Abstract
Objective
To examine the association between physical activity measured during leisure, sport and work and retinal microvascular signs.
Methods
Participants of the Atherosclerosis Risk in Communities (ARIC) Study, a population-based cross-sectional study, had retinal photographs taken at their third follow up visit (1993–1995). Retinal microvascular signs were assessed using a standardized protocol and retinal vascular caliber by a computer-assisted method. Leisure, sport and work-related physical activity levels were determined through a modified Baecke physical activity questionnaire.
Results
A higher level of physical activity during sport and work was significantly associated with a lower prevalence of arteriovenous (AV) nicking, wider venular caliber and retinopathy. In multivariate models, persons with a level of sport-related physical activity above the median were less likely to have AV nicking (odds ratio [OR] = 0.87; 95% confidence interval [CI] 0.78–0.97) and wider retinal venules (OR= 0.91; 95% CI 0.83–0.99). Persons with a level of work-related physical activity above the median were less likely to have diabetic retinopathy (OR= 0.66, 95% CI 0.51–0.85).
Conclusions
In this cross-sectional analyses, higher levels of physical activity was associated with a lower prevalence of retinal microvascular abnormalities.
Keywords: Physical activity, retinal vascular caliber, retinopathy, arteriovenous nicking
INTRODUCTION
The human eye is a unique site where the microcirculation can be visualized and quantified reliably in a non-invasive fashion. Several population based studies have shown that microvascular changes in the retina, including retinopathy, narrowing of the retinal arterioles and widening of the retinal venules may be useful as risk indicators of both cardiovascular and cerebrovascular disease [37]. Retinopathy, which includes microaneurysms, hemorrhages and exudates, has been shown to be predictive of incident stroke and coronary heart disease as well as 10-year cardiovascular mortality [33, 35, 36]. Abnormalities related to retinal arterioles such as arteriovenous (AV) nicking and generalized arteriolar narrowing are strongly associated with hypertension [37], whilst wider retinal venules are associated with diabetes, obesity, stroke, smoking and systemic inflammation [23, 31]. However, there is limited data on whether physical activity is associated with the retinal microcirculation. Lack of physical activity has long been established as an independent modifiable risk factor for hypertension, diabetes and the metabolic syndrome, as well as clinical cardiovascular events such as coronary heart disease and ischemic stroke [3, 8–10, 12].
In this current study, we examined the association between retinal microvascular abnormalities and physical activity using data from the ARIC study cohort.
MATERIAL AND METHODS
Study Population
The ARIC study is a population-based study that included 15,792 women and men 45 to 64 years of age at recruitment in 1987–89 [18]. Population samples were selected from four clinical centers that include: Washington County, Maryland; northwest suburbs of Minneapolis, Minnesota; Jackson, Mississippi (all African-American); and Forsyth County, North Carolina. Of those examined at baseline at all sites, 93% returned for a second examination in 1990–92 and 86% for the third examination in 1993–95.
Retinal photography [13] and physical activity levels were determined at the third examination. This analysis includes 12,363 participants who had gradable retinal photographs and data regarding physical activity indexes; however total numbers varied slightly for each retinal sign, which was graded independently of the others.
Comparison of persons with and without gradable retinal photographs has been previously described [13]. Persons with ungradable retinal photographs were older, and after controlling for age, were more likely to be female, to have diabetes and hypertension, and to be current cigarette smokers. The tenets of the Declaration of Helsinki were observed, and institutional review boards at each study site approved the study. Written, informed consent was obtained from all participants.
Retinal Photography and Measurement of Retinal Microvascular Signs
The retinal photography procedure was conducted at the third examination [13]. Briefly, following 5 minutes of dark adaptation, a 45-degree retinal film photograph was taken of one randomly selected eye, centered on the optic disc and macula. Trained graders who were masked to participant identity evaluated the retinal photographs for the presence or absence of retinal microvascular abnormalities according to a standardized protocol [30].
Four categories of retinal microvascular signs were defined in this study: retinopathy, AV nicking, focal retinal arteriolar narrowing and retinal vascular caliber. Retinopathy (Fig. 1) was defined as present if any of the following lesions were graded as definite or probable in any of the four quadrants: microaneurysms, blot hemorrhages, flame-shaped hemorrhages, soft exudates, hard exudates, macular edema and intraretinal microvascular abnormalities [4]. Retinopathy was further categorized according to diabetes status into diabetic retinopathy (in persons with diabetes) and nondiabetic retinopathy (in persons without diabetes). Abnormalities related to retinal arteriolar changes such as AV nicking and focal arteriolar narrowing (Fig. 2) were defined as present if graded as definite or probable.
Fig. 1.
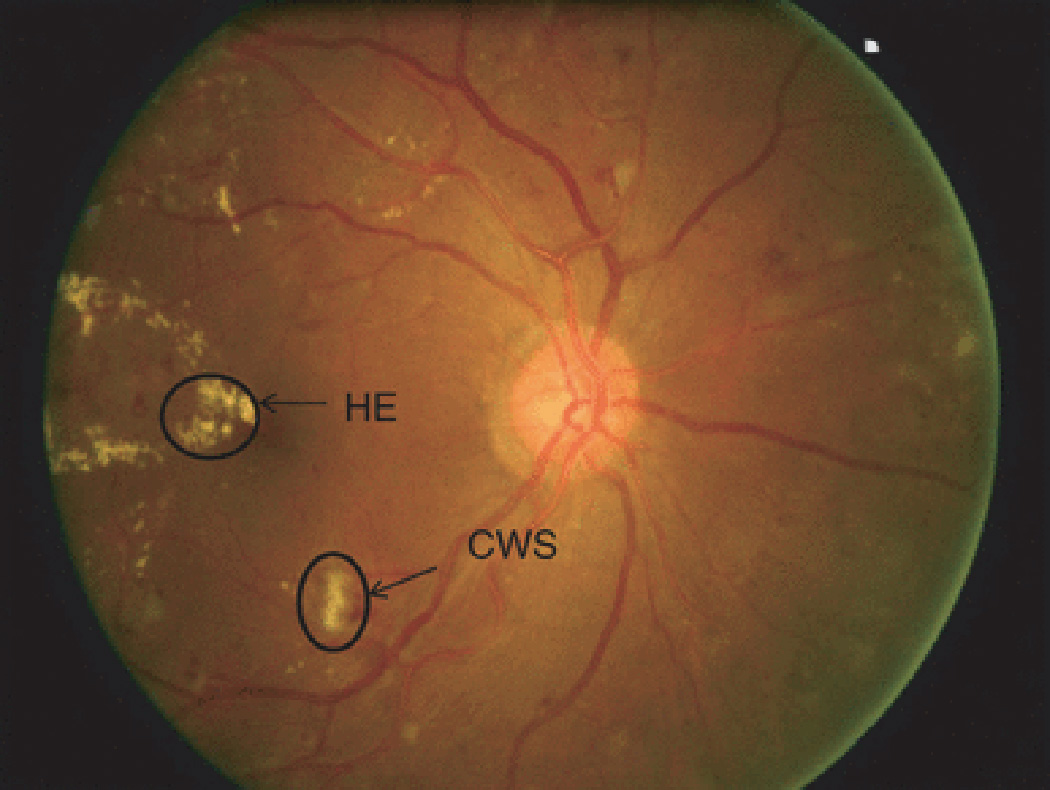
Fundus photograph showing signs of diabetic retinopathy. RH-retinal hemorrhage; HE-hard exudates; MA- microaneurysm; CWS-cotton wool spot
Fig. 2.
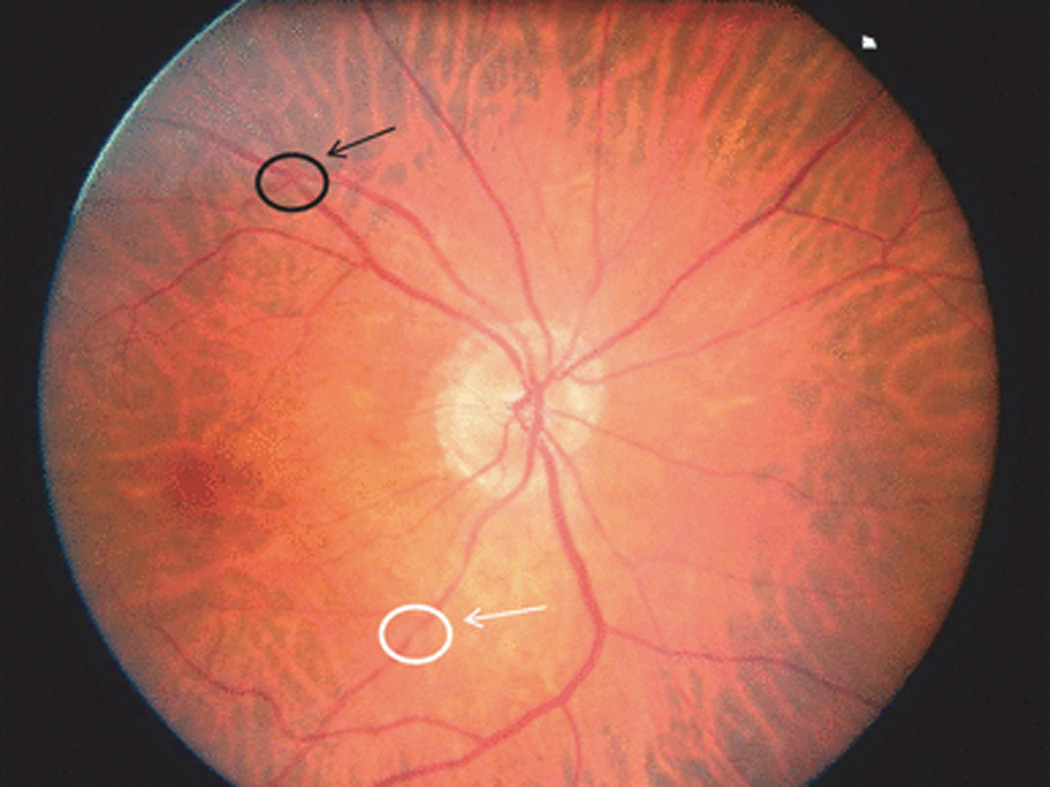
Fundus photograph showing focal arteriolar narrowing (white arrow) and arteriovenous nicking (black arrow).
Retinal arteriolar and venular calibers were measured from digitalized retinal images based on a previously described protocol. Measurements were based on retinal vessels located 1/2 to 1 disc diameter from the optic disc (Fig. 3) using computer designed software that summarizes vessel diameters as central retinal arteriolar equivalent (CRAE) and venular equivalent (CRVE), which represented the average arteriolar and venular diameter, respectively [30]. Narrower retinal arterioles were defined as the first quartile (smaller 25%) of the population distribution of CRAE, compared to the remaining three as previously described in other analyses [27]. Wider venules were defined as the fourth quartile (largest 25%) of the population distribution of CRVE compared to quartiles 1–3.
Fig. 3.
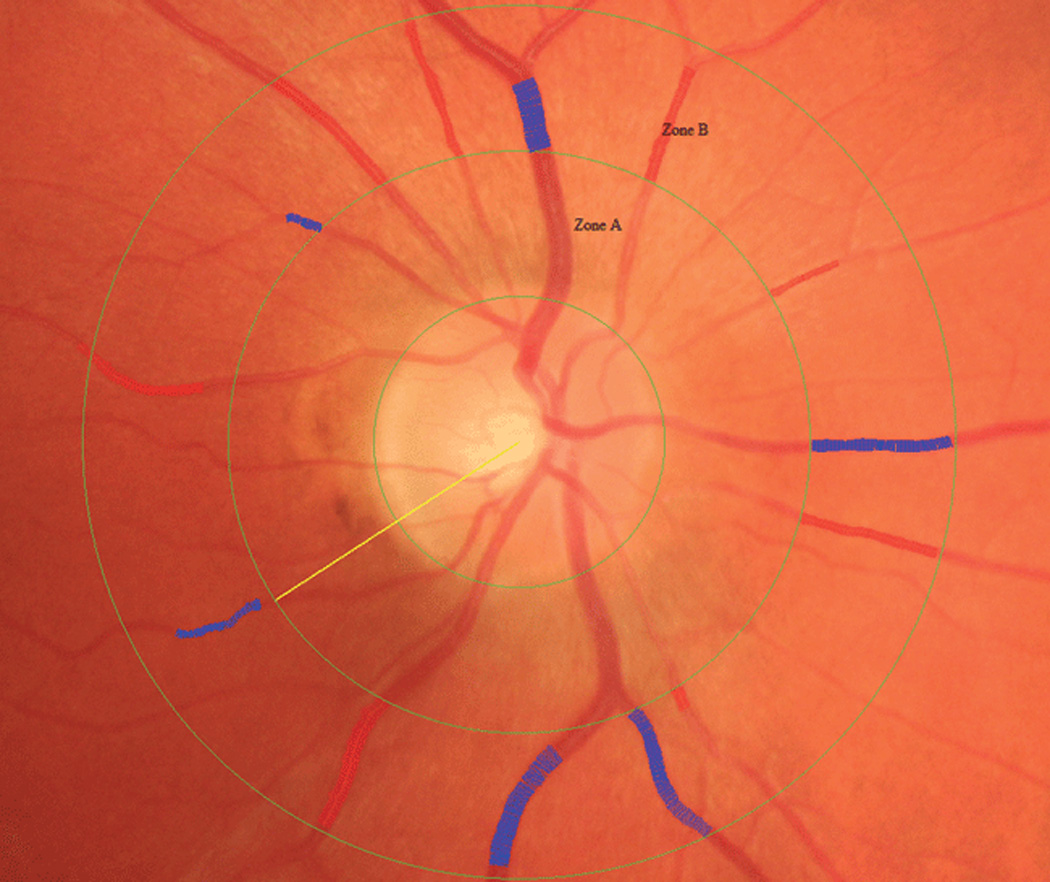
Fundus photograph showing retinal vascular caliber measurement in Zone B which is 0.5 to 1.0 disc diameter from the optic disc margin. An intensity histogram of the central width measurement is displayed alongside the image.
Reliability of the grading has been previously reported [4]. Unweighted intra- and intergrader kappa statistics ranged from 0.76 to 0.89 for retinopathy signs, 0.39 to 0.65 for focal arteriolar narrowing and AV nicking whilst intra- and inter grader reliability of coefficients ranged from 0.68 to 0.88 for retinal vessel diameters [4].
Assessment of Physical Activity
Physical activity was assessed through an interviewer administered modified Baecke Physical Activity questionnaire during the third examination [2]. The questionnaire assessed the amount of physical activity performed during leisure, sport and work times and yielded scores from 1(low) to 5 (high). A few minor modifications of the original version were made and detailed elsewhere [10]. The validity and reliability of the questionnaire has been evaluated in several other populations [26].
The leisure index consisted of four questions on walking, bicycling, television viewing and time spent performing these activities commuting to and from work or shopping. Each component of the index contributed equally to the indices. The sport index was derived from three questions regarding the frequency of overall sport and exercise participation, frequency of sweating and a subjective comparison of physical activity compared to other of a similar age. A fourth component on frequency, intensity and duration of up to four activities also contributed to the sport index. The work index was calculated from eight equally weighted items relating to how often while at work each individual sits, stands, walks, lifts heavy loads, sweats, leaves work physically tired. Individuals were also asked to compare their work related activity to others of a similar age. The last component of the work index involved a ranking (light, moderate or vigorous) of activity required for the job based on the occupational job title. Light occupations included accountants, administrators, architects, computer scientists and physicians. Moderate occupations included occupations such as cooks, firefighters, police and registered nurses. Vigorous occupations included working as farmers, heavy equipment mechanics, janitors, waiters/waitresses and carpet installers. The work index used in this analysis also included individuals who worked within the home (e.g. housekeepers).
Measurement of Covariates
Participants underwent a standardized interview, clinical examination and laboratory investigations as part of the ARIC study [17]. At each visit, blood pressures were taken with a random-zero sphygmomanometer and the mean of the last two measurements were used for analyses. Hypertension was defined as systolic blood pressure (SBP) ≥140 mmHg, diastolic blood pressure (DBP) ≥90 mmHg, or use of anti-hypertensive medication during the previous 2 weeks. Mean arterial blood pressure (MABP) was computed as 2/3 of the diastolic plus 1/3 of the systolic value. Blood collection and processing followed a standard protocol.[17] Diabetes mellitus was defined as a fasting glucose level of at least 126mg/dL, a non-fasting glucose level of at least 200mg/dL, or a self-reported history of physician-diagnosed diabetes or treatment for diabetes. Total plasma cholesterol was measured by enzymatic methods, HDL cholesterol was measured after dextran-magnesium precipitation of the non-HDL lipoproteins, and glucose was assessed by a modified hexokinase/glucose-6-phosphate dehydrogenase procedure [17]. Technicians measured height and weight with participants in scrub suits, and body mass index (BMI) was calculated as weight / height2 (kg/m2). Cigarette smoking and alcohol consumption were ascertained from interview. All variables were based on data from the third examination.
Statistical Analysis
The associations between participant characteristics and retinal microvascular signs were examined using logistic regression models to examine proportions and linear regression models for means, adjusted for age and gender. AV nicking, focal arteriolar narrowing and retinopathy were analyzed as categorical outcomes (present, absent). CRAE and CRVE were analyzed as continuous variables or recoded into quartiles. In multivariable regression models examining the association between leisure, sport and work indexes and retinal microvascular signs, we analyzed each index using the lowest index as the reference group as well as categorizing each index based on the median value (lower than the median and greater or equal to the median). Multivariable models were adjusted for age, gender, race, post secondary education, BMI, history of being a current drinker, current smoker, serum HDL cholesterol and MABP. All analyses were performed using Intercooled Stata 9.0 (StataCorp, College Station, TX).
RESULTS
Comparison of quartiles of physical activity measures by selected participant characteristics are shown in Tables 1a and 1b. In summary, more active participants, that is, participants within the higher quartiles of physical activity measures (leisure, work and sport-related respectively) were less likely to have hypertension, and diabetes but were more likely to have completed post secondary education, have higher levels of serum HDL cholesterol and a history of being alcohol drinkers compared to those less active. Participants with higher levels of sport-related physical activity were less likely to have AV nicking and more likely to have narrower venules (smaller CRVE).
Table 1.
| a: Participant characteristics by quartiles of leisure and work-related physical activity measures. | ||||||||
|---|---|---|---|---|---|---|---|---|
| LEISURE INDEX | WORK INDEX | |||||||
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | |
| (1.25–2.00) | (2.10–2.25) | (2.26–2.75) | (2.76–5.00) | (0.88–1.00) | (1.01–1.75) | (1.76–2.63) | (2.64–5.00) | |
| n = 4537 | n = 2157 | n = 3607 | n = 2191 | n = 5492 | n = 926 | n = 3019 | n = 3025 | |
| Characteristic | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
| Male | 1962 (43) | 958 (44) | 1598 (44) | 957 (44) | 3370 (61) | 471 (51) | 1454 (48) | 1501 (50)** |
| History of hypertension | 2033 (45) | 835 (39) | 1350 (37) | 733 (33)** | 2493 (45) | 363 (39) | 1000 (33) | 1090 (36)** |
| Prevalent coronary heart disease | 189 (4) | 82 (4) | 139 (4) | 86 (4) | 293 (5) | 40 (4) | 79 (3) | 76 (3)** |
| Smoking, current | 971 (21) | 369 (17) | 526 (15) | 281 (13)** | 922 (17) | 137 (15) | 440 (15) | 638 (21) |
| Education, post secondary | 3264 (72) | 1778 (82) | 3002 (83) | 1833 (84)** | 4086 (74) | 717 (77) | 2720 (90) | 2200 (73)** |
| History of Alcohol, current drinker | 2150 (47) | 1156 (54) | 1972 (55) | 1208 (55)** | 2586 (47) | 474 (51) | 1831 (61) | 1466 (49)* |
| Diabetes | 806 (18) | 311 (14) | 481 (13) | 234 (11)** | 941 (17) | 145 (16) | 401 (13) | 373 (12)** |
| African American | 1559 (34) | 336 (16) | 507 (14) | 274 (13)** | 1059 (19) | 205 (22) | 634 (21) | 818 (27)** |
| Non-diabetic retinopathy | 199 (4) | 67 (3) | 146 (4) | 73 (3)* | 212 (4) | 36 (4) | 105 (3) | 133 (4) |
| Diabetic retinopathy | 207 (5) | 61 (3) | 86 (2) | 49 (2) | 238 (4) | 28 (3) | 63 (2) | 76 (3)* |
| Arterio-venous nicking | 603 (13) | 261 (12) | 445 (12) | 291 (13) | 712 (13) | 101 (11) | 377 (13) | 377 (12) |
| Focal arteriolar narrowing | 597 (13) | 294 (14) | 457 (13) | 310 (14) | 768 (14) | 112 (12) | 374 (12) | 372 (12) |
| mean ± SD | mean ± SD | mean ± SD | mean ± SD | mean ± SD | mean ± SD | mean ± SD | mean ± SD | |
| Age, years | 59.97±5.68 | 59.98±5.81 | 60.05±5.68 | 59.91±5.71 | 62.72±5.26 | 59.94±5.63 | 57.46±4.94 | 57.68±5.94** |
| SBP, mmHg | 126.14±19.81 | 123.89±18.68 | 123.43±18.02 | 122.53±18.13** | 126.52±19.74 | 123.81±19.23 | 121.50±17.36 | 123.84±18.59** |
| DBP, mmHg | 72.40±10.74 | 71.37±10.34 | 71.30±10.25 | 71.10±9.93** | 70.53±10.18 | 71.82±10.32 | 72.78±10.34 | 72.64±10.48 |
| BMI, m/kg2 | 29.31±5.98 | 28.50±5.52 | 27.08±5.16 | 27.43±4.85** | 28.39±5.64 | 28.44±5.63 | 28.47±5.42 | 28.65±5.52 |
| Cholesterol, mmol/L | 5.38±0.99 | 5.35±0.96 | 5.36±0.97 | 5.38±0.96 | 5.46±1 | 5.31±0.94 | 5.29±0.94 | 5.3±0.96** |
| HDL, mg/dL | 1.33±0.46 | 1.33±0.46 | 1.34±0.46 | 1.41±0.5** | 1.36±0.48 | 1.34±0.46 | 1.35±0.48 | 1.34±0.45* |
| LDL, mg/dL | 3.31±0.9 | 3.29±0.89 | 3.29±0.89 | 3.25±0.89* | 3.33±0.91 | 3.26±0.87 | 3.24±0.88 | 3.27±0.89* |
| Trigylcerides, mg/dL | 1.61±1 | 1.61±0.97 | 1.62±0.99 | 1.6±1.22 | 1.69±1.06 | 1.56±0.88 | 1.57±1.06 | 1.52±0.98 |
| CRAE, µm | 163.02±16.64 | 162.30±16.85 | 161.56±16.70 | 162.46±16.58* | 161.94±16.72 | 161.44±17.39 | 161.76±16.58 | 163.67±16.62** |
| CRVE, µm | 195.04±17.10 | 192.73±16.50 | 191.86±16.34 | 191.32±16.33** | 192.10±17.05 | 193.34±16.67 | 192.82±16.23 | 194.69±16.67** |
| b: Participant characteristics by quartiles of sport-related physical activity measure. | ||||
|---|---|---|---|---|
| SPORT INDEX | ||||
| Q1 | Q2 | Q3 | Q4 | |
| (1.00–2.00) | (2.01–2.50) | (2.51–3.00) | (3.01–5.00) | |
| n = 4563 | n = 2472 | n = 4661 | n = 753 | |
| Characteristic | n (%) | n (%) | n (%) | n (%) |
| Male | 1702 (37) | 1024 (41) | 2261 (49) | 479(64)** |
| History of hypertension | 1986 (44) | 1004 (41) | 1708 (37) | 239 (32)** |
| Prevalent coronary heart disease | 183 (4) | 88 (4) | 199 (5) | 24 (3)* |
| Smoking, current | 953 (21) | 441 (18) | 663 (14) | 79 (10)** |
| Education, post secondary | 3335 (73) | 1932 (78) | 3899 (84) | 680 (90)** |
| Alcohol, current drinker | 2053 (45) | 1230 (50) | 2659 (57) | 518 (69)** |
| Diabetes | 791 (17) | 378 (15) | 584 (13) | 67 (9)** |
| African American | 1262 (28) | 540 (22) | 781 (17) | 84 (11)** |
| Non-diabetic retinopathy | 186 (4) | 116 (5) | 157 (3) | 21 (2)* |
| Diabetic retinopathy | 187 (4) | 84 (3) | 114 (2) | 14 (2) |
| Arterio-venous nicking | 613 (13) | 349 (14) | 541 (14) | 91 (12)** |
| Focal arteriolar narrowing | 588 (13) | 350 (14) | 612 (13) | 103 (14) |
| mean ± SD | mean ± SD | mean ± SD | mean ± SD | |
| Age, years | 59.41±5.60 | 60.17±5.66 | 60.43±5.75 | 59.91±5.72** |
| SBP, mmHg | 125.48±19.50 | 124.16±18.48 | 123.79±18.53 | 121.45±17.82** |
| DBP, mmHg | 72.06±10.72 | 71.41±10.49 | 71.50±10.21 | 71.64±9.92* |
| BMI, m/kg2 | 29.51±6.24 | 28.51±5.36 | 27.74±4.87 | 26.95±4.15** |
| Cholesterol, mg/dL | 5.38±0.99 | 5.38±0.99 | 5.36±0.95 | 5.3±0.94 |
| HDL, mg/dL | 1.34±0.46 | 1.35±0.46 | 1.35±0.48 | 1.39±0.48** |
| LDL, mg/dL | 3.3±0.9 | 3.3±0.9 | 3.29±0.87 | 3.24±0.85 |
| Trigylcerides, mg/dL | 1.63±1 | 1.63±0.96 | 1.6±1.09 | 1.49±1.07* |
| CRAE, µm | 162.59±16.74 | 162.18±16.78 | 161.99±16.69 | 162.75±16.38 |
| CRVE, µm | 194.51±17.20 | 193.31±15.57 | 191.76±16.30 | 191.61±16.07** |
p<0.05,
p<0.001 from test for heterogeneity across quartiles adjusted for age and gender except age and gender respectively.
SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure; BMI, Body Mass Index; HDL, High Density Lipoprotein; LDL, Low density Lipoprotein; CRAE, Central retinal Arteriolar Equivalent; CRVE, Central Retinal Venular Equivalent.
Leisure Index Level
After controlling for age, gender, race, post secondary education, BMI, MABP, diabetes and being a current drinker and smoker, a higher level of leisure-related physical activity was not associated with the presence of any retinal microvascular signs compared to the lowest level (Table 2). A higher level of physical activity was associated with a narrower CRVE as indicated by a negative beta-coefficient, however the association was not statistically significant (Table 3).
Table 2.
Association between leisure, sport and work indexes and retinal microvascular signs.
| Diabetic | Non-diabetic | Arterio-venous | Focal arteriolar | CRAE, 1st | CRVE, 4th | |||
|---|---|---|---|---|---|---|---|---|
| Retinopathy | Retinopathy | nicking | narrowing | quartile range | quartile range | |||
| n (%) | OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | ||
| LEISURE Index Level (1–5) | N= 12 492 | |||||||
| 1.0–2.0 | 4537 (36) | Ref | Ref | Ref | Ref | Ref | Ref | |
| 2.1–3.0 | 6877 (55) | 0.80 (0.62–1.02) | 0.92 (0.76–1.13) | 0.99 (0.88–1.12) | 0.98 (0.87–1.11) | 1.03 (0.94–1.14) | 1.02 (0.93–1.23) | |
| >3.0 | 1078 (9) | 1.11(0.69–1.82) | 0.86 (0.60–1.25) | 1.09 (0.89–1.34) | 0.98 (0.79–1.21) | 0.93 (0.78–1.10) | 0.98 (0.82–1.17) | |
| Less active (< median of index =2.25) | 1275 (10) | Ref | Ref | Ref | Ref | Ref | Ref | |
| More active (≥ median of index) | 1111 217 (90) | 0.88 (0.65–1.19) | 0.98 (073–1.33) | 1.09 (0.91–1.32) | 1.02 (0.84–1.24) | 0.89 (0.76–1.04) | 0.97 (0.84–1.-12) | |
| SPORT Index Level (1–5) | N = 12 449 | |||||||
| 1.0–2.0 | 4563 (37) | Ref | Ref | Ref | Ref | Ref | Ref | |
| 2.1–3.0 | 4978 (40) | 0.89 (0.69–1.15) | 0.96 (0.78–1.19) | 0.94 (0.83–1.07) | 1.06 (0.93–1.20) | 1.02 (0.92–1.13) | 0.94 (0.85–1.04) | |
| 3.1–4.0 | 2479 (20) | 0.92 (0.66–1.30) | 0.89 (0.68–1.16) | 0.93 (0.80–1.09) | 0.99 (0.85–1.16) | 0.96 (0.85–1.10) | 0.86 (0.75–0.98)* | |
| 4.1–5.0 | 429 (3) | 1.01 (0.40–2.53) | 0.68 (0.37–1.24) | 0.93 (0.68–1.28 | 1.15 (0.84–1.56) | 0.81 (0.62–1.04) | 1.12 (0.87–1.44) | |
| Less active (<median of index =2.50) | 5791 (47) | Ref | Ref | Ref | Ref | Ref | Ref | |
| More active (≥ median of index) | 6658 (53) | 0.88 (0.69–1.11) | 0.88 (0.72–1.05) | 0.87 (0.78–0.97)* | 0.98 (0.88–1.10) | 0.92 (0.84–1.01) | 0.91 (0.83–0.99)* | |
| WORK Index Level (1–5) | N =12 462 | |||||||
| 1.0–2.0 | 7105 (57) | Ref | Ref | Ref | Ref | Ref | Ref | |
| 2.1–3.0 | 3515 (28) | 0.69 (0.51–0.93)* | 1.01 (0.81–1.27) | 1.00 (0.87–1.13) | 1.14 (0.99–1.30) | 1.10 (0.98–1.22) | 0.88 (0.79–0.98)* | |
| >3.0 | 1842 (15) | 0.83 (0.56–1.18) | 1.06 (0.81–1.39) | 1.11 (0.94–1.30) | 1.09 (0.92–1.30) | 0.92 (0.80–1.06) | 1.09 (0.96–1.24) | |
| Less active (<median of index=1.75) | 6177 (50) | Ref | Ref | Ref | Ref | Ref | Ref | |
| More active (≥ 1.75) | 6285 (50) | 0.66 (0.51–0.85)* | 1.03 (0.84–1.27) | 1.10 (0.97–1.25) | 1.11 (0.98–1.20) | 1.08 (0.98–1.20) | 0.94 (0.85–1.04) | |
OR (odds ratio) and 95%CI adjusted for age, gender, race, post secondary education, BMI, diabetes, current drinker, current smoker, HDL cholesterol, MABP.
p<0.05
Table 3.
Regression analyses showing adjusted* mean difference in µm (95 % CI) for CRAE and CRVE for leisure, sport and work indexes.
| CRAE µm | CRVE µm | ||
|---|---|---|---|
| LEISURE Index Level (1–5) | |||
| 1.0–2.0 | Ref | Ref | |
| 2.1–3.0 | −0.30 (−0.96 to 0.35) | −0.45 (−1.11 to 0.21) | |
| >3.0 | 0.32 (−0.81 to 1.45) | −0.90 (−2.05 to 0.25) | |
| SPORT Index Level (1–5) | |||
| 1.0–2.0 | Ref | Ref | |
| 2.1–3.0 | 0.64 (−0.04 to 1.33) | −0.35 (−1.05 to 0.34) | |
| 3.1–4.0 | 0.39 (−0.45 to1.23) | −0.75 (−1.66 to 0.11) | |
| 4.1–5.0 | 1.59 (−0.07 to 3.25) | −0.44 (−2.13 to 1.24) | |
| WORK Index Level (1–5) | |||
| 1.0–2.0 | Ref | Ref | |
| 2.1–3.0 | −1.03 (−1.76 to −0.30)** | −1.00 (−1.74 to −0.26)** | |
| >3.0 | 0.76 (0.15 to −1.66) | 0.36 (−0.56 to1.27) | |
Adjusted for age, gender, race, post secondary education, BMI, diabetes, current drinker, current smoker, HDL, cholesterol and MABP.
p<0.05
Sport Index Level
Compared to the lowest level of sport–related physical activity, participants in the 2.1–3.0 level were less likely to have wider venules (OR=0.86; 95%CI 0.75–0.98) (Table 2). When comparing participants with sport-related physical activity levels above and below the median, physical activity levels above the median was significantly associated with a lower prevalence of AV nicking (OR=0.87; 95%CI 0.78–0.97) and wider CRVE (OR= 0.91; 95%CI 0.83–0.99) (Table 2).
Work Index Level
In the multivariable models, persons with a level of work-related physical activity above the median were less likely to have diabetic retinopathy (OR= 0.69, 95%CI 0.51–0.93) compared to those below the median (Table 2). In addition, an intermediate level of physical activity during work was significantly associated with a wider arteriolar (β coefficient = −1.03 for CRAE) and venular caliber (β coefficient = −1.00 for CRVE) compared to participants in the lowest level of work-related physical activity (Table 3).
Table 4 shows the association between median work index and retinal microvascular signs stratified by gender, hypertension status, diabetic retinopathy, race and BMI status. Higher levels of work-related physical activity were associated with a lower prevalence of diabetic retinopathy in males, those with hypertension, in African-Americans and those who were obese. Wider CRVE was less likely to occur in whites with higher work index whilst focal arteriolar narrowing was more likely to occur in participants with higher levels of work-related physical activity who were male, white and overweight.
Table 4.
Odds ratio of retinal microvascular signs by work index median stratified by gender, hypertension, diabetes, race and BMI status.
| Multivariable-adjusted OR (95%CI) for retinal microvascular signs |
||||||||
|---|---|---|---|---|---|---|---|---|
| n (%) | ||||||||
| N | Work Index ≥ median |
Diabetic Retinopathy |
Non-Diabetic Retinopathy |
Arterio-venous Nicking |
Focal arteriolar narrowing |
CRAE, 1st quartile range |
CRVE, 4th quartile range |
|
| Work Index | Work Index | Work Index | Work Index | Work Index | Work Index | |||
| ≥ median | ≥ median | ≥ median | ≥ median | ≥ median | ≥ median | |||
| Gender | ||||||||
| Female | 7006 | 3069 (44) | 0.72 (0.50–1.02) | 1.10 (0.83–1.45) | 1.01 (0.85–1.19) | 1.04 (0.88–1.23) | 1.09 (0.94–1.24) | 0.92 (0.80–1.05) |
| Male | 5590 | 3076 (55) | 0.66 (0.44–0.99)* | 0.97 (0.71–1.32) | 1.19 (0.99–1.43) | 1.21 (1.00–1.47)* | 1.07 (0.93–1.23) | 0.95 (0.81–1.10) |
| Hypertension status | ||||||||
| Normotensive | 7399 | 3948 (53) | 0.76 (0.47–1.22) | 1.04 (0.78–1.38) | 1.06 (0.89–1.26) | 1.17 (0.98–1.39) | 1.04 (0.91–1.20) | 0.88 (0.77–1.00) |
| Hypertensive | 5131 | 2162 (42) | 0.67 (0.48–0.92)** | 1.07 (0.79–1.45) | 1.13 (0.95–1.35) | 1.07 (0.90–1.27) | 1.14 (0.98–1.32) | 1.02 (0.87–1.19) |
| Diabetic status | ||||||||
| Non-diabetic | 10610 | 5316 (50) | n/a | 1.05 (0.85–1.30) | 1.04 (0.91–1.19) | 1.10 (0.97–1.26) | 1.06 (0.95–1.18) | 0.94 (0.84–1.05) |
| Diabetic | 1915 | 797 (42) | 0.69 (0.53–0.89)** | n/a | 1.34 (0.99–1.81) | 1.15 (0.83–1.60) | 1.27 (0.95–1.67) | 0.89 (0.70–1.14) |
| Race | ||||||||
| African-American | 2837 | 1481 (52) | 0.70 (0.49–0.99)* | 1.01 (0.69–1.47) | 1.09 (0.85–1.39) | 0.94 (0.71–1.24) | 1.16 (0.91–1.48) | 1.06 (0.88–1.28) |
| White | 9759 | 4664 (48) | 0.68 (0.46–1.01) | 1.07 (0.83–1.37) | 1.09 (0.95–1.26) | 1.17 (1.02–1.24)* | 1.07 (0.96–1.20) | 0.88 (0.78–0.99)* |
| BMI status | ||||||||
| Normal weight | 3467 | 1663 (48) | 0.60 (0.26–1.39) | 0.91 (0.61–1.36) | 0.91 (0.71–1.17) | 0.95 90.75–1.20) | 1.09 (0.89–1.33) | 0.92 (0.76–1.12) |
| Overweight | 4968 | 2456 (49) | 0.85 (0.52–1.40) | 1.07 (0.77–1.49) | 1.12 (0.92–1.36) | 1.26 (1.03–1.55)* | 1.07 (0.91–1.26) | 0.92 (0.78–1.08) |
| Obese | 4147 | 2024 (49) | 0.64 (0.46–0.91)* | 1.14 (0.78–1.65) | 1.19 (0.97–1.45) | 1.09 (0.89–1.34) | 1.10 (0.93–1.31) | 0.97 (0.82–1.50) |
Adjusted for age, gender, post secondary education, current smoker, current drinker, diabetes, hypertensive status, BMI, HDL cholesterol and MABP.
p<0.05
p<0.001
Multivariate models were used to show the association between retinal microvascular signs and BMI and other biochemical measures such as serum cholesterol, HDL- cholesterol, LDL-cholesterol and triglycerides. The presence of AV nicking was independently associated with higher BMI, lower HDL-cholesterol and higher triglycerides; focal arteriolar narrowing with higher BMI; a smaller CRAE with higher BMI, lower HDL and LDL-cholesterol; and a larger CRVE with higher cholesterol, LDL-cholesterol and triglycerides but lower HDL-cholesterol levels (Table 5).
Table 5.
Association of retinal microvascular signs with BMI and biochemical measures
| Multivariable-adjusted* Beta coefficient (SE) |
||||||
|---|---|---|---|---|---|---|
| Diabetic retinopathy |
Non-diabetic retinopathy |
Arterio-venous nicking |
Focal arteriolar narrowing |
Generalized arteriolar narrowing |
Generalized venular dilation |
|
| BMI, kg/m2 | 0.07 (0.33) | 0.28 (0.24) | 0.72 (0.15)** | 0.68 (0.14)** | 0.69 (0.12)** | 0.30 (0.12) |
| Cholesterol, mg/dL | −1.99 (2.34) | −0.01 (1.66) | −1.20 (1.00) | −1.41 (0.99) | −0.05 (0.81) | 3.71 (0.82)** |
| HDL-cholesterol, mg/dL | −0.36 (0.81) | −0.17 (0.77) | −0.88 (0.45)* | 1.45(0.36)** | −2.24 (0.37)* | −2.24 (0.37)** |
| LDL-cholesterol, mg/dL | −1.18 (2.08) | −0.26 (1.57) | −1.31 (0.94) | −1.51(0.93) | −1.77 (0.76)* | 4.50 (0.77)** |
| Triglycerides, mg/dL | −1.38 (7.15) | 1.22 (3.64) | 5.51 (2.41)* | 2.37 (2.39) | 1.46 (1.97) | 7.08 (1.99)** |
Adjusted for age, gender, race, post secondary education, current smoker, current drinker, diabetes, hypertensive status, BMI, HDL cholesterol and MABP.
p<0.05
p<0.001
DISCUSSION
In this large population-based study, we examined the association of physical activity in different domains (leisure, sport and work) with retinal microvascular characteristics. The findings from our cross-sectional analysis indicate that higher levels of physical activity during sport and work-related activities were associated with a lower prevalence of retinal microvascular abnormalities. In models adjusted for age, gender, race, educational status, BMI, alcohol, smoking, HDL cholesterol and MABP, we found that a higher sport index level was associated with a 9% reduction in the odds of having a larger CRVE and a 13% reduction in the odds of AV nicking. A higher level of work-related physical activity was associated with a 34% reduced risk of having diabetic retinopathy.
Epidemiologic studies that have explored this association previously are limited to those looking at physical activity and diabetic retinopathy in those with insulin-dependent diabetes mellitus. In the Wisconsin Epidemiologic Study of Diabetic Retinopathy [5], among women diagnosed with diabetes before the age of 14 years, those who participated in high school team sports were less likely to have proliferative diabetic retinopathy than those who did not. However, other epidemiologic data do not show a strong relationship between physical activity and diabetic retinopathy (Table 6).
Table 6.
Association between Physical activity and Diabetic Retinopathy in epidemiological studies
| Study | Year | Summary |
|---|---|---|
| Pittsburgh Insulin Dependent Diabetes Mellitus Morbidity and Mortality Study [22] | 1986 | Persons with Type I diabetes who participated in team sports in high school or college were less likely to develop severe eye disease compared to non-participants (OR = 0.91; p > 0.10). |
| The Epidemiology of Diabetes Complications Study [21] | 1991 | A lower risk of retinopathy was noted in persons with Type I diabetes who reported higher levels of physical activity in school years though not statistically significant when adjusted for potential confounders. |
| Wisconsin Epidemiological Study of Diabetic Retinopathy [5] | 1992 | Women (but not men), diagnosed with diabetes before the age of 14, who participated in team sports were less likely to have proliferative diabetic retinopathy after adjusting for age, duration of diabetes and other complications (OR, 0.34, p = 0.02). |
| Wisconsin Epidemiological Study of Diabetic Retinopathy [6] | 1995 | No association between physical activity measures and incidence or progression of proliferative diabetic retinopathy in persons with Type1 diabetes for a period of more than six years. |
| The Finnish Diabetic Nephropathy (FinnDiane Study) [28] | 2008 | Persons with Type I diabetes who performed low-intensity leisure-time physical activity (LTPA) as opposed to higher intensities of LTPA had a greater prevalence of proliferative retinopathy (OR, 1.49, 95 % CI, 1.15–1.93). No association with frequency of physical activities was noted. |
AV nicking has been associated with current and past blood pressure, markers of atherosclerosis, incident stroke and 12-year cardiovascular and all-cause mortality [34]. While a wider retinal arteriolar caliber has been noted to be related to diabetes, smoking and higher fibrinogen levels [31], a wider retinal venular caliber has been linked with cerebral hypoxia [7]and hyperglycemia [16] and is predictive of obesity [29], incident stroke [14] and coronary artery disease [32]. Our findings suggest that the microvasculature is perhaps sensitive to the metabolic changes associated with physical activity. It is well known that physical inactivity increases the risk of obesity [25] and there are possibly common pathophysiological mechanisms involved in the association of physical activity and the microvasculature similar to that for obesity [11]. Our analyses provided further evidence for an association between retinal microvascular changes, namely, AV nicking and wider venular caliber in the retina and a higher BMI, higher serum cholesterol and triglyceride levels. Whilst an interaction between physical activity, retinal microvessels and the metabolic syndrome cannot be excluded, we found that the association between greater physical activity and a lower frequency of retinal microvascular abnormalities was independent of established cardiovascular risk factors shown to impact on the retinal vasculature.
The underlying pathogenic processes that may explain these associations remain inconclusive. Abnormalities in the retinal microvasculature seem to reflect changes in the vessel wall, endothelial function and inflammation and are seen in up to 15% of the nondiabetic, general population [34]. Physical activity induces a reduction in inflammatory markers that are known to be associated with venous dilatation [1]. Several epidemiologic studies have reported associations of specific (serum hsCRP, plasma fibrinogen and Interleukin6) and non-specific markers of inflammation (white blood cell count, erythrocyte sedimentation rate) with larger venular caliber [15, 19, 31]. Shear stress-mediated improvement in endothelial function is known to occur with regular physical activity [24] and the endothelium plays a major role in regulating the structure and function of blood vessels. Larger retinal venular caliber is associated with markers of endothelial dysfunction, namely soluble intercellular adhesion molecule-1 and plasminogen activator inhibitor-1[31]. Additionally, it has been shown in previous ARIC analyses that AV-nicking is also associated with markers of endothelial dysfunction and inflammation [20].
The study strengths include a large sample size recruited from the general population and a standardized protocol for acquisition and grading of retinal images. The use of a validated and reproducible questionnaire (Baecke) is an added advantage as it measures the habitual long-term physical activity levels of the population in three distinct domains [2]. However, limitations should also be highlighted. Firstly, the cross-sectional nature of the study makes it difficult to evaluate any causal link between physical activity and retinal microvascular signs. Secondly, the physical activity assessment was self-reported and may be subject to recall bias and misclassification. Thirdly, the number of participants who had high physical activity indexes was small in comparison to the other levels thus reducing our ability to find any significant associations if they truly exist. This is exemplified by the reduced prevalence of wider retinal venular caliber noted with intermediate levels of sport and work related physical activity but not with the highest levels.
In conclusion, our analyses indicate that higher levels of physical activity was associated with reduced signs of retinal microvascular disease. However, more cross-sectional and longitudinal studies are needed to confirm these findings.
ACKNOWLEDGEMENTS
This study was supported by contracts N01-HC-35125, N01-HC-35126, N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022 from the National Heart, Lung, and Blood Institute, Bethesda, MD. Additional support was provided by the National Heart Foundation, the Science Technology Innovation Grant, Victoria and the Sylvia and Charles Viertel Clinical Investigator Award (TYW).The authors thank the staff and participants in the ARIC study for their important contributions.
REFERENCES
- 1.Adamopoulos S, Parissis J, Kroupis C, Georgiadis M, Karatzas D, Karavolias G, et al. Physical training reduces peripheral markers of inflammation in patients with chronic heart failure. Eur Heart J. 2001;22:791–797. doi: 10.1053/euhj.2000.2285. [DOI] [PubMed] [Google Scholar]
- 2.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 3.Barengo NC, Hu G, Lakka TA, Pekkarinen H, Nissinen A, Tuomilehto J. Low physical activity as a predictor for total and cardiovascular disease mortality in middle-aged men and women in Finland. Eur Heart J. 2004;25:2204–2211. doi: 10.1016/j.ehj.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Couper DJ, Klein R, Hubbard LD, Wong TY, Sorlie PD, Cooper LS, et al. Reliability of retinal photography in the assessment of retinal microvascular characteristics: the Atherosclerosis Risk in Communities Study. Am J Ophthalmol. 2002;133:78–88. doi: 10.1016/s0002-9394(01)01315-0. [DOI] [PubMed] [Google Scholar]
- 5.Cruickshanks KJ, Moss SE, Klein R, Klein BE. Physical activity and proliferative retinopathy in people diagnosed with diabetes before age 30 yr. Diabetes Care. 1992;15:1267–1272. doi: 10.2337/diacare.15.10.1267. [DOI] [PubMed] [Google Scholar]
- 6.Cruickshanks KJ, Moss SE, Klein R, Klein BE. Physical activity and the risk of progression of retinopathy or the development of proliferative retinopathy. Ophthalmology. 1995;102:1177–1182. doi: 10.1016/s0161-6420(95)30893-7. [DOI] [PubMed] [Google Scholar]
- 7.de Jong FJ VM, Ikram MK, Ikram MA, Hofman A, Krestin GP, van der Lugt A, et al. Arteriolar oxygen saturation, cerebral blood flow, and retinal vessel diameters: the Rotterdam Study. Ophthalmology. 2008;115:887–892. doi: 10.1016/j.ophtha.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 8.Dunstan DW, Salmon J, Owen N, Armstrong T, Zimmet PZ, Welborn TA, et al. Associations of TV viewing and physical activity with the metabolic syndrome in Australian adults. Diabetologia. 2005;48:2254–2261. doi: 10.1007/s00125-005-1963-4. [DOI] [PubMed] [Google Scholar]
- 9.Evenson KR, Rosamond WD, Cai J, Toole JF, Hutchinson RG, Shahar E, et al. Physical activity and ischemic stroke risk. The atherosclerosis risk in communities study. Stroke. 1999;30:1333–1339. doi: 10.1161/01.str.30.7.1333. [DOI] [PubMed] [Google Scholar]
- 10.Folsom AR, Arnett DK, Hutchinson RG, Liao F, Clegg LX, Cooper LS. Physical activity and incidence of coronary heart disease in middle-aged women and men. Med Sci Sports Exerc. 1997;29:901–909. doi: 10.1097/00005768-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Hamburg NM, McMackin CJ, Huang AL, Shenouda SM, Widlansky ME, Schulz E, et al. Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers. Arterioscler Thromb Vasc Biol. 2007;27:2650–2656. doi: 10.1161/ATVBAHA.107.153288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Healy GN, Dunstan DW, Shaw JE, Zimmet PZ, Owen N. Beneficial associations of physical activity with 2-h but not fasting blood glucose in Australian adults: the AusDiab study. Diabetes Care. 2006;29:2598–2604. doi: 10.2337/dc06-0313. [DOI] [PubMed] [Google Scholar]
- 13.Hubbard LD, Brothers RJ, King WN, Clegg LX, Klein R, Cooper LS, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106:2269–2280. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- 14.Ikram MK, de Jong FJ, Bos MJ, Vingerling JR, Hofman A, Koudstaal PJ, et al. Retinal vessel diameters and risk of stroke: the Rotterdam Study. Neurology. 2006;66:1339–1343. doi: 10.1212/01.wnl.0000210533.24338.ea. [DOI] [PubMed] [Google Scholar]
- 15.Ikram MK, de Jong FJ, Vingerling JR, Witteman JC, Hofman A, Breteler MM, et al. Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? The Rotterdam Study. Invest Ophthalmol Vis Sci. 2004;45:2129–2134. doi: 10.1167/iovs.03-1390. [DOI] [PubMed] [Google Scholar]
- 16.Ikram MK, Janssen JA, Roos AM, Rietveld I, Witteman JC, Breteler MM, et al. Retinal vessel diameters and risk of impaired fasting glucose or diabetes: the Rotterdam study. Diabetes. 2006;55:506–510. doi: 10.2337/diabetes.55.02.06.db05-0546. [DOI] [PubMed] [Google Scholar]
- 17.Institute. NHLaB. Atherosclerosis Risk in Communities Study. Version 2 ed. ed. Chapel Hill: Coordinating Center, School of Public Health, University of North Carolina; 1988. [Google Scholar]
- 18.Investigators TA. The Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 19.Klein R, Klein BE, Knudtson MD, Wong TY, Tsai MY. Are inflammatory factors related to retinal vessel caliber? The Beaver Dam Eye Study. Arch Ophthalmol. 2006;124:87–94. doi: 10.1001/archopht.124.1.87. [DOI] [PubMed] [Google Scholar]
- 20.Klein R, Sharrett AR, Klein BE, Chambless LE, Cooper LS, Hubbard LD, et al. Are retinal arteriolar abnormalities related to atherosclerosis?: The Atherosclerosis Risk in Communities Study. Arterioscler Thromb Vasc Biol. 2000;20:1644–1650. doi: 10.1161/01.atv.20.6.1644. [DOI] [PubMed] [Google Scholar]
- 21.Kriska AM, LaPorte RE, Patrick SL, Kuller LH, Orchard TJ. The association of physical activity and diabetic complications in individuals with insulin-dependent diabetes mellitus: the Epidemiology of Diabetes Complications Study--VII. J Clin Epidemiol. 1991;44:1207–1214. doi: 10.1016/0895-4356(91)90153-z. [DOI] [PubMed] [Google Scholar]
- 22.LaPorte RE, Dorman JS, Tajima N, Cruickshanks KJ, Orchard TJ, Cavender DE, Becker DJ, Drash AL. Pittsburgh Insulin-Dependent Diabetes Mellitus Morbidity and Mortality Study: physical activity and diabetic complications. Pediatrics. 1986;78:1027–1033. [PubMed] [Google Scholar]
- 23.Liew G, Sharrett AR, Wang JJ, Klein R, Klein BE, Mitchell P, et al. Relative importance of systemic determinants of retinal arteriolar and venular caliber: the atherosclerosis risk in communities study. Arch Ophthalmol. 2008;126:1404–1410. doi: 10.1001/archopht.126.10.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maiorana A, O'Driscoll G, Taylor R, Green D. Exercise and the nitric oxide vasodilator system. Sports medicine (Auckland, N.Z. 2003;33:1013–1035. doi: 10.2165/00007256-200333140-00001. [DOI] [PubMed] [Google Scholar]
- 25.Manson JE, Skerrett PJ, Greenland P, VanItallie TB. The escalating pandemics of obesity and sedentary lifestyle. A call to action for clinicians. Arch Intern Med. 2004;164:249–258. doi: 10.1001/archinte.164.3.249. [DOI] [PubMed] [Google Scholar]
- 26.Pereira MA, FitzerGerald SJ, Gregg EW, Joswiak ML, Ryan WJ, Suminski RR, et al. A collection of Physical Activity Questionnaires for health-related research. Med Sci Sports Exerc. 1997;29:S1–S205. [PubMed] [Google Scholar]
- 27.Rogers SL, Tikellis G, Cheung N, Tapp R, Shaw J, Zimmet PZ, et al. Retinal arteriolar caliber predicts incident retinopathy: the Australian Diabetes, Obesity and Lifestyle (AusDiab) study. Diabetes care. 2008;31:761–763. doi: 10.2337/dc07-1622. [DOI] [PubMed] [Google Scholar]
- 28.Waden J, Forsblom C, Thorn LM, Saraheimo M, Rosengard-Barlund M, Heikkila O, et al. Physical activity and diabetes complications in patients with type 1 diabetes: the Finnish Diabetic Nephropathy (FinnDiane) Study. Diabetes Care. 2008;31:230–232. doi: 10.2337/dc07-1238. [DOI] [PubMed] [Google Scholar]
- 29.Wang JJ, Taylor B, Wong TY, Chua B, Rochtchina E, Klein R, et al. Retinal vessel diameters and obesity: a population-based study in older persons. Obesity (Silver Spring) 2006;14:206–214. doi: 10.1038/oby.2006.27. [DOI] [PubMed] [Google Scholar]
- 30.Wong TY, Hubbard LD, Klein R, Marino EK, Kronmal R, Sharrett AR, et al. Retinal microvascular abnormalities and blood pressure in older people: the Cardiovascular Health Study. Br J Ophthalmol. 2002;86:1007–1013. doi: 10.1136/bjo.86.9.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong TY, Islam FM, Klein R, Klein BE, Cotch MF, Castro C, et al. Retinal vascular caliber, cardiovascular risk factors, and inflammation: the multi-ethnic study of atherosclerosis (MESA) Invest Ophthalmol Vis Sci. 2006;47:2341–2350. doi: 10.1167/iovs.05-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong TY KA, Klein R, Sharrett AR, Klein BE, Siscovick DS, Cushman M, et al. Quantitative retinal venular caliber and risk of cardiovascular disease in older persons: the cardiovascular health study. Arch Intern Med. 2006;166:2388–2394. doi: 10.1001/archinte.166.21.2388. [DOI] [PubMed] [Google Scholar]
- 33.Wong TY, Klein R, Couper DJ, Cooper LS, Shahar E, Hubbard LD, et al. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet. 2001;358:1134–1140. doi: 10.1016/S0140-6736(01)06253-5. [DOI] [PubMed] [Google Scholar]
- 34.Wong TY, Klein R, Klein BE, Tielsch JM, Hubbard L, Nieto FJ. Retinal microvascular abnormalities and their relationship with hypertension, cardiovascular disease, and mortality. Surv Ophthalmol. 2001;46:59–80. doi: 10.1016/s0039-6257(01)00234-x. [DOI] [PubMed] [Google Scholar]
- 35.Wong TY, Klein R, Nieto FJ, Klein BE, Sharrett AR, Meuer SM, et al. Retinal microvascular abnormalities and 10-year cardiovascular mortality: a population-based case-control study. Ophthalmology. 2003;110:933–940. doi: 10.1016/S0161-6420(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 36.Wong TY, Klein R, Sharrett AR, Duncan BB, Couper DJ, Tielsch JM, et al. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. Jama. 2002;287:1153–1159. doi: 10.1001/jama.287.9.1153. [DOI] [PubMed] [Google Scholar]
- 37.Wong TY, McIntosh R. Systemic associations of retinal microvascular signs: a review of recent population-based studies. Ophthalmic Physiol Opt. 2005;25:195–204. doi: 10.1111/j.1475-1313.2005.00288.x. [DOI] [PubMed] [Google Scholar]


