Abstract
Objectives
To assess the predictive accuracy of conventional cardiovascular risk factors for incident heart failure(HF) and atrial fibrillation(AF) and the added benefit of multiple biomarkers reflecting diverse pathophysiological pathways.
Background
HF and AF are interrelated cardiac diseases associated with substantial morbidity and mortality and increasing incidence. Data on prediction and prevention of these diseases in healthy individuals is limited.
Methods
In 5,187 individuals from the community-based Malmö Diet and Cancer study, we studied the performance of conventional risk factors and six biomarkers including midregional pro-atrial natriuretic peptide(MR-proANP), N-terminal pro-B-type natriuretic peptide(Nt-proBNP), midregional pro-adrenomedullin, cystatin C, C-reactive protein(CRP) and copeptin.
Results
During a mean follow-up of 14 years,112 individuals were diagnosed with HF and 284 individuals with AF. Nt-proBNP(HR=1.63 per SD,95%CI=1.29–2.06,p<0.001), CRP(HR=1.57 per SD,95%CI=1.28–1.94,p<0.001) and MR-proANP(HR=1.26 per SD,95%CI=1.02-1-56,p=0.03) predicted incident HF independently of conventional risk factors and other biomarkers. MR-proANP(HR=1.62,95%CI=1.42-1.84,p<0.001) and CRP(HR=1.18,95%CI=1.03–1.34,p=0.01) independently predicted AF. Addition of biomarkers to conventional risk factors improved C-statistics from 0.815 to 0.842 for HF and from 0.732 to 0.753 for AF and the Integrated discriminatory index for both diseases(p<0.001). Net reclassification improvement with biomarkers was observed in 22% of individuals for HF(NRI,p<0.001) and in 7% for AF(NRI,p=0.06), mainly due to up-classification of individuals who developed disease(HF:29%,AF:19%). Addition of CRP to natriuretic peptides did not improve discrimination or reclassification.
Conclusions
Conventional cardiovascular risk factors predict incident HF and AF with reasonable accuracy in middle-aged individuals free from disease. Natriuretic peptides, but not other biomarkers, improve discrimination modestly for both diseases above and beyond conventional risk factors and substantially improve classification for HF.
Keywords: Atrial Fibrillation, Heart failure, Prediction, Natriuretic peptides, Risk factors, Epidemiology
Introduction
Although the incidence of coronary heart disease has declined in recent years, the incidence of heart failure and atrial fibrillation has risen (1). These interrelated diseases (2) are major causes of morbidity and mortality (3–4), with lifetime risks as high as 20% and 25%, respectively (5,6). Accordingly, practice guidelines for heart failure and atrial fibrillation have shifted their emphasis from treatment to prevention (7,8).
The identification of individuals at risk for heart failure and atrial fibrillation remains a challenge, however. Previous studies have identified risk factors for heart failure (9–10) and atrial fibrillation (11–12) and risk scores for risk assessment have been developed (13–15). However, these scores have focused on specific subgroups (elderly, patients with hypertension, coronary heart disease or valvular heart disease) and require information on markers rarely used in the clinic today, such as radiologic evidence of cardiomegaly. Additionally, many individuals who develop heart failure and atrial fibrillation are not identified by risk factors, reflecting the etiologic heterogeneity of these diseases. Asymptomatic ventricular dysfunction frequently precedes heart failure or atrial fibrillation, but routine screening echocardiography is prohibitively expensive and not currently recommended in the general population. It has been suggested that biomarkers reflecting common pathophysiological processes (16) may perform better than standard risk factors and identify individuals who might benefit from echocardiographic screening. A number of plasma biomarkers have been related to heart failure (16) and atrial fibrillation risk (17,18). However, the predictive value of models incorporating multiple biomarkers together has not been well established.
In a population-based sample of middle-aged individuals, we sought to evaluate the predictive accuracy of conventional cardiovascular risk factors and the incremental predictive value of a panel of biomarkers reflecting diverse pathophysiological pathways implicated in heart failure and atrial fibrillation including hemodynamic stress (the midregional fragment of pro-atrial natriuretic peptides (MR-proANP), the amino-terminal fragment of pro-B-type natriuretic peptide (Nt-proBNP), the midregional fragment of pro-adrenomedullin (MR-proADM)), plasma volume and osmolarity (copeptin), inflammation (C-reactive protein (CRP)) and renal function (cystatin C). We evaluated model improvement using both the C statistic and the newer measures of integrated discrimination improvement and net reclassification improvement (19–21).
Methods
Study sample
The Malmö Diet and Cancer Study (MDCS) is a prospective cohort study which includes 28,449 men (born between 1923–1945) and women (born between 1923–1950) from the city of Malmö in southern Sweden who underwent baseline examinations between 1991 and 1996. From this cohort 6,103 individuals with a baseline examination between 1991 and 1994 were randomly selected to participate in a study of cardiovascular risk factors, the MDCS Cardiovascular Cohort (MDC-CC), of whom 5,543 underwent blood sampling under standardized fasting conditions (22). Information on all conventional risk factors was available in 5,187 individuals, which constitutes the sample examined in the current study. Blood pressure (systolic and diastolic) was measured using a mercury-column sphygmomanometer after 10 minutes of rest in the supine position. Data on current smoking, diabetes mellitus and use of antihypertensive and antidiabetic medications was ascertained from a questionnaire. Diabetes mellitus was defined as fasting blood glucose >6.0 mmol/L, self-reported physician diagnosis or use of antidiabetic medications. MDCS was approved by the Ethics Committee of Lund University, Sweden, and all individuals provided informed consent.
Ascertainment of endpoints
Cardiac disease endpoints were ascertained by linkage of Swedish personal identification numbers to the national Swedish registers (Swedish Hospital Discharge Register, Swedish Cause of Death Register) maintained by the Swedish National Board of Health and Welfare. High case validity in these registers has been previously found for heart failure (23), myocardial infarction (24) and atrial fibrillation (25). Heart failure was ascertained from the Swedish Hospital Discharge Register using diagnosis codes 427.00, 427.10 and 428.99 for International Classification of Diseases 8th Revision (ICD-8), 428 for the 9th Revision (ICD-9) and I50 and I11.0 for the 10th Revision (ICD-10) as primary diagnosis like in previous studies (23). Atrial fibrillation was defined using diagnosis codes 427.92 (ICD-8), 427D (ICD-9) and I48 (ICD-10) as in previous studies (25). Myocardial infarction was defined using diagnosis codes 410 (ICD-8 and ICD-9) and I21 (ICD-10) or as death from ischemic heart disease defined using diagnosis codes 412 and 414 (ICD-8 and ICD-9) or I22-I23 and I25 (ICD-10) as in previous studies (24). Follow up extended to January 1, 2007.
Laboratory measurements
Measurements of fasting blood glucose, HbA1c, insulin and cholesterol (HDL, triglycerides, total cholesterol) were performed in MDC-CC on fresh blood samples according to standard procedures at the Department of Clinical Chemistry, University Hospital Malmö, as described previously (22). Plasma biomarkers were measured from fasting plasma samples that had been frozen at −80° C immediately after collection and that had not previously been thawed (22). Copeptin and the midregional fragments of pro-atrial natriuretic peptide and pro-adrenomedullin were measured using immunoluminometric sandwich assays (BRAHMS, Berlin, Germany). Nt-proBNP was measured using the automated Dimension Vista (R) Intelligent Lab System method (Siemens Healthcare Diagnostics Inc., Deerfield, IL). CRP was measured by a high-sensitivity assay (Roche Diagnostics, Basel, Switzerland). Cystatin C was measured using a particle-enhanced immuno-nephelometric assay (N Latex Cystatin, Siemens Healthcare Diagnostics Inc., Deerfield, IL).
Statistical analysis
One individual whose personal identification number did not match national registers was excluded from all analyses and individuals with prevalent atrial fibrillation or prevalent heart failure were excluded from analyses of the respective disease. MR-proANP, Nt-proBNP, CRP and copeptin showed right skewed distributions and underwent natural logarithmic transformation. All biomarkers were scaled to a standard deviation of 1 for ease of comparison. Cox proportional hazards models were used to assess association of biomarkers with disease independently of conventional risk factors, with Wald tests for biomarker significance testing. The proportionality of hazards assumption was confirmed using Schoenfeld’s global test. We first assessed association between each disease and each biomarker after adjustment for conventional risk factors. All biomarkers associated with disease (p<0.05) were then included in a backward elimination model with adjustment for conventional risk factors. Performance of the final models was evaluated using the C statistic, a generalization of the area under the receiver operating characteristic (ROC) curve. Adequate calibration of all final models across quantiles was confirmed using the Grønnesby and Borgan test (26). We tested models for the integrated discrimination improvement (IDI) and for reclassification of individuals into risk categories as the net reclassification improvement (NRI), the proportion of individuals correctly reclassified across risk categories over the proportion of individuals incorrectly reclassified (20). Finally, we created multimarker risk scores by summing individual Z scores weighted by the beta estimate per standard deviation for each biomarker. Kaplan-Meier curves of cumulative incidence were created for comparison of multimarker score quartiles.
The conventional models were determined using regression analysis with backward elimination including age, sex, systolic blood pressure, diastolic blood pressure, use of antihypertensive treatment, body mass index, low density lipoprotein, high density lipoprotein, current smoking, history of diabetes mellitus and history of myocardial infarction. A history of heart failure was also included for atrial fibrillation. For heart failure we used risk category thresholds of <6%, ≥ 6 to <20% and ≥ 20% that have been used for coronary heart disease (22,27). For atrial fibrillation, we used risk category thresholds of <5%, ≥ 5 to <15% and ≥ 15% as proposed for the Framingham prediction model (14).
Secondary analyses were performed with censoring at incident myocardial infarction for heart failure and with censoring at incident myocardial infarction or heart failure for atrial fibrillation. Two-sided p-values < 0.05 were considered significant. All analyses were performed in SPSS (SPSS version 16, SPSS Inc, Chicago) or STATA (STATA version 8, StataCorp, College Station, Texas).
Results
Baseline characteristics are shown in Table 1. Atrial fibrillation was prevalent in 47 individuals (0.9 %) and heart failure in 8 individuals (0.2 %). During a median follow-up of 13.8 years, 112 individuals were diagnosed with new onset heart failure and 284 individuals were diagnosed with new onset atrial fibrillation. Of the 63 individuals (1.2 %) with both conditions at the end of follow-up 39 (62 %) were diagnosed with atrial fibrillation before heart failure, 15 (24 %) were diagnosed with heart failure before atrial fibrillation and 9 (14 %) received both diagnoses on the same day.
Table 1.
Baseline characteristics.
| Variable | Value |
|---|---|
| Age (years) | 57.6 (5.9) |
| Male | 2094 (41 %) |
| Systolic blood pressure (mm Hg) | 141.4 (19.0) |
| Diastolic blood pressure (mm Hg) | 86.9 (9.4) |
| Antihypertensive treatment | 850 (17 %) |
| Body mass index | 25.7 (3.9) |
| Low density lipoprotein (mmol/L) | 4.2 (1.0) |
| High density lipoprotein (mmol/L) | 1.4 (0.4) |
| Diabetes mellitus | 399 (8 %) |
| Current smoking | 1379 (27 %) |
| History of myocardial infarction | 75 (2 %) |
| Midregional pro-atrial natriuretic peptide (pmol/L, n=4,880) | 66.1 (50.9–85.9) |
| N-terminal pro-B-type natriuretic peptide (pg/mL, n=4,778) | 61.0 (34.0–111.0) |
| Midregional pro-adrenomedullin (nmol/L, n=4,879) | 0.5 (0.1) |
| C-reactive protein (mg/L, n=4,922) | 1.4 (0.7–2.8) |
| Cystatin C (mg/dL, n=4,777) | 0.8 (0.1) |
| Copeptin (pmol/L, n=4,873) | 5.1 (3.2–8.1) |
Shown are baseline characteristics of 5,135 individuals free of atrial fibrillation and heart failure at baseline and with information on all conventional risk factors. For continuous traits, mean with standard deviation are given for normally distributed traits and median with interquartile range for right skewed traits. For categorical traits, numbers and percentages are given.
Prediction of heart failure and atrial fibrillation with conventional risk factors
Independently significant conventional risk factors used in final models are shown in Table 2 with corresponding hazard ratios. The C statistic of conventional risk factors was 0.815 for heart failure and 0.732 for atrial fibrillation as shown in Table 3.
Table 2.
Conventional risk factors and biomarkers for prediction of incident heart failure and atrial fibrillation.
| HF model 1 | HF model 2 | AF model 1 | AF model 2 | |
|---|---|---|---|---|
| Age | 1.12 (1.07–1.16) | 1.09 (1.04–1.13) | 1.11 (1.08–1.14) | 1.09 (1.06–1.12) |
| Female sex | 0.52 (0.35–0.77) | 0.48 (0.31–0.75) | 0.61 (0.48–0.78) | 0.55 (0.42–0.70) |
| BMI | 1.05 (1.00–1.10) | 1.03 (0.98–1.09) | 1.05 (1.02–1.08) | 1.05 (1.01–1.08) |
| SBP | - | - | 1.01 (1.00–1.02) | 1.01 (1.00–1.02) |
| DBP | - | - | 0.98 (0.96–1.00) | 0.99 (0.98–1.01) |
| Antihypertensive treatment | 2.40 (1.59–3.62) | 1.91 (1.20–3.04) | 1.38 (1.04–1.84) | 1.21 (0.89–1.64) |
| LDL cholesterol | - | - | 1.05 (1.02–1.08) | 0.90 (0.78–1.02) |
| History of MI | 4.13 (2.29–7.44) | 3.07 (1.64–5.76) | 2.64 (1.57–4.44) | 1.81 (1.05–3.13) |
| History of Diabetes | 2.82 (1.81–4.39) | 2.93 (1.82–4.70) | - | - |
| Current smoking | 1.71 (1.13–2.59) | 1.56 (0.99–2.46) | - | - |
| lnMR-proANP | 1.68 (1.41–2.00) | 1.26 (1.02–1.56) | 1.67 (1.47–1.89) | 1.62 (1.42–1.84) |
| lnNt-proBNP | 1.95 (1.63–2.34) | 1.63 (1.29–2.06) | 1.45 (1.28–1.65) | - |
| MR-proADM | 1.35 (1.17–1.56) | - | 1.26 (1.12–1.41) | - |
| lnCRP | 1.67 (1.37–2.03) | 1.57 (1.28–1.94) | 1.17 (1.04–1.33) | 1.18 (1.03–1.34) |
| CystC | 1.20 (1.06–1.36) | - | 1.11 (1.00–1.24) | - |
| lnCopeptin | 1.35 (1.03–1.77) | – | 1.09 (0.95–1.26) | - |
Shown are hazards ratios for conventional risk factors and biomarkers per standard deviation with 95 % confidence intervals from Cox proportional hazards models. Results are shown for each endpoint for a model with only conventional risk factors and for single biomarkers with adjustment for conventional risk factors (model 1) and models with all individually significant biomarkers adjusted for traditional risk factors and backward elimination at p≥0.05 (model 2).
Table 3.
Discrimination and risk category reclassification using biomarkers.
| C-statistic | IDI | NRI | |
|---|---|---|---|
| Heart failure | |||
| Conventional risk factors | 0.815 | - | - |
| MR-proANP | 0.824 | 0.03 (p<0.001) | 14 % (p=0.005) |
| Nt-proBNP | 0.837 | 0.03 (p<0.001) | 16 % (p=0.003) |
| CRP | 0.823 | 0.01 (p=0.02) | 7 % (p=0.10) |
| MR-proANP, Nt-proBNP | 0.838 | 0.03 (p<0.001) | 17 % (p=0.002) |
| CRP, Nt-proBNP | 0.842 | 0.04 (p<0.001) | 19 % (p=0.003) |
| All biomarkers | 0.842 | 0.05 (p<0.001) | 22 % (p<0.001) |
| Atrial fibrillation | |||
| Conventional risk factors | 0.732 | - | - |
| MR-proANP | 0.750 | 0.02 (p<0.001) | 8 % (p=0.04) |
| CRP | 0.734 | 0.001 (p=0.25) | 2 % (p=0.32) |
| Both | 0.753 | 0.02 (p<0.001) | 7 % (p=0.06) |
Shown are measures of discrimination and reclassification for models with conventional risk factors only and models with the addition of biomarkers to conventional risk factors for heart failure (HF) and atrial fibrillation (AF). For IDI, the IDI statistics and p-values are shown. For NRI, the proportion of individuals correctly reclassified over the proportion of individuals incorrectly reclassified and p-values are shown.
Biomarkers for prediction of incident heart failure
All biomarkers when considered individually were significantly associated with heart failure after adjustment for conventional risk factors as shown in Table 2. In multivariable regression models with backward elimination including all significant biomarkers and conventional risk factors, Nt-proBNP, MR-proANP and CRP remained significant predictors of heart failure as also shown in Table 2. In secondary analyses with censoring at incident myocardial infarction Nt-proBNP and CRP but not MR-proANP remained significant predictors.
Biomarkers for prediction of incident atrial fibrillation
MR-proANP, Nt-proBNP, MR-proADM and CRP were associated with atrial fibrillation after adjustment for conventional risk factors as shown in Table 2. In multivariable regression models with backward elimination including all significant biomarkers and conventional risk factors, MR-proANP and CRP remained significant as also shown in Table 2. In a secondary analysis with censoring at myocardial infarction or heart failure, both MR-proANP and CRP remained significant predictors independently of conventional risk factors.
Biomarkers and disease discrimination
For heart failure, improvement in discrimination compared to the model with conventional risk factors was observed with inclusion individually of Nt-proBNP (C statistic = 0.837), MR-proANP (0.824) or CRP (0.823) in the model as shown in Table 3. Inclusion of all three markers together resulted in a C-statistic of 0.842.
For atrial fibrillation, improvement in discrimination compared to the model with conventional risk factors was observed with inclusion of MR-proANP (0.750) in the model and, minimally, with CRP (0.734). Inclusion of both biomarkers yielded a C-statistic of 0.753.
Biomarkers and risk category reclassification
Net reclassification improvement was observed in 22 % of individuals for heart failure with the addition to conventional risk factors of all three biomarkers (CRP, MR-proANP, Nt-proBNP; NRI p<0.001), mostly due to substantial upward reclassification to a higher risk category (29%) of individuals who were subsequently diagnosed with heart failure during follow-up. For atrial fibrillation, 7 % of individuals were reclassified by a model with both biomarkers (CRP, MR-proANP; NRI p=0.06), mostly due to substantial upward reclassification (19%) of individuals who were diagnosed with atrial fibrillation during follow-up to a higher risk category. Multimarker scores yielded similar results with 22% net reclassification improvement (NRI p<0.001) for heart failure and 6% reclassification (NRI p=0.07) for atrial fibrillation. Results for NRI are shown in Table 3 and numbers reclassified are shown in Table 4. Significant improvement of IDI was also observed for both diseases (p<0.001). The cumulative incidence of heart failure and atrial fibrillation across quartiles of the multimarker risk score is shown in Figure 1. When added to a model with Nt-proBNP and conventional risk factors, neither MR-proANP (p=0.94) nor CRP (p=0.61) improved NRI but improved IDI (MR-proANP: 0.007, p=0.02, CRP: 0.01, p=0.005) for heart failure. The addition of CRP to MR-proANP and conventional risk factors did not improve classification (p=0.77) or IDI (p=0.43) for atrial fibrillation.
Table 4.
Risk classification by models with and without biomarkers for heart failure or atrial fibrillation.
| Model with biomarkers | ||||
|---|---|---|---|---|
| Model with conventional risk factors | Category 1 | Category 2 | Category 3 | Total |
| Heart failure | ||||
| <6% | 44 / 4274 | 14 / 108 | 3 / 7 | 61 / 4389 |
| ≥ 6 to <20% | 5 / 124 | 9 / 124 | 11 / 30 | 25 / 278 |
| ≥20% | 0 / 0 | 2 / 12 | 8 / 25 | 10 / 37 |
| Total | 49 / 4398 | 25 / 244 | 22 / 62 | 96 / 4704 |
| Atrial fibrillation | ||||
| <5% | 57 / 2640 | 15 / 221 | 0 / 0 | 72 / 2861 |
| 5 to 15% | 24 / 388 | 92 / 1228 | 34 / 137 | 150 / 1753 |
| ≥15% | 0 / 0 | 13 / 64 | 21 / 105 | 34 / 169 |
| Total | 81 / 3028 | 120 / 1513 | 55 / 242 | 256 / 4783 |
Reclassification across risk groups defined as <6%, ≥ 6% to <20%, ≥ 20% for heart failure and defined as <5%, 5% to 15%, ≥ 15% for atrial fibrillation. Numbers in the cells refer to numbers of events during follow-up over total number of individuals.
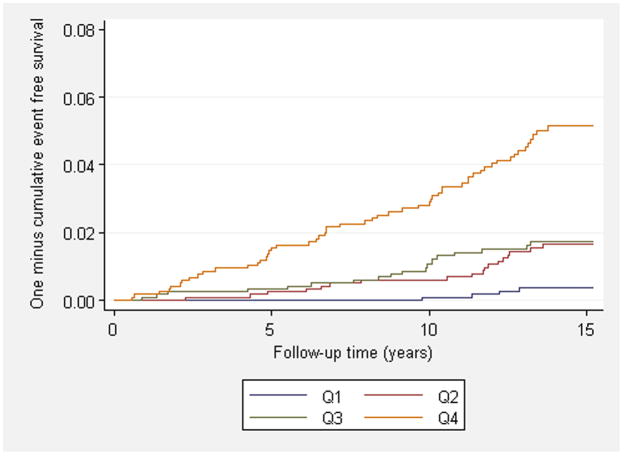
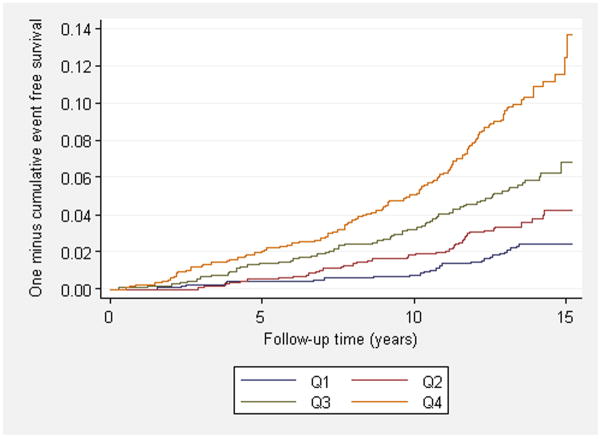
Figure 1. Cumulative incidence of heart failure and atrial fibrillation.
Panels show the cumulative incidence of heart failure (A) and atrial fibrillation (B) during follow-up across quartiles of multimarker scores. Individuals in panel A were free of heart failure at baseline and individuals in panel B were free of atrial fibrillation at baseline. Risk score distribution expressed as median (minimum, maximum) for heart failure was −3.4 (-11.2, −2.0) for the first quartile, −1.0 (−2.0, −0.6) for the second quartile, 0.8 (−0.6, 1.9) for the third quartile and 3.3 (1.9, 13.2) for the fourth quartile. Risk score distribution for atrial fibrillation was −2.2 (−12.7, −1.3) for the first quartile, −0.7 (−1.3, −0.3) for the second quartile, 0.5 (−0.3, 1.2) for the third quartile and 2.2 (1.2, 11.2) for the fourth quartile. P-values for trend were <0.001 for both heart failure and atrial fibrillation.
Discussion
In this community-based cohort, we found that conventional cardiovascular risk factors predicted heart failure and atrial fibrillation with reasonable accuracy and that the addition of biomarkers to conventional risk factors modestly improved discrimination for both diseases and substantially improved risk classification for heart failure. This improvement was mainly mediated by natriuretic peptides. These findings stand in contrast to the weaker performance of contemporary cardiovascular biomarkers (such as CRP and BNP) in improving discrimination and risk category classification for atherosclerotic events above conventional risk factors in the general population (22,28,29). One potential explanation for this difference could be a smaller value of conventional risk factors for prediction of heart failure and atrial fibrillation, compared with atherosclerotic events, providing greater room for improvement with biomarkers. Indeed, the population attributable risks for conventional risk factors for myocardial infarction (30) has been described to be substantially higher than for heart failure (10,31) and atrial fibrillation (11). Still, we observed excellent discrimination of heart failure using conventional risk factors (0.815) and acceptable discrimination of atrial fibrillation (0.753). Our models with conventional risk factors contained fewer risk factors and had similar or better predictive accuracy to that described for previous models for both AF and HF (14,15).
Findings and implications for heart failure
Prior studies have noted the association of CRP and natriuretic peptides with future heart failure (16), but there has been limited information regarding the additive value of these biomarkers for risk prediction. Furthermore, prior studies have frequently included fewer than 100 heart failure events. Thus, the present investigation extends the findings of prior studies to a larger cohort, with the use of novel predictive indices and assessment of a large panel of contemporary biomarkers.
Our data suggest that the association between Nt-proBNP, MR-proANP and CRP with heart failure risk is not mediated by interim myocardial infarction. Mild natriuretic peptide elevation may reflect asymptomatic left ventricular systolic or diastolic dysfunction (32,33). Asymptomatic left ventricular dysfunction is common in the population (32), and can progress to heart failure over time (34,35). Although we did not have concurrent echocardiographic measures, it is interesting to note that prior investigations have found the association of BNP with incident heart failure to be independent of left ventricular mass, ejection fraction, or left atrial enlargement, suggesting that natriuretic peptide elevations may reflect more subtle cardiac abnormalities. The association of CRP with heart failure has been postulated to reflect active inflammatory processes in the myocardium which, following an initial precipitating event, progressively drive remodeling and dilation of cardiac chambers (16,18). However, further data are warranted to establish the underlying mechanisms and the improvement in discrimination with CRP was modest. It is possible that better markers reflecting myocardial remodeling and other pathways exist and will improve risk prediction beyond natriuretic peptides.
Improved ability to identify individuals at risk for heart failure could be used to identify individuals who might benefit from echocardiographic testing and to guide preventive interventions such as angiotensin converting enzyme inhibitors, which have been found to prevent the development of heart failure and improve survival in individuals with asymptomatic left ventricular dysfunction (34,36). Further studies are necessary to determine whether any subgroups might benefit more from biomarker testing, what the appropriate follow up of positive biomarker results should be (e.g. echocardiography), and the cost-effectiveness of more widespread screening (37,38).
Findings and implications for atrial fibrillation
Natriuretic peptides and inflammation were also the best markers for atrial fibrillation. Our data suggest that these associations are not mediated by interim myocardial infarction and heart failure. Interestingly, MR-proANP performed slightly better than Nt-proBNP, suggesting that the former could be a better marker of the atrial stress leading to atrial fibrillation. This finding is supported by the observation in the Framingham Heart Study that N-terminal pro-atrial natriuretic peptide levels predicted atrial fibrillation more strongly than Nt-proBNP (17). CRP predicted atrial fibrillation with similar risk estimates as in a previous study (18). However, a relatively weak predictor on its own with only minimal improvement in discrimination, CRP did not substantially improve prediction when added to MR-proANP.
Unfortunately, no preventive treatments for atrial fibrillation have been established. Subgroup analyses of clinical trials have shown interesting results with angiotensin receptor inhibitors, statins and beta-blockers, and calls for additional trials to address this issue have been raised, but the current data are inconclusive (8).
Strengths and limitations
We used a large sample with long follow-up, enabling identification of large numbers of disease events. Our use of national register data could potentially reduce the impact of attendance bias on endpoint ascertainment, which could be substantial for heart failure. High case validity of atrial fibrillation, heart failure and myocardial infarction has been validated in these registers (23–25). However, as disease outcomes were ascertained from national registers on cause of death and hospitalization, it is possible that individuals who developed disease but were not hospitalized escaped detection, leading to underestimates of disease rates. It seems less likely that these effects would be differential based on the biomarkers measured here and thus would be more likely to bias our study to the null.
In conclusion, our findings provide evidence that natriuretic peptides improve prediction of incident heart failure and atrial fibrillation in the general population in addition to conventional risk factors. Whether individuals with elevated levels of these biomarkers will benefit from further testing and preventive therapy remains to be determined.
Supplementary Material
Acknowledgments
The authors would like to thank BRAHMS AG and Siemens Healthcare Diagnostics for biomarker measurements. The authors acknowledge the valuable assistance of Mary Lou Gantzer, PhD, Christine Perkins, BA, and Dejan Blagovcanin, BA, all at Siemens Healthcare Diagnostics.
Funding Sources
The Malmö Diet and Cancer study was made possible by grants from the Malmö city council. Biomarker measurements were performed and funded by BRAHMS AG and Siemens Healthcare Diagnostics. Drs Smith, Platonov, Hedblad, Engström and Melander were supported by the Swedish Heart and Lung Foundation. Dr Newton-Cheh was supported by NIH grant K23-HL-080025, a Doris Duke Charitable Foundation Clinical Scientist Development Award, and a Burroughs Wellcome Fund Career Award for Medical Scientists. Dr Platonov was supported by the Region Skane and Lund University Hospital. Dr Wang was supported by NIH grants R01-HL-086875, R01-HL-083197, R01-DK-081572 and by a grant from the American Heart Association. Drs Hedblad and Melander were supported by the Swedish Medical Research Council. Dr Melander was supported by grants from the Medical Faculty of Lund University, Malmö University Hospital, the Albert Påhlsson Research Foundation, the Crafoord Foundation, the Region Skane, the Hulda and Conrad Mossfelt Foundation, the King Gustaf V and Queen Victoria Fund, the Lennart Hanssons Memorial Fund, and the Wallenberg Foundation.
Abbreviations and acronyms
- MDCS
Malmö Diet and Cancer study
- MDC-CC
Malmö Diet and Cancer study: Cardiovascular Cohort
- CRP
C-reactive protein
- MR-proADM
Midregional pro-adrenomedullin
- MR-proANP
Midregional pro-atrial natriuretic peptide
- Nt-proBNP
N-terminal pro-B-typ natriuretic peptide
- NRI
Net Reclassification Improvement
- IDI
Integrated Discriminatory Improvement
- ROC curve
Receiver Operating Characteristic curve
Footnotes
Potential conflicts of interest
Joachim Struck, Nils G. Morgenthaler and Andreas Bergmann are employees of Brahms AG, which holds patent rights on the assays used for MR-proADM, MR-proANP and Copeptin.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Braunwald E. Shattuck lecture--cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–1369. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 2.Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol. 2003;91 (suppl):2D–8D. doi: 10.1016/s0002-9149(02)03373-8. [DOI] [PubMed] [Google Scholar]
- 3.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 4.Stewart S, Hart CL, Hole DJ, McMurray JJV. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the renfrew/paisley study. Am J Med. 2002;113:359–364. doi: 10.1016/s0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham heart study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham heart study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 7.Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 8.Benjamin EJ, Chen PS, Bild DE, et al. Prevention of atrial fibrillation: report from a National Heart, Lung, and Blood Institute workshop. Circulation. 2009;119:606–18. doi: 10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kannel WB, Belanger AJ. Epidemiology of heart failure. Am Heart J. 1991;121:951–957. doi: 10.1016/0002-8703(91)90225-7. [DOI] [PubMed] [Google Scholar]
- 10.Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 11.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 12.Psaty BM, Manolio TA, Kuller LH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 13.Kannel WB, D'Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med. 1999;159:1197–1204. doi: 10.1001/archinte.159.11.1197. [DOI] [PubMed] [Google Scholar]
- 14.Schnabel RB, Sullivan LM, Levy D, et al. Development of a risk score for atrial fibrillation (Framingham Heart study): a community-based cohort study. Lancet. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butler J, Kalogeropoulos A, Georgiopoulou V, et al. Incident heart failure prediction in the elderly. The Health ABC Heart Failure Score. Circ Heart Fail. 2008;1:125–33. doi: 10.1161/CIRCHEARTFAILURE.108.768457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358:2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 17.Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 18.Aviles RJ, Martin DO, Apperson-Hansen C, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 19.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–935. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 20.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 21.Hlatky MA, Greenland P, Arnett DK, et al. Criteria for evaluation of novel markers of cardiovascular risk. A scientific statement from the American Heart Association. Circulation. 2009;119:2408–16. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melander O, Newton-Cheh C, Almgren P, et al. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA. 2009;302:49–57. doi: 10.1001/jama.2009.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingelsson E, Arnlöv J, Sundström J, Lind L. The validity of a diagnosis of heart failure in a hospital discharge register. Eur J Heart Fail. 2005;7:787–791. doi: 10.1016/j.ejheart.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Hammar N, Alfredsson L, Rosén M, Spetz CL, Kahan T, Ysberg AS. A national record linkage to study acute myocardial infarction incidence and case fatality in sweden. Int J Epidemiol. 2001;30 (Suppl 1):S30–4. doi: 10.1093/ije/30.suppl_1.s30. [DOI] [PubMed] [Google Scholar]
- 25.Smith JG, Platonov PG, Hedblad B, Engström G, Melander O. Atrial fibrillation in the Malmö diet and cancer study: a study of occurrence, risk factors and diagnostic validity. Eur J Epidemiol. 2010;25:95–102. doi: 10.1007/s10654-009-9404-1. [DOI] [PubMed] [Google Scholar]
- 26.Grønnesby JK, Borgan O. A method for checking regression models in survival analysis based on the risk score. Lifetime Data Anal. 1996;2:315–28. doi: 10.1007/BF00127305. [DOI] [PubMed] [Google Scholar]
- 27.Wilson PWF, Smith SC, Blumenthal RS, Burke GL, Wong ND. Task Force #4—how do we select patients for atherosclerosis imaging? 34th Bethesda conference: can atherosclerosis imaging techniques improve the detection of patients at risk for ischemic heart disease. J Am Coll Cardiol. 2003;41:1898–1906. doi: 10.1016/s0735-1097(03)00361-9. [DOI] [PubMed] [Google Scholar]
- 28.Wang TJ, Gona P, Larson MG, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 29.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 30.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the Interheart study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 31.Kalogeropoulos A, Georgiopoulou V, Kritchevsky SB, et al. Epidemiology of incident heart failure in a contemporary elderly cohort: the Health, aging, and body composition study. Arch Intern Med. 2009;169:708–715. doi: 10.1001/archinternmed.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costello-Boerrigter LC, Boerrigter G, Redfield MM, et al. Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide in the general community: determinants and detection of left ventricular dysfunction. J Am Coll Cardiol. 2006;47:345–353. doi: 10.1016/j.jacc.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tschöpe C, Kasner M, Westermann D, Gaub R, Poller WC, Schultheiss H. The role of Nt-proBNP in the diagnostics of isolated diastolic dysfunction: correlation with echocardiographic and invasive measurements. Eur Heart J. 2005;26:2277–2284. doi: 10.1093/eurheartj/ehi406. [DOI] [PubMed] [Google Scholar]
- 34.Jong P, Yusuf S, Rousseau MF, Ahn SA, Bangdiwala SI. Effect of enalapril on 12-year survival and life expectancy in patients with left ventricular systolic dysfunction: a follow-up study. Lancet. 2003;361:1843–1848. doi: 10.1016/S0140-6736(03)13501-5. [DOI] [PubMed] [Google Scholar]
- 35.Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108:977–82. doi: 10.1161/01.CIR.0000085166.44904.79. [DOI] [PubMed] [Google Scholar]
- 36.The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 37.Wang TJ, Levy D, Benjamin EJ, Vasan RS. The epidemiology of "asymptomatic" left ventricular systolic dysfunction: implications for screening. Ann Intern Med. 2003;138:907–16. doi: 10.7326/0003-4819-138-11-200306030-00012. [DOI] [PubMed] [Google Scholar]
- 38.Heidenreich PA, Gubens MA, Fonarow GC, Konstam MA, Stevenson LW, Shekelle PG. Cost-effectiveness of screening with B-type natriuretic peptide to identify patients with reduced left ventricular ejection fraction. J Am Coll Cardiol. 2004;43:1019–26. doi: 10.1016/j.jacc.2003.10.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.