Abstract
AMP-activated protein kinase (AMPK) is a metabolic sensor involved in intracellular energy metabolism through the control of several homeostatic mechanisms, which include autophagy and protein degradation. Recently, we reported that AMPK activation by resveratrol promotes autophagy-dependent degradation of the amyloid-β (Aβ) peptides, the core components of the cerebral senile plaques in Alzheimer's disease. To identify more potent enhancers of Aβ degradation, we screened a library of synthetic small molecules selected for their structural similarities with resveratrol. Here, we report the identification of a series of structurally related molecules, the RSVA series, which inhibited Aβ accumulation in cell lines nearly 40 times more potently than did resveratrol. Two of these molecules, RSVA314 and RSVA405, were further characterized and were found to facilitate CaMKKβ-dependent activation of AMPK, to inhibit mTOR (mammalian target of rapamycin), and to promote autophagy to increase Aβ degradation by the lysosomal system (apparent EC50 ∼1 μM). This work identifies the RSVA compounds as promising lead molecules for the development of a new class of AMPK activating drugs controlling mTOR signaling, autophagy, and Aβ clearance.—Vingtdeux, V., Chandakkar, P., Zhao, H., d'Abramo, C., Davies, P., Marambaud, P. Novel synthetic small-molecule activators of AMPK as enhancers of autophagy and amyloid-β peptide degradation.
Keywords: Alzheimer's disease, mTOR, resveratrol, raptor, p62
Alzheimer's disease (AD) is caused by progressive neurodegeneration in different regions of the brain neocortex and hippocampus. Two lesions are invariably observed in the AD brain, the neurofibrillary tangles formed by tau aggregation and the senile plaques, which result from the deposition of the neurotoxic amyloid-β (Aβ) peptides (1, 2). Two main Aβ isoforms exist, Aβ1–40 and Aβ1–42, which are produced by sequential endoproteolysis of the amyloid-β precursor protein (APP) by β- and γ-secretases (3–5).
Recently, we and others have shown that the naturally occurring polyphenol resveratrol (trans-3,4′,5-trihydroxystilbene) controls Aβ levels by facilitating its proteolytic clearance in cell lines and in primary neurons (6–8) and lowers amyloid deposition in vivo in APP-transgenic mouse brain (7, 9). Our work (7) demonstrated that activation of the metabolic sensor AMPK is responsible for the anti-amyloidogenic effect of resveratrol. AMPK is a heterotrimeric Ser/Thr protein kinase activated by different upstream kinases via a specific Thr phosphorylation within the activation loop of the catalytic α-subunit (10). LKB1 is the main AMPK-activating kinase, and its action is facilitated when the AMP:ATP ratio rises. The Ca2+/calmodulin-dependent protein kinase kinase-β (CaMKKβ), a kinase predominantly expressed in neural tissue, is also involved in AMPK phosphorylation and activation (11, 12). AMPK targets several proteins involved in cellular energy balance, including the regulator of fatty acid biosynthesis acetyl-CoA carboxylase (ACC; ref. 13). AMPK signaling controls mechanisms relevant to protein degradation by inhibiting mammalian target of rapamycin (mTOR) and by activating macroautophagy (10). Macroautophagy (hereafter referred to as autophagy) is an evolutionary conserved lysosomal pathway involved in protein and organelle turnover via the formation of vacuoles known as autophagosomes (14). Notably, autophagy is deregulated in the AD brain and controls Aβ metabolism both in cell lines and in vivo in mouse models (15–17). Beyond its role in protein metabolism, the protein kinase mTOR is also critically involved in cellular homeostasis through its function in cell growth (18, 19). Indeed, mTOR promotes cell growth and anabolism by increasing protein and lipid synthesis via activation of p70-S6 kinase (p70S6K) and by decreasing autophagic catabolism through phosphorylation-mediated inhibition of the Atg1 protein complex (19). Overactivated mTOR is associated with several pathologies, such as cancer, diabetes, and the metabolic syndrome (18). Conversely, inhibition of mTOR prolongs life span (20) and may be involved in the longevity effect of caloric restriction (21).
Our recent work (7) revealed that resveratrol activates AMPK by increasing intracellular Ca2+ levels and by promoting AMPK phosphorylation at Thr-172 by CaMKKβ. Activation of AMPK by resveratrol results in mTOR inhibition and potentiation of autophagy and lysosomal clearance of Aβ. Notably, we (7) also demonstrated that orally administered resveratrol in mice can cross the blood-brain barrier to activate AMPK and reduce Aβ levels and deposition in the cerebral cortex. These data underscored the therapeutic potential of AMPK activation as an anti-amyloidogenic strategy in AD. In the present study, we screened in cell lines synthetic small molecules selected for their structural similarities with resveratrol with the aim to identify compounds with improved anti-amyloidogenic activities. We identified a series of structurally related molecules, the RSVA series, that inhibited Aβ accumulation in cell lines nearly 40 times more potently than did resveratrol. Two of these analogues of resveratrol, RSVA314 and RSVA405, were found to share with resveratrol the same mechanism of action by the following means: facilitating CaMKKβ-dependent activation of AMPK, inhibiting mTOR, and promoting autophagy and lysosomal degradation of Aβ. This work identifies the RSVA compounds as promising lead molecules for the development of AMPK-activating drugs controlling mTOR signaling and facilitating autophagy and Aβ degradation.
MATERIALS AND METHODS
Materials and antibodies
STO-609, A23187, compound C, and bafilomycin A1 were purchased from Calbiochem (EMD Chemicals, Gibbstown, NJ, USA). Chloroquine and DMSO were from Sigma-Aldrich (St. Louis, MO, USA). Dominant negative T172A-AMPK cDNA was kindly provided by Dr. David Carling (Medical Research Council Clinical Sciences Centre, Imperial College, London, UK). Kinase-dead K45R-AMPK cDNA was obtained from Addgene (Cambridge, MA, USA). Anti-Aβ-(1–17; 6E10), anti-Aβ1–40 (11A50-B10), and anti-Aβ1–42 (12F4) antibodies were from Covance (Emeryville, CA, USA). Antibodies directed against AMPK, pAMPK (Thr-172), ACC, pACC (Ser-79), p70S6K, p-p70S6K (Thr-389), pS6 (Ser-235/236), peEF2K (Ser-366), LKB1, LC3, pRaptor (Ser-792), raptor, CREB, pCREB (Ser-133), pc-Jun (Ser-63), c-Jun, and c-Fos were from Cell Signaling Technology (Danvers, MA, USA). Anti-actin antibody was from BD Transduction Laboratories (San Jose, CA, USA). Anti-myc (9E10) antibody was from Chemicon International (Temecula, CA, USA). Anti-p62 antibody was from Santa Cruz Biotechnologies (Santa Cruz, CA, USA).
Cell lines and drug treatments
HEK293 cells stably transfected with human APP695 (APP-HEK293) were provided by Dr. Luciano D'Adamio (Albert Einstein College of Medicine, Bronx, NY, USA). N2a cells stably transfected with human APP695 harboring the Swedish double mutation (SwAPP-N2a) were obtained from Dr. Gopal Thinakaran (University of Chicago, Chicago, IL, USA). SH-SY5Y cells stably transfected with human APP695 (APP-SY5Y) were obtained from Dr. Luc Buee (INSERM U815, Centre Jean Pierre Aubert, Lille, France; ref. 22). APP-HEK293 cells were grown in DMEM plus 10% FBS, penicillin, streptomycin, and 5 μg/ml puromycin. SwAPP-N2a and APP-SY5Y cells were maintained in 1:1 DMEM/Opti-MEM supplemented with 10% FBS, penicillin, streptomycin, and 0.2 mg/ml G418. All cell lines were tested negative for mycoplasma contaminants (23). For drug treatments, cells were treated at confluence for the indicated concentrations and incubation times. Medium was then changed, and treatments were continued for another 3 h to allow Aβ secretion. Cells were transiently transfected with T172A-AMPK or K45R-AMPK cDNAs using Lipofectamine 2000 reagent (Invitrogen, San Diego, CA, USA), as per manufacturer's instructions.
Primary neuronal cultures
Primary neurons were prepared as described previously (7). Briefly, C57BL/6 females were killed at 17.5 days of gestation. Forebrains were dissected in ice-cold Hanks' balanced salt solution (Invitrogen) plus 0.5% w/v d-glucose (Sigma) and 25 mM HEPES (Invitrogen) under a dissection microscope. Dissociation was carried out mechanically in ice-cold dissection medium containing 0.01% papain (Worthington Biochemical Corporation, Lakewood, NJ, USA), 0.1% w/v dispase (Roche Applied Science, Indianapolis, IN, USA), and 0.01% DNase (Worthington) and by incubation at 37°C twice for 15 min. Cells were then spun down at 220 g for 5 min at 4°C; resuspended in Neurobasal medium with 2% B27, 1 mM sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM Glutamax (Invitrogen); filtered through a 40-μm cell strainer (Thermo Fisher Scientific Inc., Hudson, NH, USA); counted; and plated on poly-l-ornithine- and laminin-coated plates at a density of ∼106 cells/well. Culture medium was completely replaced after 16–20 h, and new medium (30% of starting volume) was added every 3 days until the end of the culture period.
Compound library screening
The 158 compounds were selected from the Hit2Lead screening compound library (Chembridge Corp., San Diego, CA, USA) for their structural similarities with resveratrol using the similarity search program in the Hit2Lead database (http://www.hit2lead.com). These compounds were solubilized in DMSO to obtain 10 mM stock solutions and were first screened at 10 μM on APP-HEK293 cells for their ability to reduce secreted Aβ.
Cell cytotoxicity assay
Cell toxicity was quantified by lactate dehydrogenase (LDH) release assays according to the manufacturer's instructions (CytoTox 96, Non-Radioactive Cytotoxicity Assay; Promega, Madison, WI, USA). Absorbance measurements were obtained using a Tecan GENios Pro plate reader (Tecan Group, Männedorf, Switzerland) at 492 nm. For each experiment, measurements were performed in triplicate.
Human phosphoprotein array
APP-HEK293 cells were treated with RSVA314 (3 μM), RSVA405 (3 μM), or vehicle (DMSO) for 24 h. Cell lysates (250 μg total proteins/array) were applied to the phosphoprotein array following the manufacturer's instructions (Proteome Profiler Human Phospho-Kinase Array Kit; R&D Systems, Minneapolis, MN, USA).
AMPK activity assay
AMPK activity was determined using the CycLex AMPK Kinase Assay Kit (CycLex Co., Ltd., Nagano, Japan) according to the manufacturer's instructions. Briefly, recombinant active human AMPK (α1β1γ1; Sigma-Aldrich) was incubated with the indicated concentrations of AMP, RSVA314, or RSVA405 for 30 min at 30°C in a precoated plate with a substrate peptide corresponding to mouse insulin receptor substrate-1 (IRS-1). AMPK activity was measured by monitoring the phosphorylation of Ser-789 in IRS-1 using an anti-mouse phospho-Ser-789 IRS-1 monoclonal antibody and peroxidase-coupled anti-mouse IgG antibody. Conversion of the chromogenic substrate tetra-methylbenzidine was quantitated by absorbance measurement at 450 nm.
Measurement of intracellular ATP levels
Cells plated on 35-mm culture dishes in DMEM containing 10% FBS were treated for 24 h with vehicle (DMSO) or RSVAs at the indicated concentrations. Total cellular ATP was measured according to the supplier's instructions (ATP Assay Kit; Calbiochem).
Western blot (WB) analyses and Aβ ELISA
Cells were washed with PBS and solubilized in ice-cold HEPES buffer (25 mM HEPES, pH 7.4; 150 mM NaCl; and 1× Complete protease inhibitor mixture, Roche Applied Sciences) containing 1% SDS. Cell extracts (5–20 μg) were analyzed by SDS-PAGE using the antibodies listed above. Secreted and intracellular total Aβ or Aβ1–40 and Aβ1–42 were analyzed by WB or ELISA, as described previously (6, 7). For WB, 20 μl of medium was electrophoresed on 16.5% Tris-Tricine gels and transferred onto 0.2-μm nitrocellulose membranes. Membranes were microwaved for 5 min in PBS, blocked in 5% fat-free milk in TBS, and incubated overnight at 4°C with 6E10 antibody (1:1000 in SuperBlock Blocking Buffer; Thermo Fisher Scientific). A standard ECL detection procedure was then used.
Immunocytochemistry
Cells were plated on poly-l-lysine-coated glass coverslips 24 h before treatments. After treatment, cells were washed with PBS, fixed in 4% formaldehyde, and permeabilized with 0.2% Triton X-100. After blocking in 1% BSA for 30 min, the slides were incubated with anti-LC3 primary antibodies (1:50 dilution) for 2 h at room temperature and then washed 3 times with PBS and incubated with rabbit secondary antibody conjugated to Alexa fluorophores (Invitrogen). Finally, cells were stained with DAPI, and coverslips were mounted on glass slides using Vectashield (Vector Laboratories Inc., Burlingame, CA, USA) and observed using a Nikon Eclipse E2000-S microscope (Nikon, Tokyo, Japan).
RESULTS
Identification of the RSVA compounds
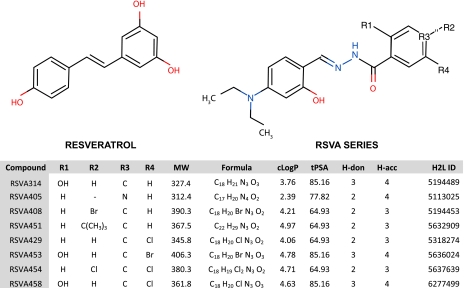
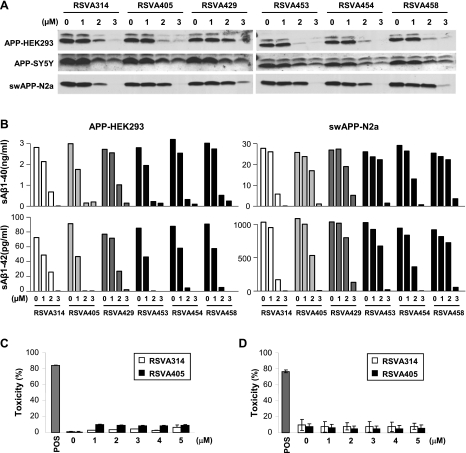
The 158 synthetic small molecules from the Hit2Lead screening compound library (see Materials and Methods) were selected for their structural similarities with resveratrol and were screened on APP-transfected HEK293 cells for their ability to reduce secreted Aβ (Supplemental Table 1). Because resveratrol lowers extracellular Aβ levels in APP-transfected cells after a 24 h incubation and with an apparent half-maximal inhibitory concentration (IC50) ∼40 μM (6, 7), the small molecules were tested at concentrations not >10 μM and for the same incubation period of 24 h. Eight compounds with a very similar molecular structure potently reduced secreted Aβ levels under these conditions [RSVA (resveratrol analogue) series, see Supplemental Table 1 and Fig. 1]. Further evaluation determined that 6 molecules in the RSVA series inhibited total extracellular Aβ accumulation in 3 different cell lines overexpressing either wild-type or Swedish mutant APP, with an apparent IC50 of 1–2 μM (Fig. 2A). Both secreted Aβ1–40 and Aβ1–42 were diminished by the 6 RSVA compounds with a comparable IC50 between 1 and 2 μM (Fig. 2B). LDH release assays in HEK293 cells and primary neurons revealed no cytotoxicity associated with the treatment with the 2 most potent compounds, RSVA314 (N′-[4-(diethylamino)-2-hydroxybenzylidene]-2-hydroxybenzohydrazide) and RSVA405 (N′-[4-(diethylamino)-2-hydroxybenzylidene] isonicotinohydrazide), at concentrations as high as 5 μM (Fig. 2C, D), indicating that the effect of the RSVA compounds on Aβ levels cannot be explained by cell toxicity.
Figure 1.
Structures and physicochemical parameters of the RSVA compounds. Structures were drawn using Marvin applet. MW, molecular weight (Da); cLogP, partition coefficient log P; tPSA, topological polar surface area; H-don, hydrogen bond donors; H-acc, hydrogen bond acceptors; H2L ID, Hit2Lead ID number.
Figure 2.
Effect of the RSVA compounds on extracellular Aβ levels in different cell lines. A, B) APP-HEK293, APP-SY5Y, and SwAPP-N2a cells were treated for 24 h with the indicated concentrations of RSVA compounds. Medium was changed, and drug treatments were continued for another 3 h to allow Aβ secretion. Secreted total Aβ was analyzed by WB (A), and secreted Aβ1–40 and Aβ1–42 were analyzed by ELISA (B). C, D) LDH release measurements in APP-HEK293 cells (C) or primary neurons (D) treated for 24 h with the indicated concentrations of RSVA314 or RSVA405. Triton-X100 (1%) was used as a positive control for cytotoxicity (POS). Values are given as percentage toxicity. Histograms in C, D illustrate the mean ± sd values of 3 independent experiments.
RSVA314 and RSVA405 are potent activators of AMPK
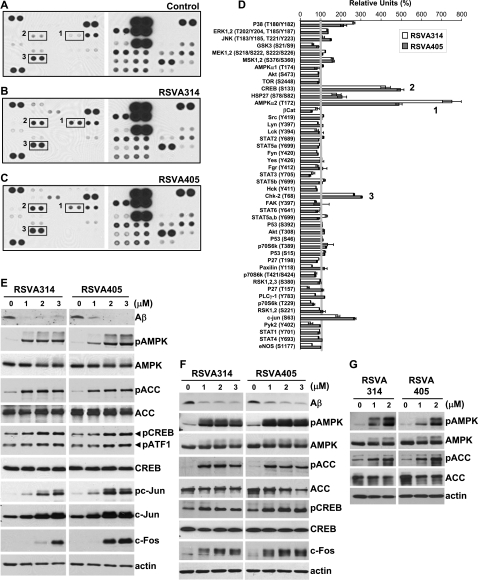
Previously, we showed that resveratrol promoted Aβ degradation by activating AMPK signaling (7). Here we sought to determine whether the RSVA compounds share with resveratrol the same mechanism of action. To this end, a phosphoprotein screen was used to assess the effects of the 2 RSVA compounds, RSVA314 and RSVA405, on 46 major kinases. We determined that, similar to resveratrol (7), phosphorylation at Thr-172 on AMPKα2 subunit was the most robust effect of RSVA314 and RSVA405 in HEK293 cells (Fig. 3A–D). Consistent with an activation of AMPK and also consistent with the effect of resveratrol (7, 24), RSVA314 and RSVA405 treatments resulted in a significant elevation of the phosphorylation at Ser-133 of the AMPK target CREB (Fig. 3A–D). A few additional positive hits were identified, which include the previously reported targets of resveratrol, p38, HSP27, c-Jun, and Chk2 (refs. 7, 25–28; Fig. 3D). WB analyses confirmed the effect of RSVA314 and RSVA405 on AMPK phosphorylation in APP-HEK293 (Fig. 3E), APP-SY5Y (Fig. 3F), and wild-type HEK293 cells (Fig. 3G), as well as on CREB phosphorylation (Fig. 3E, F). RSVA314 and RSVA405 also increased the phosphorylation of the AMPK target ACC in a dose-dependent manner and at concentrations consistent with the effect of these compounds on Aβ levels [apparent half maximal effective concentration (EC50) ≤ 1 μM; Fig. 3E–G]. In the same concentration range, RSVA314 and RSVA405 also increased the phosphorylation of ATF1, another CREB/ATF family member (Fig. 3E), and the expression levels of c-Fos (Fig. 3E, F) and c-Jun (Fig. 3E), 2 proteins transcriptionally controlled by CREB (29, 30). Together, these data indicate that RSVA314 and RSVA405 primarily target AMPK to increase its phosphorylation at Thr-172 and to promote its activation, as demonstrated by the increased ACC phosphorylation and CREB activation.
Figure 3.
RSVA314 and RSVA405 are potent activators of AMPK. A–D) APP-HEK293 cells were treated for 24 h with DMSO (control, A), RSVA314 (3 μM, B), or RSVA405 (3 μM, C). Cell extracts were then probed on human phosphoprotein arrays. Representative phospho-protein array analyses are shown in A–C. Boxes 1–3 in A–C indicate phospho-Thr-172 AMPKα2, phospho-Ser-133 CREB, and phospho-Thr-68 Chk2, respectively. Results are expressed as percentage of the control levels (D). E–G) APP-HEK293 (E), APP-SY5Y (F), and wild-type HEK293 cells (G) were treated for 24 h with the indicated concentrations of RSVA314 and RSVA405. Cell extracts were then analyzed by WB for secreted Aβ or cellular phospho-Thr-172 AMPK (pAMPK), AMPK, phospho-Ser-79 ACC (pACC), ACC, phospho-Ser-133 CREB (pCREB), CREB, phospho-Ser-63 c-Jun (pc-Jun), c-Jun, c-Fos, or actin levels.
RSVA314 and RSVA405 promote CaMKKβ-dependent phosphorylation of AMPK
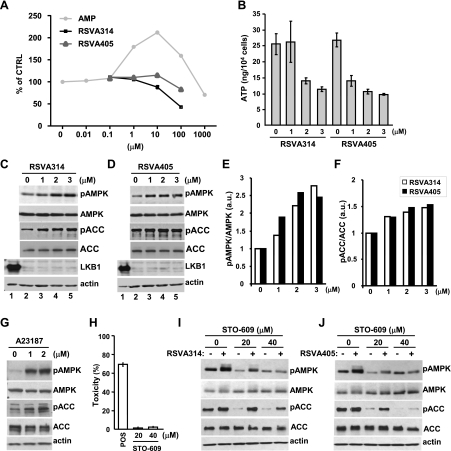
In vitro assays with purified AMPK revealed that RSVA314 and RSVA405 are not direct activators of the kinase (Fig. 4A), suggesting that, in a manner similar to resveratrol (7), these compounds act upstream from AMPK activation. AMPK is primarily activated by 2 kinases, LKB1 and CaMKKβ. RSVA314 and RSV405 noticeably decreased ATP levels (Fig. 4B), suggesting that LKB1-dependent phosphorylation of AMPK, consecutive to an elevation of the AMP:ATP ratio, may be involved in AMPK activation by the 2 compounds. We found, however, that RSVA314 and RSVA405 treatments were still able to promote AMPK and ACC phosphorylation in the LKB1-deficient HeLa cells (Fig. 4C–F), suggesting that LKB1 is dispensable for the effect of the RSVA compounds on AMPK activation. Moreover, in HEK293 cells, a relevant cell system for calcium-dependent activation of AMPK (Fig. 4G), we observed that the CaMKKβ inhibitor STO-609, used at nontoxic concentrations to the cells (Fig. 4H), potently reduced the effect of RSVA314 and RSVA405 on AMPK and ACC phosphorylation (Fig. 4I, J). Thus, these compounds activated AMPK primarily by promoting its phosphorylation by CaMKKβ.
Figure 4.
RSVA314 and RSVA405 promote CaMKKβ-dependent phosphorylation of AMPK. A) Effect of RSVA314 and RSVA405 on recombinant human AMPK (α1β1γ1) activity. AMP was used as a positive control for AMPK activation. Data are means ± sd of 3 independent measurements. B) Intracellular ATP levels in APP-HEK293 cells treated for 24 h with the indicated concentrations of RSVA compounds. Mean ± sd values of 3 independent measurements are shown. C, D) WB analyses of pAMPK, AMPK, pACC, ACC, LKB1, and actin levels in HEK293 (lane 1) and HeLa cells (lanes 2–5) treated for 24 h with the indicated concentrations of RSVA314 (C) or RSVA405 (D). E, F) Densitometric analyses and quantification of the ratios pAMPK/AMPK and pACC/ACC from experiments as in C, D. a.u., arbitrary units. G) WB analyses of pAMPK, AMPK, pACC, ACC, and actin levels in APP-HEK293 cells incubated for 24 h with the indicated concentrations of A23187. H) LDH release measurements in APP-HEK293 cells treated for 24 h with the indicated concentrations of STO-609. Values are given as percentage of toxicity. Histogram illustrates the mean ± sd values of 3 independent measurements. I, J) WB analyses of pAMPK, AMPK, pACC, ACC, and actin levels in APP-HEK293 cells incubated for 24 h with the indicated concentrations of STO-609 and in the absence (−) or presence (+) of 3 μM RSVA314 (I) or RSVA405 (J).
RSVA314 and RSVA405 lower Aβ levels by activating AMPK
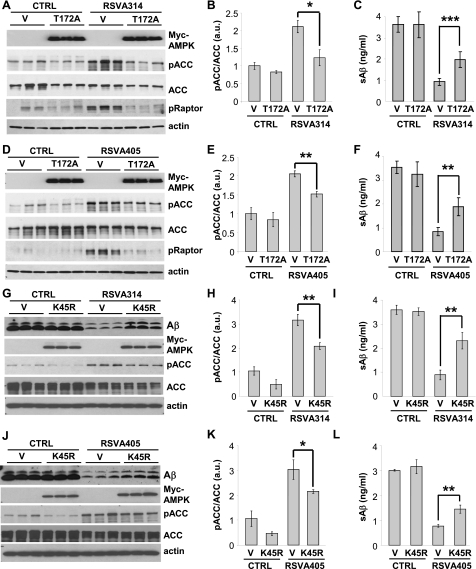
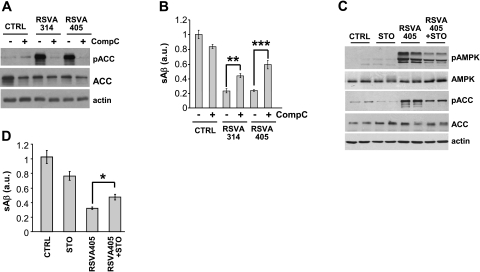
To determine whether AMPK activation is involved in the anti-amyloidogenic effect of the RSVA compounds, we then asked whether AMPK inhibition interferes with the effect of RSVA314 and RSVA405 on Aβ levels. Expression of the dominant-negative T172A-AMPK mutant (ref. 31; Fig. 5A–F) or of the kinase-dead K45R-AMPK mutant (ref. 32; Fig. 5G–L) significantly inhibited the effect of RSVA314 and RSVA405 on the phosphorylation of ACC and raptor, another direct target of AMPK (33), and on the levels of secreted Aβ (Fig. 5). Furthermore, pharmacological inhibition of AMPK using the AMPK inhibitor compound C (ref. 34; Fig. 6A, B) or the CaMKKβ inhibitor STO-609 (Fig. 6C, D) led to a significant interference of the effect of the RSVA compounds on secreted Aβ levels. These data show that RSVA314 and RSVA405 lowered Aβ levels by activating AMPK.
Figure 5.
Effect of RSVA314 and RSVA405 on Aβ levels is inhibited in cells expressing inactive AMPK. APP-HEK293 cells were transfected with control vector (V) and Myc-tagged dominant negative T172A-AMPK (T172A, A–F) or Myc-tagged kinase-dead K45R-AMPK (K45R, G–L). At 24 h post-transfection, cells were treated for 24 h with DMSO (CTRL) or 3 μM RSVA314 (A–C, G-I) or 3 μM RSVA405 (D–F, J–L). Myc-AMPK, pACC, ACC, pRaptor, actin, or secreted Aβ levels were analyzed by WB (A, D, G, J). B, E, H, K) Densitometric analyses and quantification of the ratio pACC/ACC from experiments as in A, D, G, J. C, F, I, L) ELISA measurements of secreted Aβ. Histograms show means ± sd of 3 independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001 (Student's t test).
Figure 6.
Pharmacological inhibition of AMPK interferes with the effect of RSVA314 and RSVA405 on Aβ levels. A) WB analyses of pACC, ACC, and actin levels in APP-HEK293 cells incubated for 24 h with DMSO (CTRL), 3 μM RSVA314, or 3 μM RSVA405 in the absence (−) or presence (+) of compound C (CompC, 40 μM). B) ELISA measurements of secreted Aβ in 3 independent experiments as in A. C) WB analyses of pAMPK, AMPK, pACC, ACC, and actin levels in APP-HEK293 cells incubated for 24 h in the absence or presence of STO-609 (STO, 40 μM) and RSVA405 (3 μM). D) ELISA measurements of secreted Aβ in 3 independent experiments as in C. *P < 0.05; **P < 0.01; ***P < 0.001 (Student's t test).
RSVA314 and RSVA405 inhibit mTOR, induce autophagy, and facilitate the lysosomal degradation of Aβ
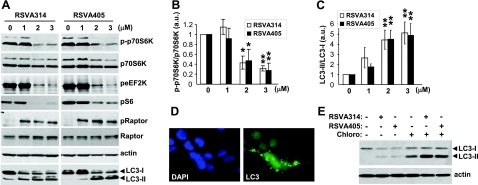
We previously reported that AMPK activation leads to Aβ degradation via mTOR inhibition and activation of autophagy (7). Autophagy is involved in protein and organelle turnover and participates in intracellular Aβ metabolism in vitro and in vivo (7, 15, 16). AMPK promotes autophagy via inhibition of mTOR (35). In this context, we asked whether the RSVA molecules inhibit mTOR signaling and activate autophagy. Figure 7 shows that treatments with RSVA314 and RSVA405 resulted in a dose-dependent inhibition of the phosphorylation of p70S6K, eukaryotic elongation factor 2 kinase (eEF2K), and S6 ribosomal protein, 3 proteins downstream from mTOR activation (Fig. 7A, B). In contrast, RSVA314 and RSVA405 treatments resulted in a robust increase in the phosphorylation of the protein raptor at Ser-792 (Fig. 7A). Raptor has recently been found to be directly phosphorylated by AMPK at residues Ser-722 and Ser-792 (33). This phosphorylation was proposed to be essential for inhibition of the raptor-containing mTOR complex 1 (mTORC1; ref. 33). These results indicate that RSVA314 and RSVA405 are potent inhibitors of mTOR signaling via a mechanism implicating AMPK activation.
Figure 7.
RSVA314 and RSVA405 inhibit mTOR signaling and induce autophagy. A–C) APP-HEK293 cells were treated for 24 h with the indicated concentrations of RSVAs. Cell extracts were then analyzed by WB for phospho-Thr-389 p70S6K (p-p70S6K), p70S6K, phospho-Ser-366 eEF2K (peEF2K), phospho-Ser-235/236 S6 (pS6), phospho-Ser-792 raptor (pRaptor), raptor, actin, and LC3 (A). B, C) Densitometric analyses and quantification of the p-p70S6K/p70S6K (B) and LC3-II/LC3-I (C) ratios. D) Immunocytochemistry analysis with anti-LC3 (green) antibodies in APP-HEK293 cells incubated for 24 h in the presence of 3 μM RSVA405. Nuclei were stained with DAPI (blue). E) WB analyses of LC3 levels in APP-HEK293 cells incubated for 24 h in the absence (−) or presence (+) of RSVA314 (3 μM), RSVA405 (3 μM), or chloroquine (Chloro, 100 μM).
In addition, we found that RSVA314 and RSVA405 led to a strong and dose-dependent conversion of the light chain 3 (LC3) protein from LC3-I to LC3-II (Fig. 7A, C), a marker of autophagy induction. Furthermore, formation of autophagosomes immunoreactive for LC3 was also observed in the presence of RSVA405 (Fig. 7D). Notably, treatment with the lysosomotropic drug chloroquine, which neutralizes lysosomal degradation, strongly potentiated LC3-II accumulation by RSVA314 and RSVA405 (Fig. 7E), demonstrating that these compounds not only increased autophagosome formation but also facilitated autophagic flux coupled to lysosomal degradation (36). Notably, RSVA314 and RSVA405 inhibited mTOR, increased raptor phosphorylation, and facilitated LC3 conversion at concentrations consistent with the effect of these compounds on Aβ levels (EC50=1–2 μM).
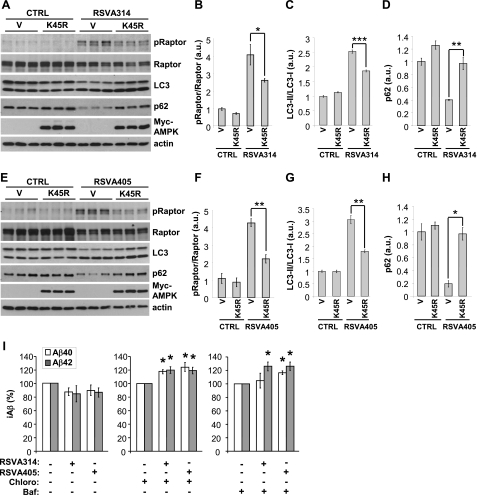
To confirm the implication of AMPK in the effects of the RSVAs on mTOR signaling and autophagy, we used cells expressing the kinase-dead K45R-AMPK mutant. Figure 8 shows that both the increase of raptor phosphorylation and LC3 conversion triggered by RSVA314 and RSV405 were significantly inhibited in cells expressing K45R-AMPK, as compared with control cells (Fig. 8A–C, E–G). p62/SQSTM1 (p62) is an autophagy receptor protein that binds LC3-II to target specific cargoes for autophagic degradation. Autophagy deficiency leads to p62 accumulation, whereas autophagy activation facilitates p62 degradation (37). Consistent with an activation of autophagy, RSVA314 and RSVA405 were found to reduce p62 levels (Fig. 8A, D, E, H). Notably, expression of K45R-AMPK resulted in a significant inhibition of the effect of the RSVAs on p62 degradation (Fig. 8A, D, E, H). Together, these data confirm that AMPK activation is required for the effect of the RSVAs on mTOR signaling and autophagy induction.
Figure 8.
RSVA314 and RSVA405 control mTOR and autophagy via AMPK, and facilitate the lysosomal degradation of Aβ. A–H) APP-HEK293 cells were transfected with control vector (V) or Myc-tagged kinase-dead K45R-AMPK (K45R). At 24 h post-transfection, cells were treated for 24 h with DMSO (CTRL) and 3 μM RSVA314 (A–D) or 3 μM RSVA405 (E–H). pRaptor, raptor, LC3, p62, Myc-AMPK, and actin levels were analyzed by WB (A, E). B–D, F–H) Densitometric analyses and quantification of the ratios pRaptor/Raptor and LC3-II/LC3-I, and of p62 levels from experiments in A, E. I) ELISA measurements of intracellular Aβ (iAβ) from APP-HEK293 cells incubated for 24 h in the absence (−) or presence (+) of RSVA314 (3 μM), RSVA405 (3 μM), chloroquine (Chloro, 100 μM), or bafilomycin A1 (Baf, 100 nM). Histograms show the means ± sd of 3 independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001 (Student's t test).
Finally, treatment with chloroquine or bafilomycin A1, another lysosomotropic compound that blocks autophagosome-lysosome fusion, resulted in a significant increase of the intracellular levels of both Aβ1–40 and Aβ1–42 in the presence of the 2 RSVA compounds (Fig. 8I). Together these data show that RSVA314 and RSVA405, via activation of AMPK, inhibited mTOR to induce autophagy and intracellular degradation of Aβ by the lysosomal system.
In summary, this work describes the identification and first characterization of novel small-molecule indirect activators of AMPK. The 2 most potent activators identified in our screen, RSVA314 and RSVA405, share with resveratrol the same mechanism of action: facilitating CaMKKβ-dependent activation of AMPK, inhibiting mTOR, and promoting autophagy and Aβ degradation by the lysosomal system. RSVA314 and RSVA405 controlled the AMPK/mTOR/autophagy pathway and reduced Aβ levels with a consistent EC50 ∼ 1 μM.
DISCUSSION
Our recent work (7) showed that AMPK activation and autophagy induction by the natural polyphenol resveratrol promotes intracellular degradation of Aβ by the lysosomal system. Specifically, we found that resveratrol inhibits the activity of mTOR, an AMPK target critically involved in autophagy repression. In the current study, we describe the identification and characterization of a series of structurally related analogues of resveratrol, the RSVA series (Fig. 1), which are 40-fold more potent than resveratrol. We show that 2 of the most potent compounds in the RSVA series, RSVA314 and RSVA405, share with resveratrol the same mechanism of action by facilitating CaMKKβ-dependent activation of AMPK, by inhibiting mTOR, and by promoting autophagy-dependent degradation of Aβ. RSVA314 and RSVA405 controlled the AMPK/mTOR/autophagy pathway and lowered Aβ levels with an apparent EC50 ∼ 1 μM. These results are important for 2 reasons: first, they strengthen the therapeutic potential of AMPK activation in AD (7), and second, they identify novel compounds as promising lead molecules for the development of drugs facilitating Aβ clearance by controlling the AMPK/mTOR/autophagy pathway.
Autophagy is deregulated in AD and is involved in Aβ metabolism both in vitro in cell lines and in vivo in mice (15, 16). The role of autophagy in APP metabolism and Aβ clearance is complex and its study has generated some apparently conflicting data suggesting that Aβ is both produced and degraded during autophagy (38, 39). In this context, a working model was proposed in which Aβ is first produced during the autophagic turnover of APP before being degraded after autophagosome fusion with the lysosomes (17). Recent work (40) further supports the role of autophagy in AD pathogenesis by showing that presenilin 1 (PS1), a protein genetically linked to familial AD (FAD), is essential for lysosomal acidification and proteolysis during autophagy induction and that PS1-FAD mutations significantly impair this mechanism. Furthermore, it was shown that Beclin 1, a key regulator of autophagy induction, is down-regulated in AD brains and is crucial for the control of APP turnover and processing during autophagy flux (41). In summary, there is overwhelming evidence that autophagy is involved in Aβ metabolism and AD pathogenesis and thus could represent a therapeutic target in AD. Our previous data (7) together with the current study support the notion that pharmacological activation of autophagy is overall beneficial by promoting a reduction of Aβ accumulation and Aβ-mediated toxity (42–44).
Like AD, several other human neurological conditions, which include Parkinson's disease, Huntington's disease, and some forms of ataxias, are referred to as protein conformation disorders (or proteinopathies) and are caused by the accumulation of misfolded proteins. Strong evidence also supports the therapeutic potential of autophagy induction in these disorders. Indeed, autophagy was found to be critical for the disposal of many aggregation-prone mutant proteins, such as α-synuclein and huntingtin, or α1-antitrypsin Z, another aggregation-prone protein involved in hepatic fibrosis (45–47). Autophagy-enhancing drugs are therefore actively tested for the treatment of several protein conformation disorders.
Beyond their therapeutic potential in AD and in other protein conformation disorders, AMPK-activating compounds are of interest for diabetes and cancer. Indeed, a large body of literature has shown that AMPK is central in the control of glucose and lipid metabolism (13, 48) and its activation is required for the antidiabetic effect of the widely prescribed drug metformin (34, 49). Metformin and the other antidiabetic drugs thiazolidinediones (e.g., rosiglitazone and troglitazone) are believed to act, at least in part, by indirectly activating AMPK (48, 50). Resveratrol, which also indirectly activates AMPK by increasing cytosolic calcium levels (7) or by facilitating AMP-dependent activation of AMPK (50), has protective effects against the deregulation of energy homeostasis observed in mouse models for type 2 diabetes, and this via a mechanism implicating AMPK (51, 52). Several small molecules that bind and directly activate AMPK, such as AICAR, A-769662, or PT1, were identified (48). These molecules bind either to the regulatory γ-subunit or to the catalytic α-subunit of AMPK to directly activate the kinase (53). RSVA314 and RSVA405 did not activate recombinant AMPK in vitro, suggesting that the RSVA compounds did not bind nor directly activated AMPK. Moreover, CaMKKβ inhibition resulted in a significant prevention of the effect of these compounds on AMPK activation in cell lines (Fig. 4), indicating that the RSVA compounds acted upstream from AMPK and thus represent a new class of potent and indirect activators of AMPK. Further investigation will be needed to identify the precise mechanism of AMPK activation by the RSVAs and to determine whether these molecules can offer new therapeutic opportunities aimed at indirectly activating AMPK and at preventing the deregulated glucose and lipid metabolism in metabolic diseases.
In mammals, mTOR is essential for the control of cell metabolism and growth, and its inhibition is of therapeutic interest in cancer. Analogs of rapamycin, which inhibit mTOR, have been approved for the treatment of several cancers (54). mTOR is the catalytic subunit of at least 2 distinct protein complexes: mTORC1 and mTORC2 (55). In mTORC1, mTOR interacts with several regulatory subunits, including raptor (regulatory-associated protein of mTOR). Raptor is required for the recruitment of several downstream substrates to mTORC1, such as p70S6K. AMPK controls mTORC1 activity by directly phosphorylating tuberous sclerosis complex 2 (TSC2), a tumor suppressor that inhibits mTORC1 (55). During energy stress, AMPK also inhibits mTORC1 by directly phosphorylating raptor at Ser-792 (33). Our data indicate that the RSVA compounds are potent inhibitors of mTOR signaling. Specifically, we showed that RSVA314 and RSVA405 promoted the inactivation of several downstream effectors of mTOR involved mRNA translation, including p70S6K, eEF2K, and S6 ribosomal protein (Fig. 7). The RSVA compounds were also found to potently activate phosphorylation of raptor at Ser-792, indicating that these molecules inhibited mTOR by activating AMPK-dependent phosphorylation of raptor. It will be interesting to determine whether RSVA314 and RSV405 also facilitated the inhibitory effect of AMPK on mTORC1 via TSC2 phosphorylation. Altogether, these results show that the RSVA compounds are potent inhibitors of mTOR and thus hold significant potential as antiproliferative drugs and may be relevant to cancer therapy.
The 8 molecules in the RSVA series have physicochemical and structural features that make them interesting lead molecule candidates for orally active drugs. With a molecular weight <500 Da, a log P <5, and <5 H-bond donors and 10 H-bond acceptors (see Fig. 1), the RSVA molecules do not violate Lipinski's rule of 5 of druglikeness (56). Furthermore, RSVA408, RSVA451, RSVA429, and RSVA454 have a topological polar surface area (tPSA) value < 70 (Fig. 1) and thus may cross the blood-brain barrier and could potentially be active in the central nervous system (CNS). Therefore, the RSVA series has physicochemical parameters predicting acceptable aqueous solubility, intestinal permeability, and brain penetration, making these molecules good candidates for experimental evaluation in vivo for oral bioavailability and CNS bioactivity.
In summary, this work describes the identification and characterization of novel small-molecule activators of AMPK that potently inhibit mTOR signaling, activate autophagy, and trigger Aβ clearance by the lysosomal system. AMPK is a key target for drug discovery mostly because of its broad spectrum of functions in energy metabolism and cell growth, but also in neuroprotection in the CNS (57–60). Our results strengthen the notion that small-molecule indirect and potent activators of AMPK can be identified and thus may offer new therapeutic perspectives in disorders where AMPK, mTOR, and autophagy are involved. Finally, the present data further support the hypothesis that AMPK represents a promising drug target for the treatment of AD (7).
Supplementary Material
Acknowledgments
The authors thank Dr. Gopal Thinakaran (University of Chicago, Chicago, IL, USA), Dr. Luciano D'Adamio (Albert Einstein College of Medicine, Bronx, NY, USA), Dr. Luc Buee (INSERM U815, Centre Jean Pierre Aubert, Lille, France), and Dr. David Carling (Medical Research Council Clinical Sciences Centre, Imperial College, London, UK) for kindly providing cell lines and cDNA.
This work was supported in part by the Institutional Clinical and Translational Science Award UL1-RR024996 (Weill Medical College of Cornell University, New York, NY, USA; Clinical and Translational Science Center Pilot Award to P.M.) and National Institutes of Health grant PO1 AT004511 (National Center for Complementary and Alternative Medicine Project 2 to P.M.).
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Selkoe D. J. (2001) Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev. 81, 741–66 [DOI] [PubMed] [Google Scholar]
- 2. Querfurth H. W., LaFerla F. M. (2010) Alzheimer's disease. N. Engl. J. Med. 362, 329–344 [DOI] [PubMed] [Google Scholar]
- 3. Marambaud P., Robakis N. K. (2005) Genetic and molecular aspects of alzheimer's disease shed light on new mechanisms of transcriptional regulation. Genes Brain Behav. 4, 134–146 [DOI] [PubMed] [Google Scholar]
- 4. Wilquet V., De Strooper B. (2004) Amyloid-beta precursor protein processing in neurodegeneration. Curr. Opin. Neurobiol. 14, 582–588 [DOI] [PubMed] [Google Scholar]
- 5. Checler F. (1995) Processing of the beta-amyloid precursor protein and its regulation in alzheimer's disease. J. Neurochem. 65, 1431–1444 [DOI] [PubMed] [Google Scholar]
- 6. Marambaud P., Zhao H., Davies P. (2005) Resveratrol promotes clearance of alzheimer's disease amyloid-beta peptides. J. Biol. Chem. 280, 37377–37382 [DOI] [PubMed] [Google Scholar]
- 7. Vingtdeux V., Giliberto L., Zhao H., Chandakkar P., Wu Q., Simon J. E., Janle E. M., Lobo J., Ferruzzi M. G., Davies P., Marambaud P. (2010) AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J. Biol. Chem. 285, 9100–9113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vingtdeux V., Dreses-Werringloer U., Zhao H., Davies P., Marambaud P. (2008) Therapeutic potential of resveratrol in alzheimer's disease. BMC Neurosci. 9, S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Karuppagounder S. S., Pinto J. T., Xu H., Chen H. L., Beal M. F., Gibson G. E. (2009) Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer's disease. Neurochem. Int. 54, 111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hardie D. G. (2007) AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat. Rev. Mol. Cell. Biol. 8, 774–785 [DOI] [PubMed] [Google Scholar]
- 11. Carling D., Sanders M. J., Woods A. (2008) The regulation of AMP-activated protein kinase by upstream kinases. Int. J. Obes. 32, S55–59 [DOI] [PubMed] [Google Scholar]
- 12. Fogarty S., Hawley S. A., Green K. A., Saner N., Mustard K. J., Hardie D. G. (2010) Calmodulin-dependent protein kinase kinase-beta activates AMPK without forming a stable complex: synergistic effects of Ca2+ and AMP. Biochem. J. 426, 109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Viollet B., Lantier L., Devin-Leclerc J., Hebrard S., Amouyal C., Mounier R., Foretz M., Andreelli F. (2009) Targeting the AMPK pathway for the treatment of type 2 diabetes. Front. Biosci. 14, 3380–3400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maiuri M. C., Zalckvar E., Kimchi A., Kroemer G. (2007) Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell. Biol. 8, 741–752 [DOI] [PubMed] [Google Scholar]
- 15. Jaeger P. A., Wyss-Coray T. (2009) All-you-can-eat: Autophagy in neurodegeneration and neuroprotection. Mol. Neurodegener. 4, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nixon R. A. (2007) Autophagy, amyloidogenesis and alzheimer disease. J. Cell Sci. 120, 4081–4091 [DOI] [PubMed] [Google Scholar]
- 17. Nixon R. A., Yang D. S., Lee J. H. (2008) Neurodegenerative lysosomal disorders: A continuum from development to late age. Autophagy 4, 590–599 [DOI] [PubMed] [Google Scholar]
- 18. Wullschleger S., Loewith R., Hall M. N. (2006) TOR signaling in growth and metabolism. Cell 124, 471–484 [DOI] [PubMed] [Google Scholar]
- 19. Chan E. Y. (2009) mTORC1 phosphorylates the ULK1-mAtg13-FIP200 autophagy regulatory complex. Sci. Signal. 2, pe51. [DOI] [PubMed] [Google Scholar]
- 20. Harrison D. E., Strong R., Sharp Z. D., Nelson J. F., Astle C. M., Flurkey K., Nadon N. L., Wilkinson J. E., Frenkel K., Carter C. S., Pahor M., Javors M. A., Fernandez E., Miller R. A. (2009) Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Selman C., Tullet J. M., Wieser D., Irvine E., Lingard S. J., Choudhury A. I., Claret M., Al-Qassab H., Carmignac D., Ramadani F., Woods A., Robinson I. C., Schuster E., Batterham R. L., Kozma S. C., Thomas G., Carling D., Okkenhaug K., Thornton J. M., Partridge L., Gems D., Withers D. J. (2009) Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 326, 140–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vingtdeux V., Hamdane M., Loyens A., Gele P., Drobeck H., Begard S., Galas M. C., Delacourte A., Beauvillain J. C., Buee L., Sergeant N. (2007) Alkalizing drugs induce accumulation of amyloid precursor protein by-products in luminal vesicles of multivesicular bodies. J. Biol. Chem. 282, 18197–18205 [DOI] [PubMed] [Google Scholar]
- 23. Zhao H., Dreses-Werringloer U., Davies P., Marambaud P. (2008) Amyloid-beta peptide degradation in cell cultures by mycoplasma contaminants. BMC Res. Notes 1, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thomson D. M., Herway S. T., Fillmore N., Kim H., Brown J. D., Barrow J. R., Winder W. W. (2008) AMP-activated protein kinase phosphorylates transcription factors of the CREB family. J. Appl. Physiol. 104, 429–438 [DOI] [PubMed] [Google Scholar]
- 25. Puissant A., Robert G., Fenouille N., Luciano F., Cassuto J. P., Raynaud S., Auberger P. (2010) Resveratrol promotes autophagic cell death in chronic myelogenous leukemia cells via JNK-mediated p62/SQSTM1 expression and AMPK activation. Cancer Res. 70, 1042–1052 [DOI] [PubMed] [Google Scholar]
- 26. Wang Z., Chen Y., Labinskyy N., Hsieh T. C., Ungvari Z., Wu J. M. (2006) Regulation of proliferation and gene expression in cultured human aortic smooth muscle cells by resveratrol and standardized grape extracts. Biochem. Biophys. Res. Commun. 346, 367–376 [DOI] [PubMed] [Google Scholar]
- 27. She Q. B., Bode A. M., Ma W. Y., Chen N. Y., Dong Z. (2001) Resveratrol-induced activation of p53 and apoptosis is mediated by extracellular-signal-regulated protein kinases and p38 kinase. Cancer Res. 61, 1604–1610 [PubMed] [Google Scholar]
- 28. Tyagi A., Singh R. P., Agarwal C., Siriwardana S., Sclafani R. A., Agarwal R. (2005) Resveratrol causes Cdc2-tyr15 phosphorylation via ATM/ATR-Chk1/2-Cdc25C pathway as a central mechanism for S phase arrest in human ovarian carcinoma ovcar-3 cells. Carcinogenesis 26, 1978–1987 [DOI] [PubMed] [Google Scholar]
- 29. Sanyal S., Sandstrom D. J., Hoeffer C. A., Ramaswami M. (2002) AP-1 functions upstream of CREB to control synaptic plasticity in Drosophila. Nature 416, 870–874 [DOI] [PubMed] [Google Scholar]
- 30. Marambaud P., Wen P. H., Dutt A., Shioi J., Takashima A., Siman R., Robakis N. K. (2003) A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell 114, 635–645 [DOI] [PubMed] [Google Scholar]
- 31. Stein S. C., Woods A., Jones N. A., Davison M. D., Carling D. (2000) The regulation of AMP-activated protein kinase by phosphorylation. Biochem. J. 345, 437–443 [PMC free article] [PubMed] [Google Scholar]
- 32. Mu J., Brozinick J. T., Jr, Valladares O., Bucan M., Birnbaum M. J. (2001) A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol. Cell 7, 1085–1094 [DOI] [PubMed] [Google Scholar]
- 33. Gwinn D. M., Shackelford D. B., Egan D. F., Mihaylova M. M., Mery A., Vasquez D. S., Turk B. E., Shaw R. J. (2008) AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 30, 214–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., Wu M., Ventre J., Doebber T., Fujii N., Musi N., Hirshman M. F., Goodyear L. J., Moller D. E. (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 108, 1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meijer A. J., Codogno P. (2007) AMP-activated protein kinase and autophagy. Autophagy 3, 238–240 [DOI] [PubMed] [Google Scholar]
- 36. Klionsky D. J., Abeliovich H., Agostinis P., Agrawal D. K., Aliev G., Askew D. S., Baba M., Baehrecke E. H., Bahr B. A., Ballabio A., Bamber B. A., Bassham D. C., Bergamini E., Bi X., Biard-Piechaczyk M., Blum J. S., Bredesen D. E., Brodsky J. L., Brumell J. H., Brunk U. T., Bursch W., Camougrand N., Cebollero E., Cecconi F., Chen Y., Chin L. S., Choi A., Chu C. T., Chung J., Clarke P. G., Clark R. S., Clarke S. G., Clave C., Cleveland J. L., Codogno P., Colombo M. I., Coto-Montes A., Cregg J. M., Cuervo A. M., Debnath J., Demarchi F., Dennis P. B., Dennis P. A., Deretic V., Devenish R. J., Di Sano F., Dice J. F., Difiglia M., Dinesh-Kumar S., Distelhorst C. W., Djavaheri-Mergny M., Dorsey F. C., Droge W., Dron M., Dunn W. A., Jr, Duszenko M., Eissa N. T., Elazar Z., Esclatine A., Eskelinen E. L., Fesus L., Finley K. D., Fuentes J. M., Fueyo J., Fujisaki K., Galliot B., Gao F. B., Gewirtz D. A., Gibson S. B., Gohla A., Goldberg A. L., Gonzalez R., Gonzalez-Estevez C., Gorski S., Gottlieb R. A., Haussinger D., He Y. W., Heidenreich K., Hill J. A., Hoyer-Hansen M., Hu X., Huang W. P., Iwasaki A., Jaattela M., Jackson W. T., Jiang X., Jin S., Johansen T., Jung J. U., Kadowaki M., Kang C., Kelekar A., Kessel D. H., Kiel J. A., Kim H. P., Kimchi A., Kinsella T. J., Kiselyov K., Kitamoto K., Knecht E., Komatsu M., Kominami E., Kondo S., Kovacs A. L., Kroemer G., Kuan C. Y., Kumar R., Kundu M., Landry J., Laporte M., Le W., Lei H. Y., Lenardo M. J., Levine B., Lieberman A., Lim K. L., Lin F. C., Liou W., Liu L. F., Lopez-Berestein G., Lopez-Otin C., Lu B., Macleod K. F., Malorni W., Martinet W., Matsuoka K., Mautner J., Meijer A. J., Melendez A., Michels P., Miotto G., Mistiaen W. P., Mizushima N., Mograbi B., Monastyrska I., Moore M. N., Moreira P. I., Moriyasu Y., Motyl T., Munz C., Murphy L. O., Naqvi N. I., Neufeld T. P., Nishino I., Nixon R. A., Noda T., Nurnberg B., Ogawa M., Oleinick N. L., Olsen L. J., Ozpolat B., Paglin S., Palmer G. E., Papassideri I., Parkes M., Perlmutter D. H., Perry G., Piacentini M., Pinkas-Kramarski R., Prescott M., Proikas-Cezanne T., Raben N., Rami A., Reggiori F., Rohrer B., Rubinsztein D. C., Ryan K. M., Sadoshima J., Sakagami H., Sakai Y., Sandri M., Sasakawa C., Sass M., Schneider C., Seglen P. O., Seleverstov O., Settleman J., Shacka J. J., Shapiro I. M., Sibirny A., Silva-Zacarin E. C., Simon H. U., Simone C., Simonsen A., Smith M. A., Spanel-Borowski K., Srinivas V., Steeves M., Stenmark H., Stromhaug P. E., Subauste C. S., Sugimoto S., Sulzer D., Suzuki T., Swanson M. S., Tabas I., Takeshita F., Talbot N. J., Talloczy Z., Tanaka K., Tanaka K., Tanida I., Taylor G. S., Taylor J. P., Terman A., Tettamanti G., Thompson C. B., Thumm M., Tolkovsky A. M., Tooze S. A., Truant R., Tumanovska L. V., Uchiyama Y., Ueno T., Uzcategui N. L., van der Klei I., Vaquero E. C., Vellai T., Vogel M. W., Wang H. G., Webster P., Wiley J. W., Xi Z., Xiao G., Yahalom J., Yang J. M., Yap G., Yin X. M., Yoshimori T., Yu L., Yue Z., Yuzaki M., Zabirnyk O., Zheng X., Zhu X., Deter R. L. (2008) Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 4, 151–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Komatsu M., Ichimura Y. (2010) Physiological significance of selective degradation of p62 by autophagy. FEBS Lett. 584, 1374–1378 [DOI] [PubMed] [Google Scholar]
- 38. Yu W. H., Cuervo A. M., Kumar A., Peterhoff C. M., Schmidt S. D., Lee J. H., Mohan P. S., Mercken M., Farmery M. R., Tjernberg L. O., Jiang Y., Duff K., Uchiyama Y., Naslund J., Mathews P. M., Cataldo A. M., Nixon R. A. (2005) Macroautophagy–a novel beta-amyloid peptide-generating pathway activated in alzheimer's disease. J. Cell Biol. 171, 87–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pickford F., Masliah E., Britschgi M., Lucin K., Narasimhan R., Jaeger P. A., Small S., Spencer B., Rockenstein E., Levine B., Wyss-Coray T. (2008) The autophagy-related protein beclin 1 shows reduced expression in early alzheimer disease and regulates amyloid beta accumulation in mice. J. Clin. Invest. 118, 2190–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee J. H., Yu W. H., Kumar A., Lee S., Mohan P. S., Peterhoff C. M., Wolfe D. M., Martinez-Vicente M., Massey A. C., Sovak G., Uchiyama Y., Westaway D., Cuervo A. M., Nixon R. A. (2010) Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by alzheimer-related PS1 mutations. Cell 141, 1146–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jaeger P. A., Pickford F., Sun C. H., Lucin K. M., Masliah E., Wyss-Coray T. (2010) Regulation of amyloid precursor protein processing by the beclin 1 complex. PloS One 5, e11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Spilman P., Podlutskaya N., Hart M. J., Debnath J., Gorostiza O., Bredesen D., Richardson A., Strong R., Galvan V. (2010) Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of alzheimer's disease. PloS One 5, e9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hung S. Y., Huang W. P., Liou H. C., Fu W. M. (2009) Autophagy protects neuron from abeta-induced cytotoxicity. Autophagy 5, 502–510 [DOI] [PubMed] [Google Scholar]
- 44. Caccamo A., Majumder S., Richardson A., Strong R., Oddo S. (2010) Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and tau: effects on cognitive impairments. J. Biol. Chem. 285, 13107–13120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sarkar S., Ravikumar B., Floto R. A., Rubinsztein D. C. (2009) Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies. Cell Death Differ. 16, 46–56 [DOI] [PubMed] [Google Scholar]
- 46. Martinez-Vicente M., Talloczy Z., Wong E., Tang G., Koga H., Kaushik S., de Vries R., Arias E., Harris S., Sulzer D., Cuervo A. M. (2010) Cargo recognition failure is responsible for inefficient autophagy in huntington's disease. Nat. Neurosci. 13, 567–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hidvegi T., Ewing M., Hale P., Dippold C., Kemp C. B., Maurice N., Mukherjee A., Goldbach C., Watkins S., Michalopoulos G., Perlmutter D. H. (2010) An autophagy-enhancing drug promotes degradation of mutant {alpha}1-antitrypsin Z and reduces hepatic fibrosis. Science 329, 229–232 [DOI] [PubMed] [Google Scholar]
- 48. Zhang B. B., Zhou G., Li C. (2009) AMPK: An emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 9, 407–416 [DOI] [PubMed] [Google Scholar]
- 49. Shaw R. J., Lamia K. A., Vasquez D., Koo S. H., Bardeesy N., Depinho R. A., Montminy M., Cantley L. C. (2005) The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310, 1642–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hawley S. A., Ross F. A., Chevtzoff C., Green K. A., Evans A., Fogarty S., Towler M. C., Brown L. J., Ogunbayo O. A., Evans A. M., Hardie D. G. (2010) Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 11, 554–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Canto C., Auwerx J. (2009) PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 20, 98–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Um J. H., Park S. J., Kang H., Yang S., Foretz M., McBurney M. W., Kim M. K., Viollet B., Chung J. H. (2009) AMPK-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes 59, 554–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zorn J. A., Wells J. A. (2010) Turning enzymes ON with small molecules. Nat. Chem. Biol. 6, 179–188 [DOI] [PubMed] [Google Scholar]
- 54. Sudarsanam S., Johnson D. E. (2010) Functional consequences of mTOR inhibition. Curr. Opin. Drug Discov. Devel. 13, 31–40 [PubMed] [Google Scholar]
- 55. Sabatini D. M. (2006) mTOR and cancer: Insights into a complex relationship. Nat. Rev. Cancer 6, 729–734 [DOI] [PubMed] [Google Scholar]
- 56. Lipinski C. A., Lombardo F., Dominy B. W., Feeney P. J. (2001) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 46, 3–26 [DOI] [PubMed] [Google Scholar]
- 57. Poels J., Spasic M. R., Callaerts P., Norga K. K. (2009) Expanding roles for AMP-activated protein kinase in neuronal survival and autophagy. Adv. Drug Deliv. Rev. 31, 944–952 [DOI] [PubMed] [Google Scholar]
- 58. Ronnett G. V., Ramamurthy S., Kleman A. M., Landree L. E., Aja S. (2009) AMPK in the brain: Its roles in energy balance and neuroprotection. J. Neurochem. 109(Suppl. 1), 17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nath N., Khan M., Paintlia M. K., Singh I., Hoda M. N., Giri S. (2009) Metformin attenuated the autoimmune disease of the central nervous system in animal models of multiple sclerosis. J. Immunol. 182, 8005–8014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li J., McCullough L. D. (2010) Effects of AMP-activated protein kinase in cerebral ischemia. J. Cereb. Blood Flow Metab. 30, 480–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.