum levels were higher than in C57/Bl6 controls and IL-17 mRNA was 100 fold increased in the aorta [38]. IL-17 expressing T cells were found in the atherosclerotic aortas of aged Apoe−/− mice [38]. When Apoe−/− mice were treated with an oral anti-CD3 antibody that induced regulatory T cells and reduced atherosclerotic lesion formation by approximately 30 %, IL-17 and other inflammatory cytokine production in splenic lymphocytes was decreased [35].
Van Es et al. were the first to perform a mechanistic study into the role of IL-17 in atherosclerosis [39]. The authors transplanted LDL-receptor deficient (Ldlr−/−) mice, another commonly used model for atherosclerosis that depends on dietary lipid uptake, with either wild-type or IL-17 receptor A deficient bone marrow to investigate the role of IL-17 signaling on bone marrow derived cells in the development of atherosclerotic lesions. Bone marrow reconstitution was effective as suggested by approximately 90% reduction in IL-17 receptor positive peripheral blood mononuclear cells (PBMCs) in mice receiving IL-17 receptor deficient bone marrow. IL-17 receptor deficiency of the bone marrow compartment resulted in a 46% decrease in aortic root atherosclerotic lesion size after twelve weeks on a diet containing 0.25% cholesterol and 15% cocoa butter. No significant differences in body weight or blood cholesterol levels were observed. Histologic analysis of the smaller lesions of the mice transplanted with IL-17 receptor deficient bone marrow revealed no change in relative collagen or neutrophil content but a slight increase in relative macrophage content and an approximately 40% decrease in lesional mast cells. PBMCs and splenocytes from mice that had received IL-17 receptor deficient bone marrow stimulated with anti-CD3 and anti-CD28 secreted significantly less IL-6 and slightly more IL-10, suggesting that the level of systemic inflammation was reduced by removing IL-17 receptor from bone marrow-derived cells.
In another study, Apoe−/− mice maintained on chow diet received weekly intra-peritoneal injections of an anti-IL-17 or control antibody for 12 weeks [40]. Atherosclerosis and inflammatory parameters were analyzed at 20 weeks of age. No significant differences in body weight or blood cholesterol levels were observed. IL-17 concentrations in serum at 20 weeks as determined by ELISA were lower in the anti-IL-17 treated group (41 versus 174 pg/ml), however, it is unclear whether the blocking antibody treatment interferes with IL-17 detection by ELISA. Relatively high serum IL-17 values (155 pg/ml) were observed at the start of the experiment at 8 weeks of age. Serum IgG or IgM against oxidized LDL, which are commonly observed in atherosclerosis remained unchanged. The relative frequencies of splenic CD3+ T cells, IFN-γ and Foxp3+CD4+ cells were decreased but IL-4 and IL-10 mRNA expression in the spleen was increased. Aortic root lesions were approximately 50% smaller in anti-IL-17 treated mice. The relative collagen contents and vascular smooth muscle cell contents of the fibrous cap were increased by anti-IL17A treatment as were vascular cell adhesion molecule (VCAM-1) positive cells. Less macrophages (MAC-2+), T cells (CD3+) and apoptotic cells (TUNEL+) cells found in the lesion area of treated mice. A number of inflammatory cytokines were reduced on mRNA level in the aorta. High doses of IL-17 had pro-inflammatory effects on cultured endothelial and smooth muscle cells, but the relevance of these findings to the in vivo situation was not explored.
Blockade of IL-17 signaling in Apoe−/− mice by adenoviral expression of soluble IL-17 receptor was investigated by Smith and colleagues [38]. Animals were injected with adenovirus expressing a soluble decoy IL-17 receptor A or control adenovirus at 4, 42 and 104 days of age. The presence of soluble IL-17 receptor was detected in plasma 7 days after virus injection. After 15 weeks on high fat diet, aortic en face lesion size was about 50% smaller in mice treated with the soluble IL-17 receptor compared to control virus treated mice. Plasma IL-6 and G-CSF, but nor IL-17F or IFN-γ levels were reduced in treated mice. Aortic macrophage infiltration as analyzed by immuno-histochemistry with anti Mac-2 and aortic CXCL1 expressionwere reduced. To test whether or not IL-17 mediated monocyte migration to the aorta, explanted aortas from 25–30 week old Apoe−/−mice were treated with 10 ng/ml IL-17 overnight and fluorescently labeled monocytes were added. Adherent cells were counted after washing after 1h of co-incubation. IL-17 increased the number of adhering cells to about 1.7 fold suggesting that IL-17 might act by increasing monocyte recruitment to the aorta.
In contrast to these three reports that suggest a pro-atherogenic role for IL-17, the results published by Taleb et al. [37] propose an atheroprotective role. Setting out to investigate the role of suppressor of cytokine signaling (SOCS)3 in atherosclerosis, they used Ldlr−/− mice transplanted with either SOCS3 deficient or wild type bone marrow. Mice that received SOCS3 deficient bone marrow had approximately 50% smaller aortic root lesions. This was associated with an increase in IL-17, but also IL-10. Anti-IL-17 treatment by intra-peritoneal antibody injection for six weeks increased atherosclerotic lesion size in SOCS3 deficient compared to wild-type mice, but did not change lesion size in wild-type mice. To test for an IL-17 effect in wild-type mice, recombinant IL-17 was injected into Ldlr−/− mice treated for six weeks with a high fat diet. This decreased the aortic root lesion size by approximately 30% in both 17 and 12 week old female Ldlr−/−mice. The number of CD3+ cells observed per histologic section in intima and adventitia was lower in IL-17 treated than control serum albumin treated mice. Also, less VCAM-1 expression was observed on endothelial cells of IL-17 treated mice. In vitro, IL-1-induced VCAM-1 expression was reduced by IL-17 studied by western blot.
Given the problems of exogenous administration of IL-17, blocking anitbodies or soluble receptor that could possibly contain other active ingredients, it is interesting that very recently atherosclerotic lesion formation in Apoe−/−Il17a−/− mice has been reported in abstract form. Two independent groups found an approximately 25% reduction of en face aortic atherosclerotic lesion size (Madhur, Harrison, Experimental Biology 2010, abstract no. 589.8, Butcher, Galkina, American Association of Immunologists, 2010, abstract no. 34.8). The reduction in lesion size was sustained when the Apoe−/−Il17a−/− mice were treated with angiotensin, suggesting IL-17 as a pro-atherogenic mediator downstream of angiotensin (Madhur, Harrison, Exp. Biology 2010).
An explanation for the controversial findings on IL-17 effects on atherosclerotic lesion size is not obvious at the moment. Erbel et al. and Smith et al. used Apoe−/− mice and Taleb and colleagues who at the moment are the only group to find atheroprotective effects of IL-17, Ldlr−/− mice, but van Es et al. also used Ldlr−/− mice in their study that found that IL-17 receptor signaling was a pro-atherogenic event. Smith and van Es investigated blocking of the IL-17 receptor A, which is also required for signaling of another IL-17 isoform, IL-17F and heterodimers of IL-17A and F [17, 18]. However, Erbel as well as Taleb specifically investigated the effects of IL-17A by antibody and protein injections, respectively. The same applies to the preliminary results from the studies on Apoe−/−Il17a−/− mice published in abstract form. At present, although the experimental designs of blocking or substituting IL-17 were different in the published studies, a mechanistic explanation for the discrepancies remains to be defined. Also, the evidence of IL-17 effects on downstream mediators of atherosclerotic plaque formation such as endothelial cells, monocytes or smooth muscle cells is mostly derived from in vitro experiments.
Aortic aneurysms are an important complication of atherosclerosis. The diseased arterial wall contains different types of inflammatory leukocytes. An imbalance of T cell cytokines favoring an increase in TH2 and a decrease in TH1 cytokines, especially IFN-γ was described in aneurysm formation [41]. TH1 and TH17 cells can influence each other’s differentiation by inducing IFN-γ and induction of IL-6 [42] and therefore might have a role in this process. Two abstracts presented at Experimental Biology 2010 suggest a role for IL-17 in aortic aneurysm formation. Xiong and co-workers (No 751.6) found less IL-17 and also less of the TH17 stabilizing cytokine IL-23 in aneurysms than in the normal aorta. Mice deficient in the IL-23 specific p19 subunit developed larger aneurysms than controls, suggesting a protective function of IL-17 against aneurysm formation. Along the same lines, Madhur and colleagues (No. 589.9) reported more abdominal aneurysm rupture and death in angiotensin-infused Apoe−/−Il17a−/− than Apoe−/− controls after 4 weeks on high fat diet. While details of these pathologic processes, especially concerning potential compensatory up-regulation of other cytokines in this setting remain to be investigated, the data suggest that IL-17 is involved in and possibly protects against aortic aneurysm progression.
Acute coronary syndrome and other thrombotic events
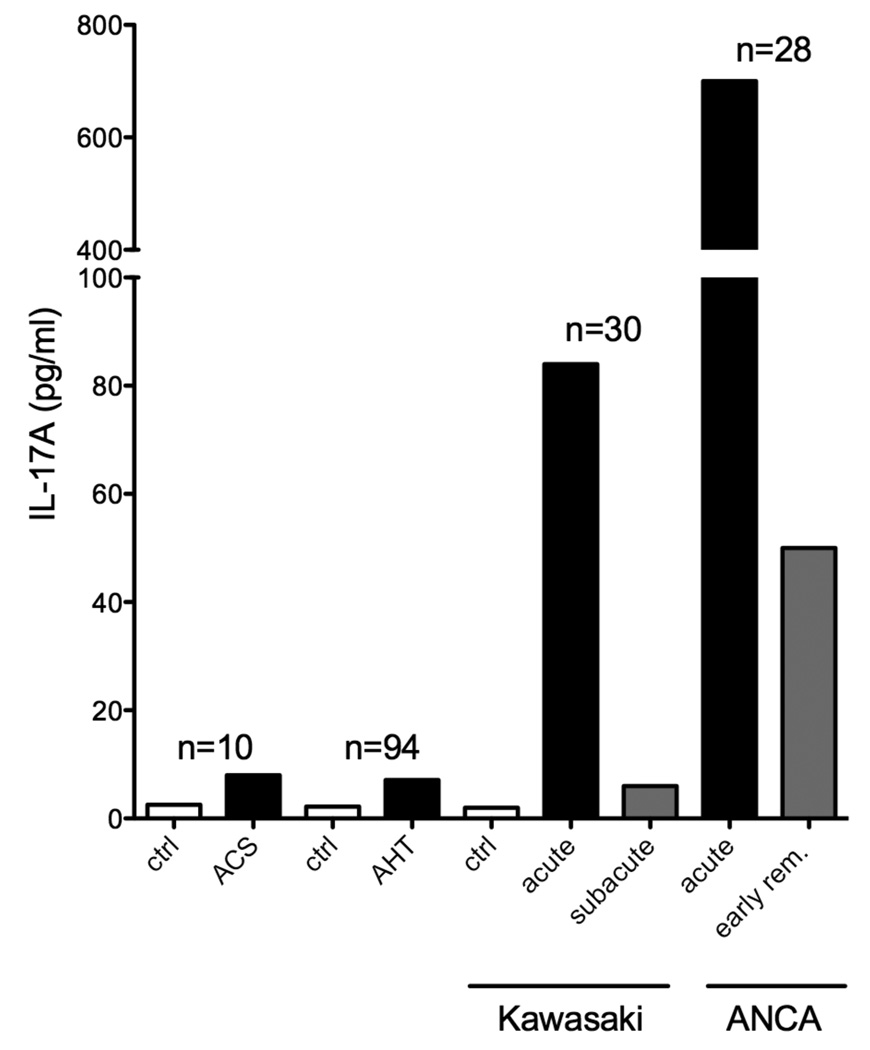
Plaque rupture and thrombosis are major complications of atherosclerotic disease, leading to acute vascular occlusion or distal embolism. In 10 patients with the acute coronary syndrome, circulating IL-17 levels were elevated compared to subjects with stable angina and healthy controls (8 versus 2.5 pg/ml, figure 2) [43]. These levels are very close to the detection limit of most assays. No other data on IL-17 in vascular thrombosis, embolism or stroke are available at the moment.
Figure 2. Systemic Interleukin 17 levels in patients with vascular disease.
Circulating IL-17 levels were elevated in a small group of patients with the acute coronary syndrome (ACS) [43]. Circulating levels were also elevated in hypertensive subjects (arterial hypertension, AHT)[48]. Among the acute vasculitides, IL-17 levels were elevated in acute and subacute Kawasaki disease [54] and ANCA vasculitis (acute and early remission) [55].
Also, there is currently no evidence for a role of IL-17 in venous thrombosis, thrombotic thrombocytopenic purpura or the hemolytic uremic syndrome. A non-significant elevation of plasma IL-17 was found in a cohort of 29 patients with immune thrombocytopenic purpura [44].
Hypertension-induced vascular damage
The adaptive immune system is increasingly recognized to play a role in hypertension-induced vascular damage [45–47].
Madhur [48] investigated aortic IL-17 production in C57Bl/6 and IL-17 deficient mice during angiotensin-II-induced hypertension. IL-17 production from isolated T cells was increased in angiotensin-II-treated animals. Mean blood pressures were lower in IL-17 deficient mice. Leukocytes were analyzed in aortic digests by flow cytometry, albeit without gating out dead cells. The number of all aortic CD45+ leukocytes and CD3+ T cells was increased by angiotensin II in wild type mice. This increase was absent in IL-17 deficient mice, arguing for a role of IL-17 in accumulation of all leukocytes including T cells during hypertensive vascular remodeling. A role for angiotensin in inducing IL-17 production was also suggested by inhibition of angiotensin converting enzyme (ACE) in neuro-inflammation in experimental autoimmune encephalitis [49]. Here, ACE inhibition decreased generation of IL-17-producing T cells and disease severity.
Aldosterone is a major downstream effector of the angiotensin pathway. Aldosterone-treated dendritic cells displayed an enhanced capacity for induction of IL-17 in CD4+ T cells [50], thus providing a plausible mechanistic link between angiotensin and IL-17 production. It remains to be determined how IL-17 increases aortic leukocyte recruitment in response to angiotensin and/or aldosterone. IL-17 may directly act chemotactic for other leukocytes, but also on vascular smooth muscle cells, thereby increasing blood pressure and increase leukocyte accumulation downstream in this pathway. To investigate this, IL-17 (100 ng/ml) was added to cultured TNF-α stimulated human aortic smooth muscle cells and analyzed by gene array [48]. A multitude of genes were differentially regulated by this regimen but only VCAM-down-regulation was confirmed by real time PCR. Clearly, more functional data and mechanisms are needed in this field.
In 94 human subjects with arterial hypertension, circulating IL-17 levels was significantly higher (7.1 pg/ml, figure 2) than in normotensive individuals (n=18, 2.2 pg/ml) [48]. Smoking and male sex were also positively correlated with IL-17 levels.
IL-17 in primary vasculitis
Primary vasculitides are systemic autoimmune disorders classified according to the size of the smallest vessels involved [51].
Large vessels
Large vessel arteriitides are giant cell arteriitis (GCA, also termed temporal arteriitis or Horton’s disease) and Takayasu arteriitis [52].
Deng and co-workers investigated peripheral blood and temporal artery T cells in patients with GCA [53]. The frequency of IL-17 producing T cells in peripheral blood and IL-17 (and IFN-γ) plasma levels (given as relative values) were elevated in patients with GCA. Interestingly, treatment with gluco-corticoids selectively decreased IL-17, but not IFN-γ plasma levels and TH17, but not TH1 or FOXP3+ cells in patient blood. Similar changes were seen in diseased human arteries transferred into SCID mice. Steroid treatment decreased IL-1β, IL-6 and IL-12 concentrations in patient’s plasma and mRNA in PBMCs and temporal artery biopsies. The authors conclude that lack of TH1-response may be the reason for reactivation of the disease after cessation of steroid treatment. However, data on TH1 and TH17 cells after cessation of treatment were not shown, and it is therefore not clear whether TH17 cells recover after cessation of treatment. Alternatively, the prominent TH17 response might alternatively reflect a higher susceptibility of TH17 to steroids, possibly due to stronger activation and/or proliferation. This may suggest a role of IL-17 in the development of GCA.
Involvement of IL-17 in Takayasu arteriitis was recently reported in abstract form by the same group (Deng et al., ATVB San Francisco 2010). Circulating IL-17 and IL-17 producing T cells were elevated in a group of 9 patients. IL-17 producing cells accumulated in the diseased aorta.
Medium size vessels
IL-17 serum concentrations were significantly elevated in a cohort of 30 patients with active Kawasaki disease (25 pg/ml, figure 2) compared to healthy controls (2 pg /ml). Patients with active disease had remarkably higher IL-17 levels (84 pg/ml) than those with sub-acute disease (6 pg /ml) [54]. There are currently no published data on IL-17 in polyarteriitis nodosa, the other idiopathic form of medium vessel vasculitis without muco-cutaneous lymph-node syndrome.
Small vessels
Primary small vessel vasculitides are subdivided depending on paucity or abundance of immune complexes (figure 1C) [2]. Pauci-immune small vessel vasculitides often are associated with antineutrophil-cytoplasmic antibodies (ANCA).
Serum IL-17 was markedly elevated to 700 pg/ml in a group of 28 patients with acute ANCA vasculitis (figure 2) [55]. In patients in early remission, IL-17 levels ranged still about 50 pg/ml. The frequency of circulating IL-17 producing CD4+ T cells was increased in two cohorts of patients with Wegener’s granulomatosis compared to healthy controls [55, 56]. Disease severity correlated with the concentration of the IL-17 promoting cytokine IL-23, but not IL-17 itself [55].
In a cohort of 14 patients with active and 21 with inactive Churg-Strauss syndrome, the frequency of IL-17-producing CD4+ T cells in peripheral blood was elevated in active versus inactive disease [57]. IL-17 levels in patients with inactive disease were not statistically different from control subjects.
Systemic lupus erythematodes (SLE)
The role of IL-17 in SLE that often involves vascular inflammation has recently been reviewed [58, 59]. The frequency of IL-17-producing CD4+ T cells was elevated in blood in a cohort of 25 patients with SLE [60]. There was a correlation of the number of IL-17 producers with disease severity and a trend towards correlation with renal involvement. SLE patients have a population of CD3+CD4−CD8− IL-17 producing cells [60, 61], similar to what has been seen in reactively IL-17 overproducing [62] and lupus prone MRL/lpr mice [63]. IL-17 producing T cells infiltrate the kidney of lupus prone MRL/lpr mice during development of the disease and were capable of transferring the disease to RAG-1−/− mice when treated with IL-23 in vitro [63]. However, the mechanism by which IL-17 promotes vascular inflammation in SLE or animal models of the disease is currently not known.
Other antibody-mediated vasculitis syndromes
Henoch-Schoenlein purpura and IgA nephropathy are characterized by deposition of IgA in the vessel wall of small to medium sized vessels [2]. IL-17 production was increased in PBMCs isolated from patients with IgA nephropathy, even more so if they suffered from nephrotic syndrome [64].
A number of recent reports demonstrated a role of IL-17 and IL-23 in different mouse models of vascular inflammation involving immunoglobulin deposition in the small vessels of the renal glomerulum. Paust and colleagues investigated nephrotoxic nephritis induced by sheep serum. Both IL-17 and IL-23 deficiency decreased renal inflammation [65]. Deficiency in either IL-23 subunit (p40 or p19) was protective in anti-glomerular basement antibody induced glomerulonephritis [66]. Mice deficient in either IL-23 subunit produced less IL-17, less TH1 cytokines and less antibodies in the spleen. The renal interstitial macrophage and CD4+ and CD8+ T cell infiltrate was smaller [66]. To investigate the effect of TH1 versus TH17 cells in glomerular inflammation, Summers and co-workers adoptively transferred TH1 and TH17 directed against ovalbumin to Rag1−/−mice, with ovalbumin planted to the glomerular basement membrane [67]. Both TH1 and TH17 transfer induced disease in a similar percentage of glomeruli. TH1 transfer predominantly attracted macrophages, TH17 transfer neutrophils. However, more severe histological damage was seen with TH1 than TH17 transfer and on day three, renal function was worse in TH1-transferred mice. Gan and colleagues studied glomerulonephritis in IL-17 deficient mice. MPO-immunized mice in which glomerulonephritis was induced by anti-glomerular basement membrane antibodies were protected when IL-17 was absent [68]. Gr1+ neutrophil and CD68+ macrophage recruitment was reduced as assessed by histology.
Cryoglobulinemic vasculitis is due to a B cell proliferative disorder, more than 90% of patients are infected with the hepatitis C virus [69]. There are currently no reports on IL-17 involvement in secondary vasculitis due to cryoglobulins. However, Antonelli and coworkers reported elevated levels of the IL-17-inducing cytokines IL-6 and IL-1β in 43 patients with chronic HCV and mixed cryoglobulinemia compared to HCV infected patients without cryoglobulinemia and healthy controls. [70] This may indicate IL-17 involvement in this disease and merits further investigation.
Other vascular disease
IL-17 is involved in sustained host–response to infection with Mycobacterium tuberculosis that can lead to vascular disease, specifically in the media of large vessels. No currently published data investigate its role in tuberculous vascular disease [71].
Systemic sclerosis is among the rheumatic diseases associated with and increased incidence of atherosclerosis [72]. Systemic IL-17 was decreased in a cohort of 444 scleroderma patients [73]. The number of TH17 cells in bronchoalveolar lavage fluid was not different from healthy controls in a small study [74].
The role of IL-17 in allo-immune vascular inflammation
Rejection of solid organ allograft is characterized by vascular pathology, both during acute rejection and chronically in transplant vasculopathy (figure 1D) [3, 75–77]. Acute vascular rejection of mismatched renal allografts was accompanied by elevated graft IL-17 in rats [78]. IL-17 protein was detected in kidneys and IL-17 mRNA in urinary mononuclear cells in human borderline changed rejected kidney allografts according to the Banff classification, but not allografts without acute rejection [78]. In a mouse model of thoracic aorta allograft rejection, application of a soluble IL-17-receptor-A-Fc-fusion protein decreased mononuclear infiltration and endothelial damage and normalized medial alpha smooth muscle actin expression on day seven. However, on day 30 no benefit of treatment was seen [79]. In a study of mismatched cardiac allograft rejection in mice deficient in the TH1 transcription factor T-bet, accelerated rejection correlated with an increase in IL-17 production and the related cytokines IL-6 and IL-12p40 [42]. Rejection was dependent on CD4+, but not CD8+ cells. Histology was characterized by a mostly neutrophilic vascular infiltration.
Chronic vascular rejection is defined as stenosis of vessels (particularly arteries and arterioles) due to progressive immune mediated host response to graft blood vessels [75]. CD4+ cells have been implicated in its progression before the advent of TH17 cells [75]. IFN-γ was considered the predominant T cell cytokine, macrophages and CD8+ cytolytic lymphocytes as main effector cells. In IL-17-deficient mice, graft coronary artery disease after heterotopic cardiac transplantations was reduced after 52 days. Most cardiac IL-17 production was in γδ-T cells according to flow-cytometry after intracellular staining [80]. Also, IL-17-deficient mice failed to develop cardiac fibrosis fifty days after mismatched organ transfer, often considered a secondary event to allograft vasculopathy [81].
In searching for a mechanism for this finding, Antonysamy and colleagues investigated generation of bone marrow dendritic cells and found that IL-17 induced CD11c expression during differentiation and enhanced their capacity to induce T cell proliferation [82]. In a chimeric model of human epicardial artery transplantation into SCID mice and transfer of human PBMCs, anti-IL-17 treatment decreased arterial IL-6, CXCL-8 and CCL20 expression. It did not decrease total T cell number but CCR6+ T cell accumulation, a receptor that is important for TH17 homing [83].
Summary
A large body of data demonstrates an increase of systemic and local IL-17 production in vascular disease both in humans and mouse models of disease. This comprises the autoimmune primary vasculitides, allo-immune vasculopathy and chronic vascular inflammation in atherosclerosis. However, the mechanistic links between IL-17 production and vascular inflammation remain to be determined in more detail. The incidence of atherosclerosis is increased in a number of autoimmune disease such as idiopathic vasculitis [84], rheumatoid arthritis and SLE [72] and it is tempting to speculate about IL-17 as a possible link between autoimmune disease and atherosclerosis, however, experimental evidence remains to be established.
Biographies

Klaus Ley holds an M.D. degree from Julius-Maximilians University in Würzburg, Germany. Before joining the La Jolla Institute for Allergy and Immunology as the founding head of the Division of Inflammation Biology in 2007, he directed the Robert M. Berne Cardiovascular Research Center at the University of Virginia. His lab works on basic and applied research in the area of leukocyte adhesion under flow and macrophage differentiation with relevance to atherosclerosis and Crohn’s disease. Dr. Ley received the 2008 Bonazinga and 2010 Malpighi awards, the highest awards of the Society for Leukocyte Biology and European Society for Microcirculation, respectively.

Sibylle von Vietinghoff, MD, completed a post-doctoral fellowship in Klaus Leys lab at the Division of Inflammation Biology, La Jolla Institute for Allergy and Immunology, CA, working on regulation of innate by adaptive immunity focusing on neutrophilic granulocytes and the vascular inflammatory infiltrate in atherosclerosis. She is currently pursuing her renal fellowship at Hannover Medical School, Germany.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no financial conflict of interest.
References
- 1.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367(9524):1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 2.Jennette JC, Falk RJ. Nosology of primary vasculitis. Curr Opin Rheumatol. 2007;19(1):10–16. doi: 10.1097/BOR.0b013e3280119877. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell RN. Graft vascular disease: immune response meets the vessel wall. Annu Rev Pathol. 2009;4:19–47. doi: 10.1146/annurev.pathol.3.121806.151449. [DOI] [PubMed] [Google Scholar]
- 4.Wick G, et al. Atherosclerosis, autoimmunity, and vascular-associated lymphoid tissue. FASEB J. 1997;11(13):1199–1207. doi: 10.1096/fasebj.11.13.9367355. [DOI] [PubMed] [Google Scholar]
- 5.Jongstra-Bilen J, Haidari M, Zhu SN, Chen M, Guha D, Cybulsky MI. Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J Exp Med. 2006;203(9):2073–2083. doi: 10.1084/jem.20060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med. 2006;203(5):1273–1282. doi: 10.1084/jem.20052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8(10):802–815. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 8.Zhu SN, Chen M, Jongstra-Bilen J, Cybulsky MI. GM-CSF regulates intimal cell proliferation in nascent atherosclerotic lesions. J Exp Med. 2009;206(10):2141–2149. doi: 10.1084/jem.20090866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rekhter MD, Gordon D. Active proliferation of different cell types, including lymphocytes, in human atherosclerotic plaques. Am J Pathol. 1995;147(3):668–677. [PMC free article] [PubMed] [Google Scholar]
- 10.Feihl F, Liaudet L, Levy BI, Waeber B. Hypertension and microvascular remodelling. Cardiovasc Res. 2008;78(2):274–285. doi: 10.1093/cvr/cvn022. [DOI] [PubMed] [Google Scholar]
- 11.Rekhter M, Nicholls S, Ferguson M, Gordon D. Cell proliferation in human arteriovenous fistulas used for hemodialysis. Arterioscler Thromb. 1993;13(4):609–617. doi: 10.1161/01.atv.13.4.609. [DOI] [PubMed] [Google Scholar]
- 12.Pickering JG, Weir L, Jekanowski J, Kearney MA, Isner JM. Proliferative activity in peripheral and coronary atherosclerotic plaque among patients undergoing percutaneous revascularization. J Clin Invest. 1993;91(4):1469–1480. doi: 10.1172/JCI116352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6(7):508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 14.Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986;6(2):131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- 15.Hansson GK, Seifert PS, Olsson G, Bondjers G. Immunohistochemical detection of macrophages and T lymphocytes in atherosclerotic lesions of cholesterol-fed rabbits. Arterioscler Thromb. 1991;11(3):745–750. doi: 10.1161/01.atv.11.3.745. [DOI] [PubMed] [Google Scholar]
- 16.Kleindienst R, Xu Q, Willeit J, Waldenberger FR, Weimann S, Wick G. Immunology of atherosclerosis. Demonstration of heat shock protein 60 expression and T lymphocytes bearing alpha/beta or gamma/delta receptor in human atherosclerotic lesions. Am J Pathol. 1993;142(6):1927–1937. [PMC free article] [PubMed] [Google Scholar]
- 17.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453(7198):1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwakura Y, Nakae S, Saijo S, Ishigame H. The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev. 2008;226:57–79. doi: 10.1111/j.1600-065X.2008.00699.x. [DOI] [PubMed] [Google Scholar]
- 19.Acosta-Rodriguez EV, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8(6):639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 20.Wilson NJ, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8(9):950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 21.Onishi RM, Gaffen SL. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 129(3):311–321. doi: 10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54(23):2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mallat Z, Taleb S, Ait-Oufella H, Tedgui A. The role of adaptive T cell immunity in atherosclerosis. J Lipid Res. 2009;50 Suppl:S364–S369. doi: 10.1194/jlr.R800092-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dansky HM, Charlton SA, Harper MM, Smith JD. T and B lymphocytes play a minor role in atherosclerotic plaque formation in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci U S A. 1997;94(9):4642–4646. doi: 10.1073/pnas.94.9.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gotsman I, Sharpe AH, Lichtman AH. T-cell costimulation and coinhibition in atherosclerosis. Circ Res. 2008;103(11):1220–1231. doi: 10.1161/CIRCRESAHA.108.182428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou X, Nicoletti A, Elhage R, Hansson GK. Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 2000;102(24):2919–2922. doi: 10.1161/01.cir.102.24.2919. [DOI] [PubMed] [Google Scholar]
- 27.Elhage R, et al. Deleting TCR alpha beta+ or CD4+ T lymphocytes leads to opposite effects on site-specific atherosclerosis in female apolipoprotein E-deficient mice. Am J Pathol. 2004;165(6):2013–2018. doi: 10.1016/s0002-9440(10)63252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buono C, Binder CJ, Stavrakis G, Witztum JL, Glimcher LH, Lichtman AH. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc Natl Acad Sci U S A. 2005;102(5):1596–1601. doi: 10.1073/pnas.0409015102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buono C, Come CE, Stavrakis G, Maguire GF, Connelly PW, Lichtman AH. Influence of interferon-gamma on the extent and phenotype of diet-induced atherosclerosis in the LDLR-deficient mouse. Arterioscler Thromb Vasc Biol. 2003;23(3):454–460. doi: 10.1161/01.ATV.0000059419.11002.6E. [DOI] [PubMed] [Google Scholar]
- 30.Gupta S, Pablo AM, Jiang X, Wang N, Tall AR, Schindler C. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J Clin Invest. 1997;99(11):2752–2761. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitman SC, Ravisankar P, Elam H, Daugherty A. Exogenous interferon-gamma enhances atherosclerosis in apolipoprotein E−/− mice. Am J Pathol. 2000;157(6):1819–1824. doi: 10.1016/s0002-9440(10)64820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tellides G, et al. Interferon-gamma elicits arteriosclerosis in the absence of leukocytes. Nature. 2000;403(6766):207–211. doi: 10.1038/35003221. [DOI] [PubMed] [Google Scholar]
- 33.Ait-Oufella H, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12(2):178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 34.Gotsman I, Gupta R, Lichtman AH. The influence of the regulatory T lymphocytes on atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27(12):2493–2495. doi: 10.1161/ATVBAHA.107.153064. [DOI] [PubMed] [Google Scholar]
- 35.Sasaki N, et al. Oral anti-CD3 antibody treatment induces regulatory T cells and inhibits the development of atherosclerosis in mice. Circulation. 2009;120(20):1996–2005. doi: 10.1161/CIRCULATIONAHA.109.863431. [DOI] [PubMed] [Google Scholar]
- 36.Eid RE, et al. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009;119(10):1424–1432. doi: 10.1161/CIRCULATIONAHA.108.827618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taleb S, et al. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J Exp Med. 2009;206(10):2067–2077. doi: 10.1084/jem.20090545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith E, et al. Blockade of Interleukin-17A Results in Reduced Atherosclerosis in Apolipoprotein E-Deficient Mice. Circulation. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Es T, et al. Attenuated atherosclerosis upon IL-17R signaling disruption in LDLr deficient mice. Biochem Biophys Res Commun. 2009;388(2):261–265. doi: 10.1016/j.bbrc.2009.07.152. [DOI] [PubMed] [Google Scholar]
- 40.Erbel C, et al. Inhibition of IL-17A attenuates atherosclerotic lesion development in ApoE-deficient mice. J Immunol. 2009;183(12):8167–8175. doi: 10.4049/jimmunol.0901126. [DOI] [PubMed] [Google Scholar]
- 41.Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2006;26(5):987–994. doi: 10.1161/01.ATV.0000214999.12921.4f. [DOI] [PubMed] [Google Scholar]
- 42.Yuan X, et al. A novel role of CD4 Th17 cells in mediating cardiac allograft rejection and vasculopathy. J Exp Med. 2008;205(13):3133–3144. doi: 10.1084/jem.20081937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang J, et al. Myeloperoxidase (MPO) and interleukin-17 (IL-17) plasma levels are increased in patients with acute coronary syndromes. J Int Med Res. 2009;37(3):862–866. doi: 10.1177/147323000903700331. [DOI] [PubMed] [Google Scholar]
- 44.Ma D, et al. Profile of Th17 cytokines (IL-17, TGF-beta, IL-6) and Th1 cytokine (IFN-gamma) in patients with immune thrombocytopenic purpura. Ann Hematol. 2008;87(11):899–904. doi: 10.1007/s00277-008-0535-3. [DOI] [PubMed] [Google Scholar]
- 45.Guzik TJ, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204(10):2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harrison DG, Vinh A, Lob H, Madhur MS. Role of the adaptive immune system in hypertension. Curr Opin Pharmacol. 10(2):203–207. doi: 10.1016/j.coph.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kvakan H, Luft FC, Muller DN. Role of the immune system in hypertensive target organ damage. Trends Cardiovasc Med. 2009;19(7):242–246. doi: 10.1016/j.tcm.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 48.Madhur MS, et al. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 55(2):500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Platten M, et al. Blocking angiotensin-converting enzyme induces potent regulatory T cells and modulates TH1- and TH17-mediated autoimmunity. Proc Natl Acad Sci U S A. 2009;106(35):14948–14953. doi: 10.1073/pnas.0903958106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herrada AA, et al. Aldosterone promotes autoimmune damage by enhancing Th17-mediated immunity. J Immunol. 184(1):191–202. doi: 10.4049/jimmunol.0802886. [DOI] [PubMed] [Google Scholar]
- 51.Jennette JC, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37(2):187–192. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 52.Weyand CM, Goronzy JJ. Medium- and large-vessel vasculitis. N Engl J Med. 2003;349(2):160–169. doi: 10.1056/NEJMra022694. [DOI] [PubMed] [Google Scholar]
- 53.Deng J, Younge BR, Olshen RA, Goronzy JJ, Weyand CM. Th17 and Th1 T-cell responses in giant cell arteritis. Circulation. 121(7):906–915. doi: 10.1161/CIRCULATIONAHA.109.872903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sohn MH, Noh SY, Chang W, Shin KM, Kim DS. Circulating interleukin 17 is increased in the acute stage of Kawasaki disease. Scand J Rheumatol. 2003;32(6):364–366. doi: 10.1080/03009740410005034. [DOI] [PubMed] [Google Scholar]
- 55.Nogueira E, et al. Serum IL-17 and IL-23 levels and autoantigen-specific Th17 cells are elevated in patients with ANCA-associated vasculitis. Nephrol Dial Transplant. doi: 10.1093/ndt/gfp783. [DOI] [PubMed] [Google Scholar]
- 56.Abdulahad WH, Stegeman CA, Limburg PC, Kallenberg CG. Skewed distribution of Th17 lymphocytes in patients with Wegener's granulomatosis in remission. Arthritis Rheum. 2008;58(7):2196–2205. doi: 10.1002/art.23557. [DOI] [PubMed] [Google Scholar]
- 57.Saito H, Tsurikisawa N, Tsuburai T, Oshikata C, Akiyama K. Cytokine production profile of CD4+ T cells from patients with active Churg-Strauss syndrome tends toward Th17. Int Arch Allergy Immunol. 2009;149 Suppl 1:61–65. doi: 10.1159/000210656. [DOI] [PubMed] [Google Scholar]
- 58.Garrett-Sinha LA, John S, Gaffen SL. IL-17 and the Th17 lineage in systemic lupus erythematosus. Curr Opin Rheumatol. 2008;20(5):519–525. doi: 10.1097/BOR.0b013e328304b6b5. [DOI] [PubMed] [Google Scholar]
- 59.Crispin JC, Tsokos GC. Interleukin-17-producing T cells in lupus. Curr Opin Rheumatol. doi: 10.1097/BOR.0b013e32833c62b0. [DOI] [PubMed] [Google Scholar]
- 60.Shah K, et al. Dysregulated balance of Th17 and Th1 cells in systemic lupus erythematosus. Arthritis Res Ther. 12(2):R53. doi: 10.1186/ar2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crispin JC, et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181(12):8761–8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22(3):285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Z, Kyttaris VC, Tsokos GC. The role of IL-23/IL-17 axis in lupus nephritis. J Immunol. 2009;183(5):3160–3169. doi: 10.4049/jimmunol.0900385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matsumoto K, Kanmatsuse K. Interleukin-17 stimulates the release of pro-inflammatory cytokines by blood monocytes in patients with IgA nephropathy. Scand J Urol Nephrol. 2003;37(2):164–171. doi: 10.1080/00365590310008929. [DOI] [PubMed] [Google Scholar]
- 65.Paust HJ, et al. The IL-23/Th17 axis contributes to renal injury in experimental glomerulonephritis. J Am Soc Nephrol. 2009;20(5):969–979. doi: 10.1681/ASN.2008050556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gan PY, et al. Th17 Cells Promote Autoimmune Anti-Myeloperoxidase Glomerulonephritis. J Am Soc Nephrol. doi: 10.1681/ASN.2009070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Summers SA, et al. Th1 and Th17 cells induce proliferative glomerulonephritis. J Am Soc Nephrol. 2009;20(12):2518–2524. doi: 10.1681/ASN.2009030337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gan PY, et al. Th17 cells promote autoimmune anti-myeloperoxidase glomerulonephritis. J Am Soc Nephrol. 21(6):925–931. doi: 10.1681/ASN.2009070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Charles ED, Dustin LB. Hepatitis C virus-induced cryoglobulinemia. Kidney Int. 2009;76(8):818–824. doi: 10.1038/ki.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Antonelli A, et al. Serum levels of proinflammatory cytokines interleukin-1beta, interleukin-6, and tumor necrosis factor alpha in mixed cryoglobulinemia. Arthritis Rheum. 2009;60(12):3841–3847. doi: 10.1002/art.25003. [DOI] [PubMed] [Google Scholar]
- 71.Cooper AM, Khader SA. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol Rev. 2008;226:191–204. doi: 10.1111/j.1600-065X.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Szekanecz Z, Koch AE. Vascular involvement in rheumatic diseases: ‘vascular rheumatology’. Arthritis Res Ther. 2008;10(5):224. doi: 10.1186/ar2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gourh P, et al. Plasma cytokine profiles in systemic sclerosis: associations with autoantibody subsets and clinical manifestations. Arthritis Res Ther. 2009;11(5):R147. doi: 10.1186/ar2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meloni F, Solari N, Cavagna L, Morosini M, Montecucco CM, Fietta AM. Frequency of Th1, Th2 and Th17 producing T lymphocytes in bronchoalveolar lavage of patients with systemic sclerosis. Clin Exp Rheumatol. 2009;27(5):765–772. [PubMed] [Google Scholar]
- 75.Libby P, Pober JS. Chronic rejection. Immunity. 2001;14(4):387–397. doi: 10.1016/s1074-7613(01)00119-4. [DOI] [PubMed] [Google Scholar]
- 76.Rahmani M, Cruz RP, Granville DJ, McManus BM. Allograft vasculopathy versus atherosclerosis. Circ Res. 2006;99(8):801–815. doi: 10.1161/01.RES.0000246086.93555.f3. [DOI] [PubMed] [Google Scholar]
- 77.Nickeleit V, Vamvakas EC, Pascual M, Poletti BJ, Colvin RB. The prognostic significance of specific arterial lesions in acute renal allograft rejection. J Am Soc Nephrol. 1998;9(7):1301–1308. doi: 10.1681/ASN.V971301. [DOI] [PubMed] [Google Scholar]
- 78.Hsieh HG, Loong CC, Lui WY, Chen A, Lin CY. IL-17 expression as a possible predictive parameter for subclinical renal allograft rejection. Transpl Int. 2001;14(5):287–298. doi: 10.1007/s001470100344. [DOI] [PubMed] [Google Scholar]
- 79.Tang JL, Subbotin VM, Antonysamy MA, Troutt AB, Rao AS, Thomson AW. Interleukin-17 antagonism inhibits acute but not chronic vascular rejection. Transplantation. 2001;72(2):348–350. doi: 10.1097/00007890-200107270-00035. [DOI] [PubMed] [Google Scholar]
- 80.Itoh S, et al. IL-17 contributes to the development of chronic rejection in a murine heart transplant model. J Clin Immunol. 30(2):235–240. doi: 10.1007/s10875-009-9366-9. [DOI] [PubMed] [Google Scholar]
- 81.Faust SM, et al. Role of T cell TGFbeta signaling and IL-17 in allograft acceptance and fibrosis associated with chronic rejection. J Immunol. 2009;183(11):7297–7306. doi: 10.4049/jimmunol.0902446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Antonysamy MA, et al. Evidence for a role of IL-17 in organ allograft rejection: IL-17 promotes the functional differentiation of dendritic cell progenitors. J Immunol. 1999;162(1):577–584. [PubMed] [Google Scholar]
- 83.Rao DA, et al. Interleukin (IL)-1 promotes allogeneic T cell intimal infiltration and IL-17 production in a model of human artery rejection. J Exp Med. 2008;205(13):3145–3158. doi: 10.1084/jem.20081661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cohen Tervaert JW. Translational mini-review series on immunology of vascular disease: accelerated atherosclerosis in vasculitis. Clin Exp Immunol. 2009;156(3):377–385. doi: 10.1111/j.1365-2249.2009.03885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]