Abstract
Immunosuppression has been sporadically discontinued by noncompliant liver allograft recipients for whom an additional 4 1/2 years of follow-up is provided. These anecdotal observations prompted a previously reported prospective drug withdrawal program in 59 liver recipients. This prospective series has been increased to 95 patients whose weaning was begun between June 1992 and March 1996, 8.4±4.4 (SD) years after liver replacement. A further 4 1/2 years follow-up was obtained of the 5 self-weaned patients. The prospectively weaned recipients (93 livers; 2 liver/ kidney) had undergone transplantation under immunosuppression based on azathioprine (AZA, through 1979), cyclosporine (CsA, 1980–1989), or tacrolimus (TAC, 1989–1994). In patients on CsA or TAC based cocktails, the adjunct drugs were weaned first in the early part of the trial. Since 1994, the T cell–directed drugs were weaned first. Three of the 5 original self-weaned recipients remain well after drug-free intervals of 14 to 17 years. A fourth patient died in a vehicular accident after 11 years off immunosuppression, and the fifth patient underwent retransplantation because of hepatitis C infection after 9 drug-free years; their allografts had no histopathologic evidence of rejection. Eighteen (19%) of the 95 patients in the prospective series have been drug free for from 10 months to 4.8 years. In the total group, 18 (19%) have had biopsy proved acute rejection; 7 (7%) had a presumed acute rejection without biopsy; 37 (39%) are still weaning; and 12 (13%, all well) were withdrawn from the protocol at reduced immunosuppression because of noncompliance (n = 8), recurrent PBC (n = 2), pregnancy (n = 1), and renal failure necessitating kidney transplantation (n = 1). No patients were formally diagnosed with chronic rejection, but 3 (3%) were placed back on preexisting immunosuppression or switched from cyclosporine (CsA) to tacrolimus (TAC) because of histopathologic evidence of duct injury. Two patients with normal liver function died during the trial, both from complications of prior chronic immunosuppression. No grafts suffered permanent functional impairment and only one patient developed temporary jaundice. Long surviving liver transplant recipients are systematically overimmunosuppressed. Consequently, drug weaning, whether incomplete or complete, is an important management strategy providing it is done slowly under careful physician surveillance. Complete weaning from CsA-based regimens has been difficult. Disease recurrence during drug withdrawal was documented in 2 of 13 patients with PBC and could be a risk with other autoimmune disorders.
The morbidity arising from the chronic use of antirejection medications is an incentive to establish the lowest possible level of immunosuppression necessary to maintain stable graft function. The finding that complete freedom from immunosuppression was sporadically possible in long-surviving recipients of liver (1) and kidney allografts (2) prompted a prospective trial of drug weaning (3). Although it was shown that significant reductions in medication or their discontinuance could be safely accomplished, the danger of consequent rejection has not been completely assessed and guidelines for judicious weaning need to be clarified. We present here further observations on 3 cohorts of liver recipients: 5 who self-weaned many years ago (1), 59 who were in the prospective weaning trial of Ramos et al. (3), and 36 who were subsequently entered.
MATERIALS AND METHODS
Case material
Historical cases
Five liver recipients who were 12 1/2 to 18 2/3 years postoperative when they were studied in May 1992 already had been drug free because of noncompliance for 5 to 11 years; all had donor leukocyte microchimerism (1). One (OT 125) of the 5 was known at that time to have thrombosis of the hepatic artery. A further 4 1/2 year follow-up is available for 4 of these 5 recipients; the fifth was killed in a vehicular accident.
Prospective series
The emphasis of this report is on 95 patients whose controlled weaning was begun between June 1992 and April 1996 after meeting the following criteria: (1) ≥5 years posttransplantation; (2) ≥2 years without a documented rejection episode; (3) evidence of medical compliance; (4) availability of a cooperative local physician for follow-up; (5) absence of acute or chronic rejection on baseline liver biopsy; and (6) exclusion of other diagnoses such as vascular or biliary tract complications or recurrence of original disease. Thirty-one patients were ≤20 years old (pediatric cohort) at entry into the study and 64 were between 21 and 68 years old.
The pretransplant diagnoses included chronic viral hepatitis (n = 13), biliary atresia (n = 25), autoimmune hepatitis (n = 4), primary sclerosing cholangitis or primary biliary cirrhosis (PBC)* (n = 16), hepatoma (n = 4), and a miscellaneous group (n = 33) including alcoholic cirrhosis, cryptogenic cirrhosis, Budd-Chiari syndrome, toxic or secondary biliary cirrhosis, Wilson’s disease, hemochromatosis, alpha 1 antitrypsin deficiency, polycystic liver disease, and cystic fibrosis. Of the 95 patients, 93 received liver allografts only. The other 2 were given a simultaneous kidney graft from the liver donor.
Baseline immunosuppression
Immunosuppression at the start of weaning consisted of AZA/PRED in 13 cases, CsA/PRED in 32, CsA/ AZA/PRED in 24, CsA/AZA in 4, CsA alone in 11, TAC in 8, TAC/ PRED in 1, and TAC/AZA in 2. This heterogeneity was due to the performance of the transplantations in 3 successive eras in which cocktail immunosuppression was based on AZA (through 1979), CsA (1980–89), and TAC (1989–1994). In the first 8 months of 1989, CsA was the baseline drug, but it was replaced by TAC during the last half of that year. Except for the TAC era, initial triple drug therapy was used throughout the 30 year period of our program (Colorado/ Pittsburgh), always involving PRED as the secondary drug, but with the variable use of AZA and ALG (or OKT3) as third or fourth agents (4). PRED was the only adjunct drug in most of the patients treated from the outset with TAC.
Complications of immunosuppression
Preexisting complications of long-term immunosuppression in the prospective series included hypertension (n = 32); renal insufficiency (n = 27); skin lesions or malignancy (n = 12); neurologic symptoms or findings (n = 4); infection (n = 6); steroid-related complications (n = 9) such as obesity, growth failure, and bone disease; and diabetes (n = 9). Thirty-seven patients had multiple complications attributable to their immunosuppression.
Weaning protocol
At the beginning of the prospective trial (3), AZA was the first drug weaned when it was part of cocktail therapy (either 2 or 3 drugs). Before complete PRED weaning, corticotropin stimulation testing was done to detect adrenal insufficiency. The baseline immunosuppressive agent (CsA, AZA, or TAC) was withdrawn last by 25% increments at 1–2-month intervals as long as hepatocellular enzyme results remained stable. After the greater difficulty of weaning from CsA based immunosuppression and also in an effort to minimize cumulative nephrotoxicity, emphasis was shifted to primary weaning of CsA (or, when applicable, TAC), followed later by AZA and/or PRED. In addition, weaning was done more gradually in the later period in an effort to reduce the incidence of rejection.
Monitoring
Liver injury tests consisting of AST, ALT, GGTP, and serum bilirubin were monitored weekly after changes in drug dosage. If liver function abnormalities developed, a liver biopsy was obtained when permitted by the patient or family. Treatment for documented cellular rejection consisted of return to the medications preceding the last dose reduction, with or without pulse steroid therapy. A switch to TAC therapy in patients previously on other baseline therapy was reserved for biopsy proved moderate or severe ACR or evidence of developing chronic rejection. The staging of acute (5) and chronic rejection (6) has been described elsewhere. The criteria of van Hoek et al. for a formal diagnosis of chronic rejection were used.
Adult and pediatric stratification
The mean time from transplantation to the start of weaning in the whole group was 8.4 years ± 4.4 (range: 1.7–25.0 yr). For adult patients, the mean was 9.13 years, and for pediatric patients it was 6.64 years (P≤0.008, t test).
Statistical analysis
Continuous variables are presented as the mean ± SD, and categorical variables as proportions. The standard two-sample t test was used to test differences between means, while differences in proportions were tested using Pearson’s chi-square test or Fisher’s exact test (f test) if expected frequencies were less than five. The one-way analysis of variance was used instead of the two-sample t test when the number of groups to be compared was greater than two.
The cumulative probability of being weaned off immunosuppressive therapy was computed using the Kaplan-Meier (product-limit) method, and probability curves compared by the log-rank (Mantel-Cox) test. Patients who died, developed rejection, refused to observe the protocol, or are still weaning were censored. All tests were two-tailed. A P value less than 0.05 was considered statistically significant.
RESULTS
Graft loss and patient survival
Historical cases (n = 5)
Patient OT 125, who was drug free for 5 years with normal hepatic function in 1992 (1) despite a known hepatic artery thrombosis had subacute deterioration of allograft function beginning in 1995. She underwent successful liver retransplantation in April 1996 at UCLA (Busuttil R). Study of the allograft at UCLA (and independently by Pittsburgh pathologists) showed that chronic hepatitis C was responsible for the graft loss, with no evidence of rejection. The allograft had been in place for 19 years, of which 9 were without immunosuppression.
Patient OT 92 was killed instantly in an automobile accident on 5 November 1995, 17 1/2 years posttransplantation and 11 years after stopping drugs. His liver function and the coroner’s report of the allograft were normal.
The other 3 patients (OT 73, 150, and 169) are 18 2/3, 18, and 16 1/2 years posttransplantation. They continue to have normal allograft function after drug free intervals of 14, 17, and 15 years, respectively.
Prospective trial (n = 95)
Two patients died during the study period of non-liver allograft–related causes, both with normal liver function. The first, who was still weaning, died of septic pulmonary complications of cystic fibrosis. The second patient, who had been returned to baseline immunosuppression following an easily controlled mild acute cellular rejection, died of a pulmonary embolus following complications of a severe toe infection. It is noteworthy that these deaths were directly or indirectly attributable to the morbidity of chronic immunosuppression, which the weaning protocol was designed to ameliorate. There were no other graft losses.
Status of prospective weaning
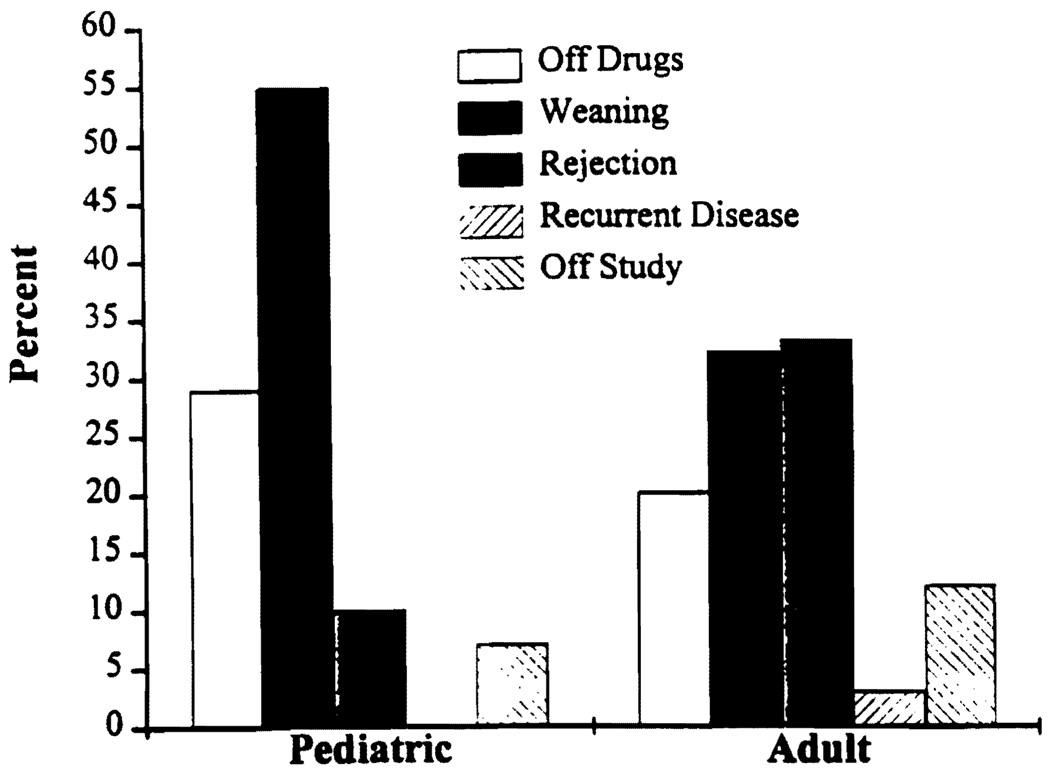
Table 1 depicts the current status of the 95 patients prospectively weaned. Figure 1 shows the results by age. The status of weaning is as follows.
TABLE 1.
Prospective weaning trial results (n = 95)
| Off Drugs | Weaning in progress |
Rejection | Weaning interruptedc |
|
|---|---|---|---|---|
| Number of patients (%) | 18 (19%) | 37 (39%) | 28 (29%) | 12 (13%) |
| Years from transplant to weaning start |
||||
| Mean±SDa | 8.3±5.4 | 8.1±4.2 | 8.9±3.4 | 8.5±6.0 |
| Median (range) | 7.6 (1.8–21.6) | 7.8 (1.7–21.3) | 8.7 (3.1–19.6) | 6.7 (1.8–25.0) |
| Time from weaning to off, rejection, or weaning interruption (months) |
Not Applicable | |||
| Mean±SD | 5.9±5.9 | 13.2±11.6 | 12.0±8.3 | |
| Median (range) | 0.28 (0–31.7) | 9.4 (0.4–42.5) | 9.5 (2.6–27.2) | |
| Follow-up (months) | ||||
| Mean±SDb | 34.5±11.2 | 33.6±18.1 | 22.8±14.4 | |
| Median (range) | 35.5 (10.1–57.2) | 19.6 (1.1–66.2) | 25.3 (0.2–42.4) |
P=0.901 (ANOVA).
As of 5-6-96 or patient death.
Because of noncompliance (n = 8) recurrent disease (PBC, n = 2), pregnancy (n = 1), and renal failure requiring renal transplant (n = 1).
FIGURE 1.
Weaning trial results by age group.
Complete
Eighteen (19%) of the 95 patients in the prospective cohort have been drug free for 0.84 to 4.8 years. Seven of these recipients already were on low levels of immunosuppression at the first visit to the weaning clinic and had drugs stopped at that time. The 11 others were weaned over a mean period of 6.6 months. Their current liver function tests are summarized in Table 2.
TABLE 2.
Mean laboratory values (±SD) in patients (n = 18) off immunosuppression
| Bilirubin (mg/dl) | AST (IU/L) | ALT (IU/L) | GGT (IU/L) | Creatinine (mg/dl)a |
|---|---|---|---|---|
| 0.7±0.37 | 39.7±22 | 39.6±26 | 45.7±69 | .78±.34 |
| (range 0.3–1.4) | (range 5–91) | (range 9–101) | (range 10–258) | (range 0.3–1.5) |
1/18 patients is dialysis dependent; the mean creatinine is for the other 17.
In progress
Currently 37 patients are in the uninterrupted process of drug weaning. The mean percentage decrease in baseline immunosuppression in these patients is shown in Table 3.
TABLE 3.
Reductions of individual immunosuppressive drugs in 37 patients with uninterrupted but still incomplete weaning
| Baseline drug (mean±SD) |
||||
|---|---|---|---|---|
| Azathioprine | Cyclosporine | Prednisone | Tacrolimus | |
| Baseline (mg/day) | 59±29 | 299±113 | 5.5±3.5 | 2.5±2.4 |
| Current dosage (mg/day) | 45±21 | 142±88 | 4.7±4.6 | 1.3±1.2 |
| % Decrease | 13±33 | 30±35 | 14±88 | 44±31 |
Interrupted by rejection
Eighteen patients had weaning interrupted because of histopathologically confirmed rejection. Seven additional patients had weaning stopped because the diagnosis of rejection was suspected clinically, although not proved by biopsy. Finally, suspicion of incipient chronic rejection on biopsy prompted resumption of immune suppression in 3 cases.
Withdrawal from protocol
Twelve patients were withdrawn from the protocol due to noncompliance (n = 8), pregnancy (n = 1), renal failure requiring kidney transplantation (n = 1), and recurrent PBC (n = 2). The 8 excluded because of noncompliance were left at whatever level of treatment had been reached at the time the decision to stop weaning was made. All 8 were well at the time and remain so.
Results by immunosuppression regimen
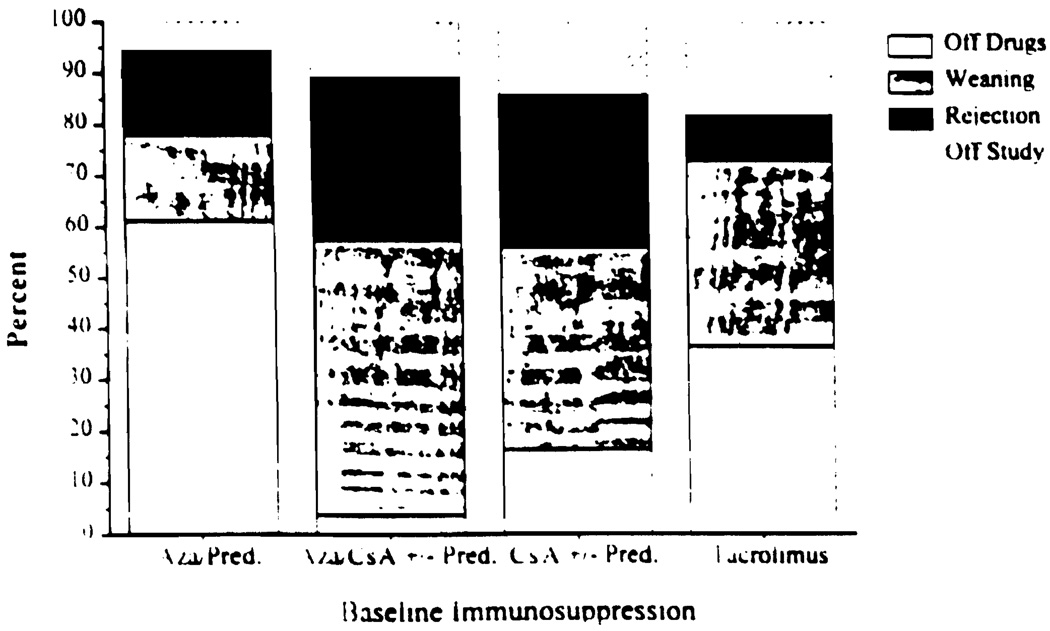
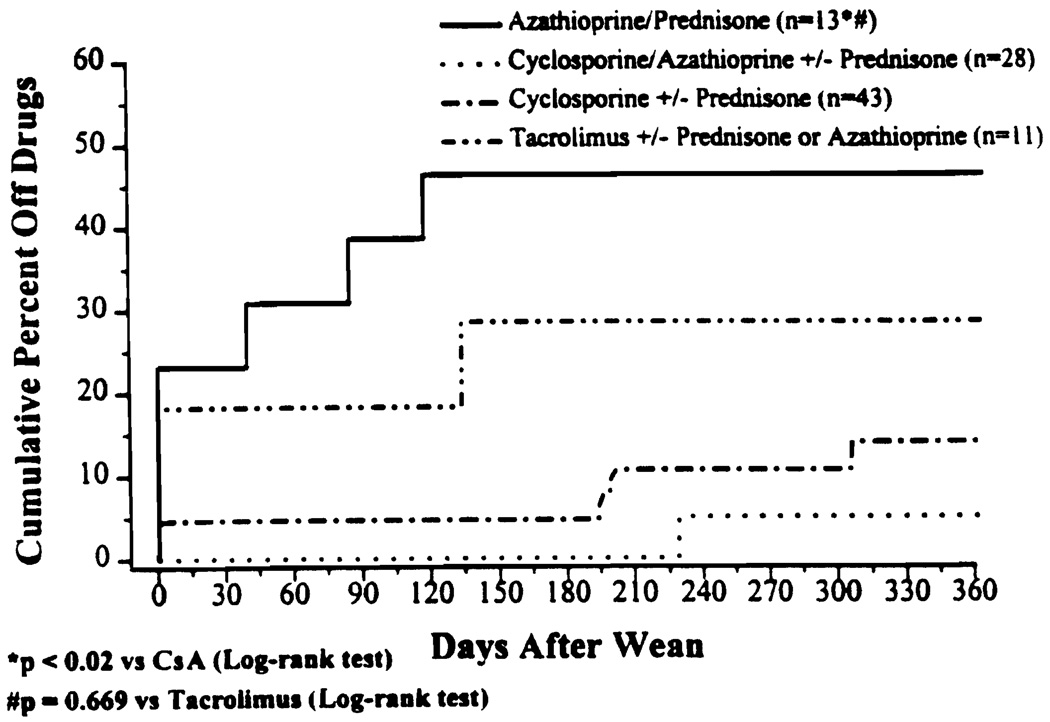
There was no significant difference in the prospective trial in the rate of success when the baseline immunosuppressant was TAC or AZA (Fig. 2). However, there was a significant difference between these groups and all of the CsA-based regimens (P≤0.003). Figure 3 demonstrates the cumulative percent of patients off immunosuppression at one year postweaning. AZA/PRED-based patients were more likely to be off drugs at one year as compared with CsA-based patients (P=0.0007, log rank). TAC-based patients enjoyed a similar advantage (P=0.0031, log rank).
FIGURE 2.
Results by baseline immunosuppression.
FIGURE 3.
Percent and timing of complete drug withdrawal by baseline immunosuppression.
Impact on preexisting complications
The drug-free patients (n = 18) did not have a significant change in renal function or an improvement in hypertension. The most common benefits were resolution of gingival hyperplasia in two children, resolution of infections in 3, resolved lymphoproliferative disorders in 3, and relief of neuropsychiatric complaints in 2.
Allograft related adverse events
Significant hepatocellular enzyme elevations (AST, ALT, GGTP) occurred in 44 (46%) prospectively weaned patients during the study period, followed by liver biopsy in 37. The biopsy findings were acute cellular rejection (n = 18), minor duct injury (n = 3) not severe enough to meet the criteria for chronic rejection (see below), hepatitis (n = 3), normal pathology (n = 7), evidence of biliary tract obstruction (n = 3), nonspecific portal inflammation (n = 2), and steatosis (n = 1).
Acute cellular rejection (n = 18)
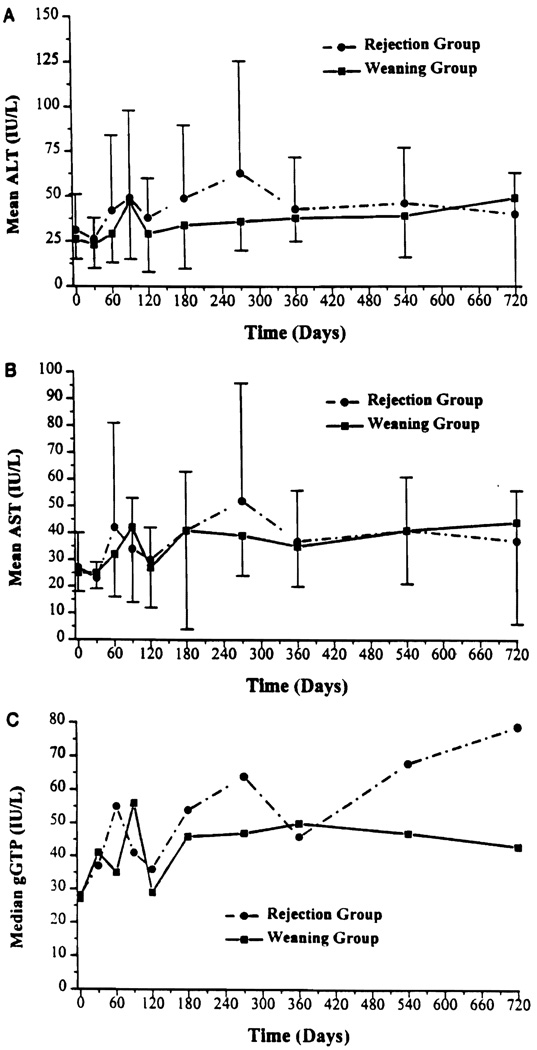
The biopsy proved acute cellular rejections documented in 18 patients (19% of the total group) occurred at a mean time of 13.2 months after weaning was started, and were graded as mild, moderate, or severe (no examples) by histopathologic criteria described in detail elsewhere (5). The characteristic mononuclear cell infiltrate was mild in most cases, as was reflected by the fact that only one recipient had an increase in serum bilirubin. There was no significant difference in the laboratory trends of AST/ALT/GGTP in the proved rejection (n = 18) versus the continuous weaning groups (n = 37) (Fig. 4, a–c).
FIGURE 4.
Mean ALT, AST, and gGTP by rejection and weaning groups.
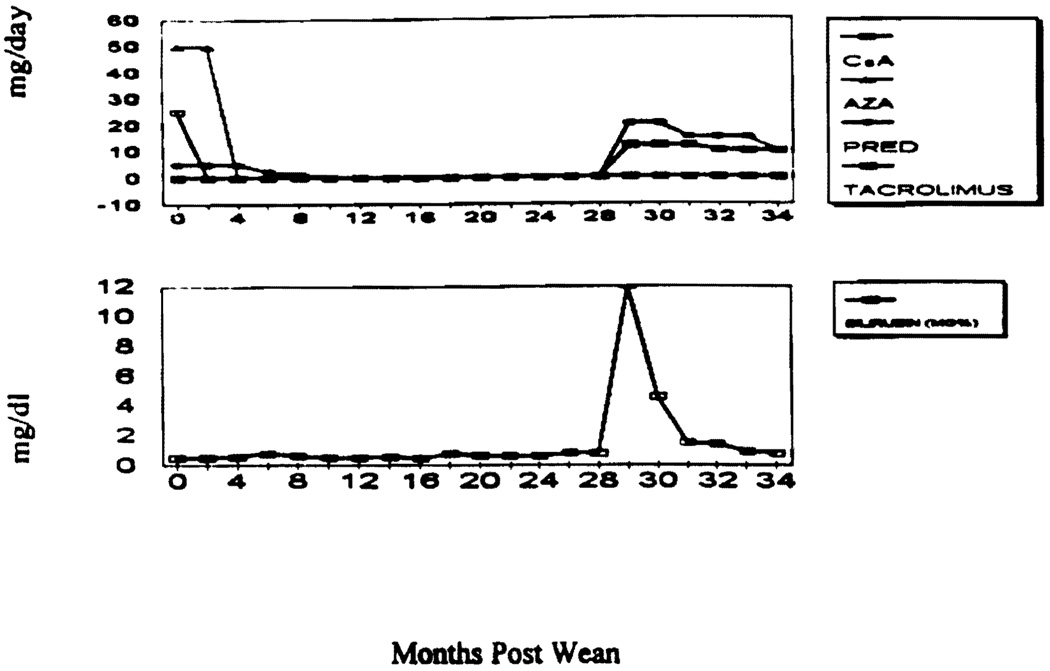
Treatment consisted of pulse steroids and resumption of the baseline medication schedules in 15 patients, with prompt return of hepatocellular function to normal. The other 3 patients, all on CsA-based regimens, were converted to TAC therapy because of a moderate acute rejection on biopsy, including the one who developed jaundice 29 months after all drugs were stopped (Fig. 5).
FIGURE 5.
Clinical course of OT 1308.
OKT3 was not given to any of the 95 patients in the trial. One patient developed herpes stomatitis following treatment for rejection. Otherwise there were no infectious complications following resumption of antirejection therapy.
Unconfirmed acute cellular rejection (n = 7, 7%)
This group included 4 of the 7 patients who did not undergo biopsy before intensifying immunosuppression, and 3 whose current biopsy revealed only early chronic duct injury that was unchanged from findings in the baseline biopsies (before weaning). Because there was normalization of liver injury tests after the increase of immunosuppression, these 7 cases were arbitrarily included in the acute rejection category.
Suspected chronic rejection (n = 3, 3%)
This diagnosis, which has been defined as the absence of detectable bile ducts in ≥50% of the portals triads seen in a biopsy sample or the presence of obliterative arteriopathy (7), was not made in any of the 95 patients. However, 3 allografts developed suspicious de novo histopathologic findings during weaning (Table 4). The previous level of immunosuppression was restored in two cases, and TAC was started in the third who had been weaned from AZA (Table 4).
TABLE 4.
Biopsy evidence arousing suspicion of incipient chronic rejection in 3 cases, and therapeutic response
| OT No./date of Txa | Bili/GGTP (mg%/IU at time of bx)b |
Current Bili/GGTP | Bx date showing chronic duct injury |
Bx findings | Drugs at time of bx |
Current drugs |
|---|---|---|---|---|---|---|
| 64 2/18/73 |
0.6/220 | 0.3/187 | 3/12/96 | Duct not seen in 4/10 triads |
Off | FK 2/2 |
| 331 9/30/83 |
0.6/476 | 0.4/90 | 2/14/95 | Duct seen in all triads, but early damage |
25 CsA | 50 CyA 2.5 PRED |
| 1192 8/5787 |
0.8/223 | 1.2/292 | 2/7/96 | Duct not seen in 2/10 triads |
5 PEED 75 b.i.d. CsA |
20 PRED 150 b.i.d. CsA |
Tx=liver transplantation.
Bx=liver biopsy.
Viral hepatitis (n = 3, 3%)
Two patients developed biopsy evidence during weaning of recurrent hepatitis attributable to non-A non-B virus (later shown to be hepatitis C). A third recipient whose original disease was biliary atresia, developed de novo C virus hepatitis infection. Weaning has continued in these patients with stabilization of liver injury tests.
Recurrent autoimmune disease (n = 2)
Thirteen patients entered the study with diagnosis of PBC. Two (15%), who were 7 and 8 years posttransplantation, were diagnosed with histopathologically obvious recurrent PBC 7 and 24 months after the initiation of weaning. Liver function tests normalized after return to the preexisting CsA-based baseline immunosuppression, including PRED. There has been no recurrence of autoimmune hepatitis in this series.
Bile duct obstruction (n = 3, 3%)
The liver function abnormalities in all 3 patients were incorrectly attributed to rejection and briefly treated as such in 2 cases. Liver enzyme values normalized in all 3 recipients after bile duct reconstruction. Weaning was resumed.
Previously reported equivocal cases
In our report 2 years ago of the first portion of the current prospective series, there were 19 liver recipients whose reasons for enzyme elevations during weaning were not considered to be unconditionally established (3). In the intervening 2 years, 10 of these patients have had progression of the suspected pathologic process (Table 5); 5 have remained unchanged with continuation or completion of weaning, one died of pulmonary complication of cystic fibrosis, and 3 were withdrawn from the protocol.
TABLE 5.
Current status after two additional years of 19 previously reported patients (2) who had enzyme elevations during weaning without a definitive explanation
| OT No. | Diagnosis | Biopsy at first enzyme elevation | Follow-up therapy (interval) | Current statusa |
|---|---|---|---|---|
| 2134 | BAb | Normal | Normal | Off drugs |
| 0666c | NANB hepatitis | Lobular reactivity | Hepatitis (23 months) | Weaning |
| 0592 | PBC | Lobular reactivity | ND | On drugs |
| 0440 | A-1-A | ND | ND | On drugs |
| 1308 | Hepatitis C | Lobular reactivity | Moderate ACR (23 months) | On drugs (Fig. 5) |
| 0202 | Wilson’s disease | Bile duct obstruction | ND | Off drugs |
| 0235 | PBC | ND | ND | Weaning |
| 64 | BA | Regenerative hyperplasia | Early duct injury (19 months) |
On drugs |
| 0042 | Wilson’s disease | ND | ND | Off drugs |
| 0105 | Cryptogenic | Lobular reactivity | ND | Off drugs |
| 1464 | Alcoholic cirrhosis | ND | ACR (4 months) | Off drugs |
| 0289 | NABA hepatitis | ND | Hepatitis (6 months) | Weaning |
| 331 | PBC | Lobular reactivity | Chronic duct injury (8 months) |
On drugs |
| 476 | Cystic fibrosis | Normal | ND | Died during weaning, liver normald |
| 1215 | Halothane hepatitis |
ND | ND | Off study; pregnancy |
| 314 | BA | ND | Hepatitis (6 months) | Weaning |
| 474 | Hepatitis B | Hepatitis | ND | On drugs |
| 516 | PBC | Lobular reactivity | Recurrent PBC (15 months) | On drugs |
| 1182 | NANB Hepatitis | Hepatitis | ND | On drugs |
A total of 18 surviving patients are all clinically well with good or completely normal liver function.
ND, not done; BA, biliary atresia; PBC, primary biliary cirrhosis; A1-A, alpha-1 antitrypsin deficiency.
Data on patients who had progression of suspected pathologic process at the time of an earlier report (2).
Pulmonary infection associated with cystic fibrosis.
DISCUSSION
The ultimate rationale for this trial came from evidence that donor/recipient nonreactivity is gradually induced by the dissemination from organ allografts and persistence thereafter of donor leukocytes including pluripotent stem cells (1, 8–12) with ultimate diminution of drug dependence. In fact, the majority of the patients entered into the weaning trial had been kept at a maintenance treatment level higher than needed. Even those who developed rejection during the weaning process are currently receiving less average immunosuppression now than at their entry.
The potential benefits of weaning are too obvious to enumerate. However, weaning carries a risk of rejection, which was proved to have occurred acutely at some time during or after weaning in 18 (19%) of the 95 patients entered. Seven more cases were arbitrarily placed in the acute rejection category without biopsy verification, including 3 in which the preweaning biopsy had revealed early duct injury too minor to qualify as chronic rejection. Including these 7 patients, there was a 25/95 (26%) global incidence of acute rejection that occurred from 0.2 to 42 months after starting the weaning. Restoration of immunosuppression to preexisting levels resulted in normalization of liver and enzyme elevations in 15 of the 18 with biopsy proved rejection and all 7 with the presumed diagnosis. In the 3 exceptional cases, the rejection was histopathologically moderate or severe and one of these patients became jaundiced, with a peak bilirubin of 12 mg%. The 3 patients were switched to TAC. We believe that the availability of TAC is a prerequisite safety net (13) for a weaning trial.
The circumstances in the patient who developed hyperbilirubinemia after rapid weaning from GsA, AZA, and PRED were similar to those in the weaning series reported by Sanborn et al. (14) in which liver recipients were taken off this triple drug therapy at an average 3.1 years posttransplantation after a minimum one year of stable graft function. As in our temporarily jaundiced patient, weaning was done rapidly because of CsA-associated renal failure. Six of the 12 patients developed rejection, and 2 died (14).
Chronic rejection was not encountered in our trial, by the criteria conventionally used to make this diagnosis (7). However, when 3 patients developed de novo histopathologic findings suggestive of early chronic rejection, accompanied in one case by an acute increase in canalicular enzymes, immunosuppression was restored to preexisting levels (n = 2) or replaced with TAC (n = 1). Although it is possible that the biopsy finding was a sampling variable, the failure to find ducts in ≥50% of portal triads may not be adequate as the gold standard for this diagnosis (7). One of us (A.J.D.) has observed that, when the incipient duct changes are associated with elevated gamma GTP, the combination frequently premonitors chronic rejection.
Three practical lessons from this experience deserve emphasis. One is the necessity for close physician surveillance during weaning with frequent assessments of liver function, repeat biopsy with the slightest suspicion of rejection, and prompt restoration of immunosuppression if this is the diagnosis (which it frequently is not). Second, weaning should not be as abrupt as was done in the early patients of this series (3) or in other trials (14–18). Third, the risk of autoimmune recurrence is a real one, as previously predicted (3). Although recurrence of autoimmune hepatitis has not yet been observed, 2 (15%) of the 13 patients with PBC developed recurrence. We have, for the time being, suspended further dose reductions in patients with the latter diagnosis.
With these provisos, we conclude that drug weaning is an important management consideration for a large number of stable long surviving liver recipients. It was disappointing to note that even complete discontinuance of immunosuppression resulted neither in appreciable recovery of renal function nor in amelioration of hypertension, underscoring the need to reduce if possible the doses of the nephrotoxic T cell–directed drugs at an earlier time. However, the long term benefits of drug reduction or discontinuation need to be assessed on an individual basis, with continual assessment of the risk of rejection and need for intervention. Weaning cannot be safely done unless rescue therapy with TAC is available. Finally, this experience has confirmed the frequency with which late hepatic allograft dysfunction is due to causes other than rejection (18, 19).
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AZA
azathioprine
- CsA
cyclosporine
- GGTP
gamma-glutamyl transpeptidase
- PRED
prednisone
- PBC
primary biliary cirrhosis
- TAC
tacrolimus
Footnotes
Presented at the 22nd Annual Meeting of the American Society of Transplant Surgeons, May 29–31, 1996, Dallas, TX.
This work was supported by: Research Grants from the Veterans Administration and Project Grant No. DK 29961 from the National Institutes of Health. Bethesda, Maryland.
REFERENCES
- 1.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole organ transplantation: the basis of graft acceptance. Hepatology. 1993;17:1127. [PMC free article] [PubMed] [Google Scholar]
- 2.Mazariegos GV, Ramos H, Shapiro R, Zeevi A, Fung JJ, Starzl TE. Weaning of immunosuppression in long-term recipients of living-related renal transplants: a preliminary study. Transplant Proc. 1995;27:207. [PMC free article] [PubMed] [Google Scholar]
- 3.Ramos HC, Reyes J, Abu-Elmagd K, et al. Weaning of immunosuppression in long-term liver transplant recipients. Transplantation. 1995;59:212. doi: 10.1097/00007890-199501270-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Evolution of liver transplantation. Hepatology. 1982;2:614. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fung JJ, Todo S, Jain A, Demetris AJ, McMichael JP, Starzl TE. The Pittsburgh randomized trial of tacrolimus versus cyclosporine for liver transplantation. J Am Coll Surg. 1996;183:117. [PMC free article] [PubMed] [Google Scholar]
- 6.Demetris AJ, Fung JJ, Todo S, et al. Conversion of liver allograft recipients from cyclosporine to FK 506 immunosuppressive therapy—a clinicopathologic study of 96 patients. Transplantation. 1992;53:1056. doi: 10.1097/00007890-199205000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Hoek B, Wiesner RH, Krom RAF, Ludwig J, Moore SB. Severe ductopenic rejection following liver transplantation: incidence, time of onset, risk factors, treatment, and outcome. Semin Liver Dis. 1992;12:41. doi: 10.1055/s-2007-1007375. [DOI] [PubMed] [Google Scholar]
- 8.Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demetris AJ, Murase N, Fujisaki S, Fung JJ, Rao AS, Starzl TE. Hematolymphoid cell trafficking, microchimerism, and GVHD reactions after liver, bone marrow, and heart transplantation. Transplant Proc. 1993;25:3337. [PMC free article] [PubMed] [Google Scholar]
- 10.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994;19:916. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starzl TE, Demetris AJ, Murase N, Trucco M, Thomson AW, Rao AS. The lost chord: microchimerism and allograft survival. Immunol Today. doi: 10.1016/s0167-5699(96)10070-0. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murase N, Starzl TE, Tanabe M, et al. Variable chimerism, graft versus host disease, and tolerance after different kinds of cell and whole organ transplantation from Lewis to Brown-Norway rats. Transplantation. 1995;60:158. doi: 10.1097/00007890-199507000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Starzl TE, Todo S, Fung J, Demetris AJ, Venkataramanan R, Jain A. FK 506 for human liver, kidney and pancreas transplantation. Lancet. 1989;2:1000. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanborn WJ, Hay JE, Porayko MK, et al. Cyclosporine with-drawal for nephrotoxicity in liver transplant recipients does not result in sustained improvement in kidney function and causes cellular and ductopenic rejection. Hepatology. 1994;19:925. [PubMed] [Google Scholar]
- 15.Schlitt HJ, Hundrieser J, Ringe B, Pichlmayr R. Donor-type microchimerism associated with graft rejection eight years after liver transplantation. N Engl J Med. 1994;330:646. doi: 10.1056/NEJM199403033300919. [DOI] [PubMed] [Google Scholar]
- 16.Anand AC, Hubscher SG, Gunson BK, McMaster P, Neuberger JM. Timing, significance, and prognosis of late acute liver allograft rejection. Transplantation. 1995;60:1098. doi: 10.1097/00007890-199511270-00007. [DOI] [PubMed] [Google Scholar]
- 17.Molleston JP, Alevy YG, Sivasai KSR, Mohanakumar T, Howard TK. Evidence that pediatric liver transplant recipients may undergo late rejection episodes in spite of donor-specific microchimerism. Transplantation. 1996;61:656. doi: 10.1097/00007890-199602270-00025. [DOI] [PubMed] [Google Scholar]
- 18.Starzl TE, Koep LJ, Halgrimson CG, et al. Fifteen years of clinical liver transplantation. Gastroenterology. 1979;77:375. [PMC free article] [PubMed] [Google Scholar]
- 19.Pappo O, Ramos H, Starzl TE, Fung JJ, Demetris AJ. Structural integrity and identification of causes of liver allograft dysfunction occurring more than 5 years after transplantation. Am J Surg Pathol. 1995;19:192. doi: 10.1097/00000478-199502000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]