Abstract
The inhibiting effect of glabridin from Chinese herb Licorice on fatigue was investigated in male BALB/c mice. Mice were divided into the following 4 experimental groups: control group (CG), low dose group (LG), middle dose group (MG) and high dose group (HG,). The control group was given 0.5% Tween 80 solution and the treatment groups (LG, MG, HG) were given various doses of glabridin (5, 10, 20 mg/kg) for 28 consecutive days. Body mass, blood lactic acid (BLA), serum blood urea nitrogen (BUN), liver glycogen and muscle glycogen concentrations in mice were determined. Results showed that glabridin significantly inhibited fatigue, which extended the exhaustive exercise time of mice, effectively delayed the elevation of blood lactic acid and increase in the storage of liver and muscle glycogen.
Keywords: glabridin, inhibits, fatigue, mice
Introduction
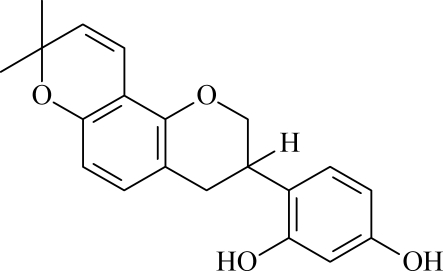
Licorice (gancao in Chinese) is the name applied to the roots and stolons of some Glycyrrhiza species (Fabaceae) and has been used by human beings for at least 4000 years (Nomura et al., 2002). It is a Chinese herb commonly used as an expectorant and to arrest coughing, reduce fever, comfort the stomach, alleviate urgency, and potentiate the effects of various other herbs (Bo et al., 2002; Sabbioni et al., 2005; Tian et al., 2008). Licorice contains glycyrrhizin, oleane triterpenoids, glucose, and flavonoids (Zhou et al., 2004). Glabridin (Figure 1) is a polyphenolic flavonoid and a main constituent in the hydrophobic fraction of licorice extract. It has been reported to exhibit multiple pharmacological activities, such as cytotoxic activity, antimicrobial activity, estrogenic and anti-proliferative activity against human breast cancer cells. It also affects melanogenesis, inflammation, low-density lipoprotein oxidation and protection of mitochondrial functions from oxidative stresses (Choi, 2005; Tian et al., 2008). These results clearly demonstrated that glabridin is an active component of Licorice.
Figure 1.
Molecular structures of glabridin
Fatigue is the symptom, which indicates that the health is about or already subjected to harm (Yu et al., 2008a). Physiologically, fatigue induced the changes of glucocorticoids in the biogenic monoamine levels (Glavin et al., 1991). It is well known that glucocorticoids are major mediators of the stress response and modulate many signaling events in the immune response. Thus, fatigue causes various disorders in relation to bio-regulatory, autonomic nervous, endocrine and immune system (Maes et al., 1998). These disorders can lead to a reduction in exercise intensity or even to the interruption of activity (Davis and Bailey, 1997), so fatigue is worthy of attention of modern people who are usually under stress. Many reports have indicated that flavonoids from plants were helpful on extensive exercise (Li et al., 2000; Hong et al., 2006; Chang et al., 2006; Wu et al., 2008; Yu et al., 2008 b). However, there is no information in detail concerning that glabridin can inhibit fatigue. The present study was designed to investigate if glabridin from Chinese herb Licorice could inhibit fatigue by swimming exercise of mice.
Materials and Methods
Chemicals
Glabridin (purity 97.21 %), was purchased from Shanghai Jiahe Pharmaceutical Co. Ltd (Shanghai, China); Reagent kits for the determination of blood lactic acid (BLA), serum blood urea nitrogen (BUN), liver glycogen and muscle glycogen were purchased from Jiancheng Biotechnology Co. (Nanjing, China); All other reagents were purchased from either Sigma Chemical Co. (St.Louis, USA) or Sinopharm Chemical Reagent Beijing Co., Ltd (Beijing, China).
Animals and diets
Male BALB/c mice weighing approximately 18 to 22 g were obtained from the Laboratory Animal Holding Unit, East China Jiaotong University (Nanchang, China). The animals were housed with a 12-hr light/dark cycle in a temperature- and humidity-control1ed room. The animals were given free access to basal diet (the Disease Control Center, Jiangxi, China) and water. The ingredient and nutrient compositions of the basal diet fed to mice are given in Table 1. After adaptation to the lighting conditions for 1 week, the healthy animals were used. The approval of this experiment was obtained from the Institutional Animal Ethics Committee of East China Jiaotong University (Nanchang, China) and the study was carried out according to the “Principles of Laboratory Animal Care” (World Health Organization (WHO) Chronicle, 1985).
Table 1.
The composition of diet fed to mice
| Ingredients | Content (%) |
| Maize meal | 52.00 |
| Wheat | 25.00 |
| Soyabean meal | 14.00 |
| Fish meal | 5.00 |
| Dicalcium phosphate | 1.50 |
| Vitamin mix | 0.50 |
| Mineral mix | 0.50 |
| Sodium chloride | 1.00 |
| Limestone flour | 0.50 |
| - | - |
Data derived from The Disease Control Center in Jiangxi, China. Vitamin mix (g/kg) is composed of the following: Vitamin A-8000 IU; Vitamin D-1000 IU; Riboflavin-8 mg; Niacin-20mg; Pantothenic acid-4 mg; Vitamin B12- 0.012 mg; Vitamin E-25 mg; Vitamin K-10 mg; Folic acid-6mg; Choline chloride-200 mg; Thiamin-2 mg. Mineral mix (g/kg), as follows: Calcium-1183 mg; Phosphorus - 391 mg; Manganese - 25 mg; Cobalt - 0.4mg; Copper - 7 mg; Iron - 30 mg; Iodine - 1.3 mg; Magnesium - 102 mg.
Experimental design
64 male BALB/c mice were randomly divided into the following 4 experimental groups based on body mass: control group (CG, control mice treated with 0.5 % Tween 80 solution), low dose group (LG, mice treated with 5mg/kg glabridin), middle dose group (MG, mice treated with 10mg/kg glabridin) and high dose group (HG, mice treated with 20 mg/kg glabridin). Glabridin was first dissolved in ethanol, diluted in 0.5 % Tween 80 (v/v) and were fed by gavage to treatment groups once a day for 28 days. Same volume of 0.5 % Tween 80 solution provied was prfor the control group. The animals were carefully monitored every day.
Measurement of exhaustive exercise time in mice
After a period of 28 days, eight mice were taken out from each group to make swimming exhaustive exercise. The mice swam with wire of 5 % body mass tied to their tails in the pool (length: 60 cm, width: 50 cm, depth: 45 cm) filled with 30 cm depth of water. Water was maintained at a constant temperature of 3 during the swimming protocol. Mice were regarded as exhausted when they were underwater for 8 secs, and their swimming time was immediately recorded (Chi et al., 2008).
Measurement of blood lactic acid (BLA) concentrations in mice
After a period of 28 days, eight mice were taken out from each group for BLA analyses. The blood samples were collected from eye sockets of mice 30 mins after intragastric administration and 30 mins after weight loading swimming (2 % body mass), respectively (Wan and Li, 2007; Zhang et al., 2005). Then BLA was tested according to the recommended procedures provided by the kit.
Measurement of serum blood urea nitrogen, liver and muscle glycogen concentrations in mice
After a period of 28 days, eight mice were taken out from each group for liver glycogen, muscle glycogen and serum blood urea nitrogen (BUN) analyses. Mice were forced to swim for 90 mins without a load. After an hour's resting, the mice were killed to collect liver, gastrocnemius muscle, and plasma samples for enzyme activity assays (Wan and Li, 2007; Zhang et al., 2005). The concentration of serum BUN, hepatic and muscle glycogen were tested according to the recommended procedures provided by the kits.
Statistical analysis
Data were presented as means ± SD. Student's t-test was used to assess significance of difference. P < 0.05 was considered significant.
Results and Discussion
Change of body mass, body mass gain, and food efficiency ratio (FER) during the experimental period are shown in Table 2. Body mass was recorded before experiment (initial) and after 28 days (final), and body mass gain was computed. FER was computed from the body mass gain and food intake for the experimental period (FER = body mass gain for the experimental period/food intake for the experimental period) (Jung et al., 2004). There was no significant difference between the control group and each treatment group (p>0.05). In the present study, glabridin had no significant effect on the body mass, body mass gain, and FER.
Table 2.
Effect of glabridin on body mass, body mass gain, and food efficiency ratio of mice
| Group | Body mass (g) | body mass gains (g) | Food efficiency ratio | |
| Initial | Final | |||
| CG | 19.22±1.34 | 7.46±2.57 | 7.46±2.57 | 0.21±0.17 |
| LG | 20.26±1.19 | 8.04±3.26 | 8.04±3.26 | 0.19±0.16 |
| MG | 19.68±2.04 | 7.78±2.31 | 7.78±2.31 | 0.24±0.20 |
| HG | 20.58±1.67 | 7.53±3.02 | 7.53±3.02 | 0.21±0.19 |
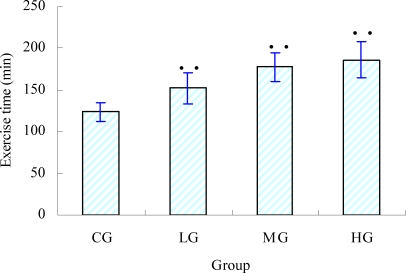
The maximum swimming time of the mice was measured to investigate the effect of glabridin inhibiting fatigue (Wang et al., 2006). Figure 1 showed that the swimming time of each treatment group (LG, MG and HG) increased significantly (p<0.05) when compared with that of the control group. The swimming time of the LG, MG and HG increased by 22.58 %, 43.55 % and 50.04 %, respectively. These results indicated that different doses of glabridin had significant effect on the endurance of the mice in the experimental.
Figure 1.
Effect of glabridin on exhaustive exercise time of mice (mean ± SD, n=8) • •p < 0.05 as compared with the control group
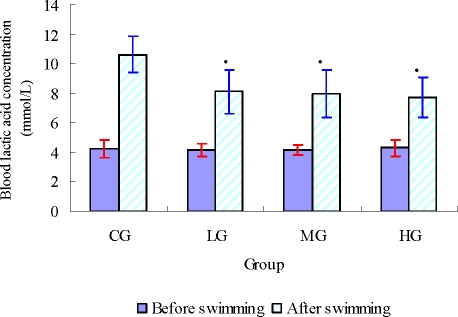
BLA is the glycolysis product of carbohydrate under an anaerobic condition, and glycolysis is the main energy source for intense exercise in a short time. Therefore, BLA is one of the important indicators for judging the degree of fatigue (Wang et al., 2006; Yu et al., 2008a). As shown in Figure 2, there was no significant difference in the concentration of BLA between treatment groups and the control group before swimming (p>0.05). After swimming, the concentration of BLA of all the treatment groups (LG, MG and HG) were significantly lower than that of control group (p<0.05). These results hinted that glabridin can effectively retard and lower the BLA produced after swimming, postpone the appearance of fatigue and accelerate the recovering from fatigue.
Figure 2.
Effect of glabridin on blood lactic acid (BLA) of mice (mean ± SD, n=8) • •p < 0.05 as compared with the control group
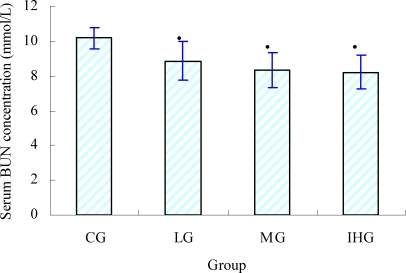
Serum BUN is the metabolism outcome of protein and amino acid. Urea is formed in the liver and is carried by the blood to the kidneys for excretion. Because urea is separated from the bloodstream by the kidneys, urea nitrogen concentration in the blood can be used as the indication of renal function. However, there are many factors other than renal disease that can cause BUN alteration (Wang et al., 2006). This includes protein breakdown, dehydration, stress, fatigue, etc. The serum BUN value was found to increase significantly after exercise. So serum BUN is the other important biochemical parameters related to fatigue (Ma et al., 2008; Jing et al., 2009). Serum BUN changes after swimming for all the groups were shown in Figure 3. The result showed that serum BUN concentration of all the treatment groups were significantly lower than that of the control group (p<0.05). It indicated that glabridin possessed the ability to lower or retard the formation of BUN after exercise.
Figure 3.
Effect of serum BUN of mice (mean ± SD, n=8) • •p < 0.05 as compared with the control group
It was known that endurance capacity of body was markedly decreased if the energy was exhausted. As glycogen was the important resource of energy during exercise, the increasing of glycogen stored in liver is an advantage to enhance the endurance of the exercise (Yu et al., 2008a). Wilber (1959) reported that severe depletion of liver glycogen was noted in all guinea pigs that swam to exhaustion. Liver glycogen depletion might be an important factor in the development of fatigue because as liver glycogen is depleted during exercise there is an inability to maintain blood glucose level, and the ensuing hypoglycemia could result in impaired nervous function (Dohm et al., 1983). Dohm et al. (1983) also demonstrated the importance of muscle glycogen levels in endurance exercise and suggested that depletion of muscle glycogen was a factor in fatigue and exhaustion (Jung et al., 2004). In the present study, the data of glycogen were shown in Table 3. After swimming, the concentration of liver glycogen of all the treatment groups were higher than that of control group (p<0.05). The concentration of muscle glycogen of all the treatment groups were higher than that of the control group (p<0.05). These data indicated that glabridin can significantly increase the concentration of liver and muscle glycogen of mice after swimming.
Table 3.
Effect of glabridin on liver and muscle glycogen of mice (mean ± SD, n=8)
| Group | The concentration of glycogen (mg/g) | |
| liver | Muscle | |
| CG | 7.58 ± 1.21 | 1.17± 0.95 |
| LG | 12.27 ± 1.06• • | 1.75 ± 0.61• • |
| MG | 17.38 ± 1.19• • | 2.29±0.44• • |
| HG | 15.43±1.36• • | 2.18±0.87• • |
p < 0.05 as compared with the control group
Conclusions
Our results suggested that glabridin from Chinese herb Licorice could significantly inhibit fatigue, which extended the exhaustive exercise time of mice, effectively delayed the increasing of blood lactic acid, increasing the storage of liver and muscle glycogen. However further studies to clarify the detailed mechanisms involved in the anti-fatigue properties of glabridin are necessary.
References
- 1.Bo T, Li KA, Liu HW. Fast determination of flavonoids in Glycyrrhizae radix by capillary zone electrophoresis. Anal Chim Acta. 2002;458:345–354. [Google Scholar]
- 2.Chang YQ, Zheng HY, Qu HG, Ma JR, Ma Y, Xi XW. Study on Antifatigue Effect of Anthoxanthin of Salix. Food Sci. 2006;27(8):251–253. [Google Scholar]
- 3.Chi AP, Chen JP, Wang ZZ, Xiong ZY, Li QX. Morphological and structural characterization of a polysaccharide from Gynostemma pentaphyllum Makino and its anti-exercise fatigue activity. Carbohy Pol. 2008;74:868–874. [Google Scholar]
- 4.Choi E. The licorice root derived isoflavan glabridin increases the function of osteoblastic MC3T3-E1 cells. Biochem Pharm. 2005;70:363–368. doi: 10.1016/j.bcp.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 5.Davis JM, Bailey SP. Possible mechanisms of central nervous system fatigue during exercise. Med Sci Sports Exerc. 1997;29:45–47. doi: 10.1097/00005768-199701000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Dohm GL, Tapscott EB, Barakat HA, Kasperek GJ. Influence of fasting on glycogen depletion in rats during exercise. J App Physiology. 1983;55:830–833. doi: 10.1152/jappl.1983.55.3.830. [DOI] [PubMed] [Google Scholar]
- 7.Glavin GB, Murison R, Overmier JB, Pare WP, Bakke HK, Henke PG, Hernandez DE. The neurobiology of stress ulcers. Brain Res Brain Res Rev. 1991;16:301–343. doi: 10.1016/0165-0173(91)90012-w. [DOI] [PubMed] [Google Scholar]
- 8.Hong XE, Gao YY, Luo LP, Xia DH. Studies on Mice Antifatigue Effect of Flavonoids from Sweet Potato Vines. Food Sci. 2006;27(2):256–258. [Google Scholar]
- 9.Jing LJ, Cui GW, Feng Q, Xiao YS. Orthogonal test design for optimization of the extraction of polysaccharides from Lycium barbarum and evaluation of its anti-athletic fatigue activity. J Medicinal Plant Res. 2009;3(5):433–437. [Google Scholar]
- 10.Jung K, Kim I, Han D. Effect of medicinal plant extracts on forced swimming capacity in mice. J Ethnopharm. 2004;93:75–81. doi: 10.1016/j.jep.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Li BC, Guo KM, Wang XJ, Hang J, Tang L, Xiong ZY. The Influence of Puerarire Radices Total Flavone on the Na+-K+-ATPase Activity of Rat Different Tissues in Endurance Training. J Beijing Univ Phys Educat. 2000;10:112–114. [Google Scholar]
- 12.Ma L, Cai DL, Li HX, Tong B. Anti-fatigue effects of salidroside in mice. J Med Coll PLA. 2008;23:88–93. [Google Scholar]
- 13.Maes M, Song C, Lin A, De JR, Van GA, Keins G, Bosmans E, De MI, Benoy I, Neels H, Demedts P, Janca A, Scharpe S, Smith RS. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10:313–318. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- 14.Nomura T, Fukai T, Akiyama T. Chemistry of phenolic compounds of licorice (Glycyrrhiza species) and their estrogenic and cytotoxic activities. Pure Appl Chem. 2002;74(7):1199–1206. [Google Scholar]
- 15.Sabbioni C, Mandrioli R, Ferranti A, Bugamelli F, Saracino MA, Forti GC, Fanali S, Raggi MA. Separation and analysis of glycyrrhizin, 18β-glycyrrhetic acid and 18α-glycyrrhetic acid in liquorice roots by means of capillary zone electrophoresis. J Chromatogr A. 2005;1081:65–71. doi: 10.1016/j.chroma.2005.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian M, Yan HY, Row KH. Simultaneous Extraction and Separation of Liquiritin, Glycyrrhizic Acid, and Glabridin from Licorice Root with Analytical and Preparative Chromatography. Biot Biop Engin. 2008;13:671–676. doi: 10.1007/s12257-008-0019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan BJ, Li YR. Research On Anti-fatigue Effect Of Herba Eclipta. J Anhui Sports Sci. 2007;28:51–54. [Google Scholar]
- 18.Wang JJ, Shieh MJ, Kuo SL, Lee CL, Pan TM. Effect of red mold rice on antifatigue and exercise-related changes in lipid peroxidation in endurance exercise. Appl Microbiol Biotechnol. 2006;70:247–253. doi: 10.1007/s00253-005-0051-5. [DOI] [PubMed] [Google Scholar]
- 19.Wilber CG. Some factors which are correlated with swimming capacity in guinea pigs. J Appl Physiology. 1959;14:199–203. doi: 10.1152/jappl.1959.14.2.199. [DOI] [PubMed] [Google Scholar]
- 20.Wu XF, Han SY, Zhu LS, Lv H, Cheng XY. Study of total flavones of buckwheat flower and leaf on anti-stress response in mice. J N Chin Coal Med Univ. 2008;10(6):740–741. [Google Scholar]
- 21.Yu B, Lu ZX, Bie XM, Lu FX, Huang XQ. Scavenging and anti-fatigue activity of fermented defatted soybean peptides. Eur Food Res Technol. 2008a;226:415–421. [Google Scholar]
- 22.Yu FR, Feng ST, Xie MR, Lian XZ. Anti-fatigue Effect of Cynomorium Songaricum Flavone on Old Rats. Chinese Journal of Rehabilitation Th Pr. 2008b;14(12):1141–1143. [Google Scholar]
- 23.Zhang C, Lu Y, Guo GX, Zhang H. Studies on Antifatigue of Buckwheat Protein. J Wuxi Univ Light Ind. 2005;24:78–82. [Google Scholar]
- 24.Zhou JH, Wu WP, Sun WJ. Evolvement in chemical research of licorice flavonoid. Drug Stanoaros of China. 2004;5(1):10–13. [Google Scholar]
- 25.World Health Organization, author. Principles of laboratory animal care. World Health Organization Chronicle. 1985;39:51–56. [Google Scholar]