Abstract
The paradoxical appearance of aggregated α-synuclein (αsyn) in naive transplanted embryonic stem cells in Parkinson's disease (PD) brains has recently been reported, highlighting the possibility of neuron to neuron transmission of αsyn in PD. Here, we demonstrate in a cellular model the presence of αsyn oligomers in the extracellular space, their uptake by neurons, retrograde axonal transport to cell soma, and detrimental effects on neighboring cells. Moreover, we demonstrate that Hsp70 chaperones αsyn in the extracellular space and reduces extracellular αsyn oligomer formation and related toxicity. These novel findings provide evidence that extracellular αsyn oligomers may represent a crucial player in the propagation of pathology in PD, with their modulation by Hsp70 representing a potential new target for therapeutic interventions. —Danzer, K. M., Ruf, W. P., Putcha, P., Joyner, D., Hashimoto, T., Glabe, C., Hyman, B. T., McLean, P. J. Heat-shock protein 70 modulates toxic extracellular α-synuclein oligomers and rescues trans-synaptic toxicity.
Keywords: Parkinson's disease, aggregation, microfluidic culture system, protein complementation
Aggregation of α-synuclein (αsyn) and related cytotoxicity play a central role in the development of Parkinson's disease (PD), dementia with Lewy bodies (LBs), multiple system atrophy, and several other neurodegenerative diseases referred to as α-synucleinopathies. Recent evidence implicates oligomeric and prefibrillar forms of αsyn, rather than mature fibrils, as the pathogenic species in PD (1–4). Because αsyn inclusion body pathology associated with PD occurs in a hierarchical distribution, with its epicenter in the brainstem, then extending to the mesolimbic cortex and associated areas (5), Braak et al. (6) have suggested that αsyn pathology spreads gradually throughout the neuraxis as PD progresses. However, the underlying mechanisms of disease progression in PD remain to be determined. Recent studies showing that grafted healthy neurons gradually develop the same pathology as the host neurons in PD brains (7, 8) have highlighted the possibility that a seeding-nucleation mechanism may exist. The presence of LBs in neurons that were transplanted years previously, but not in recently transplanted neurons, suggests that the pathology arises as a consequence of factors inherent to the PD brain. We have previously shown that recombinant αsyn oligomers are taken up by neurons in culture and trigger cell death (9, 10). Furthermore, Desplats et al. (11) recently demonstrated that αsyn can be directly transmitted from neuronal cells overexpressing αsyn to transplanted embryonic stem cells both in tissue culture and in transgenic animals, raising the possibility that a prion-like mechanism could be responsible for the host-to-graft transfer of PD pathology (12). These data raise the possibility that a specific conformation of αsyn is transmitted from host cells that promotes aggregation of αsyn and triggers toxicity in adjacent neurons. In vitro studies have shown that αsyn is secreted by living neurons and enters the surrounding medium, consistent with the fact that measureable quantities of αsyn are detected in CSF and plasma (13–15) of patients with PD, but the precise mechanism of secretion is debated (15), and the species released is unknown. Here, we identify that oligomeric αsyn species are present in the extracellular space, and transmitted as a nidus of misfolded protein to neighboring neurons, mediating αsyn toxicity. Moreover, we show that extracellular αsyn oligomerization is precisely modulated by heat-shock protein 70 (Hsp70), which therefore may represent a new target for therapeutic strategies to halt PD progression.
MATERIALS AND METHODS
Plasmid generation
Fusion constructs αsyn-hGLuc1 (S1), αsyn-hGLuc2 (S2), Venus1-αsyn (V1S), and αsyn-Venus2 (SV2) were generated by subcloning αsyn into NotI/ClaI sites of humanized Gaussia luciferase and VenusYFP constructs provided by Dr. Stephen Michnick (University of Montreal, Montreal, QC, Canada; ref. 16). The Hsp70 plasmid in this study has been described previously (17).
Adeno-associated virus (AAV) vector construction and production
The viral vectors rAAV-CBA-IRES-EGFP and rAAV-CBA-SYNUCLEIN-IRES-EGFP were described previously (18). rAAV-CBA-SYNUCLEIN-LUC1-WPRE (S1) and rAAV-CBA-SYNUCLEIN-LUC2-WPRE (S2) were constructed as follows: syn-hGLuc1 (S1) and syn-hGLuc2 (S2) were constructed by subcloning αsyn into the NotI/NheI sites of the AAV-CBA-WPRE vector (18). rAAV-CBA-VENUS1-SYNUCLEIN-WPRE (V1S) and rAAV-CBA-SYNUCLEIN-VENUS2-WPRE (SV2) were constructed as follows: the fragments Venus1-Synuclein and Synuclein-Venus2 were inserted into the EcoRV and NheI sites of the pAAV-CBA-WPRE vector (18). Recombinant virus AAV type 8 was generated by tripartite transfection (AAV-rep/cap expression plasmid, adenovirus miniplasmid, and AAV vector plasmid) into 293A cells and purified by iodixanol gradient, followed by Q sepharose column chromatography (Harvard Gene Therapy Initiative, Harvard Medical School). The purified virus was dialyzed against PBS, concentrated by Amicon spin column (Millipore, Billerica, MA, USA), and titered by dot-blot hybridization. Final titers for virus were as follows: S1, 1.5E13 genome copies (gc)/ml; S2, 1.3E13 gc/ml; V1S, 8.3E12 gc/ml; and SV2, 8.7E12 gc/ml.
Human αsyn ELISA
αSyn concentration was quantified using ELISA (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Briefly, a monoclonal antibody specific for αsyn was coated onto the wells, and αsyn bound simultaneously to the immobilized monoclonal (capture) antibody and to the solution phase rabbit polyclonal (detection) antibody specific for αsyn. After washing, a horseradish peroxidase-labeled anti-rabbit IgG (anti-rabbit IgG-HRP) was added, which bound to the detection antibody to complete the 4-member sandwich. After incubation and washing, substrate solution was added. Absorbance was read at 450 nm. The absorbance was directly proportional to the concentration of αsyn present in the original specimen. The αsyn concentration was determined by plotting sample absorbances against standards using GraphPad Prism fitting software (4-parameter algorithm; GraphPad, San Diego, CA, USA).
Human Hsp70 ELISA
Hsp70 ELISA quantitative sandwich immunoassay (Stressgen, Ann Arbor, MI, USA) was performed according to the manufacturer's manual. In brief, a mouse monoclonal antibody specific for inducible Hsp70 was precoated on the wells of the provided Hsp70 immunoassay plate. Inducible Hsp70 was captured by the immobilized monoclonal antibody and detected with an Hsp70-specific rabbit polyclonal antibody. The rabbit polyclonal antibody was subsequently bound by a horseradish peroxidase-conjugated anti-rabbit IgG secondary antibody. The assay was developed with tetramethylbenzidine (TMB) substrate, and a blue color developed in proportion to the amount of captured Hsp70. The color development was stopped with acid stop solution, which converts the end-point color to yellow. The intensity of the color was measured in a microplate reader at 450 nm. Hsp70 concentrations from the sample were quantitated by interpolating absorbance readings from a standard curve generated with the calibrated Hsp70 protein standard provided. Hsp70 concentration was determined by plotting sample absorbances against standards using Graph Pad Prism fitting software (4-parameter algorithm).
Cell culture and transfections
Unless otherwise stated, human H4 neuroglioma cells (HTB-148; American Type Culture Collection, Manassas, VA, USA) were maintained in Opti-MEM medium supplemented with 10% fetal bovine serum (both from Invitrogen) and incubated at 37°C. Cells were plated 24 h prior to transfection, growing to 80–90% confluency prior to transfection. Transfection was performed using Superfect (Qiagen, Chatsworth, CA, USA) using equimolar ratios of plasmids according to the manufacturer's instructions. For conditioned medium (CM) experiments, medium was collected 48 h post-transfection and centrifuged for 5 min at 3000 g to eliminate floating cells before being used.
Gaussia luciferase protein-fragment complementation assay
Fusion constructs S1 and S2 were generated as described previously (19). S1 and S2 were transfected into H4 cells in a 96-well plate format as described above. At 48 h after transfection, culture medium was transferred to a new 96-well plate (Costar; Corning, Corning, NY, USA). Cells were washed with PBS and replaced with serum- and phenol-red free medium. Luciferase activity from protein complementation was measured for CM and live cells in an automated plate reader at 480 nm following the injection of the cell permeable substrate, coelenterazine (20 μM; Prolume Ltd, Pinetop, AZ, USA) with a signal integration time of 2 s.
β-Galactosidase (β-gal) enzyme assay
At 48 h post-transfection, H4 cells were washed with magnesium and calcium-free phosphate buffered saline (Invitrogen). Cells were lysed using passive lysis buffer provided by Promega (Madison, WI, USA). One volume of 2× β-gal buffer (Promega) was added to lysed cells or CM and incubated for 30 min at 37°C until yellow color developed. Absorbance was read at 420 nm.
Primary cortical cell culture
Primary cortical neurons were prepared from cerebral cortices of embryonic day (E) 14–16 mouse embryos. Cortices were dissected from embryonic brain, and the meninges were removed. Cortices were dissociated by trypsinization for 5 min at room temperature, and cells were resuspended in Neurobasal (NB; Life Technologies, Inc., Gaithersburg, MD, USA) medium supplemented with 10% fetal bovine serum, 2 mM Glutamax, 100 U/ml penicillin, and 100 μg/ml streptomycin; centrifuged at 100 g for 10 min; and resuspended in NB/B-27 [NB medium containing 2% (v/v) B-27 supplement], 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM glutamine and plated at a density of 1.25 × 105 cells/cm2 on 96-well plates (Corning), 6-well plates (Costar) coated with 20 μg/ml poly-d-lysine (Sigma-Aldrich, St. Louis, MO, USA), or in microfluidic devices (see below). Cells were maintained at 37°C in 5% CO2 in a humidified incubator. Medium was changed every third day. Neurons were grown for 6–7 days in vitro (DIV) and transiently transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol.
Separation of monomeric and oligomeric αsyn using size-exclusion chromatography (SEC)
SEC was performed as described previously (20). Briefly, 500 μl of CM from H4 cells cotransfected with S1 or S2 with or without Hsp70 was injected to a Superose 6 10/300 GL gel-filtration column (GE Healthcare, Piscataway, NJ, USA). The sample was eluted from the column in 1× PBS at a flow rate of 0.5 ml/min. The eluate was monitored at 215–280 nm and fractionated into a 96-well plate with 200-μl fractionation volume. After SEC, 100 μl of each fraction was analyzed for luciferase activity using the Gaussia luciferase protein-fragment complementation assay, as described above.
Ex vivo aggregation of αsyn
At 48 h post-transfection, CM from S1-transfected H4 cells and CM from S2-transfected H4 cells was collected as described above. S1 and S2 CM were mixed in a 1:1 ratio and incubated on a rocking shaker at 100 rpm (Model 55; Reliable Scientific Inc., Nesbit, MS, USA). Aliquots of incubated samples were removed at indicated time points and tested for luciferase activity as described above. CM from S1/S2-cotransfected cells was also collected as described above and incubated on a rocking shaker at 100 rpm. Aliquots were removed and tested for luciferase activity. Coaggregation of α-syn CM and Aβ CM was performed in the same way as described above and incubated on a rocking shaker at 100 rpm for 3 d. Thereafter, luciferase activity was determined.
Dot blot
CM was collected as described previously. A sample of each condition (700 μl each) was applied to nitrocellulose membrane (pore size 0.22 μm; Protran; Whatman, Sanford, ME, USA) placed in a dot-blot apparatus (Schleicher & Schuell Minifold-I Dot-Blot System; Whatman). Samples were filtered through the membrane by gentle vacuum and developed using conditions as described previously (21). Briefly, the membrane was blocked with 10% nonfat dried milk in Tris-buffered saline (TBS; Sigma-Aldrich) containing 0.01% Tween 20 (TBS-T), at room temperature for 1 h. After 3 washes with TBS-T, the membrane was incubated with anti-oligomer antibody A11 (Invitrogen) or Syn-1 antibody (1:1000; BD Transduction, Franklin Lakes, NJ, USA) overnight at 4°C with gentle agitation. The membranes were then washed 3 times for 5 min with TBS-T, incubated with horseradish peroxidase-conjugated anti-rabbit IgG or anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) diluted 1:2000 in 5% nonfat dried milk in TBS-T, and incubated for 1 h at room temperature. The blots were washed 3 times with TBS-T and developed with Pierce ECL chemiluminescence kit (Thermo Scientific, Rockford, IL, USA).
Nanochamber devices
Microfluidic devices were adapted from Taylor et al. (22) and manufactured in-house. The devices contain 2 chambers that are connected by 300- or 600-μm channels that are 3 μm high and 8 μm wide. Initial experiments used 300-μm channels, but we found that 1000-μm channels were more convenient, as they allowed visualization of a longer section of axon. The device is attached to a poly-d-lysine-coated coverslip (MatTek Cultureware, Ashland, MA, USA), to which the cells adhere. This allows the growth of axons through the channels and ensures that only 1 or 2 axons propagate through each channel. The microfluidic devices were loaded with mouse primary cortical neurons isolated from E14 mouse embryos that were trypsinized and dissociated and placed in the chamber at an approximate density of 1 × 105 cells/device. After 7 DIV, neurons were infected with rAAV2/2 expressing either eGFP, syn-ires-GFP, V1S, or SV2. Infections were carried out as follows: 0.3 μl/device of rAAV2/8 expressing either eGFP (1.3E13 gc/ml), syn(wt)-ires-GFP (1.1E13 gc/ml), or V1S (8.3E12 gc/ml) together with 0.3 μl/device of rAAV2/8 expressing SV2 (8.7E12 gc/ml). Neurons infected with rAAV2/8 expressing eGFP, syn-ires-GFP, V1S, or SV2 were examined 3 d after infection using a Zeiss Axiovert 200 inverted microscope (Carl Zeiss, Jena, Germany) encased in a 37°C, 5% CO2 environmental chamber equipped with a Zeiss LSM 510 Meta confocal scanhead using 488-nm lasers. All images were acquired using a ×20 Plan Apochromat lens, ×25 APO-Plan Neoflu lens, or ×63 1.2 NA C-APO-Plan Neoflu water-immersion lens (Carl Zeiss) mounted on the microscope described before. Somal and axonal CM from infected neurons were also collected after 3 d expression of viral constructs, and αsyn was quantitated using ELISA.
Immunoabsorption of αSyn from CM
Culture medium from V1S/SV2-infected primary neurons (14 DIV) or S1/S2-, wild-type (wt) αsyn-, or mock-transfected human H4 cells was collected 48 h post-transfection. Medium was centrifuged for 5 min at 3000 g to eliminate floating cells and cellular debris. Supernatants (1 ml) from each condition were precleared with 50 μl of protein G Sepharose (Sigma) for 1 h at 4°C with gentle shaking and then centrifuged at 15,000 g at 4°C for 10 min. Supernatants were incubated with 5 μg of Syn-1 antibody (BD Transduction). After an overnight incubation at 4°C, protein G Sepharose (100 μl) was added and incubated overnight at 4°C with gentle shaking. Sepharose beads were removed by centrifugation (15,000 g for 10 min at 4°C), and supernatant was used for experiments.
αSyn uptake experiments
The axonal medium of 7-DIV primary cortical neurons with visible axons transversing the microgrooves was replaced with CM from H4 cells transfected with S1/S2 or nontransfected culture medium and supplemented with 2% (v/v) B-27 supplement. After 3–4 d incubation of axonal terminals with luciferase-positive CM, neuronal cell bodies were washed and lysed with 1× PBS. Lysate was then transferred to a 96-well plate and analyzed for luciferase activity.
Coculture experiments
Neurons (7 DIV) cultured in microfluidic devices were infected with rAAV2/8 expressing V1S. Human H4 cells were plated in a 35-mm dish (Costar) 24 h before infection with rAAV2/8 expressing SV2. At 14 h postinfection, H4 cells were washed, trypsinized, and transplanted to the axonal terminal side of the microfluidic device containing V1S-infected neurons. H4 cells and primary cortical neurons were cocultured for 5 d until green fluorescence of reconstituted VenusYFP was visible in both neurons and H4 cells. All images were acquired using a ×20 Plan Apochromat lens, ×25 APO-Plan Neoflu lens, or 63× 1.2 NA C-APO-Plan Neoflu water-immersion lens, mounted on the microscope described before.
Neuron to neuron transmission experiments
For uptake experiments 7-DIV neurons were cultured in poly-d-coated 6-well plates (Costar) and treated with CM from primary neurons transfected with S1/S2. Treatment was carried out by replacing 50% of the NB medium with oligomer-containing CM from transfected neurons. After 4 d incubation, cells were washed and lysed with 1× PBS using a 27.5-gauge needle. Lysates were then analyzed for luciferase activity as described above.
Apo-ONE homogeneous caspase-3/7 assay
To investigate whether CM from H4 cells transfected with S1/S2, wt αsyn, or mock transfectant had toxic properties, CM was collected as described previously and added to naive H4 cells seeded 24 h before in a 96-well plate. Medium of naive H4 cells was completely replaced by CM from different transfection conditions. After 3 d incubation, Apo-ONE homogeneous caspase-3/7 assay (Promega) was performed according to manufacturer's protocol.
Pharmacological treatments in vitro
H4 cells were plated into 96-well plates (Costar) and transfected as described above. Transfection mix was incubated for 2 h according to the manufacturer's protocol, then medium was replaced by fresh culture medium containing 1 μM 17-(allylamino)-17-demethoxygeldanamycin (17-AAG; Sigma-Aldrich) or an appropriate amount of DMSO.
CellTiter-Glo luminescent cell viability assay
Neurons (7 DIV) were infected using either complementation pair rAAV2/8 encoding S1 and S2 alone or together with rAAV2/2 expressing Hsp70 (2.5E10, 3.25E10, and 1.2E9 infectious units/6-well plate, respectively). rAAV2/8 eGFP (9.75E10 infectious units) was used as control. After expression for 4 d, CM was collected as described above and transferred to 7-DIV naive neurons plated on poly-d-coated 6-well or 96-well plates (Costar). Fifty percent of the medium of naive neurons was replaced by CM from different infection conditions. After incubation for 5 d, CellTiter-Glo luminescent cell viability assay (Promega) was performed according to manufacturer's protocol.
Uptake of recombinant αsyn oligomers
Neurons (7 DIV) cultured in microfluidic devices were treated at the axonal terminal side with 1 μM (moles of monomer) Alexa 488-labeled recombinant oligomer type C (described previously in ref. 9) dissolved in neuronal culture medium. After incubation for 3–4 d, green fluorescent oligomer type C was visible in axons and neuronal cell bodies. All images were acquired using a ×20 Plan Apochromat lens, ×25 APO-Plan Neoflu lens, or 63× 1.2 NA C-APO-Plan Neoflu water-immersion lens, mounted on the microscope described before.
Statistical analysis
Statistical analyses were carried out using Prism 4.0 (GraphPad). Values in the figures are expressed as means ± sd or means ± se.
RESULTS
Oligomeric αsyn is secreted from neuronal cells
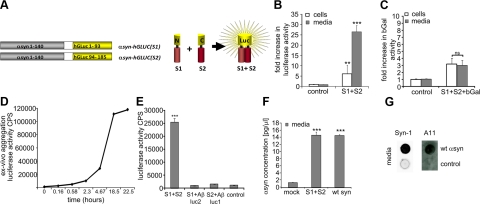
It has been previously reported that αsyn is found in CM from neuroblastoma cells and primary neurons (23). To further investigate the species of αsyn present in the extracellular space, we developed a protein-fragment complementation assay (19, 24, 25) with nonbioluminescent amino- or carboxy-terminal fragments of humanized Gaussia princeps luciferase (hGLuc) fused to αsyn (16, 26) (Fig. 1A). This highly sensitive assay can be used to monitor αsyn oligomerization in both living cells (24) and CM. Using this assay, transfection of H4 neuroglioma cells with S1 and S2 results in reconstituted luciferase activity in CM 4-fold higher than measured in intact cells (Fig. 1B), raising the possibility that oligomeric αsyn species are released from H4 cells. As a control, we used S1 or S2 plasmid alone, wt αsyn, or mock transfection to determine the background levels of the assay and normalized the results to these controls (Fig. 1B). As an additional control, we cotransfected H4 cells with S1/S2 and β-gal and detected only negligible levels of β-gal activity in CM, indicating that dying cells alone are not responsible for the presence of αsyn in the medium, and thus pointing to an active secretion process (Fig. 1C). To determine whether oligomerization of αsyn proceeds in the extracellular space, we performed an ex vivo experiment whereby CM containing S1 alone was mixed with CM containing S2 alone. Interestingly, luciferase activity reconstituted rapidly ex vivo in a time-dependent manner (Fig. 1D), suggesting that secreted αsyn is capable of further aggregation in the extracellular space. To exclude the possibility that N- or C-terminal fragments of hGLuc can reconstitute nonspecifically when coincubated, we performed a similar ex vivo experiment using CM containing Aβ fused to nonbioluminscent N-terminal fragments of hGLuc (Aβ-luc1) in combination with CM containing S2 as well as CM containing Aβ fused to C-terminal fragments of hGLuc (Aβ-luc2) in combination with CM containing S1. After 3 d incubation, neither of the combinations of αsyn and Aβ had measurable reconstituted luciferase activity compared to αsyn (Fig. 1E). These data demonstrate that S1 and S2 complementation is due to specific αsyn-αsyn interactions and is indicative of αsyn aggregation occurring also in the extracellular space.
Figure 1.
αSyn oligomers are secreted from cells. A) Principle of bioluminescent-protein complementation assay based on G. princeps luciferase, adapted from Kerppola (56) B) Intact cells and CM from H4 cells cotransfected with S1/S2 were assayed for luciferase activity 48 h post-transfection. Intact cells displayed a 6-fold increase in luciferase activity compared to control. CM had a 26-fold increase in luciferase activity vs. control. n = 4. C) β-Gal activity is not significantly (ns) increased in medium compared to cells when cotransfected with S1/S2. n = 6, P = 0.78. D) Ex vivo mixing of S1 CM with S2 CM leads to a time-dependent increase in luciferase activity. Data are representative of 3 independent experiments. E) Ex vivo aggregation is not facilitated by luciferase tags. No luciferase activity was detected when S1 CM was mixed with Aβ-luc2 CM or S2 CM was mixed with Aβ-luc1 CM for 3 d. By contrast, a significant increase in luciferase activity was detected when S1 CM was incubated with S2 CM for 3 d. n = 6. F) αSyn protein concentration in CM of S1/S2- wt αsyn- or mock-transfected cells. n = 3. G) Dot blot of CM from wt αsyn-transfected H4 cells using anti-synuclein Syn-1 or oligomer-specific antibody A11. Nontransfected medium was used as negative control. Representative blots from 4 independent experiments. Data represent means ± sd. **P < 0.01; ***P < 0.001.
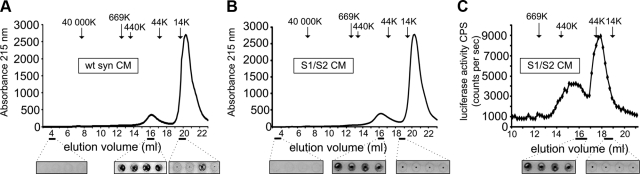
To quantify the amount of αsyn secreted from the H4 cells and to confirm that luciferase-tagged αsyn behaves the same as wt αsyn, we used a human αsyn-specific ELISA and detected 1.3, 14.5, and 14.6 pg/μl αsyn in the CM of mock-, S1/S2-, and wt αsyn-transfected cells, respectively (Fig. 1F). Additional dot-blot analysis with Syn-1 and A11 confirmed the presence of αsyn oligomers in CM for both wt αsyn-transfected cells (Fig. 1G) and S1/S2-transfected cells (Supplemental Fig. S1). To further characterize the released αsyn species, we used SEC to fractionate CM from wt αsyn and S1/S2-transfected cells. A dot-blot analysis with Syn-1 antibody was performed on each of the SEC fractions to confirm the presence of αsyn. Both CM from wt αsyn-transfected cells and CM from S1/S2-transfected cells showed a similar SEC profile with αsyn present in fractions ∼14.5 to 22 and ∼12.5 to 21 ml for wt αsyn and S1/S2 CM, respectively (Fig. 2A, B). These elution profiles are consistent when comparing luciferase-tagged S1/S2 to untagged wt αsyn.
Figure 2.
SEC from CM containing αsyn. A) CM from wt αsyn-transfected H4 cells was loaded onto a Superose 6 column. Molecular mass was estimated using standard samples: thyroglobulin (669 kDa), ferritin (440 kDa), ovalbumin (44 kDa), and ribonuclease (14 kDa). B) CM from S1/S2-transfected H4 cells was loaded onto a Superose 6 column. C) Luciferase activity in SEC fractions from S1/S2 CM. All fractions were analyzed using a dot-blot approach with anti-synuclein Syn-1 antibody. SEC profiles and dot-blot analyses are representative from 3 independent experiments.
In addition, we took advantage of the luciferase complementation assay and performed a luciferase assay on all fractions from S1/S2 CM to detect oligomeric αsyn. We detected a heterogeneous population of oligomeric αsyn in CM of S1/S2-transfected cells secreted from cells ranging from ∼30mers to dimers (13.5 to 19 ml; Fig. 2C). As before, dot-blot analysis was used to confirm αsyn in those fractions. Taken together, 3 independent biophysical assays demonstrate that αsyn in an oligomeric form is secreted from cells.
αSyn is released from axonal terminals
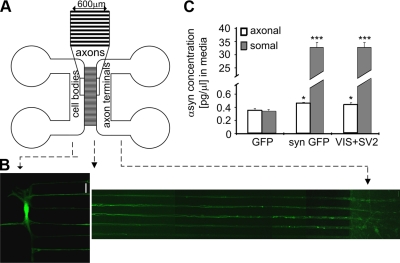
To pinpoint the location of αsyn release and to gain mechanistic insight into αsyn release, we used a novel compartmentalized microfluidic culture system (22, 27–29) that contains chambers for culturing neurons such that the cell bodies are fluidically isolated from their axonal terminals, which transverse microgrooves between the chambers (Fig. 3A). To investigate whether αsyn is secreted from axonal terminals, we took advantage of our previously reported fluorescent protein complementation assay (19) as a means to visualize αsyn oligomers and infected primary cortical neurons cultured in the somal chamber of microfluidic devices with AAV encoding either GFP or αsyn-ires-GFP, or coinfected cortical neurons in the somal chamber with AAV encoding αsyn-VenusYFP fluorescent protein-fragment complementation pairs V1S or SV2. After 3 d, we detected reconstituted VenusYFP fluorescence, αsyn-ires-GFP, and GFP in the cell body and axonal terminal regions of the transduced microfluidic devices (Fig. 3B and Supplemental Fig. S2). Pure medium (50 μl) was removed from the axonal chamber and analyzed for αsyn content using ELISA. We detected 0.46 and 0.42 pg/μl αsyn in the axonal medium for αsyn-ires-GFP and V1S/SV2, respectively, compared to significantly lower amounts (0.35 pg/μl) in GFP medium, confirming an increase in αsyn in the axonal chamber following overexpression and suggesting that αsyn is released from the small number of axons that reach the axonal terminal chamber (Fig. 3C). A high amount of αsyn was also detected in the medium of the somal chamber, due to the presence of axonal terminals and dendrodentritic synapses in this compartment. Taken together, these findings indicate that αsyn forms oligomers in primary neurons and can be released from axonal terminals.
Figure 3.
αSyn is released from axonal terminals. A) Schematic representation of microfluidic devices used to culture primary cortical neurons. B) Primary cortical neurons in microfluidic devices (7 DIV) were coinfected with rAAV2/8 expressing V1S and SV2. After 3 d, αsyn oligomers were detected in cell bodies and axons transversing the microgrooves of the devices. Scale bar = 20 μm. C) αSyn content in CM from axonal terminal compartments of microfluidic devices infected in cell body compartments with AAV expressing GFP, αsyn-ires-GFP, or V1S/SV2 5 d previously. Data represent means ± sd; n = 3. *P < 0.05; ***P < 0.001.
αSyn oligomers are transmitted from neuron to neuron and from neurons to non-neuronal cells
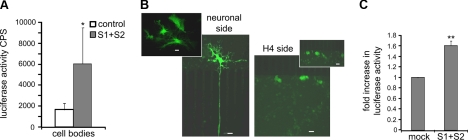
The functional significance of secreted, extracellular αsyn oligomers has yet to be explored. We and others have shown that recombinant αsyn can be taken up by cells in culture (refs. 9, 10, 30, 31 and Supplemental Fig. S3). To determine the fate of extracellular αsyn oligomers and to gain insight into the mechanism of αsyn transmission, we treated the axonal terminals of primary neurons in microfluidic devices with CM from H4 cells previously transfected with αsyn constructs S1/S2. After 3–4 d incubation, we harvested the cell bodies from S1/S2- and control-treated microfluidic devices and measured the luciferase activity indicative of αsyn oligomers in the respective fluidically isolated somal chambers. Intriguingly, we detected significantly greater luciferase activity in the cell body chamber of S1/S2 oligomer-treated devices (ranging from 2.5- to 9.5-fold) than control-treated devices (Fig. 4A). Collectively, these data provide evidence that αsyn oligomers secreted from human neuroglioma cells are taken up by the axonal terminals of mature neurons and retrogradely transported back to the cell bodies.
Figure 4.
αSyn oligomers are transmitted from neuron to neuroglioma and neuron to neuron. A) CM from H4 cells transfected with S1/S2 was added to the axonal terminal compartment of microfluidic devices containing 5-DIV primary neuronal cultures. After 5 d, axonal terminals and cell bodies were lysed and assayed for luciferase activity. Axon terminals treated with S1/S2 CM displayed increased luciferase activity in their cell bodies compared to control CM-treated axonal terminals. Data are representative of 3 independent experiments. B) H4 cells infected with AAV-V1S were transplanted, 14 h post-infection, to the axonal terminal compartment of microfluidic devices containing primary cortical neurons with cell bodies infected with AAV-SV2 14 h previously. After 4 d of coculture, VenusYFP expression was observed in primary neuronal cell bodies whose axons transversed the microgrooves to the axonal terminal compartment. In addition, VenusYFP expression was also detected in H4 cells cocultured in the axonal terminal compartment that were in proximity to the microgrooves. Scale bars = 20 μm. C) Primary cortical neurons were cotransfected with S1/S2. After 48 h, CM was removed and transferred to a naive primary cortical neuron sister culture and incubated for a further 4 d. Neurons cultured in CM from S1/S2-transfected neurons displayed significantly higher luciferase activity than neurons incubated in mock CM. Data represent means ± sd; n = 3. ** P < 0.01; 1-group Student's t test.
To further investigate the transmission of oligomeric αsyn, H4 cells were cultured independently, infected with AAV-V1S, washed twice with phosphate-buffered saline to remove residual virus, and transplanted 14 h later to the axonal terminal chamber of a microfluidic device containing primary neurons that had been infected with AAV-SV2 in the cell body chamber 14 h previously. After 4 d of coculture, robust VenusYFP expression was observed only in those neuronal cell bodies whose axons transversed the microgrooves to the axonal terminal chamber. In addition, VenusYFP expression was also detected in H4 cells cocultured in the axonal terminal chamber that were in close proximity to the microgrooves (Fig. 4B) highlighting the presence of bidirectional transmission of αsyn between neurons and non-neuronal cells. As a control, we infected neurons with V1S and SV2 alone and did not detect any significant fluorescence. To exclude the possibility that virus may diffuse through the microchannels, trypan blue was added to the medium of the axonal compartment in control experiments. No diffusion of trypan blue was observed over a period of 5 d, confirming the fluidic isolation of the axonal and somal compartments.
To avoid the possible confound of viral contamination and determine whether oligomeric αsyn is transmitted between mature neurons, we transfected primary cortical neurons with the complementation pairs S1/S2. After 48 h, CM was harvested and added to naive mature primary neurons (7 DIV) in a sister culture dish. After further 4 d incubation, S1/S2 oligomer- and mock-treated neurons were assayed for luciferase activity. Intriguingly, we found 50% more luciferase signal in neurons treated with oligomer containing medium than the background luciferase activity from mock-treated neurons to which the signal was normalized (Fig. 4C), indicating that mature neurons secreted oligomeric αsyn, which was then transmitted to, and taken up by, naive mature neurons.
Secreted αsyn oligomers induce caspase-3/7 activation
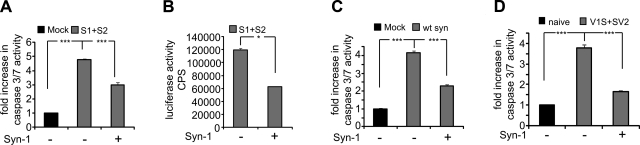
To decipher the functional consequences of αsyn transfer across membranes and determine whether secreted αsyn oligomers exert toxic effects on neighboring cells, we cultured naive H4 cells in CM derived from H4 cells transfected with S1/S2 or mock transfectant. After 4 d, naive H4 cells exposed to S1/S2 CM demonstrated increased caspase-3 and -7 activity compared to mock-transfected cells, suggesting progressive cellular degeneration. Interestingly, cells cultured with S1/S2 CM that were subjected to immunodepletion with Syn-1 antibody had a significantly less caspase-3 and -7 activity compared to nonimmunodepleted S1/S2 CM (Fig. 5A). To control for successful immunodepletion, we monitored luciferase activity in S1/S2 CM with and without immunodepletion. Immunodepletion resulted in dramatic decrease in luciferase activity, indicative of a reduction in αsyn oligomerization (Fig. 5B). To test whether CM from wt αsyn-transfected cells was also capable of initiating toxicity, we incubated naive H4 cells with wt αsyn CM and found a significant increase in caspase-3 and -7 activity compared to control, which could be ameliorated by preabsorption with Syn-1 antibody (Fig. 5C). Likewise, primary neurons incubated with CM from V1S/SV2-infected neurons also showed increased caspase-3 and -7 activity that could be reduced with preimmunodepletion (Fig. 5D). These results suggest that the uptake and transport of oligomeric αsyn from the extracellular space results in the initiation of cell death and that immunodepletion of αsyn from CM blocks this effect.
Figure 5.
Secreted αsyn oligomers are toxic to neighboring cells. A) H4 cells were transfected with S1/S2 or mock transfectant. After 48 h, CM was collected and transferred to naive H4 cells. After 3 d incubation, caspase-3/7 activity is increased in S1/S2 CM-treated cells, which can be ameliorated by immunodepletion of αsyn. n = 5. B) Reduction of luciferase activity in S1/S2 CM after immunodepletion with Syn-1 antibody. n = 3. C) H4 cells were transfected with wt αsyn or mock transfectant. After 48 h, CM was collected and transferred to naive H4 cells. After 3 d incubation, caspase-3/7 activity is increased in wt αsyn CM-treated cells, which can be ameliorated by immunodepletion of αsyn. n = 5. D) Primary neurons were infected with AAV-V1S and AAV-SV2 or left naive. After 48 h, CM was collected and transferred to naive primary neurons. After 3 d incubation, caspase-3/7 activity is increased in V1S/SV2 CM treated cells, which can be ameliorated by immunodepletion of αsyn. n = 5. Data represent means ± se. *P < 0.05, ***P < 0.0001; unpaired t test with Welch's correction.
Hsp70 inhibits extracellular oligomer formation and rescues cytotoxicity mediated by secreted αsyn oligomers
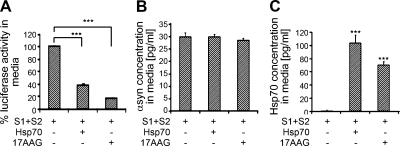
Inhibition of αsyn aggregation and related toxicity is a promising strategy for disease modifying therapeutics for PD. Bonini et al. (1) pioneered the use of molecular chaperone Hsp70 to protect Drosophila from the deleterious effects of αsyn. The same group also demonstrated that up-regulation of Hsp70 with geldanamycin could prevent the progressive degeneration of dopaminergic neurons induced by αsyn in Drosophila (32). We have previously shown that αsyn oligomerization and toxicity can be modified by the molecular chaperone Hsp70 (17, 19, 33). Here, we asked whether Hsp70 can also influence extracellular oligomer formation and/or secretion of αsyn. To ensure that Hsp70 coexpression did not change expression levels of αsyn, we quantified the amount of intracellular αsyn using αsyn ELISA. H4 cells that were cotransfected with S1/S2 and Hsp70 or treated with the Hsp90 inhibitor 17-AAG in a cotreatment paradigm had comparable levels of αsyn (Supplemental Fig. S4), consistent with our previous studies (24). Interestingly, however, when H4 cells were cotransfected with S1/S2 and Hsp70, αsyn oligomer formation in CM was reduced by 64%. Moreover, treatment with the Hsp90 inhibitor 17-AAG reduced extracellular αsyn oligomer formation by 84%, emphasizing a pharmacological relevance of up-regulating Hsp70 via inhibition of Hsp90 (Fig. 6A). By ELISA, the amount of αsyn in CM of S1/S2 with Hsp70 was the same as CM from S1/S2 without Hsp70. Likewise, levels of αsyn in CM were maintained following treatment with 17-AAG even though luciferase activity was dramatically reduced (Fig. 6B). Together, these data indicate that the observed reduction in luciferase activity is not due to reduced αsyn in the CM.
Figure 6.
Hsp70 prevents extracellular αsyn oligomer formation. A) H4 cells transfected with S1/S2 were either cotransfected with or without Hsp70 or treated with 1 μM 17-AAG or DMSO. After 48 h, luciferase activity from CM was measured. n = 3. ***P < 0.001. B) αSyn content in CM of cotransfected or treated cells was measured using ELISA. n = 5. C) Hsp70 content in CM of cotransfected or 17-AAG treated cells was measured using ELISA. n = 3. ***P < 0.0001. Data represent means ± sd.
Increasing evidence suggests that Hsp70 can be released from cells by an active mechanism that is independent from de novo Hsp70 synthesis or cell death (34–39). Moreover, it has been reported that Hsp70 can be released into the extracellular environment after stress (40) or in an exosome-dependent manner (41). Based on these observations, we used an Hsp70 ELISA to ask whether Hsp70 was also present in CM of S1/S2 + Hsp70-transfected cells or cells transfected with S1/S2 and treated with 17-AAG. Indeed, we found significantly increased levels of Hsp70 in CM from cells cotransfected with S1/S2 + Hsp 70 or treated with 17-AAG compared to cells transfected with S1/S2 alone or treated with DMSO (Fig. 6C). These data suggest that Hsp70 is released together with αsyn, providing a mechanism whereby Hsp70 can modulate αsyn oligomerization.
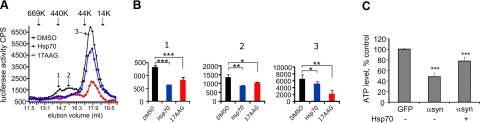
If Hsp70 is affecting extracellular oligomer formation, we hypothesized that this could be detected as a modification in released αsyn species using SEC. Indeed, treatment with 17-AAG or cotransfection with Hsp70 eliminated species found between 13.6 and 16.5 ml in SEC fractions of CM of H4 cells, and reduced the large peak representing dimeric αsyn species (Fig. 7A, B). These results suggest that up-regulation of Hsp70 changes oligomeric αsyn species in CM. Furthermore, we detected less cell death in neurons incubated with CM containing αsyn and Hsp70 compared to CM with αsyn alone (Fig. 7C).
Figure 7.
Hsp70 modifies extracellular αsyn species and rescues toxicity. A) Luciferase activity in SEC fractions from CM of S1/S2-transfected cells (black), S1/S2 + Hsp70-transfected cells (blue), or S1/S2-transfected cells treated with 17-AAG (red). Arrows indicate fractions modified by the presence of Hsp70 or treatment with 17-AAG. Data are representative of 3 independent experiments. B) Statistical analysis of fractions 14.5 ml (1), 15.5 ml (2), and 17.5 ml (3) of measured luciferase activity from CM of S1/S2-transfected cells (black), S1/S2 + Hsp70-transfected cells (blue), or S1/S2-transfected cells treated with 17AAG (red); n = 3. data represent mean ± sd. C) Primary cortical neurons were transfected with GFP, S1/S2, or S1/S2 + Hsp70. After 4 d, CM was collected and transferred to naive primary cortical neurons. After a further 5-d incubation, cytotoxicity was assessed using the cell titer glow assay, which measures ATP levels in cells. n = 4. Data represent means ± sd. *P < 0.05, **P < 0.01, ***P < 0.0001; 1-way ANOVA, Bonferroni's multiple comparison test.
DISCUSSION
In this study, we have shown that oligomeric αsyn is present in the extracellular space. Moreover, using a novel microfluidic culture system, we were able to demonstrate that αsyn can be transsynaptically transmitted from cell to cell. Because available assays have limitations regarding the determination of specific oligomeric species, we employed an innovative protein complementation assay that allows the detailed investigation of oligomeric αsyn specifically. The present study provides evidence that αsyn in an oligomeric form is present in the extracellular space, is taken up by neighboring cells, and has detrimental and toxic consequences. Moreover, the molecular chaperone Hsp70 appears to chaperone αsyn in the extracellular space and prevent extracellular oligomer formation. Finally, we demonstrate that Hsp70's rescue of oligomer-induced toxicity is accompanied by a concomitant modification of the αsyn oligomeric species in the extracellular space.
The emergence of αsyn-positive LB-like structures in mesencephalic transplants in PD patients (7, 8) has resulted in intense speculation of mechanisms involved. It has been speculated that a prion-like mechanism may be responsible for the transfer of PD pathology from host to graft and that misfolded αsyn in host cells might seed misfolding and aggregation of the protein in adjacent grafted neurons (6, 12). This hypothesis could also account for the pathological findings of Braak et al. (6) in the PD brain, which suggest that αsyn pathology spreads in a sequential and predicable manner, beginning in the gastrointestinal and olfactory systems. It has been further speculated that an unidentified neurotropic pathogen may be transmitted via retrograde and transneuronal transport to the susceptible brain regions (42). Our data raise the possibility that a candidate for the proposed, unidentified pathogen might, in analogy to prion disease, be αsyn in a specific oligomeric conformation. In addition to detecting oligomers in the extracellular space, we were able to identify a heterogenous population of extracellular αsyn oligomers ranging from dimers to ∼30-mers using SEC. Furthermore, using a protein complementation assay and novel microfluidic culture chambers, our data suggest that oligomeric αsyn is taken up and transported retrogradely to the cell soma and that bidirectional transmission of oligomeric αsyn between mature neurons and non neuronal cells may exist. Our finding that αsyn is present in the axonal medium from neurons infected with αsyn in a microfluidic chamber suggests that αsyn is released from the small amount of axons reaching the axonal chamber. Moreover, αsyn was also detected in the medium of the somal chamber due to the presence of axonal terminals and dendrodendritic synapses in this compartment. However, we cannot rule out the possibility that αsyn is released for sites other than the axonal terminals. A general effect such as pore formation may exist, which would be the same across all compartments. We believe our data provide evidence for αsyn transfer across membranes, including neuron to neuron and neuron to non-neuronal cell transmission of oligomeric αsyn and vice versa. The mechanism of transsynaptic transmission described herein supports the data of Desplats et al. (11) demonstrating that αsyn is transmitted from neuronal cells overexpressing αsyn to transplanted embryonic stem cells both in tissue culture and in transgenic animals. Our data significantly augment these recent studies by focusing on the characterization of the αsyn species released and investigating the modulation of extracellular oligomer formation by molecular chaperones. Indeed, we found that coexpression of Hsp70 results in a significant reduction in extracellular oligomer formation. Surprisingly, monomeric αsyn still appears to be present in the extracellular space together with Hsp70, thereby ruling out the possibility that Hsp70 inhibits αsyn secretion or promotes degradation. Moreover, the fact that Hsp70 was capable of eliminating or reducing specific αsyn species with a concomitant rescue of toxicity adds to the speculation that specific species of αsyn between ∼440 and ∼65 kDa in size may be toxic αsyn species. However, we cannot exclude the possibility that the species identified via SEC may represent a complex of multiple proteins, including αsyn that is dissociated in the presence of Hsp70. Regardless, our data identify Hsp70 as one critical component in the defense against the toxic effects of extracellular misfolded αsyn in neurons. The role of extracellular Hsp70 has gained much attention over the past 10 yr. Although Hsp70 does not contain a consensus secretory signal and thus cannot transverse the plasma membrane by conventional mechanisms it can be released from cells by active mechanisms that are independent of de novo Hsp70 synthesis or cell death (43). Tytell and Hightower were the first groups to discover that heat shock proteins may transit through the extracellular space to carry out their functions (44, 45). Notably, several studies have confirmed these earlier findings, demonstrating that Hsp70 is secreted from a variety of cell types in response to stress (34–38). Although it has been suggested that cellular Hsp70 release may be a consequence of nonspecific processes, such as cell death, several lines of evidence argue against this notion (34, 35, 44). A recent report has shown that Hsp70 can also be released in an exosome dependent manner (41). Moreover, extracellular Hsp70 has been demonstrated to play an important role in immune and inflammatory responses (46). The physiological stimuli that lead to Hsp70 secretion are largely unknown. Heat shock and stress have been shown to induce Hsp70 secretion and potentially lead to macrophage activation (37, 39, 40, 47). Here, we speculate that αsyn oligomers are the trigger for Hsp70 release.
Since Hsp70 is known to reduce αsyn-mediated toxicity (1, 17, 19, 32, 33, 48) it is very likely that Hsp70 is not released from dying cells but instead is actively released together with αsyn, providing a mechanism whereby Hsp70 can modulate αsyn oligomerization. However, based on our data, we cannot distinguish whether the observed actions of Hsp70 are mediated intracellularly, where an abundant supply of ATP exists, or extracellularly where ATP has been found following cellular stress (49). It is also possible that Hsp70 works extracellularly and that ATP is not required, as was recently described (50). We further speculate that if Hsp70 is associated with αsyn in the extracellular space with concomitant beneficial effects, it may also chaperone other aggregation-prone proteins in the extracellular space as a cellular defense strategy.
Intercellular and transsynaptic transmission of exogenous protein aggregates has been suggested not only for prion diseases (51). Recent evidence suggests a similar mechanism of pathological propagation in a mouse model of Alzheimer's disease, in which injection of brain extracts from human patients with Alzheimer's disease or from old amyloid precursor protein (APP) transgenic mice with plaque pathology, induced amyloid deposition in young APP mice (52). Also, extracellular tau aggregates have been shown to induce intracellular tau aggregation in a similar mode of aggregate transmission (53). Ren et al. (54) demonstrated that externally applied polyglutamine fibrils induced the aggregation of otherwise soluble polyglutamine proteins in the cytoplasm. Thus, the consideration that aggregated proteins may be transmitted from cell to cell may contribute to the dissection of the pathways underlying many progressive neurodegenerative diseases. Further investigation of the role of extracellular Hsp70 in those diseases may provide insight into the toxic species and events leading to transmission and cell death.
Our data support the release of oligomeric αsyn from neurons and its subsequent uptake by neighboring neurons. We believe our novel cellular model addresses several previously paradoxical or unexplained observations including the development of αsyn inclusions in grafts of embryonic stem cells in PD and the progressive “march” of αsyn deposits through connected neural systems from brainstem to cortex in PD. Furthermore, the effect of anti-αsyn antibodies on αsyn aggregation (55), when αsyn aggregates occurred intracellularly but the antibodies could not interact with them directly can be rationalized by the findings presented herein. Together, the present study supports the hypothesis that secreted oligomeric αsyn species are responsible for propagation of αsyn pathology and that modulation of secreted oligomeric αsyn by Hsp70 represents an exciting new strategy in disease modification attempts in PD.
Supplementary Material
Acknowledgments
The authors thank Jon Edd and Daniel Irima for help and advice on the microfluid chamber production.
This work was supported by the U.S. National Institutes of Health (NIH; NS063963 to P.J.M.), the BioMEMS Resource Center (P41 EB002503 to J.E. and D.I.), and the Massachusetts General Hospital–Massachusetts Institute of Technology Udall Center for Excellence in Parkinson Disease Research (NIH NS038372).
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Auluck P. K., Chan H. Y. E., Trojanowski J. Q., Lee V. M.-Y., Bonini N. M. (2002) Chaperone suppression of α-synuclein toxicity in a Drosophila model for Parkinson's disease. Science 295, 865–868 [DOI] [PubMed] [Google Scholar]
- 2. Bodner R. A., Outeiro T. F., Altmann S., Maxwell M. M., Cho S. H., Hyman B. T., McLean P. J., Young A. B., Housman D. E., Kazantsev A. G. (2006) Pharmacological promotion of inclusion formation: a therapeutic approach for Huntington's and Parkinson's diseases. Proc. Natl. Acad. Sci. U. S. A. 103, 4246–4251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bucciantini M., Calloni G., Chiti F., Formigli L., Nosi D., Dobson C. M., Stefani M. (2004) Prefibrillar amyloid protein aggregates share common features of cytotoxicity. J. Biol. Chem. 279, 31374–31382 [DOI] [PubMed] [Google Scholar]
- 4. Park J. Y., Lansbury P. T., Jr. (2003) Beta-synuclein inhibits formation of alpha-synuclein protofibrils: a possible therapeutic strategy against Parkinson's disease. Biochemistry 42, 3696–3700 [DOI] [PubMed] [Google Scholar]
- 5. Irizarry M. C., Growdon W., Gomez-Isla T., Newell K., George J. M., Clayton D. F., Hyman B. T. (1998) Nigral and cortical Lewy bodies and dystrophic nigral neurites in Parkinson's disease and cortical Lewy body disease contain alpha-synuclein immunoreactivity. J. Neuropathol. Exp. Neurol. 57, 334–337 [DOI] [PubMed] [Google Scholar]
- 6. Braak H., Del Tredici K., Rub U., de Vos R. A., Jansen Steur E. N., Braak E. (2003) Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging 24, 197–211 [DOI] [PubMed] [Google Scholar]
- 7. Kordower J. H., Chu Y., Hauser R. A., Freeman T. B., Olanow C. W. (2008) Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat. Med. 14, 504–506 [DOI] [PubMed] [Google Scholar]
- 8. Li J. Y., Englund E., Holton J. L., Soulet D., Hagell P., Lees A. J., Lashley T., Quinn N. P., Rehncrona S., Bjorklund A., Widner H., Revesz T., Lindvall O., Brundin P. (2008) Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat. Med. 14, 501–503 [DOI] [PubMed] [Google Scholar]
- 9. Danzer K. M., Haasen D., Karow A. R., Moussaud S., Habeck M., Giese A., Kretzschmar H., Hengerer B., Kostka M. (2007) Different species of alpha-synuclein oligomers induce calcium influx and seeding. J. Neurosci. 27, 9220–9232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Danzer K. M., Krebs S. K., Wolff M., Birk G., Hengerer B. (2009) Seeding induced by alpha-synuclein oligomers provides evidence for spreading of alpha-synuclein pathology. J. Neurochem.111, 192–203 [DOI] [PubMed] [Google Scholar]
- 11. Desplats P., Lee H. J., Bae E. J., Patrick C., Rockenstein E., Crews L., Spencer B., Masliah E., Lee S. J. (2009) Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc. Natl. Acad. Sci. U. S. A. 106, 13010–13015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brundin P., Li J. Y., Holton J. L., Lindvall O., Revesz T. (2008) Research in motion: the enigma of Parkinson's disease pathology spread. Nat. Rev. Neurosci. 9, 741–745 [DOI] [PubMed] [Google Scholar]
- 13. Borghi R., Marchese R., Negro A., Marinelli L., Forloni G., Zaccheo D., Abbruzzese G., Tabaton M. (2000) Full length alpha-synuclein is present in cerebrospinal fluid from Parkinson's disease and normal subjects. Neurosci. Lett. 287, 65–67 [DOI] [PubMed] [Google Scholar]
- 14. El-Agnaf O. M., Salem S. A., Paleologou K. E., Curran M. D., Gibson M. J., Court J. A., Schlossmacher M. G., Allsop D. (2006) Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson's disease. FASEB J. 20, 419–425 [DOI] [PubMed] [Google Scholar]
- 15. Mollenhauer B., Cullen V., Kahn I., Krastins B., Outeiro T. F., Pepivani I., Ng J., Schulz-Schaeffer W., Kretzschmar H. A., McLean P. J., Trenkwalder C., Sarracino D. A., Vonsattel J. P., Locascio J. J., El-Agnaf O. M., Schlossmacher M. G. (2008) Direct quantification of CSF alpha-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp. Neurol. 213, 315–325 [DOI] [PubMed] [Google Scholar]
- 16. Remy I., Michnick S. W. (2006) A highly sensitive protein-protein interaction assay based on Gaussia luciferase. Nat Methods 3, 977–979 [DOI] [PubMed] [Google Scholar]
- 17. Klucken J., Shin Y., Masliah E., Hyman B. T., McLean P. J. (2004) Hsp70 reduces alpha-synuclein aggregation and toxicity. J. Biol. Chem. 279, 25497–25502 [DOI] [PubMed] [Google Scholar]
- 18. St Martin J. L., Klucken J., Outeiro T. F., Nguyen P., Keller-McGandy C., Cantuti-Castelvetri I., Grammatopoulos T. N., Standaert D. G., Hyman B. T., McLean P. J. (2007) Dopaminergic neuron loss and up-regulation of chaperone protein mRNA induced by targeted over-expression of alpha-synuclein in mouse substantia nigra. J. Neurochem. 100, 1449–1457 [DOI] [PubMed] [Google Scholar]
- 19. Outeiro T. F., Putcha P., Tetzlaff J. E., Spoelgen R., Koker M., Carvalho F., Hyman B. T., McLean P. J. (2008) Formation of toxic oligomeric alpha-synuclein species in living cells. PLoS ONE 3, e1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Volles M. J., Lee S. J., Rochet J. C., Shtilerman M. D., Ding T. T., Kessler J. C., Lansbury P. T., Jr. (2001) Vesicle permeabilization by protofibrillar alpha-synuclein: implications for the pathogenesis and treatment of Parkinson's disease. Biochemistry 40, 7812–7819 [DOI] [PubMed] [Google Scholar]
- 21. Kayed R., Head E., Thompson J. L., McIntire T. M., Milton S. C., Cotman C. W., Glabe C. G. (2003) Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300, 486–489 [DOI] [PubMed] [Google Scholar]
- 22. Taylor A. (2003) Microfluidic multicompartment device for neuroscience research. Langmuir 19, 1551–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee H. J., Patel S., Lee S. J. (2005) Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J. Neurosci. 25, 6016–6024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Putcha P., Danzer K. M., Kranich L. R., Scott A., Silinski M., Mabbett S., Hicks C. D., Veal J. M., Steed P. M., Hyman B. T., McLean P. J. (2009) Brain permeable small molecule inhibitors of Hsp90 prevent alpha-synuclein oligomer formation and rescue alpha-synuclein-induced toxicity. J. Pharmacol. Exp. Ther. 332, 847–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tetzlaff J. E., Putcha P., Outeiro T. F., Ivanov A., Berezovska O., Hyman B. T., McLean P. J. (2008) CHIP targets toxic alpha-ynuclein oligomers for degradation. J. Biol. Chem. 283, 17962–17968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Remy I., Michnick S. W. (2004) A cDNA library functional screening strategy based on fluorescent protein complementation assays to identify novel components of signaling pathways. Methods 32, 381–388 [DOI] [PubMed] [Google Scholar]
- 27. Rhee S. W., Taylor A. M., Tu C. H., Cribbs D. H., Cotman C. W., Jeon N. L. (2005) Patterned cell culture inside microfluidic devices. Lab. Chip 5, 102–107 [DOI] [PubMed] [Google Scholar]
- 28. Stoothoff W., Jones P. B., Spires-Jones T. L., Joyner D., Chhabra E., Bercury K., Fan Z., Xie H., Bacskai B., Edd J., Irimia D., Hyman B. T. (2009) Differential effect of three-repeat and four-repeat tau on mitochondrial axonal transport. J. Neurochem. 111, 417–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taylor A. M., Blurton-Jones M., Rhee S. W., Cribbs D. H., Cotman C. W., Jeon N. L. (2005) A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat. Methods 2, 599–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu J., Zhang J. P., Shi M., Quinn T., Bradner J., Beyer R., Chen S., Zhang J. (2009) Rab11a and HSP90 regulate recycling of extracellular alpha-synuclein. J. Neurosci. 29, 1480–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Luk K. C., Song C., O'Brien P., Stieber A., Branch J. R., Brunden K. R., Trojanowski J. Q., Lee V. M. (2009) Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc. Natl. Acad. Sci. U. S. A. 106, 20051–20056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Auluck P. K., Bonini N. M. (2002) Pharmacological prevention of Parkinson disease in Drosophila. Nat. Med. 8, 1185–1186 [DOI] [PubMed] [Google Scholar]
- 33. McLean P. J., Klucken J., Shin Y., Hyman B. T. (2004) Geldanamycin induces Hsp70 and prevents alpha-synuclein aggregation and toxicity in vitro. Biochem. Biophys. Res. Commun. 321, 665–669 [DOI] [PubMed] [Google Scholar]
- 34. Broquet A. H., Thomas G., Masliah J., Trugnan G., Bachelet M. (2003) Expression of the molecular chaperone Hsp70 in detergent-resistant microdomains correlates with its membrane delivery and release. J. Biol. Chem. 278, 21601–21606 [DOI] [PubMed] [Google Scholar]
- 35. Febbraio M. A., Ott P., Nielsen H. B., Steensberg A., Keller C., Krustrup P., Secher N. H., Pedersen B. K. (2002) Exercise induces hepatosplanchnic release of heat shock protein 72 in humans. J. Physiol. 544, 957–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fleshner M., Campisi J., Amiri L., Diamond D. M. (2004) Cat exposure induces both intra- and extracellular Hsp72: the role of adrenal hormones. Psychoneuroendocrinology 29, 1142–1152 [DOI] [PubMed] [Google Scholar]
- 37. Guzhova I., Kislyakova K., Moskaliova O., Fridlanskaya I., Tytell M., Cheetham M., Margulis B. (2001) In vitro studies show that Hsp70 can be released by glia and that exogenous Hsp70 can enhance neuronal stress tolerance. Brain Res. 914, 66–73 [DOI] [PubMed] [Google Scholar]
- 38. Hunter-Lavin C., Davies E. L., Bacelar M. M., Marshall M. J., Andrew S. M., Williams J. H. (2004) Hsp70 release from peripheral blood mononuclear cells. Biochem. Biophys. Res. Commun. 324, 511–517 [DOI] [PubMed] [Google Scholar]
- 39. Mambula S. S., Stevenson M. A., Ogawa K., Calderwood S. K. (2007) Mechanisms for Hsp70 secretion: crossing membranes without a leader. Methods 43, 168–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vega V. L., Rodriguez-Silva M., Frey T., Gehrmann M., Diaz J. C., Steinem C., Multhoff G., Arispe N., De Maio A. (2008) Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J. Immunol. 180, 4299–4307 [DOI] [PubMed] [Google Scholar]
- 41. Lancaster G. I., Febbraio M. A. (2005) Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J. Biol. Chem. 280, 23349–23355 [DOI] [PubMed] [Google Scholar]
- 42. Hawkes C. H., Del Tredici K., Braak H. (2007) Parkinson's disease: a dual-hit hypothesis. Neuropathol. Appl. Neurobiol. 33, 599–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mambula S. S., Calderwood S. K. (2006) Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J. Immunol. 177, 7849–7857 [DOI] [PubMed] [Google Scholar]
- 44. Hightower L. E., Guidon P. T., Jr. (1989) Selective release from cultured mammalian cells of heat-shock (stress) proteins that resemble glia-axon transfer proteins. J. Cell. Physiol. 138, 257–266 [DOI] [PubMed] [Google Scholar]
- 45. Tytell M., Greenberg S. G., Lasek R. J. (1986) Heat shock-like protein is transferred from glia to axon. Brain Res. 363, 161–164 [DOI] [PubMed] [Google Scholar]
- 46. Calderwood S. K., Theriault J. R., Gong J. (2005) Message in a bottle: role of the 70-kDa heat shock protein family in anti-tumor immunity. Eur. J. Immunol. 35, 2518–2527 [DOI] [PubMed] [Google Scholar]
- 47. Barreto A., Gonzalez J. M., Kabingu E., Asea A., Fiorentino S. (2003) Stress-induced release of HSC70 from human tumors. Cell. Immunol. 222, 97–104 [DOI] [PubMed] [Google Scholar]
- 48. Auluck P. K., Meulener M. C., Bonini N. M. (2005) Mechanisms of suppression of α-synuclein neurotoxicity by geldanamycin in Drosophila. J. Biol. Chem. 280, 2873–2878 [DOI] [PubMed] [Google Scholar]
- 49. Stagg J., Smyth M. J. (2010) Extracellular adenosine triphosphate and adenosine in cancer. [E-pub ahead of print] Oncogene doi: 10.1038/onc.2010.292 [DOI] [PubMed] [Google Scholar]
- 50. Dedmon M. M., Christodoulou J., Wilson M. R., Dobson C. M. (2005) Heat shock protein 70 inhibits alpha-synuclein fibril formation via preferential binding to prefibrillar species. J. Biol. Chem. 280, 14733–14740 [DOI] [PubMed] [Google Scholar]
- 51. Caughey B. (2000) Transmissible spongiform encephalopathies, amyloidoses and yeast prions: common threads? Nat. Med. 6, 751–754 [DOI] [PubMed] [Google Scholar]
- 52. Meyer-Luehmann M., Coomaraswamy J., Bolmont T., Kaeser S., Schaefer C., Kilger E., Neuenschwander A., Abramowski D., Frey P., Jaton A. L., Vigouret J. M., Paganetti P., Walsh D. M., Mathews P. M., Ghiso J., Staufenbiel M., Walker L. C., Jucker M. (2006) Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science 313, 1781–1784 [DOI] [PubMed] [Google Scholar]
- 53. Frost B., Jacks R. L., Diamond M. I. (2009) Propagation of tau misfolding from the outside to the inside of a cell. J. Biol. Chem. 284, 12845–12852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ren P. H., Lauckner J. E., Kachirskaia I., Heuser J. E., Melki R., Kopito R. R. (2009) Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat. Cell Biol. 11, 219–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Masliah E., Rockenstein E., Adame A., Alford M., Crews L., Hashimoto M., Seubert P., Lee M., Goldstein J., Chilcote T., Games D., Schenk D. (2005) Effects of alpha-synuclein immunization in a mouse model of Parkinson's disease. Neuron. 46, 857–868 [DOI] [PubMed] [Google Scholar]
- 56. Kerppola T. K. (2006) Visualization of molecular interactions by fluorescence complementation. Nat. Rev. Mol. Cell. Biol. 7, 449–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.