Abstract
Rejection, the leading cause of liver allograft dysfunction, is usually detected by liver biopsy. The purpose of this study was to determine if there are angiographic findings that correlate with this posttransplantation complication. In a retrospective study, the angiograms of 35 patients with histologically proven allograft rejection were reviewed. The examinations were done because of suspected posttransplantation vascular complications. Abnormal hepatic arteriograms were observed in 30 (86%). Eleven (37%) of the 30 had hepatic artery thrombosis (all had acute rejection). Nineteen (63%) of the 30 had varying degrees of intrahepatic arterial narrowing (14 had acute and five had chronic rejection). Additional findings in patients with acute rejection included (1) stretching of the intrahepatic arterial tree (five cases) and (2) slow flow, poor peripheral arterial filling, and a decrease in the number of intrahepatic arteries (10 cases total). Intrahepatic branch vessel stenoses and occlusions were seen in four patients with chronic rejection.
We conclude that there is good correlation between the angiographic findings and histologic evidence of rejection. Although angiography is not advocated as a test for transplant rejection, detectable of certain findings raises the possibility of rejection.
Liver transplantation is an accepted treatment for irreversible end-stage liver disease in both children and adults [1, 2]. Survival rates continue to improve, especially as the result of the success of cyclosporine as an immunosuppressive drug [3, 4]. Four-year survival is 75% in children and 50% in adults [3].
However, rejection is still the most common cause of posttransplantation hepatic dysfunction [5], occurring in at least 37% of liver allografts [6]. Without liver biopsy, the diagnosis is often made by exclusion.
After transplantation, hepatic angiography is often performed for the evaluation of possible vascular complications. In our institution. 18% of liver transplant patients require postoperative angiography [7]. In this study, we analyzed the angiographic findings in 35 liver transplant patients with a histologic diagnosis of rejection to determine if there was a correlation between rejection and angiographic findings.
Materials and Methods
During the 65-month period ending June 1986, 590 patients (231 children and 359 adults) received 766 orthotopic liver transplants at the University Health Center of Pittsburgh. There were 263 men and 327 women, aged 4 months to 67 years.
One hundred four patients (110 transplants) underwent 123 angiographic studies. Angiography was performed from 1 day to over 11 years after transplantation. Seventy-five percent of the studies were performed within the first 2 months after transplantation. Excluding rejection, a detailed analysis of the findings in the first 87 patients has been reported [7].
The current study group consists of a subgroup of the angiographic population defined above. Included are 35 patients (19 children and 16 adults) who had a hepatic arteriogram and a histologic diagnosis of allograft rejection. Indications for angiography in these patients were suspected hepatic artery thrombosis in 33, hemobilia in one, and evaluation of portal vein patency for retransplantation in one.
Liver tissue was available for histologic evaluation in 24 cases within 6 days, in eight cases at 7–12 days, and in three cases at 13–18 days after angiography. The diagnosis of rejection was made by liver biopsy in 20 patients and by histologic examination of the hepatectomy specimen after retransplantation in 15 patients. Because of the variable interval between angiography and the diagnosis of rejection, no attempt was made to correlate the degree of rejection with the angiographic findings.
Results
Abnormal hepatic arteriograms were observed in 30 (86%) of the 35 cases. The most common arteriographic abnormality in both acute and chronic rejection was varying degrees of narrowing of the intrahepatic arterial tree in 19 (63%) of the 30 patients.
Acute Rejection
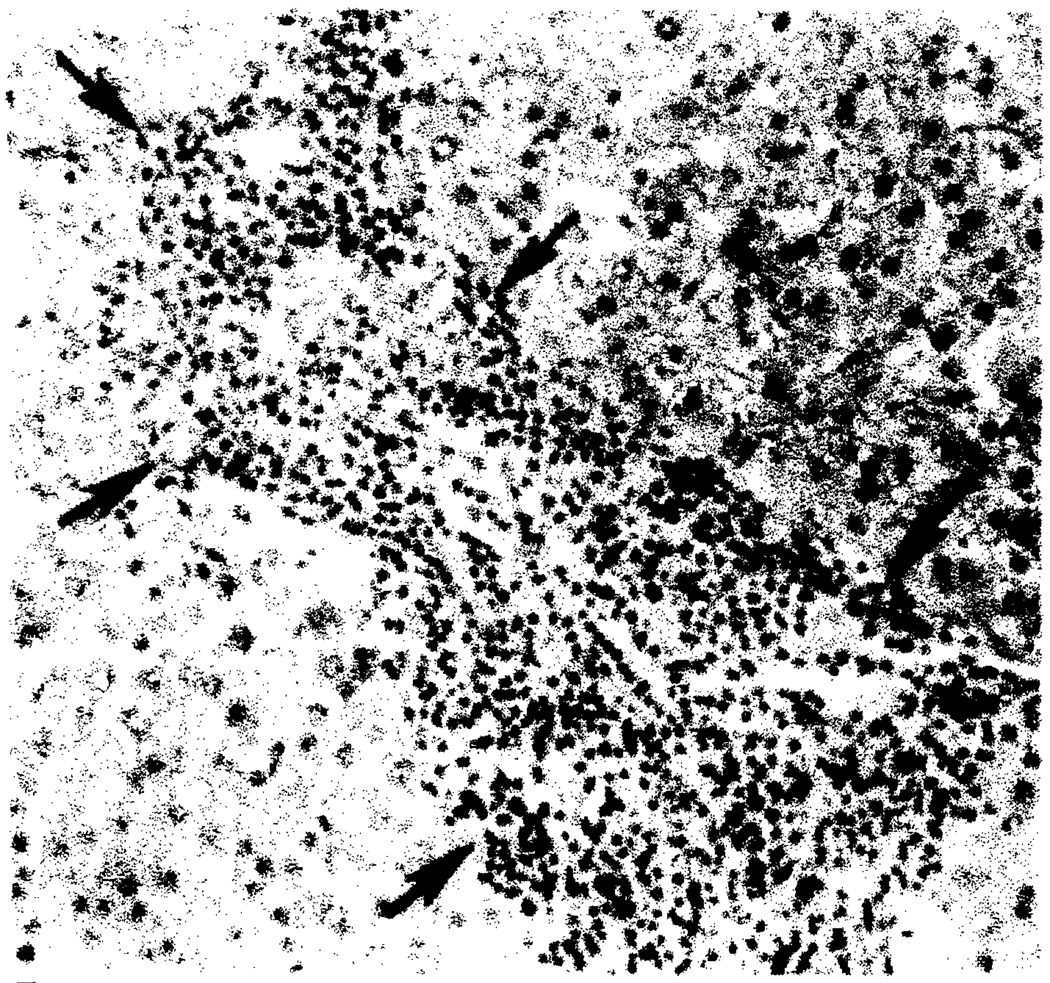
Thirty patients had acute rejection. Diffuse arterial narrowing and stretching of the intrahepatic arterial tree were observed in five patients (Fig. 1). In three patients, narrowing of the peripheral hepatic arterial branches was shown. Slow flow, arterial narrowing, poor peripheral arterial filling, and a decrease in the number of intrahepatic arteries (“pruned-tree” appearance) were seen to varying degrees in 10 patients (Fig. 2). Hepatic artery thrombosis occurred in 11 patients with acute rejection. Five patients had normal hepatic arteriograms.
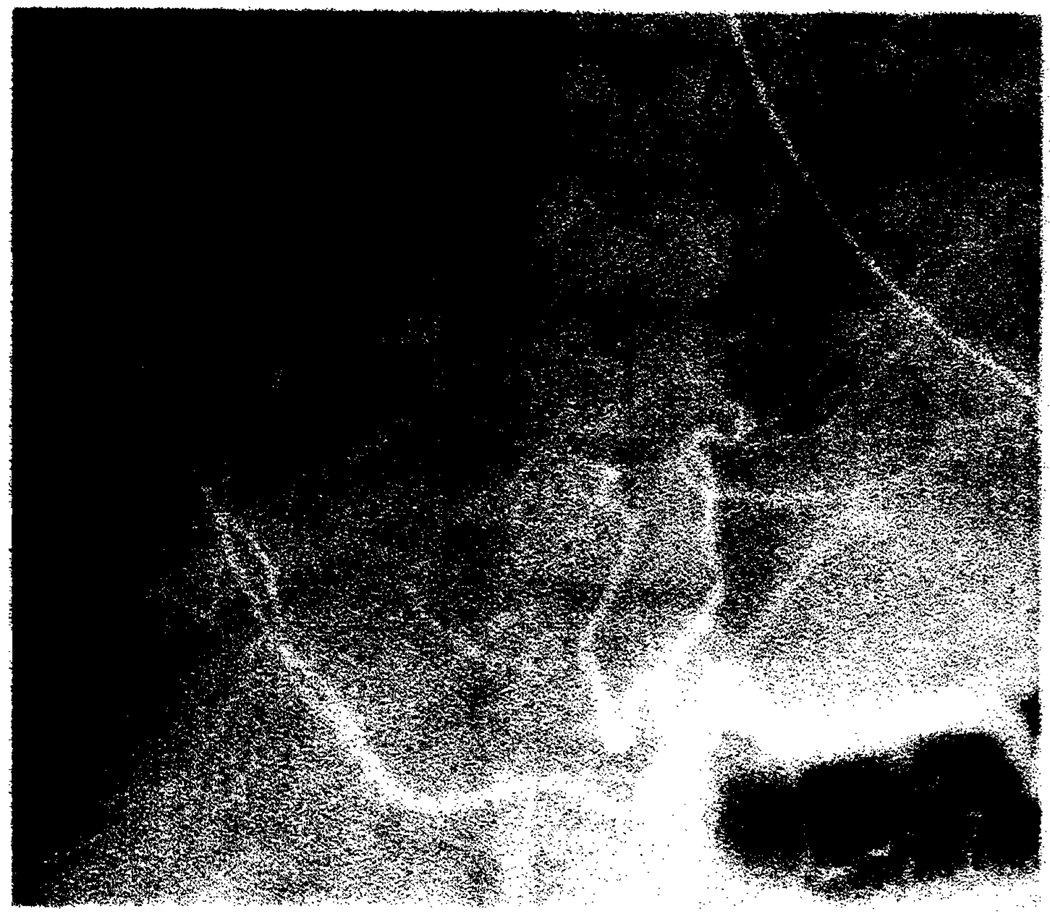
Fig. 1.
Acute rejection. Diffuse narrowing and stretching of intrahepatic arteries on celiac arteriogram 3 months after transplantation.
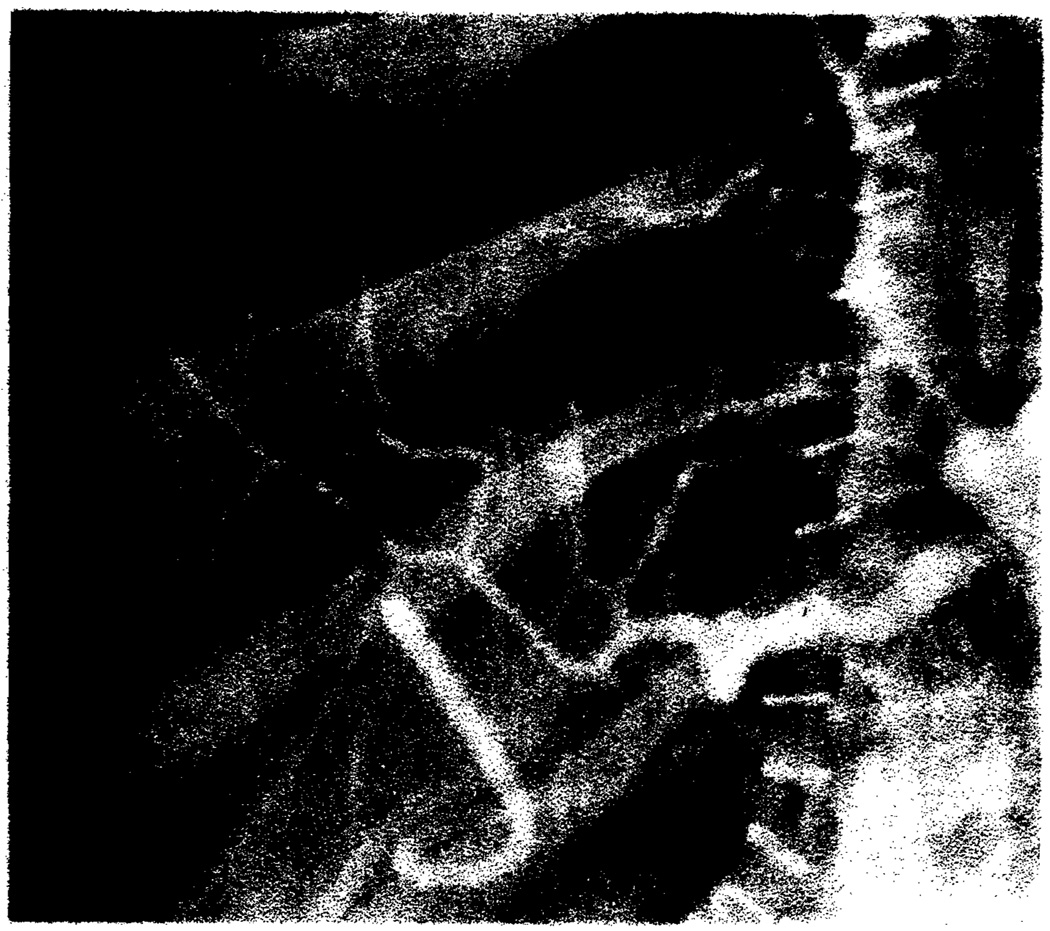
Fig. 2.
Acute rejection. Arterial narrowing, poor peripheral arterial filling, and reduced number of intrahepatic arteries on celiac arteriogram 2 weeks after transplantation.
Chronic Rejection
Five patients had chronic rejection. In four, diffuse arterial narrowing was observed that was generally more marked than seen in acute rejection (Figs. 3 and 4). These four patients also had branch vessel stenoses and occlusions (Figs. 3 and 4). One patient had narrowing of the peripheral intrahepatic arteries.
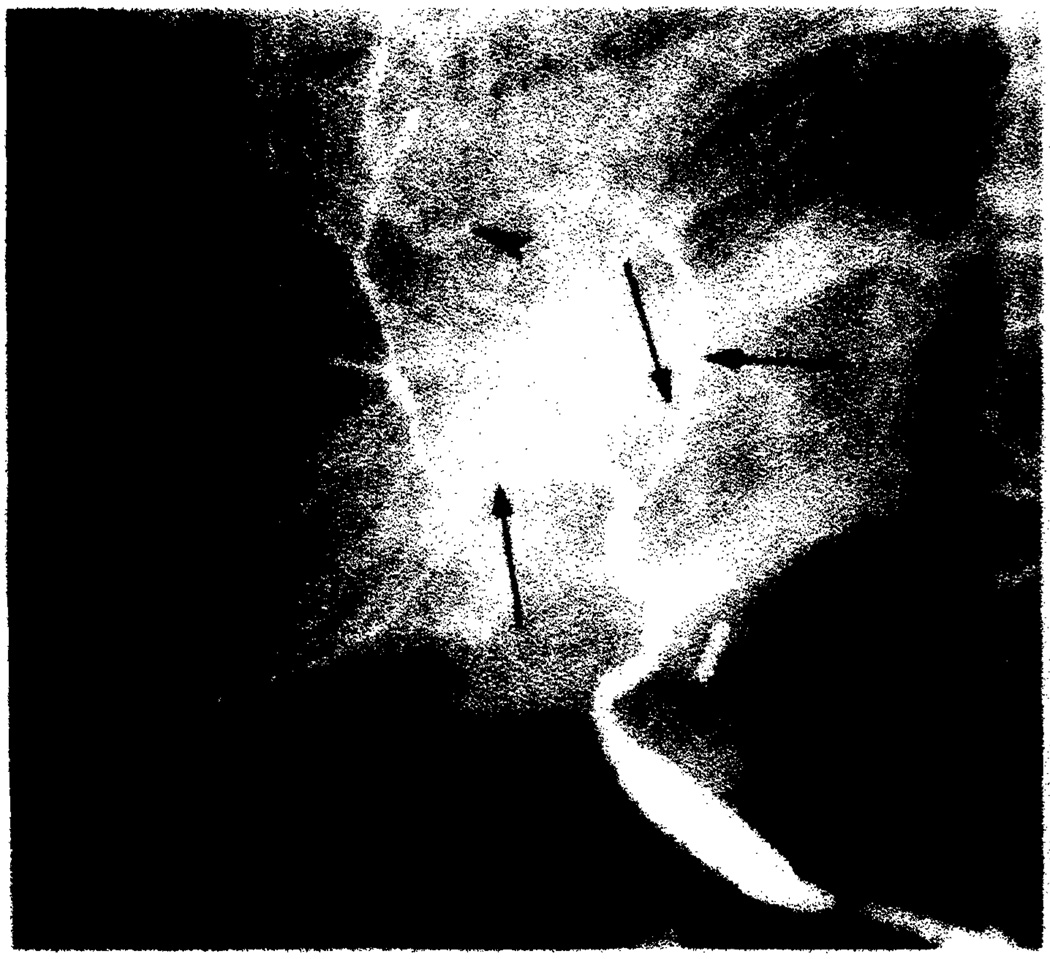
Fig. 3.
Chronic rejection. Severe diffuse arterial narrowing with branch vessel occlusions (arrowheads) and stenoses (arrows) on hepatic arteriogram 8 months after transplantation.
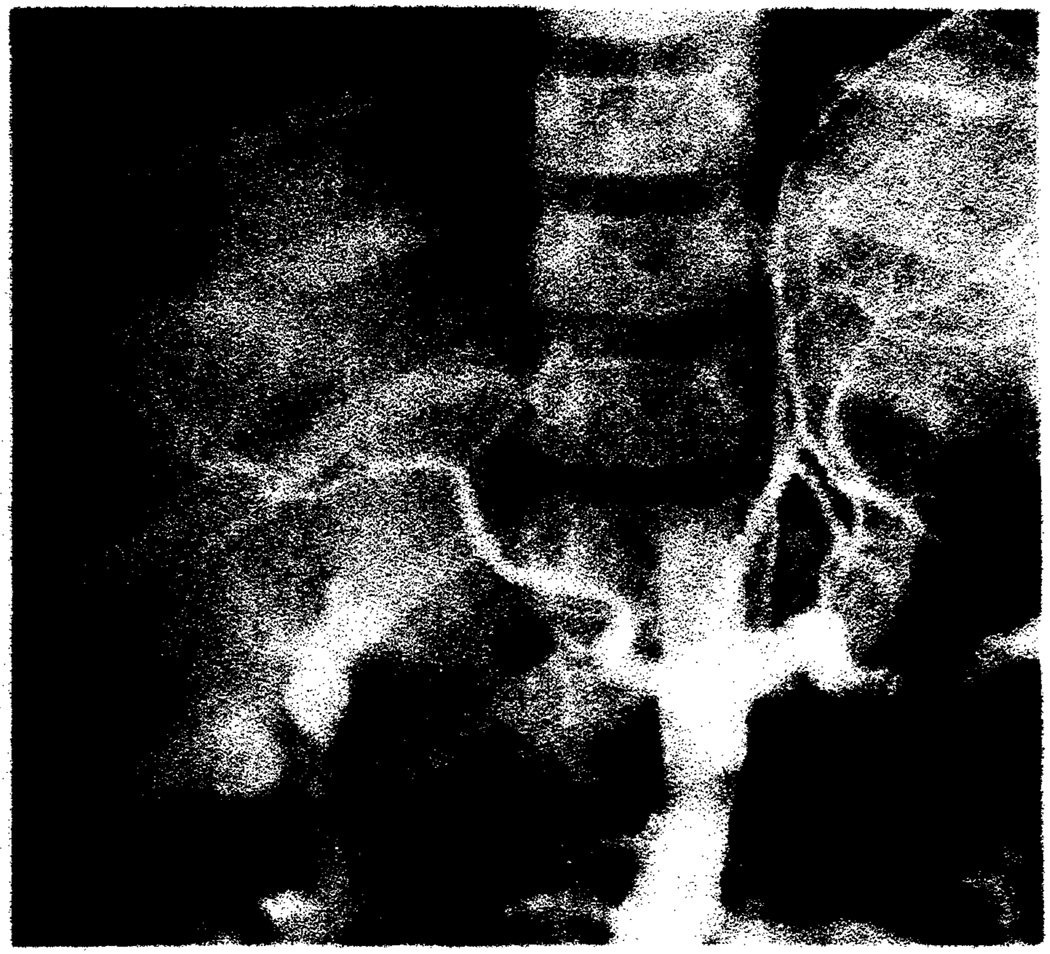
Fig. 4.
Chronic rejection. Markedly narrowed hepatic arteries with no peripheral filling and with branch vessel occlusions on celiac arteriogram 9 months after transplantation.
Discussion
After liver transplantation, rejection is one of the leading causes of hepatic dysfunction in both the early and late postoperative periods [6]. The differentiation of rejection from other causes of hepatic dysfunction is crucial for institution of proper therapy.
Postoperative graft dysfunction can be due to several other conditions including ischemic damage, biliary obstruction, hepatic artery thrombosis, viral infection, hemolysis, and drug-induced hepatic injury [5, 8, 9]. Differentiation of rejection from these condition occasionally can be suggested by clinical presentation and liver function tests [5]. However, without liver biopsy, the diagnosis of rejection is often made by exclusion [6, 8]. Biliary obstruction can be excluded by cholangiography [10, 11]. Hepatic artery patency can be evaluated with duplex sonography, although angiography may be required [12].
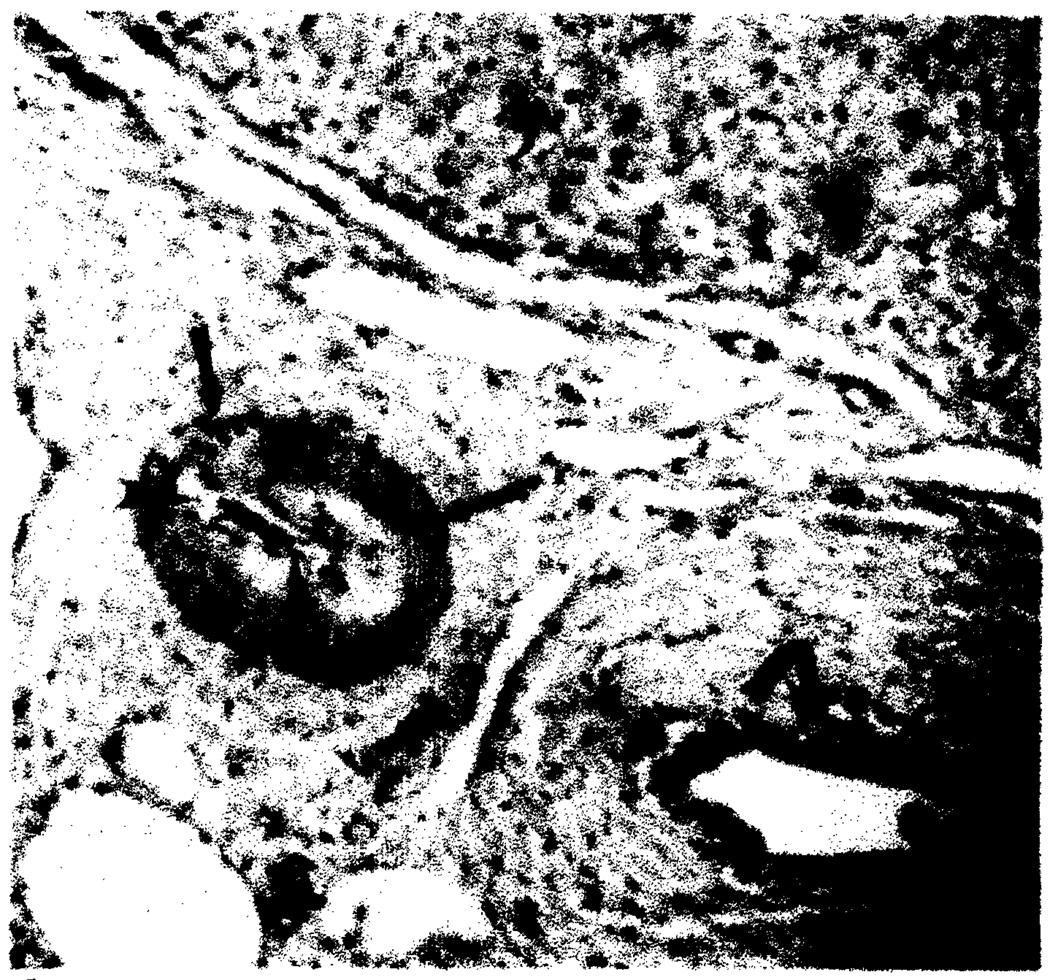
Histologically, rejection is manifested by a cellular-mediated injury of hepatocytes and bile ductules and a spectrum of vascular lesions in the hepatic arteries [6]. Rejection episodes within the first 2 months after transplantation are characterized by a marked inflammatory infiltrate within the portal tracts (Fig. 5). We postulate that this infiltrate results in microvascular injury with subsequent edema, which increases intrahepatic pressure resulting in arterial narrowing and slow arterial flow. Slow flow and arterial narrowing were observed in many of our patients. Elevated intrahepatic pressure has been shown to occur in rejecting liver allografts in the rat [13]. Blood-flow studies during acute rejection have demonstrated a marked decrease in total hepatic blood flow [8, 14]. During an acute rejection episode, the allograft is frequently swollen [9], probably caused by the infiltrative process and edema. This swelling correlates with the stretched appearance of the intrahepatic arterial tree noted angiographically (Fig. 1).
Fig. 5.
Acute rejection. Inflammatory infiltrate (arrows) expanding portal tract on needle biopsy 10 days after transplantation. (H and E, × 100)
The arterial lesions of rejection are most evident in the medium-sized hepatic arteries [6]. In the first 2 months after surgery, vasculitis and fibrinoid necrosis predominate. Later, deposition of subintimal foam cells, intimal sclerosis, and myointimal hyperplasia occur, which are the hallmarks of chronic rejection (Fig. 6). The end result is arterial narrowing and eventually occlusion (Figs. 3 and 4).
Fig. 6.
Chronic rejection (same patients as in Fig. 3). Severe luminal narrowing (arrowheads) of a branch hepatic artery (straight arrows) caused by medial thickening and deposition of medial and subintimal foam cells seen in failed allograft 8 months after transplantation. Bile duct (curved arrow). (H and E, × 100)
Similar angiographic findings have been described in renal allograft rejection [15, 16]. However, one manifestation of renal allograft rejection, microaneurysms [15, 16], was not observed in our series. Microaneurysms as seen in renal allograft rejection are a manifestation of arteritis [16]. The vasculitis seen in liver allograft rejection predominantly involves the veins; arteritis is unusual.
Eleven patients with acute rejection had hepatic artery thrombosis. Several factors have been implicated in the pathogenesis of liver transplant arterial thrombosis [17]. Our histologic observations indicate that there is also an association between acute rejection and the development of hepatic artery thrombosis in some cases. In these cases thrombus usually originates at the anastomosis. Pathologically, we have observed anastomotic intimal irregularities in these patients. We believe that with acute rejection and subsequent diminished hepatic arterial flow, thrombus forms at sites of arterial intimal defects, such as suture lines.
Severe chronic rejection can cause progressive narrowing of the intrahepatic arteries and result in occlusions (Fig. 6). This explains the diffuse arterial narrowing and the branch vessel stenoses and occlusions seen angiographically (Figs. 3 and 4). The end stage of this process is occlusion of all intrahepatic arteries with subsequent thrombosis of the hepatic artery.
Five patients with acute rejection had an essentially normal hepatic arteriogram. The rejection in these patients may have been too mild to manifest itself angiographically. Another possible explanation for the absence of findings is response to immunosuppressive therapy. With appropriate antirejection treatment, the inflammatory infiltrate seen in acute rejection can clear within several days. Therefore, hepatic angiography would be normal.
Recently, difuse intrahepatic arterial-portal vein shunting has been suggested as a feature of rejection in liver transplants [18]. However, arteriovenous shunting secondary to rejection was not observed in our series. One patient with rejection demonstrated filling of peripheral portal branches late in the hepatic arterial phase, which was thought to be consistent with hepatic vein occlusion. Retransplantation was required. Pathologic examination of the hepatectomy specimen showed hepatic vein endophlebitis and occlusion.
The angiographic findings observed in our series are not specific for rejection. They have been seen in hepatic necrosis [18]. We have also observed these findings occasionally in acute cholangitis. Hepatic necrosis can occur secondarily to severe rejection, hepatitis, ischemic injury during harvesting, or posttransplantation hepatic artery thrombosis. The underlying etiology for necrosis is difficult if not impossible to determine pathologically. Therefore, angiography is not advocated as a specific diagnostic test for rejection. Angiography is indicated in the evaluation of possible posttransplantation vascular complications [7]. The findings should be interpreted in conjunction with all available clinical and laboratory information.
ACKNOWLEDGMENT
We thank Maggie Zdrodowski for manuscript preparation.
REFERENCES
- 1.Gartner JC, Jr, Zitelli BJ, Malatack JJ, Shaw BW, Iwatsuki S, Starzl TE. Orthotopic liver transplantation in children: two-year experience with 47 patients. Pediatrics. 1984;74:140–145. [PMC free article] [PubMed] [Google Scholar]
- 2.Van Thiel DH, Shade RR, Gavaler JS, Shaw BW, Jr, Iwatsuki S, Starzl TE. Medical aspects of liver transplantations. Hepatology. 1984;4 suppl 1:79S–83S. doi: 10.1002/hep.1840040721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Iwatsuki S, Shaw BW, Jr, Gordon RD. Orthotopic liver transplantation in 1984. Transplant Proc. 1985;17:250–258. [Google Scholar]
- 4.Starzl TE, Iwatsuki S, Shaw BW, Jr, Gordon RD, Esquivel CO. Immunosuppression and other nonsurgical factors in the improved results of liver transplantation. Semin Liver Dis. 1985;5:334–343. doi: 10.1055/s-2008-1040630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esquivel CO, Jaffe R, Gordon RD, Iwatsuki S, Shaw BW, Starzl TE. Liver rejection and its differentiation from other causes of graft dysfunction. Semin Liver Dis. 1985;5:369–374. doi: 10.1055/s-2008-1040634. [DOI] [PubMed] [Google Scholar]
- 6.Demetris AJ, Lasky S, Van Thiel DH, Starzl TE, Dekker A. Pathology of hepatic transplantation: a review of 62 adult allograft recipients immunosuppressed with a cyclosporine/steroid regimen. Am J Pathol. 1985;118:151–161. [PMC free article] [PubMed] [Google Scholar]
- 7.Wozney P, Zajko AB, Bron KM, Point S, Starzl TE. Vascular complications after liver transplantation: a 5-year experience. AJR. 1986;147:657–863. doi: 10.2214/ajr.147.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pichlmayr R, Brölsch Ch, Neuhaus P, et al. Report on 68 human orthotopic liver transplantation with special reference to rejection phenomena. Transplant Proc. 1983;15:1279–1283. [Google Scholar]
- 9.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Evolution of liver transplantation. Hepatology. 1982;2:614–636. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zajko AB, Campbell WL, Bron KM, et al. Cholangiography and interventional biliary radiology in adult liver transplantation. AJR. 1985;144:127–133. doi: 10.2214/ajr.144.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zajko AB, Bron KM, Campbell WL, Behal R, Van Thial DH, Starzl T. Percutaneous transhepatic cholangiography and biliary drainage after liver transplantation: a five-year experience. Gastrointest Radiol. doi: 10.1007/BF01885124. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segel MC, Zajko AB, Bowen A, et al. Hepatic artery thrombosis after liver transplantation: radiologic evaluation. AJR. 1986;146:137–141. doi: 10.2214/ajr.146.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasir B, Kamada N, Hohmeier H, Tamaki T, Salaman JR. Intraorgan pressure changes in rejecting kidney, liver, and heart transplants in the rat. Transplantation. 1985;40:17–21. doi: 10.1097/00007890-198507000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Groth CG, Porter KA, Otte JB, et al. Studies of blood flow and ultrastructural changes in rejecting and nonrejecting canine orthotopic liver homografts. Surgery. 1968;63:658–668. [PMC free article] [PubMed] [Google Scholar]
- 15.Castaneda-Zuniga W, Sibley R, Zollikofer C, et al. Renal artery aneurysms: an angiographic sign of transplant rejection. Radiology. 1980;136:333–335. doi: 10.1148/radiology.136.2.6996031. [DOI] [PubMed] [Google Scholar]
- 16.Rankin RN. Renal transplant rejection and aneurysm formation. Radiology. 1982;143:655–657. doi: 10.1148/radiology.143.3.7043646. [DOI] [PubMed] [Google Scholar]
- 17.Tzakis AG, Gordon RD, Shaw BW, Jr, Iwatsuki S, Starzl TE. Clinical presentation of hepatic artery thrombosis after liver transplantation in cyclosporine era. Transplantation. 1985;40:667–671. doi: 10.1097/00007890-198512000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardella JF, Castaneda-Zuniga WR, Hunter D, Young A, Amplatz K. Angiographic and interventional radiologic considerations in liver transplantation. AJR. 1986;146:143–153. doi: 10.2214/ajr.146.1.143. [DOI] [PubMed] [Google Scholar]