Abstract
Over two decades of research have demonstrated that the peptide hormone endothelin-1 (ET-1) plays multiple, complex roles in cardiovascular, neural, pulmonary, reproductive, and renal physiology. Differential and tissue-specific production of ET-1 must be tightly regulated in order to preserve these biologically diverse actions. The primary mechanism thought to control ET-1 bioavailability is the rate of transcription from the ET-1 gene (edn1). Studies conducted on a variety of cell types have identified key transcription factors that govern edn1 expression. With few exceptions, the cis-acting elements bound by these factors have been mapped in the edn1 regulatory region. Recent evidence has revealed new roles for some factors originally believed to regulate edn1 in a tissue or hormone-specific manner. In addition, other mechanisms involved in epigenetic regulation and mRNA stability have emerged as important processes for regulated edn1 expression. The goal of this review is to provide a comprehensive overview of the specific factors and signaling systems that govern edn1 activity at the molecular level.—Stow, L. R., Jacobs, M. E., Wingo, C. S., Cain, B. D. Endothelin-1 gene regulation.
Keywords: preproendothelin-1, edn1 promoter, transcription factors, ET-1
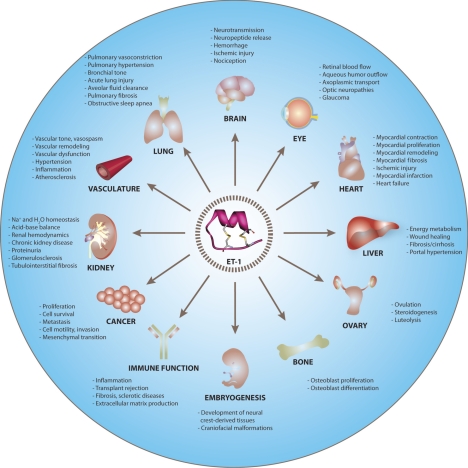
endothelin-1 (ET-1) is a peptide hormone with diverse biological actions. It was originally identified in 1988 as an endothelium-derived factor that produced prolonged vasoconstriction and an increase in arterial blood pressure (1). The vasoactive properties of ET-1 have made the peptide best known for its role in hypertension. However, studies over the past 20 yr have revealed that ET-1 plays a much more pervasive role in mammalian physiology (see Fig. 1 and references herein for specific reviews; refs. 2–27). It is now clear that ET-1 is critical for neurological function (2–4), pulmonary physiology (13), fluid and electrolyte transport (20), autoimmune disorders (26), and cancer biology (25). ET-1 is also required for the development of tissues derived from the embryonic neural crest. Mice harboring a disruption in the ET-1 gene (edn1) asphyxiate shortly after birth because of severe craniofacial malformations (28). The biological importance of ET-1 is further exemplified by its clinical relevance. Most notably, the therapeutic intervention with ET-1 receptor antagonists has dramatically improved the clinical management of patients suffering from severe pulmonary arterial hypertension (13). ET-1 receptor antagonists have also been used to successfully prevent the progression of chronic kidney disease (21), a known, independent risk factor for cardiovascular disease. The national database of registered clinical trials lists dozens of other studies targeting the ET-1 pathway for patients suffering from a range of disorders, including glaucoma, cancer, sleep apnea, severe asthma, and scleroderma (http://www.clinicaltrials.gov).
Figure 1.
Overview of ET-1 physiology. Major physiological actions of ET-1 are summarized in the text and have been recently reviewed in brain (2–4), eye (5, 6), heart (7–10), lung (3, 11–14), vasculature (3, 15–19), kidney (3, 18, 20, 21), liver (23), ovaries (24), cancer (3, 25), immune function (3, 9, 10, 26), bone (3), and embryogenesis (3, 27). Structure of ET-1 was rendered from the Research Collaboratory for Structural Bioinformatics (RCSB) Protein Data Bank (PDB 1T7H).
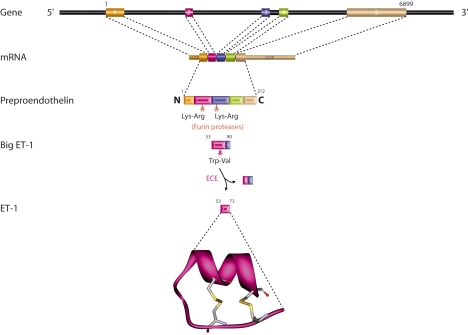
ET-1 is the most abundantly and widely expressed member of the endothelin family of proteins (ET-1, ET-2, and ET-3) (29). The diverse biological actions of ET-1 are mediated by two different ET-1 receptor subtypes, ETA and ETB (30). However, differential receptor expression patterns and signal cascades only partially account for the complex physiology of ET-1. Indeed, mechanisms that regulate ET-1 expression are also essential to maintain its normal physiological activity. Synthesis of the biologically active 21-aa ET-1 peptide is a multistep process (Fig. 2). Transcription of the human edn1 gene yields a 2.8-kb mRNA that encodes the 212-aa preproET-1 (1). A 17-aa leader sequence targets preproET-1 to the endoplasmic reticulum where it enters the secretory pathway (31). Prior to exocytosis, furin-like proteases cleave preproET-1 to a 38-aa protein called big ET-1 (32). The final cleavage step is mediated by endothelin-converting enzymes that cleave big ET-1 into active ET-1 (33, 34). It is likely that regulatory mechanisms exist for each of these post-translational processing steps; however, transcriptional regulation is thought to be the major mechanism controlling ET-1 bioavailability. For example, ET-1 localizes to both constitutive secretory vesicles (35) and specialized regulatory granules known as Weibel-Palade bodies in endothelial cells (36). Hypoxia, thrombin, and shear stress are known to stimulate ET-1 via exocytosis of Weibel-Palade bodies (37) but are also known to stimulate steady-state edn1 mRNA levels. In one study, it was noted that hypoxia-mediated accumulation of ET-1 in Weibel-Palade bodies was accompanied by a simultaneous increase in edn1 mRNA, suggesting that transcription was the initial step in ET-1 induction (38). However, the most compelling evidence comes from several independent studies that specifically addressed the level of ET-1 stimulation, and the prevailing scientific consensus is that transcription is the primary level of ET-1 regulation (39–43).
Figure 2.
Overview of ET-1 synthesis. Intron-exon structure and RNA processing pathway are indicated for the edn1 gene. Translation yields preproET-1 that is processed in sequential proteolytic steps to generate ET-1. Structure of ET-1 contains 2 disulfide bridges and was rendered from the RCSB Protein Data Bank (PDB 1T7H).
The ability of the edn1 gene to respond to various hormones and stimuli is essential for maintaining spatial, temporal and quantitatively correct ET-1 expression in the body. Ultimately, these signaling pathways converge on elements in the edn1 regulatory region to modulate gene activity. Alterations in edn1 expression patterns have been documented in the pathogenesis and progression of various human diseases, including asthma (44), atherosclerosis (19), cardiomyopathy (45, 46), proteinuria (47, 48), diabetic retinopathy (49, 50), cancer (51–53), vitiligo (54), and sclerosis (55). Genetic polymorphisms in the edn1 promoter region have been linked to an increased incidence of essential left ventricular hypertrophy (56) and asthma (57). Additionally, a common adenine insertion in the 5′- untranslated region (UTR) of edn1 resulted in increased mRNA levels and is associated with essential hypertension (58) and orthostatic intolerance (59). Specific pharmacological agents are also known to directly interfere with edn1 expression. For example, peroxisome proliferator-activated receptor γ (PPARγ) agonists are commonly prescribed for diabetic patients and are known to block the key transcription factor activator protein-1 (AP-1) from binding to the edn1 promoter (60). The consequential decrease in edn1 expression has been linked to edema, a dose-limiting side effect in patients receiving PPAR agonists (61). Thus, it is becoming apparent that control of edn1 expression is essential for various aspects of human physiology, pathology, and pharmacology. Despite the clear significance of regulated edn1 transcription, information on the edn1 promoter remains scattered in the literature among different fields. In the present review, the regulatory elements governing edn1 gene activity will be considered along with the known factors that bind to and modulate the gene promoter.
THE ENDOTHELIN-1 GENE
The mammalian edn1 gene contains 5 exons and spans ∼6.8 kb of genomic DNA (41) (Fig. 2). The primary transcriptional start site for edn1 has been mapped independently by nuclease protection (41) and 5′ extension assays (62). Both reports also indicated that edn1 transcription could initiate at an alternative start site located 65 bp upstream of the primary start site (41, 62). (For the purposes of this review, the nucleotide positions of each cis-acting element are numbered relative to the primary transcriptional start site in the human edn1 gene; NCBI accession no. NC_000006.) This alternative transcript begins upstream of the TATA box and several other cis-acting elements within the edn1 promoter, but it does not affect the preproET-1 coding sequence. Primer extension assays conducted on tissue RNA libraries provided evidence that transcription from the alternative start site occurred in a tissue-specific manner and may be important for edn1 expression in the heart and brain (62). In addition to the wild-type transcript, 3 alternatively spliced isoforms of edn1 have also been reported in humans (Supplemental Table 1). Each transcript retains the coding sequence for the mature ET-1 peptide but encodes for predicted preproET-1 isoforms that are 211, 178, and 78 aa in length. The regulatory importance of alternatively spliced edn1 mRNA remains unclear. In contrast, the proximal promoter of edn1 is highly conserved among mammalian species and has been studied in considerable depth (Fig. 3B) (63). The edn1 promoter contains both common cis-acting elements and important cell-type specific and inducible elements.
Figure 3.
Summary of edn1 promoter regulation. A) cis-acting elements of the edn1 promoter are shown along with the known regulatory pathways and factors that mediate edn1 gene activity. Although many of these signaling pathways are known to overlap, only the factors documented in the signal transduction pathway leading from the given stimulus to the edn1 gene are shown. Nucleotide positions are numbered relative to the primary transcriptional start site and correspond to the human edn1 gene (NCBI accession no. NC_000006). Signals that increase or decrease edn1 are shown in green and red, respectively. Akt, protein kinase B; AP-1, activator protein-1; ALK5, activin receptor-like kinase 5; ERK1/2, extracellular signal-regulated kinase 1/2; FOXO1, forkhead box O1; GR, glucocorticoid receptor; GSK3β, glycogen synthase kinase 3β; HIF-1, hypoxia inducible factor-1; IκB, inhibitor of κB; IKK, IκB kinase; JNK, c-Jun N-terminal kinase; MAPK, mitogen activate protein kinase; MR, mineralocorticoid receptor; NADPH, nicotinamide adenine dinucleotide phosphate; Per1, period 1; PI3K, phosphoinositide 3-kinase; PKC, protein kinase C; TAFs, TATA associated factors; TBP, TATA binding protein; TGFβ, transforming growth factor β; TNF-α, tumor necrosis factor α; RNA pol, RNA polymerase; RhoA, Ras homology kinase A; Smad, mothers against decapentaplegic homologue; Vezf1, vascular endothelial zinc finger binding protein f1. B) Alignment of the edn1 proximal promoter from Homo sapiens, Pan troglodytes, Macaca mulatta, Bos taurus, Equus caballus, Canis familiaris, Sus scrofa, Rattus norvegicus, and Mus musculus. Alignment logo was created using WebLogo software (63). Overall height of each nucleotide indicates the relative conservation at that position measured in bits, with 2.0 bits representing 100% conservation in all 9 species analyzed.
Common promoter elements
Deletion analysis of the human edn1 promoter linked to a reporter gene revealed that the minimal promoter elements were housed within the first 150 bp upstream of the transcriptional start site (64). This region of the edn1 gene is highly conserved across all mammals and contains both TATA and CAAT boxes located at positions −31 and −98 bp, respectively (41) (Fig. 3). Recognition of the TATA box presumably involves the cognate TATA box binding protein during assembly of the transcription initiation complex. CAAT box elements are very common components of eukaryotic promoters and are recognized by several different DNA binding proteins, including the CAAT transcription factor, nuclear factor-Y, CCAAT displacement protein, and CCAAT/enhancer binding protein (65). Most of these factors function to recruit RNA polymerase II. Although it can be inferred that a subset of these factors act on the edn1 gene promoter, their ability to bind to or regulate the edn1 gene has not been empirically tested. Interestingly, mutation of the edn1 CAAT box did not alter basal transcription rates in endothelial cells, but it completely blocked the transcriptional activity of edn1 in response to hypoxia (66). This result suggests that the CAAT box might be more important under conditions of promoter induction rather than simply acting as a basal promoter element.
Regulated elements in the minimal promoter
A consensus binding motif for the vascular endothelial zinc finger 1 (Vezf1) transcription factor is located at −55 bp in the edn1 promoter (Fig. 3). Vezf1 was first identified using a retroviral screen and appears to be expressed exclusively in vascular endothelial and hematopoietic cells (67). Aitsebaomo et al. (68) demonstrated that Vezf1 specifically bound to the edn1 promoter and suggested that the Vezf1 binding site was responsible for endothelial cell dependent edn1 expression. To date, the only reported regulatory cell signal for Vezf1 is the Rac1-specific GTPase p68RacGAP, which participates in a direct protein-protein interaction with Vezf1 (69). Coexpression of Vezf1 and p68RacGAP in endothelial cells revealed that p68RacGAP inhibits Vezf1 transcriptional activation of the edn1 gene in a dose-dependent manner. More recently, site-directed mutagenesis of the edn1 promoter showed that the Vezf1 binding site is involved in both basal and insulin-stimulated increases in edn1 expression in endothelial cells (70). This insulin-dependent mechanism involved phosphatidylinositol-3 kinase (PI3K)-dependent inhibition of the glycogen synthase kinase 3β ultimately leading to derepression of Vezf1. Therefore, one can imagine that the glycogen synthase kinase 3β and p68RacGAP are part of the same signal transduction pathway targeted by insulin to activate the edn1 gene. Elucidating the components of the insulin-Vezf1 pathway may provide useful targets for modulating the adverse actions of ET-1 in the vasculature of patients with hyperinsulinemia and insulin resistance.
A recent study identified a forkhead box O (FOXO) binding site located at position −60 bp in the edn1 promoter (71). Chromatin immunoprecipitation (ChIP) assays were used in this study to show that the binding site was occupied by FOXO1 in human endothelial cells. The green tea polyphenol epigallocatechin gallate dramatically reduced edn1 mRNA levels through a pathway that involved Akt-dependent phosphorylation and inactivation of FOXO1. Red wine and its polyphenols resveratrol and quercetin also inhibit edn1 expression in the vasculature (72, 73). Decreased vascular edn1 expression may explain some of the cardiovascular benefits from dietary polyphenols. Consistent with this concept was the observation that red wine polyphenols decreased circulating ET-1 levels and prevented both oxidative stress and hypertension in deoxycorticosterone acetate-salt treated rats (74). Endogenous cellular signals that regulate the edn1 FOXO motif have not yet been defined. However, it seems reasonable that FOXO-regulation of edn1 would be important under a number of biological circumstances, given the proximity of the motif to the transcriptional start site and the high degree of conservation between mammalian species (Fig. 3B).
The AP-1 binding site has long been recognized as an important response element in the edn1 promoter. This site is located at −108 bp and recruits both AP-1 subunits Fos and Jun (75) (Fig. 3). AP-1 is a transcription factor that mediates the genomic responses to growth factors, cytokines, and other proinflammatory and proliferative signals. Phorbol esters characteristically stimulate AP-1 through a pathway that involves protein kinase C (PKC), and the addition of these compounds results in potent induction of edn1 transcription (41, 76, 77). PKC-dependent activation of AP-1 has been identified as a critical mechanism for many other edn1 stimuli, including thrombin (60, 76, 78), angiotensin II (79), and high-density lipoprotein (80). However, PKC activation is not necessarily required for AP-1 action on edn1. For example, both insulin and thrombin have been shown to activate edn1 transcription through PKC-independent pathways (39, 81). An alternative pathway involving both the mitogen-activated protein kinases JNK and ERK1/2 was proposed for the leptin-dependent recruitment of AP-1 to the edn1 promoter (82, 83). Electrophoretic mobility shift assays (EMSA) showed that leptin induced the direct binding of AP-1 to the human edn1 promoter (83).
Mutational analysis of the AP-1 binding site revealed that the element is also required for basal transcription of edn1 in cultured endothelial cells (75). Basal edn1 transcription apparently requires a cooperative interaction of AP-1 with GATA-2, another transcription factor that binds to a nearby promoter element (84). The actions of specific inhibitors support an important role for AP-1 in edn1 regulation. Both PPARγ agonists (60) and homocysteine (85) inhibit edn1 expression by preventing AP-1 binding to the edn1 promoter. Similarly, resveratrol can prevent both angiotensin II (86) and cyclic strain (87) from stimulating edn1 by blocking AP-1. Finally, AP-1 participates in cooperative interactions with other transcription factors under conditions when the edn1 gene is activated. For example, AP-1 and Smad proteins synergistically induce edn1 transcription in endothelial cells treated with transforming growth factor β (TGFβ; ref. 88). In summary, the AP-1 binding site is a key cis-acting element involved in both basal and stimulated edn1 expression.
The edn1 promoter is highly responsive to hypoxia and recruits a protein complex that includes AP-1 and at least 3 other factors: p300, a scaffold protein with intrinsic histone acetyltransferase activity; the hypoxia inducible factor 1 (HIF-1); and GATA-2 (66). The binding site for HIF-1 is located at position −118 bp (66) (Fig. 3). Results from gel shift assays, DNA pulldown, and reporter gene assays have confirmed that both components of the HIF-1 heterodimer, HIF-1α and HIF-1β, bind to the edn1 promoter to regulate transcription (66, 89). Mutations in the binding sites for HIF-1, GATA-2, AP-1, or CAAT abolished the response to hypoxia (66). The hypoxia-dependent signal transduction pathway responsible for the activation of edn1 has not been thoroughly defined. However, studies using inhibitors of tyrosine kinase or PI3K suggest that the molecular mechanism underlying the hypoxic regulation of edn1 involves both kinases (90). Tyrosine kinase inhibitors inhibited HIF-1 transcriptional activity, but they had no effect on the binding of HIF-1 to the edn1 promoter region (89). Unlike many other signals that have only been studied in one cell type, hypoxic induction of edn1 has been observed in a variety of cell types, including endothelial cells (66), renal inner medullary collecting duct cells (91), pancreatic islet cells (92), cardiomyocytes (93), and glial cells (94). Hypoxia induced edn1 expression has been implicated in the pathology of fetal growth restriction (95), myocardial infarction (96), and obstructive sleep apnea (97, 98). Belaidi et al. (97) provided the most direct evidence for the hypoxic regulation of edn1 in the pathology of disease. ChIP assays showed that spontaneously hypertensive rats exposed to intermittent hypoxia had increased HIF-1 binding to the edn1 promoter in myocardial tissue extracts, and increased myocardial ET-1 was associated with hypertension and myocardial infarction.
Recently, substantial evidence has emerged showing that the HIF-1 element regulates edn1 expression under conditions independent from hypoxia. Spirig et al. (99) used the HIF-1α-specific inhibitor chetomin to demonstrate that stimulation of Toll-like receptors (TLR2/4) on dendritic cells resulted in HIF-1α-dependent edn1 expression. In another study, Patel et al. (100) used ChIP and supershifted EMSAs to show that the placenta growth factor can stimulate HIF-1α recruitment to the edn1 promoter. This signaling pathway involved the activation of both PI3K and NADPH oxidase. Treatment of pulmonary microvascular endothelial cells with inhibitors of either enzyme, LY294002 or diphenylene iodonium (DPI), blocked HIF-1α binding to the edn1 gene. Ethanol and acetaldehyde have also been shown to stimulate edn1 transcription by activating HIF-1 (101). EMSA and ChIP assays showed an increase in HIF-1α bound to the edn1 promoter in the presence of ethanol. The NADPH oxidase inhibitor DPI blocked transcription by preventing HIF-1α from binding to the edn1 promoter. However, the addition of LY294002 had no apparent effect, suggesting that the ethanol-dependent activation of edn1 does not require PI3K activation. Together, these studies show that HIF-1 regulation of edn1 is a dynamic process that extends beyond the well-known response to oxygen availability and is pertinent for a variety of other stimuli.
An inverted GATA motif is positioned at −135 bp in the edn1 promoter (102) (Fig. 3). These elements bind the GATA family of transcription factors, GATA 1–6 (103). In general, GATA-1, GATA-2, and GATA-3 factors are expressed in hematopoietic derived cells, brain, spinal cord, and inner ear. GATA-4, GATA-5, and GATA-6 are expressed in heart, gut, and other mesodermal and endodermal derived tissues (104). Mutation of the edn1 GATA motif blunted basal edn1 promoter activity in endothelial cells (102). It has long been thought that the primary GATA isoform responsible for binding to and regulating edn1 transcription in endothelial cells is GATA-2 (105, 106). A direct interaction of GATA-2 with AP-1 has been demonstrated by immunoprecipitation, and coexpression of GATA-2 and AP-1 resulted in a synergistic activation of the edn1 promoter (84). More recently, Glenn et al. (107) used supershifted EMSA and ChIP experiments to convincingly demonstrate that edn1 expression is regulated through the recruitment of endogenous GATA-4 in cardiac fibroblasts. Exogenously derived GATA-1, GATA-3, and GATA-5 factors can also stimulate edn1 expression (84, 108). Collectively, this evidence suggests that the regulation of edn1 may be a feature common to the family of GATA factors as a whole. However, the action of GATA factors on edn1 in tissues other than endothelial cells or fibroblasts has not been rigorously examined. Several signals known to activate GATA factors have been linked to edn1 expression. Hypertrophic signals, including ET-1 itself, stimulate GATA-4 action (109). The signaling pathway apparently involves activation of RhoA and p38 MAPK and eventually leads to the phosphorylation and activation of GATA-4. In contrast, several agents that inhibit edn1 transcription are known to directly interfere with GATA factors. For example, Kuwahara-Watanabe et al. (110) used EMSA experiments to show that heparin blocked edn1 transcription by preventing GATA-2 and AP-1 from binding to the edn1 promoter. Retinoids antagonize edn1 transcription, while simultaneously down-regulating GATA-2 mRNA expression levels (106, 111). High levels of retinoic acid are known to decrease embryonic edn1 expression and can result in the development of craniofacial abnormalities (112). Simple dietary supplementation with folinic acid was shown to restore edn1 mRNA levels and mitigate many of the developmental abnormalities caused by retinoic acid (113).
A novel Smad protein-binding element has been identified at position −191 bp in the edn1 promoter (88) (Fig. 3). Smad proteins mediate the genomic action of TGFβ, a potent stimulator of edn1 expression (88, 114–116). Castañares et al. (117) showed that TGFβ signaled through activin receptor-like kinase 5 (ALK5) to stimulate endogenous Smad3 and Smad4 recruitment to the edn1 promoter in endothelial cells. Overexpression of Smad2 also stimulated an increase in TGFβ-dependent edn1 expression, suggesting that other Smad proteins may regulate edn1 transcription (88). Transactivation and EMSA experiments indicated that Smad3 forms a cooperative interaction with AP-1 to synergistically activate the edn1 gene in response to TGFβ. In addition, p300 was identified in the immobilized Smad-AP-1 protein complex bound to the edn1 promoter. However, the enzymatic activity of p300 was apparently not required for TGFβ action on edn1 since an acetylase-deficient mutant of p300 was able to associate with AP-1 and Smad and stimulate robust edn1 expression. Thus, it seems reasonable to speculate that p300 serves as a molecular bridge between Smad proteins and AP-1.
Distal upstream regulatory elements
Several regulatory elements upstream of the basal promoter have been identified in the 5′ region of the mammalian edn1 gene. For the purposes of this review, only those elements that have been empirically tested will be considered. These elements include hormone response elements, an NF-κB binding site and an E-box motif. Although the locations of such elements vary between species, computer analysis indicates consensus binding sequences in many mammalian species.
In recent years, steroid hormone regulation of edn1 has come to the forefront of the ET-1 field. Aldosterone, a mineralocorticoid involved in maintaining sodium homeostasis in the body, is known to stimulate edn1 expression in vascular smooth muscle cells (118), cardiomyocytes (119), and renal epithelial cells (43, 120). Recently, Stow et al. (43) identified two nontraditional hormone response elements located at −572 bp and −690 bp in the murine edn1 gene (Fig. 3). DNA pulldown and ChIP assays demonstrated that both the mineralocorticoid receptor (MR) and the glucocorticoid receptor (GR) bind directly to the endogenous edn1 promoter in an aldosterone-dependent manner. Moreover, pharmacological inhibition of MR or GR and siRNA knockdown of either receptor confirmed the role of both proteins in the aldosterone-dependent regulation of the edn1 promoter. In the nuclei of cells treated with aldosterone, but not vehicle, the immobilized protein complex bound to the edn1 promoter contained the steroid receptor coactivator-1 protein (SRC-1) and p300. The interaction between aldosterone and ET-1 is relevant for renal physiology and sodium homeostasis because the two hormones have opposing actions on the epithelial sodium channel activity. Importantly, aldosterone-dependent induction of ET-1 may represent a negative feedback loop on aldosterone-mediated sodium reabsorption in the kidney (43). Disruption of the interaction between aldosterone and ET-1 has implications for several cardiovascular and renal diseases, including hypertension and chronic kidney disease.
The edn1 regulatory region includes an E-box motif located in the vicinity of the steroid hormone response elements at −662 bp (Fig. 3). E-box motifs characteristically bind a wide range of basic helix-loop-helix type transcription factors. Previous work detected the proto-oncogene c-Myc transcription factor bound to the edn1 E-box motif in fibroblasts (121). Regulation of edn1 was responsive to the concentration exogenously expressed c-Myc in the cell. At low concentrations of c-Myc, the edn1 gene is active, while high concentrations of c-Myc result in repression of edn1 gene transcription. Levels of endogenous c-Myc are regulated in a cell cycle-dependent manner spiking near the G1-S transition, suggesting a repression of edn1 during replication. The same E-box motif may also be involved in the recruitment of other transcription factors. For example, M. L. Gumz et al. found Per1, a circadian rhythm transcription factor, bound at the E-box motif in the murine edn1 gene to negatively regulate gene expression (unpublished results). Since Per1 is not thought to bind DNA directly, its presence implies binding of other circadian rhythm factors such as Clock or Bmal1. The significance of circadian regulation of edn1 remains to be determined.
NF-κB is a redox-sensitive transcription factor known to activate edn1 in a variety of cell types (122–127). The edn1 promoter contains a functional NF-κB binding site located at position −2090 bp (122) (Fig. 3). Interestingly, only NF-κB heterodimers (p65/p50) appear to activate edn1 in endothelial cells because coexpression of p65 and p50 subunits led to an increase in edn1, whereas expression of p50 alone led to reduction in edn1 transcription. Decreased edn1 activity was also observed following overexpression of inhibitor of κB (IκBα), a protein that binds to and stabilizes NF-κB in an inactive state. Recent ChIP evidence indicated that the edn1 promoter contained two additional NF-κB binding sites located at −891 and −1214 bp (123). Indeed, stimulation of pulmonary artery smooth muscle cells with tumor necrosis factor α (TNF-α) and interferon-γ led to the recruitment of NF-κB to all three sites. An increase in regional histone H4 acetylation, a marker of transcriptionally active chromatin, accompanied this NF-κB binding. Other investigators have reported that glucose stimulates the recruitment of NF-κB and p300 to the edn1 promoter and that binding of these factors was associated with an increase in histone H3 acetylation (124). Several key components of the NF-κB signaling pathway leading to the activation of edn1 have been reported. For example, TNF-α treatment of glioblastoma cells led to a dose-dependent association of NF-κB with the edn1 promoter through a pathway that involved PI3K activation (125). Oleic acid stimulation of edn1 required the activation of calcium-dependent PKC followed by subsequent NF-κB activation (126). Other cytokines are also able to activate NF-κB-dependent edn1 expression. For example, interleukin-1β treatment resulted in increased NF-κB activation and edn1 expression in renal inner medullary collecting duct cells (127). A subcutaneous infusion of interleukin-1β also resulted in increased renal edn1 expression in mice, and it has been proposed that cytokine-dependent edn1 expression is involved in several in vivo inflammatory processes.
EMERGING REGULATORY MECHANISMS
There are a number of stimuli known to alter edn1 transcription that have undefined molecular signaling pathways. For example, it has long been known that the edn1 promoter is responsive to intracellular calcium levels (1). Recently, Strait et al. (128) conducted ex vivo experiments on inner medullary collecting cells in conjunction with in vitro reporter gene assays to show that the rat edn1 promoter was sensitive to both intracellular calcium and calmodulin. The calcium response appears to be a tissue-specific mechanism for renal epithelial cells because it was not observed in aortic endothelial cells. These reporter assays indicated that the calmodulin-responsive element was located somewhere between −1 and −3 kb in the edn1 regulatory region. However, the exact nucleotide element governing the calcium responsiveness has not yet been mapped. Further investigation into the calcium-dependent regulation of edn1 may be useful for identifying novel cell-signaling or tissue-specific mechanisms that control edn1 transcription.
Mechanotransduction signals also regulate edn1 in vascular endothelial and renal epithelial cells (129–131). Increased flow rate and shear stress stimulate the release of ET-1 from many vascular and nonvascular cell types (131, 132). Renal epithelial cells have cilia that can sense changes in flow and respond by changing intracellular calcium levels. It was recently postulated that intracellular calcium signaling may provide a link between luminal flow and edn1 transcription (128). Endothelial cells also respond to mechanical stimuli by increasing edn1 expression. Early studies showed that cyclic strain activated PKC and eventually led to an increase in intracellular calcium and edn1 expression (133). Consistent with a PKC-dependent pathway was the finding that cyclic strain resulted in AP-1-dependent edn1 transcription in endothelial cells (130).
Extracellular hypertonicity also regulates ET-1 release from inner medullary collecting duct cells (134). Likewise, a high-salt diet increased edn1 expression in cardiomyocytes (135) and renal medullary thick ascending limb cells (136). The molecular mechanism responsible for mediating this response remains unknown. The only reported osmotic-dependent transcription factor is the tonicity-responsive enhancer binding protein (TonEBP), which is also known as the nuclear factor of activated T cells type 5 (NFAT5). NFAT proteins bind to the core consensus sequence GGAAA (137) and inspection of the human edn1 promoter region revealed nine putative binding sites located within the first 2,000 bp upstream of the transcriptional start site (Supplemental Table 2). Regulation of edn1 by TonEBP/NFAT5 has not been tested; however, the putative motif located at −155 bp was reportedly subject to regulation by NFAT2 in cardiac progenitor TC13 cells (108). New evidence indicates that NFAT2 is sensitive to mechanical stimulation and directs the expression of target genes in response to fluid shear stress and tensile strain in osteoblasts (138). Therefore, an interesting possibility is that the NFAT binding site is responsible for integrating multiple mechanical and physical signals into a single edn1 transcriptional response.
The mechanisms controlling cell-type-specific expression of edn1 are critical for maintaining the integrity of the ET-1 signaling system in vivo. The identification of Vezf1 regulation of edn1 has provided insight into endothelial cell-specific transcription. However, this discovery does not preclude the possibility that other cis-acting sites in edn1 also mediate endothelial expression. More than a decade ago, Bu et al. (139) identified a region located between −347 and −320 bp that appeared to be involved in endothelial cell edn1 expression and recruited a specific protein complex. The components of the complex were not identified, but this issue could be revisited using current technologies.
Epigenetic regulation
Tissue-specific edn1 expression is also modulated by DNA methylation. Several segments rich in CpG dinucleotides located in the first intron of edn1 are subject to methylation and gene silencing (140). For example, differential methylation patterns correlated with the differences in edn1 expression in several cell lines. The edn1 gene was hypomethylated in renal inner medullary collecting duct cells that express high levels of ET-1. In contrast, fibroblasts have a hypermethylated edn1 gene and express only a small amount of ET-1. Recently, Dickson et al. (141) reported that Vezf1 elements protect the local DNA from methylation and inactivation. Disruption of Vezf1 in mouse embryonic stem cells resulted in decreased levels of the DNA methyltransferase Dnmt3b and resulted in widespread genomic demethylation (142). Vezf1 modulation of the edn1 DNA methylation status may explain the high level of edn1 expression in endothelial cells given its tissue specificity.
Histone modification patterns are also known to influence edn1 transcriptional activity. Stow et al. (43) showed that methylation of histone H3 lysine 4 residues was associated with transcriptional induction by aldosterone in renal epithelial cells. As noted above, several stimuli leading to NF-κB-dependent expression of edn1 also involved local acetylation of histone H3 and H4 (123, 124). Pharmacological inhibition of histone deacetylases caused a dose-dependent decrease in edn1 expression in pancreatic stellate cells (143). These studies reveal that histone modification is a common theme for edn1 regulation. It is possible that the progressive loss of epigenetic markers, either histone modifications or DNA methylation, could lead to unregulated edn1 expression and contribute to altered levels of ET-1 observed in late-onset diseases such as diabetes or hypertension.
Regulation mRNA stability
Post-transcriptional modifications are known to affect mRNA levels of edn1. The edn1 mRNA transcript is highly labile with an approximate intracellular half-life of 15 min (41). The 3′-UTR of the human edn1 mRNA contains 3 AUUUA motifs at nt 1927, 1942, and 1955 (nucleotide positions correspond to NCBI accession no. NM_001955). These elements are thought to confer selective mRNA degradation via accelerated deadenylation. Site-directed mutagenesis of each element indicated that the AUUUA motif located at position 1942 was responsible for facilitating edn1 mRNA degradation (144). AUUUA motifs also regulate mRNA stability through the recruitment of AU-rich element binding proteins that either enhance degradation or result in mRNA stabilization. Recently, RNA affinity chromatography and RNA-EMSAs showed that glyceraldehyde 3-phosphate dehydrogenase (GAPDH) directly interacted with edn1 mRNA to mediate rapid degradation (145). S-glutathionylation of GAPDH, which occurs during oxidative stress, prevented its interaction with edn1 mRNA and resulted in increased edn1 mRNA levels. In addition, knockdown of a common AU-rich element binding protein AUF1 stimulated edn1 mRNA expression in vascular endothelial cells (144).
The edn1 mRNA is also subject to microRNA (miRNA)-mediated regulation. Yeligar et al. (101) reported that miRNAs play a role in regulating edn1 mRNA levels in rat liver sinusoidal epithelial cells and human microvascular epithelial cells. In this study, transfection of the microvascular cells with plasmids overexpressing miR-155 or miR-199 reduced edn1 mRNA levels and prevented ethanol-induced edn1 expression. Moreover, a striking 17-fold increase in edn1 mRNA was observed when the cells were transfected with an antimiR-199 expression vector. Computer analysis suggests that there may be other miRNAs that target the edn1 mRNA in different tissues. Thus, tissue-specific or hormone-regulated miRNAs likely play important roles mediating edn1 translational regulation and mRNA turnover.
PERSPECTIVE AND PREDICTIONS
The regulation of edn1 gene transcription is achieved through the actions of various hormones, stimuli, and cell signals that converge on cis-acting elements in the edn1 promoter. Although some response elements such as the AP-1 and HIF-1 sites function in many cells types, many factors governing edn1 apparently vary significantly among different cells. For example, the edn1 gene is highly responsive to calcium in renal epithelial cells, whereas Vezf1 appears to be specifically involved in endothelial cell edn1 gene activity. One of the challenges facing the field is to address whether transcriptional regulation that is currently viewed as tissue-specific may be applicable in other cell types or under different conditions. The focus on the promoter proximal region of the edn1 gene has very likely resulted in omission of important regulatory elements located far upstream in the promoter and possibly downstream within the edn1 locus. Only one study evaluated greater than 3 kb of the 5′ regulatory region, and it revealed thrombin-dependent DNase I hypersensitivity sites as far out as −6.7 kb (146). A systematic survey of the edn1 gene for tissue- and condition-specific enhancer elements is needed. Finally, the emerging fields of epigenetic regulation and mRNA stability require a more thorough consideration. Future studies will need to determine the significance of these mechanisms in the overall physiological control of ET-1.
Supplementary Material
Acknowledgments
Financial support for this work was provided by U.S. National Institutes of Health National Institute of Diabetes and Digestive and Kidney Disease grant 1R01DK082680.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. (1988) A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332, 411–415 [DOI] [PubMed] [Google Scholar]
- 2. Dashwood M. R., Loesch A. (2009) Endothelin-1 as a neuropeptide: neurotransmitter or neurovascular effects? J. Cell Commun. Signal. 4, 51–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khimji A. K., Rockey D. C. (2010) Endothelin—biology and disease. Cell. Signal. 22, 1615–1625 [DOI] [PubMed] [Google Scholar]
- 4. Hans G., Schmidt B. L., Strichartz G. (2009) Nociceptive sensitization by endothelin-1. Brain Res. Rev. 60, 36–42 [DOI] [PubMed] [Google Scholar]
- 5. Good T. J., Kahook M. Y. (2010) The role of endothelin in the pathophysiology of glaucoma. Expert Opin. Ther. Targets 14, 647–654 [DOI] [PubMed] [Google Scholar]
- 6. Chauhan B. C. (2008) Endothelin and its potential role in glaucoma. Can. J. Ophthalmol. 43, 356–360 [DOI] [PubMed] [Google Scholar]
- 7. Rehsia N. S., Dhalla N. S. (2010) Potential of endothelin-1 and vasopressin antagonists for the treatment of congestive heart failure. Heart Fail. Rev. 15, 85–101 [DOI] [PubMed] [Google Scholar]
- 8. Barton M., Yanagisawa M. (2008) Endothelin: 20 years from discovery to therapy. Can. J. Physiol. Pharmacol. 86, 485–498 [DOI] [PubMed] [Google Scholar]
- 9. Porter K. E., Turner N. A. (2009) Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol. Ther. 123, 255–278 [DOI] [PubMed] [Google Scholar]
- 10. Leask A. (2010) Potential therapeutic targets for cardiac fibrosis: TGFβ, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ. Res. 106, 1675–1680 [DOI] [PubMed] [Google Scholar]
- 11. Ross B., D'Orleans-Juste P., Giaid A. (2010) Potential role of endothelin-1 in pulmonary fibrosis: from the bench to the clinic. Am. J. Respir. Cell Mol. Biol. 42, 16–20 [DOI] [PubMed] [Google Scholar]
- 12. Karkoulias K., Lykouras D., Sampsonas F., Drakatos P., Canova S., Tsoukalas G., Spiropoulos K. (2010) The role of endothelin-1 in obstructive sleep apnea syndrome and pulmonary arterial hypertension: pathogenesis and endothelin-1 antagonists. Curr. Med. Chem. 17, 1059–1066 [DOI] [PubMed] [Google Scholar]
- 13. Abman S. H. (2009) Role of endothelin receptor antagonists in the treatment of pulmonary arterial hypertension. Annu. Rev. Med. 60, 13–23 [DOI] [PubMed] [Google Scholar]
- 14. Comellas A. P., Briva A. (2009) Role of endothelin-1 in acute lung injury. Transl. Res. 153, 263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dhaun N., Goddard J., Kohan D. E., Pollock D. M., Schiffrin E. L., Webb D. J. (2008) Role of endothelin-1 in clinical hypertension: 20 years on. Hypertension 52, 452–459 [DOI] [PubMed] [Google Scholar]
- 16. Thorin E., Webb D. J. (2010) Endothelium-derived endothelin-1. Pflügers Arch. 459, 951–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bohm F., Pernow J. (2007) The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc. Res. 76, 8–18 [DOI] [PubMed] [Google Scholar]
- 18. Kohan D. E. (2010) Endothelin, hypertension and chronic kidney disease: new insights. Curr. Opin. Nephrol. Hypertens. 19, 134–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ivey M. E., Osman N., Little P. J. (2008) Endothelin-1 signalling in vascular smooth muscle: pathways controlling cellular functions associated with atherosclerosis. Atherosclerosis 199, 237–247 [DOI] [PubMed] [Google Scholar]
- 20. Kohan D. E. (2009) Biology of endothelin receptors in the collecting duct. Kidney Intl. 76, 481–486 [DOI] [PubMed] [Google Scholar]
- 21. Barton M. (2008) Reversal of proteinuric renal disease and the emerging role of endothelin. Nat. Clin. Pract. Nephrol. 4, 490–501 [DOI] [PubMed] [Google Scholar]
- 22. Vollmar B., Menger M. D. (2009) The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol. Rev. 89, 1269–1339 [DOI] [PubMed] [Google Scholar]
- 23. Pitts K. R. (2009) Endothelin receptor antagonism in portal hypertension. Expert Opin. Investig. Drugs 18, 135–142 [DOI] [PubMed] [Google Scholar]
- 24. Meidan R., Levy N. (2007) The ovarian endothelin network: an evolving story. Trends Endocrinol. Metab. 18, 379–385 [DOI] [PubMed] [Google Scholar]
- 25. Bagnato A., Spinella F., Rosano L. (2008) The endothelin axis in cancer: the promise and the challenges of molecularly targeted therapy. Can. J. Physiol. Pharmacol. 86, 473–484 [DOI] [PubMed] [Google Scholar]
- 26. Ramos-Casals M., Fonollosa-Pla V., Brito-Zeron P., Siso-Almirall A. (2010) Targeted therapy for systemic sclerosis: how close are we? Nat. Rev. Rheumatol. 6, 269–278 [DOI] [PubMed] [Google Scholar]
- 27. Gitton Y., Heude E., Vieux-Rochas M., Benouaiche L., Fontaine A., Sato T., Kurihara Y., Kurihara H., Couly G., Levi G. (2010) Evolving maps in craniofacial development. Semin. Cell. Dev. Biol. 21, 301–308 [DOI] [PubMed] [Google Scholar]
- 28. Kurihara Y., Kurihara H., Suzuki H., Kodama T., Maemura K., Nagai R., Oda H., Kuwaki T., Cao W. H., Kamada N., Jishage K., Ouchi Y., Azuma S., Toyoda Y., Ishikawa T., Kumada M., Yazaki Y. (1994) Elevated blood pressure and craniofacial abnormalities in mice deficient in endothelin-1. Nature 368, 703–710 [DOI] [PubMed] [Google Scholar]
- 29. Yanagisawa M., Masaki T. (1989) Molecular biology and biochemistry of the endothelins. Trends Pharmacol. Sci. 10, 374–378 [DOI] [PubMed] [Google Scholar]
- 30. Davenport A. P. (2002) International Union of Pharmacology. XXIX. Update on endothelin receptor nomenclature. Pharmacol. Rev. 54, 219–226 [DOI] [PubMed] [Google Scholar]
- 31. Fabbrini M. S., Valsasina B., Nitti G., Benatti L., Vitale A. (1991) The signal peptide of human preproendothelin-1. FEBS Lett. 286, 91–94 [DOI] [PubMed] [Google Scholar]
- 32. Blais V., Fugere M., Denault J. B., Klarskov K., Day R., Leduc R. (2002) Processing of proendothelin-1 by members of the subtilisin-like pro-protein convertase family. FEBS Lett. 524, 43–48 [DOI] [PubMed] [Google Scholar]
- 33. Shimada K., Matsushita Y., Wakabayashi K., Takahashi M., Matsubara A., Iijima Y., Tanzawa K. (1995) Cloning and functional expression of human endothelin-converting enzyme cDNA. Biochem. Biophys. Res. Commun. 207, 807–812 [DOI] [PubMed] [Google Scholar]
- 34. Emoto N., Yanagisawa M. (1995) Endothelin-converting enzyme-2 is a membrane-bound, phosphoramidon-sensitive metalloprotease with acidic pH optimum. J. Biol. Chem. 270, 15262–15268 [DOI] [PubMed] [Google Scholar]
- 35. Harrison V. J., Barnes K., Turner A. J., Wood E., Corder R., Vane J. R. (1995) Identification of endothelin 1 and big endothelin 1 in secretory vesicles isolated from bovine aortic endothelial cells. Proc. Natl. Acad. Sci. U. S. A. 92, 6344–6348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Doi Y., Kudo H., Nishino T., Yamamoto O., Nagata T., Nara S., Morita M., Fujimoto S. (2002) Enhanced expression of endothelin-1 and endothelin-converting enzyme-1 in acute hypoxic rat aorta. Histol. Histopathol. 17, 97–105 [DOI] [PubMed] [Google Scholar]
- 37. Lowenstein C. J., Morrell C. N., Yamakuchi M. (2005) Regulation of Weibel-Palade body exocytosis. Trends Cardiovasc. Med. 15, 302–308 [DOI] [PubMed] [Google Scholar]
- 38. Doi Y., Kudo H., Nishino T., Nagata T., Fujimoto S. (2004) The enhancement of preproendothelin-1 synthesis and the acceleration of endothelin-1 processing in the acute hypoxic rat aorta. J. Cardiovasc. Pharmacol. 44(Suppl. 1), S207–S210 [DOI] [PubMed] [Google Scholar]
- 39. Oliver F. J., de la Rubia G., Feener E. P., Lee M. E., Loeken M. R., Shiba T., Quertermous T., King G. L. (1991) Stimulation of endothelin-1 gene expression by insulin in endothelial cells. J. Biol. Chem. 266, 23251–23256 [PubMed] [Google Scholar]
- 40. Saito S., Hirata Y., Imai T., Marumo F. (1995) Autocrine regulation of the endothelin-1 gene in rat endothelial cells. J. Cardiovasc. Pharmacol. 26(Suppl. 3), S84–S87 [PubMed] [Google Scholar]
- 41. Inoue A., Yanagisawa M., Takuwa Y., Mitsui Y., Kobayashi M., Masaki T. (1989) The human preproendothelin-1 gene. Complete nucleotide sequence and regulation of expression. J. Biol. Chem. 264, 14954–14959 [PubMed] [Google Scholar]
- 42. Todd-Turla K. M., Zhu X. L., Shu X., Chen M., Yu T., Smart A., Killen P. D., Fejes-Toth G., Briggs J. P., Schnermann J. B. (1996) Synthesis and secretion of endothelin in a cortical collecting duct cell line. Am. J. Physiol. Renal Physiol. 271, F330–F339 [DOI] [PubMed] [Google Scholar]
- 43. Stow L. R., Gumz M. L., Lynch I. J., Greenlee M. M., Rudin A., Cain B. D., Wingo C. S. (2009) Aldosterone modulates steroid receptor binding to the endothelin-1 gene (edn1). J. Biol. Chem. 284, 30087–30096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pegorier S., Arouche N., Dombret M. C., Aubier M., Pretolani M. (2007) Augmented epithelial endothelin-1 expression in refractory asthma. J. Allergy Clin. Immunol. 120, 1301–1307 [DOI] [PubMed] [Google Scholar]
- 45. Aharinejad S., Krenn K., Paulus P., Schafer R., Zuckermann A., Grimm M., Abraham D. (2005) Differential role of TGF-β1/bFGF and ET-1 in graft fibrosis in heart failure patients. Am. J. Transplant. 5, 2185–2192 [DOI] [PubMed] [Google Scholar]
- 46. Ergul A., Grubbs A. L., Zhang Y., Spinale F. G. (2000) Selective upregulation of endothelin converting enzyme-1a in the human failing heart. J. Card. Fail. 6, 314–320 [DOI] [PubMed] [Google Scholar]
- 47. Lehrke I., Waldherr R., Ritz E., Wagner J. (2001) Renal endothelin-1 and endothelin receptor type B expression in glomerular diseases with proteinuria. J. Am. Soc. Nephrol. 12, 2321–2329 [DOI] [PubMed] [Google Scholar]
- 48. Nakamura T., Ebihara I., Shirato I., Fukui M., Tomino Y., Koide H. (1993) Endothelin-1 mRNA expression by peripheral blood monocytes in IgA nephropathy. Lancet 342, 1147–1148 [DOI] [PubMed] [Google Scholar]
- 49. Khan Z. A., Farhangkhoee H., Mahon J. L., Bere L., Gonder J. R., Chan B. M., Uniyal S., Chakrabarti S. (2006) Endothelins: regulators of extracellular matrix protein production in diabetes. Exp. Biol. Med. (Maywood) 231, 1022–1029 [PubMed] [Google Scholar]
- 50. Strzalka-Mrozik B., Nowak A., Gola J., Kowalczyk M., Kapral M., Mazurek U. (2010) Factors associated with changes in endothelin-1 gene expression in patients with diabetic retinopathy in type 2 diabetes mellitus. Mol. Vis. 16, 1272–1279 [PMC free article] [PubMed] [Google Scholar]
- 51. Douglas M. L., Richardson M. M., Nicol D. L. (2004) Endothelin axis expression is markedly different in the two main subtypes of renal cell carcinoma. Cancer 100, 2118–2124 [DOI] [PubMed] [Google Scholar]
- 52. Pickering V., Jordan R. C., Schmidt B. L. (2007) Elevated salivary endothelin levels in oral cancer patients–a pilot study. Oral Oncol. 43, 37–41 [DOI] [PubMed] [Google Scholar]
- 53. Donckier J. E., Michel L., Delos M., Havaux X., Van Beneden R. (2006) Interrelated overexpression of endothelial and inducible nitric oxide synthases, endothelin-1 and angiogenic factors in human papillary thyroid carcinoma. Clin. Endocrinol. (Oxf.) 64, 703–710 [DOI] [PubMed] [Google Scholar]
- 54. Moretti S., Fabbri P., Baroni G., Berti S., Bani D., Berti E., Nassini R., Lotti T., Massi D. (2009) Keratinocyte dysfunction in vitiligo epidermis: cytokine microenvironment and correlation to keratinocyte apoptosis. Histol. Histopathol. 24, 849–857 [DOI] [PubMed] [Google Scholar]
- 55. Kawaguchi Y., Suzuki K., Hara M., Hidaka T., Ishizuka T., Kawagoe M., Nakamura H. (1994) Increased endothelin-1 production in fibroblasts derived from patients with systemic sclerosis. Ann. Rheum. Dis. 53, 506–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Castro M. G., Rodriguez-Pascual F., Magan-Marchal N., Reguero J. R., Alonso-Montes C., Moris C., Alvarez V., Lamas S., Coto E. (2007) Screening of the endothelin1 gene (EDN1) in a cohort of patients with essential left ventricular hypertrophy. Ann. Hum. Genet. 71, 601–610 [DOI] [PubMed] [Google Scholar]
- 57. Zhu G., Carlsen K., Carlsen K. H., Lenney W., Silverman M., Whyte M. K., Hosking L., Helms P., Roses A. D., Hay D. W., Barnes M. R., Anderson W. H., Pillai S. G. (2008) Polymorphisms in the endothelin-1 (EDN1) are associated with asthma in two populations. Genes Immun. 9, 23–29 [DOI] [PubMed] [Google Scholar]
- 58. Popowski K., Sperker B., Kroemer H. K., John U., Laule M., Stangl K., Cascorbi I. (2003) Functional significance of a hereditary adenine insertion variant in the 5′-UTR of the endothelin-1 gene. Pharmacogenetics 13, 445–451 [DOI] [PubMed] [Google Scholar]
- 59. Winker R., Garland E. M., Rudiger H. W., Diedrich A., Biaggioni I., Ponocny I., Cascorbi I., Robertson D. (2005) Influence of an insertion variant in the 5′UTR of the endothelin-1 gene on orthostatic intolerance. Am. J. Med. Sci. 330, 166–171 [DOI] [PubMed] [Google Scholar]
- 60. Delerive P., Martin-Nizard F., Chinetti G., Trottein F., Fruchart J. C., Najib J., Duriez P., Staels B. (1999) Peroxisome proliferator-activated receptor activators inhibit thrombin-induced endothelin-1 production in human vascular endothelial cells by inhibiting the activator protein-1 signaling pathway. Circ. Res. 85, 394–402 [DOI] [PubMed] [Google Scholar]
- 61. Geese W. J., Achanzar W., Rubin C., Hariharan N., Cheng P., Tomlinson L., Ordway N., Dracopoli N. C., Delmonte T., Hui L., Krishnan B., Cosma G., Ranade K. (2008) Genetic and gene expression studies implicate renin and endothelin-1 in edema caused by peroxisome proliferator-activated receptor gamma agonists. Pharmacogenet. Genomics 18, 903–910 [DOI] [PubMed] [Google Scholar]
- 62. Benatti L., Bonecchi L., Cozzi L., Sarmientos P. (1993) Two preproendothelin 1 mRNAs transcribed by alternative promoters. J. Clin. Invest. 91, 1149–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Crooks G. E., Hon G., Chandonia J. M., Brenner S. E. (2004) WebLogo: a sequence logo generator. Genome Res. 14, 1188–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lee M. E., Bloch K. D., Clifford J. A., Quertermous T. (1990) Functional analysis of the endothelin-1 gene promoter. Evidence for an endothelial cell-specific cis-acting sequence. J. Biol. Chem. 265, 10446–10450 [PubMed] [Google Scholar]
- 65. Mantovani R. (1998) A survey of 178 NF-Y binding CCAAT boxes. Nucleic Acids Res. 26, 1135–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yamashita K., Discher D. J., Hu J., Bishopric N. H., Webster K. A. (2001) Molecular regulation of the endothelin-1 gene by hypoxia. Contributions of hypoxia-inducible factor-1, activator protein-1, GATA-2, AND p300/CBP. J. Biol. Chem. 276, 12645–12653 [DOI] [PubMed] [Google Scholar]
- 67. Xiong J. W., Leahy A., Lee H. H., Stuhlmann H. (1999) Vezf1: A Zn finger transcription factor restricted to endothelial cells and their precursors. Dev. Biol. 206, 123–141 [DOI] [PubMed] [Google Scholar]
- 68. Aitsebaomo J., Kingsley-Kallesen M. L., Wu Y., Quertermous T., Patterson C. (2001) Vezf1/DB1 is an endothelial cell-specific transcription factor that regulates expression of the endothelin-1 promoter. J. Biol. Chem. 276, 39197–39205 [DOI] [PubMed] [Google Scholar]
- 69. Aitsebaomo J., Wennerberg K., Der C. J., Zhang C., Kedar V., Moser M., Kingsley-Kallesen M. L., Zeng G. Q., Patterson C. (2004) p68RacGAP is a novel GTPase-activating protein that interacts with vascular endothelial zinc finger-1 and modulates endothelial cell capillary formation. J. Biol. Chem. 279, 17963–17972 [DOI] [PubMed] [Google Scholar]
- 70. Yang Z., Li J. C. (2008) Stimulation of endothelin-1 gene expression by insulin via phosphoinositide-3 kinase-glycogen synthase kinase-3beta signaling in endothelial cells. Life Sci. 82, 512–518 [DOI] [PubMed] [Google Scholar]
- 71. Reiter C. E., Kim J. A., Quon M. J. (2010) Green tea polyphenol epigallocatechin gallate reduces endothelin-1 expression and secretion in vascular endothelial cells: roles for AMP-activated protein kinase, Akt, and FOXO1. Endocrinology 151, 103–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Corder R., Douthwaite J. A., Lees D. M., Khan N. Q., Viseu Dos Santos A. C., Wood E. G., Carrier M. J. (2001) Endothelin-1 synthesis reduced by red wine. Nature 414, 863–864 [DOI] [PubMed] [Google Scholar]
- 73. Nicholson S. K., Tucker G. A., Brameld J. M. (2009) Physiological concentrations of dietary polyphenols regulate vascular endothelial cell expression of genes important in cardiovascular health. Br. J. Nutr. 103, 1398–1403 [DOI] [PubMed] [Google Scholar]
- 74. Jimenez R., Lopez-Sepulveda R., Kadmiri M., Romero M., Vera R., Sanchez M., Vargas F., O'Valle F., Zarzuelo A., Duenas M., Santos-Buelga C., Duarte J. (2007) Polyphenols restore endothelial function in DOCA-salt hypertension: role of endothelin-1 and NADPH oxidase. Free Radic. Biol. Med. 43, 462–473 [DOI] [PubMed] [Google Scholar]
- 75. Lee M. E., Dhadly M. S., Temizer D. H., Clifford J. A., Yoshizumi M., Quertermous T. (1991) Regulation of endothelin-1 gene expression by Fos and Jun. J. Biol. Chem. 266, 19034–19039 [PubMed] [Google Scholar]
- 76. Kitazumi K., Tasaka K. (1993) The role of c-Jun protein in thrombin-stimulated expression of preproendothelin-1 mRNA in porcine aortic endothelial cells. Biochem. Pharmacol. 46, 455–464 [DOI] [PubMed] [Google Scholar]
- 77. Marsden P. A., Dorfman D. M., Collins T., Brenner B. M., Orkin S. H., Ballermann B. J. (1991) Regulated expression of endothelin 1 in glomerular capillary endothelial cells. Am. J. Physiol. Renal Physiol. 261, F117–F125 [DOI] [PubMed] [Google Scholar]
- 78. Foschi M., Sorokin A., Pratt P., McGinty A., La Villa G., Franchi F., Dunn M. J. (2001) PreproEndothelin-1 expression in human mesangial cells: evidence for a p38 mitogen-activated protein kinase/protein kinases-C-dependent mechanism. J. Am. Soc. Nephrol. 12, 1137–1150 [DOI] [PubMed] [Google Scholar]
- 79. Imai T., Hirata Y., Emori T., Yanagisawa M., Masaki T., Marumo F. (1992) Induction of endothelin-1 gene by angiotensin and vasopressin in endothelial cells. Hypertension 19, 753–757 [DOI] [PubMed] [Google Scholar]
- 80. Hu R. M., Chuang M. Y., Prins B., Kashyap M. L., Frank H. J., Pedram A., Levin E. R. (1994) High density lipoproteins stimulate the production and secretion of endothelin-1 from cultured bovine aortic endothelial cells. J. Clin. Invest. 93, 1056–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Marsen T. A., Simonson M. S., Dunn M. J. (1995) Thrombin induces the preproendothelin-1 gene in endothelial cells by a protein tyrosine kinase-linked mechanism. Circ. Res. 76, 987–995 [DOI] [PubMed] [Google Scholar]
- 82. Chao H. H., Hong H. J., Liu J. C., Lin J. W., Chen Y. L., Chiu W. T., Wu C. H., Shyu K. G., Cheng T. H. (2007) Leptin stimulates endothelin-1 expression via extracellular signal-regulated kinase by epidermal growth factor receptor transactivation in rat aortic smooth muscle cells. Eur. J. Pharmacol. 573, 49–54 [DOI] [PubMed] [Google Scholar]
- 83. Quehenberger P., Exner M., Sunder-Plassmann R., Ruzicka K., Bieglmayer C., Endler G., Muellner C., Speiser W., Wagner O. (2002) Leptin induces endothelin-1 in endothelial cells in vitro. Circ. Res. 90, 711–718 [DOI] [PubMed] [Google Scholar]
- 84. Kawana M., Lee M. E., Quertermous E. E., Quertermous T. (1995) Cooperative interaction of GATA-2 and AP1 regulates transcription of the endothelin-1 gene. Mol. Cell. Biol. 15, 4225–4231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Drunat S., Moatti N., Demuth K. (2002) Homocysteine decreases endothelin-1 expression by interfering with the AP-1 signaling pathway. Free Radic. Biol. Med. 33, 659–668 [DOI] [PubMed] [Google Scholar]
- 86. Chao H. H., Juan S. H., Liu J. C., Yang H. Y., Yang E., Cheng T. H., Shyu K. G. (2005) Resveratrol inhibits angiotensin II-induced endothelin-1 gene expression and subsequent proliferation in rat aortic smooth muscle cells. Eur. J. Pharmacol. 515, 1–9 [DOI] [PubMed] [Google Scholar]
- 87. Liu J. C., Chen J. J., Chan P., Cheng C. F., Cheng T. H. (2003) Inhibition of cyclic strain-induced endothelin-1 gene expression by resveratrol. Hypertension 42, 1198–1205 [DOI] [PubMed] [Google Scholar]
- 88. Rodriguez-Pascual F., Redondo-Horcajo M., Lamas S. (2003) Functional cooperation between Smad proteins and activator protein-1 regulates transforming growth factor-beta-mediated induction of endothelin-1 expression. Circ. Res. 92, 1288–1295 [DOI] [PubMed] [Google Scholar]
- 89. Minchenko A., Caro J. (2000) Regulation of endothelin-1 gene expression in human microvascular endothelial cells by hypoxia and cobalt: role of hypoxia responsive element. Mol. Cell. Biochem. 208, 53–62 [DOI] [PubMed] [Google Scholar]
- 90. Zhang J., Narayan V. M., Juedes N., Patel J. M. (2009) Hypoxic upregulation of preproendothelin-1 gene expression is associated with protein tyrosine kinase-PI3K signaling in cultured lung vascular endothelial cells. Int. J. Clin. Exp. Med. 2, 87–94 [PMC free article] [PubMed] [Google Scholar]
- 91. Miller R. L., Kohan D. E. (1998) Hypoxia regulates endothelin-1 production by the inner medullary collecting duct. J. Lab. Clin. Med. 131, 45–48 [DOI] [PubMed] [Google Scholar]
- 92. Kugelmeier P., Nett P. C., Zullig R., Lehmann R., Weber M., Moritz W. (2008) Expression and hypoxic regulation of the endothelin system in endocrine cells of human and rat pancreatic islets. JOP 9, 133–149 [PubMed] [Google Scholar]
- 93. Takanashi M., Miyauchi T., Kakinuma Y., Goto K., Yamaguchi I. (2004) Establishment of hypoxia inducible factor-1alpha overexpressing cells that produce endothelin-1. J. Cardiovasc. Pharmacol. 44(Suppl. 1), S268–S273 [DOI] [PubMed] [Google Scholar]
- 94. Zhang Y., Li Y., Totsune K., Kikuchi K., Murakami O., Shibahara S., Takahashi K. (2006) Hypoxia increases endothelin-1 mRNA expression but not immunoreactive endothelin in the medium of T98G glioblastoma cells under cytokine treatment. Peptides 27, 3003–3006 [DOI] [PubMed] [Google Scholar]
- 95. Thaete L. G., Jilling T., Synowiec S., Khan S., Neerhof M. G. (2007) Expression of endothelin 1 and its receptors in the hypoxic pregnant rat. Biol. Reprod. 77, 526–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Cernacek P., Stewart D. J., Monge J. C., Rouleau J. L. (2003) The endothelin system and its role in acute myocardial infarction. Can. J. Physiol. Pharmacol. 81, 598–606 [DOI] [PubMed] [Google Scholar]
- 97. Belaidi E., Joyeux-Faure M., Ribuot C., Launois S. H., Levy P., Godin-Ribuot D. (2009) Major role for hypoxia inducible factor-1 and the endothelin system in promoting myocardial infarction and hypertension in an animal model of obstructive sleep apnea. J. Am. Coll. Cardiol. 53, 1309–1317 [DOI] [PubMed] [Google Scholar]
- 98. Phillips B. G., Narkiewicz K., Pesek C. A., Haynes W. G., Dyken M. E., Somers V. K. (1999) Effects of obstructive sleep apnea on endothelin-1 and blood pressure. J. Hypertens. 17, 61–66 [DOI] [PubMed] [Google Scholar]
- 99. Spirig R., Potapova I., Shaw-Boden J., Tsui J., Rieben R., Shaw S. G. (2009) TLR2 and TLR4 agonists induce production of the vasoactive peptide endothelin-1 by human dendritic cells. Mol. Immunol. 46, 3178–3182 [DOI] [PubMed] [Google Scholar]
- 100. Patel N., Gonsalves C. S., Malik P., Kalra V. K. (2008) Placenta growth factor augments endothelin-1 and endothelin-B receptor expression via hypoxia-inducible factor-1 alpha. Blood 112, 856–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yeligar S., Tsukamoto H., Kalra V. K. (2009) Ethanol-induced expression of ET-1 and ET-BR in liver sinusoidal endothelial cells and human endothelial cells involves hypoxia-inducible factor-1alpha and microrNA-199. J. Immunol. 183, 5232–5243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wilson D. B., Dorfman D. M., Orkin S. H. (1990) A nonerythroid GATA-binding protein is required for function of the human preproendothelin-1 promoter in endothelial cells. Mol. Cell. Biol. 10, 4854–4862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Brewer A., Pizzey J. (2006) GATA factors in vertebrate heart development and disease. Expert Rev. Mol. Med. 8, 1–20 [DOI] [PubMed] [Google Scholar]
- 104. Viger R. S., Guittot S. M., Anttonen M., Wilson D. B., Heikinheimo M. (2008) Role of the GATA family of transcription factors in endocrine development, function, and disease. Mol. Endocrinol. 22, 781–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lee M. E., Temizer D. H., Clifford J. A., Quertermous T. (1991) Cloning of the GATA-binding protein that regulates endothelin-1 gene expression in endothelial cells. J. Biol. Chem. 266, 16188–16192 [PubMed] [Google Scholar]
- 106. Dorfman D. M., Wilson D. B., Bruns G. A., Orkin S. H. (1992) Human transcription factor GATA-2. Evidence for regulation of preproendothelin-1 gene expression in endothelial cells. J. Biol. Chem. 267, 1279–1285 [PubMed] [Google Scholar]
- 107. Glenn D. J., Rahmutula D., Nishimoto M., Liang F., Gardner D. G. (2009) Atrial natriuretic peptide suppresses endothelin gene expression and proliferation in cardiac fibroblasts through a GATA4-dependent mechanism. Cardiovasc. Res. 84, 209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Nemer G., Nemer M. (2002) Cooperative interaction between GATA5 and NF-ATc regulates endothelial-endocardial differentiation of cardiogenic cells. Development 129, 4045–4055 [DOI] [PubMed] [Google Scholar]
- 109. Charron F., Tsimiklis G., Arcand M., Robitaille L., Liang Q., Molkentin J. D., Meloche S., Nemer M. (2001) Tissue-specific GATA factors are transcriptional effectors of the small GTPase RhoA. Genes Dev. 15, 2702–2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kuwahara-Watanabe K., Hidai C., Ikeda H., Aoka Y., Ichikawa K., Iguchi N., Okada-Ohno M., Yokota J., Kasanuki H., Kawana M. (2005) Heparin regulates transcription of endothelin-1 gene in endothelial cells. J. Vasc. Res. 42, 183–189 [DOI] [PubMed] [Google Scholar]
- 111. Yokota J., Kawana M., Hidai C., Aoka Y., Ichikawa K., Iguchi N., Okada M., Kasanuki H. (2001) Retinoic acid suppresses endothelin-1 gene expression at the transcription level in endothelial cells. Atherosclerosis 159, 491–496 [DOI] [PubMed] [Google Scholar]
- 112. Vieux-Rochas M., Coen L., Sato T., Kurihara Y., Gitton Y., Barbieri O., Le Blay K., Merlo G., Ekker M., Kurihara H., Janvier P., Levi G. (2007) Molecular dynamics of retinoic acid-induced craniofacial malformations: implications for the origin of gnathostome jaws. PLoS One 2, e510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zhang Z., Xu Y., Li L., Han J., Zheng L., Liu P., Li Y. (2006) Prevention of retinoic acid-induced early craniofacial abnormalities by folinic acid and expression of endothelin-1/dHAND in the branchial arches in mouse. Br. J. Nutr. 96, 418–425 [PubMed] [Google Scholar]
- 114. Ohta K., Hirata Y., Imai T., Kanno K., Emori T., Shichiri M., Marumo F. (1990) Cytokine-induced release of endothelin-1 from porcine renal epithelial cell line. Biochem. Biophys. Res. Commun. 169, 578–584 [DOI] [PubMed] [Google Scholar]
- 115. Kurihara H., Yoshizumi M., Sugiyama T., Takaku F., Yanagisawa M., Masaki T., Hamaoki M., Kato H., Yazaki Y. (1989) Transforming growth factor-beta stimulates the expression of endothelin mRNA by vascular endothelial cells. Biochem. Biophys. Res. Commun. 159, 1435–1440 [DOI] [PubMed] [Google Scholar]
- 116. Schnermann J. B., Zhu X. L., Shu X., Yang T., Huang Y. G., Kretzler M., Briggs J. P. (1996) Regulation of endothelin production and secretion in cultured collecting duct cells by endogenous transforming growth factor-beta. Endocrinology 137, 5000–5008 [DOI] [PubMed] [Google Scholar]
- 117. Castanares C., Redondo-Horcajo M., Magan-Marchal N., ten Dijke P., Lamas S., Rodriguez-Pascual F. (2007) Signaling by ALK5 mediates TGF-beta-induced ET-1 expression in endothelial cells: a role for migration and proliferation. J. Cell Sci. 120, 1256–1266 [DOI] [PubMed] [Google Scholar]
- 118. Wolf S. C., Schultze M., Risler T., Rieg T., Lang F., Schulze-Osthoff K., Brehm B. R. (2006) Stimulation of serum- and glucocorticoid-regulated kinase-1 gene expression by endothelin-1. Biochem. Pharmacol. 71, 1175–1183 [DOI] [PubMed] [Google Scholar]
- 119. Doi T., Sakoda T., Akagami T., Naka T., Mori Y., Tsujino T., Masuyama T., Ohyanagi M. (2008) Aldosterone induces interleukin-18 through endothelin-1, angiotensin II, Rho/Rho-kinase, and PPARs in cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 295, H1279–H1287 [DOI] [PubMed] [Google Scholar]
- 120. Gumz M. L., Popp M. P., Wingo C. S., Cain B. D. (2003) Early transcriptional effects of aldosterone in a mouse inner medullary collecting duct cell line. Am. J. Physiol. Renal Physiol. 285, F664–F673 [DOI] [PubMed] [Google Scholar]
- 121. Shichiri M., Adachi S., Sedivy J. M., Marumo F., Hirata Y. (1997) Biphasic regulation of the preproendothelin-1 gene by c-myc. Endocrinology 138, 4584–4590 [DOI] [PubMed] [Google Scholar]
- 122. Quehenberger P., Bierhaus A., Fasching P., Muellner C., Klevesath M., Hong M., Stier G., Sattler M., Schleicher E., Speiser W., Nawroth P. P. (2000) Endothelin 1 transcription is controlled by nuclear factor-κB in AGE-stimulated cultured endothelial cells. Diabetes 49, 1561–1570 [DOI] [PubMed] [Google Scholar]
- 123. Wort S. J., Ito M., Chou P. C., Mc Master S. K., Badiger R., Jazrawi E., de Souza P., Evans T. W., Mitchell J. A., Pinhu L., Ito K., Adcock I. M. (2009) Synergistic induction of endothelin-1 by tumor necrosis factor alpha and interferon gamma is due to enhanced NF-κB binding and histone acetylation at specific κB sites. J. Biol. Chem. 284, 24297–24305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Chen S., Feng B., George B., Chakrabarti R., Chen M., Chakrabarti S. (2010) Transcriptional co-activator p300 regulates glucose induced gene expression in the endothelial cells. Am. J. Physiol. Endocrinol Metab 298, E127–E137 [DOI] [PubMed] [Google Scholar]
- 125. Terragni J., Graham J. R., Adams K. W., Schaffer M. E., Tullai J. W., Cooper G. M. (2008) Phosphatidylinositol 3-kinase signaling in proliferating cells maintains an anti-apoptotic transcriptional program mediated by inhibition of FOXO and non-canonical activation of NFκB transcription factors. BMC Cell Biol. 9, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Park J. Y., Kim Y. M., Song H. S., Park K. Y., Kim M. S., Pak Y. K., Lee I. K., Lee J. D., Park S. J., Lee K. U. (2003) Oleic acid induces endothelin-1 expression through activation of protein kinase C and NF-κB. Biochem. Biophys. Res. Commun. 303, 891–895 [DOI] [PubMed] [Google Scholar]
- 127. Boesen E. I., Sasser J. M., Saleh M. A., Potter W. A., Woods M., Warner T. D., Pollock J. S., Pollock D. (2008) Interleukin-1β, but not interleukin-6, enhances renal and systemic endothelin production in vivo. Am. J. Physiol. Renal Physiol. 295, F446–F453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Strait K. A., Stricklett P. K., Kohan J. L., Miller M. B., Kohan D. E. (2007) Calcium regulation of endothelin-1 synthesis in rat inner medullary collecting duct. Am. J. Physiol. Renal Physiol. 293, F601–F606 [DOI] [PubMed] [Google Scholar]
- 129. Malek A. M., Greene A. L., Izumo S. (1993) Regulation of endothelin 1 gene by fluid shear stress is transcriptionally mediated and independent of protein kinase C and cAMP. Proc. Natl. Acad. Sci. U. S. A. 90, 5999–6003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Cheng T. H., Shih N. L., Chen S. Y., Loh S. H., Cheng P. Y., Tsai C. S., Liu S. H., Wang D. L., Chen J. J. (2001) Reactive oxygen species mediate cyclic strain-induced endothelin-1 gene expression via Ras/Raf/extracellular signal-regulated kinase pathway in endothelial cells. J. Mol. Cell. Cardiol. 33, 1805–1814 [DOI] [PubMed] [Google Scholar]
- 131. Dschietzig T., Richter C., Bartsch C., Bohme C., Heinze D., Ott F., Zartnack F., Baumann G., Stangl K. (2001) Flow-induced pressure differentially regulates endothelin-1, urotensin II, adrenomedullin, and relaxin in pulmonary vascular endothelium. Biochem. Biophys. Res. Commun. 289, 245–251 [DOI] [PubMed] [Google Scholar]
- 132. Kohan D. E. (2006) The renal medullary endothelin system in control of sodium and water excretion and systemic blood pressure. Curr. Opin. Nephrol. Hypertens. 15, 34–40 [DOI] [PubMed] [Google Scholar]
- 133. Wang D. L., Wung B. S., Peng Y. C., Wang J. J. (1995) Mechanical strain increases endothelin-1 gene expression via protein kinase C pathway in human endothelial cells. J. Cell. Physiol. 163, 400–406 [DOI] [PubMed] [Google Scholar]
- 134. Migas I., Bäcker A., Meyer-Lehnert H., Kramer H. J. (1995) Endothelin synthesis by porcine inner medullary collecting duct cells. Effects of hormonal and osmotic stimuli. Am. J. Hypertens. 8, 748–752 [DOI] [PubMed] [Google Scholar]
- 135. Tsai Y. H., Ohkita M., Gariepy C. E. (2006) Chronic high-sodium diet increases aortic wall endothelin-1 expression in a blood pressure-independent fashion in rats. Exp. Biol. Med. (Maywood) 231, 813–817 [PubMed] [Google Scholar]
- 136. Herrera M., Garvin J. L. (2005) A high-salt diet stimulates thick ascending limb eNOS expression by raising medullary osmolality and increasing release of endothelin-1. Am. J. Physiol. Renal Physiol. 288, F58–F64 [DOI] [PubMed] [Google Scholar]
- 137. Stroud J. C., Lopez-Rodriguez C., Rao A., Chen L. (2002) Structure of a TonEBP-DNA complex reveals DNA encircled by a transcription factor. Nat. Struct. Biol. 9, 90–94 [DOI] [PubMed] [Google Scholar]
- 138. Celil Aydemir A. B., Minematsu H., Gardner T. R., Kim K. O., Ahn J. M., Lee F. Y. Nuclear factor of activated T cells mediates fluid shear stress- and tensile strain-induced Cox2 in human and murine bone cells. Bone 46, 167–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Bu X., Quertermous T. (1997) Identification of an endothelial cell-specific regulatory region in the murine endothelin-1 gene. J. Biol. Chem. 272, 32613–32622 [DOI] [PubMed] [Google Scholar]
- 140. Vallender T. W., Lahn B. T. (2006) Localized methylation in the key regulator gene endothelin-1 is associated with cell type-specific transcriptional silencing. FEBS Lett. 580, 4560–4566 [DOI] [PubMed] [Google Scholar]
- 141. Dickson J., Gowher H., Strogantsev R., Gaszner M., Hair A., Felsenfeld G., West A. G. VEZF1 elements mediate protection from DNA methylation. PLoS Genet. 6, e1000804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Gowher H., Stuhlmann H., Felsenfeld G. (2008) Vezf1 regulates genomic DNA methylation through its effects on expression of DNA methyltransferase Dnmt3b. Genes Dev. 22, 2075–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Bulow R., Fitzner B., Sparmann G., Emmrich J., Liebe S., Jaster R. (2007) Antifibrogenic effects of histone deacetylase inhibitors on pancreatic stellate cells. Biochem. Pharmacol. 74, 1747–1757 [DOI] [PubMed] [Google Scholar]
- 144. Mawji I. A., Robb G. B., Tai S. C., Marsden P. A. (2004) Role of the 3′-untranslated region of human endothelin-1 in vascular endothelial cells. Contribution to transcript lability and the cellular heat shock response. J. Biol. Chem. 279, 8655–8667 [DOI] [PubMed] [Google Scholar]
- 145. Rodriguez-Pascual F., Redondo-Horcajo M., Magan-Marchal N., Lagares D., Martinez-Ruiz A., Kleinert H., Lamas S. (2008) Glyceraldehyde-3-phosphate dehydrogenase regulates endothelin-1 expression by a novel, redox-sensitive mechanism involving mRNA stability. Mol. Cell. Biol. 28, 7139–7155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Golden C. L., Nick H. S., Visner G. A. (1998) Thrombin regulation of endothelin-1 gene in isolated human pulmonary endothelial cells. Am. J. Physiol. Lung Physiol. 274, L854–L863 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.