Abstract
Two children with the short-gut syndrome and secondary liver failure were treated with evisceration and transplantation en bloc of the stomach, small intestine, colon, pancreas, and liver. The first patient died perioperatively, but the second lived for more than 6 months before dying of an Epstein-Barr virus-associated lymphoproliferative disorder that caused biliary obstruction and lethal sepsis. There was never evidence of graft rejection or of graft-vs-host disease in the long-surviving child. The constituent organs of the homograft functioned and maintained their morphological integrity throughout the 193 days of survival.
In older investigations, maximum survival after multivisceral transplantation in untreated dogs was 9 days.1,2 The liver as part of the multivisceral graft was rejected later and less severely than after liver transplantation alone. In addition, histopathologic findings suggestive of graft-vs-host disease (GVHD) were widespread by the time of death.2
We have attempted the same operation in two children, one of whom survived for more than 6 months before dying of complications from the lymphoproliferative disease (LPD) that is associated with Epstein-Barr virus infections.3,4 Two other patients are known to have had similar procedures.5
METHODS
Technique Standardization in Pigs
Outbred Landrace pigs weighing 15 to 25 kg were anesthetized with pento-barbital sodium and ketamine hydrochloride and submitted to the same operation as shown in Fig 1 except that the retrohepatic inferior vena cava was replaced with the same segment from the donor. The donor organs were chilled with intra-aortic lactated Ringer’s solution. Graft splenectomy, cyclosporine therapy (given orally or by a brief intravenous course), and oral prednisone did not have a predictable effect on the survival of the 22 pigs that lived through operation and for at least 2 days (Table 1). Diarrhea was a frequent problem. Eight animals developed skin lesions of GVHD (Fig 2). Sepsis or GVHD usually was suspected even when the cause of death was not obvious with gross examination at autopsy. Intestinal perforation, probably secondary to rejection, was found in 5 pigs. One animal that was treated with a short course of cyclosporine lived for 104 days, had seemingly normal stools, and ate well enough to gain weight from 12.5 to 30 kg. Biopsy specimens of the jejunum, spleen, and liver obtained 2 months after operation were normal. At autopsy, there was no grossly identifiable cause of death although the clinical suspicion was of GVHD.
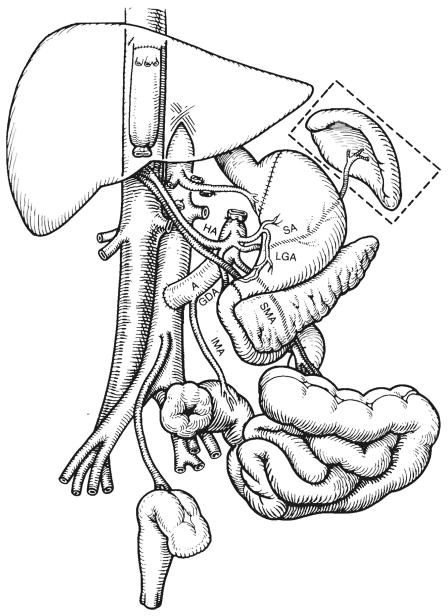
Fig 1.
The recipient operation of multivisceral transplantation. Note that the venous outflow of the graft was into a cloaca of the left and middle hepatic veins, leaving the recipient inferior vena cava intact. A indicates donor aorta; HA, hepatic artery; SA, splenic artery; LGA, left gastric artery; SMA, superior mesenteric artery; IMA, inferior mesenteric artery; and GDA, gastro-duodenal artery.
Table 1.
Fate of 22 (of 34) Pigs That Survived for at Least 2 Days After Operation
| Group | No. of Pigs | Survival, No. of Days |
|---|---|---|
| 1 No treatment | 6 | 5, 14, 9, 12, 10, 7 |
| 2 Oral cyclosporine, 20 mg/kg, and prednisone, 5 mg/d | 6 | 104, 2, 23, 16, 9, 9 |
| 3 Same as group 2; graft splenectomy | 5 | 23, 18, 7, 11, 2 |
| 4 Same as group 3; 6 mg/kg of cyclosporine intravenously, days 1–3 | 5 | 14, 17, 3, 15, 14 |
Fig 2.
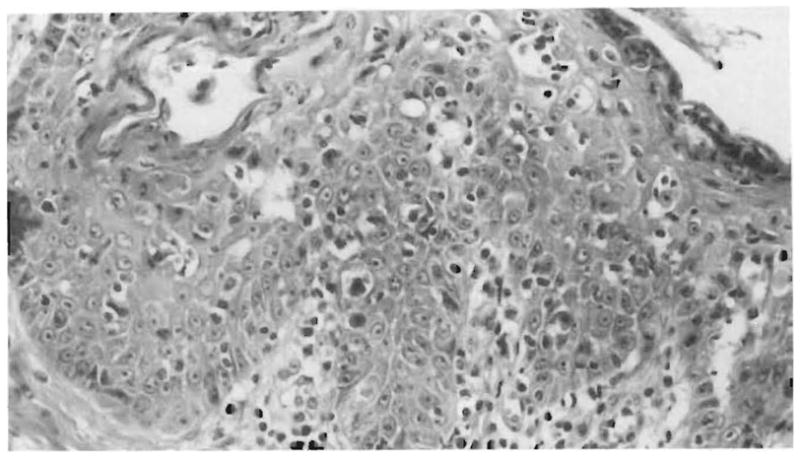
Skin biopsy from a pig 14 days after multivisceral transplantation. Note the lymphocytic infiltrate at the dermal-epidermal junction and lymphocytes in proximity to necrotic epithelial cells (satellitosis) (hematoxylineosin, × 350).
Case Reports
Case 1
A 6 10/12-year-old white girl lost most of her small bowel, necessitating total parenteral hyperalimentation (TPN), after it had been sucked out while she was sitting on the drain of a swimming pool that was being emptied. During the following year, she developed liver failure and underwent multiple surgical procedures, including a central splenorenal shunt. She was admitted to the Children’s Hospital of Pittsburgh on July 29, 1983, with heart failure, pulmonary edema, bilateral pleural effusions, anasarca, renal failure, and liver failure. Total serum bilirubin level was 503 μmol/L; prothrombin time, 21 seconds (control, 12 seconds); and serum albumin level, 19 g/L. By August 17, 1983, when a donor became available, she was anuric and receiving ventilatory assistance although still conscious.
The 27-month-old male donor had the same blood type as the recipient (A, Rh +). The graft was core-cooled with chilled intra-aortic lactated Ringer’s solution and removed in an adjacent operating room. The cold ischemia time was 1 hour. There was no warm ischemia. The organs transplanted were the same as in Fig 1 except that the kidneys were also included with the multivisceral graft and connected to the recipient bladder with ureteroneocystostomies. Exsanguinating hemorrhage was continuous throughout the recipient operation, requiring transfusion of 61 750 mL of blood. When the graft was revascularized, it had a normal appearance but two episodes of cardiac arrest occurred several minutes later, followed by intractable hypotension that continued until death of the patient 30 minutes after arrival in the intensive care unit.
Case 2
A 3½-year-old black girl underwent multivisceral transplantation at Children’s Hospital of Pittsburgh on November 1, 1987. Her disease began with perinatal volvulus and gangrene for which massive small-bowel resection was required with jejunoileal anastomosis. Liver failure secondary to TPN was progressive from the age of 2 years. By the time of transplantation, the child weighed 14 kg (50th percentile) and was 82 cm tall (fifth percentile). She had hepatosplenomegaly and scars from multiple abdominal procedures. Total bilirubin level was 470 μmol/L; prothrombin time, 18.2 seconds; serum albumin level, 20 g/L; and aspartate aminotransferase level, 303 U/L. Serum urea nitrogen, creatinine, and serum electrolyte levels were normal. The hematocrit was 0.38; white blood cell count, 3800 × 106/L; and platelets, 90 000 × 106/L. There was radiological evidence of pulmonary edema.
The Donor Operation
The donor for patient 2 was a 2-month-old white female weighing 3.25 kg who had a closed-head injury 4 days previously. Results of blood chemistry studies were normal except for an elevation of total bilirubin level to 41 μmol/L on the final day. Ten milligrams of OKT3, a mouse anti–human T-lymphocyte monoclonal antibody,6 was given intravenously 90 minutes before removing the graft. The graft was cooled without preliminary warm ischemia by infusing 500 mL of the special cold preservation fluid recently developed at the University of Wisconsin.7,8 It was stored in an ice chest and revascularized in the recipient after 6 hours 38 minutes of cold ischemia. The spleen was removed immediately.
Operation
Patient 2 had removal of the liver, pancreas, spleen, distal part of the stomach, duodenum, residual small intestine, and ascending, transverse, and descending colon. The distal sigmoid colon and rectum as well as a cuff of proximal stomach were retained. The recipient retrohepatic inferior vena cava was kept intact, and a cloaca was fashioned from the left and middle hepatic veins to which the suprahepatic inferior vena cava of the multivisceral graft was anastomosed for venous outflow (Fig 1). The donor inferior vena cava was ligated below the liver. The distal graft aorta was anastomosed to the midabdominal aorta of the recipient (Fig 1). Revascularization did not cause cardiovascular instability or bleeding; most of the total operative blood loss of 5050 mL occurred during donor organ removal.
Gastrogastrostomy was performed, as well as Heineke-Mikulicz pyloroplasty of the graft, to facilitate gastric emptying (Fig 1). Colocolostomy was performed. Five days later, the patient was reexplored and pinhole perforations were found at both the upper and lower anastomoses. The gastrogastrostomy was reinforced and left intact. Distally, the colonic anastomosis was converted to an end sigmoid colostomy with suture closure of the native colon remnant (Fig 1).
Postoperative Fluid, Electrolyte, and Nutritional Management
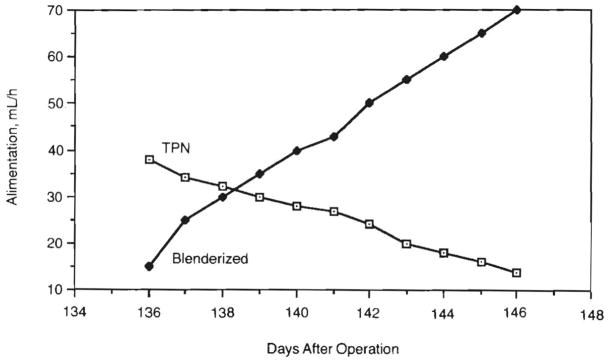
Right-sided filling pressures and serial monitoring of urinary output and concentrations guided intravenous therapy with crystalloid and colloid in the acute postoperative setting. Total parenteral nutrition was used until the gastrointestinal tract regained function. As enteral feeds were increased, the volume of TPN was reduced accordingly (Fig 3). The child could not be taught the physical act of eating.
Fig 3.
Enteral and parenteral alimentation. As a blenderized diet was increased, we were able to gradually reduce total parental hyperalimentation (TPN) to a very low level.
Enteral feedings were started 2 weeks after operation through a trans-nasal jejunostomy tube. Conversion to a nasogastric feeding tube was possible by the fifth week when gastric emptying had improved. Initially, feedings were continuous. As gastrointestinal tract function improved, intermittent feedings were tolerated, and by day 135, intermittent intake was used exclusively.
In the early postoperative period, enteral feedings were with simple solutions such as 5% glucose in an isotonic electrolyte solution (Pedialyte) and an iso-osmolar concentration of an elemental diet (Vivonex). More complex formulas (Portagen, Pregestimil) were not well tolerated. The most effective formula appeared to be an iso-osmolar elemental diet (Peptamen) containing peptides as the protein source and only a small amount of long-chain triglycerides, which allowed TPN to be gradually reduced. Shortly thereafter, it was possible to give a blenderized diet containing Peptamen, meats, and vegetables and to progressively wean her from TPN (Fig 3). Bulk fiber in the form of pectin or psyllium complexes and the bile-salt binding agent, cholestyramine, were added at various times to slow the transit time and improve fluid absorption.
Qualitative and quantitative measurements of intravenous and enteral infusates as well as output of urine, stool, and catheter drainage (gastric, biliary, and Jackson-Pratt) were done. Daily weights were taken. These data were used to guide volume replacement, electrolyte management, and nutrition.
Infectious Disease
Broad-spectrum antibiotic coverage for patient 2 was provided perioperatively with intravenous ampicillin sodium, cefotaxime sodium (Claforan), and metronidazole (Flagyl). Beginning on postoperative day 12, until the seventh postoperative week, selective bacterial decontamination that included oral and enteral colistin sulfate (Polymixin E), tobramycin sulfate, and amphotericin B were given to suppress the growth of fungi and potentially pathogenic gram-negative bacteria while not perturbing the growth of anaerobes.9 Because the donor had positive serological test results for cytomegalovirus, ganciclovir was given for 14 days from the time of operation. Acyclovir (acycloguanosine) was used after the diagnosis of LPD had been established.10
Immunosuppression
Cyclosporine was given intravenously or enterally to patient 2 to keep the blood level at or above 1000 ng/mL by radioimmunoassay.11 When LPD was diagnosed 91 days after operation, administration of cyclosporine was gradually discontinued and therapy was stopped for 15 days. Treatment was subsequently reinstituted at low doses.
The daily dosage of methylprednisolone sodium succinate (Solu-medrol) was decreased from 100 to 5 mg by day 9. A 1-week course of 2.5 mg/d of OKT36 was begun on postoperative day 23 because of the suspicion of rejection. Nine doses of 25 mg of azathioprine were given intravenously for a 13-day period beginning on postoperative day 8. As prophylaxis against GVHD, 4.5 Gy of total abdominal irradiation from an 8-MV linear accelerator were given in six divided doses from 1 to 3 weeks after transplantation.
Pharmacokinetic Studies
Patient 2 was given 50 and 38 mg of intravenous cyclosporine for a 2-hour period on postoperative days 74 and 102, respectively. This was followed the next day (postoperative days 75 and 103 respectively) with oral doses of 550 and 150 mg in olive oil. Multiple blood samples were drawn on these study dates and analyzed for cyclosporine concentration by high-performance liquid chromatography.12
Assessment of Graft Function
Liver
Findings from conventional hepatic function tests were obtained almost daily and correlated with the results of tests on liver biopsy samples.
Intestine
Absorption was estimated from serum glucose levels after Peptamen administration and by cyclosporin blood levels after gastric instillation of this drug. The percentage of nasogastric fluid absorbed was determined by measuring stool output. Albumin leakage through the intestinal mucosa was studied in the fourth postoperative month after the intravenous injection of human serum albumin labeled with 25 mCi of chromium 51 and by monitoring the amount of isotope that appeared in the stool during the next 4 days (normally <0.5% for a 3-day period).13 Stool electrolytes, glucose, and protein levels were measured.
Pancreas
Serial serum trypsin and amylase levels were obtained. Serum C peptide levels were measured and com pared with the levels of 0.24 to 0.72 pmol/mL obtained in 20 nonfasting diabetics.
Histocompatibility (HLA) Typing
With lymphocytotoxicity and two-color fluorescence testing, patient 2 showed the following phenotype: HLA - A3, 30; B35, w42(Bw6); DRw6, -(DRw52); DQw1, w3. Because the graft was flushed with OKT3 monoclonal antibody, donor lymphocytes could not be readily HLA typed because of high background lymphocytotoxicity. However, Al, A26, and probably Bw4 were identified. The recipient had cytotoxic antibodies against only 2% of the human population.
Special Pathological Studies
In addition to routine histopathologic processing, portions of all biopsy samples were snap frozen to immunostain for lymphocyte markers and other studies. Monoclonal antibodies to HLA-DR, CD2, CD3, CD4, CD8, CD20, MO2, and κ and λ light chains were used. Polyclonal antibodies to IgA, IgG, IgM, α-antichymotrypsin, lysozyme, and S100 protein were also used. For special typing studies in tissues, the monoclonal antibodies used had specificity against HLA-A3 and Bw4; they were detected with an alkaline phosphatase/antialkaline phosphatase reaction.14
RESULTS
Localization of OKT3 From Donor Before Treatment
The donor lymph nodes sampled 1 hour after the intravenous administration of 10 mg of OKT3 had a heavy concentration of mouse immunoglobulin (Fig 4). The murine globulin was not sought in extranodal donor tissues.
Fig 4.
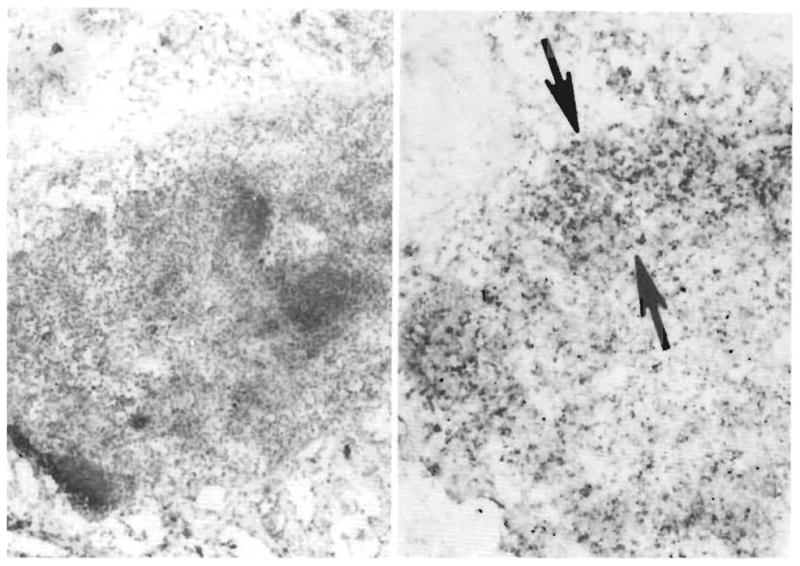
Demonstration of OKT3. Left, A small mesenteric donor lymph node is embedded in fat (frozen section, pinocyanol, × 110), Right, Some cells in the nodal sinuses, cortex, and paracortex (arrows) stain darkly for the presence of murine IgG (alkaline phosphatase/antialkaline phosphatase, × 110).
Graft-vs-host disease was not observed in specimens of the recipient colon during life or at autopsy, or in the skin biopsy sample of a transient rash 3 weeks after transplantation. At autopsy, the depletion of lymphoid tissue from the grafted viscera was remarkable, and, in addition, small nodes from the donor mesentery and the host’s periaortic region were depleted of lymphocytes. The effect, if any, of graft irradiation after transplantation was not analyzable.
Survival and Causes of Death
Patient 1 died 30 minutes after arrival in the intensive care unit. The graft in patient 1 had nonspecific findings of terminal shock. At autopsy, the heart had biventricular hypertrophy and interstitial myocarditis with focal fibrosis and adjacent chronic pericarditis. The lungs had massive and multiple pulmonary emboli as well as severe congestion with focal hemorrhages and necrotizing vasculitis. The kidneys had acute bilateral tubular necrosis.
Patient 2 lived for 192 days after transplantation; 143 days were spent on the ward and 49 days in the pediatric intensive care unit. Multifocal LPD was diagnosed by computed tomographic scan on postoperative day 91 (Figs 5, top, and 6, top). One month earlier on January 5, 1988, the Epstein-Barr profile had shown IgG viral capsid antigen to be positive at a 1:160 titer, up from 1:5 on December 24, 1987. The IgM viral capsid antigen, IgG (EA, D, R), and anti–Epstein-Barr nuclear antigen were all less than 1:5 on February 22. When a biopsy of the hepatic LPD lesions was done at 3 months, the cells tested positive for HLA-A3 but not for Bw4, consistent with recipient but not with donor origin of the lymphoid cells in the biopsy specimen. Kupffer’s cells in adjacent uninvolved liver had the same recipient HLA antigens. This was not surprising since Kupffer’s cells are known to be replaced with recipient macrophages within 100 days.15 The plasmacytoid cells in the lymphoproliferation had cytoplasmic immunoglobulin; κ and λ chains were equally represented but virtually all cells had IgG heavy chain. These findings were suggestive of polyclonality.3,4 Anti–Epstein-Barr nuclear antigen was detected in the lymphoid cells and the presence of Epstein-Barr virus DNA was confirmed using a DNA probe.
Fig 5.
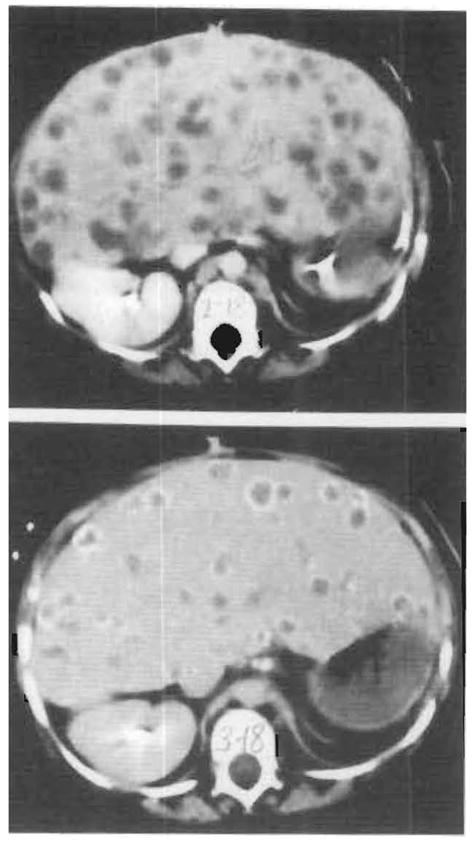
Computed tomographic scans of initial lymphoproliferative disease process. Top, One hundred nine days after transplantation. Multiple lucencies within the liver parenchyma are seen. Histological examination of the cells obtained on liver biopsy revealed a polyclonal lymphoproliferative disorder (see Fig 6, right). Bottom, One hundred thirty-seven days after transplantation. Calcific rims surround many of the parenchymal lucencies. Histological examination revealed necrotic lymphoproliferative cells (see Fig 6, center).
When immunosuppression was stopped on day 104 for 15 days, the lesions underwent total necrosis during the next 3½ weeks as proved by biopsy specimens. By day 130, there was calcification in the rims of the hepatic masses (Figs 5, bottom, and 6, center). However, by day 165 a new hilar mass had appeared, causing an obstruction of the bile duct (Figs 6, right, and 7). The obstruction was partly relieved with catheter drainage but sepsis, cardiovascular collapse, and multiple system organ failure eventually followed.
Fig 6.
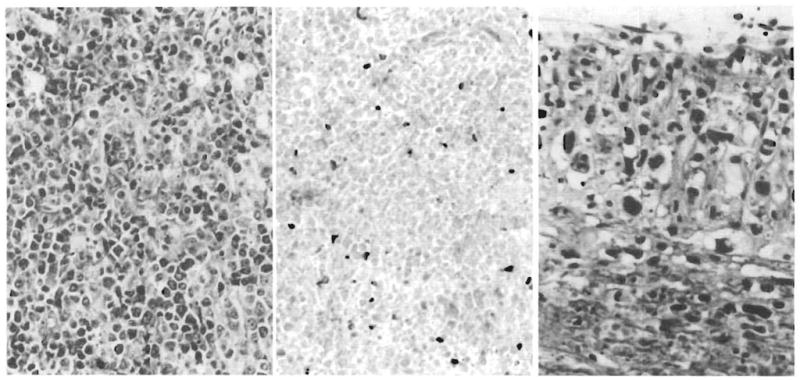
Allograft liver, lymphoproliferative disease. Left, Allograft liver biopsy specimen at 3 months. A diffuse plasmacytoid lymphoid proliferation destroys hepatic tissue (Giemsa, ×220). Center, Allograft liver biopsy specimen at 3¾ months. Necrotic lymphoproliferative disease is represented by ghost cells that have lost both nuclear and cytoplasmic staining detail. Occasional nucleated inflammatory cells persist (hematoxylin-eosin, × 240). Right, Hepatic hilar vasculitis present at autopsy. Branches of the hepatic artery, the hilar fibrous tissue, head of pancreas, and adjacent colon are permeated by a very polymorphous, anaplastic-looking infiltrate quite different from the earlier lymphoproliferative disorder (hematoxylin-eosin, × 440).
At autopsy, the liver had multiple necrotic nodules of “burned out” polymorphous LPD with calcific peripheries. The new hilar LPD lesion was morphologically very different from the earlier ones. This lesion also involved a contiguous segment of the colon wall and the head of the pancreas. The cells had large bizarre nuclei and surrounded larger blood vessels, often infiltrating and destroying the muscular walls of the hilar arteries. The cells, unlike those of the earlier LPD, were not plasmacytoid. Findings of typing for B and T lymphocytes and monocyte-dendritic cells were negative. There was destructive hilar vasculitis with left and right hepatic duct infarction and bacterial overgrowth. Hepatic parenchymal infarction was widespread. Sinusoidal fibrosis and ductular proliferation consistent with hyperalimentation effect was also noted.
Two small perforations of the intestine found at autopsy were additional foci of potentially lethal sepsis; one was recent and the other was walled off.
Organ Function and Structure
Liver
Within 48 hours of transplantation, the bilirubin level fell from 564.3 to 130 μmol/L. After this, there was an insidious rise in bilirubin level, which did not respond to pulse steroid therapy (Fig 8). The 1-week course of OKT3 beginning on postoperative day 23 did not influence the bilirubin level, but just after this, the levels began to fall. A final increase in bilirubin level was due to an obstruction of the bile duct by the LPD.
Fig 8.
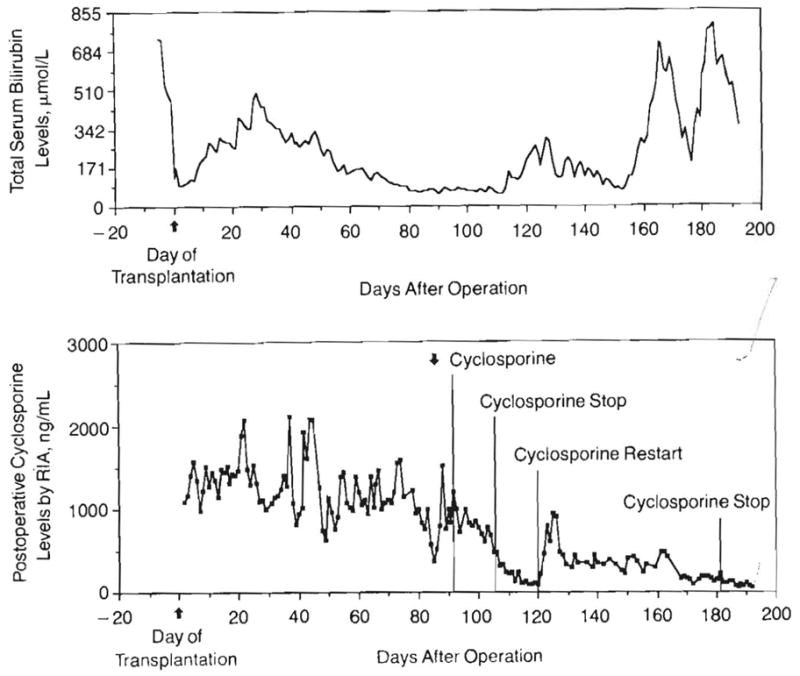
Parallel graphs of serum total bilirubin levels and cyclosporine (radioimmunoassay [RIA]) levels. Four major findings: (1) Rapid fall in total bilirubin level immediately after transplantation, (2) Progressive rise in bilirubin level from days 7 to 30. OKT3 administered for one week beginning day 23 with a decline to normal after treatment. (3) No rise in bilirubin level is seen during the early lymphproliferative disease (day 91). Cyclosporine was subsequently stopped but restarted due to fear of potential rejection or graft-vs-host disease. (4) Late, rapid rise of bilirubin level caused by the obstructing, fatal lymphoproliferative disease that was only transiently relieved by biliary catheter drainage.
Early transaminase level rises to a peak of 323 U/L returned to normal by 1 week. During the time of withdrawal of immunosuppression and necrosis of the lymphoma, the levels of serum transaminases rose during a 10-day period from 1½ to 30 times normal, reaching a peak aspartate aminotransferase value of 1473 U/L. At the same time, there was little effect on the bilirubin level. It was thought that the necrosis had occurred very abruptly.
Normal or near-normal prothrombin times suggested intact synthetic function. Serum albumin concentrations were well maintained until a short time before death.
Fifteen sequential liver biopsy specimens were examined. Purulent cholangitis was diagnosed on day 11, with gram-negative bacilli identified in the tissue. Progressive cholestasis, bile ductular proliferation, hepatocyte ballooning, and sinusidal fibrosis were believed to be evidence of hyperalimentation-induced damage (Fig 9). Features of rejection were never observed in the liver biopsy specimens. The autopsy findings reflected damage secondary to the LPD.
Fig 9.
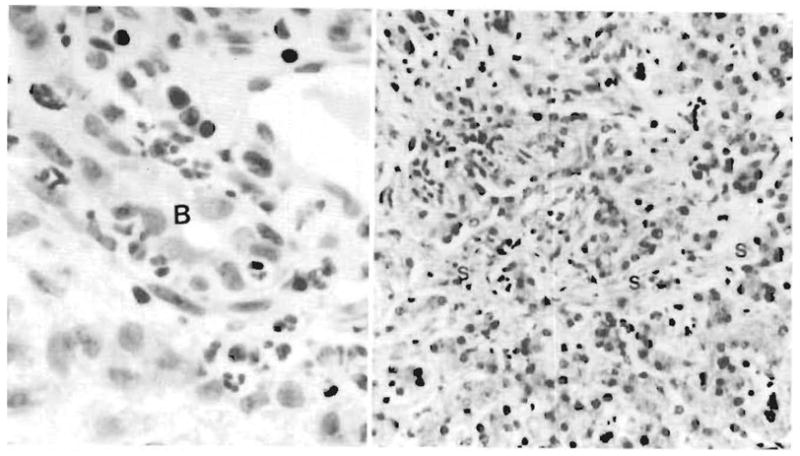
Allograft liver. Left, Biopsy specimen obtained on day 11 after transplantation reveals a cholangitis and pericholangitis. Neutrophils are present in and around a bile duct (B). Right, Allograft liver biopsy specimen obtained at 6 months. The liver has canalicular cholestasis, bile ductular proliferation, and a generalized fine sinusoidal fibrosis (S) believed to be most consistent with prolonged hyperalimentation effect (hematoxylineosin, ×200).
The Hollow Viscera
An upper gastrointestinal tract series with a small-bowel follow-through was done by administering a barium meal on day 109 (Fig 10). Transit time from stomach to colon was 2½ hours, which is normal for a child of this age. The mucosal pattern was normal (Fig 10). The mucosal barrier was intact. In the fourth postoperative month, only 0.46% of the intravenous 51C-labeled albumin was excreted into the stool over a 4-day period.
Fig 10.
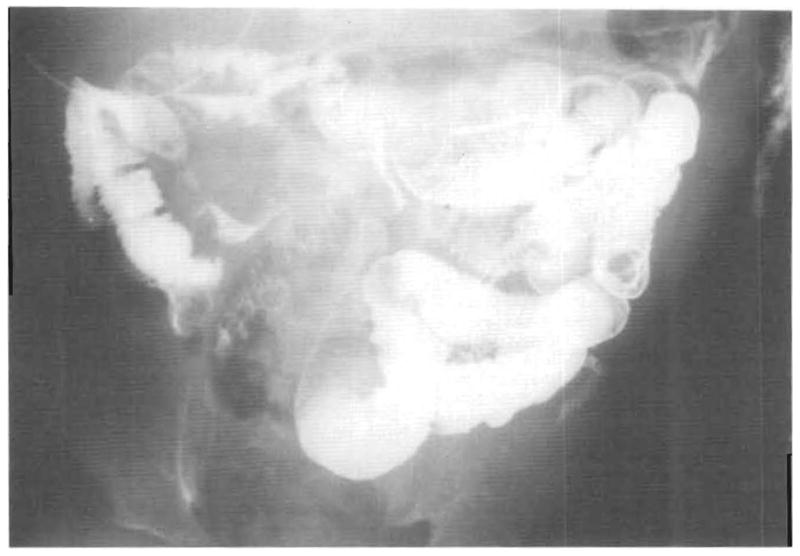
Barium meal–day 109. Note the feathery normal appearance of the small intestine mucosa.
At the end of the second month, only 5.0±7.0 (SD) g/L of total protein, 33.36 ±25.37 (SD) μmol/L of glucose, and low concentrations of sodium and potassium were detected in the stool. This suggested that the stool losses were mainly water. Two months after transplantation, stool volume became less and the percent fluid absorption improved from a low of about 10% to a high of approximately 75%. The majority of stool output was between midnight and 7 AM. Total stool output during periods of fasting (156 ±24 [SD] mL/24 h) was similar to that during feeding (159 ± 145 [SD] mL/24 h). Carbohydrate absorption was determined to be very efficient, as only small amounts of reducing sugars were noted in the stool during Peptamen administration. The amount of intravenous hyperalimentation was greatly reduced and nearly stopped when nasogastric feedings were increased with both Peptamen and blenderized diets (Fig 3). Positive nitrogen balance was present on days 4 to 17.
Immediately after operation, the patient weighed 12.6 kg. This increased to 15.6 kg at the time of nasogastric feedings. Her weight reached a maximum of 16.3 kg 3 months postoperatively. However, it dropped to 14.5 to 15.0 kg in the last month of life.
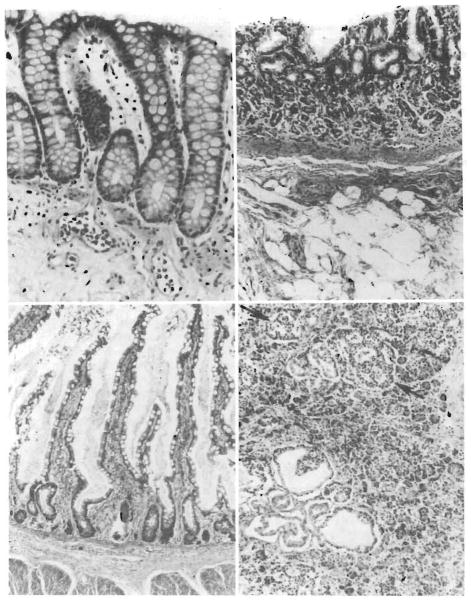
The allografted large bowel was sampled at 6 days, 3 months, and 6 months after transplantation. No suggestion of rejection was observed (Fig 11, top left). The epithelium was intact and there was a paucity of lymphoid tissue in the 6-month biopsy specimen.
Fig 11.
Gastrointestinal tract. Top left, Colonic biopsy specimen from the donor colon proximal to the colostomy, taken 1 week before death (6 months after transplant). The glands extend to the muscularis mucosae, a normal finding, and there is neither cellular infiltration nor epithelial damage. Lymphoid tissue is sparse (hematoxylin-eosin, ×280). Top right, Donor stomach at autopsy. The mucosa has mild atrophy and some fibrosis of the lamina propria (hematoxylin-eosin, ×180). Bottom left, Donor ileum at autopsy. In places, the villous architecture reveals hyperplasia rather than atrophy. The surface epithelium is intact, the lamina propria somewhat fibrotic, but there is no cellular infiltrate (hematoxylin-eosin, ×150) Bottom right, Donor pancreas at autopsy. There are foci of cystic ductular proliferation in the head of the pancreas. For the most part, islets are abundant (arrows) and there is little atrophy, interstital fibrosis or cellular infiltrate (hematoxylin-eosin, ×180).
At autopsy, the allografted gastric mucosa was mildly atrophic when compared with the remnant of host stomach (Fig 11, top right). The small intestine had intermixed zones of atrophic and hypertrophic mucosa. The submucosa was mildly fibrotic and the intestinal arteries were thick walled. There was no evidence of lymphangiectasia (Fig 11, bottom left). No solitary lymphoid follicles were visualized in the large intestine. Peyer’s patches were not seen in the ileum nor were there plasma cells.
Pancreas
Serum amylase levels were less than 42 U/L throughout the entire course. Ten weeks after operation at a time when the child was not being administered exogenous pancreatic enzymes, trypsin activity was detected in the colostomy drainage beyond a 1:256 dilution (normal for this age, >1:40). The findings were consistent with normal proteolytic activity in the feces.
Serum C peptide level was 0.72 pmol/mL on day 37. The level was 2.16 pmol/mL on day 93, three times higher than the upper limit of the nonfasting diabetic.
At autopsy, the transplanted pancreas was nearly annular and appeared virtually normal except for minor ductoinsular proliferation with microcystic changes (Fig 11, bottom right).
Cyclosporine Pharmacokinetics
The clearance of cyclosporine following intravenous administration was 3.4 mL/min per kilogram during the first study period (postoperative days 74 and 75) and 3.8 mL/min per kilogram during the second study period (postoperative days 102 and 103). The half-life of cyclosporine was 11.3 and 16 hours for the respective periods. Oral bioavailability was 2% for the first period and 8% for the second period. The intestine absorbed cyclosporine as well as in pediatric patients with liver transplants but not as well as in pediatric recipients of renal transplants (Table 2). Decreasing the volume of olive oil at the time of the second testing could have explained the improvement of absorption by elimination of a “castor oil” effect. The lower clearance and prolonged half-life of cyclosporine relative to that in pediatric patients with liver and renal transplants may be explained by diminished hepatic function during the testing periods. The bilirubin level at the time of the first study was 102 μmol/L, and at the time of the second study it was 62.9 μmol/L.
Table 2.
Cyclosporine Pharmacokinetics
| Transplant Group | Cyclosporine Clearance Rate, mL/min* per Kilogram | Half-life, h | Volume of Distribution, L/kg* | Bioavailability, % |
|---|---|---|---|---|
| Pediatric renal (n = 7) | 11.8 (9.8–15.5) | 7.3 | 4.7 (3–7.4) | 31 |
| Pediatric liver (n = 7) | 8.4 (1.9–13.9) | … | … | <5–18 |
| Multivisceral† (n = 1) | 3.4 | 11.3 IV | 2.3 | 0 |
| 9.1 PO | … | 2 | ||
| 3.8 | 16.0 IV | 4.3 | … | |
| 13.6 PO | … | 8 | ||
Numbers in parentheses indicate range.
IV indicates intravenous; and PO, oral.
Evidence of Bacterial Translocation
During the first 12 postoperative days, gram-negative bacteria were cultured from the blood on six occasions. After selective decontamination was started on day 12 and continued until death, the child had no further episodes of gram-negative bacteremia. However, she had 39 blood cultures of gram-positive organisms, preponderantly Staphylococcus epidermidis. The gram-positive infections were treated with oral and systemic vancomycin hydrochloride, or other antibiotics as dictated by results of cultures. There was evidence that the recurrent bacteremia originated in the intestine since the identical organism was cultured from the blood and stool 81% of the time. The patient also had six positive blood cultures of Candida that were treated by oral and intravenous amphotericin B. On seven occasions despite antibacterial or antifungal therapy, it was necessary to remove the central venous catheter to reverse the septic episode.
COMMENT
The problems of rejection and graft-vs-host reactions with multivisceral transplantation differ only quantitatively from those encountered with transplantation of the individual organs. The susceptibility to rejection of liver and pancreas grafts is common knowledge. In addition, complications of GVHD after clinical liver transplantation can occur16,17 and may do so more often than is realized. If the spleen is included with whole-pancreas grafts, serious or even lethal GVHD can result.18–20 Nevertheless, transplantation of both the liver and pancreas has been carried out with increasing enthusiasm and success.
Comparable progress has not been made with the experimental or clinical transplantation of the hollow intra-abdominal organs. Before the availability of cyclosporine, long-term survival with intestinal transplantation was unattainable. Afterward, this was accomplished in dogs,21,22 pigs,23,24 and rats.25–27 However, surgical failures have continued to be common and rejection has been difficult to control. Infection from enteric microorganisms leaking through the rejecting grafts has been thereby increased. The best results in a large animal model were reported by Grant et al,24 who were able to consistently obtain protracted survival after orthotopic small-bowel transplantation in out-bred pigs. Seven of 16 pigs were kept alive with carefully monitored intravenous and oral cyclosporine treatment for almost 100 days. All therapy was then stopped with subsequent long-term survival as if tolerance had been induced.
Despite these encouraging notations, a warning sign was noted in our own experience after total pancreas transplantation in which duodenum and jejunum were included as part of a composite graft.18 A protein-losing enteropathy, hypoproteinemia, and diarrhea were caused by the small segment of intestine, necessitating resection of all of the jejunum and all but a bubble of duodenum. The prospect of this complication with a transplant of the entire gastrointestinal tract was what prompted our studies on albumin leakage in the multivisceral recipient, which proved to be negative.
An even more sinister aspect of intestinal transplantation has been the GVHD that was described long ago both in untreated and immunosuppressed dogs2,28,29 and studied decisively by Monchik and Russell30 in F1 hybrid rats whose breeding permitted the lymphoid tissue in the intestinal graft to mount an attack on the immunologically defenseless recipient. Strategies to inhibit the GVHD have included ex vivo irradiation of the graft.30–34 Because GVHD in intestinal grafts is proportional to the bowel length,35 the inclusion of an even greater mass of lymphoid tissue in a multivisceral graft was a cause of anxiety.
In our long-surviving patient, both rejection and GVHD were controlled. There was no evidence of rejection in the biopsy samples of the liver and intestinal tract or in the autopsy specimens. Nor was there clinical or histopathologic evidence of GVHD. Avoidance of GVHD depends on effective immunosuppression, but management directed at the lymphoid tissue in the multivisceral graft may have contributed. The tiny donor was given a massive dose of OKT3 that had bound to donor lymphoid tissues sampled 1 hour later. The objective was to prevent or mitigate GVHD, something that was frequently reported to be feasible by Shaffer et al36 after giving antilymphocyte serum to rat donors of intestinal grafts in a unidirectional F1 hybrid model. In addition, the graft in our recipient was treated prophylactically with 4.5 Gy in six divided doses during postoperative weeks 1 through 3.
Therapy to prevent rejection and GVHD may have been too intensive. Overimmunosuppression is implicit in the development of LPD in our patient and possibly in the child reported by Williams et al5 in this issue of The Journal. The LPD in our patient’s liver biopsy samples contained HLA antigens that were recipient specific. There was necrosis and calcification of the LPD lesions after discontinuance of immunosuppression, an effect that has been noted before3,4 and that may have been aided by treatment with the antiviral agent acyclovir.10 A more ominous-appearing infiltrate developed in the hilar region after restarting cyclosporine and prednisone therapy. The decision to resume immunosuppression may have been unnecessary since it is conceivable that the kind of tolerance induction described by Grant et al24 in pigs had already occurred.
A tolerant state has been achieved often in rodents with a variety of whole-organ grafts and to a less predictable extent in large, outbred animals.37,38 It has not been determined if tolerance induction will be easier or more difficult in intestinal as opposed to other kind of grafts or if the presence of functional lymphoid tissue in the graft will simplify or complicate the task. Recent studies with bone marrow by Knobloch and Dennert39 have suggested that the immunologically active T lymphocytes responsible for GVHD could also be intrinsically immunosuppressive and necessary for graft acceptance provided they are present in appropriate numbers.
The most encouraging observation in our patient was that unequivocal function was obtained from all of the organs in the multivisceral graft. Despite early involvement with cholangitis and later with LPD and biliary obstruction, the liver provided good synthetic and other function for more than a half year. The hollow gastrointestinal tract viscera allowed near-total enteral nutrition for extended periods. Pancreatic function was present even though exogenous insulin was intermittently given with TPN. None of the organs ever had histopathologic evidence of rejection.
Much was learned about the management of the infectious problems that have plagued efforts at transplantation of the gastrointestinal tract organs. It has been recognized for 30 years that bacteria indigenous to the gastrointestinal tract translocated after hepatic transplantation when the grafts were damaged by ischemia or rejection.40,41 The problem is undoubtedly even more critical following preservation and transplantation of the small intestine.42 “Selective decontamination” was attempted in our patient with nonabsorbable antibiotics,9 hoping to prevent the overgrowth and “leak” of pathogenic gram-negative microorganisms and of Candida while preserving a normal flora of intestinal anaerobes. The effort was successful in that gram-negative bacteria were never again cultured from the blood, although Candida was not eliminated. Subsequent bacteremias were with gram-positive cocci, predominantly staphylococci, that could be controlled with appropriate antibiotics or venous catheter removal. The microorganisms in the blood usually could be predicted 1 or 2 days in advance by finding the same bacteria in the stool.
The most important conclusion from our longest surviving patient was that she came tantalizingly close to truly long-term survival with a functioning gastrointestinal tract. There were no mortality factors that were beyond rational explanation nor any management adjustments that require therapeutic tools that are not now available. It seems inevitable that further cautious trials of multivisceral and/or intestinal transplantation will be made.
Fig 7.
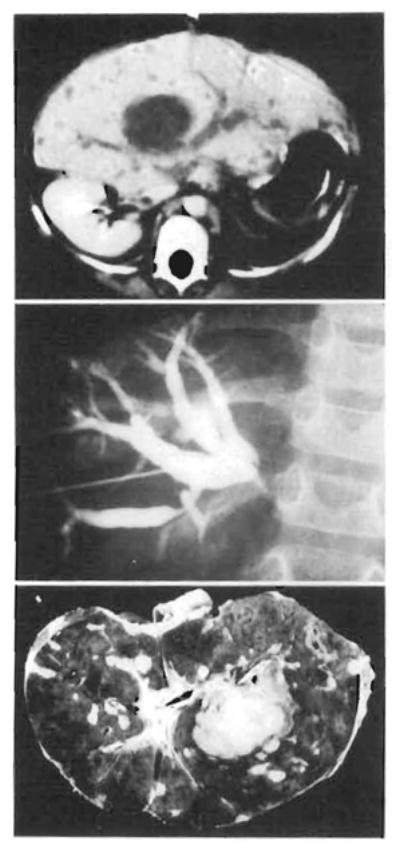
Computed tomographic scan and cholangiogram of terminal lymphoproliferative disease process and the hemisected liver at autopsy. Top, Computed tomographic scan at 165 days. The hepatic ducts are obstructed by a large hilar mass discovered on histological examination to be morphologically different than the previous lymphoproliferative disease process (see Fig 6, right). This was confirmed by transhepatic cholangiogram (center). Note the resolving lymphoproliferative disease in the periphery of the liver on the computed tomographic scan, Bottom, Transected liver at autopsy confirms previously mentioned findings and reveals widespread parenchymal necrosis with hemorrhage. Note catheters within the hepatic ducts.
Acknowledgments
This study was supported by research grants from the Veterans Administration; grant AM-29961 from the National Institutes of Health, Bethesda, Md; and the Pathology Education and Research Foundation of Pittsburgh, Pittsburgh, Pa.
Antibodies were purchased from Beckton Dickinson, Mountain View, Calif, and from Dako Corp, Santa Barbara, Calif. Monoclonal antibodies to HLA specificities were generously supplied by Karin Nelson, PhD, Genetic Systems, Seattle, Wash.
References
- 1.Starzl TE, Kaupp HA., Jr Mass homotransplantation of abdominal organs in dogs. Surg Forum. 1960;11:28–30. [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Kaupp HA, Jr, Brock DR, Butz GW, Jr, Linman JW. Homotransplantation of multiple visceral organs. Am J Surg. 1962;103:219–229. doi: 10.1016/0002-9610(62)90491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Nalesnik MA, Porter KA, et al. Reversibility of lymphomas and lymphoproliferative lesions developing under cyclosporin-steroid therapy. Lancet. 1984;1:583–587. doi: 10.1016/s0140-6736(84)90994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nalesnik MA, Makowka L, Starzl TE. The diagnosis and treatment of post-transplant lymphoproliferative disorders. Curr Probl Surg. 1988;25:365–472. doi: 10.1016/0011-3840(88)90011-1. [DOI] [PubMed] [Google Scholar]
- 5.Williams JW, Sankary HN, Foster PF, Lowe J, Goldman GM. Splanchnic transplantation: an approach to the infant dependent on parenteral nutrition who develops irreversible liver disease. JAMA. 1989;261:1458–1462. doi: 10.1001/jama.261.10.1458. [DOI] [PubMed] [Google Scholar]
- 6.Cosimi A, Colvin R, Burton R, et al. Use of monoclonal antibodies to T cell subsets for immunologic monitoring and treatment in recipients of renal allografts. N Engl J Med. 1981;305:308. doi: 10.1056/NEJM198108063050603. [DOI] [PubMed] [Google Scholar]
- 7.Jamieson NV, Sundberg R, Lindell S, et al. Successful 24 hour liver preservation: a preliminary report. Transplant Proc. 1988;20:945–947. [Google Scholar]
- 8.Wahlberg JA, Love R, Landgaard L, Southard JH, Belzer FO. Seventy-two hour preservation of the canine pancreas. Transplantation. 1987;43:5–8. doi: 10.1097/00007890-198701000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Stoutenbeek CP, van Saene HKF, Miranda DR, Zandstra DF, Binnendijk B. The effect of oral nonabsorbable antibiotics on the emergence of resistant bacteria in patients in an intensive care unit. J Antimicrob Chemother. 1987;19:513–520. doi: 10.1093/jac/19.4.513. [DOI] [PubMed] [Google Scholar]
- 10.Hanto DW, Frizzera G, Gajl-Peczalska KJ, et al. Epstein-Barr virus induced B-cell lymphoma after renal transplantation. N Engl J Med. 1982;306:913–918. doi: 10.1056/NEJM198204153061506. [DOI] [PubMed] [Google Scholar]
- 11.Donatsch P, Abisch E, Homberger M, Trabar R, Trapp M, Voges R. A radioimmunoassay to measure cyclosporin A in plasma and serum samples. J Immunoassay. 1981;2:19–32. doi: 10.1080/01971528108062989. [DOI] [PubMed] [Google Scholar]
- 12.Sawchuk RJ, Cartier LL. Liquid chromatographic determination of cyclosporin A in blood and plasma. Clin Chem. 1981;27:1368–1371. [PubMed] [Google Scholar]
- 13.Waldmann TA. Gastrointestinal protein loss demonstrated by 51Cr labelled albumin. Lancet. 1961;2:121–123. doi: 10.1016/s0140-6736(61)92646-0. [DOI] [PubMed] [Google Scholar]
- 14.Cordell JL, Falini B, Erben WN, et al. Immunoenzymatic labelling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (AP-APP complexes) J Histochem Cytochem. 1984;32:219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- 15.Kashiwagi N, Porter KA, Penn I, Brettschneider L, Starzl TE. Studies of homograft sex and of gamma globulin phenotypes after orthotopic homotransplantation of the human liver. Surg Forum. 1969;20:374–376. [PMC free article] [PubMed] [Google Scholar]
- 16.Ramsey G, Nusbacher J, Starzl TE, Lindsay GD. Isohemagglutinins of graft origin after ABO-unmatched liver transplantation. N Engl J Med. 1984;311:1167–1170. doi: 10.1056/NEJM198411013111807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burdick JF, Vogelsang GB, Smith WJ, Farmer ER. Severe graft-versus-host disease in a liver-transplant recipient. N Engl J Med. 1988;318:689–691. doi: 10.1056/NEJM198803173181107. [DOI] [PubMed] [Google Scholar]
- 18.Starzl TE, Iwatsuki S, Shaw BW, Jr, et al. Pancreaticoduodenal transplantation in humans. Surg Gynecol Obstet. 1984;159:265–272. [PMC free article] [PubMed] [Google Scholar]
- 19.Gonwa TA, Goeken NE, Schulak JA, Nghiem DD, Corry RJ. Failure of cyclosporine to prevent in vivo T cell priming in man: studies in allogeneic spleen transplantation. Transplantation. 1985;40:299–304. doi: 10.1097/00007890-198509000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Deierhoi MH, Sollinger HW, Bozdech MJ, Belzer FO. Lethal graft versus host disease in a recipient of a pancreas-spleen transplant. Transplantation. 1986;41:544–546. [PubMed] [Google Scholar]
- 21.Craddock GN, Nordgren SR, Reznick RK, et al. Small bowel transplantation in the dog using cyclosporine. Transplantation. 1983;35:284–288. doi: 10.1097/00007890-198304000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Diliz-Perez HS, McClure J, Bedetti C, et al. Successful small bowel allotransplantation in dogs with cyclosporine and prednisone. Transplantation. 1984;37:126–129. doi: 10.1097/00007890-198402000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ricour C, Revillion Y, Amaud-Battandier F, et al. Successful small bowel allografts in piglet using cyclosporine. Transplant Proc. 1983;15 (suppl 1):3019–3026. [Google Scholar]
- 24.Grant D, Duff J, Zhong R, et al. Successful intestinal transplantation in pigs treated with cyclosporine. Transplantation. 1988;45:279–284. doi: 10.1097/00007890-198802000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Kirkman RL, Lear PA, Madara JL, Tilney NL. Small intestine transplantation in the rat: immunology and function. Surgery. 1984;96:280–287. [PubMed] [Google Scholar]
- 26.Hatcher PA, Deaton DH, Bollinger R. Transplantation of the entire small intestine in inbred rats using cyclosporine. Transplantation. 1987;43:478–484. doi: 10.1097/00007890-198704000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Lee KKW, Schraut WH. Structure and function of orthotopic small bowel allografts in rats treated with cyclosporine. Am J Surg. 1986;151:55–60. doi: 10.1016/0002-9610(86)90011-5. [DOI] [PubMed] [Google Scholar]
- 28.Lillehei RC, Goott B, Miller FA. The physiologic response of the small bowel of the dog to ischemia including prolonged in vitro preservation of the bowel with successful replacement and survival. Ann Surg. 1959;150:543–560. doi: 10.1097/00000658-195910000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lillehei RC, Longerbeam JK, Goott B, Goldberg S, Scott WR. Gastrointestinal transplantation. Surg Clin North Am. 1962;42:1197–1218. doi: 10.1016/s0039-6109(16)36786-x. [DOI] [PubMed] [Google Scholar]
- 30.Monchik GJ, Russell PS. Transplantation of the small bowel in the rat: technical and immunologic considerations. Surgery. 1971;70:693–702. [PubMed] [Google Scholar]
- 31.Cohen Z, MacGregor AB, Moore KTH, Falk RE, Langer B, Cullen JB. Canine small bowel transplantation: a study of immunologic responses. Arch Surg. 1976;111:248–253. doi: 10.1001/archsurg.1976.01360210042008. [DOI] [PubMed] [Google Scholar]
- 32.Williams JW, McClellan T, Peters TG, et al. Effect of pretransplant graft irradiation on canine intestinal transplantation. Surg Gynecol Obstet. 1988;167:197–204. [PubMed] [Google Scholar]
- 33.Deltz E, Ulrich K, Schach T, et al. Graft-versus-host reaction in small bowel transplantation and possibilities for its circumvention. Am J Surg. 1986;151:379–386. doi: 10.1016/0002-9610(86)90473-3. [DOI] [PubMed] [Google Scholar]
- 34.Lee KKW, Schraut WH. In vitro allograft irradiation prevents graft versus host disease in small bowel transplantation. J Surg Res. 1985;38:364–372. doi: 10.1016/0022-4804(85)90050-2. [DOI] [PubMed] [Google Scholar]
- 35.Deltz E, Muller-Hermelink HK, Ulrich K, Thiede A, Muller-Ruchholtz W. Development of graft versus host reaction in various target organs after small intestinal transplantation. Transplant Proc. 1981;13:1215–1216. [PubMed] [Google Scholar]
- 36.Shaffer D, Maki T, DeMichele SJ, et al. Studies in small bowel transplantation: prevention of graft versus host disease with preservation of allograft function by donor pretreatment with antilymphocyte serum. Transplantation. 1988;45:262–269. [PubMed] [Google Scholar]
- 37.Starzl TE. Experience in Renal Transplantation. Philadelphia, Pa: WB Saunders Co; 1964. pp. 164–170. [Google Scholar]
- 38.Starzl TE. Experience in Hepatic Transplantation. Philadelphia, Pa: WB Saunders Co; 1969. pp. 203–206.pp. 227–233. (with the assistance of Putnam CW) [Google Scholar]
- 39.Knobloch C, Dennert G. Loss of F1 hybrid resistance to bone marrow grafts after injection of parenteral lymphocytes. Transplantation. 1988;45:175–183. doi: 10.1097/00007890-198801000-00037. [DOI] [PubMed] [Google Scholar]
- 40.Brettschneider L, Tony JL, Boose DS, et al. Specific bacteriologic problems after orthotopic liver transplantation in dogs and pigs. Arch Surg. 1968;97:313–322. doi: 10.1001/archsurg.1968.01340020177021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Starzl TE. Experience in Hepatic Transplantation. Philadelphia, Pa: WB Saunders Co; 1969. pp. 322–347. (with the assistance of Putnam CW) [Google Scholar]
- 42.Toledo-Pereyra LH, Raij L, Simmons RL, Najarian JS. Role of endotoxin and bacteria in long term survival of preserved small bowel allografts. Surgery. 1974;73:474–481. [PubMed] [Google Scholar]