Abstract
It has been reported that the water extract of the whole unripe fruit of Momordica charantia can significantly reduce blood glucose levels. However the safety of its use during pregnancy has not been fully investigated. The aim of this investigation is to determine the safety of this extract during pregnancy. The water extract of the unripe fruit was given to pregnant Sprague Dawley rats on days 7, 8, 9, 10, 11, 12, 13 and 14 of gestation. The litter size was determined for each group and the litters were examined for gross malformations. The gross and histological examinations of various organs of the litters were also carried out. Results show that 8.65% of the litters from experimental animals were malformed as against 1.62% of control. It also showed that 31.2% of all the malformed litters had multiple congenital malformations. It also showed that the experimental rats had nine resorption sites while control had none. This demonstrates that the water extract of Momordica charantia is teratogenic in Sprague Dawley rats and should be used with caution in man.
Keywords: Momordica charantia teratogenicity
Introduction
The placenta provides a selective barrier between the fetus and the mother. This barrier protects the fetus from some harmful substances crossing from the mother to the fetus. Chemicals (including drugs) are among some harmful agents that sometimes breach this barrier.
Since teratogenicity of thalidomide was highlighted a lot of research has been undertaken to determine the safety of different drugs during pregnancy. To emphasize the importance of this, most drug manufacturers always include the caution “not to be used in pregnancy unless prescribed by a physician”, in their directions for use of drugs.
Herbal preparations have been in use before the onset of pharmaceutical products. However, their use has been dramatically increased recently; hence such terms as “herbal medicine and alternative medicine” are gradually finding their way into medicinal curricula. The use of herbs for the management of different illnesses is appealing because it is cheap and readily available. It also reduces the stress of the hospital environment. Herbalists have been practising their trade for ages. This is usually done with some degree of secrecy. Furthermore, the complications that might be associated with their use are not usually highlighted.
Extracts from different parts of Momordica charantia have been reported to have medicinal values. Different extracts have been reported used for the treatment of infections (Chen et al., 2009, Sonibare et al., 2009), hyperglycemia (Uche-Nwachi and Mitchell, 2004; Han et al., 2008; Shih et al., 2009; Gbolade, 2009) and wound healing (Teoh et al., 2009; Alam et al., 2009; Lii et al., 2009; Ono et al., 2009).
One of the commonest uses is in the control of blood glucose in diabetics. The mode of action in the hypoglycemic property of Momordica charantia, has been extensively studied with varying results. Krawinkel and Keding (2006), reported that, the mechanism of action, whether it is via regulation of insulin release or altered glucose metabolism and its insulin-like effect is still under debate while Shih et al. (2009), reported that Momordica charantia extract acts by decreasing insulin resistance and increases glucose transporter protein 4 (GLUT4) in skeletal muscles. Herbalists usually administer the water extracts to diabetics to control their blood glucose.
The incidence of diabetes has been on the increase. Many diabetic patients in rural communities use herbal hypoglycemic agents for the control of their blood sugar. One of the herbs most frequently used is coralli/bitter gourd (Momordica charantia). Momordica charantia has been native to the tropics. According to Seaforth (1998), the herb is found in some parts of the Amazon, East Africa, Asia, and the Caribbean and has been used as a folklore medicine to treat various ailments including diabetes. Several investigators including Welinhinda et al. (1986), Ahmed et al. (1998), Ahmad et al, (1999), Sitasawad et al (2000), Lin et al. (2001), Miura et al. (2001), Vikrant et al (2001), Biyani et al, (2003) and Uche-Nwachi and Mitchell (2004), Gbolade et al (2009), Shih et al (2009), Han et al (2008), also had reported that the hypoglycemic property of the aqueous extract of Momordica charantia. Singh et al. (1989), Day et al (1990), Higashino et al (1992), and Ali et al (1993) had reported that extracts of the entire unripe fruit have been used as a hypoglycemic agents. However, other investigators, including Chongkol et al (1987), Karuanayake et al, (1990), Cakici et al (1994), and Platel and Sirnivasan (1995), reported that the hypoglycemic property of Momordica charantia depended on what part of the fruit used and the method of extraction employed. Uche-Nwachi and Mitchell (2004) reported that, while the unripe whole fruit extract had comparable hypoglycemic property to insulin and oral hypoglycemic drugs, that the ripe fruit did not possess this property. They also found that the extract lost its hypoglycemic property if not refrigerate. In spite of findings of these investigators, not much has been done to determine the safety of the use of Momordica charantia extracts in pregnancy. The aim of this investigation is to determine its possible teratogenic effect and its safety in pregnancy.
Materials and Methods
Animals
Fifty three Sprague Dawley female rats weighing 280–380 grams were selected from the Animal House in the Faculty of Medical Sciences, University of the West Indies. The animals were acclimatized for two weeks, under standard conditions of temperature and illumination (12 hr light and 12 hrs dark) cycle. Animals had access to food (standard rat diet) and water ad libitum, and were cared for according to the Animal House Committee regulations on “the Care of Experimental Animals”.
Confirmation of pregnancy
Vaginal smears were taken every two hours during the day, to determine the estrus cycle based upon vaginal cytology. Rats with proestrus vaginal cytology were grouped in threes. Each group was placed in a cage with a virile male for mating. Mating was confirmed by the presence of spermatozoa in the vaginal smear. This was the sperm positive date and corresponds to day zero in the dating of the animals. The pregnant rats were weighed daily. A significant weight gain on the 10th day of gestation was also confirmatory of pregnancy.
Preparation of Extracts
Unripe Momordica charantia was obtained from the local market. The water extract was prepared using 5kg of the whole unripe fruit including the seeds and 300ml of distilled water. This was blended using a commercial blender until pulp was formed. This was then strained using a cheese cloth to remove the coarse fibers.
Treatment
The experimental animals were divided into groups of three. A total of 8 groups (1–8) were acclimatized in separate cages before they were mated with virile males at different days in gestation. The rats in group 1 cage were commenced on the extract on the 7th day of gestation, while the animals on cage 8 were commenced on the 14th day of gestation. The remaining cages (2–7), were started on successive days of gestation. Using the dating method, two milliliters of the extract was administered orally from days 7, 8, 9, 10,11,12,13 and 14, which corresponded to cages 1–8 of gestation respectively (Uche-Nwachi et al., 2004).
Control groups made of 29 female rats were divided into groups of 4. Each group was mated with a viral male. This control group received distilled water orally in place of the extract. The aim was to get the same numbers of pups from the control and experimental groups for statistical analysis. Rats with no significant weight gain were sacrificed on day 22 to observe any resorption sites. After delivery the litter size and average pup weight from each group (control and experimental) was recorded. Malformed pups were also recorded. Pups were weighed daily and were individually marked when weaned. Pups were allowed to mature up to 45 days of age, when they were sacrificed via ether asphyxiation (Miettinen et al., 2002). Important organs which will determine the viability of the pups (brain, heart, lungs, liver, spleen) and organs from the same embryonic origin (kidney, ovaries, and testis), were harvested and observed for the presence of defects. The organs were weighed, and then fixed in Bouin's fluid. Paraffin sections, (0.5µ thick) were cut and stained with hematoxylin and eosin. A one-way ANOVA was used for the statistical analysis of the organ weight differences between experimental and control pups.
Results
Birth weights
Results showed that the mean birth weight of the experimental pups was 6.34g vs. 7.06g (p < 0.05), and that 16 of the pups from the experimental animals (8.65%) were malformed as against 3 from the control (1.62%) {Table 1}. These result also showed that while 5 pups from the experimental groups had multiple congenital malformations, none from the control group had such malformations (Table 1). The result further showed that while the control rats had no resorption sites, the experimental rats had 9 resorption sites (Table 1, Figure 4a).
Table 1.
Mean litter size, body weight, resorption sites and congenital malformations in the pups from control and experimental animals
| Control | Experimental (MC) | |
| Dams | 29 | 24 |
| Total number of pups | 185 | 185 |
| Males | 83 | 83 |
| Females | 102 | 102 |
| Mean litter size | 6.4 | 7.7 |
| Mean birth weight | 7.06 g | 6.34 g |
| Gross congenital malformation | 3 | 16 |
| % of congenital malformation | 1.62 | 8.65 |
| Males with malformations | 1 | 10 |
| Females with malformations | 2 | 6 |
| Multiple malformations | 0 | 5 |
| Resorption sites | 0 | 9 |
Figure 2d.
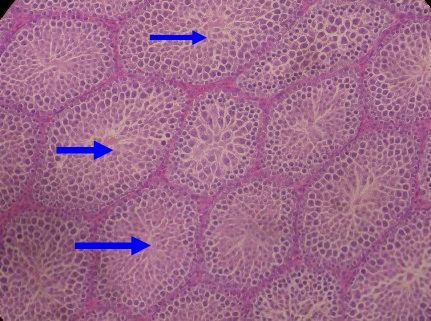
Photomicrograph (H&E), of the undescended testis from experimental rats x40. Note the absence of canalization of the seminiferous tubules (arrows)
Malformations
The result also showed that while only 1.62% of the control pups were malformed, 14.8% of the pups from day 7, 14.3% from day 8, 7.4% from day 9, 12.2% from day 11, 2.9% from day 12, 21.4% from day 13 had malformations. It also showed that there were no malformations on days 10 and 14 (Table 2).
Table 2.
Distribution of malformations and resorption sites among the different days in gestation, the extracts was initiated
| Day of gestation |
Litter Size |
Normal Pups |
Pups Affected |
Malformation types | Malformation % |
Resorption Sites |
| Control | 185 | 183 | Female | Distended uterine horn and uterine atrophy |
1.62 | 0 |
| Female | Ovarian atrophy, | |||||
| Male | Unilateral testicular agenesis |
|||||
| Day 7 | 27 | 23 | Female | Unilateral ovarian hypertrophy, Bilateral |
14.8 | 0 |
| Female | ovarian atrophy Bilateral ovarian |
|||||
| Female | atrophy, enlarged left lung and splenomegaly Cryptorchidism |
|||||
| Male | ||||||
| Day 8 | 7 | 6 | Female | Uterine horn atrophy | 14.3 | 9 |
| Day 9 | 27 | 25 | Male | Bilateral testicular atrophy and hepatomegaly |
7.4 | 0 |
| Male | Hepatomegaly | |||||
| Day 10 | 14 | 14 | 0 | 0 | 0 | |
| Day 11 | 41 | 36 | Male | Cryptorchidism | 12.2 | 0 |
| Male | Cryptorchidism and splenomegaly |
|||||
| Male | Bilateral testicular atrophy, Hepatic atrophy and renal hypertrophy, Bilateral |
|||||
| Male | testicular atrophy and splenomegaly, Anencephaly and |
|||||
| Male | spinabifida | |||||
| Day 12 | 34 | 33 | Male | Unilateral testicular hypertrophy |
2.9 | 0 |
| Day 13 | 14 | 11 | Female | Distended uterine horns |
21.4 | 0 |
| Female | Distended uterine horns |
|||||
| Male | Hepatic atrophy | |||||
| Day 14 | 22 | 22 | 0 | 0 | 0 |
Mean organ weight
The result also showed that the mean weights of the kidney, the liver, the spleen, and the brain were significantly lower in the experimental pups, while the heart was significantly increased when compared with control (p<0.05) {Table 3}.
Table 3.
Significant organ weight difference (P< 0.05)
| Day | Organ | Mean organ weight of control (g) |
Mean organ weight experimental (g) |
P value Significant at P<0.05 |
| 8 | Brain | 1.88 | 1.77 | 0.002 |
| 9 | Heart | 0.78 | 0.87 | 0.010 |
| 10 | Heart | 0.75 | 0.84 | 0.042 |
| 11 | Liver | 10.65 | 8.69 | 0.001 |
| 12 | Liver | 11.07 | 9.54 | 0.004 |
| 13 | Brain | 1.90 | 1.82 | 0.020 |
| 14 | Kidney | 1.89 | 1.59 | 0.003 |
| 14 | Lungs | 1.23 | 1.05 | 0.008 |
| 14 | Liver | 10.00 | 8.53 | 0.019 |
| 14 | Spleen | 0.82 | 0.66 | 0.038 |
Histology
Histological analysis also showed that the undescended testes had uncannalized seminiferous tubules (Fig 1d), and that the distended uterine horns had wide lumina (Figure 2c). Further analysis also showed that the atrophic ovary had no viable follicles (Fig 3b). It also showed multiple resorption sites in the experimental animals (Figure 4a).
Figure 1a.
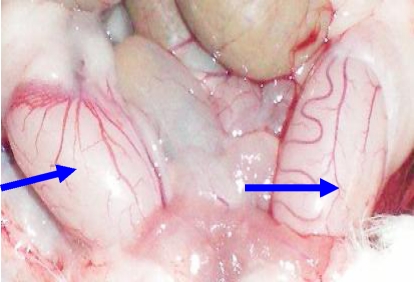
Photograph of normal testes from control rats x40. Note the size and shapes of the testes (arrows).
Figure 1b.
Photograph of undescended testes, (arrows) x40. Note the size and position of the testes inside the abdominal cavity.
Figure 1c.
Photomicrograph (H&E), of normal testis from control rats x40. Note the canalization of the seminiferous tubules (arrows).
Figure 2a.
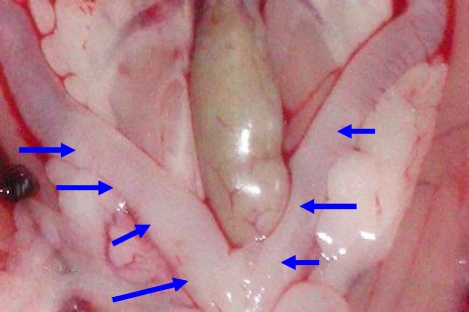
Photograph of distended uterine horns from experimental rats (arrows) x40.
Figure 2b.
Photograph of normal uterine horns from control rats (arrows) x40.
Figure 2c.
Photomicrograph (H&E), of a cross section of distended uterine horn from experimental rat x40. Note the luminal size (arrow)
Figure 2d.
Photomicrograph of a cross section of a normal uterine horn from control rat, x40. Note the size of the lumen (arrow), when compared with Fig 2c above.
Figure 3a.
Photomicrograph (H&E), of a normal ovary from control rat, x40. Note the presence of corpora lutea (red arrows), and developing follicles (blue arrow).
Figure 3b.
Photomicrograph (H&E), of the ovary from experimental rat x 40. Note the absence of corpus luteum and the absence of viable follicles.
Figure 4a.
Photograph of resorption sites in the uterine horns of experimental rats (arrows) x 40.
Figure 4b.
Photomicrograph (H&E), of early resorption from the uterine horn of experimental rat x40. Note the cavity of the horn filled with resorbed debris (arrow).
Figure 4c.
Photomicrograph (H&E), of late resorption site from the uterine horn x40. Note the re-epithelization of the endometrium (arrow).
Discussion
According to, Chan et al (1984), Leung and Yeung, (1987), Aguwa and Mittal. (1983), Grover and Yadav (2004), Momordica charantia extracts have abortifacient properties. It has also been reported to reduce fertility in both males and females by Farnsworth and Waller (1982), and Basch et al. (2003). This present study showed that, there were 9 resorption sites in the experimental animals while the control had none (Table 1). The present study also showed that 8.7 % of the experimental pups had malformations while only 1.6% of the control had malformations (Table 1).
It was also demonstrated in this study that, the teratogenicity of the water extract of Momordica charantia was dependent on the day of gestation it was administered. The present study also showed that reproductive organs were affected most in the malformed liters (Table 2), and that administering the extract from day 7, 11, and 13, showed the greatest number of malformations (Table 2). Furthermore, our result also showed that there was significant reduction in the weights of the brain, liver, kidney, lung, and spleen in the pups from the experimental animals, while there was a significant increase in the weight of the heart (Table 3). This implies that most organs from the pups of the experimental animals were affected in one way or another. The alterations in morphology and weight obviously may affect the respective functions of the affected organs.
We therefore conclude that the water extract from the unripe fruit of Momordica charantia is teratogenic and that this was dependent on the period in gestation it was administered, and that the reproductive organs of the pups were most affected. We therefore advise that this preparation should be used with utmost caution during pregnancy in man.
Acknowledgements
We are grateful to the technical staff of Anatomy Unit of the Faculty of Medical Sciences, and the staff of Histopathology Laboratory in the Department of Para clinical Sciences for their assistance. We are also grateful to Camille Mitchell for her assistance in the histology laboratory.
References
- 1.Aguwa CN, Mittal GC. Abortifacient effects of the roots of Momodica Charantia Angustisepala. J Ethnopharmacol. 1983;7:169–173. doi: 10.1016/0378-8741(83)90018-1. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad N, Hassan MR, Halder H, Bennoor KS. Effect of Momordica charantia extracts on fasting and postprandial serum glucose levels in NIDDM patients. Bangladesh Med Res Counc Bull. 1999;25(1):11–13. [PubMed] [Google Scholar]
- 3.Ahmed I, Adeghate E, Sharma AK, Pallot DJ, Singh J. Effects of Momordica charantia fruit juice on islet morphology in the pancreas of the streptozotocin-diabetic rat. Diabetes Res Clin Pract. 1998;40(3):145–151. doi: 10.1016/s0168-8227(98)00022-9. [DOI] [PubMed] [Google Scholar]
- 4.Alam S, Asad M, Asdaq SM, Prasad VS. Antinuclear activity of methalonic extract of Momordica charantia L. in rats. J Ethnopharmacol. 2009 Jun 25;123(3):464–469. doi: 10.1016/j.jep.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 5.Ali L, Khan AK, Mamun MI, Mosihuzzaman M, Nahar N, Nur-e-Alam M, Rokeya B. Studies on hypoglycemic effects of fruit pulp, seed, and whole plant of Momordica chrantia on normal and diabetic model rats. Planta Med. 1993;59(5):408–412. doi: 10.1055/s-2006-959720. [DOI] [PubMed] [Google Scholar]
- 6.Basch E, Gabardi S, Ulbricht C. Bitter Melon (Momordica Charantia) a review of efficacy and safety. Am J Health Syst Pharm. 2003;60(4):356–359. doi: 10.1093/ajhp/60.4.356. [DOI] [PubMed] [Google Scholar]
- 7.Biyani MK, Banavalikar MM, Suthar AC, Shahani S, Sivakami S, Jaspreet V. Antihyperglycemic Effects of Three Extracts From Momordica Charantia. J Ethnopharmacol. 2003;88(1):107–111. doi: 10.1016/s0378-8741(03)00184-3. [DOI] [PubMed] [Google Scholar]
- 8.Cakici I, Hurmoglu C, Tunctan B, Abacioblu N, Kanzik I, Sener B. Hypoglycemic effect of Momordica charantia extracts in normoglycaemic or cyproheptadine-induced hyperglycemic mice. J Ethnopharmacol. 1994;44(2):117–121. doi: 10.1016/0378-8741(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 9.Chan WY, Tam PP, Yeung HW. The termination or early pregnancy in the mouse by beta-momorcharin. Contraception. 1984;29(1):91–100. doi: 10.1016/0010-7824(84)90062-3. [DOI] [PubMed] [Google Scholar]
- 10.Chen JC, Liu WQ, Lu L, Qiu MH, Zheng YT, Yang LM, Zang XM, Zhou L, Li ZR. Kuguacins F-S, cucurbitane triterpenoids from Momordica charantia. Phytochemistry. 2009 Jan;70(1):133–140. doi: 10.1016/j.phytochem.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Chongkol T, Rachanee M, Malyn U, Kampanat P, Chitkavi P. The Hypoglycemic Activity of Momordica charantia Linn. In normal and alloxan-induced Diabetic Rabbits. J National Res Council Thilan. 1987;19(1):1–11. [Google Scholar]
- 12.Day C, Cartwright T, Provost J, Bailey CJ. Hypoglycemic effect of Momordica charantia extracts. Planta Med. 1990;56(5):426–429. doi: 10.1055/s-2006-961003. [DOI] [PubMed] [Google Scholar]
- 13.Farnsworth NR, Waller DP. Current status of plant products reported to inhibit sperm. Res Front Fertile Regul. 1982;2(1):1–16. [PubMed] [Google Scholar]
- 14.Gbolade AA. Inventory of antidiabetic plants in selected districts of Lagos State, Nigeria. J Ethnopharmacol. 2009 Jan 12;121(1):135–139. doi: 10.1016/j.jep.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Grover JK, Yadav SP. Pharmacological actions and potential uses of Momodica Charantia. J Ethnopharmacol. 2004;93(1):123–132. doi: 10.1016/j.jep.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 16.Han C, Hui Q, Wang Y. Hypoglycemic activity of saponin fraction extracted from Momordica charantia in PEG/salt aqueous two-phase systems. Nat Prod Res. 2008;22(13):1112–1119. doi: 10.1080/14786410802079675. [DOI] [PubMed] [Google Scholar]
- 17.Higashino, Suzuki A, Tanaka Y, Pootakham K. Hypoglycemic effects of Siamese Momordica charantia and Phyllanthus urinaria extracts in streptozotocin-induced diabetic rats. Nippon Yakurigaku Zasshi. 1992;100(5):415–421. doi: 10.1254/fpj.100.415. [DOI] [PubMed] [Google Scholar]
- 18.Karuanayake EH, Jeevathayaparan S, Tennekoon KH. Effect of Momordica charantia fruit juice on streptozotocin-induced diabetes in rats. J Ethnopharmacol. 1990;30(2):199–204. doi: 10.1016/0378-8741(90)90008-h. [DOI] [PubMed] [Google Scholar]
- 19.Krawinkel MB, Keding GB. Bitter gourd ( Momordica charantia): A dietary approach to hypoglycemia. Nutr Rev. 2006;64(7 Pt 1):331–337. doi: 10.1301/nr.2006.jul.331-337. [DOI] [PubMed] [Google Scholar]
- 20.Leung SO, Yeung KN. The immunosuppressive activities of two abortifacient isolated from the seeds of bitter melon(Momordica Charantia) Immunopharmacology. 1987;133:159–171. doi: 10.1016/0162-3109(87)90054-3. [DOI] [PubMed] [Google Scholar]
- 21.Lii CK, Chen HW, Yun WT, Liu KL. Suppressive effects of wild bitter gourd (Momordica charantia Linn. var. abbreviate ser.) fruit extract on inflammatory responses in RAW264.7 macrophages. J Ethnopharmacol. 2009 Mar 18;122(2):227–233. doi: 10.1016/j.jep.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 22.Lin X, Shen X, Long Z, Yang Q. Effects of cactus, Alove veral, Momordica charantia on Reducing the Blood Glucose of Diabetic Mice. Wei Sheng Yan Jiu. 2001;30(4):203–205. [PubMed] [Google Scholar]
- 23.Miettinen HM, Alaluusua S, Tuomisto J, Vilukesela M. Effect of in utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure on rat molar development: the role of exposure time. Toxicol Appl Pharmacol. 2002;184(1):57–66. [PubMed] [Google Scholar]
- 24.Miura T, Itoh C, Iwamoto N, Kato M, Kawai M, Park SR, Suzuki I. Hypoglycemic activity of the fruit of the Momordica charantia in type 2 diabetic mice. J Nutr sci Vitaminol. 2001;47(5):340–344. doi: 10.3177/jnsv.47.340. [DOI] [PubMed] [Google Scholar]
- 25.Ono T, Tsuji T, Sakai M, Yukizaki C, Ino H, Akagi I, Hiramtsu K, Matsumoto Y, Sugiura Y, Uto H, Tsubouchi H, Gohda E. Induction of hepatocyte growth factor production in human dermal fibroblasts and their proliferation by the extract of bitter gourd melon pulp. Cytokine. 2009;46(1):119–126. doi: 10.1016/j.cyto.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Platel K, Sirnivasan K. Effect of the dietary intake of freeze dried bitter gourd (Momordica chrantia) in streptozotocin induced diabetic rats. Nahrung. 1995;39(4):262–268. doi: 10.1002/food.19950390403. [DOI] [PubMed] [Google Scholar]
- 27.Seaforth CE. Natural Products in the Caribbean Folk Medicine. Trinidad and Tobago: Falaah Productions limited; 1998. p. 50. [Google Scholar]
- 28.Shih CC, Lin CH, Lin WL, Wu JB. Momordica charantia extract on insulin resistance and the skeletal muscle GLUT4 protein in fructose-fed rats. J Ethnopharmacol. 2009 May 4;123(1):82–90. doi: 10.1016/j.jep.2009.02.039. [DOI] [PubMed] [Google Scholar]
- 29.Sitasawad SL, Shewade Y, Bhone R. Role of bitter gourd fruit juice in stz-induced diabetic state in vivo and vitro. J Ethnopharmacol. 2000;73(1–2):71–79. doi: 10.1016/s0378-8741(00)00282-8. [DOI] [PubMed] [Google Scholar]
- 30.Sonibare MA, Moody JO, Adesanya EO. Use of medicinal plants for the treatment of measles in Nigeria. J Ethnopharmacol. 2009 Mar 18;122(2):268–272. doi: 10.1016/j.jep.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Teoh SL, Latiff AA, Das S. The effect of topical extract of Momordica charantia (bitter gourd) on wound healing in nondiabetic rats and in rats with diabetes induced by streptozotocin. Clin Exp Dermatol. 2009 Mar 23; doi: 10.1111/j.1365-2230.2008.03117.x. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 32.Uche-Nwachi EO, Mitchell CV. Effect of water extract of the whole unripe fruit of Momordica Charantia, on the blood glucose concentrations of alloxan-diabetic Sprague Dawley Rats. J Caribbean veterinary medical association. 2004;4:11–17. [Google Scholar]
- 33.Vikrant V, Grover JK, Tandon N, Rathi SS, Gupta N. Treatment with extracts of Momordica charantia and Eugenia jambolana prevents hyperglycemia and hyperinsulinemia in fructose fed rats. J Ethnopharmacol. 2001;76(2):139–143. doi: 10.1016/s0378-8741(01)00218-5. [DOI] [PubMed] [Google Scholar]
- 34.Welihinda J, Karunanayake EH, Sheriff MH, Jayasinghe KS. Effect of Momordica on the glucose tolerance in maturity onset diabetes. J Ethno pharmacology. 1986;17(3):227–282. doi: 10.1016/0378-8741(86)90116-9. [DOI] [PubMed] [Google Scholar]