Abstract
Vibrio cholerae-derived zonula occludins toxin (Zot) is a multifunctional protein that reversibly disassembles intestinal tight junctions (tjs). Zot structure-function analysis has mapped this activity to aa 288–293, named AT1002. AT1002 reduced transepithelial electrical resistance across rat small intestine, ex vivo, as did Zot and its processed mature form, ΔG. AT1002 increased in vivo permeability to sugar tracers, whereas scrambled control peptides did not. Binding and barrier assays in proteinase activated receptor (PAR)2-expressing and PAR2-null cells established AT1002 activity to be PAR2 dependent. Coincident with the increased intestinal permeability, confocal microscopy of AT1002-exposed rat intestinal IEC6 cells revealed displacement of ZO-1 and occludin from intercellular boundaries. In coimmunoprecipitation assays, AT1002 decreased ZO-1-occludin and ZO-1-claudin 1 interactions coincident with PKCα-dependent ZO-1 serine/threonine phosphorylation. Further, AT1002 increased serine phosphorylation of myosin 1C and, at the same time, transiently diminished its association with ZO-1. The COOH-terminal domain of ZO-1 was required for its association with myosin 1C. These data indicate that the NH2-terminal portion of active Zot contains a PAR2-activating motif, FCIGRL, that increases PKCα-dependent ZO-1 and myosin 1C serine/threonine phosphorylation. These modifications provoke selective disengagement of ZO-1 from its binding partners, occludin, claudin 1, and myosin 1C, coincident with opening of tjs.—Goldblum, S. E., Rai, U., Tripathi, A., Thakar, M., De Leo, L., Di Toro, N., Not, T., Ramachandran, R., Puche, A. C., Hollenberg, M. D., Fasano, A. The active Zot domain (aa 288–293) increases ZO-1 and myosin 1C serine/threonine phosphorylation, alters interaction between ZO-1 and its binding partners, and induces tight junction disassembly through proteinase activated receptor 2 activation.
Keywords: intestinal permeability, protein-protein interaction, zonula occludens 1
The intestinal epithelial paracellular pathway is regulated in part through dynamic alterations within intercellular tight junctions (tjs) (1). The physiological regulation of these complex multiprotein structures remains largely undefined (1). Vibrio cholera elaborates a 44.8-kDa protein, zonula occludens toxin (Zot), that reversibly opens intestinal tjs and increases paracellular permeability (2). Structure-function analyses indicate that the biologically active portion of Zot can be mapped to aa 288–293 (3). V. cholerae-mediated proteolytic cleavage at aa 288 releases the biologically active fragment into the intestinal micromilieu. Interestingly, the newly formed NH2 terminus of cleaved Zot (FCIGRL aa 288–293) is structurally similar to a motif (SLIGRL) contained in the tethered ligand for proteinase activated receptor (PAR)2, a receptor that has been implicated in the regulation of tj permeability (4).
Multiple proteins have been localized to the tj, including the integral membrane proteins, occludin, claudins, and junctional adhesion molecule, and the membrane-associated guanylate kinase (MAGuK) family of cytoplasmic scaffolding proteins, ZO-1, -2, and -3 (5–8). This tj multiprotein complex is tethered to the actin cytoskeleton. Of these tj components, the central scaffolding protein, ZO-1 (9), directly and indirectly couples the integral plasma membrane proteins, occludin (10, 11) and claudins, to the other cytoplasmic tj proteins (12) and the actin cytoskeleton (11). The assembly and regulation of intercellular tjs have been explained in part by PKC activation (13). In epithelial cells, PKC activation is coincident with increases in tj-mediated paracellular permeability (14).
Although much is known about tj molecular architecture and its assembly during morphogenesis (15, 16), the physiological regulation of mature, already formed tjs remains incompletely understood. Early signaling studies demonstrated that the effect of Zot on intestinal tjs is mediated through PKCα-dependent actin polymerization (14). Our studies have demonstrated that the hexapeptide immediately downstream of the Zot cleavage site in V. cholerae is the active domain (3). Of interest, this 6-mer, AT1002, has a proteinase activated receptor (PAR) activating peptide (AP)-like structure. Accordingly, we elected to use Zot and its active derivatives to gain insight into the intracellular signaling operative in physiological regulation of intact intercellular tjs.
MATERIALS AND METHODS
Materials
Tris glycine gels were obtained from Invitrogen (Carlsbad, CA, USA). Protein concentrations were assayed with a Lowry kit (Pierce Chemical, Rockford, IL, USA). Zot and ΔG were expressed and purified as we have described previously (3). The Zot active domain, NH2-FCIGRL-COOH (i.e., AT1002), together with NH2-GRLFCI-COOH, NH2-FMIGRL-COOH, and NH2-YMLAHI-COOH, was synthesized by the Biopolymer Laboratories, University of Maryland (Baltimore, MD, USA) as described previously (3). The PKC inhibitor, CGP41251, and its inactive analog, CGP42700, were gifts from Ciba Geigy (Basel, Switzerland). Rabbit polyclonal anti-occludin, anti-ZO-1, anti-claudin-1, horseradish peroxidase (HRP)-conjugated anti-occludin, FITC-conjugated anti-ZO-1, and anti-phosphothreonine antibodies were purchased from Zymed (San Francisco, CA, USA). Mouse polyclonal anti-phosphoserine antibodies were purchased from Calbiochem (San Diego, CA, USA). Anti-PKCα antibody was obtained from Cell Signaling Technology (Danvers, MA, USA). Rabbit polyclonal anti-myosin 1C and tetramethylrhodamine isothiocyanate (TRITC)-conjugated goat anti-rabbit antibodies were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA). Secondary polyclonal anti-rabbit and anti-mouse antibodies were purchased from Amersham (Little Chalfont, UK). PKCα-targeting and control small interfering RNAs (siRNAs) were designed by Invitrogen.
Cell lines
The transformed human intestinal Caco2 cell line (American Type Culture Collection, Rockville, MD, USA) was maintained in minimal essential Eagle's medium supplemented with 10% FBS, 1% nonessential amino acids, 100 U/ml penicillin, and 100 U/ml streptomycin. Rat intestinal IEC6 cells (American Type Culture Collection) were maintained in DMEM with 4.5 g/L glucose, containing 5% FBS, 10 μg/ml insulin, 4 mM l-glutamine, 50 U/ml penicillin, and 50 U/ml streptomycin. For both Caco2 and IEC6 cells, only passages 20–45 were studied. Rat kidney-derived KNRK cells expressing rat PAR2 were cultured in DMEM containing 10% FBS and passaged without use of trypsin for subculturing, as described previously for use in a radioligand binding assay (17). MDCK type II cells and cells stably expressing full-length green fluorescent protein (GFP)-ZO-1, the ZO-1 NH2-terminal domain (aa 1–876), and the ZO-1 COOH-terminal domain (aa 1046–1748; kindly provided by Dr. A. S. Fanning, University of North Carolina, Chapel Hill, NC, USA) were cultured in DMEM containing 10% FBS and selected with G418 (0.4 mg/ml; Invitrogen; refs. 18, 19).
Ex vivo small intestine micro-Snapwell barrier function assay
The Snapwell system was used to define the dose and time requirements for changes in transepithelial electrical resistance (TEER) in response to Zot, the recombinant active Zot domain, ΔG, and the AT1002 peptide. White adult male Wistar rats (250–330 g) were euthanized by decapitation under methafane inhalation anesthesia in accordance with the Animal Care and Use Committees at the University of Maryland School of Medicine (Baltimore, MD, USA) and Children Hospital Istituto di Recovero e Cura a Carattere Scientifico Burlo Garofolo (Trieste, Italy). The small intestines (jejunum and ileum) were harvested and opened along the mesenteric border and washed free of intestinal contents, and sections were mounted on filter supports between two Plexiglas discs with an opening of 3 mm and placed in modified Snapwell chambers (Corning, Corning, NY, USA) (20). Increasing concentrations of the 3 Zot-related proteins, Zot, ΔG, and AT1002, were each introduced to the luminal aspect of the intestine for increasing exposure times. TEER, measured using a planar electrode (Endohm SNAP electrode attached to an Evom-g WPI analyzer; World Precision Instruments, Sarasota, FL, USA), was expressed in ohm square centimeters. The difference between TEER at time 0 and TEER at each indicated time point, ΔTEER, was expressed as TEERtx − TEERt0 where t is time and x is the indicated time point. In selected experiments, small intestinal segments isolated from either wild-type (WT) C57BL/6J or PAR2−/− mice were similarly studied.
AT1002 binding to PAR2 in PAR2-expressing KNRK and Caco2 cells
AT1002 contains a sequence (FCIGRL aa 288–293) that is structurally similar to a motif contained in the tethered ligand for PAR2 (SLIGRL in rats; SLIGKV in humans), a receptor that has been coupled to the regulation of tj permeability (4). First, we asked whether AT1002 might bind to PAR2 expressed on cultured cells. The ability of AT1002 to competitively displace the radiolabeled PAR2 receptor probe, 3H-labeled 2-furoyl-LIGRLO-NH2, was evaluated as described previously (17). PAR2-expressing KNRK cells were routinely grown at 37°C in 75-cm2 plastic T-flasks (Nunc; VWR Scientific, Mississauga, ON, Canada) using 10 ml of DMEM supplemented with 10% FBS under an atmosphere of 5% CO2 in air. For the binding assays, cells were cultured to ∼80% confluence and passaged by resuspension in calcium-free isotonic saline EDTA-containing cell dissociation buffer (Invitrogen). To study the ability of the peptide, FCIGRL, to compete for the PAR2 binding of the radiolabeled receptor probe [[3H]propionyl-2-furoyl-LIGRLO-NH2 ([3H]propionyl-2fLI)], cells were harvested using calcium-free dissociation medium, pooled, and resuspended at ∼107 cells/ml in binding buffer (isotonic, 115 mM NaCl buffered at pH 7.4 with 25 mM HEPES and supplemented with 0.1% w/v BSA) as described previously (17). The binding of [3H]propionyl-2fLI to the PAR2-expressing KNRK cells was measured by a Microfuge centrifugation method (Beckman Coulter, Fullerton, CA) that pellets cell-bound ligand below an oil-water interface (17). KNRK cells (transfected or not with rat PAR2) (∼107 cells/ml in a total volume of 50 μl) were incubated for 10 min in 1-ml tubes (8.8×4.5 cm) (Bio-Rad, Mississauga, ON, Canada) containing 50 μl of binding buffer, with or without unlabeled peptide (e.g., 100–500 μM unlabeled SLIGRL-NH2 or FCIGRL). The binding reaction was initiated by the addition of 10 μl of [3H]propionyl-2fLI at ∼30 nM in a final volume of 0.1 ml. After 1 h, 80 μl of the cell suspension was transferred to the oil-loaded (150 μl of dibutyl/diisononyl phthalate, 6:4, v/v) microcentrifuge tubes and centrifuged (15,000 rpm, 5 min, RT) to pellet the cells below the oil-buffer interface to separate free from cell-bound radioactivity. The tips of the microcentrifuge tube were cut below the oil-buffer interface, the cell harvest was solubilized in nonionic surfactant-containing scintillation fluid (5 ml of Ecolite; MP Biomedicals, Irvine, CA, USA), and the bound 3H-labeled ligand probe was counted. An aliquot of the cell-free supernatant was used to measure the total amount of radioactivity added to each incubation mixture. The specific binding of radiolabeled probe was calculated by subtracting from the total amount bound the amount of radioactivity bound in the presence of 100–500 μM unlabeled ligand. To estimate the apparent Ki of binding for FCIGRL, the ability of this peptide to compete for the binding of [3H]propionyl-2fLI was measured at a radiolabeled ligand concentration ∼125 nM in a final volume of 0.1 ml. The concentration of unlabeled FCIGLR that reduced specific binding by 50% (IC50) was estimated from the binding competition curve and was used as an estimate of the Ki of the unlabeled ligand.
Standard and confocal immunofluorescence microscopy
Postconfluent Caco2 and IEC6 cells were treated for 1.5 h with 30 nM Zot, 800 nM ΔG, 250 μM AT1002, or the BSA control. Cells were washed 4 times (PBS without Ca2+ and Mg2+), fixed (95% ethanol, 4°C, 0.5h), dehydrated [acetone, room temperature (RT), 3 min], blocked (2% BSA, 0.5 h), and incubated overnight at 4°C with FITC-conjugated anti-ZO-1 or anti-occludin antibodies. Tissues were processed for either standard immunofluorescence microscopy (Nikon Eclipse 2000-E; Nikon, Tokyo, Japan) or scanning laser confocal microscopy (Zeiss LSM410; Carl Zeiss, Oberkochen, Germany). For colocalization of ZO-1 with myosin 1C, MDCK cells stably expressing GFP-labeled full-length human ZO-1 (18, 19) were seeded at 50,000 cells/well in the wells of 8-well chamber slides (Lab-Tek, Rochester, NY, USA) and cultured for 3 d in DMEM supplemented with G418 (250 μg/ml). The cells were fixed (4% paraformaldehyde, 15 min, RT), washed 4 times (100 mM glycine), permeabilized (0.1% Triton X-100, 2 min), again washed, blocked (1% BSA, 30 min, RT), and incubated overnight at 4°C with an antibody raised in rabbits against the nuclear isoform of murine myosin 1C (1:500; Sigma-Aldrich; ref. 21). After washing 4 times, the cells were incubated for 60 min at RT with TRITC-conjugated goat anti-rabbit IgG (1:1000). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). After multiple washes, the monolayers were processed for standard fluorescence microscopy. For colocalization, overlay images of GFP-ZO-1, myosin 1C, and DAPI were merged.
In vivo measurement of intestinal permeability by dual sugar test
To establish the minimal effective dose of AT1002 required to increase intestinal permeability, BALB/c mice were orally administered 100 μl of a solution containing 12 mg of lactulose (LA) and 8 mg of l-rhamnose (RA) in the presence of increasing concentrations of AT1002 (22). At 1 h after administration of the solution, blood samples (80 μl) were collected from 3 mice for each AT1002 concentration. The effect of 3 escalating doses of FCIGRL on intestinal permeability was then studied over the course of 13 h in 4 mice/dose, performing 6 blood collections before and at increasing times after AT1002 administration. To establish the specificity of the FCIGRL sequence on intestinal permeability, we compared its permeating activity with equivalent concentrations of 3 scrambled peptides: NH2-GRLFCI-COOH, NH2-FMIGRL-COOH, and NH2-YMLAHI-COOH, using the FCIGRL-active oral dose (250 μg) in 4 mice for each scrambled sequence. Blood samples were centrifuged, and the sera were deproteinized and stored at −20°C. Then 50 μl of serum or 10 μl of aqueous sugar standard was added to 200 μl of derivatization reagent in a 300-μl glass insert, mixed, and placed in a 4-ml WISP vial (Waters Corp., Milford, MA, USA), and heated overnight at 65°C. The reaction mixture was allowed to cool to RT, after which 5 μl was injected into a PerkinElmer model 200 HPLC system (PerkinElmer, Wellesley, MA, USA). The separation column was a Spherisorb ODS II column (150 × 4.6 mm i.d. with 3-μm particles; Waters) equipped with a precolumn (10 × 4.6 mm i.d.), filled with the same packing material. As mobile phase, 30% acetonitrile was used under isocratic condition. The flow rate of the mobile phase was set at 1 ml/min and run at RT. Data were collected online by a model 900 interface and processed by AMD Duron PC software (PerkinElmer/Nelson, San Jose, CA, USA). Each result was expressed as the mean ± se LA/RA ratio. A normal value of ≤0.03 represents the mean value of 10 mice previously tested under basal conditions.
Coimmunoprecipitation assays
Postconfluent Caco2 and IEC6 cells each were treated for increasing exposure times with 30 nM Zot, 800 nM ΔG, 100 μM AT1002, or equivalent concentrations of BSA. Cells were lysed with modified RIPA buffer as described previously (23). Cell lysates were incubated with rabbit anti-ZO-1 antibody, followed by protein G cross-linked to agarose; the ZO-1 immmunoprecipitates were processed for immunoblotting with specific antibodies raised against occludin, claudin-1, ZO-2, and myosin 1C, followed by HRP-conjugated secondary antibody; and the blots were developed with ECL. To control for protein loading and transfer, the blots were stripped and reprobed with the immunoprecipitating antibody as described previously (23). In selected experiments, cells were pretreated with the PKC inhibitor, CGP41251 (5 μM), or its inactive analog, CGP42700 (5 μM). In other experiments, MDCK cells and MDCK cells stably expressing full-length ZO-1, the NH2-terminal domain (aa 1–876), or the COOH-terminal domain (aa 1046–1748) (18, 19) were lysed with modified RIPA buffer, and the lysates were immunoprecipitated with anti-ZO-1 antibodies. The ZO-1 immunoprecipitates were processed for myosin 1C immunoblotting.
Assay of transepithelial flux
Transwell chambers with polycarbonate filter supports (24 mm diameter, 0.4 mm pore size; Corning) inserted in the wells of 24-well plates were seeded with 8.0 × 104 Caco2 cells/chamber and cultured for 21 d (37°C), as described previously (20). Baseline barrier integrity of each monolayer was established by measuring TEER with the Endohm system. Caco2 monolayers with TEER values of 200 Ω · cm2 were considered electrically mature and suitable for transport studies. ΔG (800 nM) in PBS or PBS alone each was introduced into the upper compartment and incubated for 20 min. The contents of the upper compartment were removed and replaced with a mixture containing 800 nM ΔG or PBS each together with [14C]mannitol in PBS for 1.5 h at 37°C. The upper and lower compartments were collected into 4.5 ml of scintillation fluid (Optifluor; Packard Instruments, Meriden, CT, USA) and counted in a liquid scintillation analyzer (TriCarb 1500; Packard Instruments). Percentage transport was calculated as [(dpm of treatment group − dpm of background)/(dpm of BSA control − dpm of background)] × 100%. Again, in selected experiments, cells were pretreated with CGP41251 (5 μM) or CGP42700 (5 μM).
Phosphoserine and phosphothreonine immunoblotting
Postconfluent IEC6 cells were treated with AT1002 or equivalent concentrations of BSA and lysed with modified RIPA buffer, and the cell lysates were immunoprecipitated with anti-ZO-1 antibody as described above. The ZO-1 immunoprecipitates were processed for immunoblotting with either anti-phosphoserine or anti-phosphothreonine antibodies followed by secondary antibody and developed with ECL. To control for protein loading and transfer, the blots were stripped and reprobed with the immunoprecipitating antibody, as described (23).
Knockdown of PKCα through RNA interference
A mixture of 3 siRNAs designed to target human PKCα, as well as an irrelevant control siRNA not corresponding to any known sequence in the human genome (Invitrogen), each was introduced into Caco2 cells as described previously (24). First, 5 × 105 cells were centrifuged (200 g, 10 min), after which the pellet was resuspended in 100 μl of Nucleofector solution (Amaxa Biosystems, Gaithersburg, MD, USA) and incubated with 300 nM siRNAs. The Caco2 cell-siRNA mixture was transferred to an Amaxa-certified cuvette and subjected to programmed electroporation (program B-024; Amaxa Biosystems). The PKCα siRNA-transfected cells were cultured for increasing times and lysed, and the lysates were processed for immunoblotting with anti-PKCα antibody (Cell Signaling Technology). To confirm equivalent protein loading and transfer, blots were stripped and reprobed for β-tubulin. Caco2 cells transfected with either PKCα-targeting or control siRNAs were processed for ZO-1 fluorescence microscopy as described above.
Statistical analysis
All values are expressed as means ± se. The differences in the in vivo experiments were analyzed using the nonparametric Mann-Whitney test. For the remaining experiments, the differences were analyzed by a 2-tailed Student's t test for either paired or unpaired varieties and by 1-way ANOVA using Dunnett's test (SPSS, Chicago, IL, USA). Statistical significance was set at P < 0.05.
RESULTS
Three Zot-related proteins, Zot, ΔG, and AT1002, open intestinal tjs
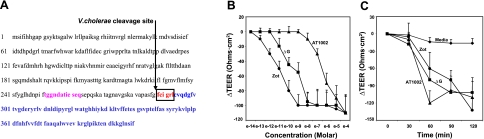
Figure 1A displays the amino acid sequences of full-length Zot, ΔG, and AT1002. The Zot protein is composed of 399 aa. ΔG is a truncated form of Zot that retains aa 264–399. The 6-mer AT1002 (aa 288–293) corresponds to the NH2 terminus PAR-AP motif of mature Zot (25). Full-length Zot, ΔG, and AT1002 were compared for reversible barrier-disrupting activity in the Snapwell system. The mean ± se baseline TEER values for the 3 experimental groups were Zot, 41.3 ± 6.7; ΔG, 33.8 ± 8.8; and AT1002, 48.9 ± 9.1 Ω · cm2. There were no differences between groups. Rat tissue was exposed for 90 min to increasing concentrations of each of the 3 Zot-derived proteins, after which TEER was measured (Fig. 1B). Each Zot-related protein decreased TEER in a dose-dependent manner. The minimal threshold concentrations for full-length Zot, ΔG, and AT1002 to decrease TEER were 10−12, 10−11, and 10−7 M, respectively. In other studies over time of the same 3 Zot-derived proteins, the baseline TEER values for the medium control and three experimental groups were medium, 35.5 ± 7.7; Zot, 31.9 ± 9.9; ΔG, 43.3 ± 8.1; and AT1002, 40.6 ± 8.7 Ω · cm2. Again, there were no differences between groups. After rat tissue was exposed for increasing exposure times up to 120 min to fixed concentrations of full-length Zot (30 nM), ΔG (800 nM), or AT1002 (50 μM), each decreased TEER in a time-dependent manner (Fig. 1C). The stimulus-to-response lag time for each of these 3 proteins was ≤60 min. On removal of each agonist, the TEER returned to baseline (data not shown).
Figure 1.
Zot-derived proteins regulate rat intestinal tj. A) Complete amino acid sequences of full-length Zot protein (aa 1–399), ΔG (aa 264–399), and AT1002 (aa 288–294). V. cholerae-anchoring Zot membrane-spanning domain is depicted in magenta, active domain in blue, and PAR2-AP motif in red. B) Sections of rat intestine were mounted in a modified micro-Snapwell device and exposed for 90 min to increasing concentrations of Zot (●), ΔG (■), or AT1002 (▴), after which TEER was measured. Data are mean ± se TEER values (n=4). Baseline TEER values (Ω · cm2) for the 3 experimental groups: Zot, 41.3 ± 6.7; ΔG, 33.8 ± 8.8; and AT1002, 48.9 ± 9.1. C) Sections of rat intestine were mounted in micro-Snapwell devices and exposed for increasing exposure times to 10−9 M Zot, 10−7 M ΔG, 10−5 M AT1002, or medium alone, after which TEER was measured. Data are mean ± se TEER values (n=4). Baseline TEER values (Ω · cm2) for the medium control and 3 experimental groups (n=4): medium, 35.5 ± 7.7; Zot, 31.9 ± 9.9; ΔG, 43.3 ± 8.1; and AT1002, 40.6 ± 8.7.
AT1002 effect on TEER is PAR2 dependent
AT1002 binds to PAR2
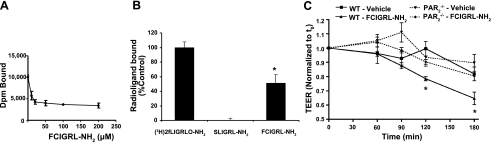
We asked whether AT1002 binds to PAR2. First, we tested whether AT1002 might compete for the binding of the radiolabeled PAR2 receptor probe, 3H-labeled 2-furoyl-LIGRLO-NH2, to surface-expressed PAR2 (Fig. 2A). When the 3H-labeled PAR2 ligand, in the presence of increasing concentrations of unlabeled AT1002, was coincubated with PAR2-expressing KNRK cells, AT1002 competed for the binding of 3H-labeled PAR2 ligand in a concentration-dependent manner. The apparent Ki for AT1002 estimated from the binding-inhibition curve (IC50) was ∼6 μM. AT1002 was able to compete for ∼50% of the bound 3H-labeled PAR2 ligand compared with that bound to cells incubated with the radiolabeled ligand alone (Fig. 2A, B). Further, the peptide representing the tethered ligand of PAR2 (SLIGRL-NH2) was, as anticipated, able to competitively displace 100% of radioligand binding, whereas the reverse-sequence peptide that cannot activate PAR2 (LRGILS-NH2) failed to compete for binding (not shown). Non-PAR2-transfected KNRK cells that do not express PAR2 on the cell surface fail to bind the radiolabeled receptor probe (17). Thus, FCIGRL was able to interact directly with PAR2.
Figure 2.
FCIGRL-NH2(AT1002) bioactivity is PAR2 dependent. A) A fixed concentration of 3H-labeled 2-furoyl-LIGRLO-NH2 in the presence of increasing concentrations of AT1002 (i.e., FCIGRL-NH2) was incubated with PAR2-expressing KNRK cells, and specific binding of the radiolabeled probe was calculated. B) 3H-labeled 2-furoyl-LIGRLO-NH2 in the presence of equivalent concentrations of either the tethered PAR2 ligand sequence, SLIGRL-NH2, the reverse-sequence peptide, LRGILS-NH2 (data not shown), or AT1002 (i.e., FCIGRL-NH2), was incubated with KNRK cells, and specific binding of the radiolabeled probe was determined. C) Effect of FCIGRL-NH2 on intestinal permeability is PAR2 dependent. Intestinal segments from WT and PAR2−/− mice were mounted in micro-Snapwell chambers and treated with medium only (normalized to 1) or FCIGRL-NH2 (250 μM) in duplicate, and TEER was measured at the indicated time points. Results represent means ± se for 4 mice/treatment group. Baseline TEER values (Ω · cm2): WT-vehicle, 57.6 ± 7.7; WT-FCIGRL-NH2, 40.5 ± 5.8; PAR2−/−-vehicle, 46.4 ± 5.9; and PAR2−/−-FCIGRL-NH2, 40.0 ± 8.9. *P < 0.05 vs. all other groups at same time point.
FCIGRL-NH2 requires PAR2 to increase intestinal permeability
Intestinal segments from WT or PAR2−/− mice (The Jackson Laboratory, Bar Harbor, ME, USA) were mounted in a micro-Snapwell apparatus and incubated for 180 min with FCIGRL-NH2 or medium alone (Fig. 2C). The mean ± se baseline TEER values for the 2 control and 2 experimental groups were WT-vehicle, 57.6 ± 7.7; WT-FCIGRL-NH2, 40.5 ± 5.8; PAR2−/−-vehicle, 46.4 ± 5.9; and PAR2−/−-FCIGRL-NH2, 40.0 ± 8.9 Ω · cm2. The baseline TEER values for WT vs. PAR2−/− intestines were not significantly different. As anticipated, AT1002 decreased TEER in WT intestinal segments compared with the vehicle control. In contrast, PAR2−/− intestinal segments were refractory to stimulation by AT1002 (Fig. 2C). Thus, PAR2 contributes to increased permeability induced by the ZOT-active peptide.
AT1002 provokes ZO-1 redistribution in intestinal epithelial cells
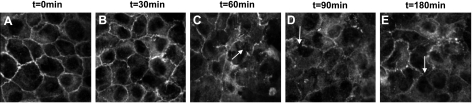
To establish whether these Zot/ΔG/AT1002-induced decreases in TEER were coincident with departure of ZO-1 from the tj, 3-dimensional confocal microscopy was applied to rat epithelial cells. Postconfluent IEC6 cell monolayers were exposed for increasing times (0–180 min) to AT1002 (250 μM) or medium alone, after which the monolayers were probed for ZO-1 and processed for confocal microscopy (Fig. 3). In the control monolayers, ZO-1 staining was restricted exclusively to the intercellular boundaries (Fig. 3A). After AT1002 exposure times as brief as 30 min, ZO-1 staining at the cell-cell interface was diminished, whereas ZO-1 movement into the cytoplasm increased (Fig. 3B). The departure of ZO-1 from the intercellular boundaries with movement into the cytosol increased over time (Fig. 3C–E). Similar results were obtained with Zot (data not shown). These combined data indicate that ZO-1 departs from the cell-cell interface in response to the Zot/AT1002 stimulus.
Figure 3.
AT1002 induces ZO-1 departure from intestinal epithelial intercellular boundaries. Postconfluent IEC6 monolayers were exposed to medium alone (A) or exposed for increasing times to AT1002 (250 μM; B–E), probed for ZO-1, and processed for confocal immunofluorescence microscopy. Untreated monolayers showed typical ZO-1 staining at the cell boundaries (A). Exposure to AT1002 was associated with a loss of ZO-1 at the intercellular junctions (C–E, arrows). ×600.
Effect of AT1002 on in vivo intestinal permeability in mice
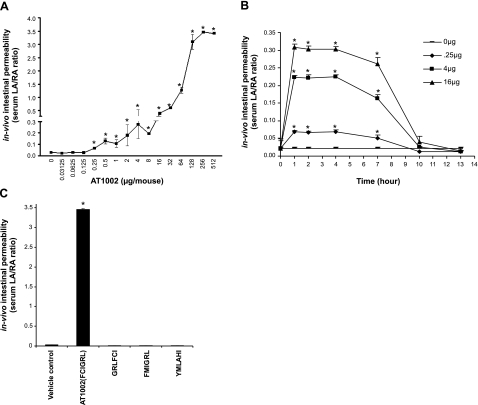
AT1002 displayed comparable dose- and time-dependent TEER-reducing activity in the rat intestine system as did Zot and ΔG (Fig. 1B, C), in vitro. To establish in vivo biological activity for this 6-mer, small intestinal permeability was assessed in mice by means of a dual sugar test with HPLC quantification (Fig. 4). The two sugars, LA and RA, were administered as permeability tracers for the intestinal paracellular and transcellular pathways, respectively. Mice were orally challenged with 100 μl of solution containing 12 mg of LA and 8 mg of RA, sampled at increasing times, and the results are expressed as the mean ± se serum LA/RA ratio. In mice administered AT1002 at doses of >0.25 μg/mice, the serum LA/RA ratio increased compared with that of the simultaneous controls with further dose-dependent increments (Fig. 4A). The AT1002 effect on intestinal permeability plateaued at doses of ≥256 μg/mice. In mice challenged with 3 escalating doses of AT1002, 0.25, 4, and 16 μg/mouse, intestinal permeability was measured over a 12-h period (Fig. 4B). After AT1002 administration, serum LA/RA ratios were dose dependently increased at 1 h, sustained through ≥7 h, and returned to basal levels by ≥10 h. To establish whether the effect of AT1002 (FCIGRL) on intestinal permeability was specific, its permeating activity was compared with that elicited by equivalent doses of 3 scrambled peptide controls (Fig. 4C). The reversal of the first 3 amino acids, substituting the cysteine in position 2 with a methionine, or generating an irrelevant peptide, each totally aborted the permeating activity (Fig. 4C). These results indicate that AT1002 specifically and reversibly increases intestinal permeability in a dose- and time-dependent manner. Further, our combined data indicate that Zot and its derivatives, when used at appropriate concentrations, exert comparable changes in intestinal permeability both ex vivo and in vivo. Accordingly, for the experiments described below, Zot and its derivatives have been used singularly or in combination in an interchangeable manner.
Figure 4.
Effect of AT1002 on in vivo intestinal permeability in mice. A) Mice were orally administered 100 μl of solution containing 12 mg of lactulose LA and 8 mg of RA with increasing concentrations of AT1002. Small intestinal permeability was assessed after 1 h using the dual sugar test with HPLC quantitation (n=5). LA and RA were used as permeability tracers. B) In other experiments, mice were administered LA, RA, and 3 escalating doses of AT1002 (0.25, 4, and 16 μg/mouse); intestinal permeability was measured at 1, 2, 4, 7, 10, and 13 h (n=4). C) Mice were administered AT1002 (250 μg) or equimolar concentrations of 1 of 3 scrambled peptide controls. At 7 h, intestinal permeability was measured (n=4). Intestinal permeability is expressed as mean ± se serum LA/RA ratio (normal values ≤0.03). *P < 0.05 vs. simultaneous controls.
Zot/ΔG alters localization of ZO-1 and occludin in Caco2 cells
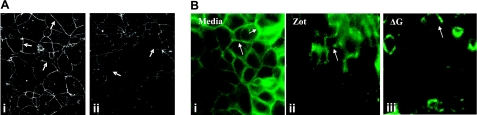
In rat tissues, Zot decreases TEER (Fig. 1B, C) coincident with departure of ZO-1 from intercellular tjs in rat IEC6 cells (Fig. 3). We asked whether these findings could be extended to the human system. Caco2 cells are human colonic adenocarcinoma cells that physiologically mimic the mature small intestine villous epithelium (26). Caco2 monolayers were exposed for 90 min to Zot (30 nM) or the BSA control after which they were analyzed by confocal microscopy. As observed in IEC6 cells (Fig. 3), Zot caused ZO-1 displacement from the cell boundaries (Fig. 5A). We then asked whether occludin was retained within or, like ZO-1, also departed from the tj, in response to the Zot or ΔG stimulus (Fig. 5B). Immunofluorescence microscopy of Zot-treated (Fig. 5Bii) and ΔG-treated (Fig.5Biii) Caco2 cell monolayers revealed markedly diminished occludin staining at cell-cell boundaries compared with the medium control (Fig. 5Bi). Like ZO-1, occludin also exits the intercellular tjs in response to the Zot stimulus.
Figure 5.
Effect of Zot and ΔG on ZO-1 and occludin localization in Caco2 cells. A) Postconfluent Caco2 cells were exposed to medium alone (i) or 30 nM Zot (ii) and processed for confocal immunofluorescence microscopy of ZO-1. Arrows indicate ZO-1 staining (i) that is substantially reduced in Zot-exposed monolayers (ii). ×400. B) Postconfluent Caco2 monolayers were exposed for 90 min to BAS control (i), 30 nM Zot (ii), or 800 nM ΔG (iii), after which they were processed for occludin immunostaining. ×400. Photomicrographs are representative of ≥3 independent experiments.
Disassociation of ZO-1 and occludin
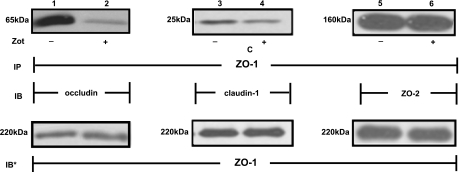
Because the Zot/ΔG stimulus profoundly diminished ZO-1 and occludin localization to the intercellular boundaries, we asked whether ZO-1 physically disengaged from its integral membrane-binding partner, occludin (Fig. 6). When total cell lysates were immunoprecipitated with anti-ZO-1 antibody, occludin was coimmunoprecipitated (Fig. 6, lane 1). After Zot treatment, coimmunoprecipitation of occludin was markedly diminished (Fig. 6, lane 2). These data indicate that Zot reduces ZO-1 association with occludin. We then asked whether the Zot-induced ZO-1-occludin disassociation was specific for occludin. We tested whether ZO-1 might also disengage from either of two of its other established binding partners, claudin 1 and ZO-2 (Fig. 6). Zot reduced ZO-1 coimmunoprecipitation of claudin 1 (Fig. 6, lanes 3, 4) but not of ZO-2 (Fig. 6, lanes 5, 6). Therefore, Zot induces ZO-1 disassociation from its 2 integral membrane binding partners, occludin and claudin 1, but not from the cytoplasmic protein, ZO-2.
Figure 6.
Zot effect on ZO-1 protein-protein interactions. Postconfluent intestinal epithelial Caco-2 cells were treated for 90 min with 30 nM Zot (lanes 2, 4, 6) or BSA (lanes 1, 3, 5), after which they were lysed; lysates were immunoprecipitated (IP) with anti-ZO-1 antibodies, and ZO-1 immunoprecipitates were processed for occludin (lanes 1, 2), claudin-1 (lanes 3, 4), and ZO-2 (lanes 5, 6) immunoblotting (IB). To ensure equal loading and transfer, PVDF membranes were stripped and reprobed with the immunoprecipitating anti-ZO-1 antibody (IB*). Immunoblots are representative of 3 independent experiments.
Effect of PKC inhibition on Zot-induced tj disassembly
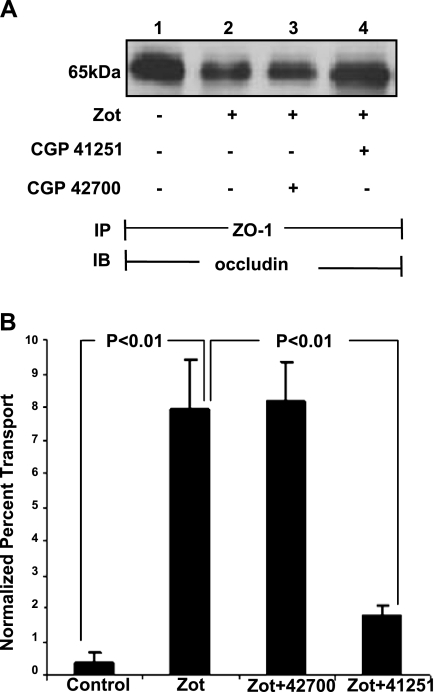
We previously demonstrated that the Zot-induced actin polymerization and reductions in TEER are mediated through PKC activation (14). We asked whether the ability of Zot to diminish the ZO-1-occludin association might also be PKC dependent (Fig. 7A). Zot reduced ZO-1 coimmunoprecipitation of occludin (Fig. 7A, lane 2), and prior PKC inhibition with CGP41251 diminished the Zot effect (Fig. 7A, lane 4), whereas the inactive analog, CGP42700, did not (Fig. 7A, lane 3). In an in vitro barrier assay, Zot also increased the transepithelial flux of mannitol (Fig. 7B). Prior PKC inhibition with CGP41251 protected against the Zot-induced increase in mannitol transfer, whereas the inactive analog, CGP42700, again, did not (Fig. 7B). These data indicate that the influence of Zot on tj protein-protein interactions and barrier integrity is PKC dependent.
Figure 7.
PKC inhibition protects against Zot-induced tj disassembly and increases in paracellular permeability. A) Postconfluent Caco2 monolayers were preincubated for 15 min with a 10 nM concentration of the PKC inhibitor, CGP41251, or the inactive analog, CGP42700, after which they were exposed for 90 min to 30 nM Zot or medium alone and lysed. Lysates were immunoprecipitated (IP) with anti-ZO-1 antibodies; ZO-1 immunoprecipitates were processed for immunoblotting (IB) with anti-occludin antibodies. B) Caco2 cells cultured to confluence in transwell chambers were preincubated for 15 min with the PKC inhibitor, CGP41251 (10 nM), or the inactive analog, CGP42700, followed by exposure for 90 min to 30 nM Zot or medium alone in the presence of the permeability tracer, [14C]mannitol. At 90 min, the top and bottom compartments were collected and counted in a liquid scintillation counter. Mean ± se percentage transport was calculated as [(dpm of treated group −dpm of background)/(dpm of BSA control − dpm of background)] × 100% (n=4).
AT1002 provokes ZO-1 departure from tjs through PKCα activation
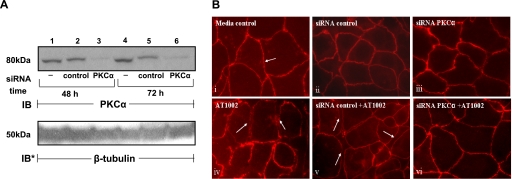
We previously found that Zot activates PKCα (14) and that prior broad-spectrum PKC inhibition protects against Zot-induced actin reorganization (14), disengagement of ZO-1 from occludin (Fig. 6), and increased transepithelial flux of a permeability tracer (Fig. 7). To establish whether PKCα is the operative PKC isoform, Caco2 cells were transfected with PKCα-targeting or control siRNAs and lysed, and the lysates were processed for PKCα immunoblotting (Fig. 8A). In cells transfected with PKCα-targeting siRNAs, PKCα protein was knocked down ≥95% compared with control siRNA-transfected cells (Fig. 8A, lanes 3 vs. 2, 6 vs. 5). In other experiments, AT1002 provoked partial departure of ZO-1 from the intercellular boundaries of control siRNA-transfected cells with cell retraction from neighboring cells (Fig. 8Bv vs. ii). Prior knockdown of PKCα protected against the AT1002-induced ZO-1 departure and intercellular gap formation (Fig. 8Bvi). These data indicate that ZO-1 disassociation from the tj multiprotein complex in response to the Zot stimulus is mediated through PKCα activation.
Figure 8.
Prior PKCα knockdown protects vs. AT1002-induced ZO-1 translocation. A) Caco2 cells were transfected with PKCα-targeting or control siRNAs and after 48 and 72 h were lysed; lysates were processed for PKCα immunoblotting. To indicate protein loading and transfer, blots were stripped and reprobed for β-tubulin. IB, immunoblot; IB*, immunoblot after stripping. Blot is representative of 2 independent experiments. B) Postconfluent Caco2 cells were exposed for 1 h to AT1002 (250 μM; iv) or medium alone (i). Other cells were transfected with either control (ii, v) or PKCα-targeting siRNAs (iii, vi), after which they were treated with AT1002 (v, vi) or medium alone (ii, iii). Cells were processed for ZO-1 immunofluorescence microscopy. Arrows indicate ZO-1 staining at the cell-cell boundaries and areas from which ZO-1 has departed. ×600.
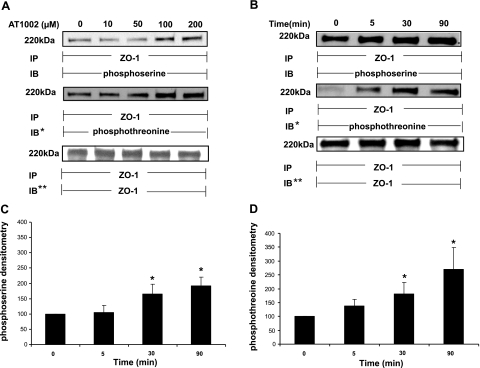
AT1002 increases serine/threonine phosphorylation of ZO-1
The Zot derivative, AT1002, was active in vivo (Fig. 4), and both Zot-induced ZO-1-occludin disengagement and increases in paracellular permeability were PKC dependent (Fig. 7A, B). As a first step to establish whether changes in the phosphorylation status of ZO-1 itself might be involved, phosphoserine and phosphothreonine immunoblotting were enlisted (Fig. 9). Rat IEC6 intestinal epithelial cells were exposed for 0.5 h to increasing concentrations of AT1002, lysed, and immunoprecipitated with anti-ZO-1 antibody. The ZO-1 immunoprecipitates were resolved by SDS-PAGE, transferred to PVDF, and probed with either anti-phosphoserine or anti-phosphothreonine (Fig. 9A) antibodies. AT1002 at ≥100 μM increased serine phosphorylation of ZO-1 with plateauing of the phosphoserine signal at higher concentrations. AT1002 at concentrations as low as 50 μM increased threonine phosphorylation of ZO-1. When cells were exposed for increasing times to a fixed concentration of AT1002 (Fig. 9B), at times ≥30 min, the peptide increased ZO-1 serine phosphorylation compared with the medium control (Fig. 9B, C). Quantitative densitometry of these blots established the mean increases in the phosphoserine signal normalized to ZO-1 to be 65% at 0.5 h and >90% at 1.5 h (Fig. 9C). Threonine phosphorylation of ZO-1 was evident after exposure times as brief as 5 min and achieved significance at ≥30 min (Fig. 9B, D). Quantitative densitometry of these blots demonstrated mean increases of phosphothreonine signal normalized to ZO-1 to be 38, 82, and 170% at 5, 30, and 90 min, respectively (Fig. 9D). These data indicate that AT1002 increased serine and threonine phosphorylation of ZO-1 at concentrations and times compatible with those required for reduction in TEER ex vivo (Fig. 1B, C), increased intestinal permeability in vivo (Fig. 4A, B), and altered protein-protein interactions within the tj multiprotein complex (Fig. 6B).
Figure 9.
AT1002 increases ZO-1 serine/threonine phosphorylation. A) Postconfluent IEC6 monolayers were treated for 30 min with increasing concentrations of AT1002 or medium alone and lysed. ZO-1 was immunoprecipitated (IP) from 500 μg of total cell lysate; ZO-1 immunoprecipitates were processed for phosphoserine immunoblotting (IB) and then were stripped and reprobed for phosphothreonine immunoblotting (IB*). To ensure equal loading and transfer, immunoblots were stripped and reprobed with immunoprecipitating anti-ZO-1 antibody (IB**). B) IEC6 monolayers were treated for increasing times with AT1002 (100 μM) or medium alone and lysed. ZO-1 was immunoprecipitated from 500 μg of total cell lysate, and the ZO-1 immunoprecipitates were processed for phosphoserine immunoblotting (IB) and then were stripped and reprobed for phosphothreonine immunoblotting (IB*). To ensure equal loading and transfer, the immunoblots were stripped and reprobed with the immunoprecipitating anti-ZO-1 antibody (IB**). Molecular mass (kDa) is indicated at left. Blots are representative of ≥3 independent experiments. C, D) For the phosphoserine (C) and phosphothreonine blots (D), densitometric quantification of each phosphoserine and phosphothreonine signal was normalized to the ZO-1 signal for the same lane on the same stripped and reprobed blot. Vertical bars represent mean ± se fold change in arbitrary densitometry units of phosphoserine/threonine signal normalized to arbitrary densitometry units of ZO-1 signal, each relative to the simultaneous control (n=4). *P < 0.05 vs. medium control.
Myosin 1C serine phosphorylation and association with ZO-1
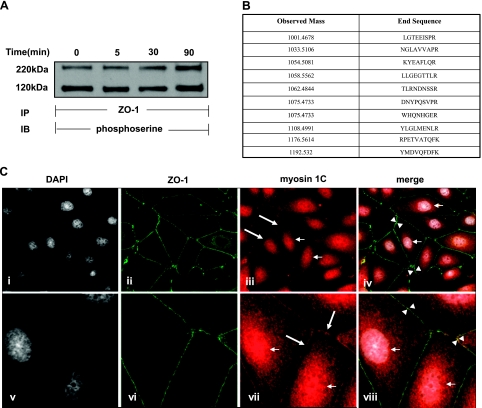
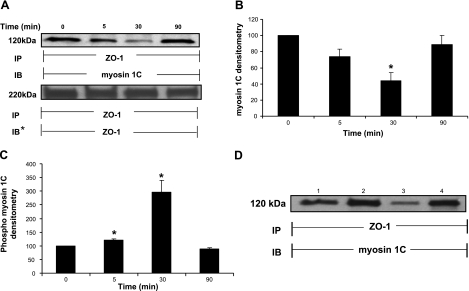
When IEC6 cell monolayers were treated with 100 μM AT1002 for increasing exposure times, immunoprecipitation of ZO-1 revealed a phosphoserine-containing binding partner that migrated with an estimated Mr of ∼120,000 (Fig. 10A). Coomassie Blue staining of the gel revealed the ∼120-kDa band (data not shown). To identify the ∼120-kDa protein, the band was excised and subjected to tandem mass spectrometry (MS/MS) (Fig. 10B). MS/MS analysis revealed the ∼120-kDa protein to be myosin 1C (21). To extend our finding that ZO-1 interacts with myosin 1C in an in vivo intact cell system, MDCK cells stably expressing GFP-ZO-1 were probed for myosin 1C and studied for colocalization with double-label confocal microscopy (Fig. 10C). ZO-1 (Fig. 10Cii, vi) and myosin 1C (Fig. 10Ciii, vii) each could be immunolocalized to the intercellular boundaries. In addition, myosin 1C staining could be immunolocalized to the nucleus (Fig. 10Ciii, vii) (21). When Fig. 10Ci, ii, iii and v, vi, vii were merged, colocalization of ZO-1 and myosin 1C was evident at points of intercellular contact (Fig. 10Civ, viii; arrows). Stripping and reprobing the same blot displayed in Fig. 10A with anti-myosin 1C antibody (Fig. 11A) confirmed the identity of the ∼120 kDa coimmunoprecipitated protein. After exposure for 5, 30, and 90 min to a fixed concentration of AT1002, immunoprecipitation of ZO-1 coimmunoprecipitated less myosin 1C at 5 and 30 min compared with that seen with the medium control (Fig. 11A, B). At 5 min, the decrease in coimmunoprecipitation of myosin 1C approached but did not reach statistical significance (P=0.063). By 90 min, coimmunoprecipitation of myosin 1C returned to the pretreatment baseline. Quantitative densitometry demonstrated the mean decreases in myosin 1C coimmunoprecipitation at 5 and 30 min to be 26 and 56%, respectively (Fig. 11B). These data indicate that, in response to the AT1002 stimulus, the ZO-1-myosin 1C association was transiently disrupted over ≥30 min, with reengagement of these 2 binding partners by 90 min. Having demonstrated increased serine/threonine phosphorylation of ZO-1, we asked whether its binding partner, myosin 1C, might also be modified. When the phosphoserine signal for the ∼120 kDa band, identified as myosin 1C (Fig. 10A), was normalized to myosin 1C coimmunoprecipitating with ZO-1 (Fig. 11A, B), the phosphorylation state of myosin 1C increased temporarily, coincident with its disengagement from ZO-1 (Fig. 11C). To establish which portion of ZO-1 constitutively interacts with myosin 1C, coimmunoprecipitation assays in which ZO-1 was immunoprecipitated from lysates of MDCK cells, cells stably expressing full-length ZO-1, cells stably expressing the NH2-terminal domain (aa 1–876), and cells stably expressing the COOH-terminal domain (aa 1046–1748) were performed (Fig. 11D). MDCK cells were used to control for endogenous ZO-1 (Fig. 11D, lane 1). Immunoprecipitation of ZO-1 from lysates prepared from cells expressing full-length (Fig. 11D, lane 2) and COOH-terminal ZO-1 (Fig. 11D, lane 4) coimmunoprecipitated comparable amounts of myosin 1C, whereas myosin 1C coimmunoprecipitated from cells expressing the ZO-1 NH2-terminal domain was markedly reduced (Fig. 11D, lane 3). These data indicate that the COOH-terminal portion of ZO-1 is required for full ZO-1-myosin 1C association. Therefore, myosin 1C is a substrate for Zot-induced serine phosphorylation and constitutively directly/indirectly associates with the COOH-terminal portion of another substrate for Zot-induced serine phosphorylation, ZO-1, from which it transiently disengages in response to the Zot stimulus.
Figure 10.
Association of ZO-1 and myosin 1C. A) IEC6 monolayers were treated with AT1002 (100 μM) or medium alone for increasing times and lysed. ZO-1 was immunoprecipitated (IP) from 500 μg of total cell lysate; ZO-1 immunoprecipitates were processed for phosphoserine immunoblotting (IB). B) MS/MS spectroscopy of the excised band migrating with a molecular mass of ∼120 kDa (see A). C) MDCK cells stably expressing GFP-ZO-1 were fixed and counterstained with DAPI (i, v), incubated with medium alone (ii, vi), or probed with anti-myosin 1C antibody followed by TRITC-conjugated anti-rabbit IgG (iii, vii). In iv and viii, GFP-ZO-1, myosin 1C, and DAPI were merged. View: ×600 (i–iv); ×1000 (v–viii). Small arrows indicate myosin 1C localization to nucleus. Large arrows indicate myosin 1C localization to intercellular boundaries. Arrowheads indicate areas of colocalization of ZO-1 with myosin 1C (yellow-orange).
Figure 11.
AT1002 alters the interaction between ZO-1 and myosin 1C. A) IEC6 monolayers were treated with AT1002 (100 μM) or medium alone for increasing times and lysed. ZO-1 was immunoprecipitated (IP) from 500 μg of total cell lysate; ZO-1 immunoprecipitates were processed for immunoblotting (IB) with anti-myosin 1C antibodies. To ensure equal loading and transfer, blots were stripped and reprobed with the immunoprecipitating anti-ZO-1 antibody (IB*). B) Quantitative densitometry of coimmunoprecipitated myosin 1C. Bars represent mean ± se fold change of arbitrary densitometry units of ZO-1 signal normalized to arbitrary densitometry units of myosin 1C signal, each relative to the simultaneous control (n=4). *P < 0.05 vs. medium control. C) Quantitative densitometry of phosphoserine signal for myosin 1C coimmunoprecipitating with ZO-1. Bars represent mean ± se fold change of arbitrary densitometry units of phosphoserine signal normalized to arbitrary densitometry units of myosin 1C signal coimmunoprecipitating with ZO-1, each relative to the simultaneous control (n=4), *P < 0.05 vs. medium control. D) Lysates from MDCK cells (lane 1) and cells stably expressing full-length ZO-1 (lane 2), ZO-1 NH2-terminal domain (aa 1–876; lane 3), or ZO-1 COOH-terminal domain (aa 1046–1748; lane 4) were each immunoprecipitated with anti-ZO-1 antibodies; ZO-1 immunoprecipitates were processed for myosin 1C immunoblotting. Blots are representative of ≥3 independent experiments.
DISCUSSION
Since our initial studies delineating the roles of actin reorganization and PKC activation in barrier responsiveness to Zot (14), much information has been generated to identify the molecular components of the tj and the prerequisite events leading to tj assembly (1). However, the mechanisms through which the mature, already formed, tj multiprotein complex is dynamically regulated or disassembled remain poorly understood. In the current studies, we now have established that Zot/ΔG/AT1002 induces disassembly of mature tjs through PAR2 activation (Fig. 2). Multiple biological effects coupled to PAR2 activation are reportedly PKC-mediated (27). Accordingly, we asked whether PKC activation might be an early step in the AT1002-mediated cascade of events leading to tj disassembly. AT1002 increased PKC-dependent serine/threonine phosphorylation of ZO-1 (Fig. 9) and diminished its associations with both occludin and claudin 1 but not ZO-2 (Fig. 6) coincident with ZO-1 and occludin displacement from the junctional complex (Figs. 3 and 5). More specifically, prior knockdown of PKCα protected against Zot-induced departure of ZO-1 from the tj (Fig. 8). AT1002 has been reported to induce ZO-1 departure from cell-cell boundaries (28). Interestingly, the Zot stimulus also increased serine phosphorylation of myosin 1C (Fig. 11C), temporally coincident with its transient disassociation from ZO-1 (Fig. 11A, B) and increased intestinal permeability (Figs. 1 and 4).
Our in vitro, ex vivo (Fig. 1), and in vivo (Fig. 4) experiments demonstrate that Zot, its mature 12-kDa form, ΔG, and the NH2-terminal mature Zot PAR-AP 6-mer motif, AT1002 (FCIGRL), each reversibly provokes tj disassembly, with bioactivity increasing with the molecular mass of the compound (Zot>ΔG>AT1002; Fig. 1). Although AT1002 exerts a tj response comparable to those seen with full-length Zot and ΔG, 100- to 10,000-fold higher concentrations are required (Fig. 1). It is conceivable that AT1002 might disrupt barrier integrity through cytotoxicity. However, we have previously reported that exposure to AT1002 at concentrations up to 70-fold and exposure times up to 2-fold to those used in the current studies failed to induce cell injury (28). PAR2-AP (SLIGRL) and the NH2 terminus of the Zot extracellular domain cleaved by V. cholerae (FCIGRL aa residues 288–293) (25) share structural similarities. Activation of intestinal PAR2 increases intestinal paracellular permeability (29). PAR2 is activated by proteolytic cleavage of its extracellular NH2-terminal domain, creating a new NH2 terminus that acts as a “tethered ligand” (30). Exogenous addition of the peptide SLIGRL (PAR2-AP) also activates PAR2, independently of receptor cleavage (31). PAR2 signaling shares elements with signaling events initiated by the Zot/zonulin stimulus, including activation of PKC (31) and actin polymerization (32). Recent studies have shown that PAR2 colocalizes with multiple PKC isoforms and that PAR2 activation promotes translocation of PKC catalytic subunits from the cytosol to the plasma membrane (i.e., activation) (27, 33). Taken together, the PAR2-AP Zot motif and the previously established Zot-induced signaling are compatible with PAR2 as the target receptor for Zot. Our AT1002 competitive binding studies (Fig. 2A, B) together with the barrier-unresponsiveness of small intestinal fragments isolated from PAR2-null mice to AT1002 exposure (Fig. 2C), indicate that PAR2, alone or in concert with other receptors, engages the FCIGRL motif in Zot and mediates its barrier-disrupting activity.
Of the many PKC family members, only a selected number, including PKCα and PKCζ, have been associated with tj regulation (34). In the gastrointestinal epithelial cell lines, IEC-18 and LLC-PK1, the PKC-activating phorbol ester, 12-O-tetradecanoylphorbol-13-acetate, decreased TEER with a concomitant increase in the passage of polyethylene glycol molecular weight 4000 (PEG-4000), a paracellular transport tracer (13). In contrast, the proinflammatory cytokines, TNF-α and IFNγ, cooperatively provoke intestinal tj disassembly through myosin light chain kinase activation (35). While providing information on the link between phosphorylation events and changes in epithelial permeability, studies using phorbol esters do not necessarily reflect the physiological events involved in the modulation of mature tjs.
Intracellular signals either directly modifying the tj multiprotein complex itself or influencing the tj-actin cytoskeletal linkage offer two mechanisms (one charge and size dependent and the other size and charge independent) through which tjs may be regulated (34). Claudins have been experimentally connected to selective and charge-dependent paracellular permeability of small molecules (<4 Å) such as Na+ and Cl− (36). The claudins are 20- to 27-kDa integral membrane proteins, each of which contains two extracellular loops with variably charged amino acid residues that dictate charge selectivity and short cytoplasmic tails (37). Although the mechanisms used by claudins remain to be established, occludin phosphorylation at its COOH-terminal cytosolic domain has been linked to the paracellular charge- and size-independent passage of molecules larger than 4 Å (34). Occludin comprises four transmembrane domains, two extracellular loops, a short intracellular turn, and NH2- and COOH-terminal cytoplasmic domains. The extracellular loops participate in homotypic adhesion (38); deletion of the second loop interferes with occludin localization (39).
Although changes in ZO-1 phosphorylation reportedly occur during tj disassembly (37), controversy as to whether such modifications occur exists (34). We now have provided, for the first time, evidence that the PKC-mediated serine/threonine phosphorylation of ZO-1 is operative during the reversible opening of mature intercellular tjs. ZO-1 is a 225-kDa phosphoprotein that belongs to the MAGuK family of scaffolding and signaling molecules that share several protein-binding domains, including three PDZ domains, a src homology (SH) 3 domain, and a GUK domain (40). These structural features allow ZO-1 to play a central role in organization of the tj multiprotein complex; it tethers integral membrane tj proteins to both other MAGuK members and to the actin cytoskeleton (18, 39, 41). The ZO-1 PDZ1 domain also appears to interact with the claudins (40). The ZO-1 unique 5 (U5) motif located between the SH3 and GUK domains interacts with integral membrane protein occludin (42). Finally, a unique 220-aa proline-rich region in the COOH-terminal portion of ZO-1 interacts directly with the actin cytoskeleton (39). Interestingly, ZO-1 contains serine and threonine residues within each of the domains described above. Identification of the specific serine and threonine residues phosphorylated in response to the AT1002 stimulus to participate in the altered protein-protein interactions described here are currently under investigation.
The ZO-1 coimmunoprecipitation experiments revealed that, in addition to occludin, another phosphoserine-containing protein with apparent Mr of ∼120,000 constitutively associates with and transiently disengages from ZO-1 in response to the AT1002 stimulus (Fig. 11B). We have now identified this phosphoserine-containing protein as myosin-1C (Fig. 10B). Full expression of the constitutive association of myosin 1C with ZO-1 requires the ZO-1 COOH terminus (Fig. 11D). Colocalization of ZO-1 with myosin 1C in MDCK cells extended these findings to an in vivo intact cell system (Fig. 10Civ, viii). These results indicate that ZO-1-myosin-1C association is diminished during reversible tj disassembly after hyperphosphorylation of both binding partners. Type I myosins are highly conserved, actin-based molecular motors that localize to the actin-rich cortex and participate in several cell functions, including endocytosis, polarized morphogenesis, and cell migration (43). Further, type 1 myosins are reportedly involved in modulating actin assembly and polymerization due to phosphorylation of the myosin motor domain (43–45). Functional activities of many nonmuscle myosin isoforms are regulated by heavy chain phosphorylation. Depending on the myosin isoform, the serine or threonine residues located within the head (myosin I or myosin VI) or within the COOH-terminal tail domains (myosin II or myosin V) can be phosphorylated by more or less specific endogenous kinases (46). Furthermore, myosin contractility seems to be pivotal in the actin polymerization process (47). Based on this evidence and on our previous reports showing that Zot-induced tj disassembly requires PKC-driven actin polymerization (14), it is conceivable that Zot-induced myosin 1C phosphorylation (Fig. 11C) and its coincident disassociation from ZO-1 (Fig. 11A, B) participate in the intracellular signaling that promotes actin polymerization and tj disassembly. This observation is further supported by recent evidence showing that myosin-1C colocalizes with polymerized F-actin in the intestinal cell periphery and microvilli (48).
In summary, our combined data support the following hypothetical model: engagement of PAR2 by the mature NH2-terminal Zot 6-aa PAR-AP-like motif, alone or in partnership with one or more other receptors, activates PKCα and possibly other PKC isoforms, which, in turn, increases serine-threonine phosphorylation of ZO-1, coincident with loss of ZO-1-occludin and ZO-1-claudin 1 association. At the same time, Zot increases myosin 1C phosphorylation and transiently decreases its association with ZO-1 COOH-terminal domain. The concomitant serine/threonine phosphorylation events and PKCα-dependent disengagement of ZO-1 from the 2 key membrane-spanning tj proteins, occludin and claudin 1, indicate that phosphorylation of tj proteins is a prerequisite for their disengagement. The concomitant actin polymerization provides the “tj disassembly force” by temporarily removing myosin-1C from the junctional complex, leading to ZO-1 departure from the tj complex, loss of occludin association with its membrane boundaries, and, ultimately, tj disassembly.
Acknowledgments
This work was supported in part by the U.S. National Institutes of Health (grant DK048373 to A.F.).
REFERENCES
- 1. González-Mariscal L. (2006) Tight Junctions. Landes Bioscience and Springer Science + Business Media, New York [Google Scholar]
- 2. Fasano A., Baudry B., Pumplin D. W., Wasserman S. S., Tall B. D., Ketley J. M., Kaper J. B. (1991) Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc. Natl. Acad. Sci. U. S. A. 88, 5242–5246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Di Pierro M., Lu R., Uzzau S., Wang W., Margaretten K., Pazzani C., Maimone F., Fasano A. (2001) Zonula occludens toxin structure-function analysis. Identification of the fragment biologically active on tight junctions and of the zonulin receptor binding domain. J. Biol. Chem. 276, 19160–19165 [DOI] [PubMed] [Google Scholar]
- 4. Hollenberg M. D. (2003) Proteinase-mediated signaling: Proteinase-activated receptors (PARs) and much more. Life Sci. 74, 237–246 [DOI] [PubMed] [Google Scholar]
- 5. Balda M. S., Matter K. (2000) Transmembrane proteins of tight junctions. Semin. Cell Dev. Biol. 11, 281–289 [DOI] [PubMed] [Google Scholar]
- 6. Fanning A. S., Mitic L. L., Anderson J. M. (1999) Transmembrane proteins in the tight junction barrier. J. Am. Soc. Nephrol. 10, 1337–1345 [DOI] [PubMed] [Google Scholar]
- 7. Meyer T. N., Schwesinger C., Denker B. M. (2002) Zonula occludens-1 is a scaffolding protein for signaling molecules. Gα12 directly binds to the Src homology 3 domain and regulates paracellular permeability in epithelial cells. J. Biol. Chem. 277, 24855–24858 [DOI] [PubMed] [Google Scholar]
- 8. Umeda K., Ikenouchi J., Katahira-Tayama S., Furuse K., Sasaki H., Nakayama M., Matsui T., Tsukita S., Furuse M., Tsukita S. (2006) ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell 126, 741–754 [DOI] [PubMed] [Google Scholar]
- 9. Mitic L. L., Schneeberger E. E., Fanning A. S., Anderson J. M. (1999) Connexin-occludin chimeras containing the ZO-binding domain of occludin localize at MDCK tight junctions and NRK cell contacts. J. Cell Biol. 146, 683–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fanning A. S., Jameson B. J., Jesaitis L. A., Anderson J. M. (1998) The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 273, 29745–29753 [DOI] [PubMed] [Google Scholar]
- 11. Anderson J. M., Fanning A. S., Lapierre L., Van Itallie C. M. (1995) Zonula occludens (ZO)-1 and ZO-2: membrane-associated guanylate kinase homologues (MAGuKs) of the tight junction. Biochem. Soc. Trans. 23, 470–475 [DOI] [PubMed] [Google Scholar]
- 12. Balda M. S., Anderson J. M., Matter K. (1996) The SH3 domain of the tight junction protein ZO-1 binds to a serine protein kinase that phosphorylates a region C-terminal to this domain. FEBS Lett. 399, 326–332 [DOI] [PubMed] [Google Scholar]
- 13. Mullin J. M., Laughlin K. V., Ginanni N., Marano C. W., Clarke H. M., Peralta Soler A. (2000) Increased tight junction permeability can result from protein kinase C activation/translocation and act as a tumor promotional event in epithelial cancers. Ann. N. Y. Acad. Sci. 915, 231–236 [DOI] [PubMed] [Google Scholar]
- 14. Fasano A., Fiorentini C., Donelli G., Uzzau S., Kaper J. B., Margaretten K., Ding X., Guandalini S., Comstock L., Goldblum S. E. (1995) Zonula occludens toxin modulates tight junctions through protein kinase C-dependent actin reorganization, in vitro. J. Clin. Invest. 96, 710–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sheth B., Fesenko I., Collins J. E., Moran B., Wild A. E., Anderson J. M., Fleming T. P. (1997) Tight junction assembly during mouse blastocyst formation is regulated by late expression of ZO-1 α+ isoform. Development 124, 2027–2037 [DOI] [PubMed] [Google Scholar]
- 16. Anderson J. M., Van Itallie C. M., Peterson M. D., Stevenson B. R., Carew E. A., Mooseker M. S. (1989) ZO-1 mRNA and protein expression during tight junction assembly in Caco-2 cells. J. Cell Biol. 109, 1047–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Al-Ani B., Saifeddine M., Kawabata A., Renaux B., Mokashi S., Hollenberg M. D. (1999) Proteinase-activated receptor 2 (PAR2): development of a ligand-binding assay correlating with activation of PAR2 by PAR1- and PAR2-derived peptide ligands. J. Pharmacol. Exp. Ther. 290, 753–760 [PubMed] [Google Scholar]
- 18. Fanning A. S., Ma T. Y., Anderson J. M. (2002) Isolation and functional characterization of the actin binding region in the tight junction protein ZO-1. FASEB J. 16, 1835–1837 [DOI] [PubMed] [Google Scholar]
- 19. Utepbergenov D. I., Fanning A. S., Anderson J. M. (2006) Dimerization of the scaffolding protein ZO-1 through the second PDZ domain. J. Biol. Chem. 281, 24671–24677 [DOI] [PubMed] [Google Scholar]
- 20. El Asmar R., Panigrahi P., Bamford P., Berti I., Not T., Coppa G. V., Catassi C., Fasano A. (2002) Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology 123, 1607–1615 [DOI] [PubMed] [Google Scholar]
- 21. Pestic-Dragovich L., Stojiljkovic L., Philimonenko A. A., Nowak G., Ke Y., Settlage R. E., Shabanowitz J., Hunt D. F., Hozak P., de Lanerolle P. (2000) A myosin I isoform in the nucleus. Science 290, 337–341 [DOI] [PubMed] [Google Scholar]
- 22. Katouzian F., Sblattero D., Not T., Tommasini A., Giusto E., Meiacco D., Stebel M., Marzari R., Fasano A., Ventura A. (2005) Dual sugar gut-permeability testing on blood drop in animal models. Clin. Chim. Acta 352, 191–197 [DOI] [PubMed] [Google Scholar]
- 23. Young B. A., Wang P., Goldblum S. E. (1998) The counteradhesive protein SPARC regulates an endothelial paracellular pathway through protein tyrosine phosphorylation. Biochem. Biophys. Res. Commun. 251, 320–327 [DOI] [PubMed] [Google Scholar]
- 24. Liu A., Garg P., Yang S., Gong P., Pallero M. A., Annis D. S., Liu Y., Passaniti A., Mann D., Mosher D. F., Murphy-Ullrich J. E., Goldblum S. E. (2009) Epidermal growth factor-like repeats of thrombospondins activate phospholipase Cγ and increase epithelial cell migration through indirect epidermal growth factor receptor activation. J. Biol. Chem. 284, 6389–6402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Uzzau S., Cappuccinelli P., Fasano A. (1999) Expression of Vibrio cholerae zonula occludens toxin and analysis of its subcellular localization. Microb. Pathog. 27, 377–385 [DOI] [PubMed] [Google Scholar]
- 26. Sambury Y., De Angelis I., Ranaldi G., Scarino M. L., Stammati A., Zucco F. (2005) The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 21, 1–26 [DOI] [PubMed] [Google Scholar]
- 27. Amadesi S., Cottrell G. S., Divino L., Chapman K., Grady E. F., Bautista F., Karanjia R., Barajas-Lopez C., Vanner S., Vergnolle N., Bunnett N. W. (2006) Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cε- and A-dependent mechanisms in rats and mice. J. Physiol. 575, 555–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gopalakrishnan S., Pandey N., Tamiz A. P., Vere J., Carrasco R., Somerville R., Tripathi A., Ginski M., Paterson B. M., Alkan S. S. (2009) Mechanism of action of ZOT-derived peptide AT-1002, a tight junction regulator and absorption enhancer. Int. J. Pharm. 365, 121–130 [DOI] [PubMed] [Google Scholar]
- 29. Cenac N., Garcia-villar R., Ferrier L., Larauche M., Vergnolle N., Bunnett N. W., Coelho A. M., Fioramonti J., Bueno L. (2003) Proteinase-activated receptor-2-induced colonic inflammation in mice: possible involvement of afferent neurons, nitric oxide, and paracellular permeability. J. Immunol. 170, 4296–4300 [DOI] [PubMed] [Google Scholar]
- 30. Bueno L., Fioramonti J. (2008) Protease-activated receptor 2 and gut permeability: a review. Neurogastroenterol. Motil. 20, 580–587 [DOI] [PubMed] [Google Scholar]
- 31. Macfarlane S. R., Seatter M. J., Kanke T., Hunter G. D., Plevin R. (2001) Proteinase-activated receptors. Pharmacol. Rev. 53, 245–282 [PubMed] [Google Scholar]
- 32. Sharlow E. R., Paine C. S., Babiarz L., Eisinger M., Shapiro S., Seiberg M. (2000) The protease-activated receptor-2 upregulates keratinocyte phagocytosis. J. Cell Sci. 113, 3093–3101 [DOI] [PubMed] [Google Scholar]
- 33. Sato S., Ito Y., Kondo M., Ohashi T., Ito S., Nakayama S., Shimokata K., Kume H. (2005) Ion transport regulated by protease-activated receptor 2 in human airway Calu-3 epithelia. Br. J. Pharmacol. 146, 397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Benais-Pont G., Matter K., Balda M. S. (2001) Intracellular signaling in classical and new tight junction functions. In Tight Junctions (Cereijido M., Anderson J. M. eds) pp. 367–394, CRC Press, Boca Raton, FL, USA [Google Scholar]
- 35. Su L., Shen L., Clayburgh D. R., Nalle S. C., Sullivan E. A., Meddings J. B., Abraham C., Turner J. R. (2009) Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology 136, 551–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Van Itallie C. M., Holmes J., Bridges A., Gookin J. L., Coccaro M. R., Proctor W., Colegio O. R., Anderson J. M. (2008) The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J. Cell Sci. 121, 298–305 [DOI] [PubMed] [Google Scholar]
- 37. Fanning A. S., Little B. P., Rahner C., Utepbergenov D., Walther Z., Anderson J. M. (2007) The unique-5 and -6 motifs of ZO-1 regulate tight junction strand localization and scaffolding properties. Mol. Biol. Cell 18, 721–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Medina R., Rahner C., Mitic L., Anderson J., Van Itallie C. M. (2000) Occludin localization at the tight junction requires the second extracellular loop. J. Membr. Biol. 178, 235–247 [DOI] [PubMed] [Google Scholar]
- 39. Songyang Z., Fanning A. S., Fu C., Xu J., Marfatia S. M., Chishti A. H., Crompton A., Chan A. C., Anderson J. M., Cantley L. C. (1997) Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science 275, 73–77 [DOI] [PubMed] [Google Scholar]
- 40. Itoh M., Furuse M., Morita K., Kubota K., Saitou M., Tsukita S. (1999) Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J. Cell Biol. 147, 1351–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fanning A. S. (2006) ZO proteins and tight junction assembly. In Tight Junctions (González-Mariscal L. ed), Landes Bioscience and Springer Science + Business Media, New York [Google Scholar]
- 42. Van Itallie C. M., Anderson J. M. (1997) Occludin confers adhesiveness when expressed in fibroblasts. J. Cell Sci. 110, 1113–1121 [DOI] [PubMed] [Google Scholar]
- 43. Machesky L. M. (2000) The tails of two myosins. J. Cell Biol. 148, 219–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lechler T., Shevchenko A., Li R. (2000) Direct involvement of yeast type I myosins in Cdc42-dependent actin polymerization. J. Cell Biol. 148, 363–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shen L., Balck E. D., Witkowski E. D., Lencer W. I., Guerriero V., Schneeberger E. E., Turner J. R. (2006) Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J. Cell Sci. 119, 2095–2106 [DOI] [PubMed] [Google Scholar]
- 46. Redowicz M. J. (2001) Effect of Rho family GTP-binding proteins on Amoeba proteus. J. Muscle Res. Cell. Motil. 22, 163–17311519739 [Google Scholar]
- 47. Hirata H., Tatsumi H., Sokabe M. (2008) Mechanical forces facilitate actin polymerization at focal adhesions in a zyxin-dependent manner. J. Cell Sci. 121, 2795–2804 [DOI] [PubMed] [Google Scholar]
- 48. Wagner M. C., Molitoris B. A. (1997) ATP depletion alters myosin I beta cellular location in LLC-PK1 cells. Am. J. Physiol. Cell Physiol. 272, C1680–C1690 [DOI] [PubMed] [Google Scholar]