Abstract
BACKGROUND:
Various studies have been performed throughout the world on the rate of restenosis using bare metal stents (BMS) and drug-eluting stents (DES). The prohibitive costs associated with DES generally dictate the type of stent used, especially in developing countries. Therefore, there was a need for a study to assess the effect of various risk factors on restenosis in BMS and DES in the Indian context. A study was performed in the premier institution of the Indian Armed Forces, the Army Hospital (Research and Referral), New Delhi, India, under the aegis of the Indian Council of Medical Research (New Delhi). The profile of patients in the armed forces is inherently diverse in terms of demography, ethnicity, genetics, etc, which reflects the diverse and varied nature of the population in India.
METHODS AND RESULTS:
A total of 130 patients were included in the present study. Follow-up after stent implantation was scheduled for six to nine months following the procedure to assess symptoms, drug compliance, and treadmill test and coronary angiography results, and to ascertain the incidence of restenosis. However, only 80 patients returned for follow-up and, therefore, the final analysis was based on these patients. They were segregated into BMS (n=41) and DES (n=39) groups. Restenosis occurred in 29 patients (36.3%). Nine of 39 patients with DES (23.1%) and 20 of 41 patients with BMS (48.8%) developed restenosis. There was a statistically significant relationship between restenosis and female sex, clinical presentation before intervention and at the time of follow-up evaluation (unstable angina), hypertension, positive stress test and compliance with medical therapy (P<0.05). No statistically significant relationship was observed between restenosis and age, diabetes, smoking, obesity and diet (P>0.05).
CONCLUSIONS:
DES appear to reduce the restenosis rate and clinical end points, and appear to be more cost effective than BMS. Patient-related factors (eg, sex, hypertension and unstable angina) are important variables that affect the restenosis rate. Noninvasive stress testing had high positive and negative predictive values. Therefore, based on the present study, noninvasive stress testing is suggested before routine angiography at follow-up, which will reduce the need for repeat coronary angiography.
Keywords: Armed forces, Bare metal stents, Drug-eluting stents, Predictors of restenosis, Real-world registry, Restenosis
The number of percutaneous coronary interventions performed each year has increased considerably and, along with it, the use of drug-eluting stents (DES) as a potential solution for restenosis has increased. The expenses involved in the use of DES are prohibitively high; thus, cost is a major limiting factor for its use in all cases, especially in developing countries.
Many trials demonstrating the performance of DES have been performed throughout the world. However, there was a need to conduct a study that would take into account the various factors affecting patients in developing countries, such as India, giving a realistic and credible analysis of the interplay of various factors on the performance of DES and bare metal stents (BMS).
The present study was conducted at Army Hospital (Research and Referral), New Delhi, India, under the aegis of the Indian Council of Medical Research (New Delhi). The present study was the first of this nature conducted in an armed forces institution in India. The advantage accrued was that the patient cohort, mainly formed by serving personnel, ex-servicemen and their relatives, both of officer and soldier rank, by default consisted of a diverse and varied population that was representative of the diverse and varied population in India.
The objective of the present study was to compare restenosis in the use of BMS and DES. Simultaneously, taking advantage of the diverse background of the cohort, the effect of various risk factors on restenosis was assessed to suggest a cost-effective stent therapy.
METHODS
The present study was conducted as a ‘real-world’ registry. A total of 130 patients were included, of which only 80 returned for follow-up. Therefore, the final analysis was based on these patients. The reason for loss of follow-up was that the population in the armed forces is roving due to postings, transfers and the exigencies of service. An attempt was made at each stage to solicit follow-up, starting with counselling of patients and attendants during the initial phase of treatment and during discharge. This was followed up by postal and telephone intimation whenever possible. They were segregated into BMS (n=41) and DES (n=39) groups. The selection criteria included a history of unstable angina (UA), chronic stable angina (CSA), myocardial infarction (MI) or the presence of high-risk factors for coronary artery disease (CAD) accompanied by evidence of ischemia on an exercise test. Patients with triple-vessel disease and severe left ventricular dysfunction were excluded. A number of variables that play a vital role in stent therapy and affect the restenosis rate as assessed by previous studies and trials were analyzed. These included demographic, clinical, angiographic and procedural factors, stent characteristics and drug compliance. Restenosis was assessed by visual estimation and quantitative coronary angiography. Follow-up of stent implantation to ascertain incidence of restenosis was scheduled six to nine months after the procedure. Statistical significance was derived using Pearson’s χ2 test and Fisher’s exact test.
RESULTS
Analysis of restenosis
Of the 80 patients included in the study, restenosis was present in 29 patients (36.3%) and absent in 51 patients (63.8%).
Restenosis related to type of stent:
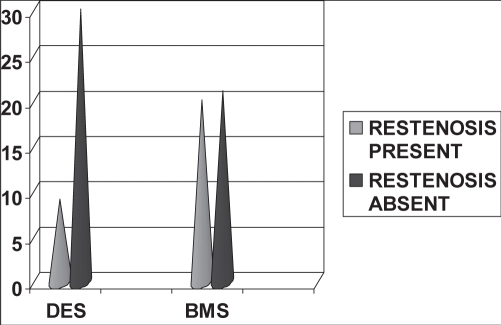
Restenosis was present in 20 patients in the BMS group (48.8%) and nine patients in the DES group (23.1%). The difference in the restenosis rate between the two stent groups was statistically significant (P<0.05) (Figure 1 and Table 1).
Figure 1).
Type of stents and restenosis. BMS Bare metal stents; DES Drug-eluting stents
TABLE 1.
Type of stents and restenosis
|
Stent type, n |
Total, n | ||
|---|---|---|---|
| Drug eluting | Bare metal | ||
| Restenosis present | 9 | 20 | 29 |
| Restenosis absent | 30 | 21 | 51 |
| Total | 39 | 41 | 80 |
χ2 at 1 differential = 5.71, P=0.017
In-stent/in-segment restenosis:
Of 29 patients with restenosis, 24 (82.8%) developed in-stent restenosis and four (13.8%) developed in-segment restenosis. Mean (± SD) in-stent restenosis length was 80.21±8.78 mm. Mean in-segment restenosis length was 76.25±14.9 mm.
Type of restenosis:
In the 29 patients with restenosis, there were 32 lesions, which included the following: 16 focal lesions (50%), seven diffuse lesions (21.9%), seven proliferative lesions (21.9%) and two occlusive lesions (6.3%). Five patients (17.24%) underwent target vessel revascularization (TVR) and 20 (68.9%) underwent target lesion revascularization. Four patients (13.8%) were on conservative management.
Stent size:
The stent size varied according to the size of the lesion and ranged from 12 mm to 33 mm in length, and from 2.75 mm to 3.5 mm in width. The mean stent length in the restenosis group was 20.9±8.24 mm and the mean width was 2.98±0.28 mm. The mean stent length in the group without restenosis was 20.01±7.31 mm and the mean width was 3.10±0.50 mm. There was no statistically significant difference in the mean length and width between the two groups (P>0.05).
Restenosis and risk factors for CAD
Sex and restenosis:
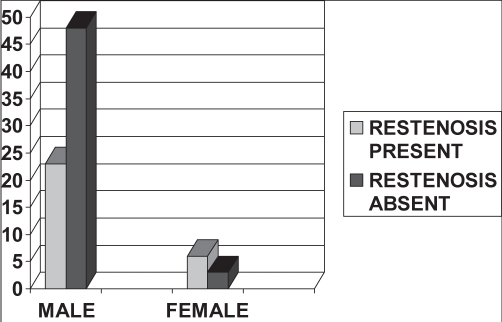
Of the total 80 patients, 71 were men. Of the 29 patients who developed restenosis, 23 of 71 (32.4%) were men and six of nine (66.7%) were women. This suggests that although fewer women presented with CAD, the rate of restenosis after they underwent percutaneous treatment was higher than in men. This difference was statistically significant (P=0.04) (Figure 2).
Figure 2).
Sex and restenosis
Age and restenosis:
The distribution of CAD in various age groups (30 years of age and younger, 31 to 45 years, 46 to 60 years, and older than 60 years) was considered. There was one patient in the 30 years of age and younger group, 21 in the group of patients 31 to 45 years of age, 33 in the group of patients 46 to 60 years of age, and 25 patients in the group of patients older than 60 years of age. The majority of patients were 40 to 60 years of age. Eight of 21 patients (38.1%) 31 to 45 years of age had restenosis, whereas 15 of 33 patients (45.5%) 46 to 60 years of age had restenosis. Only six of 25 patients (24%) older than 60 years of age had restenosis. This shows that there was a higher rate of restenosis in the group of patients 46 to 60 years of age and there was an increase in the rate of restenosis with increasing age. This trend was not maintained above 60 years of age. The mean age of patients who presented with restenosis was 52 years, and the mean age of patients who did not have restenosis was 54 years. The age difference was not statistically significant (P>0.05).
Occupation and restenosis:
The patients were categorized according to their occupation and employment. Fourteen patients were ex-servicemen, 42 were serving personnel and 24 were family members. The rate of restenosis was higher in family members. Of 24 family members, 12 (50%) developed restenosis, compared with 15 of 42 serving personnel (35.7%) and two of 14 ex-servicemen (14.3%); however, the difference was not statistically significant (P=0.08).
Obesity and restenosis:
Of 80 patients, 27 were obese (33.8%). Of the 29 patients who developed restenosis, 11 (37.9%) were obese and 18 (62.1%) were not obese. This difference was not statistically significant (P=0.55).
Diet and restenosis:
Based on their dietary habits, patients were categorized as vegetarians or nonvegetarians. Sixty patients were vegetarians. It was found that 22 of 29 patients who developed restenosis were vegetarians (75.9%) whereas seven were nonvegetarians (24.1%), which suggests that the risk of restenosis was higher in the vegetarian group, although the difference was not statistically significant (P=0.89).
Family history of CAD and restenosis:
Of the 29 patients who developed restenosis, three (10.3%) had a positive family history of CAD. There was no significant relationship found between family history and risk of restenosis (P=0.66).
Hypertension and restenosis:
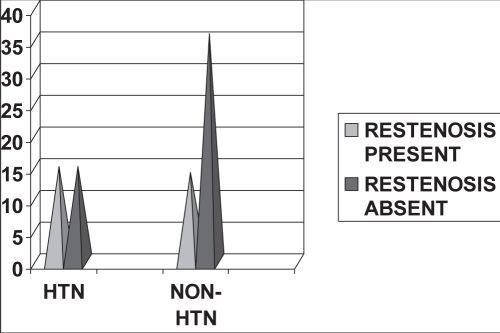
Of the 29 patients who developed restenosis, 15 were hypertensive (51.7%) and the rest were nonhypertensive. This difference was statistically significant (P=0.048) (Figure 3 and Table 2).
Figure 3).
Hypertension (HTN) and restenosis
TABLE 2.
Hypertension and restenosis
|
History of hypertension, n |
Total, n | ||
|---|---|---|---|
| No | Yes | ||
| Restenosis present | 14 | 15 | 29 |
| Restenosis absent | 36 | 15 | 51 |
| Total | 50 | 30 | 80 |
χ2 at 1 differential = 3.92, P=0.048
Diabetes and restenosis:
Of the 29 patients who developed restenosis, two (6.9%) were diagnosed with diabetes. The present study did not show a significant relationship between diabetes and the risk of restenosis (P=0.7).
Hyperlipidemia and restenosis:
Of the 29 patients who developed restenosis, only nine (31%) had dyslipidemia, which was found to be not statistically significant (P=0.16). Because reassessment of the patients’ lipid profiles was not performed during follow-up, it could not be determined whether dyslipidemia was a risk factor for restenosis based on the present study.
Smoking and restenosis:
Of the 29 patients who had restenosis, 10 patients (34.5%) had a positive history of smoking and 19 (65.5%) were nonsmokers. The difference was found to be not statistically significant (P=0.27).
Exercise stress test before intervention and restenosis:
Sixty-two patients (77.5%) underwent a stress test before percutaneous coronary intervention. Patients who had presented with an MI or who had ongoing chest pain (UA) were not subjected to a stress test before percutaneous coronary intervention. Of these 62 patients, 24 (38.7%) developed restenosis; 23 of the 24 (95.8%) patients had a positive stress test before intervention. This relationship was found to be statistically significant (P=0.02).
Segment of coronary tree and restenosis:
Of a total of 80 patients, 24 with proximal left anterior descending (LAD) artery lesions (30%) and 17 with mid-LAD artery lesions (21.3%) underwent percutaneous coronary intervention. None of the patients had distal LAD artery lesions (the procedure was not attempted in distal LAD artery lesions). Five of the 80 patients (6.3%) had critical diagonal 1 disease, but there was no significant relationship between diagonal 1 disease and the risk of restenosis. Of the 41 patients who had LAD artery lesions, three had type A lesions (7.3%), 37 had type B lesions (90.2%) and one had a type C lesion (2.5%). Of the 41 patients, 14 developed restenosis. There was no statistically significant relationship between the type of lesion and restenosis (P=0.17), although most of the patients who had type B lesions developed restenosis. A similar analysis was conducted in the left circumflex artery and its branches, and in the right coronary artery; no significant relationship between these arteries and restenosis was observed.
Lesion characteristics and restenosis:
The restenosis rate was the highest in tubular lesions (37.9%) and in chronic total occlusion (CTO) (13.8%). Restenosis was seen more often in proximal lesions (10.3%), and ostial and bifurcation lesions (6.9%).
Left ventricular ejection fraction and restenosis:
Mean left ventricular ejection fraction (LVEF) in patients with restenosis was 52.76±12%, and 53.6±8% in patients in whom restenosis was absent. There was no significant difference in LVEF between patients with and without restenosis.
Predilation/direct stenting and restenosis:
Fifty-eight patients (72.5%) underwent predilation and 22 (27.5%) underwent direct stenting. The restenosis rate was higher with direct stenting (45.5%) than with predilation (32.8%), but the difference was not statistically significant (P=0.29).
Medical history and restenosis:
Eight patients (10%) had a history of CSA and 25 (31.3%) had a history of MI. There was no statistically significant relationship between positive medical history and risk of restenosis (P>0.05).
Symptomatology before intervention and restenosis:
Of the 29 patients who developed restenosis, 10 (34.5%) initially presented with CSA. Seventeen patients (21.2%) initially presented with UA. When they were followed up, 10 (58.8%) had developed restenosis; this was found to be statistically significant (P=0.029). The restenosis rate was higher when the initial presentation was UA compared with CSA.
Medical treatment before intervention and restenosis:
Of the 29 patients who developed restenosis, 28 (96.6%) were on acetylsalicylic acid therapy, 19 (65.5%) were on clopidogrel and 23 (79.3%) were on statins before intervention, but no statistically significant relationship between medication use and risk of restenosis was established (P>0.05). No significant relationship between glycoprotein IIb/IIIa therapy and reduction in restenosis could be established because the sample size was small.
Medical treatment during follow-up and restenosis:
All 80 patients were prescribed medical therapy after percutaneous coronary intervention. Seventy-three patients (91.3%) continued regular medical therapy, whereas seven (8.7%) showed poor drug compliance. In patients undergoing regular therapy, 50 patients (68.5%) did not develop restenosis, whereas 23 (31.5%) developed restenosis; the difference was statistically significant (P=0.008). Six of the seven patients on irregular therapy (85.7%) developed restenosis. Thus, a higher restenosis rate was seen in patients with poor drug compliance.
Symptomatology at follow-up and restenosis:
During follow-up, it was observed that 10 patients (12%) had CSA. Restenosis was found in 100% of patients who presented with UA and CSA compared with 50% of patients with atypical symptoms. There was a statistically significant relationship between rest-enosis and symptomatology (P<0.05).
Exercise stress test at follow-up and restenosis:
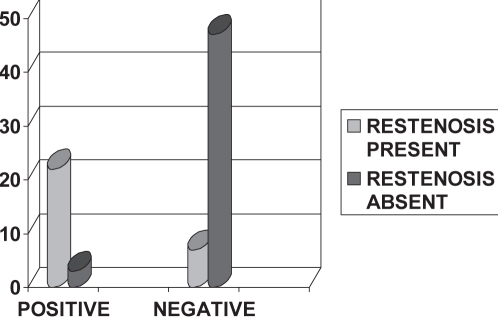
At the time of follow-up, 79 patients underwent an exercise treadmill test (ETT). The ETT results were positive in 25 patients (31.6%) and negative in 54 patients (68.3%). When the 29 patients who developed restenosis were assessed, 22 (75.8%) had a positive ETT. Only seven patients (2.4%) who had negative stress tests showed restenosis on follow-up angiography. Of 54 patients whose ETT was negative, 47 (87%) did not develop restenosis. This relationship was found to be statistically significant (P=0.000) and suggests that ETT is a good predictor of restenosis (Figure 4 and Table 3).
Figure 4).
Stress test at follow-up and restenosis
TABLE 3.
Stress test at follow-up and restenosis
|
Stress test at follow-up, n |
Total, n | ||
|---|---|---|---|
| Positive | Negative | ||
| Restenosis present | 22 | 7 | 29 |
| Restenosis absent | 3 | 47 | 50 |
| Total | 25 | 54 | 79 |
χ2 at 1 differential = 43.36, P=0.000
Major adverse cardiac events:
Of the 29 patients who developed restenosis, repeat revascularization was performed in 25 patients (86.5%) and four (13.79%) continued to be on conservative management. Target lesion revascularization was performed in 20 patients (80%) and five (20%) underwent TVR. Repeat revascularization was performed in 18 patients (72.5%) with BMS and seven (28%) with DES. The difference in major adverse cardiac events (MACEs) between the two groups was 44.5%.
DISCUSSION
Restenosis rates vary and depend on many modifiable risk factors and angiographic characteristics of the patient population. Patient-related variables, such as age and sex, have not been consistently shown to predict restenosis (1). There is much debate in the literature about the sex variation in restenosis after coronary stent placement. In a study by Macdonald et al (2), age, sex, smoking, diabetes and history of MI were not associated with restenosis. Our study suggests that age is not a predictor of restenosis. There was a trend of increase in restenosis with age (46 to 60 years of age) that was not maintained above the age of 60 years.
In our study, the restenosis rate was significantly higher in women. Watanabe et al (3) showed that female sex was an independent predictor of mortality even after adjustment for age and there was a poorer cardiovascular profile among women. Jacobs et al (4), based on the National Heart Lung and Blood Institute dynamic registry, concluded that this relationship may not exist and the influence of the sex difference on the outcome of angioplasty has decreased over the years with better treatment modalities.
The association of diabetes with restenosis was initially observed in the National Heart Lung and Blood Institute percutaneous transluminal coronary angioplasty registry (5). Subsequent reports confirmed the risk of restenosis in diabetic patients to be 1.3 times the risk in nondiabetic patients using multivariable regression analysis. Insulin-dependent diabetes mellitus had a stronger relationship with restenosis (5–7). MacDonald et al (2) did not find evidence of diabetes as a risk factor for restenosis. In a meta-analysis by Gilbert et al (8), it was shown that although diabetes was a risk factor for restenosis after stenting, the apparent effect of diabetes on restenosis rates published in the literature was overrated and reduced to approximately one-half after adjusting for the difference in age. Our small-sized study suggests that diabetes is not a strong predictor of restenosis.
The present study suggests that hypertension is an important predictor of restenosis. A similar result was shown in the study conducted by Bach et al (9).
The effect of lipids on the risk of restenosis has been controversial with balloon angioplasty and even less well established in the era of stenting. Although various studies (9–11) have related dyslipidemia with restenosis, they were conducted in a relatively small number of patients. Large prospective studies have not confirmed any such associations (12,13). Randomized trials such as the Fluvastatin Angiographic Restenosis (FLARE) study (14) confirmed this lack of relationship. In our study, we did not find a significant relationship between dyslipidemia and restenosis – the limitation being the lower number of patients. Reassessment of the lipid profile was not performed at follow-up.
Smoking is a major risk factor for atherosclerosis. It has been positively related with the risk of restenosis in a study conducted by Bach et al (9). Contrary to that, Schillinger et al (15) concluded that smoking 10 or more cigarettes daily was associated with a reduced rate of intermediate-term restenosis after lower-limb endovascular interventions in peripheral arterial athero-sclerotic disease. The protective effect exerted by smoking is presumably due to the slowing of proliferation of vascular smooth cells at the treated segment by heme oxygenase-1-derived carbon monoxide. Interestingly, our study also suggests a similar ‘smoker’s paradox’. Although smoking has a protective effect after intervention, patients who smoke present at a younger age and have a higher risk of recurrent coronary events.
We assessed the lifestyle of the patients and categorized them as serving personnel, ex-servicemen and family members. It was observed that restenosis was higher in family members than in the other two groups, although the difference was not statistically significant. This can be explained by the sedentary lifestyle of family members compared with the physically fit army personnel. In a study conducted by Belardinelli et al (16), the beneficial effect of exercise training on restenosis was observed. Although the angiographic restenosis rate was not significantly reduced by physical conditioning, the trained patients had a lower rate of anginal attacks and hospital readmission as well as better psychological well-being. Thus, exercise training improves the outcome of patients with a higher coronary risk factor profile at baseline even in the presence of angiographic restenosis.
In our study, vegetarians had a higher rate of restenosis than nonvegetarians. A plausible explanation is that a vegetarian diet has a higher level of folic acid, which has been reported to be related to an increased restenosis rate in different studies (17).
Obesity is an important risk factor for CAD, but there is conflicting evidence regarding its role in restenosis. There is no plausible explanation for the paradoxical predilection of obese patients for CAD without restenosis, but possible mechanisms are greater coronary diameter, which reduces the chance of restenosis, and age (18). Tarastchuk et al (19) found that waist circumference was an independent predictor of MACEs in men. Similarly, in the multicentre randomized TAXUS IV trial (20), obesity was found to be an important risk factor for clinical and angiographic restenosis and composite MACEs, but DES (paclitaxel-eluting stents) attenuated the increased risk associated with obesity. In our study, we found that obese patients had a lower rate of restenosis.
Genetic predisposition has been noted to influence restenosis in other studies (5,21). Although based on a small cohort size, our study suggests that a family history of CAD has no impact on restenosis.
When we examined history of MI, we found that it had no relation to restenosis, but the patients who presented with UA had a higher rate of restenosis on follow-up. In the TARGET follow-up study, Moliterno et al (22) showed that restenosis was higher in patients with acute coronary syndrome. Restenosis was found to be 1.2 to 1.7 times higher in acute coronary syndrome patients than in those with chronic stable symptoms (1,5,23,24).
As shown by previous studies, independent variables that predict restenosis include exercise-induced angina at follow-up and electrocardiographically positive ETT (25). A pooled analysis of 15 treadmill studies involving 2250 patients with angiographic follow-up revealed a 50% positive predictive value and 76% negative predictive value of ETT (26–30). In our study, patients with a positive initial ETT (before intervention) had a higher rate of restenosis. Patients with a positive ETT at follow-up (after intervention) also had a higher incidence of restenosis. The negative predictive value of the test was also high. Thus, in our study, we noted that ETT was an important predictor of restenosis.
In the Multi-Hospital Eastern Atlantic Restenosis Trial (M-HEART) (31), LAD artery lesions were found to be more prone to restenosis (45%) than lesions in the the left circumflex artery (31%) and right coronary artery (32%). A plausible explanation is an increase in elastic recoil of the surrounding muscular interventricular septum, which leads to an under-treatment of LAD artery lesions. Serruys et al (32) suggested that restenosis was a ubiquitous phenomenon without any predilection for a particular site in the coronary tree. In our study, we did not find any relationship between the vessel segment and restenosis. Specific situations such as aorto-ostial lesions, bifurcation lesions, CTO, lesions in venous grafts and type C lesions confer a higher than usual risk of restenosis (33,34). Similarly, in our study, we found a higher restenosis rate in bifurcation lesions and CTO.
There was no statistically significant difference in the LVEF between patients with or without restenosis in our study.
Süselbeck and Singh (35) observed that direct stenting was as effective as stenting following predilation. Mehilli et al (36), in the ISAR-DIRECT trial, showed no reduction in thrombotic and restenotic complications in direct stenting compared with conventional stenting. Contrarily, in our study, we found a higher restenosis rate with direct stenting, although it was not statistically significant.
In the Antiplatelet Trialists’ Collaboration (37), data showed that antiplatelet therapy reduced the occurrence of restenosis by 4%. The M-HEART II (38) trial showed that thromboxane A2 inhibitors did not significantly reduce the risk of restenosis. In our study, during follow-up, patients who had better drug compliance were found to have a lower rate of restenosis. When we analyzed the effects of individual drugs such as acetylsalicylic acid, clopidogrel, statins, angiotensin-converting enzyme inhibitors and beta-blockers on restenosis, we found that none of them provided a statistically significant clinical benefit.
Long lesion length has been associated with increased rest-enosis in many large prospective clinical studies (24,31,39). Hamasaki et al (40) observed an increase in the restenosis rate in lesions longer than 15 mm. Kereiakes and Linnemeier (41) reported the stent length as the most important predictor for angiographic restenosis. Our study did not show any statistically significant relationship between the length and diameter of the stent and restenosis.
The reasons for the prohibitively high cost of DES include developmental and research costs, acquisition of exclusive and expensive licenses from pharmaceutical companies and building of new manufacturing facilities. The prospective randomized controlled Basel Stent Kosten Effektivitäts Trial (BASKET) (42) was conducted to compare the cost-effectiveness of two available DES (sirolimus- and paclitaxel-eluting stents) with that of third-generation BMS. It attempted to answer whether DES were cost effective in an everyday setting. Kaiser et al (42) showed that the use of DES reduced the rate of MACEs by 44% due to a lower rate of TVR. In DES-treated patients, stent cost per patient was higher by a mean of €1702. Although the follow-up costs were slightly lower in patients with DES, the overall cost at six months was still €905 higher. The incremental cost-effectiveness ratio of DES compared with BMS to avoid one major event was €18,311 and the cost per quality-adjusted life-year gained was more than €50,000. It was concluded that DES would prove to be more cost effective if used in certain subgroups such as the elderly and those at higher risk (42).
In our study, we found that the rate of restenosis was lower in DES (23.1%) than in BMS (48.8%) and the difference was statistically significant. MACE (repeat revascularization) rates were 72.5% in the BMS group and 28% in the DES group; ie, a difference of 44.5% was noted. Thus, although the initial cost of therapy was higher in DES, the reduction in the repeat revascularization resulted in subsequent reduction in cost, thereby making it a more cost-effective therapy than BMS.
CONCLUSION
A total of 130 patients were included in the study conducted at the Army Hospital (Research and Referral); however, 50 patients were excluded from the final analysis because they did not report for follow-up. A number of variables including demographic, clinical and angiographic factors, procedural factors, stent characteristics and medications were considered in the overall backdrop of the pattern of restenosis.
In our study, demographic factors, such as age and occupation, were not found to influence restenosis and there was a significant relationship observed between female sex and restenosis.
Diabetes, although extensively discussed in previous studies as an important variable accentuating restenosis, could not be established as a causative factor due to the small cohort size in our study. Hypertension, UA and poor drug compliance emerged as important risk factors for restenosis in our study.
Although restenosis was found to be independent of the segmental distribution, it occurred more often in complicated lesions (eg, CTO and bifurcation lesions).
Procedural factors, such as predilation and direct stenting, were not found to have a significant impact on the restenosis rate in our study.
Contrary to the results mentioned in current literature, we did not find a statistically significant relationship between restenosis and longer stent length, smaller lesion diameter and stent type.
After dividing the patients into BMS and DES groups, we analyzed the difference in restenosis among the various types of BMS (cobalt-chromium/stainless steel and thick/thin strut). However, we did not find any significant difference. Due to a ban on TAXUS stents (Boston Scientific Corporation, USA) by the Drug Controller General of India, most of the patients in the DES group were implanted with sirolimus-coated stents; hence, a comparative analysis could not be conducted.
In our study, we found the most common pattern of restenosis to be focal. Patients with DES had a higher rate of focal restenosis.
Despite the addition of DES and BMS to the arsenal of coronary intervention procedures, the ‘perfect’ solution to eradicate restenosis after coronary intervention is still not available. DES appear to reduce restenosis and clinical end points and appear to be more cost effective than BMS. Patient-related factors (eg, sex, hypertension and UA) are important variables that affect restenosis and, hence, the appropriate selection of devices and patients is crucial. Noninvasive stress testing has high positive and negative predictive values. It is suggested based on the present study that noninvasive stress testing should be performed during follow-up before routine angiography, thereby reducing the need for repeat coronary artery grafting. Possibly, a less expensive DES will end the search for a stent that is cost effective and less prone to restenosis.
Acknowledgments
This study was conducted at the Army Hospital (Research and Referral), and was funded by and conducted under the aegis of the Indian Council of Medical Research.
REFERENCES
- 1.Rupprecht HJ, Brennecke R, Bernhard G, Erbel R, Pop T, Meyer J. Analysis of risk factors for restenosis after PTCA. Cathet Cardiovasc Diagn. 1990;19:151–9. doi: 10.1002/ccd.1810190302. [DOI] [PubMed] [Google Scholar]
- 2.Macdonald RG, Henderson MA, Hirshfeld JW, Jr, et al. Patient-related variables and restenosis after percutaneous transluminal coronary angioplasty – a report from the M-HEART group. Am J Cardiol. 1990;66:926–31. doi: 10.1016/0002-9149(90)90927-s. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe CT, Maynard C, Ritchie JL. Comparison of short-term outcomes following coronary artery stenting in men versus women. Am J Cardiol. 2001;88:848. doi: 10.1016/s0002-9149(01)01890-2. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs AK, Johnston JM, Haviland A, et al. Improved outcomes for women undergoing contemporary percutaneous coronary intervention: A report from the national heart, lung, and blood institute dynamic registry. J Am Coll Cardiol. 2002;39:1608. doi: 10.1016/s0735-1097(02)01835-1. [DOI] [PubMed] [Google Scholar]
- 5.Holmes DR, Jr, Vlietstra RE, Smith HC, et al. Restenosis after percutaneous transluminal coronary angioplasty (PTCA): A report from the PTCA registry of the national heart, lung, and blood institute. Am J Cardiol. 1984;53:77C–81C. doi: 10.1016/0002-9149(84)90752-5. [DOI] [PubMed] [Google Scholar]
- 6.Abizaid A, Mehran R, Bucher TA, et al. Does diabetes influence clinical recurrence after coronary stent implantation? J Am Coll Cardiol. 1997;29(Suppl A):A-188. [Google Scholar]
- 7.Aronson D, Bloomgarden Z, Rayfield EJ. Potential mechanisms promoting restenosis in diabetic patients. J Am Coll Cardiol. 1996;27:528–35. doi: 10.1016/0735-1097(95)00496-3. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert J, Raboud J, Zinman B. Meta-analyis of the effect of diabetes on restenosis rates among patients receiving coronary angioplasty stenting. Diabetes Care. 2004;27:990–4. doi: 10.2337/diacare.27.4.990. [DOI] [PubMed] [Google Scholar]
- 9.Bach R, Jung F, Kohsiek I, et al. Factors affecting the restenosis rate after percutaneous transluminal coronary angioplasty. Thromb Res. 1994;74:S55–67. doi: 10.1016/s0049-3848(10)80007-6. [DOI] [PubMed] [Google Scholar]
- 10.Desmarais RL, Sarembock IJ, Ayers CR, Vernon SM, Powers ER, Gimple LW. Elevated serum lipoprotein(a) is a risk factor for clinical recurrence after coronary balloon angioplasty. Circulation. 1995;9:1403–9. doi: 10.1161/01.cir.91.5.1403. [DOI] [PubMed] [Google Scholar]
- 11.Chiarugi L, Prisco D, Antonucci E, et al. Lipoprotein (a) and anticardiolipin antibodies are risk factors for clinically relevant restenosis after elective balloon percutaneous transluminal coronary angioplasty. Atherosclerosis. 2001;154:129–35. doi: 10.1016/s0021-9150(00)00439-1. [DOI] [PubMed] [Google Scholar]
- 12.Rozenman Y, Gilon D, Welber S, et al. Plasma lipoproteins are not related to restenosis after successful coronary angioplasty. Am J Cardiol. 1993;72:1206–7. doi: 10.1016/0002-9149(93)90997-q. [DOI] [PubMed] [Google Scholar]
- 13.Violaris AG, Melkert R, Serruys PW. Influence of serum cholesterol and cholesterol subfractions on restenosis after successful coronary angioplasty. A quantitative angiographic analysis of 3336 lesions. Circulation. 1994;90:2267–79. doi: 10.1161/01.cir.90.5.2267. [DOI] [PubMed] [Google Scholar]
- 14.Serruys PW, Foley DP, Jackson G, et al. A randomized placebo-controlled trial of fluvastatin for prevention of restenosis after successful coronary balloon angioplasty; final results of the fluvastatin angiographic restenosis (FLARE) trial. Eur Heart J. 1999;20:58–69. doi: 10.1053/euhj.1998.1150. [DOI] [PubMed] [Google Scholar]
- 15.Schillinger M, Exner M, Mlekusch W, et al. Effect of smoking on restenosis during the 1st year after lower-limb endovascular interventions. Radiology. 2004;231:831–8. doi: 10.1148/radiol.2313031088. [DOI] [PubMed] [Google Scholar]
- 16.Belardinelli R, Paolini I, Cianci G, Piva R, Georgiou D, Purcaro A. Exercise training intervention after coronary angioplasty: The ETICA trial. J Am Coll Cardiol. 2001;37:1891–900. doi: 10.1016/s0735-1097(01)01236-0. [DOI] [PubMed] [Google Scholar]
- 17.Lange H, Suryapranata H, De Luca G, et al. Folate therapy and in-stent restenosis after coronary stenting. N Engl J Med. 2004;350:2673–81. doi: 10.1056/NEJMoa032845. [DOI] [PubMed] [Google Scholar]
- 18.Rana JS, Mittleman MA, Ho KK, Cutlip DE. Obesity and clinical restenosis after coronary stent placement. Am Heart J. 2005;150:821–6. doi: 10.1016/j.ahj.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 19.Tarastchuk JCE, Guérios EE, Bueno Rda R, et al. Obesity and coronary intervention: Should we continue to use body mass index as a risk factor? Arq Bras Cardiol. 2008;90:284–9. doi: 10.1590/s0066-782x2008000500001. [DOI] [PubMed] [Google Scholar]
- 20.Nikolsky E, Kosinski E, Mishkel GJ, et al. Impact of obesity on revascularization and restenosis rates after bare-metal and drug-eluting stent implantation (from the TAXUS-IV trial) Am J Cardiol. 2005;95:709–15. doi: 10.1016/j.amjcard.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 21.Kastrati A, Dirschinger J, Schömig A. Genetic risk factors and restenosis after percutaneous coronary interventions. Herz. 2000;25:34–46. doi: 10.1007/BF03044122. [DOI] [PubMed] [Google Scholar]
- 22.Moliterno DJ, Yakubov SJ, DiBattiste PM, et al. Outcomes at 6 months for the direct comparison of tirofiban and abciximab during percutaneous coronary revascularisation with stent placement: The TARGET follow-up study. Lancet. 2002;360:355–60. doi: 10.1016/S0140-6736(02)09605-8. [DOI] [PubMed] [Google Scholar]
- 23.Leimgruber PP, Roubin GS, Hollman J, et al. Restenosis after successful coronary angioplasty in patients with single-vessel disease. Circulation. 1986;73:710–7. doi: 10.1161/01.cir.73.4.710. [DOI] [PubMed] [Google Scholar]
- 24.Bourassa MG, Lespérance J, Eastwood C, et al. Clinical, physiologic, anatomic and procedural factors predictive of restenosis after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1991;18:368–76. doi: 10.1016/0735-1097(91)90588-z. [DOI] [PubMed] [Google Scholar]
- 25.Acute Coronary Care in the Thrombolytic Era. Ann Intern Med. 1988;109:848. (Book review) [Google Scholar]
- 26.Bengtson JR, Mark DB, Honan MB, et al. Detection of restenosis after elective percutaneous transluminal coronary angioplasty using the exercise treadmill test. Am J Cardiol. 1990;65:28–34. doi: 10.1016/0002-9149(90)90021-r. [DOI] [PubMed] [Google Scholar]
- 27.Hillegass WB, Bengtson JR, Ancukiewicz M, et al. Pre-discharge exercise testing does not predict clinical events or restenosis after successful angioplasty. Circulation. 1992;86:I–137. (Abst) [Google Scholar]
- 28.Laarman G, Luijten HE, van Zeyl LG, et al. Assessment of “silent” restenosis and long-term follow-up after successful angioplasty in single vessel coronary artery disease: The value of quantitative exercise electrocardiography and quantitative coronary angiography. J Am Coll Cardiol. 1990;16:578–85. doi: 10.1016/0735-1097(90)90346-q. [DOI] [PubMed] [Google Scholar]
- 29.Wijns W, Serruys PW, Simoons ML, et al. Predictive value of early maximal exercise test and thallium scintigraphy after successful percutaneous transluminal coronary angioplasty. Br Heart J. 1985;53:194–200. doi: 10.1136/hrt.53.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azpitarte J, Tercedor L, Melgares R, Prieto JA, Romero JA, Ramírez JA. The value of exercise electrocardiography testing in the identification of coronary restenosis: A probability analysis. Int J Cardiol. 1995;48:239–47. doi: 10.1016/0167-5273(94)02240-j. [DOI] [PubMed] [Google Scholar]
- 31.Hermans WR, Rensing BJ, Kelder JC, de Feyter PJ, Serruys PW. Postangioplasty restenosis rate between segments of the major coronary arteries. Am J Cardiol. 1992;69:194–200. doi: 10.1016/0002-9149(92)91304-m. [DOI] [PubMed] [Google Scholar]
- 32.Platko WP, Hollman J, Whitlow PL, Franco I. Percutaneous transluminal angioplasty of saphenous vein graft stenosis: Long-term follow-up. J Am Coll Cardiol. 1989;14:1645–50. doi: 10.1016/0735-1097(89)90010-7. [DOI] [PubMed] [Google Scholar]
- 33.Savage MP, Douglas JS, Jr, Fischman DL, et al. Stent placement compared with balloon angioplasty for obstructed coronary bypass grafts. Saphenous vein de novo trial investigators. N Engl J Med. 1997;337:740–7. doi: 10.1056/NEJM199709113371103. [DOI] [PubMed] [Google Scholar]
- 34.Süselbeck T, Singh D. Direct stenting appears as effective as stenting following predilatation. Evid Based Cardiovasc Med. 2006;10:119–20. doi: 10.1016/j.ebcm.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 35.Mehilli J, Kastrati A, Dirschinger J, et al. Intracoronary stenting and angiographic results: Restenosis after direct stenting versus stenting with predilation in patients with symptomatic coronary artery disease (ISAR-DIRECT trial) Catheter Cardiovasc Interv. 2004;61:190–5. doi: 10.1002/ccd.10706. [DOI] [PubMed] [Google Scholar]
- 36.Collaborative overview of randomised trials of antiplatelet therapy – II: Maintenance of vascular graft or arterial patency by antiplatelet therapy. BMJ. 1994;308:159. [PMC free article] [PubMed] [Google Scholar]
- 37.Savage MP, Goldberg S, Bove AA, et al. Effect of thromboxane A2 blockade on clinical outcome and restenosis after successful coronary angioplasty. Multi-hospital eastern atlantic restenosis trial (M-HEART II) Circulation. 1995;92:3194–200. doi: 10.1161/01.cir.92.11.3194. [DOI] [PubMed] [Google Scholar]
- 38.Hirshfeld JW, Jr, Schwartz JS, Jugo R, et al. Restenosis after coronary angioplasty: A multivariate statistical model to relate lesion and procedure variables to restenosis. The M-HEART investigators. J Am Coll Cardiol. 1991;18:647–56. doi: 10.1016/0735-1097(91)90783-6. [DOI] [PubMed] [Google Scholar]
- 39.Rensing BJ, Hermans WR, Deckers JW, de Feyter PJ, Tijssen JG, Serruys PW. Lumen narrowing after percutaneous transluminal coronary balloon angioplasty follows a near Gaussian distribution: A quantitative angiographic study in 1,445 successfully dilated lesions. J Am Coll Cardiol. 1992;19:939–45. doi: 10.1016/0735-1097(92)90274-q. [DOI] [PubMed] [Google Scholar]
- 40.Hamasaki N, Nosaka H, Kimura T, et al. Influence of lesion length on late angiographic outcome and restenotic process after successful stent implantation. J Am Coll Cardiol. 1997;29:239A. (Abst) [Google Scholar]
- 41.Kereiakes D, Linnemeier TJ. Usefulness of stent length in predicting in-stent restenosis (the MULTI-LINK stent trials) Am J Cardiol. 2000;86:336. doi: 10.1016/s0002-9149(00)00928-0. [DOI] [PubMed] [Google Scholar]
- 42.Kaiser C, Brunner-La Rocca H, Buser PT, et al. Incremental cost-effectiveness of drug-eluting stents compared with a third-generation bare-metal stent in a real-world setting: Randomised Basel Stent Kosten Effektivitäts Trial (BASKET) Lancet. 2005;366:921–9. doi: 10.1016/S0140-6736(05)67221-2. [DOI] [PubMed] [Google Scholar]