Abstract
Altered glucose metabolism due to insulin resistance is a common feature of essential hypertension in humans and in animal models. Elevated endogenous aldehydes in genetic (spontaneously hypertensive rats) and acquired (fructose-induced hypertensive rats) models of essential hypertension may be due to increased production of the reactive aldehyde methylglyoxal, resulting from altered glucose metabolism. Excess methylglyoxal binds sulfhydryl groups of membrane proteins, altering calcium channels and increasing cytosolic free Ca2+ and blood pressure. It has been demonstrated that methylglyoxal, when given in drinking water to Wistar-Kyoto rats, leads to an increase in kidney aldehyde conjugates, cytosolic free Ca2+ concentration, decreased serum nitric oxide, renal vascular hyperplasia and hypertension. N-acetylcysteine (NAC) in the diet of these animals prevented hypertension and associated biochemical and morphological changes. NAC normalizes blood pressure by directly binding to excess methylglyoxal, thus normalizing Ca2+ channels, cytosolic Ca2+ and nitric oxide. NAC also leads to increased levels of tissue glutathione, a storage form of cysteine. Glutathione acts as a cofactor in the enzymatic catabolism of methylglyoxal. Cysteine and other antioxidants, such as vitamins B6, C and E, and lipoic acid, prevented hypertension and associated biochemical and morphological changes in both genetic and acquired rat models of hypertension. The antihypertensive effect of dietary antioxidants may be due to an increase in tissue cysteine and glutathione, which improves glucose metabolism and decreases tissue methylglyoxal. A diet rich in these antioxidants may be effective in preventing and controlling hypertension in humans.
Keywords: Dietary antioxidants, Hypertension, Methylglyoxal
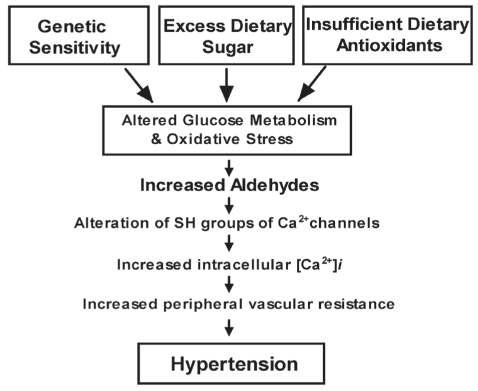
More than 600 million people suffer from high blood pressure worldwide. These people are more likely to experience strokes, heart disease and kidney failure. Essential hypertension in humans may develop through a combination of genetic and lifestyle factors. Diet has long been under investigation as an effector of blood pressure. A diet high in sugar or low in antioxidant vitamins can lead to insulin resistance, altered glucose metabolism, low tissue glutathione, hypertension and oxidative stress. Persons with a genetic predisposition for impaired glucose metabolism are particularly vulnerable to the effect of a high-sugar diet. Abnormalities in glucose use are estimated to exist in 25% of the general population and in up to 80% of persons with essential hypertension (1–3). The end result of either genetic sensitivity, a high-sugar diet and/or a diet low in antioxidant vitamins is altered glucose metabolism. Altered glucose metabolism leads to increased levels of the highly reactive ketoaldehyde methylglyoxal (Figure 1).
Figure 1).
Role of aldehydes in the development of hypertension. [Ca2+]i Free Ca2+ concentration; SH Sulfhydryl
The purpose of the present review is to discuss the mechanism by which methylglyoxal is formed and its role in causing hypertension. We will discuss the use of dietary antioxidants to lower methylglyoxal and prevent hypertension.
ENDOGENOUS FORMATION OF METHYLGLYOXAL
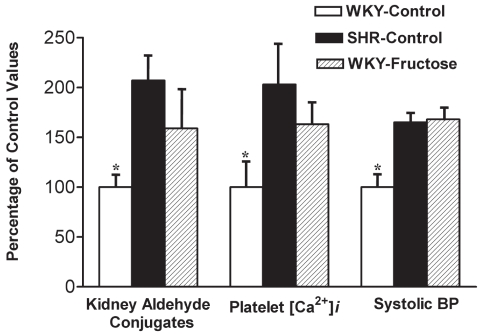
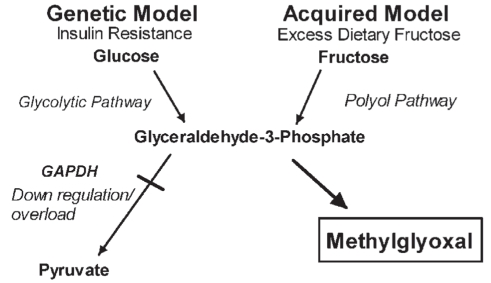
Spontaneously hypertensive rats (SHRs), a genetic model of human essential hypertension, and fructose-treated Wistar-Kyoto (WKY) hypertensive rats show insulin resistance, increased platelet cytosolic free Ca2+ and kidney aldehyde conjugates (Figure 2) (4,5). Altered Ca2+ handling in platelets is similar to that found in vascular tissue (6). We suggest that the tissue aldehyde conjugates found in these animal models are formed primarily from methylglyoxal produced as a consequence of altered glucose metabolism (Figure 3). Of the two principal pathways of intracellular glucose metabolism, complete oxidation and nonoxidative disposal (glycolytic pathway), only the latter has been shown to be reduced in insulin resistance (2). Under normal physiological conditions, glucose is metabolized to glyceraldehyde-3-phosphate (G3P), which is converted to 1,3-diphosphoglycerate by the enzyme G3P dehydrogenase (GAPDH), with further metabolism to pyruvate via the glycolytic pathway. Insulin has been shown to regulate GAPDH at the messenger RNA level through stimulation of gene expression in adipocytes and hepatoma cell culture (7). In insulin-resistant states, such as type 2 diabetes and essential hypertension, in which GAPDH may be down-regulated, there will be a buildup of G3P. Thus, glucose metabolism is slowed through the glycolytic pathway, leading to its conversion to fructose via the polyol pathway. Fructose is further metabolized to D-glyceraldehyde and dihydroxyacetone phosphate. Both D-glyceraldehyde and dihydroxyacetone phosphate are converted to G3P (8,9). This buildup of G3P by both the glycolytic and polyol pathways will result in its conversion to methylglyoxal, especially if further metabolism by GAPDH is impaired (10–13). The effect of GAPDH activity on methylglyoxal levels was demonstrated by Beisswenger et al (14). Koningic acid was used to inhibit GAPDH, resulting in a 79% reduction of GAPDH activity with a sixfold increase in methylglyoxal in human red blood cells in culture (14). SHRs show evidence of insulin resistance before they develop hypertension (15). This inherent insulin resistance may lead to decreased activity of GAPDH and excess methylglyoxal.
Figure 2).
The bar graph shows kidney aldehyde conjugates, platelet free Ca2+ concentration ([Ca2+]i) and systolic blood pressure (BP) in spontaneously hypertensive rats (SHRs) and fructose-induced hypertensive Wistar-Kyoto (WKY) rats. Starting at seven weeks of age, rats were divided into three groups of six animals each. For a period of 14 weeks, animals in the WKY-control and SHR-control groups were given a normal diet and normal drinking water; the WKY-fructose group was given a normal diet and 4% fructose in drinking water. All values (mean ± SD) are expressed as a percentage of the WKY-control group at the completion of the study at 21 weeks of age. *Values are significantly different (P<0.05) from other groups. Data from references 4 and 5
Figure 3).
Formation of excess methylglyoxal due to altered glucose metabolism in genetic and acquired models of hypertension. GAPDH Glyceraldehyde-3-phosphate dehydrogenase
Production of excess methylglyoxal can also occur as a consequence of increased dietary sugar. In the course of normal metabolism, fructose bypasses the step in glucose metabolism at phosphofructokinase, where control is exerted on the rate of glucose catabolism. It is rapidly taken up in the liver and converted to intermediate metabolites in the glycolytic pathway. If there is excess dietary sucrose or fructose, this process will deplete cells of inorganic phosphate, leading to inhibition of phosphofructokinase. Inhibition of this enzyme blocks the processing of glucose through the glycolytic pathway. This results in the shunting of glucose through the polyol pathway resulting in the formation of more fructose and, again, a buildup of G3P with further metabolism to methylglyoxal. A diet rich in sucrose or fructose can overload the GAPDH enzyme system, increasing the buildup of G3P and leading to excess methylglyoxal formation.
A recent study (16) reported higher levels of methylglyoxal in vascular smooth muscle cells in SHRs in culture compared with normotensive WKY rats. Methylglyoxal induces aldose reductase in rat aortic vascular smooth muscle cells. This leads to increased flux of glucose through the polyol pathway, with increased formation of methylglyoxal, which may participate in the development of diabetic vascular complications (17–19). Human type 2 diabetic patients have significantly elevated plasma levels of methylglyoxal (20). We have shown elevated levels of plasma methylglyoxal and glyoxal even in young complication-free patients with type 1 diabetes (21). The polyol pathway is especially active in kidney, cardiac muscle, skeletal muscle, vascular smooth muscle, and retinal and neuronal tissue. For this reason, the pathological effects of methylglyoxal would be observed more markedly in these tissues.
ROLE OF METHYLGLYOXAL IN HYPERTENSION
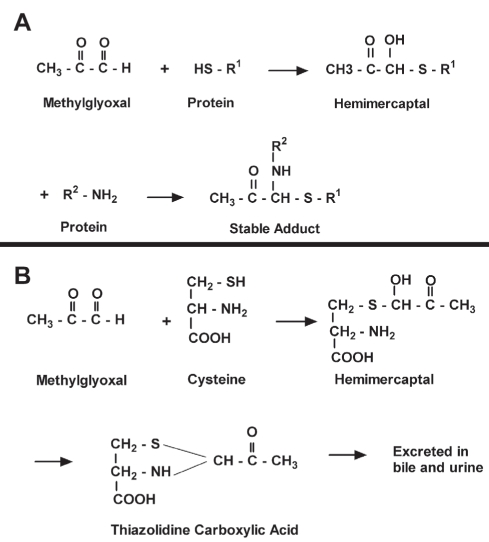
Methylglyoxal and other endogenous aldehydes are compounds of unusually high electrophilic reactivity. They react non-enzymatically with amino (NH2) and sulfhydryl (SH) groups of membrane proteins, metabolic enzymes and membrane ion channels and inhibit their function. The following reactions of aldehydes are known to occur: reaction with the SH group of a protein (cysteine), leading to formation of hemimercaptals; reaction with a free NH2 group of a protein (mainly the epsilon-NH2 group of lysine or arginine), forming a Schiff base or NH2 compounds; and further stabilization of the hemimercaptals or Schiff base adducts through cross-linking with another free NH2 group on the protein (Figure 4A). Aldehydes react 100 times faster with SH groups than with NH2 groups under comparable conditions (22). Methylglyoxal has been shown to bind to thiol groups of epithelial brush border enzymes, causing loss of activity (23).
Figure 4).
A Reaction of methylglyoxal with the free sulfhydryl (HS or SH) group of a protein, with a further reaction with a free amino (NH2) group of protein forming a stable adduct, thus permanently altering its function. B Protective effect of endogenous cysteine from methylglyoxal by forming a thiazolidine-carboxylic acid derivative, which is excreted in bile and urine
In diabetes mellitus, aldehyde glyoxal and methylglyoxal modify the free NH2 groups of lysine and arginine of proteins forming aldehyde conjugates, also called advanced glycation end products (AGEs). Elevated levels of AGEs are implicated in diabetic complications including nephropathy. We have shown that plasma protein AGEs, carboxymethyl cysteine and carboxyethyl cysteine were elevated and related to nephropathy in patients with diabetes (24). Recently, we demonstrated elevated levels of plasma methylglyoxal and methylglyoxal-derived hydroimidazolone AGEs with arginine in type 1 diabetic patients (25). These studies show high reactivity of methylglyoxal with free NH2 and cysteine groups of tissue proteins.
Under normal physiological conditions, tissue levels of methylglyoxal are maintained at a low level through further catabolism to D-lactate by the glutathione-dependent glyoxalase enzyme system. D-lactate is further converted to pyruvate by 2-hydroxyacid dehydrogenase, thereby rejoining mainstream glycolysis (26). Methylglyoxal will not accumulate as long as the glutathione concentration is adequate to maintain the glyoxalase system. Both SHRs and fructose-induced hypertensive rats show high levels of tissue aldehydes with low levels of glutathione (4,16,27–35). Methylglyoxal also binds to soluble SH compounds, such as reduced glutathione (GSH) and cysteine. Cysteine reacts with methylglyoxal forming a hemimercaptal, which is further converted to a thiazolidine-carboxylic acid, which is excreted in bile and urine (Figure 4B) (22,36,37). Excess methylglyoxal has been shown to lower glutathione in rat vascular smooth muscle cells in culture and in human red blood cells in vitro (16,38).
In essential human hypertension and hypertensive animals, the cytosolic free Ca2+ concentration ([Ca2+]i) in vascular smooth muscle cells is elevated. This increase leads to abnormal contractile activity, increased resistance in peripheral vessels and hypertension (39–45). Protein disulphide bonds and SH groups have been shown to be involved in the functioning of L-type Ca2+ channels in rabbit cardiac sarcolemmal and skeletal muscle transverse-tubule membranes (46,47). Oxidation of the SH groups of sarcolemmal Ca2+ channels and Ca2+ release channels of the sarcoplasmic reticulum may increase cytosolic [Ca2+]i, leading to contraction (48). Vascular Ca2+ channels could operate in a similar manner. Methylglyoxal has the chemical properties necessary to cause vascular Ca2+ channel alterations, leading to increased cytosolic [Ca2+]i, peripheral vascular resistance and hypertension (22,46).
Endogenous nitric oxide (NO) plays an important role in the regulation of blood pressure. NO of endothelial origin normally maintains vascular smooth muscle in a partially relaxed state (49). In human essential hypertensive patients and SHRs, NO production is impaired (50–53). A decrease in NO formation would promote an increase in peripheral vascular resistance and hypertension. Methylglyoxal strongly binds to arginine, which is the precursor to NO synthesis (54,55). Aldehydes may also inhibit NO synthase and guanylate cyclase, both thiol-dependent enzymes (54,56). In in vitro studies, methylglyoxal treatment of rat mesenteric arteries increased intracellular staining of methylglyoxal in endothelial cells and adventitia by fivefold, accompanied by an eightfold increase in the oxidative stress marker nitrotyrosine. This was associated with significantly reduced efficacy of NO-dependent relaxation. Antioxidant pretreatment prevented methylglyoxal-induced impairment of vasoreactivity. This impairment was also not observed in mesenteric arteries of glyoxalase transgenic rats (57).
Increased oxidative stress is present in both human hypertensive patients and SHRs. Methylglyoxal inhibits antioxidant enzymes through binding of SH groups at their active sites and leads to increased oxidative stress (16). The antioxidant enzyme glutathione peroxidase acts on GSH and hydrogen peroxide (H2O2) to produce oxidized glutathione (GSSG) and H2O. This enzyme also scavenges other peroxides. Glutathione reductase plays an important antioxidant defense role by reducing GSSG to GSH. Both these enzymes contain SH groups at their active site (58). In rat vascular smooth muscle cells in culture, these enzymes have been shown to be inhibited by methylglyoxal, leading to oxidative stress, low levels of GSH and increased levels of GSSG (16). Increased oxidative stress can lead to a further increase in reactive aldehydes through lipid peroxidation (22,59–63).
GAPDH, a key glycolytic enzyme, is sensitive to inhibition by agents of oxidative stress such as superoxide radicals and H2O2 (64). Of all the glycolytic enzymes, only GAPDH has been shown to be inhibited by H2O2 in cardiac muscle (65). Inhibition of GAPDH will lead to increased levels of methylglyoxal. GAPDH consists of four identical polypeptides (monomers) forming a tetramer. Four SH groups are present on each polypeptide, derived from cysteine residues within the polypeptide chain. One of the SH groups is found at the active site (cysteine-149) (66). Cysteine residues of the enzyme are highly reactive and are sensitive to modification by reactive aldehydes such as methylglyoxal. After in vitro incubation with endogenous aldehydes, including methylglyoxal, GAPDH was significantly inhibited (67,68). Incubation of platelets with methylglyoxal also produced significant inhibition of GAPDH (64).
In various in vitro studies, methylglyoxal has been shown to adversely affect metabolic functions at concentrations found in diabetic conditions, leading to cell damage and death. In rat renal cortical mitochondria, it inhibited the tricarboxylic acid cycle and electron transport chain (69). Methylglyoxal has been shown to cause apoptosis when incubated with human mesothelial cells and rat mesangial cells (70,71). Chronic oral administration of methylglyoxal leads to increased glomerular basement membrane thickness due to collagen accumulation in the kidneys of mice (72). This may explain the morphological changes we observed in kidney and vascular tissue, in which we have also found higher levels of aldehyde conjugates (Figures 5 to 8).
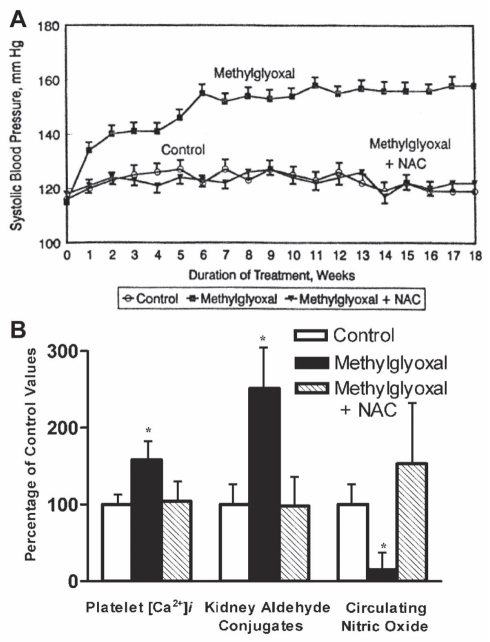
Figure 5).
A The line graph shows the effect of N-acetylcysteine (NAC) on systolic blood pressure in methylglyoxal-treated Wistar-Kyoto (WKY) rats. Starting at seven weeks of age, WKY rats were divided into three groups of six animals each. For the next 18 weeks, animals in the WKY-control group were given a normal diet and normal drinking water; the WKY-methylglyoxal group was given a normal diet and methylglyoxal in drinking water; and the WKY-methylglyoxal + NAC group was given 1.5% NAC in the diet and methylglyoxal in the drinking water. Methylglyoxal was given in the drinking water at a concentration of 0.2% during weeks 0 to 5; 0.4% at weeks 6 to 10; and 0.8% at weeks 11 to 18. Data are presented as mean ± SD of the six animals in each group for each week. Values are significantly different (P<0.05) from one to 18 weeks in the methylglyoxal groups compared with other groups of the same age. B The bar graph shows the effect of NAC on platelet free Ca2+ concentration ([Ca2+]i), kidney aldehyde conjugates and circulating nitric oxide in methylglyoxal-treated WKY rats. The experimental groups and treatment period were the same as in A. All values (mean ± SD) are expressed as a percentage of the control group values at the completion of the study at 25 weeks of age. *Values are significantly different (P<0.05) from other groups. Data from reference 84
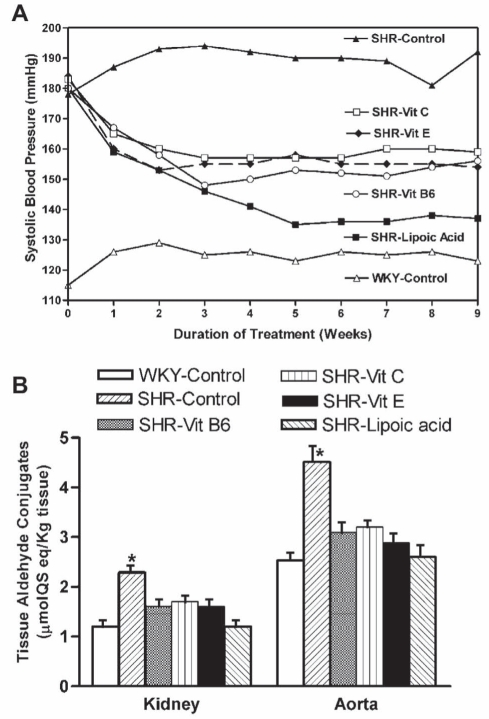
Figure 8).
A The line graph shows the effect of vitamin (Vit) B6, Vit E, Vit C and lipoic acid supplemented in the diet on systolic blood pressure in spontaneously hypertensive rats (SHRs). Starting at 12 weeks of age, animals were divided into six groups of six animals each. For nine weeks, animals in the Wistar-Kyoto (WKY)-control and SHR-control group were given a normal diet; the SHR-Vit B6 group was given a diet supplemented with 20 mg Vit B6; the SHR-Vit C group was given a diet supplemented with 100 mg Vit C; the SHR-Vit E group was given a diet supplemented with 3.4 mg Vit E; and the SHR-lipoic acid group was given a diet supplemented with 50 mg of lipoic acid per 100 g of diet. All animals were given normal drinking water. Values are given as the mean of the six animals in each group. SDs did not vary more than 6 mmHg in each case. Values are significantly different (P<0.05) from one to nine weeks in the SHR-control group compared with other groups of the same age. B The bar graph shows the effect of an antioxidant-supplemented diet on kidney and aortic aldehyde conjugates in SHRs. The experimental groups and treatment period were the same as in A. Data are presented as mean ± SD of the six animals in each group at the completion of the study at 21 weeks of age. *Values are significantly different (P<0.05) from other groups. QS Quinine sulphate. Data from references 27 to 30
Thus, methylglyoxal has the potential to elevate cytosolic [Ca2+]i, decrease NO in kidney and vascular tissue, inhibit antioxidant enzymes, deplete glutathione, cause oxidative stress in vascular tissue and produce hypertension (16,39,47,48,73–84). In both genetic (SHRs) and acquired (fructose-, threonine-, ethanol- and salt-induced) rat models of hypertension, we have shown elevated levels of aldehyde conjugates in vascular tissue, elevated cytosolic [Ca2+]i and adverse renal vascular changes (4,27–33,85–88).
To determine the direct effect of methylglyoxal on blood pressure, it was given in drinking water to WKY rats for 18 weeks (Figure 5A). Methylglyoxal-treated rats displayed a continuous increase in systolic blood pressure, which reached a plateau at six weeks of treatment. Methylglyoxal treatment resulted in significantly higher kidney aldehyde conjugates, platelet cytosolic Ca2+ and decreased circulating NO (Figure 5B). Rats treated with methylglyoxal also showed smooth muscle cell hyperplasia, thickening of the wall and narrowing of the lumen in small arteries and arterioles of the kidney (Figure 6) (84).
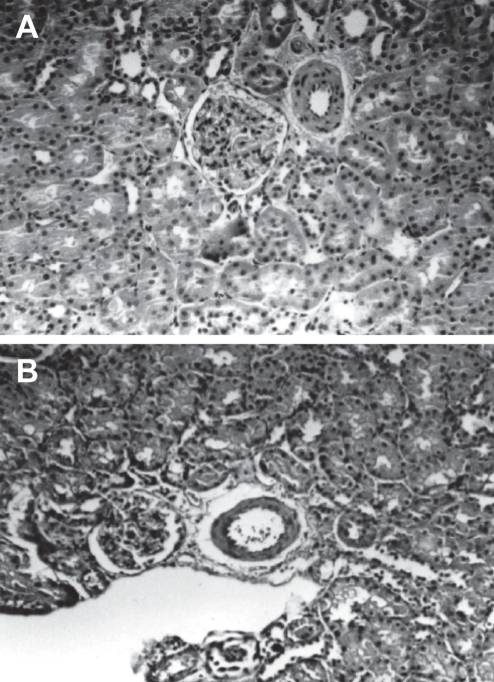
Figure 6).
A Light micrograph of a kidney from a Wistar-Kyoto (WKY) rat given methylglyoxal in drinking water for 18 weeks, showing smooth muscle cell hyperplasia with some narrowing of the lumen in the arteriole (hematoxylin and eosin stain, original magnification ×100). B Light micrograph of a kidney from a WKY rat given methylglyoxal in drinking water and N-acetylcysteine in the diet for 18 weeks, showing an almost normal-appearing arteriole (hematoxylin and eosin stain, original magnification ×100). Reproduced from reference 84
ANTIHYPERTENSIVE EFFECTS OF GLUTATHIONE, CYSTEINE, VITAMINS B6, E AND C, AND LIPOIC ACID VIA MODULATION OF METHYLGLYOXAL
Under normal physiological conditions, tissue levels of methylglyoxal are maintained at a low level. This is accomplished through further catabolism or by binding to soluble SH compounds, such as cysteine, and excretion in bile and urine. Cysteine is also a precursor of glutathione, which is essential as a cofactor in methylglyoxal catabolism. It has antioxidant activity, which acts to reduce oxidative stress and improve insulin-mediated glucose metabolism. In our studies, due to the unstable nature of cysteine, we used N-acetylcysteine (NAC), a commercially available analogue. Oral administration of NAC leads to increased tissue cysteine levels after deacylation, primarily in the kidney. Cysteine is stored in the tissues as glutathione and is released when required. Glutathione represents 90% of the total nonprotein low molecular weight thiol in the body (89).
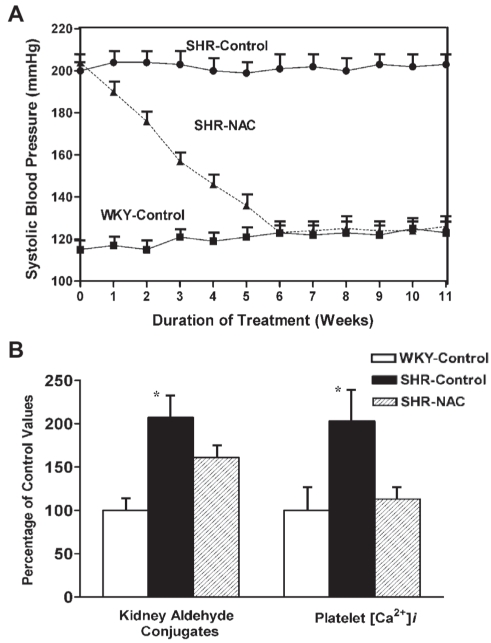
We investigated the effectiveness of NAC in lowering tissue aldehyde conjugates and blood pressure in methylglyoxal-treated WKY rats. NAC, 1.5% in the diet, prevented the increase in tissue aldehyde conjugates, cytosolic Ca2+ and hypertension. It also increased circulating NO and prevented adverse renal vascular changes (Figure 5) (84). We further investigated the antihypertensive effect of dietary cysteine supplementation in SHRs and fructose-induced WKY hypertensive rats. When SHRs were given NAC, their systolic blood pressure decreased significantly at two to 10 weeks. At six to 10 weeks, there was no significant difference in mean blood pressure in SHRs on NAC compared with WKY control rats of similar age. In addition to normalizing blood pressure, NAC also lowered kidney aldehyde conjugates and platelet [Ca2+]i in SHR rats (Figure 7) (5,88). Our study of fructose-treated WKY rats showed similar results (4).
Figure 7).
A The line graph shows the effect of N-acetylcysteine (NAC) on systolic blood pressure in spontaneously hypertensive rats (SHRs). Starting at 12 weeks of age, animals were divided into three groups of six animals each. For the next 11 weeks, the SHR-control and Wistar-Kyoto (WKY)-control groups were given a normal diet and normal drinking water and the SHR-NAC group was given 1.5% NAC in the diet and normal drinking water. Data are presented as mean ± SD of the six animals in each group. Values are significantly different (P<0.05) from one to 11 weeks in the SHR-control group compared with the other groups of the same age. B The bar graph shows the effect of NAC on kidney aldehyde conjugates and platelet free Ca2+ concentration ([Ca2+]i) in SHRs and WKY rats. The experimental groups and treatment period were the same as in A. All values (mean ± SD) are expressed as a percentage of the WKY-control group at the completion of the study at 23 weeks of age. *Values are significantly different (P<0.05) from other groups. Data from reference 5
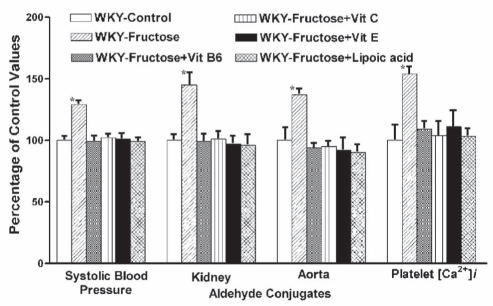
Because we found NAC to be so effective in lowering aldehydes and preventing hypertension, we decided to look at the possible effects of other antioxidants that normally occur in our diet that are known to increase tissue cysteine and glutathione. We studied the effect of dietary antioxidants on systolic blood pressure in the genetic model of hypertension, the SHR. They were given either vitamins C, E and B6, or lipoic acid for a period of nine weeks (27–30). We started this study when the rats were 12 weeks of age and already hypertensive. SHRs and normotensive WKY rats on regular chow were used as controls. Supplementation of vitamins B6, C and E significantly decreased blood pressure, while lipoic acid effectively normalized it. Tissue aldehyde conjugates were elevated in hypertensive animals compared with controls. These antioxidants significantly lowered tissue aldehydes. Treatment with these antioxidants also lowered cytosolic Ca2+ and attenuated renal vascular changes (Figure 8). We have also shown that these antioxidants prevent hypertension and normalize tissue aldehyde conjugates and platelet [Ca2+]i in fructose-treated WKY rats – an acquired model of hypertension (Figure 9) (31–33). The antihypertensive effect of dietary vitamin B6 in SHRs and fructose-treated rats is probably due to increased synthesis of cysteine from methionine (30,90). The antioxidant vitamins and lipoic acid increase tissue glutathione, improve glucose metabolism and decrease oxidative stress. We suggest that these dietary antioxidants prevent hypertension and associated biochemical and morphological changes by lowering endogenous methylglyoxal.
Figure 9).
The bar graph shows the effect of vitamin (Vit) B6, Vit C, Vit E and lipoic acid supplemented in the diet on systolic blood pressure, kidney and aortic aldehyde conjugates, and platelet free Ca2+ concentration ([Ca2+]i) in fructose-induced hypertensive Wistar-Kyoto (WKY) rats. Starting at seven weeks of age, rats were divided into six groups of six animals each. For 14 weeks, animals in the control group were given a normal diet and normal drinking water; fructose group, a normal diet and 4% fructose in drinking water; fructose + Vit B6 group, a diet supplemented with 20 mg of Vit B6 and 4% fructose in drinking water; fructose + Vit C group, a diet supplemented with 100 mg of Vit C and 4% fructose in drinking water; fructose + Vit E group, a diet supplemented with 3.4 mg of Vit E and 4% fructose in drinking water; and fructose + lipoic acid group, a diet supplemented with 50 mg of lipoic acid and 4% fructose in drinking water per 100 g of diet. Data (mean ± SD) are expressed as a percentage of the WKY-control group at the completion of the study at 21 weeks of age. *Values are significantly different (P<0.05) from other groups. Data from references 31 to 33
MODULATION OF METHYLGLYOXAL – A NEW MODALITY TO PREVENT HYPERTENSION
There is an increasing body of evidence demonstrating that essential hypertension, coronary artery disease, diabetes mellitus and hyperlipidemia develop due to the interaction of genetic and environmental factors. Diet and lifestyle factors can strongly influence the progression of hereditable disorders. Persons with a genetic susceptibility toward impaired glucose metabolism will be particularly sensitive to the adverse effects of a high-sucrose or high-fructose diet. A high-sucrose or high-fructose diet in these individuals leads to altered glucose metabolism, insulin resistance, increased levels of tissue methylglyoxal, oxidative stress and hypertension. This can be corrected nutritionally by lowering sucrose or fructose intake, and supplementation with an adequate mix of antioxidants such as vitamins B6, C and E, cysteine and lipoic acid. In most people, the present recommended daily allowance of such vitamins may be sufficient to maintain normal glucose metabolism and insulin balance, and additional vitamin supplementation may have no effect on blood pressure. However, for sugar-sensitive persons, particularly those consuming a high-sucrose or high-fructose diet, vitamin supplementation above the recommended daily allowance may be necessary to lower methylglyoxal, preventing insulin resistance, oxidative stress and hypertension. The anti-hypertensive effect documented in the Dietary Approach to Stop Hypertension (DASH) 1 and DASH 2 studies, which recommend a diet rich in fruit, vegetables, grain products and low-fat dairy goods, and low in total fat and salt intake for the control of mild hypertension, may be due to the antioxidants found in these nutrients.
CONCLUSION
The end result of either a genetic sensitivity, a high-sugar diet and/or a diet low in antioxidant vitamins is altered glucose metabolism. Altered glucose metabolism results in elevated levels of methylglyoxal, leading to hypertension and its associated biochemical and morphological changes. A diet rich in antioxidants such as cysteine, lipoic acid and vitamins B6, C and E can maintain methylglyoxal at a low level and, thus, prevent hypertension.
Acknowledgments
The authors thank the Canadian Institutes of Health Research for their financial support.
REFERENCES
- 1.Reaven GM. Insulin resistance, hyperinsulinemia, and hypertriglyceridemia in the etiology and clinical course of hypertension. Am J Med. 1991;90(2A):7S–12S. doi: 10.1016/0002-9343(91)90028-v. [DOI] [PubMed] [Google Scholar]
- 2.Ferrannini E, Buzzigoli G, Bonadonna R, et al. Insulin resistance in essential hypertension. N Eng J Med. 1987;317:350–7. doi: 10.1056/NEJM198708063170605. [DOI] [PubMed] [Google Scholar]
- 3.Levy J, Zemel MB, Sowers JR. Role of cellular calcium metabolism in abnormal glucose metabolism, and diabetic hypertension. Am J Med. 1989;87(6A):7S–16S. doi: 10.1016/0002-9343(89)90489-0. [DOI] [PubMed] [Google Scholar]
- 4.Vasdev S, Ford CA, Longerich L, Gadag V, Wadhawan S. Role of aldehydes in fructose induced hypertension. Mol Cell Biochem. 1998;181:1–9. doi: 10.1023/a:1006844222963. [DOI] [PubMed] [Google Scholar]
- 5.Vasdev S, Mian T, Ford CA, Longerich L, Parai S. Role of aldehydes in spontaneously hypertensive rats and disulfiram-induced hypertensive rats. Nutr Metab Cardiovasc Dis. 1996;6:130–40. [Google Scholar]
- 6.Bolli P, Erne P, Hulthen UL, et al. Parallel reduction of calcium-influx-dependent vasoconstriction and platelet-free calcium concentration with calcium entry and β-adrenoreceptor blockade. J Cardiovasc Pharmacol. 1984;6:S996–S1001. [PubMed] [Google Scholar]
- 7.Alexander MC, Lomanto M, Nasrin N, Ramaika C. Insulin stimulates glyceraldehydes-3-phosphate dehydrogenase gene expression through CIS-acting DNA sequences. Proc Natl Acad Sci. 1988;85:5092–6. doi: 10.1073/pnas.85.14.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kashiwagi A, Obata T, Suzaki M, et al. Increase in cardiac muscle fructose content in streptozotocin-induced diabetic rats. Metabolism. 1992;41:1041–6. doi: 10.1016/0026-0495(92)90283-g. [DOI] [PubMed] [Google Scholar]
- 9.Murray RK, Granner DK, Mayes PA, Rodwell VW. Harper’s Biochemistry. 25th edn. Norwalk: Appleton and Lange; 2000. pp. 192–3.pp. 225 [Google Scholar]
- 10.Vander Jagt DL, Hunsaker LA. Enzymology and Molecular Biology of Carbonyl Metabolism 4. New York: Plenum Press; 1993. pp. 279–88. [Google Scholar]
- 11.Kondoh Y, Kawase M, Ohmori S. D-lactate concentrations in blood, urine and sweat before and after exercise. Eur J Appl Physiol. 1992;65:88–93. doi: 10.1007/BF01466280. [DOI] [PubMed] [Google Scholar]
- 12.Thornalley PJ. Modification of the glyoxalase system in disease processes and prospects for therapeutic strategies. Biochem Soc Trans. 1993;21:531–4. doi: 10.1042/bst0210531. [DOI] [PubMed] [Google Scholar]
- 13.Phillips SA, Mirlees D, Thornalley PJ. Modification of the glyoxalase system in streptozotocin-induced diabetic rats. Effect of the aldose reductase inhibitor statil. Biochem Pharmacol. 1993;46:805–11. doi: 10.1016/0006-2952(93)90488-i. [DOI] [PubMed] [Google Scholar]
- 14.Beisswenger PJ, Howell SK, Smith K, Szwergold BS. Glyceraldehyde-3-phosphate dehydrogenase activity as an independent modifier of methylglyoxal levels in diabetes. Biochim Biophys Acta. 2003;1637:98–106. doi: 10.1016/s09254439(02)00219-3. [DOI] [PubMed] [Google Scholar]
- 15.Lembo G, Iaccarino G, Vecchione C, Rendina V, Trimarco B. Insulin modulation of vascular reactivity is already impaired in prehypertensive spontaneously hypertensive rats. Hypertens. 1995;26:290–3. doi: 10.1161/01.hyp.26.2.290. [DOI] [PubMed] [Google Scholar]
- 16.Wu L, Juurlink BHJ. Increased methylglyoxal and oxidative stress in hypertensive rat vascular smooth muscle cells. Hypertens. 2002;39:809–14. doi: 10.1161/hy0302.105207. [DOI] [PubMed] [Google Scholar]
- 17.Chang KC, Paek KS, Kim HJ, Lee YS, Yabe-Nishimura C, Seo HG. Substrate-induced up-regulation of aldose reductase by methylglyoxal, a reactive oxoaldehyde elevated in diabetes. Mol Pharmacol. 2002;61:1184–91. doi: 10.1124/mol.61.5.1184. [DOI] [PubMed] [Google Scholar]
- 18.Vander Jagt DL, Hunsaker LA. Methylglyoxal metabolism and diabetic complications: Roles of aldose reductase, glyoxalase-I, betaine aldehyde dehydrogenase and 2-oxoaldehyde dehydrogenase. Chem Biol Interact. 2003;143–144:341–51. doi: 10.1016/s0009-2797(02)00212-0. [DOI] [PubMed] [Google Scholar]
- 19.Vander Jagt DL, Hassebrook RK, Hunsaker LA, Brown WM, Royer RE. Metabolism of the 2-oxoaldehyde methylglyoxal by aldose reductase and by glyoxalase-I: Roles for glutathione in both enzymes and implications for diabetic complications. Chem Biol Interact. 2001;130–132:549–62. doi: 10.1016/s0009-2797(00)00298-2. [DOI] [PubMed] [Google Scholar]
- 20.Beisswenger PJ, Howell SK, Touchette AD, Lal S, Szwergold S. Metformin reduces systemic methylglyoxal levels in type 2 diabetes. Diabetes. 1999;48:198–202. doi: 10.2337/diabetes.48.1.198. [DOI] [PubMed] [Google Scholar]
- 21.Han Y, Randell E, Vasdev S, et al. Plasma methylglyoxal and glyoxal are elevated and related to early membrane alteration in young, complication-free patients with type 1 diabetes. Mol Cell Biochem. 2007;305:123–31. doi: 10.1007/s11010-007-9535-1. [DOI] [PubMed] [Google Scholar]
- 22.Schauenstein E, Esterbauer H, Zollner H. Aldehydes in biological systems. In: Lagnado JR, editor. Aldehydes in Biological Systems: Their Natural Occurrence and Biological Activities. London: Pion Limited; 1977. pp. 1–7. [Google Scholar]
- 23.Baskaran S, Balasubramanian KA. Effect of methylglyoxal on protein thiol and amino groups in isolated rat enterocytes and colonocytes and activity of various brush border enzymes. Ind J Biochem Biophys. 1990;27:13–7. [PubMed] [Google Scholar]
- 24.Mostafa AA, Randell EW, Vasdev SC, et al. Plasma protein advanced glycation end products, carboxymethyl cysteine, and carboxymethyl cysteine, are elevated and related to nephropathy in patients with diabetes. Mol Cell Biochem. 2007;302:35–42. doi: 10.1007/s11010-007-9422-9. [DOI] [PubMed] [Google Scholar]
- 25.Han Y, Randell E, Vasdev S, et al. Plasma advanced glycation endproduct, methylglyoxal-derived hydroimidazolone is elevated in young, complication-free patients with type 1 diabetes. Clin Biochem. 2009;42:562–9. doi: 10.1016/j.clinbiochem.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 26.McLellan AC, Phillips SA, Thornalley PJ. The assay of SD-lactoylglutathione in biological systems. Anal Biochem. 1993;211:37–43. doi: 10.1006/abio.1993.1229. [DOI] [PubMed] [Google Scholar]
- 27.Vasdev S, Gill V, Parai S, Longerich L, Gadag V. Dietary vitamin E supplementation lowers blood pressure in spontaneously hypertensive rats. Mol Cell Biochem. 2002;238:111–7. doi: 10.1023/a:1019915306581. [DOI] [PubMed] [Google Scholar]
- 28.Vasdev S, Ford CA, Parai S, Longerich L, Gadag V. Dietary vitamin C supplementation lowers blood pressure in spontaneously hypertensive rats. Mol Cell Biochem. 2001;218:97–103. doi: 10.1023/a:1007234027421. [DOI] [PubMed] [Google Scholar]
- 29.Vasdev S, Ford CA, Parai S, Longerich L, Gadag V. Dietary alpha-lipoic acid supplementation lowers blood pressure in spontaneously hypertensive rats. J Hypertens. 2000;18:567–73. doi: 10.1097/00004872-200018050-00009. [DOI] [PubMed] [Google Scholar]
- 30.Vasdev S, Ford CA, Parai S, Longerich L, Gadag V. Dietary B6 supplementation attenuates hypertension in spontaneously hypertensive rats. Mol Cell Biochem. 1999;200:155–62. doi: 10.1023/a:1007088512834. [DOI] [PubMed] [Google Scholar]
- 31.Vasdev S, Gill V, Parai S, Longerich L, Gadag V. Dietary vitamin E and C supplementation prevents fructose induced hypertension in rats. Mol Cell Biochem. 2002;241:107–14. doi: 10.1023/a:1020835229591. [DOI] [PubMed] [Google Scholar]
- 32.Vasdev S, Ford CA, Parai S, Longerich L, Gadag V. Dietary lipoic acid supplementation prevents fructose-induced hypertension in rats. Nutr Met Cardiovasc Dis. 2000;10:339–46. [PubMed] [Google Scholar]
- 33.Vasdev S, Longerich L, Gill V. Prevention of fructose-induced hypertension by dietary vitamins. Clin Biochem. 2004;37:1–9. doi: 10.1016/j.clinbiochem.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Wu L, Juurlink HJ. The impaired glutathione system and its up-regulation by sulforaphane in vascular smooth muscle cells from spontaneously hypertensive rats. J Hypertens. 2001;19:1819–25. doi: 10.1097/00004872-200110000-00016. [DOI] [PubMed] [Google Scholar]
- 35.Cabassi A, Dumont EC, Girouard H, et al. Effects of chronic N-acetylcysteine treatment on the actions of peroxynitrite on aortic vascular reactivity in hypertensive rats. J Hypertens. 2001;19:1233–44. doi: 10.1097/00004872-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Schauenstein E, Esterbauer H. Formation and properties of reactive aldehydes. Ciba Found Symp. 1978;(67):225–44. doi: 10.1002/9780470720493.ch15. [DOI] [PubMed] [Google Scholar]
- 37.Lieber CS. Mechanism of ethanol induced hepatic injury. Pharmacol Ther. 1990;46:1–41. doi: 10.1016/0163-7258(90)90032-w. [DOI] [PubMed] [Google Scholar]
- 38.Beard KM, Shangari N, Wu B, O’Brien PJ. Metabolism, not autoxidation, plays a role in α-oxoaldehyde- and reducing sugar-induced erythrocyte GSH depletion: Revelance for diabetes mellitus. Mol Cell Biochem. 2003;252:331–8. doi: 10.1023/a:1025544309616. [DOI] [PubMed] [Google Scholar]
- 39.Ives HE. Ion transport defects and hypertension. Where is the link? Hypertens. 1989;14:590–7. doi: 10.1161/01.hyp.14.6.590. [DOI] [PubMed] [Google Scholar]
- 40.Aoki K, Kawaguchi Y, Sato K, Kondo S, Yamamoto M. Clinical and pharmacological properties of calcium antagonists in essential hypertension in humans and spontaneously hypertensive rats. J Cardiovasc Pharmacol. 1982;4:S298–S302. [PubMed] [Google Scholar]
- 41.Filo RS, Bohr DF, Ruegg JC. Glycerinated skeletal and smooth muscle: Calcium and magnesium dependence. Science. 1965;147:1581–3. doi: 10.1126/science.147.3665.1581. [DOI] [PubMed] [Google Scholar]
- 42.Bohr DF. Vascular smooth muscle: Dual effect of calcium. Science. 1963;139:597–9. doi: 10.1126/science.139.3555.597. [DOI] [PubMed] [Google Scholar]
- 43.Blaustein MP. Sodium ions, calcium ions, blood pressure regulation, and hypertension: A reassessment and a hypothesis. Am J Physiol. 1977;232:C165–C173. doi: 10.1152/ajpcell.1977.232.5.C165. [DOI] [PubMed] [Google Scholar]
- 44.Bolton TB. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979;59:606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- 45.Robinson BF. Altered calcium handling as a cause of primary hypertension. J Hypertens. 1984;2:453–60. doi: 10.1097/00004872-198410000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Zaidi NF, Lagenaur CF, Abramson JJ, Pessah I, Salama G. Reactive disulfides trigger Ca2+ release from sarcoplasmic reticulum via an oxidation reaction. J Biol Chem. 1989;264:21725–36. [PubMed] [Google Scholar]
- 47.Murphy BJ, Washkurak AW, Tuana BS. Dihydropyridine binding to the L-type Ca2+ channel in rabbit heart sarcolemma and skeletal muscle transverse-tubules: Role of disulfide, sulfhydryl and phosphate groups. Biochim Biophys Acta. 1990;1052:333–9. doi: 10.1016/0167-4889(90)90230-b. [DOI] [PubMed] [Google Scholar]
- 48.Oba T, Yamaguchi M. Sulfhydryls on frog skeletal muscle membrane participate in contraction. Am J Physiol. 1990;259:C709–C714. doi: 10.1152/ajpcell.1990.259.5.C709. [DOI] [PubMed] [Google Scholar]
- 49.Scherrer U, Sartori C. Defective nitric oxide synthesis: A link between metabolic insulin resistance, sympathetic overactivity and cardiovascular morbidity. Eur J Endocrinol. 2000;142:315–23. doi: 10.1530/eje.0.1420315. [DOI] [PubMed] [Google Scholar]
- 50.Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1996;27:567–74. doi: 10.1016/0735-1097(95)00522-6. [DOI] [PubMed] [Google Scholar]
- 51.Taddei S, Salvetti A. Direct effects of insulin on the arteriolar tone: Abnormality in insulin-resistant states? Nutr Metab Cardiovasc Dis. 1996;6:178–86. [Google Scholar]
- 52.Ibarra M, Meneses A, Ransanz V, Castillo C, Hong E. Changes in endothelium-dependent vascular responses associated with spontaneous hypertension and age in rats. Arch Med Res. 1995;26:S177–S183. [PubMed] [Google Scholar]
- 53.Lusher TF, Tanner FC, Dohi Y. Age, hypertension and hypercholesterolaemia alter endothelium-dependent vascular regulation. Pharmacol Toxicol. 1992;70:S32–S39. doi: 10.1111/j.1600-0773.1992.tb01620.x. [DOI] [PubMed] [Google Scholar]
- 54.Degenhardt TP, Thorpe SR, Baynes JW. Chemical modification of proteins by methylglyoxal. Cell Mol Biol. 1998;44:1139–45. [PubMed] [Google Scholar]
- 55.Selwood T, Thornalley PJ. Binding of methylglyoxal to albumin and formation of fluorescent adducts. Inhibition by arginine, N-acetylarginine and aminoguanidine. Biochem Soc Trans. 1993;21:170S. doi: 10.1042/bst021170s. [DOI] [PubMed] [Google Scholar]
- 56.Brandwein HJ, Lewicki JA, Murad F. Reversible inactivation of guanylate cyclase by mixed disulfide formation. J Biol Chem. 1981;256:2958–62. [PubMed] [Google Scholar]
- 57.Brouwers O, Niessen PM, Haenen G, et al. Hyperglycaemia-induced impairment of endothelium-dependent vasorelaxation in rat mesenteric arteries is mediated by intracellular methylglyoxal levels in a pathway dependent on oxidative stress. Diabetologia. 2010;53:989–1000. doi: 10.1007/s00125-010-1677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stadtman TC. Selenum biochemistry. Ann Rev Biochem. 1990;59:111–27. doi: 10.1146/annurev.bi.59.070190.000551. [DOI] [PubMed] [Google Scholar]
- 59.Brooks BR, Klamerth OJ. Interaction of DNA with bifunctional aldehydes. European J Biochem. 1968;5:178–82. doi: 10.1111/j.1432-1033.1968.tb00355.x. [DOI] [PubMed] [Google Scholar]
- 60.Thornalley P, Wolff S, Crabbe J, Stern A. The autoxidation of glyceraldehyde and other simple monosaccharides under physiological conditions catalysed by buffer ions. Biochim Biophys Acta. 1984;797:276–87. doi: 10.1016/0304-4165(84)90131-4. [DOI] [PubMed] [Google Scholar]
- 61.Poli G, Dianzani MU, Cheeseman KH, Slater TF, Lang J, Esterbauer H. Separation and characterization of the aldehydic products of lipid peroxidation stimulated by carbon tetrachloride or ADP-iron in isolated rat hepatocytes and rat liver microsomal suspensions. Biochem J. 1985;227:629–38. doi: 10.1042/bj2270629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Benedetti A, Comporti M, Esterbauer H. Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochim Biophys Acta. 1980;620:281–96. doi: 10.1016/0005-2760(80)90209-x. [DOI] [PubMed] [Google Scholar]
- 63.Dargel R. Lipid peroxidation – a common pathogenetic mechanism? Exp Toxic Pathol. 1992;44:169–81. doi: 10.1016/S0940-2993(11)80202-2. [DOI] [PubMed] [Google Scholar]
- 64.Leoncini G, Maresca M, Buzzi E. Inhibition of the glycolytic pathway by methylgloxal in human platelets. Cell Biochem Function. 1989;7:65–70. doi: 10.1002/cbf.290070111. [DOI] [PubMed] [Google Scholar]
- 65.Chatham JC, Gilbert HF, Radda GK. The metabolic consequences of hydroperoxide perfusion on the isolated rat heart. Eur J Biochem. 1989;184:657–62. doi: 10.1111/j.1432-1033.1989.tb15063.x. [DOI] [PubMed] [Google Scholar]
- 66.Murray RK, Granner DK, Mayes PA, Rodwell VW. Harper’s Biochemistry. 25th edn. Norwalk: Appleton and Lange; 2000. p. 193. [Google Scholar]
- 67.Leoncini G, Maresca M, Bonsignore A. The effect of methylglyoxal on the glycolytic enzymes. FEBS Lett. 1980;117:17–8. doi: 10.1016/0014-5793(80)80903-3. [DOI] [PubMed] [Google Scholar]
- 68.Novotny MV, Yancey MF, Stuart R, Wiesler D, Peterson RG. Inhibition of glycolytic enzymes by endogenous aldehydes: A possible relation to diabetic neuropathies. Biochem Biophys Acta. 1994;1226:145–50. doi: 10.1016/0925-4439(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 69.Rosca MG, Monnier VM, Szweda LI, Weiss MF. Alterations in renal mitochondrial respiration in response to the reactive oxoaldehyde methylglyoxal. Am J Physiol Renal Physiol. 2001;283:F52–F59. doi: 10.1152/ajprenal.00302.2001. [DOI] [PubMed] [Google Scholar]
- 70.Amore A, Cappelli G, Cirina P, et al. Glucose degradation products increase apoptosis of human mesothelial cells. Nephrol Dial Transplant. 2003;18:677–88. doi: 10.1093/ndt/gfg003. [DOI] [PubMed] [Google Scholar]
- 71.Liu BF, Miyata S, Hirota Y, et al. Methylglyoxal induces apoptosis through activation of p38 mitogen-activated protein kinase in rat mesangial cells. Kidney Intern. 2003;63:947–57. doi: 10.1046/j.1523-1755.2003.00829.x. [DOI] [PubMed] [Google Scholar]
- 72.Golej J, Hoeger H, Radner W, Unfried G, Lubec G. Oral administration of methylglyoxal leads to kidney collagen accumulation in the mouse. Life Sci. 1998;63:801–7. doi: 10.1016/s0024-3205(98)00336-1. [DOI] [PubMed] [Google Scholar]
- 73.Hebbel RP, Shaley O, Foker W, Rank BH. Inhibition of erythrocyte Ca2+-ATPase by activated oxygen through thiol- and lipid-dependent mechanisms. Biochem Biophys Acta. 1986;862:8–16. doi: 10.1016/0005-2736(86)90463-3. [DOI] [PubMed] [Google Scholar]
- 74.Pierce GN, Ward R, Philipson LD. Role for sulfur-containing groups in the Na+-Ca2+ exchange of cardiac sarcolemmal vesicles. J Membrane Biol. 1986;94:217–25. doi: 10.1007/BF01869717. [DOI] [PubMed] [Google Scholar]
- 75.Needleman P, Jakschik B, Johnson EM., Jr Sulfhydryl requirement for relaxation of vascular smooth muscle. J Pharmacol Exp Ther. 1973;187:324–31. [PubMed] [Google Scholar]
- 76.Bellomo G, Mirabelli F, Richelmi P, Orrenius S. Critical role of sulfhydryl group(s) in ATP-dependent Ca2+ sequestration by the plasma membrane fraction from rat liver. FEBS. 1983;163:136–9. doi: 10.1016/0014-5793(83)81180-6. [DOI] [PubMed] [Google Scholar]
- 77.Jones DP, Thor H, Smith MT, Jewell SA, Orrenius S. Inhibition of ATP-dependent microsomal Ca2+ sequestration during oxidative stress and its prevention by glutathione. J Biol Chem. 1983;258:6390–3. [PubMed] [Google Scholar]
- 78.Di Monte D, Bellomo G, Thor H, Nicotera P, Orrenius S. Menadione-induced cytotoxicity is associated with protein thiol oxidation and alteration in intracellular Ca2+ homeostasis. Arch Biochem Biophys. 1984;235:343–50. doi: 10.1016/0003-9861(84)90207-8. [DOI] [PubMed] [Google Scholar]
- 79.Zaidi NF, Lagenaur CF, Hilkirt RJ, Xiong H, Abramson JJ, Salama G. Disulfide linkage of biotin identifies a 106-kDa Ca2+ release channel in sarcoplasmic reticulum. J Biol Chem. 1989;264:21737–47. [PubMed] [Google Scholar]
- 80.Kindmark H, Kohler M, Nilsson T, et al. Measurements of cytoplasmic free Ca2+ concentration in human pancreatic islets and insulinoma cells. FEBS. 1991;291:310–4. doi: 10.1016/0014-5793(91)81309-v. [DOI] [PubMed] [Google Scholar]
- 81.Fridolf T, Ahrén B. Dual action of the neuropeptide galanin on the cytoplasmic free calcium concentration in RIN m5F cells. Biochem Biophys Res Commun. 1993;191:1224–9. doi: 10.1006/bbrc.1993.1348. [DOI] [PubMed] [Google Scholar]
- 82.Elliott AC, Trebilcock R, Yates AP, Best L. Stimulation of HIT-T15 insulinoma cells by glyceraldehyde does not require its metabolism. Eur J Biochem. 1993;231:359–65. doi: 10.1111/j.1432-1033.1993.tb17769.x. [DOI] [PubMed] [Google Scholar]
- 83.Hirosumi J, Ouchi Y, Watanabe M, Kusunoki J, Nakamura T, Orimo H. Effect of superoxide and lipid peroxide on cytosolic free calcium concentration in cultured pig aortic endothelial cells. Biochem Biophys Res Commun. 1988;152:301–7. doi: 10.1016/s0006-291x(88)80714-9. [DOI] [PubMed] [Google Scholar]
- 84.Vasdev S, Ford CA, Longerich L, Parai S, Gadag V. Aldehyde induced hypertension in rats: Prevention by N-acetyl cysteine. Artery. 1998;23:10–36. [PubMed] [Google Scholar]
- 85.Vasdev S, Wadhawan S, Ford CA, Parai S, Longerich L, Gadag V. Dietary vitamin B6 supplementation prevents ethanol induced hypertension in rats. Nutr Met Cardiovasc Dis. 1999;9:55–63. [PubMed] [Google Scholar]
- 86.Vasdev S, Gill V, Longerich L, Parai S, Gadag V. Salt-induced hypertension in WKY rats: Prevention by α-lipoic acid supplementation. Mol Cell Biochem. 2003;254:319–26. doi: 10.1023/a:1027354005498. [DOI] [PubMed] [Google Scholar]
- 87.Vasdev S, Ford CA, Longerich L, Parai S. A nutritional approach to prevent high blood pressure. In: Pierce G, editor. Atherosclerosis, Hypertension, and Diabetes. Boston: Kluwer Academic Publishers; 2003. pp. 187–96. [Google Scholar]
- 88.Vasdev S, Longerich L, Ford CA. Role of aldehydes in hypertension. In: Sharma BK, et al., editors. Adaptation Biology. New Delhi: Narosa Publishing House; 1997. pp. 326–39. [Google Scholar]
- 89.Meister A, Anderson ME, Hwang O. Intracellular cysteine and glutathione delivery systems. J Am Coll Nutr. 1986;5:137–51. doi: 10.1080/07315724.1986.10720121. [DOI] [PubMed] [Google Scholar]
- 90.Lal KJ, Dakshinamurti K, Thliveris J. The effect of vitamin B6 on the systolic blood pressure of rats in various animal models of hypertension. J Hypertens. 1996;14:355–63. doi: 10.1097/00004872-199603000-00013. [DOI] [PubMed] [Google Scholar]