Abstract
Patients with interleukin 12 (IL-12)p40 or IL-12 receptor β1 (IL12Rβ1) deficiencies are prone to develop infections caused by mycobacteria and salmonella; other infections have only been rarely observed. In this report we describe 2 unrelated patients with complete autosomal recessive IL12Rβ1 deficiency who suffered from sepsis attributable to Klebsiella pneumoniae. A Mexican boy suffered from disseminated bacilli Calmette-Guérin disease and infections caused by K pneumoniae and Candida albicans and had a fatal outcome. A Turkish girl living in France suffered from disseminated Nocardia nova infection and K pneumoniae sepsis. Therefore, Klebsiella infections should be considered in patients with IL12Rβ1 deficiency. Conversely, IL12Rβ1 deficiency should be considered in patients with unexplained klebsiellosis.
Keywords: IL-12 deficiency, IL12Rβ1 deficiency, Klebsiella pneumoniae, Nocardia nova, Candida albicans, Mycobacterium bovis BCG
Mendelian susceptibility to mycobacterial diseases (Online Mendelian Inheritance in Man ID 209950) is a rare genetic condition predisposing mainly to mycobacteria and salmonella infections in humans and is often associated with mutations in genes that control interleukin 12 (IL-12)/IL-23– dependent, interferon γ (IFN-γ)–mediated immunity.1 Mutations in 6 genes (IFNGR1, IFNGR2, STAT1, NEMO, IL12B, and IL12RB1) account for up to 13 genetic conditions.1,2 IL-12p70 is a heterodimeric cytokine (p35 and p40) produced by macrophages and dendritic cells in response to microbial or T-cell stimulation.3 IL-12 induces the production of IFN-γ in T and natural killer cells after recognition by its receptor, consisting of β1 and β2 chains.
Some patients with mendelian susceptibility to mycobacterial diseases display defects in IL-12 production caused by mutations in the IL12B gene encoding the IL-12p40 subunit (common to IL-12 and IL-23), whereas others have defective responses to IL-12 caused by mutations in IL12RB1 (encoding the β1 chain, common to IL-12 and IL-23 receptors).4,5 Table 1 summarizes published documented infections in patients with mutations in the IL12B or IL12RB1 gene; rare infections other than mycobacteriosis and salmonellosis, such as paracoccidioidomycosis or leishmaniasis, suggest that these immunologic defects might confer susceptibility to a broader range of microorganisms. We describe here 2 unrelated patients with homozygous IL12RB1 mutations who displayed unusual infections caused by Klebsiella pneumoniae.
TABLE 1.
Infections in Patients With Mutations in IL12RB1 (Protein IL-12Rβ1) or IL12B (Protein IL-12p40)
| Infection | Gene | Mutation | Patients/Kindreds | Reference No. |
|---|---|---|---|---|
| Patients with single infection | ||||
| M bovis BCG | IL12RB1 | 783+1 G→C | 1/1 | 19 |
| IL12RB1 | 853 C→T | 4/2 | 20 | |
| 1791+2 T→G | ||||
| IL12B | g.315_316insA | 3/2 | 4 | |
| IL12B | g.297del8 | 3/1 | 21 | |
| M avium | IL12RB1 | Not identified | 2/2 | 5 and 19 |
| IL12RB1 | R213W | 1/1 | 22 | |
| IL12RB1 | C65_68del CTGC | 1/1 | 23 | |
| Salmonella group D | IL12RB1 | C186S | 1/1 | 24 |
| S enteritidis | IL12RB1 | 700+362_1619–944 del | 1/1 | 5 and 25 |
| IL12RB1 | Not identified | 1/1 | 26 | |
| IL12B | g.482+82_856–854 del | 2/2 | 4 | |
| IL12RB1 | R173P | 1/1 | 27 | |
| M tuberculosis | IL12RB1 | 1791+2T→G | 2/1 | 5 and 28 |
| IL12RB1 | R213W | 1/1 | 29 | |
| Patients with ≥ 2 infections | ||||
| BCG + Salmonella | IL12RB1 | K305X | 1/1 | 19 |
| IL12RB1 | 1791+2 T→G | 1/1 | 28 | |
| IL12RB1 | 783+1 G→A | 1/1 | 30 | |
| IL12B | g.482+82_856–854 del | 2/2 | 4 | |
| IL12B | g.315_316insA | 3/2 | 4 | |
| EM + Salmonella | IL12RB1 | Q214R | 1/1 | 5 and 19 |
| IL12RB1 | Q32X | 1/1 | 31 | |
| IL12RB1 | Q376X | 1/1 | 31 | |
| IL12RB1 | 1021+1 G→C | 1/1 | 32 | |
| IL12RB1 | del exon 8–13 | 1/1 | 33 | |
| M tuberculosis + Salmonella | IL12RB1 | 1791+2 T→G | 2/1 | 5 and 28 |
| IL12B | g.315_316insA | 1/1 | 4 | |
| Patients with other infections | ||||
| BCG + Salmonella + Paracoccidioides brasiliensis | IL12RB1 | L77F | 1/1 | 34 |
| BCG + Nocardia asteroides | IL12B | g.315_316insA | 1/1 | 4 |
| Salmonella + Kingella kingae | IL12B | C186S | 1/1 | 5 |
| Salmonella + Leishmania sp | IL12RB1 | r.467_483del | 1/1 | 35 |
ins indicates insertion; del, deletion.
CASE REPORTS
Patient 1
Patient 1 was a mestizo (European/Amerindian) boy born in Veracruz, Mexico, in 2002 to nonrelated parents. His older sibling received bacille Calmette-Guérin (BCG) vaccination at birth without any adverse reaction and remains healthy; in contrast, patient 1 was vaccinated against BCG during his first month of life and developed axillary BCGitis at the age of 8 months. Despite antimycobacterial treatment given at standard doses (including isoniazid [at 9 months], isoniazid plus rifampin [at 6 months], and isoniazid, rifampin, and ethambutol [at 3 months]), lymphadenitis extended to his cervical nodes. During this time, a bone marrow culture was positive for nontyphoidal Salmonella, and he was treated with chloramphenicol. Antimycobacterial treatment for a total of 26 months resulted in intermittent improvements but no cure.
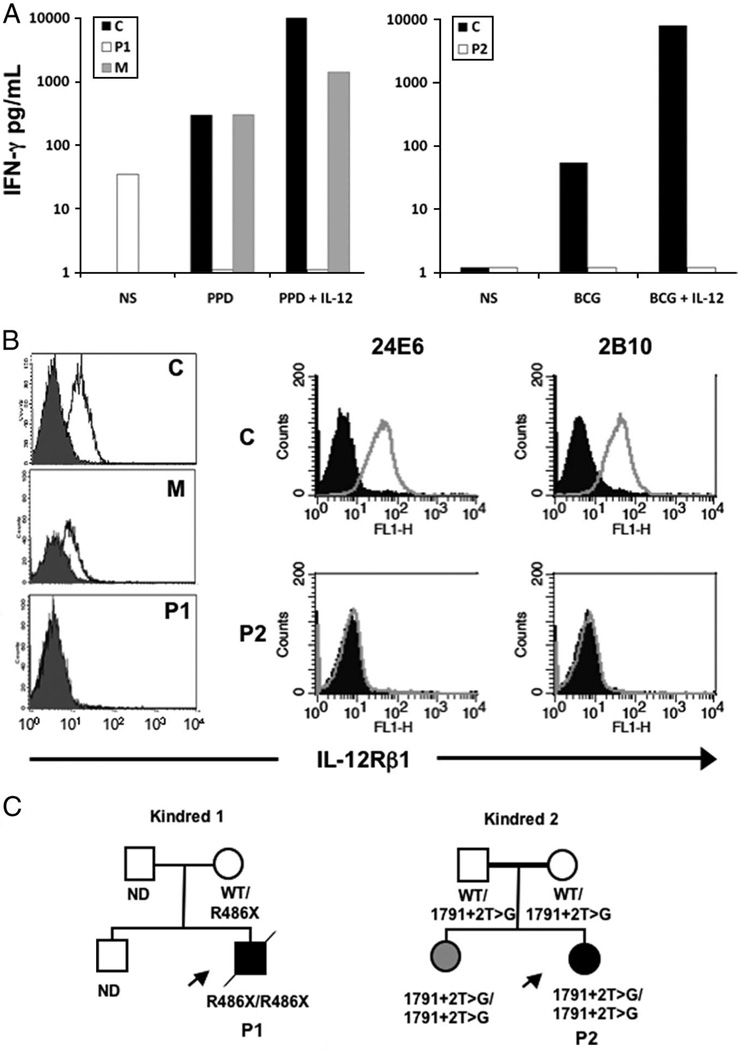
At the age of 3½ years, after 6 months without any treatment, he was admitted to the hospital with hepatosplenomegaly and multiple lymphadenitis with failure to thrive; HIV serology results were negative, and results of polymerase chain reaction and serology for Epstein-Barr virus (immunoglobulin G for viral capsid antibody) were positive. A biopsy of the cervical lymph node revealed a loss of lymph node architecture with noncaseating lymphoepithelioid granulomas and numerous Langerhans cells and tested positive for acid-fast bacilli. Daily treatment with isoniazid, rifampin, ethambutol, and ciprofloxacin was initiated. Despite this treatment, the patient was readmitted to the hospital 8 months later at the age of 4½ years with fever, weight loss, progressive dyspnea, thrush, generalized lymphadenitis, and the presence of abscesses at the posterior upper thorax wall, on the scapula, and in the lumbar region. The dorsolumbar abscess required surgical debridement, and the culture obtained from the excised material was positive for Mycobacterium bovis, which was resistant to isoniazid, rifampin, and pyrazinamide. A magnetic nuclear resonance scan of the spinal cord showed severe inflammation of the spinal meninges, and a cerebrospinal fluid sample was found to contain 78 mg/mL protein, 17 mg/mL glucose, and 18 leukocytes (mononuclear cells) per mL. The IL-12/IFN-γ axis was studied in this patient, as described elsewhere,6 and showed no response to IL-12 in terms of IFN-γ production by blood cells from (Fig 1A), which suggests that the IL-12 receptor was nonfunctional. The IL-12 receptor β1 (IL12Rβ1) chain was undetectable by flow cytometry on T-cell blasts (Fig 1B). IL12RB1 sequencing identified a homozygous R486X mutation in the patient and a heterozygous mutation in his mother (Fig 1C). Treatment with ciprofloxacin, streptomycin, ethambutol, prothionamide, and IFN-γ, together with clindamycin for the soft tissue abscess, was initiated. However, the patient’s condition worsened, and 2 months later he developed fever, a systemic inflammatory response, neurologic symptoms, aplasia, and paralysis. Three consecutive cultures of cerebrospinal fluid and urine tested positive for Candida albicans (consistent with the isolation of blastoconidia from oral lesions), so the patient was treated with amphotericin B and fluconazole.
FIGURE 1.
A, IFN-γ produced by stimulated whole-blood samples was undetectable in the patients, and they did not respond to IL-12. NS indicates not stimulated; P1, patient 1; P2, patient 2; M, mother of patient 1; PPD, purified protein derivative from M tuberculosis; C, healthy control. B, IL12Rβ1 was not expressed on PHA-T blasts, as assessed with 1 (patient 1, left) and 2 (patient 2, right) monoclonal antibodies. FL1-H indicates ●●●●●●; open histograms, IL12Rβ1; closed histograms, isotype controls. C, Family trees of the studied patients showing the mutations found. ND indicates not done; WT, wild type; arrows, patients; diagonal line, the person concerned is dead.
In the terminal phase of his illness, blood cultures of 3 blood samples tested positive for K pneumoniae serotype 2, an extended-spectrum β-lactamase producer; the patient was then treated with piperacillin plus tazobactam, and a negative blood culture was obtained after 72 hours. Despite antibiotic treatment, patient 1 suffered wasting and multiple organ failure; he died 3 months after admission at the age of 4 years 8 months.
Patient 2
Patient 2 was a girl born in 2007 to consanguineous parents of Turkish origin living in France. Patient 2 was the second child and was born at term with a normal weight and height. Her elder sister, born in 2004, had been vaccinated* with BCG in infancy and developed localized BCGitis with spontaneous improvement. Patient 2 was not vaccinated against BCG but did receive 3 injections of a pentavalent vaccine against Haemophilus influenzae type b, diphtheria, Bordetella pertussis, tetanus toxoid, and poliovirus, 3 injections of a conjugate vaccine against pneumococcus, and 1 injection of a live vaccine against measles, mumps, and rubella; there were no adverse events.
At 14 months of age, patient 2 presented with inguinal adenitis and a daily peak fever of 38.5°C. She was admitted to the hospital 2 weeks later with multiple adenopathies (inguinal, pelvic, and abdominal), which were explored by abdominal ultrasound. Laboratory results revealed a high serum C-reactive protein concentration (166 mg/L), a high white blood cell count (44.50 × 103/µL), a high polymorphonuclear cell count (20.47 × 103/µL), and a high thrombocyte count (646 × 103/µL). Histologic analysis of the inguinal adenopathy revealed multiple Ziehl-Neelsen stain–positive rods identified as Nocardia nova after microbiologic culture. The day after surgical biopsy, before the initiation of antibiotic treatment, the patient presented severe septicemia, with the isolation of K pneumoniae from 1 blood culture. However, Klebsiella was not isolated from a lymph node culture, and Nocardia was not isolated from blood samples. Intravenous treatment (imipenem and amikacin) against both pathogens was administered for 7 days followed by parenteral ceftriaxone and oral cotrimoxazole treatment for 2 weeks. The patient’s clinical status improved within 48 hours. Patient 2 was also treated for oral candidiasis during parenteral antibiotic treatment. She was treated with cotrimoxazole and clarithromycin for 1 year, and she received IFN-γ treatment for the first 6 weeks of treatment. She is now well at the age of 3 years and has no fever or biological signs of inflammation. The immune response of this patient was investigated, and complement-, B-, and T-cell responses were found to be normal. However, whole blood cells from the patient did not respond to IL-12 in terms of IFN-γ production, and T-blast cells did not express IL12Rβ1 on their surface (Fig 1, A and B). Investigation of her IL12RB1 gene revealed the presence of a homozygous splice mutation, 1791+2T→G. The patient’s parents are heterozygous for this mutation, and her sister is homozygous for the mutation (Fig 1C).
DISCUSSION
The 2 patients described here displayed an absence of expression of IL12Rβ1. They presented with disseminated BCG and salmonella disease (patient 2) or with intraabdominal N nova infection (patient 2), and both later developed Klebsiella sepsis. Patient 1 also developed Candida sepsis, probably favored by use of a central line. To our knowledge, patient 2 is the first IL12Rβ1-deficient patient with nocardiosis to be described. A patient with IL-12p40 deficiency and nocardiosis was previously described,4 which suggests that Nocardia, a bacterium that is phylogenetically and biochemically closely related to Mycobacterium, may cause clinical disease in patients with impairment of IL-12 and IL-23 immunity. C albicans may become pathogenic in immunocompromised patients, disseminating and even causing death in many such cases.7,8 In a large worldwide cohort of patients with IL12Rβ1 deficiency, up to 25% had mucocutaneous disease caused by C albicans (de Beaucoudrey et al, unpublished data, ●●●●), and the importance of this infection in such patients is being evaluated (Rodriguez-Gallego et al, unpublished data, ●●●●).
K pneumoniae is a Gram-negative bacterium that frequently causes severe, systemic, nosocomial infections in immunocompromised patients.9 Immunodeficiency, extended use of antibiotics, and neutropenia are risk factors for Klebsiella infection.9 Because the patients described here were not neutropenic, we believe that the absence of the IL12Rβ1 chain contributed to the establishment of Klebsiella infections. Therefore, the cases of the 2 patients described here and a third patient who presented also with IL12Rβ1 deficiency and multiple K pneumoniae infections (M. Levin, personal communication, ●●●●) suggest that this deficiency may be associated with a genuine susceptibility to klebsiellosis. It is interesting to note that klebsiellosis has never been reported in patients with either form of IFN-γR deficiency, who also have an incidence of salmonellosis only one-tenth that of IL-12p40- and IL12Rβ1-deficient patients,1,2 which suggests that the pathogenesis of salmonellosis and klebsiellosis involves an IL-12p40/IL12Rβ1-dependent but IFN-γ–independent mechanism.
Protective immunity to Klebsiella depends on the innate immune recognition of bacterial structures by receptors expressed on myeloid cells, and also on adaptive T-cell responses. The IL-12 and IL-23 produced by macrophages and dendritic cells induce, respectively, the production of IFN-γ and IL-17 cytokines (including IL-17A, IL-17F, and IL-22) by T cells.10 IL-23– driven IL-22 production was recently shown to be essential for the clearance of Salmonella11 and Klebsiella12 in the mouse model. Moreover, IL-17 plays an important role in eliminating Klebsiella infections in mice through the recruitment and activation of neutrophils and the production of antimicrobial peptides.13 It is interesting to note that in mouse models, IL-12 and IFN-γ also seem to be important for controlling Klebsiella infection in the lung, whereas the clearance of this infection from blood is apparently independent of IFN-γ.14,15
CONCLUSIONS
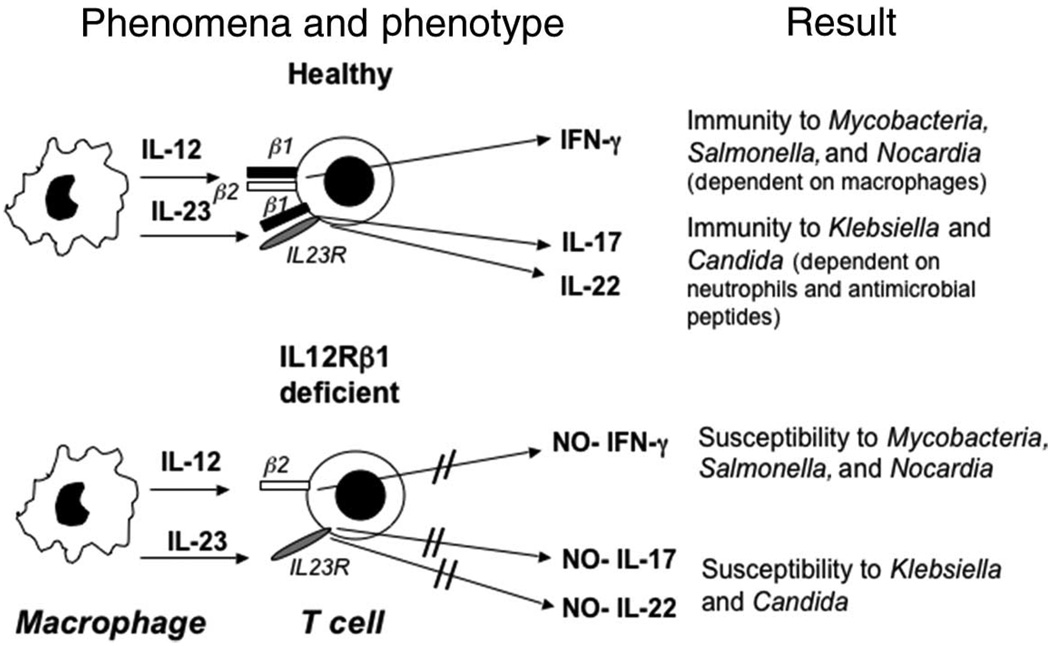
It was recently demonstrated that many patients with mutations in IL12B or IL12RB1 display only a small proportion of IL-17–producing circulating T cells.16 Thus, we hypothesize that both patients 1 and 2 presented with K pneumoniae infection probably attributable to a limited capacity to produce IL-17 cytokines (Fig 2). Impaired IL-23– dependent IL-17 immunity in IL-12p40- and IL12Rβ1-deficient patients may also explain why salmonellosis is 10 times more frequent in these patients than in IFN-γR-deficient patients, whose IL-23/IL-17 circuit is intact. It may also contribute to the vulnerability to chronic mucosal candidiasis.17 Genetic and immunologic investigations in patients with isolated klebsiellosis, salmonellosis, and chronic mucosal candidiasis will be required to test this hypothesis.18
FIGURE 2.
Simplified model of the immune response in a healthy individual and in a patient with a mutation in IL12RB1, lacking IL12Rβ1 expression. The hypothetical consequences of nonfunctional IL-12 receptor are depicted. This model is based on the clinical cases reported here and published articles on animal models of infection.
ACKNOWLEDGMENTS
Dr Pedraza and the Mexican group are supported by Consejo Nacional de Ciencia y Tecnologia (Mexico) grant 69992. The Laboratory of Human Genetics of Infectious Diseases is supported by Rockefeller University Center for Clinical and Translational Science grant 5UL1RR024143-03, other grants from the Rockefeller University, the March of Dimes, the Dana Foundation, the ANR, the Institut National de la Santé et de la Recherche Médicale, and the PHRC. Dr Casanova was an International Scholar of the Howard Hughes Medical Institute.
We thank the patients and their relatives for their voluntary participation in this study. We also thank Jenny Then, Pedro Valencia, Margarita Nava, and Christian Copin for medical care of the patients; Ludovic de Beaucoudrey, Jacqueline Feinberg, Yolanda Gonzalez, Martha Torres, and Miriam Bobadilla for helping with immunologic, genetic, and microbiologic studies for the patients.
ABBREVIATIONS
- IL
interleukin
- IFN
interferon
- BCG
bacille Calmette-Guérin
- IL12Rβ1
interleukin 12 receptor β1
Footnotes
Vaccines received: BCG (Monovax [Sanofi Aventis, Bridgewater, NJ]), pentavalent (Infanrix quinta [GlaxoSmithKline, King of Prussia, PA]), pneumococcus (Prevnar [Wyeth Lederle, Pearl River, NY]), and measles-mumps-rubella (Priorix, GlaxoSmithKline).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Filipe-Santos O, Bustamante J, Chapgier A, et al. Inborn errors of IL-12/23- and IFN-gamma-mediated immunity: molecular, cellular, and clinical features. Semin Immunol. 2006;18(6):347–361. doi: 10.1016/j.smim.2006.07.010. [published correction appears in Semin Immunol. 2007; 19(2):136–137] [DOI] [PubMed] [Google Scholar]
- 2.Dorman SE, Picard C, Lammas D, et al. Clinical features of dominant and recessive interferon gamma receptor 1 deficiencies. Lancet. 2004;364(9451):2113–2121. doi: 10.1016/S0140-6736(04)17552-1. [DOI] [PubMed] [Google Scholar]
- 3.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3(2):133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 4.Picard C, Fieschi C, Altare F, et al. Inherited interleukin-12 deficiency: IL12B genotype and clinical phenotype of 13 patients from six kindreds. Am J Hum Genet. 2002;70(2):336–348. doi: 10.1086/338625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fieschi C, Dupuis S, Catherinot E, et al. Low penetrance, broad resistance, and favorable outcome of interleukin 12 receptor beta 1 deficiency: medical and immunological implications. J Exp Med. 2003;197(4):527–535. doi: 10.1084/jem.20021769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feinberg J, Fieschi C, Doffinger R, et al. Bacillus Calmette Guérin triggers the IL-12/IFN-gamma axis by an IRAK-4- and NEMO-dependent, non-cognate interaction between monocytes, NK, and T lymphocytes. Eur J Immunol. 2004;34(11):3276–3284. doi: 10.1002/eji.200425221. [DOI] [PubMed] [Google Scholar]
- 7.Dimopoulos G, Karabinis A, Samonis G, Falagas ME. Candidemia in immunocompromised and immunocompetent critically ill patients: a prospective comparative study. Eur J Clin Microbiol Infect Dis. 2007;26(6):377–384. doi: 10.1007/s10096-007-0316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winkelstein JA, Marino MC, Johnston RB, et al. Chronic granulomatous disease: report on a national registry of 368 patients. Medicine (Baltimore) 2000;79(3):155–169. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11(4):589–603. doi: 10.1128/cmr.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang SC, Tan XY, Luxenberg DP, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203(10):2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulz SM, Köhler G, Schütze N, et al. Protective immunity to systemic infection with attenuated Salmonella enterica serovar enteritidis in the absence of IL-12 is associated with IL-23-dependent IL-22, but not IL-17. J Immunol. 2008;181(11):7891–7901. doi: 10.4049/jimmunol.181.11.7891. [DOI] [PubMed] [Google Scholar]
- 12.Aujla SJ, Chan YR, Zheng M, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14(3):275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuzaki G, Umemura M. Interleukin-17 as an effector molecule of innate and acquired immunity against infections. Microbiol Immunol. 2007;51(12):1139–1147. doi: 10.1111/j.1348-0421.2007.tb04008.x. [DOI] [PubMed] [Google Scholar]
- 14.Greenberger MJ, Kunkel SL, Strieter RM, et al. IL-12 gene therapy protects mice in lethal Klebsiella pneumonia. J Immunol. 1996;157(7):3006–3012. [PubMed] [Google Scholar]
- 15.Moore TA, Perry ML, Getsoian AG, Newstead MW, Standiford TJ. Divergent role of gamma interferon in a murine model of pulmonary versus systemic Klebsiella pneumoniae infection. Infect Immun. 2002;70(11):6310–6318. doi: 10.1128/IAI.70.11.6310-6318.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Beaucoudrey L, Puel A, Filipe-Santos O, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med. 2008;205(7):1543–1550. doi: 10.1084/jem.20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puel A, Doffinger R, Natividad A, et al. Auto-antibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;207(2):291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casanova JL, Abel L. Primary immunodeficiencies: a field in its infancy. Science. 2007;317(5838):617–619. doi: 10.1126/science.1142963. [DOI] [PubMed] [Google Scholar]
- 19.Altare F, Durandy A, Lammas D, et al. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280(5368):1432–1435. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- 20.Lee PP, Jiang LP, Wang XC, et al. Severe mycobacterial infections in two pairs of Chinese siblings with interleukin-12 receptor beta 1 deficiency. Eur J Pediatr. 2008;167(2):231–232. doi: 10.1007/s00431-007-0430-2. [DOI] [PubMed] [Google Scholar]
- 21.Elloumi-Zghal H, Barbouche MR, Chemli J, et al. Clinical and genetic heterogeneity of inherited autosomal recessive susceptibility to disseminated Mycobacterium bovis bacille Calmette-Guérin infection. J Infect Dis. 2002;185(10):1468–1475. doi: 10.1086/340510. [DOI] [PubMed] [Google Scholar]
- 22.Sakai T, Matsuoka M, Aoki M, Nosaka K, Mitsuya H. Missense mutation of the interleukin-12 receptor beta 1 chain-encoding gene is associated with impaired immunity against Mycobacterium avium complex infection. Blood. 2001;97(9):2688–2694. doi: 10.1182/blood.v97.9.2688. [DOI] [PubMed] [Google Scholar]
- 23.Haerynck F, Holland SM, Rosenzweig SD, Casanova JL, Schelstraete P, De Baets F. Disseminated Mycobacterium avium infection in a patient with a novel mutation in the interleukin-12 receptor-beta1 chain. J Pediatr. 2008;153(5):721–722. doi: 10.1016/j.jpeds.2008.05.050. [DOI] [PubMed] [Google Scholar]
- 24.Ehlayel M, de Beaucoudrey L, Fike F, et al. Simultaneous presentation of 2 rare hereditary immunodeficiencies: IL-12 receptor beta 1 deficiency and ataxia-telangiectasia. J Allergy Clin Immunol. 2008;122(6):1217–1219. doi: 10.1016/j.jaci.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Fieschi C, Bosticardo M, de Beaucoudrey L, et al. A novel form of complete IL-12/IL-23 receptor beta 1 deficiency with cell surface-expressed nonfunctional receptors. Blood. 2004;104(7):2095–2101. doi: 10.1182/blood-2004-02-0584. [DOI] [PubMed] [Google Scholar]
- 26.Carvalho BT, Iazzetti AV, Ferrarini MA, Campos SO, Iazzetti MA, Carlesse FA. Salmonella septicemia associated with interleukin 12 receptor beta 1 (IL-12Rbeta 1) deficiency [in Portuguese] J Pediatr (Rio J) 2003;79(3):273–276. [PubMed] [Google Scholar]
- 27.Sanal O, Turul T, De Boer T, et al. Presentation of interleukin-12/-23 receptor beta 1 deficiency with various clinical symptoms of salmonella infections. J Clin Immunol. 2006;26(1):1–6. doi: 10.1007/s10875-006-7830-3. [DOI] [PubMed] [Google Scholar]
- 28.Caragol I, Raspall M, Fieschi C, et al. Clinical tuberculosis in 2 of 3 siblings with interleukin-12 receptor beta 1 deficiency. Clin Infect Dis. 2003;37(2):302–306. doi: 10.1086/375587. [DOI] [PubMed] [Google Scholar]
- 29.Altare F, Ensser A, Breiman A, et al. Interleukin-12 receptor beta 1 deficiency in a patient with abdominal tuberculosis. J Infect Dis. 2001;184(2):231–236. doi: 10.1086/321999. [DOI] [PubMed] [Google Scholar]
- 30.Tanir G, Dogu F, Tuygun N, et al. Complete deficiency of the IL-12 receptor beta1 chain: three unrelated Turkish children with unusual clinical features. Eur J Pediatr. 2006;165(6):415–417. doi: 10.1007/s00431-005-0078-8. [DOI] [PubMed] [Google Scholar]
- 31.de Jong R, Altare F, Haagen IA, et al. Severe mycobacterial and salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998;280(5368):1435–1438. doi: 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- 32.Ozbek N, Fieschi C, Yilmaz BT, et al. Interleukin-12 receptor beta 1 chain deficiency in a child with disseminated tuberculosis. Clin Infect Dis. 2005;40(6):e55–e58. doi: 10.1086/427879. [DOI] [PubMed] [Google Scholar]
- 33.Scheuerman O, de Beaucoudrey L, Hoffer V, Feinberg J, Casanova JL, Garty BZ. Mycobacterial disease in a child with surface-expressed non-functional interleukin-12Rbeta1 chains. Isr Med Assoc J. 2007;9(7):560–561. [PubMed] [Google Scholar]
- 34.Moraes-Vasconcelos D, Grumach AS, Yamaguti A, et al. Paracoccidioides brasiliensis disseminated disease in a patient with inherited deficiency in the beta1 subunit of the interleukin (IL)-12/IL-23 receptor. Clin Infect Dis. 2005;41(4):e31–e37. doi: 10.1086/432119. [DOI] [PubMed] [Google Scholar]
- 35.Sanal O, Turkkani G, Gumruk F, et al. A case of interleukin-12 receptor beta-1 deficiency with recurrent leishmaniasis. Pediatr Infect Dis J. 2007;26(4):366–368. doi: 10.1097/01.inf.0000258696.64507.0f. [DOI] [PubMed] [Google Scholar]