Abstract
Although changes in diet and physical activity are undoubtedly key causal factors related to the increase in obesity, there is growing interest in the possibility that endocrine disrupting chemicals (EDCs) may affect obesity-related pathways by altering cell signaling involved in weight and lipid homeostasis. Proposed mechanisms that could underlie associations between EDCs and obesity include effects on thyroid and steroid hormones, and activation of peroxisome proliferator-activated receptors (PPARs) which play a major role in adipocyte differentiation and energy storage. Most evidence supporting the hypothesis that EDCs affect obesity comes from laboratory studies. We summarize the limited epidemiologic literature on the topic, including prospective studies of human prenatal exposure to EDCs. We also present findings from a cross-sectional study of levels of six phthalate metabolites and body mass index (BMI) and waist circumference (WC), using data from the U.S. National Health and Nutrition Examination Survey (NHANES). We found positive associations between BMI and WC among adult males for most phthalate metabolites. For example, in males aged 20–59, the adjusted mean BMI across quartiles of mono-benzyl phthalate (MBzP) was 26.7, 27.2, 28.4, 29.0 (p-trend=0.0002). In females, BMI and WC increased with quartiles of mono-ethyl phthalate (MEP) in 12–19 year olds (adjusted mean BMI=22.9, 23.8, 24.1, 24.7, p-trend =0.03), and a similar but less strong pattern was seen in 20–59 year olds. In contrast, higher levels of mono-2-ethylhexyl phthalate (MEHP) were associated with lower BMI in adolescent girls and females aged 20–59. This exploratory analysis found several associations between phthalate metabolites and obesity, including notable differences by gender. However, the cross-sectional data is a limitation. Additional prospective studies of the association between exposures to EDCs, especially during development, and obesity are warranted. As this field of research advances, there are challenging methodologic questions that must be considered by both epidemiologists and toxicologists.
Keywords: Epidemiology, obesity, endocrine disrupting chemicals, phthalates, NHANES
Introduction
The rising prevalence of obesity is of great public health concern. Among U.S. adults, this figure has doubled from 15% in the early 1970s to the most recent estimate of 34% for 2003–2006, and has tripled among young adults to the current 24% (CDC, 2008). Similar increases have been seen in children and adolescents. A recent review of European data showed obesity prevalence in adults to range from 4 to 37% depending on country, with higher proportions in Central, Eastern, and Southern Europe compared to Western and Northern Europe (Berghofer, 2008). Rates in some less developed countries are also growing dramatically, particularly in Latin America, the Caribbean, the Middle East, and North Africa (Martorell, 2000).
Obesity is closely linked to other components of the metabolic syndrome and numerous numerous adverse health effects, including type II diabetes, heart disease, certain cancers, and, ultimately, mortality (Guh, 2009). The increase in obesity is generally attributed to changes in diet and physical activity, but other factors may also play a role (Keith, 2006). The idea that changes in the diet and physical activity cannot account for all of the increase in obesity is strengthened by evidence showing that most energy expenditure is devoted to basal metabolism and other physiological processes. Furthermore, complex endocrine and other chemical signaling mechanisms exist which change resting and non-resting energy expenditure to promote constant weight in a person (Hamilton, 2007; Goodman, 2009). It is notoriously difficult for an overweight person to lose and maintain weight loss, pointing to the notion that every individual has a weight “set point” (Leibel, 1995).
In addition to sleep deprivation, more stable home temperatures, and an increase in maternal age at birth (Keith, 2006), another proposed contributor to the rise in obesity is exposure to endocrine disrupting chemicals (EDCs), the focus of this paper. EDCs are chemicals that alter the normal functioning of hormones and other signaling molecules in the body (Diamanti-Kandarakis, 2009). While much early work on endocrine disruption focused on reproductive and carcinogenic effects, the hypothesis that chemicals may affect weight homeostasis emerged more recently from different lines of research.
Where adipose tissue was once considered an inert storage depot, the discovery of the hormone leptin in the 1990s led to the recognition that adipose itself is an active endocrine organ, secreting various hormones and adipokines and expressing many receptors (Ahima and Flier, 2000; Newbold, 2009). Evidence was also mounting in support of the concept of “the developmental basis of adult disease,” which suggests that exposures in utero could lead to chronic diseases later in life (Heindel, 2003; Oken and Gillman, 2003). In addition, reports started to emerge describing excess weight gain in animals treated developmentally with certain EDCs, including diethylstilbestrol (DES) and bisphenol A (BPA) (Howdeshell, 1999; Newbold, 2005). A 2002 review articulated the hypothesis for the first time, citing as evidence chemicals for which weight gain is a known side effect (e.g. certain pharmaceuticals) and arguing that weight gain may have gone largely unnoted by toxicologists mainly concerned about weight loss as a sign of toxicity (Baillie-Hamilton, 2002).
The term “obesogens” was coined in 2006, and defined as “molecules that inappropriately regulate lipid metabolism and adipogenesis to promote obesity” (Grun and Blumberg, 2006). Others have recently proposed identifying a subclass of EDCs that broadly affect metabolic signaling such as weight homeostasis, and calling these chemicals “metabolic disruptors” (Casals-Casas, 2008). The list of chemicals studied as possible obesogens continues to grow, and includes DES, BPA, phthalates, organotins, polybrominated diphenyl ethers (PBDEs), polyfluoroalkyl chemicals (PFCs), organochlorine (OC) pesticides, and polychlorinated biphenyls (PCBs).
The amount of research in this area has risen dramatically over the last five years, though the majority of studies have been in the toxicology literature. This article reviews the still sparse epidemiologic literature on associations between EDC exposure and body weight, discusses some of our own work on the association between phthalates and obesity, and concludes with a consideration of challenges for the field as it moves forward.
Plausible Mechanisms for Endocrine Disruptor Effects on Obesity
While there are numerous potential targets of endocrine disruption on weight homeostasis, the mechanisms are at the early stages of investigation. A distinction should be drawn between non-developmental and in utero exposures, which may have quite different effects on these pathways.
Regarding possible mechanisms that may pertain to non-developmental exposures, a variety of chemicals have been shown to bind peroxisome proliferator activated receptors (PPARs). PPARs are receptors that play critical roles in adipogenesis and lipid metabolism (Grun and Blumberg, 2009). PPAR-gamma is described as a master regulator of fat cell development, with activation required for adipocyte differentiation and maturation (Evans, 2004). The thiazolidinediones are PPAR-gamma agonist medications used to improve insulin resistance, but are also associated with weight gain. Among environmental contaminants, some organotins (Grun and Blumberg, 2006) and phthalates (Hurst and Waxman, 2003) are PPAR-gamma agonists and have been linked to weight gain in animal studies.
Sex hormones also influence fat quantity and distribution (Pasquali, 2006; Lovejoy and Sainsbury, 2009). In adulthood, androgens are related to lower BMI in men (Gapstur, 2002), whereas estrogen levels tend to be inversely correlated with obesity and waist-to-hip ratio in women (Bjorntorp, 1996). Several phthalates are suspected antiandrogens in humans (Main, 2006; Pan, 2006; Swan, 2008). Numerous xenoestrogens and antiestrogens have been identified (Newbold, 2009).
Another possible mechanism is perturbation of thyroid function, which is key to maintenance of basal metabolism. A large cross-sectional study in Denmark found that small reductions in thyroid hormone were associated with significant increases in BMI after excluding people with thyroid dysfunction (Knudsen, 2005). A number of EDCs, including phthalates, BPA, and PBDEs, are suspected thyroid disruptors and seem to have the net effect of reducing circulating thyroid levels (Zoeller, 2007).
In utero exposures may be particularly important due to sensitivity during development. Certain environmental agents or stressors during critical windows of development can lead to obesity later in life. The “thrifty phenotype” or Barker hypothesis postulates that an undernourished fetal environment causes permanent metabolic programming aimed at conserving nutrients, leaving the individual susceptible to obesity and other metabolic disorders when faced with the unrestricted diet typical of many developed countries (Gluckman, 2008). This programming likely involves epigenetic alterations in the methylation patterns and histone structure that help determine gene expression, alterations which may lead to obesity due to permanent changes in the function of specific tissues (Heindel and vom Saal, 2009). Maternal malnutrition, smoking, diabetes, and chronic stress have all been consistently associated with childhood obesity in epidemiologic studies, and provide evidence for this phenomenon (Huang, 2007).
In utero exposure to EDCs may alter development of adipose tissue itself in terms of number, size, and distribution of adipocytes formed, and may affect the larger regulatory systems involved in body weight homeostasis. Recent reviews have summarized a number of hypotheses about mechanisms (Grun and Blumberg, 2009; Heindel and vom Saal, 2009; Rubin and Soto, 2009). First, estrogen exposure during development has been shown to increase the number of adipocytes and alter subsequent functioning of these cells, unlike its anti-adipogenic effect with exposure in adulthood (Heindel and vom Saal, 2009). Exposure to estrogenic chemicals such as BPA and DES may impact obesity through this pathway (Newbold, 2005; Rubin and Soto, 2009). Studies of developmental DES exposure in mice found changes in expression of genes involved in fat distribution at 19 days of age (Newbold, 2009). Second, while in utero exposure to PPAR-gamma agonists could have lasting impacts on the pathways of adipocyte development (Grun and Blumberg, 2009), exposure to PPAR-alpha agonists has also been discussed in the developmental context. As activation of the PPAR-alpha isoform increases fatty acid metabolism, exposure during development to PPAR-alpha agonists such as perfluorooctanoic acid (PFOA) may result in a starved fetal environment and subsequent obesity (Hines, 2009). Third, thyroid disruption is another possible target here as well: in utero thyroid levels are known to be involved in programming of future body weight (Grun and Blumberg, 2009).
Finally, a much less explored area is possible EDC effects on the neuroendocrine pathways involved in the hypothalamic-pituitary-adrenal axis that controls appetite and satiety (Grun and Blumberg, 2009). Alterations in these signaling systems could involve developmental programming and/or result in shorter-term effects with non-developmental exposures.
Epidemiologic studies on EDCs and obesity
There are only limited data on the possible role of EDCs in the development of obesity in humans. For mechanistic reasons, we distinguish between studies of in utero vs. later-life exposure. Similarly, measuring exposure to persistent and non-persistent compounds present different challenges.
Because of the evidence for programming of obesity during the prenatal period, measurement of EDCs during pregnancy may be the most relevant etiologic time frame for evaluating associations with childhood and adult obesity. While there is a large and growing literature examining associations between chemical exposure and birth weight (Wigle, 2008)—and growth retardation may be one plausible causal pathway to obesity in later life—we focus here on studies that relate developmental exposure to obesity later in life. To our knowledge, there have been no such studies for non-persistent chemicals. Several pregnancy cohort studies examined persistent chemicals in relation to childhood or adult BMI or height. Exposure assessment in these studies varied from direct assessment of levels of chemicals in maternal serum collected in late pregnancy (Patandin, 1998; Gladen, 2000; Gladen, 2004; Ribas-Fito, 2006;) or in cord blood (Gladen, 2000; Smink, 2008; Verhulst, 2009), to estimation of pregnancy levels by means of statistical modeling using measurements from serum collected later in the mother’s life (Blanck, 2002; Karmaus, 2009). Studies also varied in terms of outcome assessment, with some relying on self-reported weight and height, while others examined and measured weight, height, and skinfold thicknesses in study participants. Age at assessment of outcomes in these studies ranged from 12 months to adulthood.
In general, findings from these studies are inconsistent. Three studies reported positive associations between levels of dichlorodiphenyldichloroethylene (DDE), a metabolite of the OC pesticide dichlorodiphenyltrichloroethane (DDT), and BMI, weight, or height, but with some variation across subgroups. In a study of pregnancy exposures in relation to anthropometric measures in early childhood, Verhulst et al reported a stronger association between prenatal DDE and BMI in children of smokers (Verhulst, 2009). Gladen et al found effects of transplacental DDE exposure on height and weight adjusted for height in adolescent males but not females (Gladen, 2000). In a study of female offspring of a cohort of women exposed to high levels OCs in fish, Karmaus et al found a positive association with BMI in adulthood, but exposure was estimated by extrapolating current DDE levels to levels that would be expected during pregnancy (Karmaus, 2009). Other studies reported either negative (Ribas-Fito, 2006) or null associations between DDE and growth outcomes (Gladen, 2004). A study of 400 Spanish children found a two fold higher risk of obesity in children whose cord blood levels of hexachlorobenzene, another OC pesticide, were in the highest quartile compared to the lowest (Smink, 2008). Numerous confounding variables were included in the models, and similar results were found when the analysis was restricted to offspring of normal weight mothers. Male offspring of women who worked in greenhouses during pregnancy had higher BMI z scores, body fat percentage, skinfold thickness and hormonal differences measured between ages 6–10 (Wohlfahrt-Veje, 2009). No differences in BMI were found in female offspring, but exposed females had earlier breast development than unexposed females (Wohlfahrt-Veje, 2009). Specific pesticide exposures were not reported. Mixed results have been reported for PCB exposure in relation to BMI or height (Patandin, 1998; Gladen, 2000; Blanck, 2002; Smink, 2008; Karmaus, 2009; Verhulst, 2009). Two studies reported small positive associations with BMI in young children (Verhulst, 2009) and in adolescent females (Gladen, 2000), but other studies have suggested either negative (Blanck, 2002) or null associations between PCBs and BMI (Karmaus, 2009). The diversity of findings in these studies makes it difficult to draw conclusions about developmental EDC exposure and obesity. Differences in control of confounding variables, methods of exposure assessment, and definition and measurement of outcome variables all may have contributed to the inconsistencies between studies.
With post-natal and adult exposures, the epidemiologic literature is even more limited, and often involves cross-sectional studies not necessarily designed to answer this research question. For persistent compounds, there are difficult methodological problems with reverse causality (see methodological challenges section below). Body weight may be an important covariate in studies of the potential role of persistent chemicals in many health outcomes, such as cancer and cardiovascular disease. Some of these studies have evaluated associations between persistent organic pollutants such as PCBs and dioxins and BMI, but the findings are difficult to interpret (Longnecker, 2006; Wolff, 2007) A handful of other studies have considered this question incidentally, such as a birth cohort study that found that mothers who were overweight or obese before pregnancy had higher plasma levels of PFOS and PFOA (Fei, 2007), and an occupational health study that found BMI to be slightly higher in workers in the highest category of PFOA exposure, although there was no adjustment for confounding (Olsen, 1998). For non-persistent compounds, only a few studies have examined the association between post-natal exposure to phthalates and body size. A small, cross-sectional study of 90 girls aged 6–8 found slightly higher levels of most phthalate metabolites among girls with BMI ≥85% compared to thinner girls, although none of the comparisons reached statistical significance (Wolff, 2007). For example, the geometric means (ug/g creatinine) were 144 vs. 102 for mono-ethyl phthalate (MEP), 56.1 vs. 43.0 for mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), and 47.6 vs 43.3 for mono-n-butyl phthalate (MBP) in girls ≥85% versus girls <85%. Stahlhut evaluated the relationship between phthalate metabolites and waist circumference (WC, a measure of central obesity), and homeostasis model assessment (HOMA, a measure of insulin resistance), in adult males using cross-sectional data from the National Health and Nutrition Examination Survey (NHANES). The authors found mostly positive associations between WC, HOMA and several phthalates (Stahlhut, 2007), but did not report results for females.
Gender Differences in the Association between Phthalates and Obesity in NHANES
We examined the association between six urinary phthalate metabolites and BMI and WC in data from NHANES 1999–2002. Details of this analysis have been described previously (Hatch, 2008). Briefly, NHANES interviewed and examined participants to assess behavioral and physical characteristics. As part of a biomonitoring program conducted by the U.S. National Center for Environmental Health, blood and urine were collected on a random sample of one-third of the participants. Methods of laboratory analysis of phthalates are reported elsewhere (Silva, 2004). We analyzed six metabolites detectable in at least 80% of the study population: MEP, mono-2-ethylhexyl (MEHP), MBP, mono-benzyl (MBzP), MEHHP, and mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP). The latter two are further metabolites of MEHP. After exclusions, a total of 4369 subjects were available for the analyses of MEP, MEHP, MBP, and MBzP, and 2286 subjects for the analyses of MEHHP and MEOHP (measured only during 2001–2002).
We used multivariable linear regression models to examine the association between level of phthalates (categorized into quartiles) and BMI and WC for males and females within age-specific categories. Separate regression models were run for each phthalate. We also created a summary variable to estimate exposure to the four primary metabolites (MEP, MEHP, MBP, and MBzP) simultaneously. The summary score variable was computed by the following method: 1) each individual was ranked according to level of each of the four phthalates (from 1 to 4369), 2) the mean of the 4 ranks was calculated, and 3) the resulting distribution of mean ranks for the study population was divided into quartiles.
We controlled for the following potential confounding variables: age, creatinine, race/ethnicity, height, socioeconomic status, diet (percent of calories from total fat, dairy, fruit and vegetable consumption), physical activity (METS/month and TV/video/computer use), smoking, menopausal status, and parity. Tests for trend were performed by treating phthalate category as a linear predictor in the models.
Associations between phthalate metabolites and the outcomes, BMI and WC, differed markedly by gender. For MBzP, there was a strong positive relationship with BMI and WC among adult males aged 20–59 (adjusted mean BMIs from quartile 1 to 4 were 26.7, 27.2, 28.4, and 29.0 respectively (p-trend=0.0002)), but no discernible trends among females. Similarly, for MBP, the patterns in adult males and females were different, with suggestive positive trends among males for both BMI and WC, but inverse trends among females. While MEHP was inversely related to BMI and WC in adolescent and adult females, there were no major trends between MEHP and either BMI or WC among males. Results for MEHHP and MEOHP were similar (as expected due to their high correlation). There were positive associations between both metabolites and BMI and WC among 20–59 year old males, and no important trends in BMI or WC among adult females; for 60–80 year olds the trends were inverse in both genders. Associations seen with MEP were a bit different than with the other metabolites: a positive relationship between MEP quartile and BMI was apparent for adult males (20–59 and 60–80), and for adolescent and adult females (20–59). For example, among adolescent girls, adjusted mean BMIs increased from 22.9, 23.8, 24.1, to 24.7 (p-trend =0.03) across quartiles of MEP, and adjusted mean WCs were 77.4, 79.7, 80.1, and 81.6 (p-trend=0.02). In general, there were few associations of note among children aged 6 to 11 for any of the metabolites.
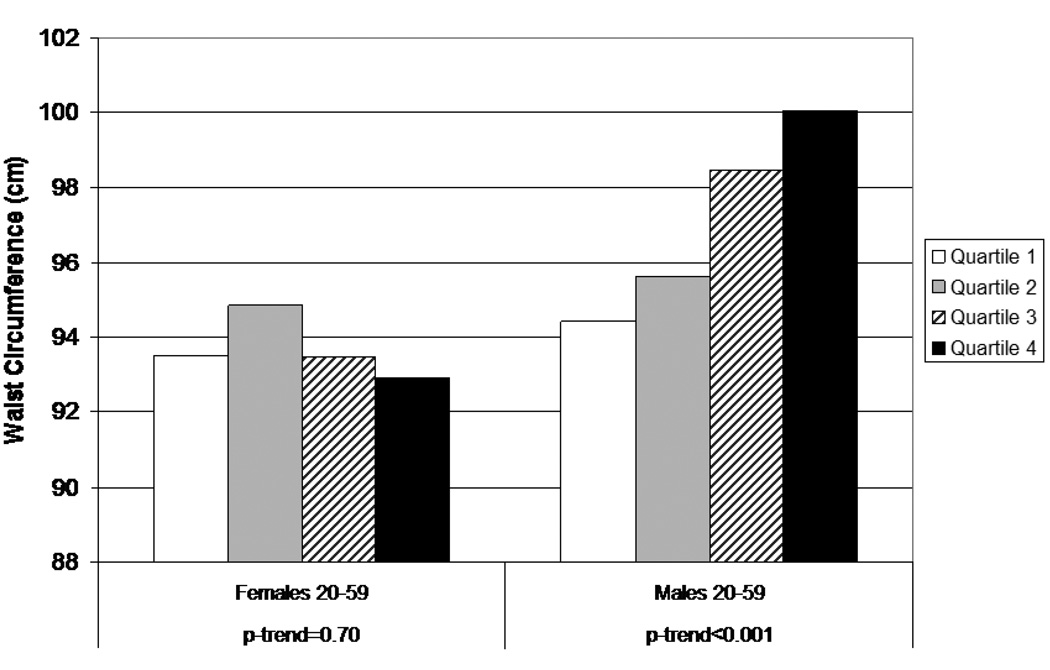
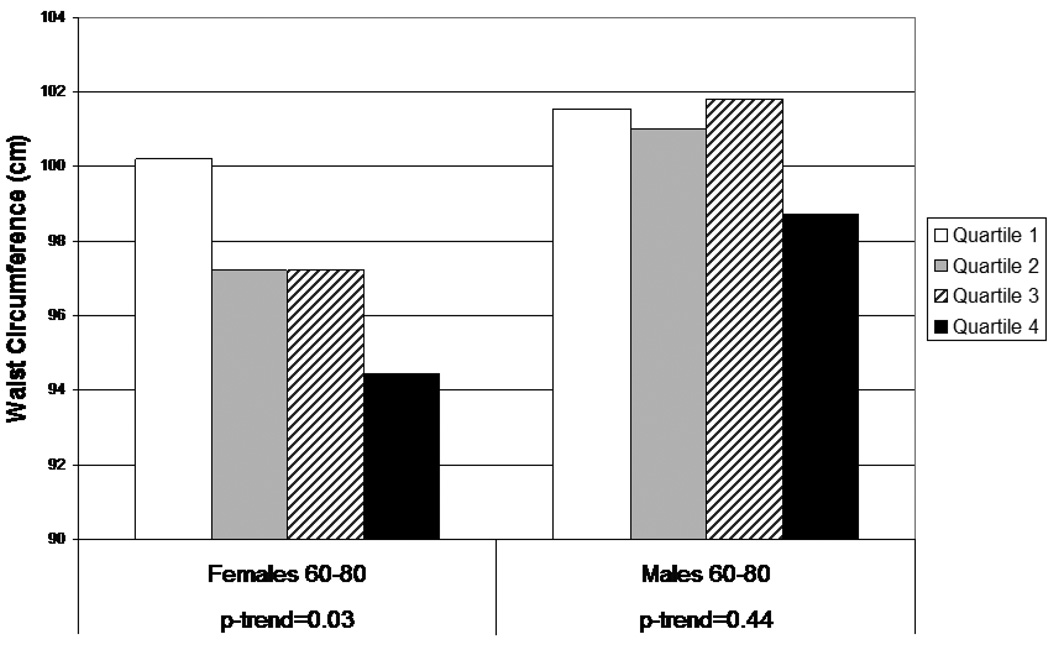
Results for WC using the summary score variable are shown in Figures 1 and 2. For adults aged 20–59 (Figure 1), the notable difference by gender is again apparent, with a marked positive trend among males (p<0.001) and little evidence of any pattern among females. For older adults (60–80) (Figure 2), subjects in the highest quartile of the summary score variable had smaller WCs than those in the other three quartiles, and there appeared to be a decreasing trend across quartiles in females. Similar results were found for BMI, not surprisingly because of the high correlation between BMI and WC.
Figure 1.
Waist circumference by quartile of combined phthalate score (MEP, MBP, MBzP, MEHP), females and males, aged 20–59
Figure 2.
Waist circumference by quartile of combined phthalate score (MEP, MBP, MBzP, MEHP), females and males, aged 60–80
The striking contrast in results between males and females, particularly among 20–59 year olds, is biologically plausible. Several phthalates are anti-androgens, and thus it is possible that effects may differ according to levels of endogenous hormones. As discussed previously, higher androgen levels are associated with smaller WC in males, whereas higher androgen levels in females are associated with higher BMI, greater risk for metabolic syndrome, and conditions such as polycystic ovarian disease (Barber, 2006). Therefore, women with the highest levels of MEHP (the metabolite with the strongest inverse relationship with BMI and WC among females) may have lower levels of androgens or a higher estrogen/androgen ratio, which could explain the inverse relationship between MEHP and BMI. Our results appear more consistent with an antiandrogenic action of certain phthalates than with action on PPAR-gamma or thyroid hormones, which might be expected to have similar effects in males and females. However, other explanations for the associations may be involved, particularly for MEP. Table 1 lists three possible biologic mechanisms that may be involved in the association between phthalate metabolites and the occurrence of obesity (for original references see Hatch, 2008, Table 4).
Table 1.
Biological Effects of Phthalates Potentially Related to Obesity
| PPAR-γ activation |
Thyroid effect | Anti-androgenic effect |
|
|---|---|---|---|
| DEP/MEP | − | − | +/− |
| DBP/MBP | +/− | +/− | ++ |
| BBzP/MBzP | + | ++ | ++ |
| DEHP/MEHP | ++ | +/− | + |
++ strong, consistent effects
+ moderate, consistent effects
+/− inconsistent effects
− no effect
Source: Hatch et al (2008)
Our exploratory study found several associations between phthalate metabolites and BMI and WC with magnitudes of potential clinical relevance. This study has a number of strengths, including the opportunity to evaluate results separately by gender, the assessment of multiple potential confounding variables (including physical activity and several aspects of diet), and a broad range of exposure levels in the population. Increased exposure to phthalates via diet is unlikely to be an explanation of our findings. There are numerous limitations. Cross-sectional studies cannot make causal inferences about the direction of the association between phthalates and obesity. Although phthalates are rapidly metabolized and do not accumulate in adipose tissue, heavier individuals may conceivably metabolize phthalates differently than individuals with less body fat. In addition, some exposure to phthalates, especially DEP, occurs through dermal absorption. Thus, heavier individuals are apt to have a greater body surface area, and may absorb more phthalates from use of lotions or other cosmetics. Confounding by unmeasured factors, such as medication use or fasting time before urine sample is a possibility, as is residual confounding. Single spot-urine measurement, used to estimate exposure, may not be a good proxy for long-term exposure, although studies of the consistency of phthalate measurements over time have shown moderate to high sensitivity to classify individuals into the highest tertile of exposure (Hauser, 2004). We were unable to look at exposure to other EDCs, which may have different effects than single exposures (Kortenkamp, 2007). We evaluated combinations of phthalates using the simplistic approach of combining rankings for individual phthalates into a single exposure variable, but the correct metric is unknown. We could not evaluate exposures throughout the life course, particularly during prenatal and neonatal development.
Methodologic Challenges in Epidemiologic Research on Obesogens
The complexity of the etiology of obesity and related disorders poses numerous challenges for the field as it moves forward. While rodent models and in vitro assays play a critical role in advancing this research, the many differences between rodent and human physiology in regards to adipogenesis must be considered (Ben-Jonathan, 2009). As this review highlights, human studies of the association between EDCs and obesity are few and suffer from methodologic limitations. Data are particularly lacking for chemicals that the emerging animal literature points to as being of notable concern, including organotins and BPA. Epidemiologists must pay close attention to the difficulties in measuring diet and exercise, the “Big Two” risk factors that may cause residual confounding if they covary with exposure. Also difficult are the logistical and conceptual challenges in studying the effect of mixtures of early life exposures on outcomes much later in life. Another area in need of additional study is the effect that pharmacokinetic differences in metabolism of chemicals may have on observed associations. Cross-sectional studies may be subject to reverse causality; therefore, it is important to investigate whether overweight individuals metabolize or excrete chemicals differently than non-overweight people. Many persistent chemicals accumulate in lipid and their absorption and excretion are related to body fat and weight loss/gain in complicated ways (Longnecker, 2006; Webster, 2006; Wolff, 2007). Non-persistent chemicals are often measured in urine, adjusting for creatinine, but creatinine, a byproduct of muscle activity, may also be associated with muscularity and body size (Hauser, 2004; Gerchman, 2009). Finally, we must advance our approach to outcome measurement beyond the use of height, weight, BMI, and WC. Additional techniques to assess obesity need to be further developed and employed in human studies, including related but perhaps more sensitive biomarkers of effect, such as adiponectin, leptin, and other relevant hormones. It would be particularly useful to incorporate some of these biomarkers into large hypothesis-generating data sets such as NHANES. We are encouraged by plans to incorporate anthropometric measures beyond BMI and WC into the National Children’s Study (Trasande, 2009).
Conclusions
Despite the challenges, the obesogen hypothesis appears to be an intriguing line of research. The toxicological research so far has expanded the notion of endocrine disruptors beyond its original primary focus on reproductive and carcinogenic outcomes to potential effects on a recently recognized endocrine organ: adipose tissue. Nevertheless, important aspects of the early thinking about EDC research remain, particularly the probable sensitivity of in utero exposure and the importance of mixtures. The epidemiologic research conducted so far, while often exploratory and cross-sectional, has found some associations that if real would be of clinical significance. The inconsistencies of the results draw attention to important and interesting methodological questions. Follow-up studies of different populations with varying designs are warranted. Of particular interest will be prospective studies that evaluate prenatal and neonatal exposures to multiple endocrine disrupting chemicals.
Acknowledgements
The authors would like to acknowledge Lynn Moore and Martha Singer for their extensive contributions to the dietary variables in the study, Mustafa Qureshi for computer programming assistance, and Janice Weinberg for statistical advice. Some results described in this paper were previously published in Environmental Health 7:27, 2008. This work was supported in part by a grant from the National Institute of Environmental Health Sciences: R21 ES013724. Jessica Nelson was supported in part by Award Number T32ES014562 from NIEHS. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIEHS or the National Institutes of Health.
References
- Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab. 2000;11(8):327–332. doi: 10.1016/s1043-2760(00)00301-5. [DOI] [PubMed] [Google Scholar]
- Baillie-Hamilton PF. Chemical toxins: a hypothesis to explain the global obesity epidemic. J Altern Complement Med. 2002;8(2):185–192. doi: 10.1089/107555302317371479. [DOI] [PubMed] [Google Scholar]
- Barber TM, McCarthy MI, et al. Obesity and polycystic ovary syndrome. Clinical Endocrinology. 2006;65(2):137–145. doi: 10.1111/j.1365-2265.2006.02587.x. [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N, Hugo ER, et al. Effects of bisphenol A on adipokine release from human adipose tissue: Implications for the metabolic syndrome. Mol Cell Endocrinol. 2009;304(1–2):49–54. doi: 10.1016/j.mce.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghofer A, Pischon T, et al. Obesity prevalence from a European perspective: a systematic review. BMC Public Health. 2008;8:200. doi: 10.1186/1471-2458-8-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorntorp P. The regulation of adipose tissue distribution in humans. Int J Obes Relat Metab Disord. 1996;20(4):291–302. [PubMed] [Google Scholar]
- Blanck HM, Marcus M, et al. Growth in girls exposed in utero and postnatally to polybrominated biphenyls and polychlorinated biphenyls. Epidemiology. 2002;13(2):205–210. doi: 10.1097/00001648-200203000-00016. [DOI] [PubMed] [Google Scholar]
- Casals-Casas C, Feige JN, et al. Interference of pollutants with PPARs: endocrine disruption meets metabolism. Int J Obes (Lond) 2008;32 Suppl 6:S53–S61. doi: 10.1038/ijo.2008.207. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Third National Report on Human Exposure to Environmental Chemicals. Atlanta: CDC; 2005. [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Health United States. Atlanta: CDC; 2008. [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RM, Barish GD, et al. PPARs and the complex journey to obesity. Nat Med. 2004;10(4):355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, et al. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ Health Perspect. 2007;115(11):1677–1682. doi: 10.1289/ehp.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gapstur SM, Gann PH, et al. Serum androgen concentrations in young men: a longitudinal analysis of associations with age, obesity, and race. The CARDIA male hormone study. Cancer Epidemiol Biomarkers Prev. 2002;11(10 Pt 1):1041–1047. [PubMed] [Google Scholar]
- Gerchman F, Tong J, et al. Body Mass Index is Associated with Increased Creatinine Clearance by a Mechanism Independent of Body Fat Distribution. J Clin Endocrinol Metab. 2009 doi: 10.1210/jc.2008-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladen BC, Klebanoff MA, et al. Prenatal DDT exposure in relation to anthropometric and pubertal measures in adolescent males. Environ Health Perspect. 2004;112(17):1761–1767. doi: 10.1289/ehp.7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladen BC, Ragan NB, et al. Pubertal growth and development and prenatal and lactational exposure to polychlorinated biphenyls and dichlorodiphenyl dichloroethene. J Pediatr. 2000;136(4):490–496. doi: 10.1016/s0022-3476(00)90012-x. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, et al. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359(1):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman H. Basic Medical Endocrinology. Academic Press; 2009. [Google Scholar]
- Grun F, Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006;147(6 Suppl):S50–S55. doi: 10.1210/en.2005-1129. [DOI] [PubMed] [Google Scholar]
- Grun F, Blumberg B. Endocrine disrupters as obesogens. Mol Cell Endocrinol. 2009;304(1–2):19–29. doi: 10.1016/j.mce.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guh DP, Zhang W, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton MT, Hamilton DG, et al. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56(11):2655–2667. doi: 10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- Hatch EE, Nelson JW, et al. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999–2002. Environ Health. 2008;7:27. doi: 10.1186/1476-069X-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, et al. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect. 2004;112(17):1734–1740. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ. Endocrine disruptors and the obesity epidemic. Toxicol Sci. 2003;76(2):247–249. doi: 10.1093/toxsci/kfg255. [DOI] [PubMed] [Google Scholar]
- Heindel JJ, vom Saal FS. Role of nutrition and environmental endocrine disrupting chemicals during the perinatal period on the aetiology of obesity. Mol Cell Endocrinol. 2009;304(1–2):90–96. doi: 10.1016/j.mce.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Hines EP, White SS, et al. Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female CD-1 mice: Low doses induce elevated serum leptin and insulin, and overweight in mid-life. Mol Cell Endocrinol. 2009;304(1–2):97–105. doi: 10.1016/j.mce.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Hotchkiss AK, et al. Exposure to bisphenol A advances puberty. Nature. 1999;401(6755):763–764. doi: 10.1038/44517. [DOI] [PubMed] [Google Scholar]
- Huang JS, Lee TA, et al. Prenatal programming of childhood overweight and obesity. Matern Child Health J. 2007;11(5):461–473. doi: 10.1007/s10995-006-0141-8. [DOI] [PubMed] [Google Scholar]
- Hurst CH, Waxman DJ. Activation of PPARalpha and PPARgamma by environmental phthalate monoesters. Toxicol Sci. 2003;74(2):297–308. doi: 10.1093/toxsci/kfg145. [DOI] [PubMed] [Google Scholar]
- Karmaus W, Osuch JR, et al. Maternal levels of dichlorodiphenyl-dichloroethylene (DDE) may increase weight and body mass index in adult female offspring. Occup Environ Med. 2009;66(3):143–149. doi: 10.1136/oem.2008.041921. [DOI] [PubMed] [Google Scholar]
- Keith SW, Redden DT, et al. Putative contributors to the secular increase in obesity: exploring the roads less traveled. Int J Obes (Lond) 2006;30(11):1585–1594. doi: 10.1038/sj.ijo.0803326. [DOI] [PubMed] [Google Scholar]
- Knudsen N, Laurberg P, et al. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab. 2005;90(7):4019–4024. doi: 10.1210/jc.2004-2225. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A. Ten Years of Mixing Cocktails - a Review of Combination Effects of Endocrine Disrupting Chemicals. Environmental Health Perspectives. 2007;115 Supplement 1:98–105. doi: 10.1289/ehp.9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibel RL, Rosenbaum M, et al. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332(10):621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- Longnecker MP. Pharmacokinetic variability and the miracle of modern analytical chemistry. Epidemiology. 2006;17(4):350–351. doi: 10.1097/01.ede.0000222510.59457.7b. [DOI] [PubMed] [Google Scholar]
- Lovejoy JC, Sainsbury A. Sex differences in obesity and the regulation of energy homeostasis. Obes Rev. 2009;10(2):154–167. doi: 10.1111/j.1467-789X.2008.00529.x. [DOI] [PubMed] [Google Scholar]
- Main KM, Mortensen GK, et al. Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environ Health Perspect. 2006;114(2):270–276. doi: 10.1289/ehp.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martorell R, Khan LK, et al. Obesity in women from developing countries. Eur J Clin Nutr. 2000;54(3):247–252. doi: 10.1038/sj.ejcn.1600931. [DOI] [PubMed] [Google Scholar]
- Newbold RR, Padilla-Banks E, et al. Environmental estrogens and obesity. Mol Cell Endocrinol. 2009;304(1–2):84–89. doi: 10.1016/j.mce.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold RR, Padilla-Banks E, et al. Developmental exposure to estrogenic compounds and obesity. Birth Defects Res A Clin Mol Teratol. 2005;73(7):478–480. doi: 10.1002/bdra.20147. [DOI] [PubMed] [Google Scholar]
- Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;11(4):496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Gilliland FD, et al. An epidemiologic investigation of reproductive hormones in men with occupational exposure to perfluorooctanoic acid. J Occup Environ Med. 1998;40(7):614–622. doi: 10.1097/00043764-199807000-00006. [DOI] [PubMed] [Google Scholar]
- Pan G, Hanaoka T, et al. Decreased serum free testosterone in workers exposed to high levels of di-n-butyl phthalate (DBP) and di-2-ethylhexyl phthalate (DEHP): a cross-sectional study in China. Environ Health Perspect. 2006;114(11):1643–1648. doi: 10.1289/ehp.9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali R. Obesity and androgens: facts and perspectives. Fertil Steril. 2006;85(5):1319–1340. doi: 10.1016/j.fertnstert.2005.10.054. [DOI] [PubMed] [Google Scholar]
- Patandin S, Koopman-Esseboom C, et al. Effects of environmental exposure to polychlorinated biphenyls and dioxins on birth size and growth in Dutch children. Pediatr Res. 1998;44(4):538–545. doi: 10.1203/00006450-199810000-00012. [DOI] [PubMed] [Google Scholar]
- Ribas-Fito N, Gladen BC, et al. Prenatal exposure to 1,1-dichloro-2,2-bis (p-chlorophenyl)ethylene (p,p'-DDE) in relation to child growth. Int J Epidemiol. 2006;35(4):853–858. doi: 10.1093/ije/dyl067. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Soto AM. Bisphenol A: Perinatal exposure and body weight. Mol Cell Endocrinol. 2009;304(1–2):55–62. doi: 10.1016/j.mce.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Barr DB, et al. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect. 2004;112(3):331–338. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smink A, Ribas-Fito N, et al. Exposure to hexachlorobenzene during pregnancy increases the risk of overweight in children aged 6 years. Acta Paediatr. 2008;97(10):1465–1469. doi: 10.1111/j.1651-2227.2008.00937.x. [DOI] [PubMed] [Google Scholar]
- Stahlhut RW, van Wijngaarden E, et al. Concentrations of Urinary Phthalate Metabolites are Associated with Increased Waist Circumference and Insulin Resistance in Adult U.S. Males. Environ Health Perspect. 2007;115:876–882. doi: 10.1289/ehp.9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res. 2008;108(2):177–184. doi: 10.1016/j.envres.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, Cronk C, et al. Environment and obesity in the National Children's Study. Environ Health Perspect. 2009;117(2):159–166. doi: 10.1289/ehp.11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst SL, Nelen V, et al. Intrauterine exposure to environmental pollutants and body mass index during the first 3 years of life. Environ Health Perspect. 2009;117(1):122–126. doi: 10.1289/ehp.0800003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster TF. Pharmacokinetics of POPs: Simple models with different implications for halflives and steady state levels. Organohalogen Compounds. 2006;68:344–347. [Google Scholar]
- Wigle DT, Arbuckle TE, et al. Epidemiologic evidence of relationships between reproductive and child health outcomes and environmental chemical contaminants. J Toxicol Environ Health B Crit Rev. 2008;11(5–6):373–517. doi: 10.1080/10937400801921320. [DOI] [PubMed] [Google Scholar]
- Wohlfahrt-Veje C, andersen HR, Jensen TK, Grandjean P, Skakkebaek N, Main KM. Long-term effects of prenatal pesticide exposure on several endocrine functions in children. 5th Copenhagen Workshop on Endocrine Disruptors; Copenhagen. 2009. [Google Scholar]
- Wolff MS, Anderson HA, et al. Pharmacokinetic variability and modern epidemiology--the example of dichlorodiphenyltrichloroethane, body mass index, and birth cohort. Cancer Epidemiol Biomarkers Prev. 2007;16(10):1925–1930. doi: 10.1158/1055-9965.EPI-07-0394. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Teitelbaum SL, et al. Pilot study of urinary biomarkers of phytoestrogens, phthalates, and phenols in girls. Environ Health Perspect. 2007;115(1):116–121. doi: 10.1289/ehp.9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller RT. Environmental chemicals impacting the thyroid: targets and consequences. Thyroid. 2007;17(9):811–817. doi: 10.1089/thy.2007.0107. [DOI] [PubMed] [Google Scholar]