Abstract
The hexane, acetone, dichloromethane and methanol extracts of Combretum vendae A.E. van Wyk (Combretaceae), Commiphora harveyi (Engl.) Engl. (Burseraceae), Khaya anthotheca (Welm.) C.DC (Meliaceae), Kirkia wilmsii Engl. (Kirkiaceae), Loxostylis alata A. Spreng. ex Rchb. (Anacardiaceae), Ochna natalitia (Meisn.) Walp. (Ochnaceae) and Protorhus longifolia (Bernh. Ex C. Krauss) Engl. (Anacardiaceae) were screened for their antimicrobial activity. The test organisms included bacteria (Enterococcus faecalis, Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus), and fungi (Aspergillus fumigatus, Candida albicans, Cryptococcus neoformans, Microsporum canis and Sporothrix schenckii). A simple bioautographic procedure, involving spraying suspensions of the bacteria or fungi on thin layer chromatography (TLC) plates developed in solvents of varying polarities was used to detect the number of antibacterial and antifungal compounds present in the extracts. All the extracts had antimicrobial activity against at least one of the test microorganisms. This activity was denoted by white spots against a red-purple background on the TLC plates after spraying with tetrazolium violet. Twenty seven TLC plates; 9 for each solvent system and 3 different solvent systems per organism were tested in the bioautographic procedure. Of the bacteria tested, S. aureus was inhibited by the most compounds separated on the TLC plates from all the tested plants. Similarly, growth of the fungus C. neoformans was also inhibited by many compounds present in the extracts. Loxostylis alata appeared to be the plant extract with the highest number of inhibition bands when compared with other plants tested against both bacteria and fungi. This species was selected for in depth further study.
Keywords: Bioautography, Medicinal plants, Antifungal, Antibacterial, Synergism
Introduction
Despite the existence of conventional antimicrobial agents, resistant or multi-resistant strains of pathogenic microorganisms are continuously appearing, imposing the need for a thorough search for and development of new drugs (Silver and Bostian, 1993). Fungi and bacteria cause important human and animal diseases, especially in tropical and subtropical regions, and commonly occur in immunocompromised or immunodeficient patients. Over the last decade, there has been a renewed interest in plants; and the pharmaceutical industry considers plants as a viable option for the discovery of new leads (Soejarto, 1996). In fact, it is also estimated that natural products are implicated in the development of 44% of all new drugs, generally as leads for the preparation of semi-synthetic derivatives (Hostettmann et al., 2001).
In an effort to discover new lead compounds, many research groups screen plant extracts to detect secondary metabolites with relevant biological activities. In this regard, several bioassays were developed for screening purposes (Hostettmann, 1991).
Once the technique has been mastered, bioautography is a highly efficacious assay for the detection of antimicrobial compounds because it allows localization of activity even in a complex matrix, and therefore facilitates the target-directed isolation of the active constituents (Rahalison et al., 1991). Bioautography has enabled rapid progress for quick detection of new antimicrobial compounds from plants and other natural products. This technique allows the localization of antimicrobial activity directly on a chromatographic plate where the organism is applied (Navarro et al., 1998). The method is fast, cheap, and permits a better bioassay-directed fractionation of bioactive compounds (Hamburger and Cordell, 1987). Bioautography is particularly important to avoid the time-consuming isolation of inactive compounds. TLC bioautographic methods combine chromatographic separation and in situ activity determination facilitating the localization and target-directed isolation of active constituents in a mixture (Shahverdi et al., 2007).
A number of bioautographic assays have been developed, which can be divided into three groups (Rios et al., 1988). These include direct bioautography; where the microorganisms grow directly on thin-layer chromatography (TLC) plates, contact bioautography; where the antimicrobial compounds are transferred from the TLC plate to an inoculated agar plate through direct contact, and agar overlay or immersion bioautography; where a seeded agar medium is applied onto the TLC plate. The latter technique can be considered as a hybrid of direct and contact bioautography (Islam et al., 2003).
In the ongoing tree screening project of the Phytomedicine Programme, University of Pretoria (www.up.ac.za/phyto), plant species that had excellent activity against Candida albicans depicted by low minimum inhibitory concentrations (≤ 0.08 mg/ml) in a broth microdilution assay (Eloff, 1998) were selected for evaluation of their potential antimicrobial activity against other animal fungal and bacterial organisms. The antibacterial and antimicrobial activity of different extracts of the same plant species have been described elsewhere (Suleiman et al., 2009). To select the most promising plant species for further investigation, the number of antimicrobial compounds plays a critically important role to simplify bioassay-guided isolation. In this contribution the use of bioautography will be used to select the most promising plant species.
Materials and methods
Plant collection
Combretum vendae, Commiphora harveyi, Khaya anthotheca and Loxostylis alata leaves were collected at the University of Pretoria Botanical Garden, South Africa. Kirkia wilmsii and Ochna natalitia were collected at the Lowveld National Botanical Garden in Nelspruit, South Africa. All plant leaves were collected in summer (November 2006) between 9:30 am and 12:30 pm. Samples of the plants were identified by tree name tags and were authenticated by Ms Lorraine Middleton and Magda Nel at the Botanical Garden of the University of Pretoria. Voucher specimens of the plants were deposited at the Schweikert Herbarium of the Department of Plant Sciences, University of Pretoria, South Africa. The voucher specimen numbers and traditional use of the selected species are provided elsewhere (Suleiman et al., 2009)
Plant storage
Immediately after collection and transportation to our laboratory, leaves were separated from stems and dried at room temperature with good ventilation. The dried leaves were milled to a fine powder in a Macsalab mill (Model 200 LAB), Eriez®, Bramley, and stored at room temperature in closed containers in the dark until use.
Plant extraction
Plant samples from each species were separately extracted by weighing four aliquots of 1 g of finely ground plant material and extracting with 10 ml of either acetone, hexane, dichloromethane (DCM) or methanol (technical grade-Merck) in centrifuge tubes. Tubes were vigorously shaken for 1 hour in a Labotec model 20.2 shaking machine at a moderate speed. Extracting plant powdered material at low speed for a longer period allows greater penetration of the solvent into the plant tissues which allows more of the plant compounds to be extracted (Silva et al., 1998). After centrifuging at 3500 x g for 10 min, the supernatant was decanted into pre-weighed labelled containers. The whole process was repeated three times to exhaustively extract the plant material and the extracts were combined. The solvent was removed under a stream of air in a fume cupboard at room temperature to quantify the extraction.
Microorganisms and medium
The fungal organisms used in this study were moulds (Aspergillus fumigatus and Microsporum canis), yeasts (Candida albicans and Cryptococcus neoformans) and a thermally dimorphic fungus (Sporothrix schenckii). All fungal organisms were isolated from clinical cases that were not treated prior to sampling in the Department of Veterinary Tropical Diseases, Faculty of Veterinary Science, University of Pretoria. A. fumigatus was isolated from a chicken, C. albicans from a Goldian finch, C. neoformans from a cheetah, M. canis from a cat suffering from dermatophytosis and Sporothrix schenckii from a horse with cutaneous lymphangitis. These fungi represent the most common and important disease-causing fungi of animals (Masoko et al., 2005). Sabouraud dextrose (SD) agar (Oxoid, Basingstoke, UK) was used as the maintenance growth medium for all the fungal strains used, and the fungi were cultured in SD broth.
The bacteria used were the Gram-positive bacteria: Staphylococcus aureus (ATCC 29213) and Enterococcus faecalis (ATCC 29212), and the Gram-negative bacteria: Pseudomonas aeruginosa (ATCC 27853) and Escherichia coli (ATCC 25922). All bacterial cultures were maintained on Mueller Hinton (MH) agar (Oxoid, Basingstoke, UK) at 4°C and cultured in MH broth at 37°C.
Phytochemical analysis
Chemical constituents of the extracts were separated on aluminium-backed thin layer chromatography (TLC) plates (Merck, silica gel 60 F254). The TLC plates were developed under saturated conditions with one of the three eluent systems developed in our laboratory, i.e., ethyl acetate/methanol/water (40:5.4:5): [EMW] (polar/neutral); chloroform/ethyl acetate/formic acid (5:4:1): [CEF] (intermediate polarity/acidic); benzene/ethanol/ammonia hydroxide (18:2:0.2): [BEA] (non-polar/basic) (Kotze and Eloff, 2002). Separated chemical compounds were detected using acidified vanillin (0.1 g vanillin: 28 ml methanol:1ml sulphuric acid) as a spray. After spraying, the chromatograms were heated at 110°C in an incubator to allow for optimal colour development.
Bioautography
Ten µl (10 mg/ml) of each extract were loaded onto TLC plates in a narrow band and eluted using the three different mobile solvent systems (CEF, BEA and EMW). The developed plates were dried under a stream of fast moving air for 5 days to remove traces of solvent on the plates. One week old cultures of fungal organisms grown on SD agar were each transferred into 250 ml of freshly prepared SD broth using a sterile swab. Densities of fungal cultures used for A. fumigatus, C. albicans, C. neoformans, M. canis and S. schenckii were approximately 8 ×106, 3 ×106, 3 ×106, 2 ×105 and 1 ×105 cells/ml respectively, In the case of bacteria, overnight cultures grown on MH broth were used and the densities of bacterial organism used for E. faecalis, E. coli, P. aeruginosa and S. aureus were approximately 2 ×1010, 3 ×1011, 5 ×1013 and 3 ×1012 cfu/ml, respectively. The prepared chromatograms were sprayed with the fungal or bacterial suspension until wet. This process was carried out in a biosafety Class II cabinet (Labotec, SA) for fungi, and Laminar flow cabinet (Labotec, SA) for bacteria. Thereafter, the plates were incubated overnight at 35°C and 100% relative humidity in the dark and then sprayed with a 2 mg/ml solution of p-iodonitrotetrazolium violet (Sigma®) (INT) (Begue and Klein, 1972) and further incubated overnight or longer in the case of S. schenckii and M. canis. White bands indicate where reduction of INT to the coloured formazan did not take place due to the presence of compounds that inhibited the growth of tested organisms.
Results and discussion
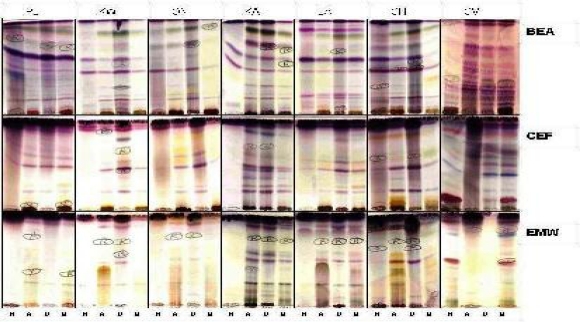
Phytochemical screening revealed the presence of varied chemical components in the different extracts of the plants. This is notable from the different colour changes depicted by individual compounds due to their reaction with the spray reagent used (vanillin/sulphuric acid) (Figure 1).
Figure 1.
Chromatograms of extracts of hexane (H), acetone (A), dichloromethane (D) and methanol (M) developed in Benzene/Ethanol/Ammonia hydroxide: 90:10:1 [BEA] (non-polar/basic), Chloroform/Ethyl acetate/Formic acid: 5:4:1 [CEF] (intermediate polar/acidic) and Ethyl acetate/Methanol/Water: 40:5.4:4 [EMW] (polar/neutral) and sprayed with vanillin in concentrated sulphuric acid. PL = P. longifolia, KW = Kirkia wilmsii, ON = Ochna natalitia, KA= Khaya anthotheca, LA = Loxostylis alata, CH = Commiphora harveyi, CV = Combretum vendae.
For example, terpenes exhibit red or blue colouration on the chromatograms when sprayed with vanillin/sulphuric acid (Gibbons and Gray, 1998). Similarities exist between chemical compositions of the components of extracts separated using the same solvent system (Figure 1). Dellar et al. (1994) reported the isolation of antifungal sesquiterpenes aristolen-2-one and prostatherol from 2 species of Prostanthera (Labiatae). Studies into the effects of terpenoids on isolated bacterial membranes revealed their site of action to be at the phospholipid bilayer. They affect bacterial processes that include the inhibition of electron transport, protein translocation, phosphorylation steps and other enzyme-dependent reactions (Knobloch et al. 1986). Perhaps similar mechanisms of action were responsible for the antimicrobial actions of the plant extracts under study.
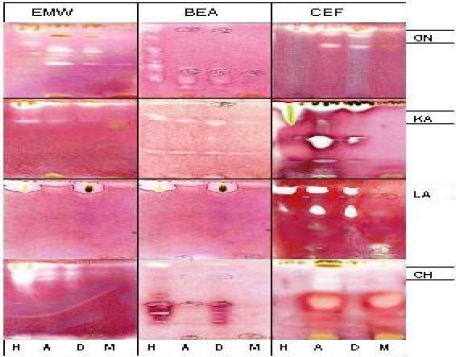
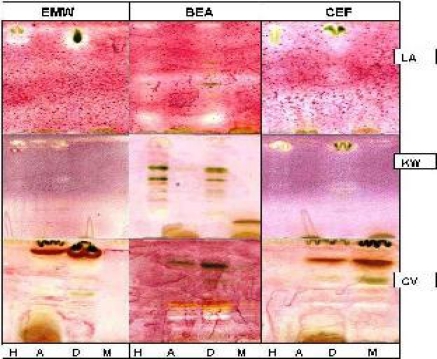
The appearance of white areas against a purple-red background on the chromatograms denotes inhibition of growth of the bacteria (Figure 2) or fungi (Figure 3) due to presence of compound(s) that inhibit their growth. Actively growing microorganisms have the ability to reduce INT to a purple-red colour (Begue and Klein, 1972). In the presence of active plant compounds on the chromatograms, the growth of the organism is inhibited.
Figure 2.
Hexane (H), acetone (A), dichloromethane (D), and methanol (M) extracts of Ochna natalitia (ON), Khaya anthotheca (KA) Loxostylis alata (LA) and Commiphora harveyi (CH) separated on TLC plates using EMW, BEA and CEF, sprayed with bacterial organisms and 24 hrs later by INT. White areas indicate inhibition of bacterial growth by compounds of the plant extract after 60 mins of incubation at 37°C. ON and KA were sprayed with E. coli while LA and CH were sprayed with S. aureus.
Figure 3.
Hexane (H), acetone (A), dichloromethane (D), and methanol (M) extracts of Loxostylis alata (LA), Kirkia wilmsii (KW) and Combretum vendae (CV) separated on TLC plates using EMW, BEA and CEF, sprayed with fungal organisms and 24 hours later by INT. White areas indicate inhibition of fungal growth by compounds of the plant extract after 24 hrs of incubation at 37 °C. LA, KW and CV were sprayed with A. fumigatus, C. albicans and S.schenkii, respectively.
An important factor in quantifying the movement of a compound on a stationary phase e.g. silica with a certain solvent system is the Rf (retardation factor) value and is the ratio of the distance moved by the compound from its origin to the movement of the solvent from the origin. Rf Due to the difference in polarity of the different solvent systems, compounds that had relatively high Rf values in polar solvents e.g. EMW, had low Rf values in non-polar solvents like BEA. By using these three solvent systems, compounds with a wide range of polarities can be separated. Because the Rf value is constant for the same compound under defined conditions, the presence of clear bands with the same Rf value may mean that the same compounds are probably responsible for the antimicrobial activity in the same extract tested against different microorganisms. This would suggest non-selective antimicrobial activity. The Rf values of the antimicrobial compounds present in different extracts eluted with different solvents against S. aureus, E. faecalis, E. coli, P. aeruginosa, A. fumigatus, S. schenckii, M. canis, C. neoformans and C. albicans are presented in Tables 1, 2, 3, 4, 5, 6, 7, 8 and 9, respectively. In some cases, no inhibition of microbial growth was observed. The absence of activity could be due to evaporation of the active compounds, photo-oxidation or due to very little amount of the active compound (Masoko and Eloff, 2005). It is also possible that synergism plays a major role in extracts that were active when the minimum inhibitory concentration of the mixture was determined, but the separated compounds had no antimicrobial activity based on bioautography. When all the compounds with activity based on bioautography were isolated and characterized the frequently had a much lower activity than could be expected indicating the presence of synergism (Eloff et al., 2008).
Table 1.
Inhibition of growth on bioautographic TLC plates by 24 extracts of 7 South African tree leaves against S. aureus
| Plant | Solvent system | Extracts | Rf values | Active bands | Total |
| Hexane | 0.92, 0.83, 0.47, 0.26 | 4 | |||
| Acetone | 0.13 | 1 | |||
| BEA | DCM | 0.92, 0.83, 0.47, 0.26, 0.13 |
5 | ||
| Hexane | 0.53, 0.51 | 2 | |||
| L. alata | CEF | Acetone | 0.1 | 1 | 21 |
| DCM | 0.53, 0.51, 0.2 | 3 | |||
| Hexane | 0.31, 0.2, | 2 | |||
| EMW | Acetone | 0.83 | 1 | ||
| DCM | 0.31,0.2 | 2 | |||
| Hexane | 0.172 | 1 | |||
| Acetone | 0.27 | 1 | |||
| P. longifolia | BEA | DCM | 0.26 | 1 | 8 |
| Hexane | 0.31 | 1 | |||
| CEF | Acetone | 0.34 | 1 | ||
| Hexane | 0.72, 0.81 | 2 | |||
| EMW | Acetone | 0.63 | 1 | ||
| K. wilmsii | BEA | Acetone | 0.23 | 1 | 5 |
| Methanol | 0.62 | 1 | |||
| Acetone | 0.23 | 1 | |||
| Acetone | 0.54, 0.08 | 2 | |||
| CEF | Methanol | 0.08 | 1 | ||
| Hexane | 0.95, 0.32 | 2 | |||
| C. harveyi | BEA | DCM | 0.95, 0.32, 0.21 | 3 | 11 |
| Hexane | 0.41 | 1 | |||
| CEF | DCM | 0.41 | 1 | ||
| Methanol | 0.32 | 1 | |||
| Hexane | 0.31 | 1 | |||
| EMW | DCM | 0.31 | 1 | ||
| Methanol | 0.72 | 1 | |||
| O. natalitia | BEA | DCM | 0.1 | 1 | 5 |
| Hexane | 0.65 | 1 | |||
| CEF | Acetone | 0.76 | 1 | ||
| DCM | 0.97 | 1 | |||
| EMW | Acetone | 0.97 | 1 | ||
| Hexane | 0.94 | 1 | |||
| K. anthotheca | BEA | DCM | 0.94 | 1 | 4 |
| Hexane | 0.2 | 1 | |||
| CEF | DCM | 0.2 | 1 | ||
| Hexane | 0.21 | 1 | |||
| Acetone | 0.21 | 1 | |||
| DCM | 0.12 | 1 | |||
| C. vendae | BEA | Methanol | 0.32 | 1 | 8 |
| Hexane | 0.74 | 1 | |||
| Acetone | 0.41 | 1 | |||
| CEF | DCM | 0.65 | 1 | ||
| Methanol | 0.41 | 1 |
Table 2.
Inhibition of growth on bioautographic TLC plates by 24 extracts of 7 South African tree leaves against E. faecalis.
| Plant | Solvent system |
Extracts | Rf values | Active bands | Total |
| Hexane | 0.78, 0.82 | 2 | |||
| BEA | DCM | 0.82 | 1 | ||
| L. alata | Acetone | 0.16 | 1 | 6 | |
| DCM | 0.67 | 1 | |||
| EMW | Acetone | 0.45 | 1 | ||
| BEA | Acetone | 0.56 | 1 | ||
| P. longifolia | CEF | Acetone | 0.98 | 1 | 2 |
| Hexane | 0.34 | 1 | |||
| K. wilmsii | BEA | Acetone | 0.56 | 1 | 3 |
| Methanol | 0.67 | 1 | |||
| Hexane | 0.91 | 1 | |||
| O. natalitia | CEF | Acetone | 0.91, 0.57 | 2 | 3 |
| BEA | Acetone | 0.24 | 1 | ||
| K. anthotheca | Hexane | 0.94 | 1 | 3 | |
| CEF | Acetone | 0.76 | 1 | ||
| C. vendae | BEA | Hexane | 0.56, 0.67 | 2 | 3 |
Table 3.
Inhibition of growth on bioautographic TLC plates by 24 extracts of 7 South African tree leaves against E. coli
| Plant | Solvent system |
Extracts | Rf values | Active bands | Total |
| BEA | Hexane | 0.62 | 1 | ||
| DCM | 0.62, 0.21 | 2 | 5 | ||
| L. alata | CEF | DCM | 0.67 | 1 | |
| EMW | DCM | 0.98 | 1 | ||
| Acetone | 0.76 | 1 | |||
| BEA | DCM | 0.95 | 1 | ||
| Acetone | 0.33 | 1 | |||
| P. longifolia | CEF | DCM | 0.33 | 1 | 6 |
| Hexane | 0.23 | 1 | |||
| EMW | DCM | 0.23 | 1 | ||
| CEF | Methanol | 0.87 | 1 | ||
| K. wilmsii | Acetone | 0.24 | 1 | 2 | |
| BEA | DCM | 0.95 | 1 | ||
| O. natalitia | Hexane | 0.95 | 1 | 3 | |
| EMW | DCM | 0.27 | 1 | ||
| Hexane | 0.93 | 1 | |||
| K. anthotheca | CEF | Acetone | 0.93, 0.67 | 2 | 5 |
| DCM | 0.93 | 1 | |||
| EMW | Methanol | 0.34 | 1 | ||
| Hexane | 0.40, 0.2 | 2 | |||
| BEA | Acetone | 0.13 | 1 | ||
| DCM | 0.26 | 1 | |||
| C. vendae | Hexane | 0.87 | 1 | 8 | |
| Acetone | 0.98 | 1 | |||
| DCM | 0.98 | 1 | |||
| Methanol | 0.56 | 1 |
Table 4.
Inhibition of growth on bioautographic TLC plates by 24 extracts of 7 South African tree leaves against P. aeruginosa
| Plant | Solvent system |
Extracts | Rf values | Active bands | Total |
| L. alata | EMW | Acetone | 0.89 | 1 | 1 |
| Hexane | 0.90 | 1 | |||
| P. longifolia | CEF | Acetone | 0.90 | 1 | 3 |
| DCM | 0.90 | 1 | |||
| Hexane | 0.1 | 1 | |||
| BEA | DCM | 0.1 | 1 | ||
| Hexane | 0.67 | 1 | |||
| K. wilmsii | CEF | Acetone | 0.67 | 1 | 8 |
| DCM | 0.67 | 1 | |||
| Hexane | 0.85 | 1 | |||
| EMW | Acetone | 0.85 | 1 | ||
| DCM | 0.85 | 1 | |||
| O. natalitia | CEF | Methanol | 0.56 | 1 | 1 |
| BEA | DCM | 0.27 | 1 | ||
| K. anthotheca | Hexane | 0.93 | 1 | 3 | |
| CEF | Acetone | 0.42 | 1 | ||
| Hexane | 0.67 | 1 | |||
| C. vendae | CEF | Acetone | 0.54 | 1 | 3 |
| DCM | 0.45 | 1 |
Table 5.
Inhibition of growth on bioautographic TLC plates by 24 extracts of 7 South African tree leaves against A. fumigatus
| Plant | Solvent system |
Extracts | Rf values | Active bands | Total |
| Hexane | 0.13, 0.54, 0.92 | 3 | |||
| BEA | DCM | 0.13, 0.54, 0.92 | 3 | ||
| Hexane | 0.63 | 1 | 10 | ||
| L. alata | CEF | DCM | 0.63 | 1 | |
| Hexane | 0.81 | 1 | |||
| EMW | DCM | 0.81 | 1 | ||
| BEA | DCM | 0.2 | 1 | ||
| P. longifolia | CEF | DCM | 0.83 | 1 | 3 |
| EMW | DCM | 0.90 | 1 | ||
| Hexane | 0.33 | 1 | 3 | ||
| C. harveyi | BEA | DCM | 0.33, 0.46 | 2 | |
| Hexane | 0.26 | 1 | |||
| BEA | Acetone | 0.21 | 1 | ||
| DCM | 0.21 | 1 | 8 | ||
| CEF | Hexane | 0.56 | 1 | ||
| K. anthotheca | DCM | 0.53 | 1 | ||
| Hexane | 0.93 | 1 | |||
| EMW | Acetone | 0.93 | 1 | ||
| DCM | 0.93 | 1 | |||
| CEF | Hexane | 0.94 | 1 | 2 | |
| C. vendae | EMW | Acetone | 0.81 | 1 |
Table 6.
Inhibition of growth on bioautographic TLC plates by 24 extracts of 7 South African tree leaves against S. schenckii
| Plant | Solvent system | Extracts | Rf values | Active bands | Total |
| BEA | DCM | 0.89, 0.43 | 2 | ||
| Methanol | 0.43 | 1 | |||
| L. alata | Hexane | 0.58 | 1 | ||
| CEF | Acetone | 0.58 | 1 | 7 | |
| DCM | 0.58 | 1 | |||
| EMW | Hexane | 0.92 | 1 | ||
| P. longifolia | BEA | DCM | 0.62, 0.46 | 2 | |
| Methanol | 0.46 | 1 | 3 | ||
| Acetone | 0.31 | 1 | |||
| K. wilmsii | EMW | Methanol | 0.31 | 1 | 2 |
| Hexane | 0.96, 0.55, 0.44 | 3 | |||
| C. harveyi | BEA | Methanol | 0.96 | 1 | |
| Hexane | 0.84 | 1 | 8 | ||
| CEF | Acetone | 0.68 | 1 | ||
| Hexane | 0.94 | 1 | |||
| EMW | Methanol | 0.12 | 1 | ||
| O. natalitia | EMW | Acetone | 0.44 | 1 | 1 |
| Hexane | 0.96 | 1 | |||
| K. anthotheca | CEF | Acetone | 0.96 | 1 | 3 |
| DCM | 0.96 | 1 | |||
| DCM | 0.89 | 1 | |||
| BEA | Methanol | 0.89 | 1 | ||
| Acetone | 0.58, 0.32 | 2 | 6 | ||
| C. vendae | CEF | DCM | 0.58, 0.32 | 2 |
Table 7.
Inhibition of growth on bioautographic TLC plates by 24 extracts of 7 South African tree leaves against M. canis
| Plant | Solvent system | Extracts | Rf values | Active bands | Total |
| Acetone | 0.31, 0.79 | 2 | |||
| BEA | DCM | 0.31 | 1 | ||
| L. alata | Hexane | 0.27, 0.42 | 2 | 6 | |
| EMW | Acetone | 0.94 | 1 | ||
| Hexane | 0.87 | 1 | |||
| P. longifolia | CEF | Acetone | 0.87, 0.41 | 2 | |
| DCM | 0.41 | 1 | 5 | ||
| EMW | Acetone | 0.91 | 1 | ||
| DCM | 0.77 | 1 | |||
| K. wilmsii | BEA | 2 | |||
| CEF | Acetone | 0.57 | 1 | ||
| O. natalitia | BEA | Acetone | 0.73 | 1 | 1 |
| Hexane | 0.91 | 1 | |||
| K. anthotheca | BEA | Acetone | 0.63 | 1 | 3 |
| EMW | Hexane | 0.29 | 1 | ||
| Hexane | 0.33 | 1 | |||
| BEA | Acetone | 0.29, 0.33 | 2 | ||
| C. vendae | DCM | 0.33 | 1 | 6 | |
| Acetone | 0.67 | 1 | |||
| CEF | DCM | 0.67 | 1 |
Table 8.
Inhibition of growth on bioautographic TLC plates by 24 extracts of 7 South African tree leaves against C. neoformans
| Plant | Solvent system | Extracts | Rf values | Active bands | Total |
| Hexane | 0.23, 0.38 | 2 | |||
| BEA | Acetone | 0.92 | 1 | ||
| DCM | 0.21 | 1 | |||
| Hexane | 0.90 | 1 | |||
| L. alata | CEF | Acetone | 0.90 | 1 | 11 |
| DCM | 0.90 | 1 | |||
| Methanol | 0.90 | 1 | |||
| Hexane | 0.77 | 1 | |||
| EMW | Acetone | 0.26 | 1 | ||
| DCM | 0.85 | 1 | |||
| Hexane | 0.26, 0.41 | 2 | |||
| CEF | Acetone | 0.26, 0.41, 0.52 | 3 | ||
| P. longifolia | DCM | 0.26 | 1 | 8 | |
| Hexane | 0.98 | 1 | |||
| EMW | Acetone | 0.98 | 1 | ||
| Hexane | 0.84 | 1 | |||
| BEA | Acetone | 0.14 | 1 | ||
| K. wilmsii | DCM | 0.96 | 1 | 5 | |
| CEF | Acetone | 0.72 | 1 | ||
| EMW | DCM | 0.16 | 1 | ||
| Hexane | 0.93 | 1 | |||
| C. harveyi | BEA | Acetone | 0.18 | 1 | |
| Acetone | 0.90 | 1 | 5 | ||
| CEF | DCM | 0.90 | 1 | ||
| Methanol | 0.90 | 1 | |||
| Hexane | 0.97 | 1 | |||
| BEA | 2 | ||||
| O. natalitia | EMW | Hexane | 0.13 | 1 | |
| BEA | Hexane | 0.92 | 1 | ||
| Hexane | 0.94 | 1 | |||
| K. anthotheca | CEF | Acetone | 0.94 | 1 | 4 |
| EMW | Hexane | 0.21 | 1 | ||
| Hexane | 0.96 | 1 | |||
| BEA | Acetone | 0.96 | 1 | ||
| C. vendae | DCM | 0.13 | 1 | 5 | |
| CEF | DCM | 0.67 | 1 | ||
| EMW | Acetone | 0.12 | 1 |
Table 9.
Inhibition of growth on bioautographic TLC plates by 24 extracts of 7 South African tree leaves against C. albicans
| Plant | Solvent system | Extracts | Rf values | Active bands | Total |
| Hexane | 0.87, 0.21 | 2 | |||
| BEA | DCM | 0.21 | 1 | ||
| L. alata | Hexane | 0.93 | 1 | 6 | |
| CEF | Acetone | 0.93 | 1 | ||
| EMW | Hexane | 0.26 | 1 | ||
| BEA | Methanol | 0.21 | 1 | ||
| P. longifolia | CEF | Acetone | 0.76 | 1 | 3 |
| EMW | Hexane | 0.42 | 1 | ||
| BEA | Hexane | 0.93 | 1 | ||
| K. wilmsii | CEF | Hexane | 0.74 | 1 | 2 |
| Hexane | 0.54 | 1 | |||
| BEA | Acetone | 0.54 | 1 | ||
| C. harveyi | DCM | 0.30 | 1 | 6 | |
| Hexane | 0.93 | 1 | |||
| CEF | Acetone | 0.93 | 1 | ||
| DCM | 0.93 | 1 | |||
| BEA | Methanol | 0.34 | 1 | ||
| O. natalitia | CEF | Acetone | 0.94 | 1 | 3 |
| EMW | Acetone | 0.56 | 1 | ||
| BEA | Hexane | 0.59 | 1 | ||
| Acetone | 0.59 | 1 | |||
| K. anthotheca | DCM | 0.59 | 1 | 5 | |
| CEF | Hexane | 0.93 | 1 | ||
| Acetone | 0.93 | 1 | |||
| Hexane | 0.34 | 1 | |||
| BEA | Acetone | 0.47 | 1 | ||
| DCM | 0.59 | 1 | |||
| C. vendae | Hexane | 0.71 | 1 | 8 | |
| Acetone | 0.63 | 1 | |||
| CEF | DCM | 0.29 | 1 | ||
| Methanol | 0.88, 0.29 | 2 |
Most of the antimicrobial agents detected in this study were present in extracts of relatively non-polar solvents. These findings agreed with previously published results (Masoko and Eloff, 2005, 2006) that the substances responsible for the antimicrobial activity were mainly non-polar in nature. However, the acetone and methanol fractions of Punica granatum and Delonix regia in contrast to the benzene fraction had good activity against methicillin resistant S. aureus (Aqil et al., 2005).
Loxostylis alata had the highest number of inhibition bands (35 and 40) against bacteria and fungi, respectively, while O. natalitia had the lowest number of inhibition bands (18 and 11 respectively) against bacteria and fungi., C. vendae, P. longifolia, K. anthotheca, C. harveyi, K. wilmsii and O. natalitia had 22, 19, 15, 11, 18 and 12 inhibition bands, respectively, against bacteria, and 27, 22, 38, 22, 11 and 7 bands, respectively, against fungi.
In another study, the methanol extract of the stem bark of Khaya anthotheca was reported to be very active against the fungus Candida krusei (Hamza et al., 2006). Many compounds isolated from Combretum and Terminalia spp. (Combretaceae) have antimicrobial actvity against bacteria and fungi (Eloff et al., 2008). Perhaps similar chemical constituents may be responsible for the antimicrobial action of Combretum vendae a member of the same family. Some triterpenoid and phenolic compounds isolated from Commiphora opobalsamum (Burseraceae) have antimicrobial activity (Abbas et al., 2007).
It is important to realize that bioautography is not a quantitative measure of antimicrobial activity. It only indicates the number of compounds that were separated with antimicrobial activity. The fact that of the bacteria tested, S. aureus had the highest number of inhibition bands does not mean that this was the most susceptible organism. Similarly, C. neoformans with the most inhibition bands against fungi does not necessarily have the highest susceptibility among fungal organisms. Some of the compounds are active against both bacteria and fungi, while others are selective in their activity. It is possible that compounds that have activity against all the tested organisms possess a broad antimicrobial action or they may even be general metabolic toxins that could be toxic to animals as well.
The absence of bioactivity in some of the plant extracts with this screening method does not preclude that they may be active, as synergistic or additive interactions of plant extracts or phytocompounds is the basis of activity of several herbal formulations (Ahmad and Aqil, 2007).
Conclusion
Although some experience is required to obtain good bioautograms especially with fungi, the method is very useful in isolating compounds with antimicrobial activity because the Rf of the active compound can be used in bioassay guided fractionation in stead of requiring labour intensive deterimination of activity of fractions. This also helps in ensuring that the compound isolated at the end is the same compound that was present in the extract and is not an artifact of the isolation procedure. If an extract had a high antimicrobial activity and this was caused by an interaction of compounds that were not active individually attemps to isolate single active compounds would be futile, this is an important procedure to select species for further work. Based on these results Loxostylis alata was selected for in depth study. One should however keep the possibility that volatile compounds may have evaporated from the chromatogram in mind when using this approach
Acknowledgements
The NRF provided funding and a PhD bursary to the senior author. Dr Jackie Picard, Department of Veterinary Tropical Diseases provided the microbial cultures and the use of the facilities for this work.
References
- 1.Abbas FA, Massarany SM, Khan S, Al-howiriny TA, Mossa JS, Abourashed EA. Phytochemical and biological studies on Saudi Commiphora opobalsamum L. Nat Prod Res. 2007;21:383–391. doi: 10.1080/14786410600942025. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad I, Aqil F. In vitro efficacy of bioactive extracts of 15 medicinal plants against ESβL-producing multidrug-resistant enteric bacteria. Microbiol Res. 2007;162:264–275. doi: 10.1016/j.micres.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Aqil F, Khan MSA, Owais M, Ahmad I. Effect of certain bioactive plant extracts on clinical isolates of β-lactamase producing methicillin resistant Staphylococcus aureus. J Basic Microbiol. 2005;45:106–114. doi: 10.1002/jobm.200410355. [DOI] [PubMed] [Google Scholar]
- 4.Begue WJ, Klein RM. The use of tetrazolium salts in bioautographic procedure. J Chromato. 1972;88:182–184. doi: 10.1016/s0021-9673(00)92965-0. [DOI] [PubMed] [Google Scholar]
- 5.Dellar JE, Cole MD, Gray AI, Gibbons S, Waterman PG. Antimicrobial sesquiterpenes from Prostanthera aff. melissifolia and P. rotundifolia. Phytochem. 1994;36:957–960. doi: 10.1016/s0031-9422(00)90471-0. [DOI] [PubMed] [Google Scholar]
- 6.Eloff JN. A sensitive and quick method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998;64:711–714. doi: 10.1055/s-2006-957563. [DOI] [PubMed] [Google Scholar]
- 7.Eloff JN, Katerere DR, McGaw LJ. The biological activity and chemistry of the southern African Combretaceae. J Ethnopharmacol. 2008;119:686–699. doi: 10.1016/j.jep.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 8.Gibbons S, Gray AI. Isolation by planar chromatography. In: Cannel RJP, editor. Natural Products Isolation. New Jersey: Humana Press; 1998. pp. 209–245. [Google Scholar]
- 9.Hamburger MO, Cordell GA. A direct bioautographic assay of compounds possessing antimicrobial activity. J Nat Prod. 1987;50:19. doi: 10.1021/np50049a003. [DOI] [PubMed] [Google Scholar]
- 10.Hamza OJM, van den Bout-van den Beukel CJP, Matee MIN, Moshi MJ, Mikx FHM, Selemani HO, Mbwambo ZH, Van der Ven AJAM, Verweij PE. Antifungal activity of some Tanzanian plants used traditionally for the treatment of fungal infections. J Ethnopharmacol. 2006;108:124–132. doi: 10.1016/j.jep.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 11.Hostettmann K. Methods in Plant Biochemistry. Vol. 6. San Diego: Academic Press; 1991. Assays for bioactivity. 360 pp. [Google Scholar]
- 12.Hostettmann K, Wolfender J, Terreaux C. Modern screening techniques for plant extracts. Pharm Biol. 2001;39(Supplement):18–32. doi: 10.1076/phbi.39.s1.18.0008. [DOI] [PubMed] [Google Scholar]
- 13.Islam N, Parveen S A, Nakazawa N, Marston A, Hostettmann K. Bioautography with the fungus Valsa ceratosperma in the search for antimycotic agents. Pharm Biol. 2003;41:637–640. [Google Scholar]
- 14.Knobloch K, Weigand N, Weis HM, Vigenschow H. Progress in Essential Oil Research. Berlin: Walter de Gruyther; 1986. p. 429. [Google Scholar]
- 15.Kotze M, Eloff JN. Extraction of antibacterial compounds from Combretum microphyllum (Combretaceae) S Afr J Bot. 2002;68:62–67. [Google Scholar]
- 16.Masoko P, Eloff JN. The diversity of antifungal compounds of six South African Terminalia species (Combretaceae) determined by bioautography. Afr J Biotechnol. 2005;4:1425–1431. [Google Scholar]
- 17.Masoko P, Eloff JN. Bioautography indicates the multiplicity of antifungal compounds from twenty-four southern African Combretum species (Combretaceae) Afr J Biotechnol. 2006;5:1625–1647. [Google Scholar]
- 18.Masoko P, Picard J, Eloff JN. Antifungal activities of six South African Terminalia species (Combretaceae) J Ethnopharmacol. 2005;99:301–308. doi: 10.1016/j.jep.2005.01.061. [DOI] [PubMed] [Google Scholar]
- 19.Navarro V, Rojas G, Delgado G, Lozoya X. Antimicrobial compounds detected in Bocconia arborea extracts by direct bioautographic method. Arch Med Res. 1998;29:191–194. [PubMed] [Google Scholar]
- 20.Rahalison L, Hamburger M, Hostettmann K, Monod M, Frenk E. A bioautographic agar overlay method for the detection of antifungal compounds from higher plants. Phyto Anal. 1991;2:199–203. [Google Scholar]
- 21.Rios JL, Recio MC, Villar A. Screening methods for natural products with antimicrobial activity: A review of the literature. J Ethnopharmacol. 1988;23:127–149. doi: 10.1016/0378-8741(88)90001-3. [DOI] [PubMed] [Google Scholar]
- 22.Shahverdi AR, Abdolpour F, Monsef-Esfahani HR, Farsam H. A TLC bioautographic assay for the detection of nitrofurantoin resistance reversal compound. J Chromato B. 2007;850:528–530. doi: 10.1016/j.jchromb.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Silva GL, Lee I, Kinghorn D. Special problems with the extraction of plants. In: Cannel RJP, editor. Natural Products Isolation. New Jersey: Humana Press; 1998. pp. 343–363. [Google Scholar]
- 24.Silver LL, Bostian KA. Discovery and development of new antibiotics: the problem of antibiotic resistance. Antimicro Agents Chemother. 1993;37:377–383. doi: 10.1128/aac.37.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soejarto DD. Biodiversity prospecting and benefit-sharing: perspective from the field. J Ethnopharmacol. 1999;51:1–15. doi: 10.1016/0378-8741(95)01345-8. [DOI] [PubMed] [Google Scholar]
- 26.Suleiman MM, McGaw LJ, Naidoo V, Eloff JN. The antimicrobial activity of leaf extracts of selected South African trees. In Press South African Journal of Botany. 2009 doi: 10.1016/j.sajb.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]