Abstract
The effect of cyclosporine on liver regeneration has been investigated in 25 dogs that underwent an end-to-side portacaval shunt (Eck fistula) followed by 4 days continuous infusion of the drug into the left branch of the portal vein. Three different cyclosporine infusion rates were used: 0.06, 0.6, and 4.0 mg/kg/day. Control animals received the intravenous vehicle of cyclosporine at the same rate as the treated animals; a second control group received insulin, 0.42 units/kg/day. Hepatocyte 3H-thymidine-labeled mitoses (index of hyperplasia) and hepatocyte volume (index of hypertrophy) were studied in the left (infused) and right (control) lobes in each animal. Cyclosporine vehicle had no measurable effect on hepatocytes that suffered typical atrophy and moderate increase in mitotic index after the Eck fistula. Cyclosporine infusion stimulated cell renewal significantly and restored hepatocyte size in the infused lobes with a dose-response relation. Similar positive effects were observed in the right (nonperfused) lobes, although they were less than those in the left (infused) lobes. This was because of an unmistakable spillover of cyclosporine from the infused lobes, especially in the large-dose group. No sign of hepatotoxicity was detected at any cyclosporine infusion rate. Cyclosporine has a remarkable hepatotropic effect that may be helpful in the context of liver transplantation.
Cyclosporine, a cyclic undecapeptide of fungal origin, is an inhibitor of T-lymphocyte activation1 and currently is the most important immunosuppressive drug used in organ transplantation. The lack of serious hepatotoxicity of cyclosporine at therapeutic dosages is a distinctive feature of this drug,2–5 although both minor and major changes in hepatic function have been described.2,3
A more complete understanding of the effects of cyclosporine on the liver is desirable in the context of hepatic transplantation. An allograft liver is subject to injury during the events of donor death, procurement, cold preservation, reperfusion in the recipient, and ultimately when it becomes the target of the recipient’s immunologic response.6,6a The ability of the liver to repair itself from such damage probably is an important prognostic determinant. In addition, the transplanted liver is expected to, and can, adjust its volume to the size requirement of the recipient.7,8 This requires intact growth-control mechanisms including the capacity for regeneration.
We have demonstrated that cyclosporine does not adversely affect hepatic regeneration after a partial hepatectomy in rats but that it actually enhances this response.9 This finding has been confirmed repeatedly.10–14 In this study the cyclosporine effect on regeneration has been examined with a standardized canine experimental model (Eck fistula) that has been used in the past to study the impact of hepatotropic substances on liver health and the capacity for regeneration.15, 16
MATERIAL AND METHODS
Animals and surgical procedures
Twenty-five adult female beagle dogs weighing between 8.3 and 13 kg (median, 10.5 kg) were purchased from Hazleton Research Products, Cumberland, Va. and conditioned at the University Animal Research Facility for at least 7 days before operation. The surgical procedures always were performed between 8:30 and 11:30 AM to eliminate the variables caused by diurnal rhythms.17 The anesthetic used during the original operation and all subsequent manipulations was sodium pentobarbital (25 to 30 mg/kg intravenously) followed by orotracheal administration of halothane (0.5% to 1.5%) mixed with nitrous oxide and oxygen (N2O/O2, 66%/33%). The dogs’ blood pressure and arterial blood gases were monitored with the aid of an arterial catheter.
An Eck fistula model was used that in effect splits the liver into two fragments. These fragments differ only by what is infused into the tied-off main right and left portal vein branches.15, 18 A large side-to-sidc portacaval shunt was constructed with continuous vascular suture, and this was converted to a functional end-to-side shunt by ligation of the portal vein above the anastomosis (Fig. 1). The main right and left portal veins also were ligated. A 2.4 mm (internal diameter) soft infusion catheter was inserted 1 cm into the left branch (Fig. 1) and led through the abdominal wall to a small battery-charged and calibrated infusion pump (Cormed II AIF, Cormed Inc., Medina N.Y.). The pump was incorporated into a light body cast (Chatam Medical Arts, Los Angeles, Calif.) that allowed free movement and self-feeding of the dog during the postoperative period. Cyclosporine was infused continuously into the left hepatic lobe through this catheter. It was previously established18 that saline control infusions into the tied-off right portal vein had no effect on the right hepatic fragment. The particular advantage of this model is that regeneration-inducing substances can be evaluated by their selective infusion in one of the portal vein branches. The effects in the infused hepatic lobes can be compared with those in the uninfused lobes. Each animal serves as its own control because in this model the noninfused and the treated hepatocytes are in the same liver.
Fig. 1.
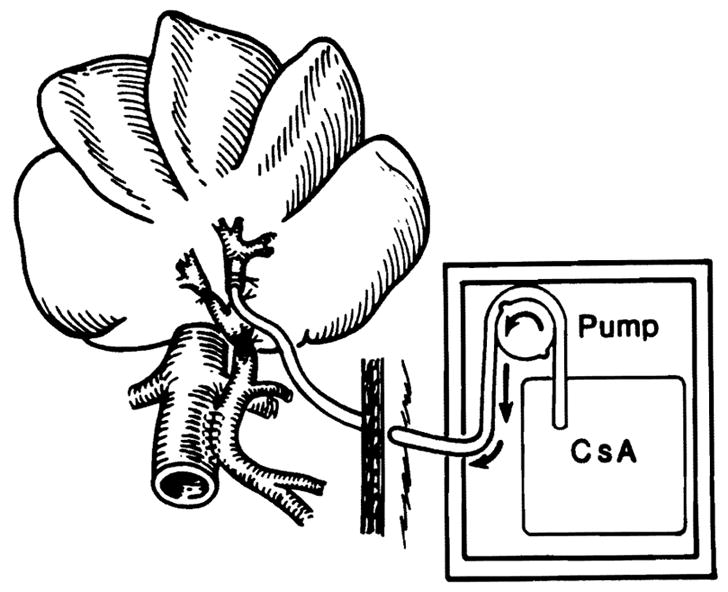
Surgical model of Eck fistula (portacaval shunt). The model in effect splits the liver into two fragments that differ only by what is infused into the tied off left portal vein branch. Therefore treated (left) and control (right) hepatocytes are present in the same liver.
During the first 24 hours after surgery, the dogs were given sugar water (sucrose, 82 gm/L) followed thereafter by regular diet. Blood samples were obtained daily from a front leg vein, and the blood was processed immediately. Cyclosporine blood levels were determined by whole-blood high-performance liquid chromatography.20 Alanine aminotransferase (ALT), total bilirubin, albumin, and creatinine levels also were measured.
Four days after the portacaval shunt had been created, the animals were administered 0.2 mCi/kg of intravenous (CH3-3H)thymidine with specific activity of 80 to 90 Ci/mmol (New England Nuclear, Boston, Mass.). Two hours later under general anesthesia, the abdomen was reentered and blood for measurement of cyclosporine level was collected from the vena cava below the liver and the left hepatic vein. The cyclosporine concentration in the left hepatic vein was thought to reflect drug that had escaped first-pass clearance by the left hepatic lobes, whereas the level in the vena cava reflected cyclosporine after systemic distribution of whatever cyclosporine had passed through the left hepatic lobes.
Biopsy specimens were obtained from left and right lobes of the liver. These were immediately fixed in 10% normal buffered formalin. The dogs were then killed with an anesthetic overdose to which potassium chloride was added. The cyclosporine infusion into the left portal vein was not interrupted until this time.
Experimental groups
Group 1 (n = 5)
The vehicle used to solubilize cyclosporine is a nonionic surfactant (Cremophor EL) and ethanol (95%) in normal saline solution (66, 33%). This vehicle was infused continuously into the left portal vein at a rate equivalent to that of a cyclosporine infusion rate of 0.06 mg/kg/day (one animal), 0.6 mg/kg/day (two animals), and 4 mg/kg/day (two animals). Because there was no effect at any infusion rate, the results were pooled.
Group 2 (n=6)
Cyclosporine (SANDIMMUNE intravenous solution, Sandoz Pharmaceuticals Inc., East Hanover, N.J.) was given at a dose of 0.06 mg/kg/day beginning within 30 minutes of completion of the portacaval shunt. The infusion volume never exceeded 28 ml during a 24-hour period. The solution administered to each dog was prepared in the morning and placed in a 40 ml pump reservoir bag. A fresh reservoir was used every day.
Group 3 (n = 6)
Group 3 was the same as group 2 except that the cyclosporine dose was 0.6 mg/kg/day.
Group 4 (n = 6)
Group 4 was the same as group 2 except that the cyclosporine dose was 4.0 mg/kg/day.
Group 5 (n = 2)
The animals were given a continuous left portal branch infusion for 4 days of 0.42 units/kg/day of purified insulin. The objective was to see if previous observations with intralobar insulin infusion in dogs with Eck fistulas18 could be repeated.
Pathologic studies
After fixation in formalin, the liver tissue was processed and stained by standard hematoxylin-eosin staining technique. Autoradiography was carried out with Kodak NTB2 liquid emulsion and an exposure time of at least 30 days. The number of mitoses, as an index of hepatocyte regeneration, was determined by counting the number of 3H-thymidine-labeled nuclei per 1000 hepatocytes. The size of individual hepatocytes (index of hypertrophy) was determined by tracing out a large number of midzonal liver cells projected on standard-thickness paper, cutting out the individual silhouettes, and weighing each. This method has been shown to be accurate for determining hepatocyte cell size and has been validated by planimetry and studies of unicellular organisms, the size of which has been determined directly.15, 16, 21 In normal, unaltered dogs, about 1.5 ± 0.5 mitoses per 1000 hepatocytes are present in the liver, with the size of midzonal hepatocytes being about 0.16 ± 0.01 size units.15, 18, 19 The exceptional reproducibility of these values and the very tight standard deviations make it easy to identify changes caused by drugs or other experimental variables.
Statistical analysis
All data are reported as mean values ± SD. For analysis of variance (ANOVA), the two main populations were control dogs (receiving vehicles) versus cyclosporine-treated dogs. A value of the F test greater than 3.0 (p <0.0l) was considered significant. The Student t test was employed in individual experimental groups to compare differences between right and left lobes. A p value less than 0.05 was considered significant.
RESULTS
Control studies
Cyclosporine vehicle
Cyclosporine vehicle had no measurable effect at any of the infusion rates. In the animals infused only with cyclosporine carrier, the hepatocytes had the typical changes of Eck fistula, which were identical in the right and left lobes.18, 22–25 The rate of mitoses was nearly three times greater than normal (Table I, group 1). Hepatocyte atrophy was apparent from the abnormally small-size units (Table II, group 1). The hepatocytes were irregular in shape, depleted of glycogen, and shrunken. Moreover, the liver was grossly smaller than usual and the reticulin framework was condensed around the central veins. The Kupffer cells were enlarged and filled with ceroid and lipofuscin. No morphologic changes suggesting hepatotoxicity were seen in the livers of the animals infused continuously with the cyclosporine vehicle at any infusion rate.
Table I.
Hepatocyte mitoses by autoradiography after intraportal continuous infusion of cyclosporine into the left portal vein of dogs with Eck fistula
| Group | No. of experiments | Cyclosporine infusion* | Mitosis (no. of labeled hepatocytes per 1000 hepatocytes [mean ± SD])† |
p Value | |
|---|---|---|---|---|---|
| Right lobes | Left lobes | ||||
| 1 | 5 | 0 (Controls)‡ | 4.11 ± 0.28 | 4.16 ± 0.58 | NS |
| 2 | 6 | 0.06 mg/kg/day | 4.0 ± 0.40 | 4.87 ± 0.52 | NS |
| 3 | 6 | 0.6 mg/kg/day | 4.37 ± 0.45 | 5.19 ± 0.46 | <0.003 |
| 4 | 6 | 4.0 mg/kg/day | 5.44 ± 0.65 | 6.33 ± 0.84 | <0.04 |
| 5 | 2 | 0 (Insulin, 0.42 units/kg/day) | 4.5 ± 1.0 | 14.3 ± 1.1 | <0.001 |
ANOVA (all cyclosporine-treatcd versus control animals): between groups, F = 10.0445; within groups, p = 0.0004
In 17 normal dogs the mitoses in left and right lobes were 1.6 ± 0.4 and 1.6 ± 0.5, respectively. The increase in mitoses caused by Eck fistula is known to be significant.18,19
Control dogs received only the vehicles of cyclosporine.
Table II.
Hepatocyte size after intraportal continuous infusion of cyelosporine in dogs with Eck fistula
| Group | No. of experiments | Dog weight (mean kg ± SD) | Treatment (cyclosporine infusion rate)* | Cell size units (mean ± SD)† |
p Value | |
|---|---|---|---|---|---|---|
| Right lobes | Left lobes | |||||
| 1 | 5 | 10.2 ± 1.8 | 0 (Controls) | 0.1085 ± 0.01 | 0.1059 ± 0.01 | NS |
| 2 | 6 | 10.2 ± 0.9 | 0.06 mg/kg/day | 0.1090 ± 0.01 | 0.1106 ± 0.01 | NS |
| 3 | 6 | 10.1 ± 1.1 | 0.6 mg/kg/day | 0.1129 ± 0.006 | 0.1242 ± 0.01 | <0.04 |
| 4 | 6 | 10.5 ± 1.3 | 4.0 mg/kg/day | 0.1381 ± 0.02 | 0.1602 ± 0.01 | <0.05 |
| 5 | 2 | 10.4 ± 0.8 | 0 (Insulin, 0.42 units/kg/day) | 0.1048 ± 0.01 | 0.1588 ±0.01 | <0.001 |
ANOVA (all cyclosporine-treated versus control animals): between groups, F = 18.103; within groups, p =0.001.
In normal dogs, hepatocyte size units are 0.16 ± 0.01.15
Insulin control
When insulin was given into the left portal vein, the left lobes were spared the atrophy and organelle deterioration that occurred on the right side (Table II, group 5); in addition, there was a striking proliferative response (Table I, group 5). These results were identical to those obtained 14 years ago.18
Cyclosporine infusion
Histopathologic studies
None of the liver specimens obtained from cyclosporine-treated animals showed any evidence of hepatocellular damage. On the contrary, the cyclosporine infusions benefited the infused and the noninfused lobes. The ANOVA of all liver samples from cyclosporine-treated animals versus the samples from animals infused with carrier only showed a significant increase in the hepatocyte mitotic index (F test = 10.04; p = 0.0004) (Table I) and in hepatocyte size (F test = 18.10; p = 0.001) (Table II). A dose-response relation was evident, the most striking results being with the largest milligram per kilogram per day infusion rate.
The directly infused left lobes had a significantly greater proliferative response and larger hepatocytes than had the right lobes (Tables I and II). However, there was an unmistakable spillover to the right lobes in the larger-dose groups 3 and 4. The same spillover probably occurred in all of the cyclosporine-treated animals because the right (nonperfused) lobes had changes similar to, although less than, those in the left (infused) lobes (Tables I and II). The increase in hepatocyte proliferation in either the right or left lobes was minor at any cyclosporine dose compared with the proliferative response to insulin (Table I). In contrast, maintenance of hepatocyte size and preservation of organelle quality was a striking feature of the cyclosporine-infused lobes (Table II).
Cyclosporine blood levels
Cyclosporine was undetectable in either the hepatic vein or systemic venous blood except with the largest dose. Only one dog of group 3 had a detectable level of cyclosporine in the left hepatic vein at the time of death. When 4 mg/kg/day was given into the left portal branch, the blood level rose to immunosuppressive levels at both sampling sites (Table III).
Table III.
Cyclosporine blood levels after 4 days of continuous intraportal infusion
| Infusion rate (mg/kg/day) | Left hepatic vein | Peripheral blood |
|---|---|---|
| 0 | — | — |
| 0.06 | Undetectable | Undetectable |
| 0.6 | Undetectable* | Undetectable |
| 4 | 278.9 ± 46.4 ng/ml | 263.5 ± 52.4 ng/ml |
Exception was one of the six animals whose hepatic vein level was 70 ng/ml. Undetectable means a cyclosporine concentration <50 ng/ml determined by a whole-blood high-performance liquid chromatographic method.
Hepatic and renal function tests
Neither the cyclosporine doses nor the infusion of vehicle had an effect on bilirubin, albumin, ALT, or creatinine levels (Fig. 2). Transaminasemia developed during 4 days of the experiment in control animals and in treated animals, but this is a known response to Eck fistula.
Fig. 2.
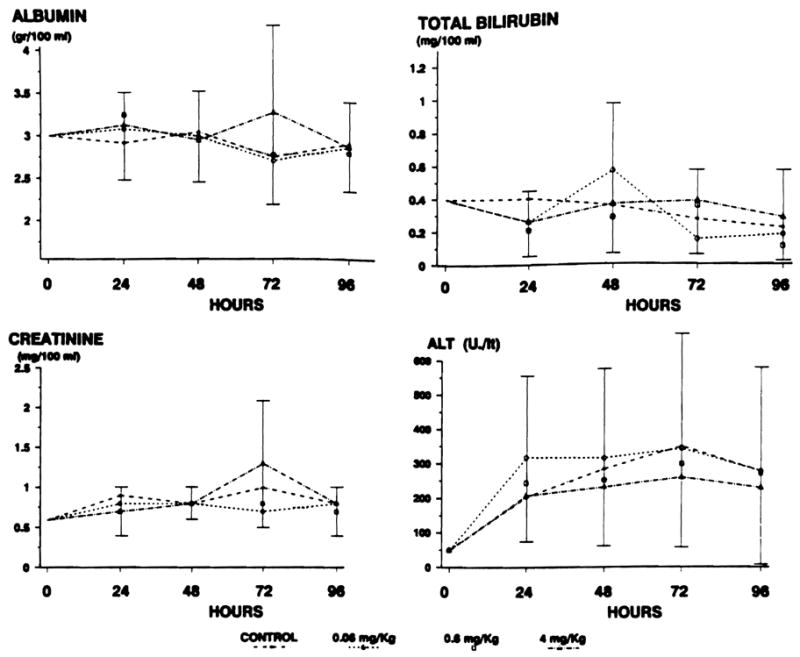
Hepatic and renal function test results. No difference between groups is observed. Increase in ALT is a known response to Eck fistula.
DISCUSSION
After liver transplantation, cyclosporine is administered orally as soon as the recipient is able to eat. Whatever cyclosporine is absorbed can reach the general circulation only after passing through the liver by the portal vein. It is known that greater than 90% of the drug undergoes extensive hepatic metabolism26 and is excreted solely into bile. Cyclosporine apparently enters hepatocytes by simple diffusion in a concentration- and time-dependent manner27 because the uptake of the drug is not dependent on an energy supply for hepatocytes. The bioavailability of the drug averages approximately 30% of the orally administered dose.28, 29
The portal route of drug delivery that follows oral ingestion was simulated in this study. The lowest infused dose appeared to be below the therapeutic range considered desirable clinically because none of the infused cyclosporine could be detected in the hepatic vein. The largest dose was definitely in the therapeutic range and it may even have been excessive. The different infusion rates of cyclosporine chosen for this study were based on the pharmacokinetics of the drug described in animals and human beings.20, 26, 28, 29
There appeared to be no harmful consequences of delivering cyclosporine straight to the liver at any of these doses, and in fact the portion of the liver exposed to cyclosporine on first pass had more rapid cell renewal and healthier hepatocytes. Similar but ever more striking findings have been obtained with the same Eck fistula model when insulin18 or a cytosolic extract from regenerating livers30 was infused. The extremely potent effect of insulin described previously18 was confirmed in the control experiments of group 5.
The influence of insulin or other substances in increasing hepatocyte size, morphologic integrity, function, and the capacity for regeneration has been referred to as hepatotropic.15, 16, 18, 19, 30, 31 According to this definition, cyclosporine is hepatotropic. The literature supporting this conclusion is summarized in Table IV. Until now, all of the evidence of a hepatotropic influence of cyclosporine has been obtained with partial liver resection in rats9–14; our studies reported herein have confirmed that the hyperplasia component of regeneration is actually augmented by cyclosporine.
Table IV.
Cyclosporine and liver regeneration: Literature survey
| Reference | Model | Cyclosporine dose | Results |
|---|---|---|---|
| Makowka et al.,9 1986 | Rat, 70% Hx | 25 mg/kg/day p.o. for 7 days pre-Hx, 5 mg/kg IV (bolus) post-Hx | Cyclosporine did not inhibit enzymatic parameters of hepatic regeneration and actually potentiated these |
| Kahn et al.,10 1988 | Rat, 70% Hx | Same as Makowka et al.9 | Confirmed Makowka et al., 1986 |
| Kim et al.,11, 12 1988 | Rat, 70% Hx | 10 mg/kg p.o. 1 day before and immediately and 24 and 48 hours post-Hx | Cyclosporine potentiated mitoses whereas azathioprine and prednisone suppressed |
| Grant et al.,13 1988 | Rat, 70% Hx | 10 mg/kg/day p.o. for 10 days post-Hx, 20 mg/kg/day p.o. for 10 days post-Hx | Cyclosporine, 10 mg/kg/day, does not impair hepatic regeneration; cyclosporine, 20 mg/kg/day, impaired hepatic regeneration by decreasing food intake |
| Kahn et al.,14 1989 | Rat, 70% Hx | Same as Makowka et al.9 | Cyclosporine pretreatment primed the liver for a stronger hepatic regeneration after Hx, increased enzymatic parameters, and increased estrogen receptor activity |
| Mazzaferro et al. (present study) | Dog, Eck fistula | 0.06, 0.6, and 4.0 mg/kg/day continuously for 4 days in left portal vein | Cyclosporine infused into portal vein increases mitotic index and prevents hepatocyte atrophy |
Hx, Hepatectomy; p.o., by mouth; IV, intravenous.
In this study we have added the additional observation that hepatocyte size and morphology can be influenced favorably by cyclosporine. The central observation was that physiologic doses of cyclosporine were able to prevent the atrophy and organelle disruption caused by Eck fistula in addition to augmenting the low-grade hyperplasia that is characteristic of the liver with Eck fistula.15, 16, 18, 31
The explanation for these effects is far from clear. It is tempting to work backward from what is known about the pathogenesis of the Eck fistula injury. Much evidence has indicated that the most important factor in this injury is the loss of endogenous insulin and other less important splanchnic substances that are diverted away from the liver by portacaval shunt.15, 16, 18, 19, 31–33 Although all of these studies emphasized the overriding role of insulin, multiple splanchnic factors other than insulin apparently also can play a cumulative hepatotropic role.15–19, 31–34 The ameliorating effects of cytosolic extracts on the liver with Eck fistula are not thought to be caused by insulin.30
Cyclosporine could be acting directly on the liver. However, it could equally well be mobilizing other splanchnic or nonsplanchnic hepatotropic factors, or its effects could be mediated in ways including immune modulation that are barely conceivable at the present time. The molecular action of cyclosporine is not known, but it is thought to bind to cytoplasmic proteins including cyclophilin and calmodulin.35–38 T-lymphocytes, brain, kidney, and hepatocytes have the highest concentration of cyclophilin in the body, fueling suspicion that hepatotoxicity could be common with cyclosporine.38 Our study provides some assurance that this will not be a major concern.
One puzzling feature of our results was the ease with which cyclosporine seemed to leak through the left liver lobes and affected the right portion of the liver. In contrast, other hepatotropic substances such as insulin18 (group 5) and biologically active cytosol30 are so completely removed with first passage that little effect can be seen in the contralateral lobes. The fact that cyclosporine easily overcame the first-passage clearance capacity of the infused lobes accounts for its systemic effect as an immunosuppressant after oral intake and subsequent portal delivery to the liver.
Acknowledgments
We acknowledge the help of Del Kahn, Michele Barone, John Prelich, and Sandra Oks for their assistance during the experiments; Bruno Begliomini for statistical analysis; Professor Leandro Gennari from Italian National Cancer Institute; and Ms. Donna Ross for editing the manuscript.
Supported by research grants from the Veterans Administration and project Grant No. DK 29961 from the National Institutes of Health, Bethesda, Md.
References
- 1.Borel JF. The histology of cyclosporine A and its significance. In: White DJG, editor. Cyclosporine A: Proceedings of an International Conference on Cyclosporine A. Amsterdam: Elsevier Biomedical Press; 1982. pp. 5–17. [Google Scholar]
- 2.Calne RY, White DJG, Thiru S, et al. Cyclosporin A in patients receiving renal allograft from cadaver donors. Lancet. 1978;2:1323–7. doi: 10.1016/s0140-6736(78)91970-0. [DOI] [PubMed] [Google Scholar]
- 3.Klintmalm GBG, Iwatsuki S, Starzl TE. Cyclosporin A hepatotoxicity in sixty-six renal allograft recipients. Transplantation. 1981;32:488–9. doi: 10.1097/00007890-198112000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomson AW, Whiting PH, Simpson JG. Pathobiology of cyclosporine A in experimental animals. In: White DJG, editor. Cyclosporine A: Proceedings of an International Conference on Cyclosporine A. Amsterdam: Elsevier Biomedical Press; 1982. pp. 177–90. [Google Scholar]
- 5.Galinsky RE, Alexander DP, Franklin MR. Effect of cyclosporine on hepatic oxidative and conjugative metabolism in rats. Drug Metab Dispos. 1987;15:731–3. [PubMed] [Google Scholar]
- 6.Starzl TE, Demetris AJ, Van Thiel DH. Medical progress: liver transplantation. Part I. N Engl J Med. 1989;321:1014–22. doi: 10.1056/NEJM198910123211505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Starzl TE, Demetris AJ, Van Thiel DH. Medical progress: liver transplantation. Part II. N Engl J Med. 1989;321:1092–9. doi: 10.1056/NEJM198910193211606. [DOI] [PubMed] [Google Scholar]
- 7.Kam I, Lynch S, Svanas G, et al. Evidence that host size determines liver size: studies in dogs receiving orthotopic liver transplants. Hepatology. 1987;7:362–6. doi: 10.1002/hep.1840070225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Thiel DH, Gavaler JS, Kam I, et al. Rapid growth of an intact human liver transplanted into a recipient larger than the donor. Gastroenterology. 1987;93:1414–9. doi: 10.1016/0016-5085(87)90274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makowka L, Svanas G, Esquivel CO, et al. Effect of cyclosporine on hepatic regeneration. Surg Forum. 1986;37:352–4. [PMC free article] [PubMed] [Google Scholar]
- 10.Kahn D, Lai HS, Romovacek H, Makowka L, Van Thiel DH, Starzl TE. Cyclosporin A augments the regenerative response after partial hepatectomy in the rat. Transplant Proc. 1988;20(suppl):850–2. [PMC free article] [PubMed] [Google Scholar]
- 11.Kim YI, Salvini P, Auxilia F, Calne RY. Effect of cyclosporin A on hepatocyte proliferation after partial hepatectomy in rats: comparison with standard immunosuppressive agent. Am J Surg. 1988;155:245–9. doi: 10.1016/s0002-9610(88)80705-0. [DOI] [PubMed] [Google Scholar]
- 12.Kim YI, Calne RY, Nagasue N. Cyclosporin A stimulates proliferation of the liver cells after partial hepatectomy in rats. Surg Gynecol Obstet. 1988;166:317–22. [PubMed] [Google Scholar]
- 13.Grant D, Black R, Zhong R, Wall W, Stiller C, Duff J. The effect of cyclosporine on liver regeneration. Transplant Proc. 1988;20(suppl):877–9. [PubMed] [Google Scholar]
- 14.Kahn D, Porter LE, Eagon PK, et al. Cyclosporine augments the hepatic regenerative response in rat. Dig Dis Sci. doi: 10.1007/BF01537420. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Starzl TE, Porter KA, Francavilla A. The Eck fistula in animals and humans. Curr Probl Surg. 1983;20:688–767. doi: 10.1016/s0011-3840(83)80010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Starzl TE, Terblanche J. Hepatotropic substances. In: Popper H, Schaffner F, editors. Progress in liver diseases. Vol. 6. New York: Grune & Stratton; 1979. pp. 135–52. [PubMed] [Google Scholar]
- 17.Bucher NLR. Regeneration of mammalian liver. Int Rev Cytol. 1963;15:245–300. doi: 10.1016/s0074-7696(08)61119-5. [DOI] [PubMed] [Google Scholar]
- 18.Starzl TE, Watanabe K, Porter KA, Putnam CW. Effect of insulin, glucagon, and insulin/glucagon infusion on liver morphology and cell division after complete portacaval shunt in dogs. Lancet. 1976;1:821–5. doi: 10.1016/s0140-6736(76)90477-3. [DOI] [PubMed] [Google Scholar]
- 19.Starzl TE, Francavilla A, Halgrimson CG, et al. The origin, hormonal nature and action of hepatotropic substance in portal venous blood. Surg Gynecol Obstet. 1973;137:179–99. [PMC free article] [PubMed] [Google Scholar]
- 20.Ptachcinski RJ, Venkataramanan R, Burckart GJ. Clinical pharmacokinetics of cyclosporine. Clin Pharmacol. 1986;11:107–32. doi: 10.2165/00003088-198611020-00002. [DOI] [PubMed] [Google Scholar]
- 21.Porter R, Whelan J, editors. Ciba Foundation Symposium. 55. Amsterdam: Elsevier, Excerpta Medica, North-Holland; 1978. Hepatotropic factors. [Google Scholar]
- 22.Weinbren K, Washington SLA. Hyperplastic nodules after portacaval anastomosis in rats. Nature. 1976;264:440–2. doi: 10.1038/264440a0. [DOI] [PubMed] [Google Scholar]
- 23.Kerr JFR. Shrinkage necrosis: a distinct mode of cellular death. J Pathol. 1971;105:13–20. doi: 10.1002/path.1711050103. [DOI] [PubMed] [Google Scholar]
- 24.Dubuisson L, Bioulac P, Saric J, Balabaud C. Hepatocyte ultrastructure in rats with portacaval shunt. Dig Dis Sci. 1982;27:1003–10. doi: 10.1007/BF01391746. [DOI] [PubMed] [Google Scholar]
- 25.Weinbren K, Hadjis NS. Compensatory hyperplasia of the liver. In: Blumgart LH, editor. Surgery of the liver and biliary tract. New York: Churchill Livingstone; 1988. pp. 49–59. [Google Scholar]
- 26.Maurer G, Loosli HR, Schreier E, Kellen B. Disposition of cyclosporine in several animal species and man. Drug Metab Dispos. 1984;12:120–6. [PubMed] [Google Scholar]
- 27.Ziegler K, Polzin G, Frimmer M. Hepatocellular uptake of cyclosporin A by simple diffusion. Biochem Biophys Acta. 1988;938:44–50. doi: 10.1016/0005-2736(88)90120-4. [DOI] [PubMed] [Google Scholar]
- 28.Venkataramanan R, Mabucky K, Buckart GJ, Ptachcinski RJ. Clinical pharmacokinetics in organ transplant patients. Clin Pharmakokinet. 1989;16:134–61. doi: 10.2165/00003088-198916030-00002. [DOI] [PubMed] [Google Scholar]
- 29.Kahan BD, Grevel J. Optimization of cyclosporine therapy in renal transplantation by a pharmacokinetic strategy. Transplantation. 1988;46:631–44. doi: 10.1097/00007890-198811000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Starzl TE, Jones AF, Terblanche J, Usui S, Porter KA, Mazzoni G. Growth-stimulating factor in regenerating canine liver. Lancet. 1979;1:127–30. doi: 10.1016/s0140-6736(79)90519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Starzl TE, Lee IY, Porter KA. The influence of portal blood upon lipid metabolism in normal and diabetic dogs and baboons. Surg Gynecol Obstet. 1975;140:381–96. [PMC free article] [PubMed] [Google Scholar]
- 32.Ricordi C, Lacy PA, Callery MP, Park PW, Flye MW. Trophic factor from pancreatic islets in combined hepatocyte-islet allografts enhances hepatocellular survival. Surgery. 1989;105:218–23. [PubMed] [Google Scholar]
- 33.Starzl TE, Francavilla A, Porter KA, Benichou J. The effect upon the liver of evisceration with or without hormone replacement. Surg Gynecol Obstet. 1978;146:524–31. [PMC free article] [PubMed] [Google Scholar]
- 34.Starzl TE, Porter KA, Busuttil RW, Pichlmayr R. Portacaval shunt in three patients with alpha-1 -antitrypsin deficiency: nine to twelve and one third years later. Hepatology. doi: 10.1002/hep.1840110133. (in press) [DOI] [PubMed] [Google Scholar]
- 35.Le Grue, Friedman AW, Kahan BD. Binding of cyclosporine by human lymphocytes and phospholipid vesicles. J Immunol. 1983;131:712–8. [PubMed] [Google Scholar]
- 36.Hait WN, Harding MW, Handschumacher RE. Calmodulin, cyclophilin and cyclosporin A. Science. 1986;233:987–8. doi: 10.1126/science.3016900. [DOI] [PubMed] [Google Scholar]
- 37.Colombani PM, Robb A, Hess AD. Cyclosporin A binding to calmodulin: a possible site of action on T-lymphocytes. Science. 1985;228:337–9. doi: 10.1126/science.3885394. [DOI] [PubMed] [Google Scholar]
- 38.Handschumacher RE, Harding MW, Rice J, Drugger RJ, Speicher D. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984;226:544–6. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]


