Abstract
The neonatal rat ventricular myocyte model of hypertrophy has provided tremendous insight with regard to signaling pathways regulating cardiac growth and gene expression. Many mediators thus discovered have been successfully extrapolated to the in vivo setting, as assessed using genetically engineered mice and physiological interventions. Studies in neonatal rat ventricular myocytes demonstrated a role for the small G-protein RhoA and its downstream effector kinase, Rho-associated coiled-coil containing protein kinase (ROCK), in agonist-mediated hypertrophy. Transgenic expression of RhoA in the heart does not phenocopy this response, however, nor does genetic deletion of ROCK prevent hypertrophy. Pharmacologic inhibition of ROCK has effects most consistent with roles for RhoA signaling in the development of heart failure or responses to ischemic damage. Whether signals elicited downstream of RhoA promote cell death or survival and are deleterious or salutary is, however, context and cell-type dependent. The concepts discussed above are reviewed, and the hypothesis that RhoA might protect cardiomyocytes from ischemia and other insults is presented. Novel RhoA targets including phospholipid regulated and regulating enzymes (Akt, PI kinases, phospholipase C, protein kinases C and D) and serum response element-mediated transcriptional responses are considered as possible pathways through which RhoA could affect cardiomyocyte survival.
Keywords: RhoA, Cardiomyocyte Ischemia, ROCK
Gαq and RhoA Signaling Pathways in Cardiac Hypertrophy
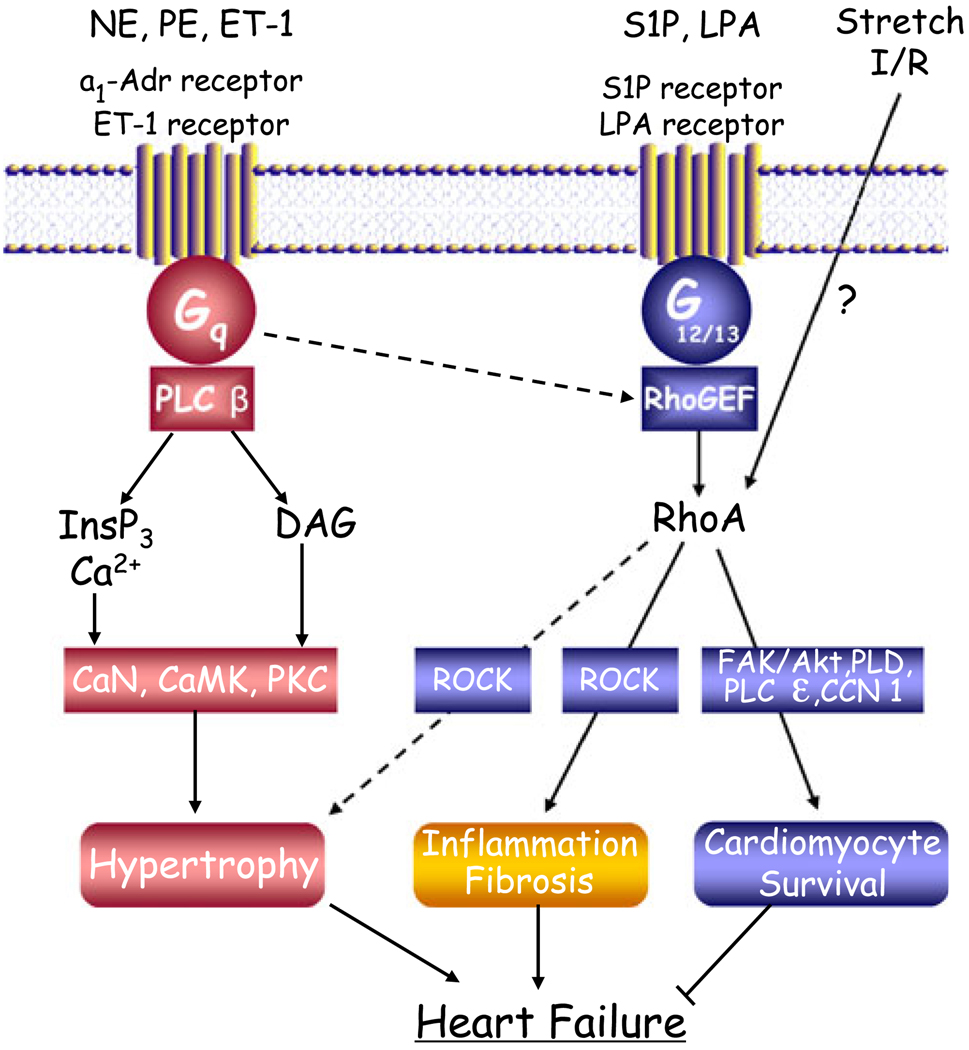
There is considerable evidence that G-protein coupled receptors (GPCRs) that interact with the heterotrimeric G0protein, Gαq, mediate cardiac hypertrophy (see Fig. 1). In neonatal rat ventricular myocytes (NRVMs), GPCR agonists such as norepinephrine (NE), phenylephrine (PE), and endothelin 1 (ET-1), acting through α1-adrenergic and ET-1 receptors coupled to Gαq, induce cardiac hypertrophy as evidenced by fetal gene expression, myofilament organization, increased protein synthesis, and cardiomyocyte enlargement [1–7]. In vivo studies subsequently demonstrated that transgenic expression of Gαq induces hypertrophy, as does Gαq expression in NRVMs [8–10]. Development of hypertrophy in mice subjected to transverse aortic constriction (TAC) was subsequently shown to be prevented by transgenic expression of a peptide inhibitor that blocks GPCR coupling to Gαq [11], or by genetic deletion of the alpha (α) subunit of Gαq and its homolog Gα11 [12]. The best known effector of Gαq is phospholipase C (PLC) [13, 14], and accordingly, signals generated through phosphoinositide hydrolysis, including activation of protein kinase C (PKC) and of Ca2+-regulated enzymes such as calcineurin and CaMKII have been considered to serve as downstream mediators of GPCR effects on hypertrophic gene expression and cell enlargement [15, 16].
Fig. 1.
GPCRs signaling in hypertrophy, survival, and failure. Dashed lines indicate pathways that the authors do not consider to be predominant. Abbreviations: NE norepinephrine, PE phenylephrine, ET-1 endothelin-1, S1P sphingosine 1-phosphate, LPA lysophosphatidic acid, I/R ischemia/reperfusion, InsP3 inositol-1,4,5-triphosphate, DAG diacylglycerol, PLC phospholipase C, RhoGEF rho guanine nucleotide exchange factor, PKC, protein kinase C, CaMK, calcium–calmodulin-dependent kinase, CaN, calcineurin, ROCK rho-associated coiled-coil containing protein kinase, FAK focal adhesion kinase, PLD phospholipase D
Studies carried out in vitro and subsequently in vivo also suggested that a low-molecular-weight G-protein, RhoA, plays a role in development of cardiac hypertrophy. Early studies from our group and others showed that in the NRVMs model of hypertrophy, agonist-induced increases in cell size, protein expression, and actin organization (all hallmarks of hypertrophy) could be attenuated by treatment with the C3 exoenzyme, which ribosylates and inhibits RhoA function [17, 18], or by expression of a dominant-negative form of RhoA [19]. Hypertrophic effects of RhoA were demonstrated to be transduced through activation of Rho-associated coiled-coil containing protein kinase (ROCK), a well-characterized RhoA effector [20–24]. RhoA and ROCK have also been demonstrated to be transducers of hypertrophy induced by static or pulsatile stretch of NRVMs [25, 26]. Our laboratory showed involvement of RhoA in MAP kinase translocation to the nucleus and in cardiomyocyte enlargement induced by stretch [25], while others have demonstrated that stretch-induced regulation of hypertrophy-associated gene expression is abolished following transfection with RhoA antisense oligonucleotides [26].
RhoA and ROCK have also been implicated in hypertrophy induced by pressure overload (TAC) or in vivo agonist infusion. There is rapid activation of RhoA and ROCK in adult rat hearts subjected to pressure overload [27]. Moreover, recent work using a similar pressure overload model showed that ROCK inhibition reduced the hypertrophic response and collagen deposition (a result of fibrosis), as well as improving cardiac function [28]. Treatment with the ROCK inhibitor fasudil (HA-1077) also blunted the hypertrophic response to angiotensin II (Ang II) infusion in rats, a treatment associated with ROCK activation as assessed by phosphorylation of ezrin/radixin/moesin (ERM) proteins [29]. These findings support the involvement of RhoA/ROCK signaling in development of hypertrophy in vivo.
The relative importance of, and relationship between, Gαq and RhoA signaling pathways in agonist and TAC-induced hypertrophy has not been extensively analyzed. Is RhoA a downstream target of Gαq signaling or does RhoA initiate a distinct and parallel hypertrophic signaling pathway? We originally proposed that RhoA could be activated downstream of Gαq in NRVM hypertrophic pathways [19], although we had no specific mechanistic insights into how this would occur. RhoA is activated by guanine nucleotide exchange factors (GEFs), proteins that catalyze exchange of guanosine 5c-diphosphate (GDP) for guanosine 5c-triphosphate (GTP) on RhoA [30]. The GTP-bound RhoA is the active form that interacts with and regulates effectors such as ROCK to elicit downstream responses [31, 32]. While it has been clear for many years that certain GPCR agonists can cause RhoA activation, the GEFs acting downstream of GPCRs have only recently been identified. Among these are GEFs such as the p63 rho GEF (RhoGEF), shown to bind and be regulated by Gαq [33–35]. Discovery of Gαq-regulated GEFs provides a means by which GPCRs that stimulate Gαq could also lead to RhoA activation and RhoA-mediated hypertrophy. Perhaps the newly discovered protective effects of cardiac α1 adrenergic receptors (Simpson, unpublished observations) reflect activation of a RhoA signaling pathway.
Notably, however, the best described hypertrophic agonists (NE, PE, and ET-1) are not nearly as efficacious at activating RhoA as are another set of ligands, including sphingosine 1-phosphate (S1P), lysophosphatidic acid (LPA), thrombin, and thromboxane A2. The receptors for this latter group of ligands couple not only to Gαq but also with high efficiency to the newest member of the heterotrimeric G-protein family, Gα12 and its family member Gα13 [36, 37]. Indeed, initial insights into how GPCRs activate RhoA emerged from seminal papers demonstrating that a particular GEF, the p115RhoGEF, interacts directly with Gα12 and Gα13 [38, 39]. It is now clear that the interaction of Gα12 or Gα13 with other RhoGEFs including leukemia-associated RhoGEF(LARG) and PDZ-RhoGEF leads to their activation [30, 40, 41]. There is also an A kinase-anchoring protein (AKAP-Lbc) that contains a RhoGEF domain and mediates RhoA activation in cardiomyocytes in response to agonists such as LPA and PE, [42, 43]. It is now well accepted that much as the effector for Gαs-coupled receptors is adenylate cyclase and that for Gαq-coupled receptors is PLCβ, RhoGEFs and RhoA activation serve as the primary effector for signaling by GPCRs that couple to Gα12/13 proteins. Ligands such as S1P and LPA, by activating GPCRs that couple to Gα12 or Gα13 and hence to RhoGEFs, are very effective initiators of RhoA signaling pathways.
Interestingly, recent studies challenge the hypothesized role of Gα12/13, RhoA, and ROCK in development of hypertrophy. Expression of Gα12 in NRVMs has been shown to induce a hypertrophic response [18, 44, 45]. Moreover a recent study of transgenic mice engineered to express an inhibitor of Gα12/13 (RGS domain of p115GEF) in a cardiac-specific manner did not show decreases in hypertrophy in response to pressure overload [46]. Genetic deletion of Gα12/13 also fails to block hypertrophy in vivo (S. Offermanns, personal communication). These findings contrast with those of comparable experiments cited above in which TAC-induced hypertrophy was inhibited when Gαq signaling was prevented [12, 47]. Recent studies also show no inhibition of pressure overload-induced hypertrophy in mice in which ROCK1, the RhoA target suggested to mediate hypertrophy in NRVMs, is genetically deleted [48, 49]. Several lines of evidence from our laboratory also argue that RhoA signaling does not lead to cardiac hypertrophy. For example, we observe no difference in hypertrophy induced by pressure overload in mice in which S1P2 or S1P3 receptors are genetically deleted, although we know that stimulation of S1P receptors leads to robust RhoA activation in cardiomyocytes [50, 51] and that RhoA activation occurs through S1P2 and/or S1P3 receptors [52]. We have also observed that cardiac-specific inducible RhoA expression does not lead to hypertrophy in mice followed for up to 1 year (Xiang et. al, manuscript in preparation). Thus, reevaluation with new models indicates that RhoA signaling is neither sufficient for the induction of cardiac hypertrophy nor necessary for that induced by pressure overload in vivo. If RhoA is not a critical player in development of in vivo hypertrophy, is there an alternative physiological role for RhoA activation and the agonists/interventions that induce RhoA activation in the heart?
Heart Failure
Cardiac hypertrophic responses can become maladaptive if the initial cardiac insult persists. The mechanisms responsible for the transition from compensatory to maladaptive hypertrophy and remodeling are not well understood, although various molecular mechanisms have been suggested to underlie this transition. A role for RhoA activation in the transition from hypertrophy to dilation and heart failure is suggested by several in vivo findings. One is that a lethal dilated cardiomyopathy develops in cardiac-specific RhoA transgenic mice [53]. More recent studies using a tyrosine phosphatase knockout mouse also showed RhoA-mediated cardiac dilation, suggesting a role for RhoA in the development of cardiomyopathy [54]. Genetically altered mouse models have also implicated ROCK in the development of heart failure. Thus, whereas ROCK1 null and heterozygous null mice show no difference in development of hypertrophy following pressure overload or Ang II infusion, they have significantly less fibrosis and reduced expression of a variety of extracellular matrix (ECM) proteins and fibrogenic cytokines [48, 55]. Similarly, there is improved cardiac function in the Gαq transgenic model of dilated cardiomyopathy when these mice are crossed with mice in which ROCK1 is deleted [49]. Taken together these studies implicate RhoA/ROCK signaling in the transition from compensatory hypertrophy to heart failure.
Both expression and activity of RhoA and ROCK have been noted to increase in a variety of cardiovascular disease models, including myocardial infarction and pressure overload [27, 56–58]. ROCK1 is activated not only by RhoA binding but also through its cleavage, which is increased in human heart failure patients [59]. A maladaptive role of RhoA/ROCK signaling in the cardiovascular system in vivo is supported by several studies demonstrating that inhibitors of RhoA/ROCK diminish diastolic contractile dysfunction induced by pressure-overload or reperfusion injury [28, 60, 61]. Key to interpreting these findings, however, is that the sites for maladaptive ROCK signaling are not clearly defined. In studies using pharmacologic inhibitors of ROCK, as well as in conventional knockout mouse models, ROCK function would be inhibited not only in cardiomyocytes but also in fibroblasts, endothelial, and inflammatory cells. RhoA/ROCK signaling pathways are well-established mediators of changes in migration, proliferation, and gene expression in these cell types [62–66]. Accordingly, RhoA- and ROCK-mediated responses in noncardiomyocytes likely contribute to the detrimental effects of RhoA signaling in cardiovascular disease.
Ischemic Injury
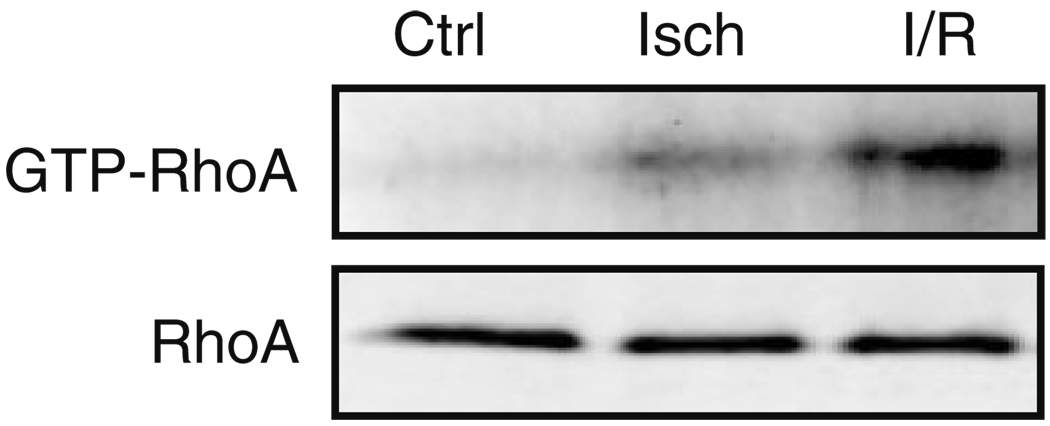
Ischemia/reperfusion (I/R) damage occurs when interrupted blood flow is followed by restored circulation, resulting in oxidative stress, mitochondrial dysfunction, inflammation, and tissue damage. I/R also activates numerous intracellular signaling pathways, some deleterious, but others protective [67]. We have observed marked activation of RhoA in response to I/R in isolated perfused mouse hearts (Fig. 2). Whether this occurs through activation of a RhoGEF in response to released mediators or as a direct result of oxidative stress is not known. Interestingly, a recent paper provided evidence that RhoA can be directly activated by reactive oxygen species, via a mechanism involving critical cysteine residues present in a redox-sensitive motif [68]. Published studies using an in vivo rat I/R model demonstrate increased expression of RhoA and activity of ROCK following 30 min of coronary occlusion followed by 24 h of reperfusion [60]. In this model, infarct size was reduced by inhibiting ROCK with Y-27632. A similar study carried out using an in vivo mouse I/R model also showed decreased infarct size and significantly less inflammation in mice treated with ROCK inhibitors compared with control, suggesting a deleterious role of RhoA/ROCK signaling in ischemic injury [61, 69].
Fig. 2.
RhoA is activated by ischemia/reperfusion in the perfused mouse heart. Isolated adult mouse hearts were retrograde perfused using the Langendorff method. Hearts were subjected to continuous perfusion (Ctrl), ischemia (Isch) for 30 min, or ischemia for 30 min and reperfusion for 60 min (I/R). Hearts were frozen, homogenized, and assessed for total RhoA in the lysate and activated RhoA based on pull-down with GST-rhotekin
What are the mechanisms by which ROCK inhibition could decrease I/R injury? A recent study showed that infarct size was not diminished by Y-27632 when wortmannin or nitro-l-arginine methyl ester were also present, suggesting that protective PI3K/Akt/NO signaling pathways are necessary [61]. Another study demonstrated that I/R decreased expression of the antiapoptotic Bcl-2 protein and that this did not occur in Y-27632-treated hearts [60]. Consistent with a role of ROCK in apoptosis, Y-27632-treated animals showed reduced TUNEL-positive nuclei in the infarcted regions [61]. Inflammatory responses induced by I/R are also abrogated by inhibition of ROCK with Y-27632 suggesting that RhoA/ROCK effects on inflammatory gene expression contribute to cardiovascular injury after I/R[61, 69]. Finally ROCK inhibition with Y-27632 or fasudil (HA-1077) was shown to decrease fibrosis following myocardial infarction in both mouse and rat models [56, 58], indicating that ROCK contributes either directly or indirectly to proliferation of cardiac fibroblasts in ischemic disease. Thus, there are numerous sites and mechanisms through which Rho/ROCK signaling could be deleterious and account for the salutary effect of ROCK inhibitors on I/R injury and development of heart failure.
RhoA Regulation of Cardiomyocyte Death and Survival
Cardiomyocyte loss by apoptosis and/or necrosis plays a crucial role in development of heart failure [70–72]. Our previous finding that cardiac-specific RhoA transgenic mice show spontaneous dilated cardiomyopathy [53] led us to hypothesize that cardiomyocyte cell death could be induced by sustained activity of RhoA. In subsequent work, we demonstrated that enhanced and sustained RhoA/ROCK signaling in NRVMs induces cardiomyocyte apoptosis [50]. Specifically, we demonstrated that expression of constitutively activated RhoA for 48–72 h activated a mitochondrial death pathway in association with a striking up-regulation, activation, and mitochondrial association of the proapoptotic Bcl family member, Bax [50].
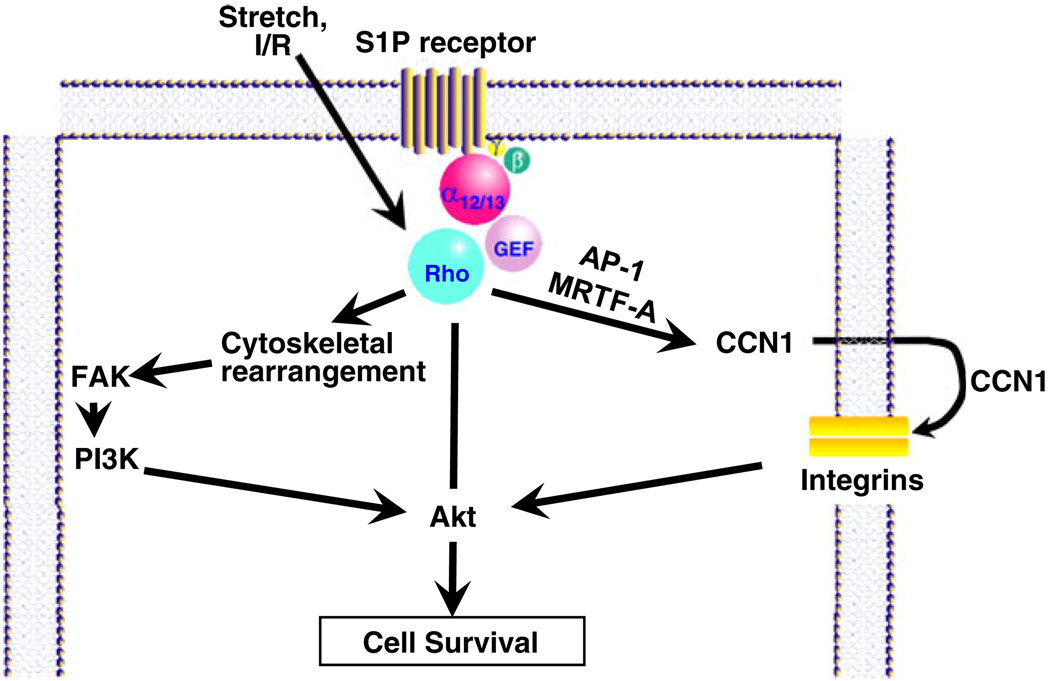
Conversely, we found that more acute RhoA activation protected cardiomyocytes from apoptotic insult [51]. Expression of activated RhoA in NRVMs for less than 24 h did not induce apoptosis but rather protected cells against both peroxide and glucose deprivation-induced apoptosis. Protection was dependent on ROCK activity, cytoskeletal integrity, and the activation of focal adhesion kinase (FAK). FAK, which is known to be activated through integrin engagement with the ECM, has a number of distinct phosphorylation sites that enable binding to signaling molecules including Src, PI3K, and p130Cas [73, 74]. We demonstrated that the role of FAK as a protein scaffold is responsive to RhoA signaling in NRVMs, recruiting the p85 subunit of PI3K and activating the survival kinase Akt. Mechanical stretch, which has been shown to activate RhoA in cardiomyocytes, was also found to elicit FAK and Akt activation [26, 51]. Interestingly, cardiomyocyte-specific ablation of FAK increased infarct size and cardiomyocyte apoptosis in response to I/R [75], consistent with a role for FAK as a protective downstream target of RhoA signaling in cardiomyocytes (Fig. 3).
Fig. 3.
RhoA-mediated protective signaling through Akt
In summary, although there is much evidence that activation of ROCK is deleterious in the heart, RhoA may have the capacity to confer protection in cardiomyocytes by signaling through Akt or other effectors. Recent studies in cardiac and noncardiac cells have identified new targets through which activated RhoA can signal. These, as well as more established targets that have not been fully investigated, are described below as potential mediators of cardiomyocyte protection through RhoA signaling.
RhoA and Phospholipid Signaling
Phosphoinositide Synthesis
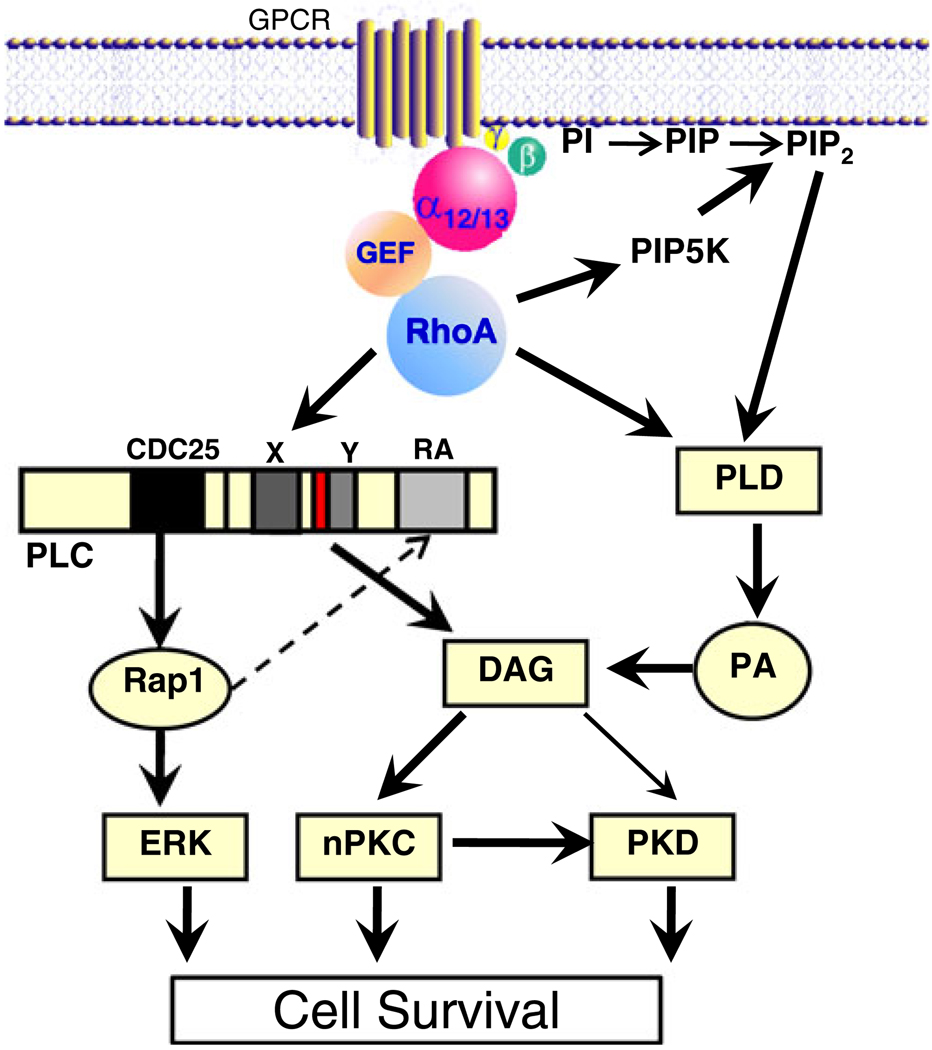
As described above, RhoA acts indirectly, through its well-known effects on cytoskeletal remodeling and FAK, to stimulate phosphoinositide signaling. There are, in addition, other phospholipid signaling pathways that are modulated through RhoA, several of which appear to be the direct result of RhoA interactions with phospholipid metabolizing enzymes (Fig. 4). One of the earliest effects described in mammalian cells was the regulation of phosphatidylinositol 4,5-bisphosphate (PIP2) synthesis via effects of RhoA on the synthetic enzyme PIP-5 kinase [76, 77]. RhoA-mediated changes in the synthesis and hence the level of PIP2 can affect the ability of the cell to respond to integrins or GPCRs that signal via PLC- mediated PIP2 hydrolysis [76, 78]. In addition to serving as a substrate for PLC, PIP2 subserves myriad cellular functions including regulation of ion channels and cytoskeletal proteins and recruitment of signaling molecules to the cell membrane. Thus, one hypothesis is that effects of RhoA on PIP2 levels can affect cell survival [79, 80].
Fig. 4.
RhoA-mediated protective signaling through phospholipid regulatory enzymes
PLC Activation
PLC epsilon (PLCε), the newest member of the PLC family, is uniquely positioned to serve as an integrator of signaling from GPCRs and small GTPases [81, 82]. This isoform of PLC is directly regulated by binding of the small GTPases Rap1 and RhoA [83–88]. There is, in contrast, no regulation by Gαq, the direct activator of the canonical PLC, PLCβ [89–91]. Accordingly PLCε is regulated by agonists that couple to Gα12/13 and RhoA rather than those that couple to Gαq [83, 90]. Another critical feature of PLCε is that it contains an N-terminal CDC25 homology domain that functions as an exchange factor for small GTPases [85, 87, 92]. This allows the enzyme to function not only as a phospholipase (generating diacylglycerol [DAG] and inositol trisphosphate) but also as an activator of Rap1 (Fig. 4).
Two important functions downstream of Rap1 activation may be relevant to cardiomyocyte signaling. One is that active Rap1 could feedback on and thus continue to activate PLCε, contributing to sustained DAG production [83, 87, 93]. DAG plays an important role in activation of PKC and studies from the Smrcka laboratory demonstrate that the novel PKC isoform, PKCε, is in fact activated through PLCε in the heart [94]. Rap1 also activates ERK, and we have shown that PLCε contributes to sustained agonist-induced ERK activation [83]. There is considerable evidence that ERK signaling is protective in many cell types including cardiomyocytes [95–97], as is PKCε [98–102]. Thus, sustained activation of ERK or PKCε, resulting from activation of PLCε, could contribute to RhoA-mediated cardiomyocyte protection. Smrcka's laboratory also demonstrated that there is a significant level of PLCε expression in the heart and that PLCε is increased in human failing hearts and in animal models of pressure overload and isoproterenol-induced hypertrophy [103]. Their analysis of PLCε knockout mice revealed decreased β-adrenergic receptor-induced contractility. In addition, they saw enhanced pathological hypertrophy and fibrosis in PLCε knockout mice, leading to the suggestion that PLCε protects against development of pathologic hypertrophy [103].
Phospholipase D Activation
While PLC activation is often considered to be the major mechanism for DAG generation, another phospholipase, phospholipase D (PLD) may be equally or more important. In contrast to PLC, PLD uses the more abundant phospholipid, phosphatidylcholine, as its substrate and initially produces phosphatidic acid (PA), which is then converted to DAG through the actions of lipid phosphatases (Fig. 4). Two mammalian PLD isozymes, PLD1 and PLD2, have been identified [33, 104–106]. There is abundant evidence that PLD1 (but not PLD2) is activated by the Rho family GTPases RhoA, Rac1, and Cdc42, with RhoA being the most efficacious [105, 107–109]. RhoA regulates PLD1 [108, 110–113] through direct interaction with its C-terminus [108, 110, 111, 114]. Activation of PLD1 could also occur indirectly through increased RhoA-mediated synthesis of PIP2, another critical cofactor for PLD activation [78, 112, 115].
Roles for PLD/PA/DAG signaling in the myocardium, particularly in myocardial protection, have been suggested. Like the kinase pathways activated during I/R and serving protective functions [67, 116–119], there is considerable evidence that oxidative stress activates and regulates PLD activity [120–126]. Activation of PLD in response to oxidative stress is associated with various cardiac pathologies, including coronary heart diseases [127–129]. PLD has been reported to be involved in cardioprotection by ischemic preconditioning, a phenomenon in which brief episodes of I/R render the myocardium insensitive to a subsequent prolonged ischemic episode [130, 131]. Pharmacologically induced activation of PLD was shown to reduce infarct size, while inhibition of PLD blocked the beneficial effects of preconditioning in isolated rabbit and rat hearts [130, 131]. Interestingly, adenosine-induced protection against I/R injury was suggested to be mediated through RhoA and a direct interaction with PLD1, as it was blocked by a mutant PLD1 that did not bind RhoA [132]. Thus, PLD1 activity appears to be involved in cardioprotection, although mechanisms for its activation and protective function have not been elucidated.
Protein Kinase Activation and DAG
As mentioned above, DAG generated through the actions of PLC or PLD activates PKC. PKC has been implicated in cardiac metabolism, contractile function, hypertrophy, heart failure, fibrosis, inflammation, and responses to ischemic injury. The predominant isozyme of PKC in the ventricle is PKCα, an isoform shown by Molkentin’s group to play a role in heart failure susceptibility and cardiac contractility [133, 134]. The novel PKC isozymes, PKCε and PKCδ, have been suggested to play divergent roles in I/R injury. PKCε has been shown to confer cardioprotection against I/R injury and to contribute to the protective effects of preconditioning [98–100, 135] and postconditioning [101, 136] in various animal models and in the human myocardium [102]. While some data also implicate PKCδ in cardioprotection [137], most evidence suggests that PKCδ is proapoptotic and has detrimental effects in the heart [138–142]. In the setting of I/R injury, it has been shown that either PKCε activation or PKCδ inhibition reduce I/R damage, whereas PKCε inhibition or PKCδ activation increase injury [102, 140, 143]. Additionally, combined PKCε activation and PKCδ inhibition have been shown to exert additive protection against I/R injury in isolated rat hearts [144].
Protein Kinase D Activation
Protein kinase D (PKD) is activated in the adult myocardium [145] and in other tissues through effects of novel PKCs [146–148]. There are a growing number of functions attributed to PKD signaling in the heart, including regulation of contractile function through phosphorylation of troponin-I [149, 150], and phosphorylation of HDAC-5, a class II HDAC, that regulates cardiac hypertrophy [151–153]. In other systems, PKD has also been shown to function, via nuclear factor κB signaling, as a mediator of cell survival [154–156]. Notably, PKD activity has been reported to be regulated by RhoA. Expression of a constitutively activated RhoA increased basal PKD kinase activity in COS-7 cells [157] and induced PKD activation-loop phosphorylation in HeLa cells [158]. RhoA-induced PKD activation was suggested to be mediated through ROCK and PKCε since treatment with the ROCK inhibitor Y-27632 or knockdown of PKCε (but not PKCδ) by siRNA inhibited RhoA-induced PKD phosphorylation in HeLa cells [158]. A Rho/ROCK/PKC signaling pathway has also been reported to be upstream of PKD-induced protection against oxidative stress in intestinal epithelial cells [159]. A functional role of PKD for cardiac protection has not, to our knowledge, been demonstrated.
RhoA and Gene Expression
RhoA and SRF
Rho GTPases are best known for their role in regulation of cytoskeletal dynamics through effectors that control cell adhesion, morphology, and motility [160–169]. Rho GTPases also play a critical, albeit generally less appreciated role, in transcriptional regulation, as first noted based on RhoA-dependent regulation of serum response factor (SRF) target genes [170]. The SRF protein is constitutively localized to the nucleus and bound to serum response element (SRE) sequences, and no direct modifications of the protein are required for its function [171–174]. Rather SRF associates with other transcription factors to provide combinatorial control of its target genes [175]. Two major classes of coactivators, regulated by separate signaling pathways, are known to activate SRF: the ternary complex factors (TCFs) and the myocardin-related transcription factors (MRTFs) [176–179].
The TCF family is activated by MAP kinase-mediated phosphorylation [176, 179, 180]. However, Treisman's group showed that RhoA effects on SRF activity were mediated through a TCF independent pathway [170]. RhoA activation was also shown to stimulate c-fos SRE transcription in a TCF-independent manner [181]. In cardiomyocytes, we reported that RhoA affects ANF gene expression through TCF independent SRE sites [182]. Myocardin, MRTF-A and MRTF-B comprise the second, more recently characterized SRF coactivator family [179, 183, 184]. The activity of MRTF-A and MRTF-B depends on RhoA signaling and actin dynamics [179, 185–189]. Association of MRTF-A with G-actin results in its sequestration in the cytoplasm. Serum stimulation and other signals that activate RhoA promote actin polymerization [188, 190, 191], leading to MRTF-A translocation into the nucleus and SRF target gene activation [179, 188, 192]. Immediate-early genes, SRF itself, skeletal α-actin, and myosin light chain-2 (MLC-2v), are among the genes regulated in this manner [180, 193–195]. Also notable among the SRF-regulated genes are the growth factor inducible immediate early genes CCN1 (Cyr61) and CCN2 (CTGF), which belong to the CCN family of matricellular proteins [196–199].
RhoA and CCN1/Cyr61
CCN1 was first identified as an immediate early gene upregulated in response to growth factors and subsequently determined to be secreted from the cell, where it serves a function intermediate between that of ECM proteins and growth factors [200–202]. CCN1 is a pleiotropic molecule, acting via cell surface integrin engagement to regulate cell migration, proliferation, and survival [197, 198, 203, 204]. Mechanical stretch induces CCN1 expression [205–207], and recent studies showed that MRTF-A and CREB binding proteins are required for mechanical strain-induced transcriptional activation of the CCN1 gene in vitro and in vivo [207, 208](see Fig. 3). Mechanical overload-induced CCN1 gene expression in vivo was also associated with RhoA-mediated nuclear localization of MRTF-A and enrichment of SRE sites on the CCN1 promoter with MRTF-A and acetylated histone H3.
CCN1 in the Heart
Little is known about the regulation or role of CCN1 in the heart, but several papers report that CCN1 expression is highly expressed in the myocardium of patients with heart failure or ischemic myopathy [209–211]. CCN1 expression has also been shown to increase in mouse heart in response to pressure overload and myocardial infarction and in cardiomyocytes stimulated by GPCR agonists [211]. Multiple signaling pathways including activation of ERK and PKC can contribute to induction of CCN1 expression [211]. In addition, there is considerable evidence that signaling through RhoA plays a major role in CCN1 induction in response to S1P and other agonists in cardiomyocytes (Zhao et. al., manuscript in preparation) as in other cell types [204, 205, 208, 212, 213]. A paper by Yoshida et al. [214] provides intriguing evidence supporting the hypothesis that CCN1 is cardioprotective. These investigators observed that CCN1 addition to isolated cardiomyocytes attenuated the response to oxidative stress and that this occurred via CCN1 effects on integrin β1-mediated FAK and Akt activation. Thus, RhoA-mediated increases in CCN1 expression and release are a potential mechanism by which the cell can further activate integrins, FAK, and protective Akt signals (see Fig. 3).
Conclusion
One of the challenges faced by maturing scientists is that of remembering what we published and defended in the past and squaring it with our more recent findings and made by our colleagues. The solace is that the old theories advanced the field to the stage where they can now be revisited and revised using more sophisticated approaches. The notion that RhoA serves as a mediator of cardiac hypertrophy, one that we proposed and others espoused a decade ago, is not wrong, but the role of RhoA in this response appears minor by comparison with that of other pathways shown to be essential and efficacious hypertrophic mediators. Pharmacologic inhibitors of ROCK have been developed in the last decade and have proven to be remarkable tools for further discovery, including demonstration that vascular tone is regulated by biochemically defined RhoA/ROCK pathways. These inhibitors have since been shown to be useful in treating a plethora of cardiovascular pathologies, from hypertension to atherosclerosis, and from heart failure to ischemic damage. While targets for the effects of ROCK inhibitors may be known, the cellular site of their action is not. Indeed, a notion we propose here is that whereas chronic RhoA signaling through ROCK may be a villain in inflammatory cells, fibroblasts, endothelial cells, and vascular smooth muscle, more acute activation of RhoA, at least within the cardiomyocyte, may serve to promote survival. There is evidence that RhoA is protective in a number of contexts, and there are multiple potential direct targets for RhoA that could mediate such responses. We suggest that RhoA is activated in the myocyte along with other protective pathways and that its effects on the cytoskeleton, phospholipids, or gene expression could be used to aid the ailing myocyte. If salutary pathways can be uncovered, they would be potential targets for cardioprotection. Accordingly, a prudent approach to treating conditions such as ischemic heart diseases might be to avoid the use of RhoA/ROCK inhibitors during the earliest phases of ischemic injury.
Footnotes
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Shigeki Miyamoto, Department of Pharmacology, University of California, 9500 Gilman Dr., La Jolla, San Diego, CA 92093-0636, USA.
Dominic P. Del Re, Department of Pharmacology, University of California, 9500 Gilman Dr., La Jolla, San Diego, CA 92093-0636, USA
Sunny Y. Xiang, Department of Pharmacology, University of California, 9500 Gilman Dr., La Jolla, San Diego, CA 92093-0636, USA
Xia Zhao, Department of Pharmacology, University of California, 9500 Gilman Dr., La Jolla, San Diego, CA 92093-0636, USA.
Geir Florholmen, Department of Pharmacology, University of California, 9500 Gilman Dr., La Jolla, San Diego, CA 92093-0636, USA.
Joan Heller Brown, Email: jhbrown@ucsd.edu, Department of Pharmacology, University of California, 9500 Gilman Dr., La Jolla, San Diego, CA 92093-0636, USA.
References
- 1.Simpson P. Norepinephrine-stimulated hypertrophy of cultured rat myocardial cells is an α1-adrenergic response. Journal of Clinical Investigation. 1983;72:732. doi: 10.1172/JCI111023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shubeita HE, McDonough PM, Harris AN, Knowlton KU, Glembotski CC, Brown JH, et al. Endothelin induction of inositol phospholipid hydrolysis, sarcomere assembly, and cardiac gene expression in ventricular myocytes: a paracrine mechanism for myocardial cell hypertrophy. The Journal of Biological Chemistry. 1990;265:20555. [PubMed] [Google Scholar]
- 3.Simpson P, McGrath A, Savion S. Myocyte hypertrophy in neonatal rat heart cultures and its regulation by serum and by catecholamines. Circulation Research. 1982;51:787. doi: 10.1161/01.res.51.6.787. [DOI] [PubMed] [Google Scholar]
- 4.Knowlton KU, Michel MC, Itani M, Shubeita HE, Ishihara K, Brown JH, et al. The α1a-adrenergic receptor subtype mediates biochemical, molecular, and morphologic features of cultured myocardial cell hypertrophy. The Journal of Biological Chemistry. 1993;268:15374. [PubMed] [Google Scholar]
- 5.Bogoyevitch MA, Glennon PE, Andersson MB, Clerk A, Lazou A, Marshall CJ, et al. Endothelin-1 and fibroblast growth factors stimulate the mitogen-activated protein kinase signaling cascade in cardiac myocytes. The Journal of Biological Chemistry. 1994;269:1110. [PubMed] [Google Scholar]
- 6.McDonough PM, Brown JH, Glembotski CC. Phenylephrine and endothelin differentially stimulate cardiac PI hydrolysis and ANF expression. The American Journal of Physiology. 1993;264:H625. doi: 10.1152/ajpheart.1993.264.2.H625. [DOI] [PubMed] [Google Scholar]
- 7.Ito H, Hirata Y, Adachi S, Tanaka M, Tsujino M, Koike A, et al. Endothelin-1 is an autocrine/paracrine factor in the mechanism of angiotensin II-induced hypertrophy in cultured rat cardiomyocytes. Journal of Clinical Investigation. 1993;92:398. doi: 10.1172/JCI116579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Angelo DD, Sakata Y, Lorenz JN, Boivin GP, Walsh RA, Liggett SB, et al. Transgenic Gαq overexpression induces cardiac contractile failure in mice. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:8121. doi: 10.1073/pnas.94.15.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorn GW, Brown JH. Gq signaling in cardiac adaptation and maladaptation. Trends in Cardiovascular Medicine. 1999;9:26. doi: 10.1016/s1050-1738(99)00004-3. [DOI] [PubMed] [Google Scholar]
- 10.Adams JW, Sakata Y, Davis MG, Sah VP, Wang Y, Liggett SB, et al. Enhanced Gαq signaling: A common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:10140. doi: 10.1073/pnas.95.17.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akhter SA, Luttrell LM, Rockman HA, Iaccarino G, Lefkowitz RJ, Koch WJ. Targeting the receptor-Gq interface to inhibit in vivo pressure overload myocardial hypertrophy. Science. 1998;280:574. doi: 10.1126/science.280.5363.574. [DOI] [PubMed] [Google Scholar]
- 12.Wettschureck N, Rutten H, Zywietz A, Gehring D, Wilkie TM, Chen J, et al. Absence of pressure overload induced myocardial hypertrophy after conditional inactivation of Gαq/Gα11 in cardiomyocytes. Natural Medicines. 2001;7:1236. doi: 10.1038/nm1101-1236. [DOI] [PubMed] [Google Scholar]
- 13.Smrcka AV, Hepler JR, Brown KO, Sternweis PC. Regulation of polyphosphoinositide-specific phospholipase C activity by purified Gq. Science. 1991;251:804. doi: 10.1126/science.1846707. [DOI] [PubMed] [Google Scholar]
- 14.Taylor SJ, Chae HZ, Rhee SG, Exton JH. Activation of the β1 isozyme of phospholipase C by α subunits of the Gq class of G proteins. Nature. 1991;350:516. doi: 10.1038/350516a0. [DOI] [PubMed] [Google Scholar]
- 15.Dorn GW, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. Journal of Clinical Investigation. 2005;115:527. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nature Reviews. Molecular Cell Biology. 2006;7:589. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 17.Thorburn J, Xu S, Thorburn A. MAP kinase- and Rho-dependent signals interact to regulate gene expression but not actin morphology in cardiac muscle cells. The EMBO Journal. 1997;16:1888. doi: 10.1093/emboj/16.8.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maruyama Y, Nishida M, Sugimoto Y, Tanabe S, Turner JH, Kozasa T, et al. Gα12/13 mediates α1-adrenergic receptor-induced cardiac hypertrophy. Circulation Research. 2002;91:961. doi: 10.1161/01.res.0000043282.39776.7c. [DOI] [PubMed] [Google Scholar]
- 19.Sah VP, Hoshijima M, Chien KR, Brown JH. Rho is required for Gαq and α1-adrenergic receptor signaling in cardiomyocytes. Dissociation of Ras and Rho pathways. Journal of Biological Chemistry. 1996;271:31185. doi: 10.1074/jbc.271.49.31185. [DOI] [PubMed] [Google Scholar]
- 20.Amano M, Fukata Y, Kaibuchi K. Regulation and functions of Rho-associated kinase. Experimental Cell Research. 2000;261:44. doi: 10.1006/excr.2000.5046. [DOI] [PubMed] [Google Scholar]
- 21.Noma K, Oyama N, Liao JK. Physiological role of ROCKs in the cardiovascular system. American Journal of Physiology. Cell Physiology. 2006;290:C661. doi: 10.1152/ajpcell.00459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaulet H, Desgranges C, Renault MA, Dupuch F, Ezan G, Peiretti F, et al. Extracellular nucleotides induce arterial smooth muscle cell migration via osteopontin. Circulation Research. 2001;89:772. doi: 10.1161/hh2101.098617. [DOI] [PubMed] [Google Scholar]
- 23.Hoshijima M, Sah VP, Wang Y, Chien KR, Brown JH. The low molecular weight GTPase Rho regulates myofibril formation and organization in neonatal rat ventricular myocytes: Involvement of Rho kinase. The Journal of Biological Chemistry. 1998;273:7725. doi: 10.1074/jbc.273.13.7725. [DOI] [PubMed] [Google Scholar]
- 24.Yanazume T, Hasegawa K, Wada H, Morimoto T, Abe M, Kawamura T, et al. Rho/ROCK pathway contributes to the activation of extracellular signal-regulated kinase/GATA-4 during myocardial cell hypertrophy. The Journal of Biological Chemistry. 2002;277:8618. doi: 10.1074/jbc.M107924200. [DOI] [PubMed] [Google Scholar]
- 25.Kawamura S, Miyamoto S, Brown JH. Initiation and transduction of stretch-induced RhoA and Rac1 activation through caveolae: Cytoskeletal regulation of ERK translocation. The Journal of Biological Chemistry. 2003;278:31111. doi: 10.1074/jbc.M300725200. [DOI] [PubMed] [Google Scholar]
- 26.Torsoni AS, Marin TM, Velloso LA, Franchini KG. RhoA/ROCK signaling is critical to FAK activation by cyclic stretch in cardiac myocytes. American Journal of Physiology. Heart and Circulatory Physiology. 2005;289:H1488. doi: 10.1152/ajpheart.00692.2004. [DOI] [PubMed] [Google Scholar]
- 27.Torsoni AS, Fonseca PM, Crosara-Alberto DP, Franchini KG. Early activation of p160ROCK by pressure overload in rat heart. American Journal of Physiology. Cell Physiology. 2003;284:C1411. doi: 10.1152/ajpcell.00098.2002. [DOI] [PubMed] [Google Scholar]
- 28.Phrommintikul A, Tran L, Kompa A, Wang B, Adrahtas A, Cantwell D, et al. Effects of a Rho kinase inhibitor on pressure overload induced cardiac hypertrophy and associated diastolic dysfunction. American Journal of Physiology. Heart and Circulatory Physiology. 2008;294:H1804. doi: 10.1152/ajpheart.01078.2007. [DOI] [PubMed] [Google Scholar]
- 29.Higashi M, Shimokawa H, Hattori T, Hiroki J, Mukai Y, Morikawa K, et al. Long-term inhibition of Rho-kinase suppresses angiotensin II-induced cardiovascular hypertrophy in rats in vivo: Effect on endothelial NAD(P)H oxidase system. Circulation Research. 2003;93:767. doi: 10.1161/01.RES.0000096650.91688.28. [DOI] [PubMed] [Google Scholar]
- 30.Rossman KL, Der CJ, Sondek J. GEF means go: Turning on RHO GTPases with guanine nucleotide-exchange factors. Nature Reviews. Molecular Cell Biology. 2005;6:167. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 31.Loirand G, Guerin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circulation Research. 2006;98:322. doi: 10.1161/01.RES.0000201960.04223.3c. [DOI] [PubMed] [Google Scholar]
- 32.Brown JH, Del Re DP, Sussman MA. The Rac and Rho hall of fame: A decade of hypertrophic signaling hits. Circulation Research. 2006;98:730. doi: 10.1161/01.RES.0000216039.75913.9e. [DOI] [PubMed] [Google Scholar]
- 33.Kodaki T, Yamashita S. Cloning, expression, and characterization of a novel phospholipase D complementary DNA from rat brain. The Journal of Biological Chemistry. 1997;272:11408. doi: 10.1074/jbc.272.17.11408. [DOI] [PubMed] [Google Scholar]
- 34.Rojas RJ, Yohe ME, Gershburg S, Kawano T, Kozasa T, Sondek J. Gαq directly activates p63RhoGEF and Trio via a conserved extension of the Dbl homology-associated pleckstrin homology domain. The Journal of Biological Chemistry. 2007;282:29201. doi: 10.1074/jbc.M703458200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lutz S, Freichel-Blomquist A, Yang Y, Ruemenapp U, Jakobs KH, Schmidt M, et al. The guanine nucleotide exchange factor p63Rho. Journal of Biological Chemistry. 2005 doi: 10.1074/jbc.M411322200. [DOI] [PubMed] [Google Scholar]
- 36.Riobo NA, Manning DR. Receptors coupled to heterotrimeric G proteins of the G12 family. Trends in Pharmacological Sciences. 2005;26:146. doi: 10.1016/j.tips.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Strathmann MP, Simon MI. Gα12 and Gα13 subunits define a fourth class of G protein α subunits. Proceedings of the National Academy of Sciences of the United States of America. 1993;88:5582. doi: 10.1073/pnas.88.13.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kozasa T, Jiang X, Hart MJ, Sternweis PM, Singer WD, Gilman AG, et al. p115 RhoGEF, a GTPase activating protein for Gα12 and Gα13. Science. 1998;280:2109. doi: 10.1126/science.280.5372.2109. [DOI] [PubMed] [Google Scholar]
- 39.Hart MJ, Jiang X, Kozasa T, Roscoe W, Singer WD, Gilman AG, et al. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Gα13. Science. 1998;280:2112. doi: 10.1126/science.280.5372.2112. [DOI] [PubMed] [Google Scholar]
- 40.Lezoualc'h F, Metrich M, Hmitou I, Duquesnes N, Morel E. Small GTP-binding proteins and their regulators in cardiac hypertrophy. Journal of Molecular and Cellular Cardiology. 2008;44:623–632. doi: 10.1016/j.yjmcc.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 41.Aittaleb M, Boguth CA, Tesmer JJ. Structure and function of heterotrimeric g protein-regulated rho guanine nucleotide exchange factors. Molecular Pharmacology. 2009;77:111–125. doi: 10.1124/mol.109.061234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diviani D, Soderling J, Scott JD. AKAP-Lbc anchors protein kinase A and nucleates Gα12-selective Rho-mediated stress fiber formation. The Journal of Biological Chemistry. 2001;276:44247. doi: 10.1074/jbc.M106629200. [DOI] [PubMed] [Google Scholar]
- 43.Appert-Collin A, Cotecchia S, Nenniger-Tosato M, Pedrazzini T, Diviani D. The A-kinase anchoring protein (AKAP)-Lbc-signaling complex mediates α1 adrenergic receptor-induced cardiomyocyte hypertrophy. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10140. doi: 10.1073/pnas.0701099104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Finn SG, Plonk SG, Fuller SJ. Gα13 stimulates gene expression and increases cell size in cultured neonatal rat ventricular myocytes. Cardiovascular Research. 1999;42:140. doi: 10.1016/s0008-6363(98)00294-6. [DOI] [PubMed] [Google Scholar]
- 45.Arai K, Maruyama Y, Nishida M, Tanabe S, Takagahara S, Kozasa T, et al. Differential requirement of Gα12, Gα13, Gαq, and G βγ for endothelin-1-induced c-Jun NH2-terminal kinase and extracellular signal-regulated kinase activation. Molecular Pharmacology. 2003;63:478. doi: 10.1124/mol.63.3.478. [DOI] [PubMed] [Google Scholar]
- 46.Nishida M, Sato Y, Uemura A, Narita Y, Tozaki-Saitoh H, Nakaya M, et al. P2Y6 receptor-Gα12/13 signalling in cardiomyocytes triggers pressure overload-induced cardiac fibrosis. The EMBO Journal. 2008;27:3104. doi: 10.1038/emboj.2008.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Esposito G, Prasad SV, Rapacciuolo A, Mao L, Koch WJ, Rockman HA. Cardiac overexpression of a G(q) inhibitor blocks induction of extracellular signal-regulated kinase and c-Jun NH(2)-terminal kinase activity in in vivo pressure overload. Circulation. 2001;103:1453. doi: 10.1161/01.cir.103.10.1453. [DOI] [PubMed] [Google Scholar]
- 48.Zhang YM, Bo J, Taffet GE, Chang J, Shi J, Reddy AK, et al. Targeted deletion of ROCK1 protects the heart against pressure overload by inhibiting reactive fibrosis. The FASEB Journal. 2006;20:916. doi: 10.1096/fj.05-5129com. [DOI] [PubMed] [Google Scholar]
- 49.Shi J, Zhang YW, Summers LJ, Dorn GW, Wei L. Disruption of ROCK1 gene attenuates cardiac dilation and improves contractile function in pathological cardiac hypertrophy. Journal of Molecular and Cellular Cardiology. 2008;44:551. doi: 10.1016/j.yjmcc.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Del Re DP, Miyamoto S, Brown JH. RhoA/Rho kinase upregulate Bax to activate a mitochondrial death pathway and induce cardiomyocyte apoptosis. The Journal of Biological Chemistry. 2007;282:8069. doi: 10.1074/jbc.M604298200. [DOI] [PubMed] [Google Scholar]
- 51.Del Re DP, Miyamoto S, Brown JH. Focal adhesion kinase as a RhoA-activable signaling scaffold mediating Akt activation and cardiomyocyte protection. The Journal of Biological Chemistry. 2008;283:35622. doi: 10.1074/jbc.M804036200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ishii I, Ye X, Friedman B, Kawamura S, Contos JJA, Kingsbury MA, et al. Marked perinatal lethality and cellular signaling deficits in mice null for the two sphingosine 1-phosphate receptors, S1P2/LPB2/EDG-5 and S1P3/LPB3/EDG-3. The Journal of Biological Chemistry. 2002;277:25152. doi: 10.1074/jbc.M200137200. [DOI] [PubMed] [Google Scholar]
- 53.Sah VP, Minamisawa S, Tam SP, Wu TH, Dorn GW, Ross J, Jr, et al. Cardiac-specific overexpression of RhoA results in sinus and atrioventricular nodal dysfunction and contractile failure. Journal of Clinical Investigation. 1999;103:1627. doi: 10.1172/JCI6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kontaridis MI, Yang W, Bence KK, Cullen D, Wang B, Bodyak N, et al. Deletion of Ptpn11 (Shp2) in cardiomyocytes causes dilated cardiomyopathy via effects on the extracellular signal-regulated kinase/mitogen-activated protein kinase and RhoA signaling pathways. Circulation. 2008;117:1423. doi: 10.1161/CIRCULATIONAHA.107.728865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rikitake Y, Oyama N, Wang CY, Noma K, Satoh M, Kim HH, et al. Decreased perivascular fibrosis but not cardiac hypertrophy in ROCK1+/− haploinsufficient mice. Circulation. 2005;112:2959. doi: 10.1161/CIRCULATIONAHA.105.584623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kobayashi N, Horinaka S, Mita S, Nakano S, Honda T, Yoshida K, et al. Critical role of Rho-kinase pathway for cardiac performance and remodeling in failing rat hearts. Cardiovascular Research. 2002;55:757. doi: 10.1016/s0008-6363(02)00457-1. [DOI] [PubMed] [Google Scholar]
- 57.Satoh S, Ueda Y, Koyanagi M, Kadokami T, Sugano M, Yoshikawa Y, et al. Chronic inhibition of Rho kinase blunts the process of left ventricular hypertrophy leading to cardiac contractile dysfunction in hypertension-induced heart failure. Journal of Molecular and Cellular Cardiology. 2003;35:59. doi: 10.1016/s0022-2828(02)00278-x. [DOI] [PubMed] [Google Scholar]
- 58.Hattori T, Shimokawa H, Higashi M, Hiroki J, Mukai Y, Tsutsui H, et al. Long-term inhibition of Rho-kinase suppresses left ventricular remodeling after myocardial infarction in mice. Circulation. 2004;109:2234. doi: 10.1161/01.CIR.0000127939.16111.58. [DOI] [PubMed] [Google Scholar]
- 59.Chang J, Xie M, Shah VR, Schneider MD, Entman ML, Wei L, et al. Activation of Rho-associated coiled-coil protein kinase 1 (ROCK-1) by caspase-3 cleavage plays an essential role in cardiac myocyte apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:14495. doi: 10.1073/pnas.0601911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bao W, Hu E, Tao L, Boyce R, Mirabile R, Thudium DT, et al. Inhibition of Rho-kinase protects the heart against ischemia/reperfusion injury. Cardiovascular Research. 2004;61:548. doi: 10.1016/j.cardiores.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 61.Hamid SA, Bower HS, Baxter GF. Rho kinase activation plays a major role as a mediator of irreversible injury in reperfused myocardium. American Journal of Physiology. Heart and Circulatory Physiology. 2007;292:H2598. doi: 10.1152/ajpheart.01393.2006. [DOI] [PubMed] [Google Scholar]
- 62.Sahai E, Alberts AS, Treisman R. RhoA effector mutants reveal distinct effector pathways for cytoskeletal reorganization. SRF activation and transformation. EMBO Journal. 1998;17:1350. doi: 10.1093/emboj/17.5.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nelson CM, Pirone DM, Tan JL, Chen CS. Vascular endothelial-cadherin regulates cytoskeletal tension, cell spreading, and focal adhesions by stimulating RhoA. Molecular Biology of the Cell. 2004;15:2943. doi: 10.1091/mbc.E03-10-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao Z, Rivkees SA. Rho-associated kinases play a role in endocardial cell differentiation and migration. Developmental Biology. 2004;275:183. doi: 10.1016/j.ydbio.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 65.Rikitake Y, Liao JK. Rho-kinase mediates hyperglycemia-induced plasminogen activator inhibitor-1 expression in vascular endothelial cells. Circulation. 2005;111:3261. doi: 10.1161/CIRCULATIONAHA.105.534024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alblas J, Ulfman L, Hordijk P, Koenderman L. Activation of Rhoa and ROCK are essential for detachment of migrating leukocytes. Molecular Biology of the Cell. 2001;12:2137. doi: 10.1091/mbc.12.7.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiological Reviews. 2008;88:581. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aghajanian A, Wittchen ES, Campbell SL, Burridge K. Direct activation of RhoA by reactive oxygen species requires a redox-sensitive motif. PLoS ONE. 2009;4:38045. doi: 10.1371/journal.pone.0008045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wolfrum S, Dendorfer A, Rikitake Y, Stalker TJ, Gong Y, Scalia R, et al. Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24:1842. doi: 10.1161/01.ATV.0000142813.33538.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Adams JW, Brown JH. G-proteins in growth and apoptosis: Lessons from the heart. Oncogene. 2001;20:1626. doi: 10.1038/sj.onc.1204275. [DOI] [PubMed] [Google Scholar]
- 71.Foo RS, Mani K, Kitsis RN. Death begets failure in the heart. Journal of Clinical Investigation. 2005;115:565. doi: 10.1172/JCI24569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dorn GW. Apoptotic and non-apoptotic programmed cardiomyocyte death in ventricular remodelling. Cardiovascular Research. 2009;81:465. doi: 10.1093/cvr/cvn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Parsons JT. Focal adhesion kinase: The first ten years. Journal of Cell Science. 2003;116:1409. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 74.Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Current Opinion in Cell Biology. 2006;18:516. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 75.Hakim ZS, DiMichele LA, Rojas M, Meredith D, Mack CP, Taylor JM. FAK regulates cardiomyocyte survival following ischemia/reperfusion. Journal of Molecular and Cellular Cardiology. 2009;46:241. doi: 10.1016/j.yjmcc.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chong LD, Traynor-Kaplan A, Bokoch GM, Schwartz MA. The small GTP-binding protein Rho regulates a phosphatidylinositol 4-phosphate 5-kinase in mammalian cells. Cell. 1994;79:507. doi: 10.1016/0092-8674(94)90259-3. [DOI] [PubMed] [Google Scholar]
- 77.Ren X-D, Bokoch GM, Traynor-Kaplan A, Jenkins GH, Anderson RA, Schwartz MA. Physical association of the small GTPase Rho with a 68-kDa phosphatidylinositol 4-phosphate 5-kinase in Swiss 3T3 cells. Molecular Biology of the Cell. 1996;7:435. doi: 10.1091/mbc.7.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schmidt M, Bienek C, Rumenapp U, Zhang C, Lummen G, Jakobs KH, et al. A role for Rho in receptor- and G protein-stimulated phospholipase C. Reduction in phosphatidylinositol 4, 5-bisphosphate by Clostridium difficile toxin b. Naunyn-Schmiedeberg's Archives of Pharmacology. 1996;354:87. doi: 10.1007/BF00178707. [DOI] [PubMed] [Google Scholar]
- 79.Mejillano M, Yamamoto M, Rozelle AL, Sun H-Q, Wang X, Yin HL. Regulation of apoptosis by phosphatidylinositol 4,5 bisphosphate, inhibition of caspases, and caspase inactivation of phosphatidylinositol phosphate 5 kinases. Journal of Biological Chemistry. 2000 doi: 10.1074/jbc.M007271200. In Press. [DOI] [PubMed] [Google Scholar]
- 80.Howes AL, Arthur JF, Zhang T, Miyamoto S, Adams JW, Dorn GW, II, et al. Akt-mediated cardiomyocyte survival pathways are compromised by G α q-induced phosphoinositide 4, 5-bisphosphate depletion. The Journal of Biological Chemistry. 2003;278:40343. doi: 10.1074/jbc.M305964200. [DOI] [PubMed] [Google Scholar]
- 81.Wing MR, Bourdon DM, Harden TK. PLC-ε: A shared effector protein in Ras-, Rho-, and G αβγ-mediated signaling. Molecular Interventions. 2003;3:273. doi: 10.1124/mi.3.5.273. [DOI] [PubMed] [Google Scholar]
- 82.Bunney TD, Katan M. Phospholipase Cε: Linking second messengers and small GTPases. Trends in Cell Biology. 2006;16:640. doi: 10.1016/j.tcb.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 83.Citro S, Malik S, Oestreich EA, Radeff-Huang J, Kelley GG, Smrcka AV, et al. Phospholipase Cε is a nexus for Rho and Rap-mediated G protein-coupled receptor-induced astrocyte proliferation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15543. doi: 10.1073/pnas.0702943104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wing MR, Snyder JT, Sondek J, Harden TK. Direct activation of phospholipase C-ε by Rho. The Journal of Biological Chemistry. 2003;278:41253. doi: 10.1074/jbc.M306904200. [DOI] [PubMed] [Google Scholar]
- 85.Kelley GG, Reks SE, Smrcka AV. Hormonal regulation of phospholipase Cε through distinct and overlapping pathways involving G12 and Ras family G-proteins. The Biochemical Journal. 2004;378:129. doi: 10.1042/BJ20031370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Song C, Hu CD, Masago M, Kariyai K, Yamawaki-Kataoka Y, Shibatohge M, et al. Regulation of a novel human phospholipase C, PLCε, through membrane targeting by Ras. The Journal of Biological Chemistry. 2001;276:2752. doi: 10.1074/jbc.M008324200. [DOI] [PubMed] [Google Scholar]
- 87.Jin TG, Satoh T, Liao Y, Song C, Gao X, Kariya K, et al. Role of the CDC25 homology domain of phospholipase Cε in amplification of Rap1-dependent signaling. The Journal of Biological Chemistry. 2001;276:30301. doi: 10.1074/jbc.M103530200. [DOI] [PubMed] [Google Scholar]
- 88.Schmidt M, Evellin S, Weernink PA, von Dorp F, Rehmann H, Lomasney JW, et al. A new phospholipase-C-calcium signalling pathway mediated by cyclic AMP and a Rap GTPase. Nature Cell Biology. 2001;3:1020. doi: 10.1038/ncb1101-1020. [DOI] [PubMed] [Google Scholar]
- 89.Kelley GG, Reks SE, Ondrako JM, Smrcka AV. Phospholipase Cε: A novel Ras effector. The EMBO Journal. 2001;20:743. doi: 10.1093/emboj/20.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lopez I, Mak EC, Ding J, Hamm HE, Lomasney JW. A novel bifunctional phospholipase C that is regulated by Gα 12 and stimulates the Ras/mitogen-activated protein kinase pathway. The Journal of Biological Chemistry. 2001;276:2758. doi: 10.1074/jbc.M008119200. [DOI] [PubMed] [Google Scholar]
- 91.Hains MD, Wing MR, Maddileti S, Siderovski DP, Harden TK. Gα12/13- and Rho-dependent activation of phospholipase C-ε by lysophosphatidic acid and thrombin receptors. Molecular Pharmacology. 2006;69:2068. doi: 10.1124/mol.105.017921. [DOI] [PubMed] [Google Scholar]
- 92.Satoh T, Edamatsu H, Kataoka T. Phospholipase Ce guanine nucleotide exchange factor activity and activation of Rap1. Methods in Enzymology. 2007;407:281. doi: 10.1016/S0076-6879(05)07024-2. [DOI] [PubMed] [Google Scholar]
- 93.Kelley GG, Kaproth-Joslin KA, Reks SE, Smrcka AV, Wojcikiewicz RJ. G-protein-coupled receptor agonists activate endogenous phospholipase Cε and phospholipase Cβ3 in a temporally distinct manner. The Journal of Biological Chemistry. 2006;281:2639. doi: 10.1074/jbc.M507681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oestreich EA, Malik S, Goonasekera SA, Blaxall BC, Kelley GG, Dirksen RT, et al. Epac and phospholipase Cε regulate Ca2+ release in the heart by activation of protein kinase cε and calcium–calmodulin kinase II. The Journal of Biological Chemistry. 2009;284:1514. doi: 10.1074/jbc.M806994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yujiri T, Sather S, Fanger GR, Johnson GL. Role of MEKK1 in cell survival and activation of JNK and ERK pathways defined by targeted gene disruption. Science. 1998;282:1911. doi: 10.1126/science.282.5395.1911. [DOI] [PubMed] [Google Scholar]
- 96.Lips DJ, Bueno OF, Wilkins BJ, Purcell NH, Kaiser RA, Lorenz JN, et al. MEK1-ERK2 signaling pathway protects myocardium from ischemic injury in vivo. Circulation. 2004;109:1938. doi: 10.1161/01.CIR.0000127126.73759.23. [DOI] [PubMed] [Google Scholar]
- 97.Purcell NH, Wilkins BJ, York A, Saba-El-Leil MK, Meloche S, Robbins J, et al. Genetic inhibition of cardiac ERK1/2 promotes stress-induced apoptosis and heart failure but has no effect on hypertrophy in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14074. doi: 10.1073/pnas.0610906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Inagaki K, Churchill E, Mochly-Rosen D. Epsilon protein kinase C as a potential therapeutic target for the ischemic heart. Cardiovascular Research. 2006;70:222. doi: 10.1016/j.cardiores.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 99.Liu GS, Cohen MV, Mochly-Rosen D, Downey JM. Protein kinase C-ε is responsible for the protection of preconditioning in rabbit cardiomyocytes. Journal of Molecular and Cellular Cardiology. 1999;31:1937. doi: 10.1006/jmcc.1999.1026. [DOI] [PubMed] [Google Scholar]
- 100.Xuan YT, Guo Y, Zhu Y, Wang OL, Rokosh G, Messing RO, et al. Role of the protein kinase C-ε-Raf-1-MEK-1/2-p44/42 MAPK signaling cascade in the activation of signal transducers and activators of transcription 1 and 3 and induction of cyclooxygenase-2 after ischemic preconditioning. Circulation. 2005;112:1971. doi: 10.1161/CIRCULATIONAHA.105.561522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang WH, Lu FH, Zhao YJ, Wang LN, Tian Y, Pan ZW, et al. Post-conditioning protects rat cardiomyocytes via PKCε-mediated calcium-sensing receptors. Biochemical and Biophysical Research Communications. 2007;361:659. doi: 10.1016/j.bbrc.2007.07.077. [DOI] [PubMed] [Google Scholar]
- 102.Sivaraman V, Hausenloy DJ, Kolvekar S, Hayward M, Yap J, Lawrence D, et al. The divergent roles of protein kinase Cε and δ in simulated ischaemia-reperfusion injury in human myocardium. Journal of Molecular and Cellular Cardiology. 2009;46:758. doi: 10.1016/j.yjmcc.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 103.Wang H, Oestreich EA, Maekawa N, Bullard TA, Vikstrom KL, Dirksen RT, et al. Phospholipase Cε modulates β-adrenergic receptor-dependent cardiac contraction and inhibits cardiac hypertrophy. Circulation Research. 2005;97:1305. doi: 10.1161/01.RES.0000196578.15385.bb. [DOI] [PubMed] [Google Scholar]
- 104.Yoshimura S, Nakashima S, Ohguchi K, Sakai H, Shinoda J, Sakai N, et al. Differential mRNA expression of phospholipase D (PLD) isozymes during cAMP-induced differentiation in C6 glioma cells. Biochemical and Biophysical Research Communications. 1996;225:494. doi: 10.1006/bbrc.1996.1201. [DOI] [PubMed] [Google Scholar]
- 105.Hammond SM, Jenco JM, Nakashima S, Cadwallader K, Gu Q, Cook S, et al. Characterization of two alternately spliced forms of phospholipase D1. Activation of the purified enzymes by phosphatidylinositol 4, 5-bisphosphate, ADP-ribosylation factor, and Rho family monomeric GTP-binding proteins and protein kinase C-α. The Journal of Biological Chemistry. 1997;272:3860. doi: 10.1074/jbc.272.6.3860. [DOI] [PubMed] [Google Scholar]
- 106.Redina OE, Frohman MA. Organization and alternative splicing of the murine phospholipase D2 gene. The Biochemical Journal. 1998;331(Pt 3):845. doi: 10.1042/bj3310845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Colley WC, Sung TC, Roll R, Jenco J, Hammond SM, Altshuller Y, et al. Phospholipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeletal reorganization. Current Biology. 1997;7:191. doi: 10.1016/s0960-9822(97)70090-3. [DOI] [PubMed] [Google Scholar]
- 108.Bae CD, Min DS, Fleming IN, Exton JH. Determination of interaction sites on the small G protein RhoA for phospholipase D. The Journal of Biological Chemistry. 1998;273:11596. doi: 10.1074/jbc.273.19.11596. [DOI] [PubMed] [Google Scholar]
- 109.Sung TC, Zhang Y, Morris AJ, Frohman MA. Structural analysis of human phospholipase D1. The Journal of Biological Chemistry. 1999;274:3659. doi: 10.1074/jbc.274.6.3659. [DOI] [PubMed] [Google Scholar]
- 110.Xie Z, Ho WT, Spellman R, Cai S, Exton JH. Mechanisms of regulation of phospholipase D1 and D2 by the heterotrimeric G proteins G13 and Gq. The Journal of Biological Chemistry. 2002;277:11979. doi: 10.1074/jbc.M109751200. [DOI] [PubMed] [Google Scholar]
- 111.Du G, Altshuller YM, Kim Y, Han JM, Ryu SH, Morris AJ, et al. Dual requirement for rho and protein kinase C in direct activation of phospholipase D1 through G protein-coupled receptor signaling. Molecular Biology of the Cell. 2000;11:4359. doi: 10.1091/mbc.11.12.4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schmidt M, Rumenapp U, Bienek C, Keller J, von Eichel-Streiber C, Jakobs KH. Inhibition of receptor signaling to phospholipase D by Clostridium difficile toxin B. Role of Rho proteins. The Journal of Biological Chemistry. 1996;271:2422. doi: 10.1074/jbc.271.5.2422. [DOI] [PubMed] [Google Scholar]
- 113.Schmidt M, Voss M, Oude Weernink PA, Wetzel J, Amano M, Kaibuchi K, et al. A role for Rho-kinase in Rho-controlled phospholipase D stimulation by the m3 muscarinic acetylcholine receptor. The Journal of Biological Chemistry. 1999;274:14648. doi: 10.1074/jbc.274.21.14648. [DOI] [PubMed] [Google Scholar]
- 114.Yamazaki M, Zhang Y, Watanabe H, Yokozeki T, Ohno S, Kaibuchi K, et al. Interaction of the small G protein RhoA with the C terminus of human phospholipase D1. The Journal of Biological Chemistry. 1999;274:6035. doi: 10.1074/jbc.274.10.6035. [DOI] [PubMed] [Google Scholar]
- 115.Schmidt M, Nehls C, Rumenapp U, Jakobs KH. m3 Muscarinic receptor-induced and Gi-mediated heterologous potentiation of phospholipase C stimulation: Role of phosphoinositide synthesis. Molecular Pharmacology. 1996;50:1038. [PubMed] [Google Scholar]
- 116.Miyamoto S, Murphy AN, Brown JH. Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II. Cell Death and Differentiation. 2008;15:521. doi: 10.1038/sj.cdd.4402285. [DOI] [PubMed] [Google Scholar]
- 117.Miyamoto S, Murphy AN, Brown JH. Akt mediated mitochondrial protection in the heart: metabolic and survival pathways to the rescue. Journal of Bioenergetics and Biomembranes. 2009;41:169. doi: 10.1007/s10863-009-9205-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lecour S. Activation of the protective survivor activating factor enhancement (SAFE) pathway against reperfusion injury: Does it go beyond the RISK pathway? Journal of Molecular and Cellular Cardiology. 2009;47:32. doi: 10.1016/j.yjmcc.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 119.Lecour S. Multiple protective pathways against reperfusion injury: A SAFE path without Aktion? Journal of Molecular and Cellular Cardiology. 2009;46:607. doi: 10.1016/j.yjmcc.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 120.Taher MM, Mahgoub MA, Abd-Elfattah AS. Redox regulation of signal transduction in vascular smooth muscle cells: thiol oxidizing agents induced phospholipase D. Biochemistry and Molecular Biology International. 1998;46:619. doi: 10.1080/15216549800204142. [DOI] [PubMed] [Google Scholar]
- 121.Natarajan V, Vepa S, Verma RS, Scribner WM. Role of protein tyrosine phosphorylation in H2O2-induced activation of endothelial cell phospholipase D. The American Journal of Physiology. 1996;271:L400. doi: 10.1152/ajplung.1996.271.3.L400. [DOI] [PubMed] [Google Scholar]
- 122.Parinandi NL, Scribner WM, Vepa S, Shi S, Natarajan V. Phospholipase D activation in endothelial cells is redox sensitive. Antioxidants Redox Signaling. 1999;1:193. doi: 10.1089/ars.1999.1.2-193. [DOI] [PubMed] [Google Scholar]
- 123.Banno Y, Wang S, Ito Y, Izumi T, Nakashima S, Shimizu T, et al. Involvement of ERK and p38 MAP kinase in oxidative stress-induced phospholipase D activation in PC12 cells. NeuroReport. 2001;12:2271. doi: 10.1097/00001756-200107200-00045. [DOI] [PubMed] [Google Scholar]
- 124.Kim JH, Lee S, Park JB, Lee SD, Kim JH, Ha SH, et al. Hydrogen peroxide induces association between glyceraldehyde 3-phosphate dehydrogenase and phospholipase D2 to facilitate phospholipase D2 activation in PC12 cells. Journal of Neurochemistry. 2003;85:1228. doi: 10.1046/j.1471-4159.2003.01755.x. [DOI] [PubMed] [Google Scholar]
- 125.Dai J, Meij JT, Padua R, Panagia V. Depression of cardiac sarcolemmal phospholipase D activity by oxidant-induced thiol modification. Circulation Research. 1992;71:970. doi: 10.1161/01.res.71.4.970. [DOI] [PubMed] [Google Scholar]
- 126.Dai J, Meij JT, Dhalla V, Panagia V. Involvement of thiol groups in the impairment of cardiac sarcoplasmic reticular phospholipase D activity by oxidants. Journal of Lipid Mediators and Cell Signalling. 1995;11:107. doi: 10.1016/0929-7855(94)00031-7. [DOI] [PubMed] [Google Scholar]
- 127.Asemu G, Dent MR, Singal T, Dhalla NS, Tappia PS. Differential changes in phospholipase D and phosphatidate phosphohydrolase activities in ischemia-reperfusion of rat heart. Archives of Biochemistry and Biophysics. 2005;436:136. doi: 10.1016/j.abb.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 128.Bruhl A, Faldum A, Loffelholz K. Degradation of phosphatidylethanol counteracts the apparent phospholipase D-mediated formation in heart and other organs. Biochimica et Biophysica Acta. 2003;1633:84. doi: 10.1016/s1388-1981(03)00090-8. [DOI] [PubMed] [Google Scholar]
- 129.Kurz T, Kemken D, Mier K, Weber I, Richardt G. Human cardiac phospholipase D activity is tightly controlled by phosphatidylinositol 4, 5-bisphosphate. Journal of Molecular and Cellular Cardiology. 2004;36:225. doi: 10.1016/j.yjmcc.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 130.Cohen MV, Liu Y, Liu GS, Wang P, Weinbrenner C, Cordis GA, et al. Phospholipase D plays a role in ischemic preconditioning in rabbit heart. Circulation. 1996;94:1713. doi: 10.1161/01.cir.94.7.1713. [DOI] [PubMed] [Google Scholar]
- 131.Tosaki A, Maulik N, Cordis G, Trifan OC, Popescu LM, Das DK. Ischemic preconditioning triggers phospholipase D signaling in rat heart. The American Journal of Physiology. 1997;273:H1860. doi: 10.1152/ajpheart.1997.273.4.H1860. [DOI] [PubMed] [Google Scholar]
- 132.Mozzicato S, Joshi BV, Jacobson KA, Liang BT. Role of direct RhoA-phospholipase D1 interaction in mediating adenosine-induced protection from cardiac ischemia. The FASEB Journal. 2004;18:406. doi: 10.1096/fj.03-0592fje. [DOI] [PubMed] [Google Scholar]
- 133.Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, et al. PKC-α regulates cardiac contractility and propensity toward heart failure. Natural Medicines. 2004;10:248. doi: 10.1038/nm1000. [DOI] [PubMed] [Google Scholar]
- 134.Liu Q, Chen X, MacDonnell SM, Kranias EG, Lorenz JN, Leitges M, et al. Protein kinase Cα, but not PKCβ or PKCγ, regulates contractility and heart failure susceptibility: Implications for ruboxistaurin as a novel therapeutic approach. Circulation Research. 2009;105:194. doi: 10.1161/CIRCRESAHA.109.195313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Saurin AT, Pennington DJ, Raat NJ, Latchman DS, Owen MJ, Marber MS. Targeted disruption of the protein kinase Cε gene abolishes the infarct size reduction that follows ischaemic preconditioning of isolated buffer-perfused mouse hearts. Cardiovascular Research. 2002;55:672. doi: 10.1016/s0008-6363(02)00325-5. [DOI] [PubMed] [Google Scholar]
- 136.Zatta AJ, Kin H, Lee G, Wang N, Jiang R, Lust R, et al. Infarct-sparing effect of myocardial postconditioning is dependent on protein kinase C signalling. Cardiovascular Research. 2006;70:315. doi: 10.1016/j.cardiores.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 137.Mayr M, Metzler B, Chung YL, McGregor E, Mayr U, Troy H, et al. Ischemic preconditioning exaggerates cardiac damage in PKC-δ null mice. American Journal of Physiology. Heart and Circulatory Physiology. 2004;287:H946. doi: 10.1152/ajpheart.00878.2003. [DOI] [PubMed] [Google Scholar]
- 138.Churchill EN, Szweda LI. Translocation of δPKC to mitochondria during cardiac reperfusion enhances superoxide anion production and induces loss in mitochondrial function. Archives of Biochemistry and Biophysics. 2005;439:194. doi: 10.1016/j.abb.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 139.Churchill EN, Murriel CL, Chen CH, Mochly-Rosen D, Szweda LI. Reperfusion-induced translocation of δPKC to cardiac mitochondria prevents pyruvate dehydrogenase reactivation. Circulation Research. 2005;97:78. doi: 10.1161/01.RES.0000173896.32522.6e. [DOI] [PubMed] [Google Scholar]
- 140.Inagaki K, Chen L, Ikeno F, Lee FH, Imahashi K, Bouley DM, et al. Inhibition of δ-protein kinase C protects against reperfusion injury of the ischemic heart in vivo. Circulation. 2003;108:2304. doi: 10.1161/01.CIR.0000101682.24138.36. [DOI] [PubMed] [Google Scholar]
- 141.Murriel CL, Churchill E, Inagaki K, Szweda LI, Mochly-Rosen D. Protein kinase Cδ activation induces apoptosis in response to cardiac ischemia and reperfusion damage: A mechanism involving BAD and the mitochondria. The Journal of Biological Chemistry. 2004;279:47985. doi: 10.1074/jbc.M405071200. [DOI] [PubMed] [Google Scholar]
- 142.Murriel CL, Mochly-Rosen D. Opposing roles of δ and εPKC in cardiac ischemia and reperfusion: targeting the apoptotic machinery. Archives of Biochemistry and Biophysics. 2003;420:246. doi: 10.1016/j.abb.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 143.Chen L, Hahn H, Wu G, Chen CH, Liron T, Schechtman D, et al. Opposing cardioprotective actions and parallel hypertrophic effects of δPKC and εPKC. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:11114. doi: 10.1073/pnas.191369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Inagaki K, Hahn HS, Dorn GW, Mochly-Rosen D. Additive protection of the ischemic heart ex vivo by combined treatment with δ-protein kinase C inhibitor and ε- protein kinase C activator. Circulation. 2003;108:869. doi: 10.1161/01.CIR.0000081943.93653.73. [DOI] [PubMed] [Google Scholar]
- 145.Haworth RS, Roberts NA, Cuello F, Avkiran M. Regulation of protein kinase D activity in adult myocardium: novel counter-regulatory roles for protein kinase Cε and protein kinase A. Journal of Molecular and Cellular Cardiology. 2007;43:686. doi: 10.1016/j.yjmcc.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 146.Rey O, Reeve JR, Jr, Zhukova E, Sinnett-Smith J, Rozengurt E. G protein-coupled receptor-mediated phosphorylation of the activation loop of protein kinase D: Dependence on plasma membrane translocation and protein kinase Cε. The Journal of Biological Chemistry. 2004;279:34361. doi: 10.1074/jbc.M403265200. [DOI] [PubMed] [Google Scholar]
- 147.Brandlin I, Eiseler T, Salowsky R, Johannes FJ. Protein kinase Cμ regulation of the JNK pathway is triggered via phosphoinositide-dependent kinase 1 and protein kinase Cε. The Journal of Biological Chemistry. 2002;277:45451. doi: 10.1074/jbc.M205299200. [DOI] [PubMed] [Google Scholar]
- 148.Tan M, Xu X, Ohba M, Ogawa W, Cui MZ. Thrombin rapidly induces protein kinase D phosphorylation, and protein kinase C δ mediates the activation. The Journal of Biological Chemistry. 2003;278:2824. doi: 10.1074/jbc.M211523200. [DOI] [PubMed] [Google Scholar]
- 149.Haworth RS, Cuello F, Herron TJ, Franzen G, Kentish JC, Gautel M, et al. Protein kinase D is a novel mediator of cardiac troponin I phosphorylation and regulates myofilament function. Circulation Research. 2004;95:1091. doi: 10.1161/01.RES.0000149299.34793.3c. [DOI] [PubMed] [Google Scholar]
- 150.Cuello F, Bardswell SC, Haworth RS, Yin X, Lutz S, Wieland T, et al. Protein kinase D selectively targets cardiac troponin I and regulates myofilament Ca2+ sensitivity in ventricular myocytes. Circulation Research. 2007;100:864. doi: 10.1161/01.RES.0000260809.15393.fa. [DOI] [PubMed] [Google Scholar]
- 151.Huynh QK, McKinsey TA. Protein kinase D directly phosphorylates histone deacetylase 5 via a random sequential kinetic mechanism. Archives of Biochemistry and Biophysics. 2006;450:141. doi: 10.1016/j.abb.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 152.Vega RB, Harrison BC, Meadows E, Roberts CR, Papst PJ, Olson EN, et al. Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Molecular and Cellular Biology. 2004;24:8374. doi: 10.1128/MCB.24.19.8374-8385.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dequiedt F, Van LJ, Lecomte E, Van D, Seufferlein VT, Vandenheede JR, et al. Phosphorylation of histone deacetylase 7 by protein kinase D mediates T cell receptor-induced Nur77 expression and apoptosis. The Journal of Experimental Medicine. 2005;201:793. doi: 10.1084/jem.20042034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Storz P, Toker A. Protein kinase D mediates a stress-induced NF-kappaB activation and survival pathway. The EMBO Journal. 2003;22:109. doi: 10.1093/emboj/cdg009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Storz P, Doppler H, Toker A. Protein kinase D mediates mitochondrion-to-nucleus signaling and detoxification from mitochondrial reactive oxygen species. Molecular and Cellular Biology. 2005;25:8520. doi: 10.1128/MCB.25.19.8520-8530.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Avkiran M, Rowland AJ, Cuello F, Haworth RS. Protein kinase d in the cardiovascular system: Emerging roles in health and disease. Circulation Research. 2008;102:157. doi: 10.1161/CIRCRESAHA.107.168211. [DOI] [PubMed] [Google Scholar]
- 157.Yuan J, Slice LW, Rozengurt E. Activation of protein kinase D by signaling through Rho and the α subunit of the heterotrimeric G protein G13. The Journal of Biological Chemistry. 2001;276:38619. doi: 10.1074/jbc.M105530200. [DOI] [PubMed] [Google Scholar]
- 158.Cowell CF, Yan IK, Eiseler T, Leightner AC, Doppler H, Storz P. Loss of cell-cell contacts induces NF-kappaB via RhoA-mediated activation of protein kinase D1. Journal of Cellular Biochemistry. 2009;106:714. doi: 10.1002/jcb.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Song J, Li J, Lulla A, Evers BM, Chung DH. Protein kinase D protects against oxidative stress-induced intestinal epithelial cell injury via Rho/ROK/PKC-δ pathway activation. American Journal of Physiology. Cell Physiology. 2006;290:C1469. doi: 10.1152/ajpcell.00486.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Laudanna C, Campbell JJ, Butcher EC. Role of Rho in chemoattractant-activated leukocyte adhesion through integrins. Science. 1996;271:981. doi: 10.1126/science.271.5251.981. [DOI] [PubMed] [Google Scholar]
- 161.Kaibuchi K, Kuroda S, Amano M. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annual Review of Biochemistry. 1999;68:459. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]
- 162.Seasholtz TM, Majumdar M, Kaplan DD, Brown JH. Rho and Rho kinase mediate thrombin-stimulated vascular smooth muscle cell DNA synthesis and migration. Circulation Research. 1999;84:1186. doi: 10.1161/01.res.84.10.1186. [DOI] [PubMed] [Google Scholar]
- 163.Schmitz AA, Govek EE, Bottner B, Van Aelst L. Rho GTPases: Signaling, migration, and invasion. Experimental Cell Research. 2000;261:1. doi: 10.1006/excr.2000.5049. [DOI] [PubMed] [Google Scholar]
- 164.Ridley AJ. Rho GTPases and cell migration. Journal of Cell Science. 2001;114:2713. doi: 10.1242/jcs.114.15.2713. [DOI] [PubMed] [Google Scholar]
- 165.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 166.Sahai E, Marshall C. RHO-GTPases and cancer. Nature Reviews. 2002;2:133. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 167.Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Developmental Biology. 2004;265:23. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 168.Jaffe AB, Hall A. Rho GTPases: Biochemistry and biology. Annual Review of Cell and Developmental Biology. 2005;21:247. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 169.Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochimica et Biophysica Acta. 2007;1773:642. doi: 10.1016/j.bbamcr.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hill CS, Wynne J, Treisman R. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995;81:1159. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- 171.Gauthier-Rouviere C, Cavadore JC, Blanchard JM, Lamb NJ, Fernandez A. p67SRF is a constitutive nuclear protein implicated in the modulation of genes required throughout the G1 period. Cell Regulation. 1991;2:575. doi: 10.1091/mbc.2.7.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hill CS, Treisman R. Transcriptional regulation by extracellular signals: mechanisms and specificity. Cell. 1995;80:199. doi: 10.1016/0092-8674(95)90403-4. [DOI] [PubMed] [Google Scholar]
- 173.Bence K, Ma W, Kozasa T, Huang X-Y. Direct stimulation of Bruton's tyrosine kinase by Gq-protein α-subunit. Nature. 1997;389:296. doi: 10.1038/38520. [DOI] [PubMed] [Google Scholar]
- 174.Fromm C, Coso OA, Montaner S, Xu N, Gutkind JS. The small GTP-binding protein Rho links G protein-coupled receptors and Gα12 to the serum response element and to cellular transformation. Proceedings of the National Academy of Sciences of the United States of America. 1997;91:10098. doi: 10.1073/pnas.94.19.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shore P, Sharrocks AD. The MADS-box family of transcription factors. European Journal of Biochemistry. 1995;229:1. doi: 10.1111/j.1432-1033.1995.tb20430.x. [DOI] [PubMed] [Google Scholar]
- 176.Treisman R. Ternary complex factors: Growth factor regulated transcriptional activators. Current Opinion in Genetics & Development. 1994;4:96. doi: 10.1016/0959-437x(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 177.Cen B, Selvaraj A, Burgess RC, Hitzler JK, Ma Z, Morris SW, et al. Megakaryoblastic leukemia 1, a potent transcriptional coactivator for serum response factor (SRF), is required for serum induction of SRF target genes. Molecular and Cellular Biology. 2003;23:6597. doi: 10.1128/MCB.23.18.6597-6608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cen B, Selvaraj A, Prywes R. Myocardin/MKL family of SRF coactivators: Key regulators of immediate early and muscle specific gene expression. Journal of Cellular Biochemistry. 2004;93:74. doi: 10.1002/jcb.20199. [DOI] [PubMed] [Google Scholar]
- 179.Posern G, Treisman R. Actin' together: Serum response factor, its cofactors and the link to signal transduction. Trends in Cell Biology. 2006;16:588. doi: 10.1016/j.tcb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 180.Johansen FE, Prywes R. Serum response factor: Transcriptional regulation of genes induced by growth factors and differentiation. Biochimica et Biophysica Acta. 1995;1242:1. doi: 10.1016/0304-419x(94)00014-s. [DOI] [PubMed] [Google Scholar]
- 181.Ueyama T, Sakoda T, Kawashima S, Hiraoka E, Hirata K, Akita H, et al. Activated RhoA stimulates c-fos gene expression in myocardial cells. Circulation Research. 1997;81:672. doi: 10.1161/01.res.81.5.672. [DOI] [PubMed] [Google Scholar]
- 182.Morissette MR, Sah VP, Glembotski CC, Brown JH. The Rho effector, PKN, regulates ANF gene transcription in cardiomyocutes through a serum response element. The American Journal of Physiology. 2000;278:H1769. doi: 10.1152/ajpheart.2000.278.6.H1769. [DOI] [PubMed] [Google Scholar]
- 183.Wang DZ, Li S, Hockemeyer D, Sutherland L, Wang Z, Schratt G, et al. Potentiation of serum response factor activity by a family of myocardin-related transcription factors. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:14855. doi: 10.1073/pnas.222561499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Parmacek MS. Myocardin-related transcription factors: Critical coactivators regulating cardiovascular development and adaptation. Circulation Research. 2007;100:633. doi: 10.1161/01.RES.0000259563.61091.e8. [DOI] [PubMed] [Google Scholar]
- 185.Sotiropoulos A, Gineitis D, Copeland J, Treisman R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell. 1999;98:159. doi: 10.1016/s0092-8674(00)81011-9. [DOI] [PubMed] [Google Scholar]
- 186.Wang D, Chang PS, Wang Z, Sutherland L, Richardson JA, Small E, et al. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell. 2001;105:851. doi: 10.1016/s0092-8674(01)00404-4. [DOI] [PubMed] [Google Scholar]
- 187.Wang Z, Wang DZ, Pipes GC, Olson EN. Myocardin is a master regulator of smooth muscle gene expression. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:7129. doi: 10.1073/pnas.1232341100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329. doi: 10.1016/s0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- 189.Lockman K, Hinson JS, Medlin MD, Morris D, Taylor JM, Mack CP. Sphingosine 1-phosphate stimulates smooth muscle cell differentiation and proliferation by activating separate serum response factor co-factors. The Journal of Biological Chemistry. 2004;279:42422. doi: 10.1074/jbc.M405432200. [DOI] [PubMed] [Google Scholar]
- 190.Geneste O, Copeland JW, Treisman R. LIM kinase and Diaphanous cooperate to regulate serum response factor and actin dynamics. The Journal of Cell Biology. 2002;157:831. doi: 10.1083/jcb.200203126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Muehlich S, Wang R, Lee SM, Lewis TC, Dai C, Prywes R. Serum-induced phosphorylation of the serum response factor coactivator MKL1 by the extracellular signal-regulated kinase 1/2 pathway inhibits its nuclear localization. Molecular and Cellular Biology. 2008;28:6302. doi: 10.1128/MCB.00427-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Vartiainen MK, Guettler S, Larijani B, Treisman R. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science. 2007;316:1749. doi: 10.1126/science.1141084. [DOI] [PubMed] [Google Scholar]
- 193.Arsenian S, Weinhold B, Oelgeschlager M, Ruther U, Nordheim A. Serum response factor is essential for mesoderm formation during mouse embryogenesis. The EMBO Journal. 1998;17:6289. doi: 10.1093/emboj/17.21.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Du KL, Chen M, Li J, Lepore JJ, Mericko P, Parmacek MS. Megakaryoblastic leukemia factor-1 transduces cytoskeletal signals and induces smooth muscle cell differentiation from undifferentiated embryonic stem cells. The Journal of Biological Chemistry. 2004;279:17578. doi: 10.1074/jbc.M400961200. [DOI] [PubMed] [Google Scholar]
- 195.Selvaraj A, Prywes R. Expression profiling of serum inducible genes identifies a subset of SRF target genes that are MKL dependent. BMC Molecular Biology. 2004;5:13. doi: 10.1186/1471-2199-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004;363:62. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- 197.Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. Journal of Cell Science. 2006;119:4803. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- 198.Chen CC, Lau LF. Functions and mechanisms of action of CCN matricellular proteins. International Journal of Biochemistry & Cell Biology. 2008 doi: 10.1016/j.biocel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Brigstock DR. The connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocrine Reviews. 1999;20:189. doi: 10.1210/edrv.20.2.0360. [DOI] [PubMed] [Google Scholar]
- 200.Yang GP, Lau LF. Cyr61, product of a growth factor-inducible immediate early gene, is associated with the extracellular matrix and the cell surface. Cell Growth & Differentiation. 1991;2:351. [PubMed] [Google Scholar]
- 201.O'Brien TP, Yang GP, Sanders L, Lau LF. Expression of cyr61, a growth factor-inducible immediate-early gene. Molecular and Cellular Biology. 1990;10:3569. doi: 10.1128/mcb.10.7.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kireeva ML, Mo FE, Yang GP, Lau LF. Cyr61, a product of a growth factor-inducible immediate-early gene, promotes cell proliferation, migration, and adhesion. Molecular and Cellular Biology. 1996;16:1326. doi: 10.1128/mcb.16.4.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Chen Y, Du XY. Functional properties and intracellular signaling of CCN1/Cyr61. Journal of Cellular Biochemistry. 2007;100:1337. doi: 10.1002/jcb.21194. [DOI] [PubMed] [Google Scholar]
- 204.Walsh CT, Stupack D, Brown JH. G protein-coupled receptors go extracellular: RhoA integrates the integrins. Molecular Interventions. 2008;8:165. doi: 10.1124/mi.8.4.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tamura I, Rosenbloom J, Macarak E, Chaqour B. Regulation of Cyr61 gene expression by mechanical stretch through multiple signaling pathways. American Journal of Physiology. Cell Physiology. 2001;281:C1524. doi: 10.1152/ajpcell.2001.281.5.C1524. [DOI] [PubMed] [Google Scholar]
- 206.Chaqour B, Goppelt-Struebe M. Mechanical regulation of the Cyr61/CCN1 and CTGF/CCN2 proteins. The FEBS Journal. 2006;273:3639. doi: 10.1111/j.1742-4658.2006.05360.x. [DOI] [PubMed] [Google Scholar]
- 207.Yang R, Amir J, Liu H, Chaqour B. Mechanical strain activates a program of genes functionally involved in paracrine signaling of angiogenesis. Physiological Genomics. 2008;36:1. doi: 10.1152/physiolgenomics.90291.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hanna M, Liu H, Amir J, Sun Y, Morris SW, Siddiqui M, et al. Mechanical regulation of the proangiogenic factor CCN1/CYR61 gene requires the combined activities of the myocardin-related transcription factor (MRTF)-A and CREB binding protein (CBP) histone acetyl transferase. The Journal of Biological Chemistry. 2009;284:23125. doi: 10.1074/jbc.M109.019059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Gabrielsen A, Lawler PR, Yongzhong W, Steinbruchel D, Blagoja D, Paulsson-Berne G, et al. Gene expression signals involved in ischemic injury, extracellular matrix composition and fibrosis defined by global mRNA profiling of the human left ventricular myocardium. Journal of Molecular and Cellular Cardiology. 2007;42:870. doi: 10.1016/j.yjmcc.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 210.Wittchen F, Suckau L, Witt H, Skurk C, Lassner D, Fechner H, et al. Genomic expression profiling of human inflammatory cardiomyopathy (DCMi) suggests novel therapeutic targets. Journal of Molecular Medicine. 2007;85:257. doi: 10.1007/s00109-006-0122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hilfiker-Kleiner D, Kaminski K, Kaminska A, Fuchs M, Klein G, Podewski E, et al. Regulation of proangiogenic factor CCN1 in cardiac muscle: impact of ischemia, pressure overload, and neurohumoral activation. Circulation. 2004;109:2227. doi: 10.1161/01.CIR.0000127952.90508.9D. [DOI] [PubMed] [Google Scholar]
- 212.Han JS, Macarak E, Rosenbloom J, Chung KC, Chaqour B. Regulation of Cyr61/CCN1 gene expression through RhoA GTPase and p38MAPK signaling pathways. European Journal of Biochemistry. 2003;270:3408. doi: 10.1046/j.1432-1033.2003.03723.x. [DOI] [PubMed] [Google Scholar]
- 213.Young N, Van B., Jr Roles of sphingosine-1- phosphate (S1P) receptors in malignant behavior of glioma cells. Differential effects of S1P2 on cell migration and invasiveness. Experimental Cell Research. 2007;313:1615. doi: 10.1016/j.yexcr.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yoshida Y, Togi K, Matsumae H, Nakashima Y, Kojima Y, Yamamoto H, et al. CCN1 protects cardiac myocytes from oxidative stress via β1 integrin-Akt pathway. Biochemical and Biophysical Research Communications. 2007;355:611. doi: 10.1016/j.bbrc.2007.01.195. [DOI] [PubMed] [Google Scholar]