Abstract
Elevated generation of reactive oxygen species (ROS) by endothelial enzymes, including NADPH-oxidase, is implicated in vascular oxidative stress and endothelial proinflammatory activation involving exposure of vascular cell adhesion molecule-1 (VCAM-1). Catalase and superoxide dismutase (SOD) conjugated with antibodies to platelet/endothelial cell adhesion molecule 1 (PECAM-1) bind specifically to endothelium and inhibit effects of corresponding ROS, H2O2, and superoxide anion. In this study, anti-PECAM/SOD, but not anti-PECAM/catalase or nontargeted enzymes, including polyethylene glycol (PEG)-SOD, inhibited 2- to 3-fold VCAM expression caused by tumor necrosis factor (TNF), interleukin-1β, and lipopolysaccharide. Anti- PECAM/SOD, but not nontargeted counterparts, accumulated in vascular endothelium after intravenous injection, localized in endothelial endosomes, and inhibited by 70% lipopolysaccharide-caused VCAM-1 expression in mice. Anti-PECAM/SOD colocalized with EEA-1-positive endothelial vesicles and quenched ROS produced in response to TNF. Inhibitors of NADPH oxidase and anion channel ClC3 blocked TNF-induced VCAM expression, affirming that superoxide produced and transported by these proteins, respectively, mediates inflammatory signaling. Anti-PECAM/SOD abolished VCAM expression caused by poly(I:C)-induced activation of toll-like receptor 3 localized in intracellular vesicles. These results directly implicate endosomal influx of superoxide in endothelial inflammatory response and suggest that site-specific interception of this signal attained by targeted delivery of anti-PECAM/SOD into endothelial endosomes may have anti-inflammatory effects.—Shuvaev, V. V., Han, J., Yu, K. J., Huang, S., Hawkins, B. J., Madesh, M., Nakada, M., and Muzykantov, V. R. PECAM-targeted delivery of SOD inhibits endothelial inflammatory response.
Keywords: drug delivery, oxidative stress, superoxide anion, endocytosis
Reactive oxygen species (ROS) mediate many cellular physiological and pathological processes, including redox-sensitive signaling and oxidative stress (1–6). Endothelial cells lining the vascular lumen represent an important source and target of ROS produced by enzymes, including mitochondrial respiratory chain (7), xanthine oxidase (8), and NADPH oxidases (9). Elevated endothelial ROS flux is implicated in ischemia, inflammation, stroke, acute lung injury, acute respiratory distress syndrome, acute myocardial infarction, atherosclerosis, hypertension, and diabetes, among other maladies (8, 10).
One particularly intriguing yet not fully understood aspect is the role of endothelial ROS in inflammation. Inflammatory agonists [e.g., cytokines tumor necrosis factor (TNF) or interleukin-1β (IL-1β)] cause endothelial activation manifested, among other signs, by expression of adhesion molecules [e.g., vascular cell adhesion molecule-1 (VCAM-1)] mediating leukocyte migration (11). Agonists, including cytokines, stimulate NADPH oxidases to produce ROS (11) involved in proinflammatory signaling (12). Transmembrane NADPH oxidases flux superoxide in the extracellular milieu and cellular compartments, including endosomes (13). Superoxide transforms into H2O2 in a rapid reaction that is further accelerated by superoxide dismutase (SOD). In endothelium, extracellular isoform of SOD bound to glycocalyx rapidly quenches superoxide released into the milieu by plasmalemmal NADPH oxidase (14). Superoxide diffuses poorly through cellular membranes but can utilize for this purpose anion chloride channel ClC3 in the plasmalemma and endosomal membranes (15, 16). Superoxide released by NADPH oxidase into endosomes has been implicated in inflammatory signaling in some cell types in vitro (17). Yet, the role of ROS in endothelial inflammatory activation is not fully understood, in part due to inadequate means for site-specific interventions in ROS-mediated processes. For example, administration of polyethylene glycol (PEG)-modified SOD, as well as SOD gene delivery, elevates tissue level of the enzyme activity and provides protective effects in animal models of oxidative stress (18–22). However, these and other nontargeted approaches cannot provide site-specific antioxidant interventions in given cell types or in subcellular compartments, such as endothelial endosomes.
Previous studies from our and other labs indicate that this problem can be solved by immunotargeting antioxidant enzymes to specific endothelial epitopes (23, 24). SOD and catalase conjugated with antibodies to platelet-endothelial adhesion molecule-1 (anti-PECAM/SOD and anti-PECAM/catalase) are delivered specifically to endothelial cells and degrade superoxide and H2O2, respectively (25, 26). Anti-PECAM/SOD and anti-PECAM/catalase, but not nontargeted enzymes, alleviate vascular oxidative stress: anti-PECAM/catalase attenuates lung ischemia/reperfusion injury (27, 28), while anti-PECAM/SOD inhibits angiotensin II-induced vasoconstriction in mice (28). In this work, we characterized delivery of these targeted antioxidants into endothelial endosomes and employed this new molecular intervention to study the role of endosomal ROS in endothelial response to proinflammatory agonists and to design site-specific antioxidant treatment.
METHODS AND MATERIALS
Cell culture and treatment
Human umbilical endothelial cells (HUVECs) were maintained in M199 medium (Gibco, Grand Island, NY, USA) with 15% FBS supplemented with 100 μg/ml heparin (Sigma, St. Louis, MO, USA), 2 mM l-glutamine (Gibco), 15 μg/ml endothelial cell growth supplement (Upstate, Lake Placid, NY, USA), 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco). For cytokine treatment, cells were incubated overnight with 0.5% FBS, and 10 ng/ml TNF or IL-1β was added to cells for indicated time. Lipopolysaccharide (LPS; 0.5 μg/ml) was added to cells in complete medium. Preliminary experiments showed increased VCAM expression starting after 3–4 h (Fig. 1). In protection experiments, cells were pretreated with antioxidant enzymes (75 μg/ml of SOD or anti-PECAM/SOD and 100 μg/ml of catalase or anti-PECAM/catalase) for 1 h prior to 4-h stimulation, and the antioxidant enzymes were present in the medium throughout the experiment. In experiments with Toll-like receptor 3 (TLR3), ligand polyinosine-polycytidylic acid [poly(I:C)] cells were incubated overnight with 0.5% FBS, and antioxidant enzymes were added along with the agent for 5 h. Pharmacological agents were used at the following concentrations: diphenyleneiodonium (DPI; 20 μM), apocynin (100–500 μM), 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid disodium salt (DIDS; 25 μM), phloretin (30 μM). Stock solutions of all inhibitors were prepared in DMSO. Inhibitors were added 15 min prior to 6-h cell stimulation by TNF.
Figure 1.
Kinetics of endothelial cell activation by proinflammatory agents. A–C) HUVECs were treated with TNF (10 ng/ml; A), IL-1β (10 ng/ml; B), or LPS (0.5 μg/ml; C) for indicated times, and samples were subjected to Western blot analysis of VCAM. D) Quantification of VCAM expression level normalized by actin and to TNF effect according to relative extent of VCAM expression (inset).
Conjugate preparation
Conjugation via amino chemistry was used to prepare anti-PECAM/enzyme conjugates as described previously (28). Heterobifunctional cross-linker 4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC) was used to introduce maleimide reactive group into the enzyme molecule. The reaction was performed at 20- to 100-fold molar excess of SMCC at room temperature for 1 h. In parallel, sulfhydryl groups were introduced into the antibody molecule through the primary amine using N-succinimidyl-S-acetylthioacetate (SATA) at 20-fold molar excess at room temperature for 30 min. Sulfhydryl groups were deprotected using 50 mM hydroxylamine for 2 h. Antibody was conjugated with catalase or SOD at 1:1 or 1:2 IgG to enzyme molar ratio, respectively. At each step, unreacted components were removed using spin protein columns (G-25 Sephadex; Roche Applied Science, Indianapolis, IN). The effective diameter of the obtained conjugates was measured by a dynamic light-scattering apparatus (90Plus Particle Sizer; Brookhaven Instruments Corp., Holtsville, NY, USA). Conjugates in 7% sucrose were frozen by liquid nitrogen and stored at −80°C. For binding studies, in vitro and in vivo SOD and catalase were radiolabeled with Na125I using Iodogen (Pierce Biotechnology, Rockford, IL, USA), as recommended by the manufacturer, prior to the conjugation. Anti-PECAM mouse monoclonal antibody (clone mAb 62) to human PECAM (25, 29) was used throughout in vitro studies and a rat monoclonal antibody against murine PECAM (clone MEC-13.3; BD Biosciences, San Jose, CA, USA) was used for animal studies.
Western blot analysis
Cells in 24-well culture dishes (∼105 cells/well) were washed twice with PBS and lysed in 100 μl of sample buffer for sodium dodecyl sulfate polyacrylamide gel electrophoresis. Cell proteins were subjected to 4–15% gradient gel. Gels were transferred to PVDF membrane (Millipore, Billerica, MA, USA), and the membrane was blocked with 3% nonfat dry milk in TBS-T (100 mM Tris, pH 7.5; 150 mM NaCl; and 0.1% Tween 20) for 1 h, followed by incubations with primary and secondary antibodies in the blocking solution. The blot was detected using ECL Plus reagents (GE Healthcare, New York, NY, USA).
Fluorescence microscopy
Cells were grown on microscope glass coverslips, treated with mouse anti-human PECAM immunoconjugates, and fixed with ice-cold 2% paraformaldehyde for 15 min. For internalization studies, cells were treated first with Alexa Fluor 594-labeled goat anti-mouse IgG, then permeabilized with 0.2% Triton X-100 for 15 min and treated with Alexa Fluor 488 goat anti-mouse IgG (30). For colocalization studies, cells were permeabilized and treated with rabbit polyclonal antibodies to early endosome antigen-1 (anti-EEA-1), washed, and treated with a mixture of secondary goat Alexa Fluor 488 anti-mouse IgG and Alexa Fluor 594 anti-rabbit IgG. Endosomal ROS generation by TNF-stimulated cells was detected by preincubation of the cells with redox-sensitive nonpermeable dye OxyBURST Green H2HFF BSA (Invitrogen, Carlsbad, CA, USA) for 5 min, followed by stimulation with TNF for 20 min before washing, fixation, and microscopy analysis (12). Samples were mounted using ProLong Gold antifade reagent with DAPI (Invitrogen), and fluorescence images were acquired using a Nikon Eclipse TE2000-U fluorescence microscope equipped with a Plan Apo ×40/1.0 oil objective (Nikon, Tokyo, Japan. Microscope controlling and image processing were carried out using Image-Pro Plus 4.5.1.27 (Media Cybernetics, Bethesda, MD, USA).
ClC3 silencing
HUVECs at 90% confluence were transfected with 60 nM validated ClC3 siRNA oligonucleotides 1 or 2 (cat no. 4390824, ID S3135 and S3137, respectively; Ambion, Austin, TX, USA) using the siRNA transfection reagent (Santa Cruz Biotechnology, Santa Cruz, CA, USA) according to the manufacturer's protocol. Nonsilencing siRNA (cat. no. AM4635, Ambion) was used as a negative control. At 48 h after transfection, HUVECs were treated as described above and harvested for Western blot analysis.
Analysis of blood level and endothelial targeting of radiolabeled enzymes in mice
All animal experiments were performed according to the protocol approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Anti-PECAM/SOD was injected IV in normal C-57BL/65 mice (The Jackson Laboratory, Bar Harbor, ME) via tail vein. In vivo distribution was performed as described earlier (28, 29) using proteins labeled with 125I. Ten micrograms of radiolabeled conjugates or nonconjugated counterpart was injected intravenously, and 1 h later, lungs were harvested, rinsed with saline, blotted dry, and weighed. Tissue radioactivity in organ and 100-μl samples of blood was determined in a Wallac 1470 Wizard γ counter (PerkinElmer, Wellesley, MA, USA).
Immunofluorescence microscopy of lung tissue sections
Animals were injected intravenously with anti-PECAM/SOD or control IgG/SOD via tail vein. After 1 h, lungs of these animals were inflated with 4% paraformaldehyde and 0.5% gluteraldhyde in 0.1 M sodium cacodylate buffer and fixed for 4 h. The mouse lungs were then treated with graded sucrose solution for cryosectioning of 10 μm thickness. For immunofluorescent staining, the cryosections were treated sequentially with 0.2% Triton X-100/PBS for 30 min, 0.375% glycine/PBS for 30 min, and 2% BSA/5% normal goat serum for 1 h. These sections were then stained with the primary antibodies against mouse PECAM and EEA-1, followed by the Alexa 488- or Alexa 594-conjugated secondary anti-rabbit IgG antibodies for PECAM and EEA-1, respectively. Conjugates were stained in a 1-step procedure with the Alexa 488-conjugated secondary anti-rat IgG antibodies. Fluorescence and transmission images of these sections were acquired using a Nikon Eclipse TE2000-U fluorescence microscope equipped with a Plan Apo ×100 1.4-NA oil objective. Microscope controlling and image processing were carried out using MetaMorph 7.6 (Molecular Devices Corp., Sunnyvale, CA, USA).
Endotoxemia model in mice
LPS was injected via tail vein. Conjugates or nonconjugated antioxidant enzymes (150 μg of SOD) were injected 10 min prior to LPS. In 5 h after LPS challenge, lungs were perfused and harvested. VCAM expression level was analyzed by Western blot. Preliminary experiments showed that VCAM expression was well detectable at doses of LPS of 50 μg/kg (Supplemental Fig. S3). Thus, a concentration of 50 μg/kg of LPS was chosen for protective studies.
Statistical analyses
Statistical significance was estimated by Student's t test.
RESULTS
Anti-PECAM/SOD inhibits proinflammatory activation of endothelial cells by cytokines and LPS
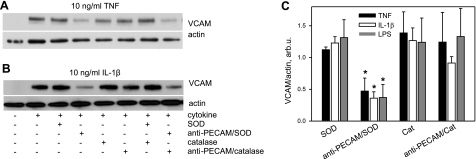
Endothelial cells treated with anti-PECAM/SOD or anti-PECAM/catalase vs. free SOD or catalase were exposed to TNF, IL-1β, or LPS. After 4 h, we assessed expression of VCAM, a marker of proinflammatory endothelial activation (31, 32). Western blot analysis showed increased VCAM expression at this time after cytokine treatment in control cells (Fig. 1). Anti-PECAM/SOD, but not SOD, catalase, or anti-PECAM/catalase, attenuated cytokine-induced VCAM expression (Fig. 2). Quantitative analysis showed that anti-PECAM/SOD inhibited VCAM expression caused in response to TNF, IL-1β, and LPS by 50–70% (47±20, 36±10, and 37±20% of control levels, respectively). In contrast, no VCAM suppression was provided by SOD (112±4, 123±11, and 131±28% for TNF, IL-1β and LPS, respectively) and anti-PECAM/catalase (124±47, 91±9, and 133±44% for TNF, IL-1β, and LPS, respectively).
Figure 2.
Anti-PECAM/SOD inhibits VCAM expression in endothelial cells activated by cytokines and LPS. A, B) Confluent human endothelial cells were treated with native SOD or catalase vs. anti-PECAM/SOD or anti-PECAM/catalase for 1 h before the addition of TNF (A), IL-1β (B), or LPS. Four hours later, VCAM expression level was assessed by Western blot analysis, with normalization per actin level in the samples. Representative images are shown. C) Summarized results of ≥3 independent experiments. Data are shown as percentage of maximal activation by cytokine or LPS alone in control cells (n≥3). *P < 0.05.
Combination of anti-PECAM/SOD and anti-PECAM/catalase did not produce a greater effect than anti-PECAM/SOD (Fig. 2). These findings imply that cytokine activation of endothelium involves signaling by the cell-generated superoxide, and thatcytokines induce flux of signaling superoxide in the endothelial compartments accessible to PECAM-targeted SOD, but not to free SOD added to the medium.
Endothelial uptake of anti-PECAM/SOD and anti-PECAM/catalase
To assess the uptake, cells were incubated with radiolabeled enzymes for 1 h at 37°C and washed prior to isotope measurement (Supplemental Fig. S1A). In this study, uptake of 125I-catalase and PEG-modified 125I-catalase uptake did not exceed 0.1%, while anti-PECAM/125I-catalase and anti-PECAM/125I-SOD underwent effective endothelial uptake (∼4.5% of added). This 50-fold difference in binding was likely due to the fact that nontargeted proteins, including PEG-modified derivatives, are taken up via nonspecific fluid phase uptake, while endothelial cells bind and internalize anti-PECAM conjugates via more specific and efficient CAM-mediated endocytosis (33). Indeed, uptake of anti-PECAM/125I-SOD and anti-PECAM/125I-catalase in PECAM-negative REN cells was very low and undistinguishable from PEG-catalase uptake by endothelial cells (∼0.1%). Consistent with the uptake data, PEG-SOD and PEG-catalase had no effect on TNF-induced VCAM expression (Supplemental Fig. S1B, C).
Endothelial endocytosis of anti-PECAM/SOD
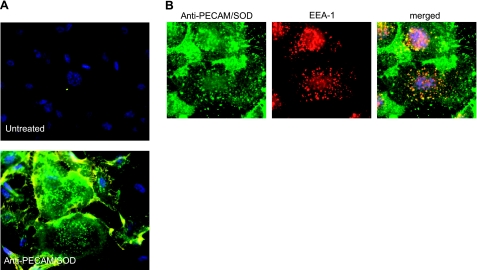
We identified surface-bound and internalized anti- PECAM/SOD in endothelial cells using fluorescence microscopy, as described previously (30). After incubation with the conjugate for 30 min at 37°C, cells were washed and stained with secondary antibodies labeled with red and green fluorophores, prior to and after cell permeabilization, respectively. Double-labeled yellow surface-bound fraction of anti-PECAM/SOD was evident in intercellular contacts, whereas green-labeled intracellular fraction of anti-PECAM/SOD manifests as vesicular structures in cytosol, particularly around the perinuclear region (Fig. 3A). Staining of permeabilized cells with antibodies recognizing the conjugate (green) and the endosomal marker EEA-1 (red) revealed colocalization of the vast majority of EEA-1-positive vesicles with the conjugate, confirming endosomal delivery of anti-PECAM/SOD as early as 15 min after adding the conjugate to cells (Fig. 3B).
Figure 3.
Endothelial endocytosis and endosomal delivery of anti-PECAM/SOD. A) Binding and uptake of anti-PECAM/SOD. Cells were treated with anti-PECAM/SOD for 1 h, washed, fixed, stained with Alexa Fluor 594-labeled antibody to rat IgG (common component of all conjugates used in the study), permeabilized, and stained with Alexa Fluor 488-labeled antibody to rat IgG. In such a dual-labeling assay, green and yellow colors depict internalized and surface-bound conjugates, respectively. Nuclei are stained with DAPI (blue). B) Endosomal localization of anti-PECAM/SOD. Cells were pretreated with the conjugate for 15 min, fixed, permeabilized, and stained for anti-PECAM/SOD (green), endosomal marker EEA-1 (red), and nuclei (DAPI, blue).
Targeted delivery of circulating anti-PECAM/SOD in the pulmonary vasculature in vivo
Next, we studied endothelial delivery and effect of anti-PECAM/SOD in intact animals. First, we determined blood and pulmonary levels of labeled antioxidant enzymes injected intravenously in mice. Pulmonary vasculature, comprising ∼25–30% of the total endothelial surface in the body, is the site of a preferential accumulation of circulating endothelial ligands, including PECAM antibodies and conjugates (23). Accordingly, anti-PECAM/125I-SOD and anti-PECAM/125I-catalase accumulated in lungs after i.v. injection: lung-to-blood ratio of conjugates was 20–40 times higher than those of other formulations, including catalase, PEG-catalase and IgG/SOD conjugate (Table 1). Blood level of PEG-catalase was significantly higher than that of native unmodified enzyme, consistent with the literature (34), yet its lung-to-blood ratio was very low (0.2), reflecting lack of specific endothelial delivery. Similarly to in vitro studies with PECAM-negative cells (Supplemental Fig. S1A), lack of pulmonary uptake of anti-PECAM/enzymes in the PECAM-1−/− knockout mice confirmed the specific nature of endothelial targeting in vivo (Table 1).
Table 1.
PECAM-1-targeted pulmonary accumulation of antioxidant enzymes after i.v. injection in mice
| Treatment | Blood (% ID/g) | Lung (% ID/g) | Lung/blood ratio |
|---|---|---|---|
| Catalase | 5.0 ± 0.9 | 2.7 ± 0.6 | 0.6 |
| PEG-catalase | 20.0 ± 12.0 | 4.0 ± 1.3 | 0.2 |
| Anti-PECAM/catalase | |||
| WT | 6.6 ± 0.5* | 84.3 ± 14.3** | 12.8 |
| KO | 20.8 ± 1.1 | 9.7 ± 0.7 | 0.5 |
| Anti-PECAM/SOD | 6.3 ± 0.6# | 63.1 ± 13.5## | 10.0 |
| IgG/SOD | 10.4 ± 1.1 | 6.1 ± 1.2 | 0.6 |
Catalase and SOD conjugated with PECAM, control nonimmune IgG, or PEG or in their native unconjugated form were injected into mice via tail vein. Animals were sacrificed after 1 h, and tissue distribution of antioxidant enzymes was analyzed by tracing 125I-labeled catalase or SOD. % ID, percentage of injected dose; WT, normal wild-type C-57BL/65 mice; KO, PECAM-knockout mice (target negative control). Values are means ± sd.
P < 0.01,
P < 0.001 vs. KO;
P < 0.01,
P < 0.001 vs. IgG/SOD.
Imaging of anti-PECAM/SOD in endothelial endosomes in vivo
Fluorescent microscopy revealed strong positive staining in the lung sections obtained from mice injected with anti-PECAM/SOD (Supplemental Fig. S2). Staining with antibodies against the conjugate (green) and PECAM (red) showed endothelial localization of anti-PECAM/SOD in the lungs (Supplemental Fig. S2). Dual staining with antibodies against the conjugate and the EEA-1 indicates that a significant fraction of anti-PECAM/SOD in the pulmonary endothelium is localized to endosomes (Fig. 4A). In contrast, IgG/SOD immunofluorescence is much weaker than that of anti-PECAM/SOD and does not colocalize with anti-EEA-1 fluorescence (Fig. 4B). These results are consistent with much higher pulmonary uptake of anti-PECAM/SOD vs. IgG/SOD (Table 1).
Figure 4.
Pulmonary localization of anti-PECAM/SOD in vivo. Anesthetized mice were injected intravenously with 150 μg of anti-PECAM/SOD or control IgG/SOD. At 1 h postinjection, lungs were harvested and fixed, and tissue cryosections were immunostained as described in Materials and Methods. Colocalization of anti-PECAM/SOD (A) and IgG/SOD (B) conjugates with endosomal marker EEA-1. Scale bars = 30 μm. Arrows on phase-contrast images indicate capillaries.
Anti-PECAM/SOD inhibits LPS-induced proinflammatory activation in the lungs in vivo
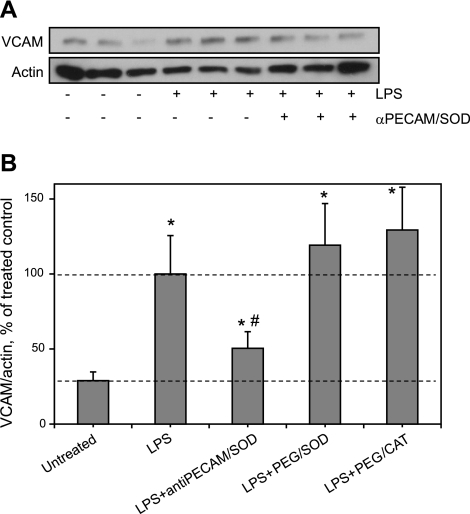
To test the effect of anti-PECAM/SOD on proinflammatory endothelial activation in vivo, we adopted a widely used model of endotoxemia (35). In accord with the literature, intravenously injected LPS caused a dose-dependent increase of VCAM level in the mouse lungs in 5 h (Supplemental Fig. S3). Intravenously injected anti-PECAM/SOD reduced LPS-induced VCAM expression in lungs by 70% (Fig. 5). In contrast, neither PEG-SOD nor PEG-catalase alleviate LPS-induced elevation in VCAM expression (Fig. 5B), despite a high level of PEG-modified proteins in blood (Table 1).
Figure 5.
Anti-PECAM/SOD inhibits elevation of pulmonary VCAM in LPS-challenged animals. Anti-PECAM/SOD or PEG-modified enzymes were injected in tail vein followed by LPS challenge (50 μg/kg). After 5 h, lungs were harvested for VCAM level analysis by Western blot (A; representative image is shown) and normalized per actin level for quantitative analysis, presented as a percentage of maximal LPS-induced VCAM level (B; analysis of ≥3 independent experiments); n ≥ 3. *P < 0.05 vs. untreated group; #P < 0.05 vs. LPS group.
Anti-PECAM/SOD quenches superoxide produced in endothelial endosomes by NADPH oxidase and transmitted for proinflammatory signaling via chloride channel ClC3
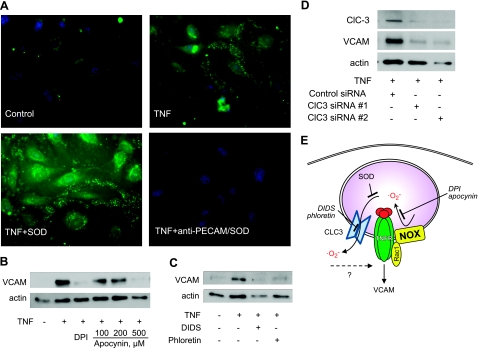
To address the mechanism of this anti-inflammatory effect of targeted delivery of SOD into endothelial endosomes, we performed an additional series of experiments in cell culture. First, we visualized ROS in endothelial cells using the membrane impermeable redox-sensitive probe OxyBURST Green conjugated to albumin. OxyBURST Green enters a wide spectrum of endosomes via nonspecific fluid-phase uptake and thus detects endosomal ROS, in particular superoxide (12). Within 20 min, TNF caused an elevation of fluorescence in redox-active endosomes, compared to quiescent endothelial cells (Fig. 6A, top panels). Anti- PECAM/SOD, but not SOD, quenched TNF-induced ROS fluorescence (Fig. 6A, bottom panels). These data confirm delivery of anti-PECAM/SOD to redox-active endosomes.
Figure 6.
Mechanism of anti-inflammatory effects of anti-PECAM/SOD. A) Anti-PECAM/SOD, but not naked SOD, quenches endosomal superoxide. Endothelial cells were pretreated with membrane nonpermeable dye OxyBURST Green and SOD (bottom left panel) or anti-PECAM/SOD (bottom right panel) and activated with TNF (except top left panel). Cells were fixed, and endosomal ROS generation was visualized by fluorescence microscopy. B–D) Nox2 is the source and ClC3 is the transmitter for endosomal superoxide in TNF-induced endothelial expression of VCAM (analysis by Western blot analysis as in Fig. 1). B) Inhibition of NADPH oxidases by DPI (20 μM) and apocynin (100–500 μM). C) Inhibition of anion channels by DIDS (25 μM) and phloretin (30 μM). D) Inhibition by ClC3 silencing by two types of ClC3 siRNA vs. control siRNA. E) Schematic representation of endosomal superoxide signaling in endothelial cells and its antagonists.
Cytokines and proinflammatory agents cause receptor-mediated activation of transmembrane NADPH oxidase, in particular, Nox2, the major NADPH-oxidase type in HUVECs, localized in plasmalemma and endosomal vesicles (36). NADPH oxidase inhibitors DPI and apocynin blocked TNF-induced VCAM expression in endothelial cells (Fig. 6B), affirming the key role of superoxide produced by NADPH oxidase in this cellular response. DIDS and phloretin, anion channel inhibitors, also blocked TNF-induced VCAM expression (Fig. 6C), implicating the role of superoxide transport via anion channels to the cytosol in the proinflammatory signaling. To validate this finding and avoid off-target effects of pharmacological inhibitors, we specifically knocked down protein expression of ClC3, an anion channel that has been implicated in superoxide transport through cell membrane (15). Silencing of ClC3 by two types of ClC3 siRNA blocked TNF-induced VCAM expression (Fig. 6D). These findings imply that anti-PECAM/SOD delivered into endosomes intercepts superoxide produced in these vesicles in response to cytokine stimulation and thus inhibits proinflammatory signaling (Fig. 6E).
Anti-PECAM/SOD inhibits endothelial cell activation via intracellular toll-like receptor 3
TNF receptor engagement by the ligand on the cell surface leads to activation of NADPH oxidases both in the plasmalemmal lipid rafts and, subsequently, in the endosomes where these transmembrane proteins get internalized via caveolar endocytosis (37). In contrast, viral dsRNA [and its synthetic analog poly(I:C)] is an activating ligand of the intracellular TLR3 localized in the cytosolic vesicles and endosomes, where poly(I:C) gets delivered from the plasmalemma by accessory binding protein (38). Poly(I:C) stimulated VCAM expression in endothelial cells (Supplemental Fig. S4). This effect was practically blocked by anti-PECAM/SOD but not SOD, catalase, or anti-PECAM/catalase (Fig. 7).
Figure 7.
Anti-PECAM/SOD blocks TLR3-mediated endothelial cell activation by poly(I:C). A) Effect of antioxidant enzymes on poly(I:C) activation of VCAM protein level determined by Western blotting. B) Quantitative analysis of 3 independent experiments.
DISCUSSION
SOD mimetics, superoxide quenchers, mutant SOD2/3 binding to negatively charged glycocalyx, and cell transfection by SOD confer protective antioxidant effects and inhibit endothelial activation by cytokines, implicating superoxide in proinflammatory signaling (19, 39–42). However, these means, as well as enzyme delivery using membrane-permeating peptides (43), provide no specific endothelial targeting and act throughout diverse cellular compartments (44). As a result, our knowledge of the role of ROS in inflammatory activation of the endothelium remains limited (17, 45). Further, translation of these antioxidant delivery means into therapeutic domain is hindered by concerns of efficacy, specificity, safety and spatiotemporal control of the interventions.
Therefore, optimization of antioxidant delivery for more effective and specific containment of oxidative stress is an important goal. For example, modification by PEG (46), PEG-containing pluronic (47), and encapsulation into nanocarriers (48, 49) prolong circulation of SOD and catalase, enhance their bioavailability and provide protective effects in animal models, including cerebral oxidative stress, perhaps, due to better access to neurons (50). However, none of these formulations provides endothelial delivery. Indeed, endothelial uptake of PEG-catalase was no better than that of naked enzyme (Table 1 and Supplemental Fig. S1A). Despite high blood level and high solubility, PEG enzymes have no effect on endothelial VCAM-1 expression (Fig. 5 and Supplemental Fig. S1B, C). Therefore, the ROS involved in endothelial activation by cytokines is localized in the intracellular compartment inaccessible to either naked or PEG-conjugated enzymes (Fig. 2). Studies in cell cultures have implicated superoxide released by NADPH oxidases into “redox-active signaling endosomes” in cytokine activation of cell types other than endothelium (12, 13, 17, 51, 52). This notion provided the context for testing effects of targeted delivery of anti-PECAM/catalase and anti-PECAM/SOD on endothelial inflammatory signaling (27, 28). Of note, endothelial uptake of anti-PECAM-conjugated enzymes exceeds that of PEG-conjugated enzymes by 20–50 times in vitro and in vivo (Supplemental Fig. S1A and Table 1).
We found that superoxide produced by endosomal NADPH oxidase mediates endothelial activation in response to diverse proinflammatory agonists. Only anti-PECAM/SOD inhibits this response, while anti-PECAM/catalase and nontargeted enzymes have no effect (Figs. 2 and 7C, D), implicating intracellular superoxide as the signaling ROS. Anti-PECAM/SOD accumulates in endothelial endosomes (Figs. 3B and 4A) and quenches cytokine-induced ROS (Fig. 6A). Pharmacological inhibitors and genetic silencing (Fig. 6B, D) showed that NADPH oxidase is the source of signaling superoxide, while the ClC3 anion channel serves as either a transmembrane transmitter of superoxide to adjacent cytosolic sensors activating inflammatory response (1, 53), or as such a sensor itself. It is plausible that superoxide released into endosomes is relatively protected against cellular SOD, which compartmentalize in mitochondria (SOD1), cytosol (SOD2), and extracellular glycocalyx (SOD3).
Endocytosis and intraendosomal signaling have recently been implicated in inflammatory endothelial activation. Inhibition of lipid rafts and caveolin-1 suppresses IL-1R1-mediated endocytosis and subsequent NF-κB activation, implicating caveolar pathways (37). TNF signaling via its receptors also seems dependent on lipid rafts (17). In contrast, a group of TLRs involved in antiviral defense (e.g., dsRNA receptor, TLR3) localized in intracellular vesicles are activated when their ligands get internalized via clathrin-mediated endocytosis (38). Interestingly, anti-PECAM carriers enter endothelium via a specific endocytic pathway (CAM-mediated endocytosis), bypassing classical clathrin- and caveolar pathways (33). Therefore, present results imply that anti-PECAM/SOD containing endosomes merge with redox-active signaling endosomes of both IL-1R/TNFR caveolar and TLR3 clathrin pathways. Since TLR3 is an intracellular receptor that activates preferentially endosomal NADPH oxidase, this result affirms that quenching of superoxide in TLR3-containing endosomes attained by internalized anti-PECAM/SOD blocks proinflammatory endothelial signaling. Notably, anti-PECAM/SOD more profoundly inhibits poly(I:C)-induced vs. cytokine-induced activation (compare data in Figs. 1C and 7B). This result suggests that TLR3-containing endosomes fuse with anti-PECAM/SOD-containing endosomes more effectively than cytokine-signaling endosomes. It is tempting to speculate that targeting SOD to endothelial determinants enriched in the lipid rafts and caveoli (54, 55) may further enhance the specificity and efficacy of inhibition of TNF- and IL-1β-induced proinflammatory activation.
In summary, by using targeted ROS antagonists in concert with pharmacological and genetic means, we found that in endothelial cells activated by inflammatory agents, superoxide produced by NADPH oxidase in the endosomes triggers cellular activation. This inflammatory pathway is inhibited by targeted delivery of anti-PECAM/SOD to endothelial endosomes. It is tempting to postulate that this targeted intervention may help to manage inflammation.
Supplementary Material
Acknowledgments
The authors are grateful to Drs. T. Dziubla and E. Simone for experiments with isotope-labeled PEG enzymes, Dr. M. Christofidou-Solomidou for help in visualization of SOD conjugates in mice, and E. Arguiri for technical assistance in animal studies. The authors thank Drs. A. Fisher, S. Albelda, and H. Ischiropoulos for their expert advice and stimulating discussions.
This work was supported, in whole or in part, by U.S. National Institutes of Health grants RO1 HL073940 and HL087036 and Project 4 of PO1 HL079063 (to V.R.M.), K99HL094536 (to B.G.H.), and HL086699 and 1S10RR022511 (to M.M.).
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Rhee S. G. (2006) Cell signaling. H2O2, a necessary evil for cell signaling. Science 312, 1882–1883 [DOI] [PubMed] [Google Scholar]
- 2. Janssen-Heininger Y. M., Mossman B. T., Heintz N. H., Forman H. J., Kalyanaraman B., Finkel T., Stamler J. S., Rhee S. G., van der Vliet A. (2008) Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic. Biol. Med. 45, 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yun J., Rocic P., Pung Y. F., Belmadani S., Carrao A. C., Ohanyan V., Chilian W. M. (2009) Redox-dependent mechanisms in coronary collateral growth: the “redox window” hypothesis. Antioxid. Redox Signal. 11, 1961–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rudolph T. K., Freeman B. A. (2009) Transduction of redox signaling by electrophile-protein reactions. Sci. Signal. 2, re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gow A. J., Farkouh C. R., Munson D. A., Posencheg M. A., Ischiropoulos H. (2004) Biological significance of nitric oxide-mediated protein modifications. Am. J. Physiol. Lung Cell. Mol. Physiol. 287, L262–L268 [DOI] [PubMed] [Google Scholar]
- 6. Forman H. J., Maiorino M., Ursini F. (2010) Signaling functions of reactive oxygen species. Biochemistry 49, 835–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ichimura H., Parthasarathi K., Quadri S., Issekutz A. C., Bhattacharya J. (2003) Mechano-oxidative coupling by mitochondria induces proinflammatory responses in lung venular capillaries. J. Clin. Invest. 111, 691–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McCord J. M., Roy R. S., Schaffer S. W. (1985) Free radicals and myocardial ischemia. The role of xanthine oxidase. Adv. Myocardiol. 5, 183–189 [PubMed] [Google Scholar]
- 9. Zimmerman M. C., Dunlay R. P., Lazartigues E., Zhang Y., Sharma R. V., Engelhardt J. F., Davisson R. L. (2004) Requirement for Rac1-dependent NADPH oxidase in the cardiovascular and dipsogenic actions of angiotensin II in the brain. Circ. Res. 95, 532–539 [DOI] [PubMed] [Google Scholar]
- 10. Pratico D. (2005) Antioxidants and endothelium protection. Atherosclerosis 181, 215–224 [DOI] [PubMed] [Google Scholar]
- 11. Thomas S. R., Witting P. K., Drummond G. R. (2008) Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxid. Redox Signal. 10, 1713–1765 [DOI] [PubMed] [Google Scholar]
- 12. Li Q., Harraz M. M., Zhou W., Zhang L. N., Ding W., Zhang Y., Eggleston T., Yeaman C., Banfi B., Engelhardt J. F. (2006) Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol. Cell. Biol. 26, 140–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frey R. S., Ushio-Fukai M., Malik A. B. (2009) NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid. Redox Signal. 11, 791–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gongora M. C., Qin Z., Laude K., Kim H. W., McCann L., Folz J. R., Dikalov S., Fukai T., Harrison D. G. (2006) Role of extracellular superoxide dismutase in hypertension. Hypertension 48, 473–481 [DOI] [PubMed] [Google Scholar]
- 15. Hawkins B. J., Madesh M., Kirkpatrick C. J., Fisher A. B. (2007) Superoxide flux in endothelial cells via the chloride channel-3 mediates intracellular signaling. Mol. Biol. Cell 18, 2002–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mumbengegwi D. R., Li Q., Li C., Bear C. E., Engelhardt J. F. (2008) Evidence for a superoxide permeability pathway in endosomal membranes. Mol. Cell. Biol. 28, 3700–3712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oakley F. D., Abbott D., Li Q., Engelhardt J. F. (2009) Signaling components of redox active endosomes: the redoxosomes. Antioxid. Redox Signal. 11, 1313–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Epperly M. W., Dixon T., Wang H., Schlesselman J., Franicola D., Greenberger J. S. (2008) Modulation of radiation-induced life shortening by systemic intravenous MnSOD-plasmid liposome gene therapy. Radiat. Res. 170, 437–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Epperly M. W., Guo H. L., Jefferson M., Nie S., Gretton J., Bernarding M., Bar-Sagi D., Archer H., Greenberger J. S. (2003) Cell phenotype specific kinetics of expression of intratracheally injected manganese superoxide dismutase-plasmid/liposomes (MnSOD-PL) during lung radioprotective gene therapy. Gene Ther. 10, 163–171 [DOI] [PubMed] [Google Scholar]
- 20. Epperly M. W., Sikora C. A., DeFilippi S. J., Gretton J. E., Bar-Sagi D., Archer H., Carlos T., Guo H., Greenberger J. S. (2002) Pulmonary irradiation-induced expression of VCAM-I and ICAM-I is decreased by manganese superoxide dismutase-plasmid/liposome (MnSOD-PL) gene therapy. Biol. Blood Marrow Transplant. 8, 175–187 [DOI] [PubMed] [Google Scholar]
- 21. Zhang X., Epperly M. W., Kay M. A., Smith T., Franicola D., Greenberger B., Komanduri P., Greenberger J. S. (2008) Radioprotection in vitro and in vivo by mini circle plasmid containing the human manganese superoxide dismutase (MnSOD) transgene. Hum. Gene Ther. 19, 820–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Supinski G. S., Callahan L. A. (2006) Polyethylene glycol-superoxide dismutase prevents endotoxin-induced cardiac dysfunction. Am. J. Respir. Crit. Care Med. 173, 1240–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ding B. S., Dziubla T., Shuvaev V. V., Muro S., Muzykantov V. R. (2006) Advanced drug delivery systems that target the vascular endothelium. Mol. Interv. 6, 98–112 [DOI] [PubMed] [Google Scholar]
- 24. Muro S., Muzykantov V. R. (2005) Targeting of antioxidant and anti-thrombotic drugs to endothelial cell adhesion molecules. Curr. Pharm. Des. 11, 2383–2401 [DOI] [PubMed] [Google Scholar]
- 25. Muzykantov V. R., Christofidou-Solomidou M., Balyasnikova I., Harshaw D. W., Schultz L., Fisher A. B., Albelda S. M. (1999) Streptavidin facilitates internalization and pulmonary targeting of an anti-endothelial cell antibody (platelet-endothelial cell adhesion molecule 1): a strategy for vascular immunotargeting of drugs. Proc. Natl. Acad. Sci. U. S. A. 96, 2379–2384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shuvaev V. V., Tliba S., Nakada M., Albelda S. M., Muzykantov V. R. (2007) Platelet-endothelial cell adhesion molecule-1-directed endothelial targeting of superoxide dismutase alleviates oxidative stress caused by either extracellular or intracellular superoxide. J. Pharmacol. Exp. Ther. 323, 450–457 [DOI] [PubMed] [Google Scholar]
- 27. Kozower B. D., Christofidou-Solomidou M., Sweitzer T. D., Muro S., Buerk D. G., Solomides C. C., Albelda S. M., Patterson G. A., Muzykantov V. R. (2003) Immunotargeting of catalase to the pulmonary endothelium alleviates oxidative stress and reduces acute lung transplantation injury. Nat. Biotechnol. 21, 392–398 [DOI] [PubMed] [Google Scholar]
- 28. Shuvaev V. V., Christofidou-Solomidou M., Bhora F., Laude K., Cai H., Dikalov S., Arguiri E., Solomides C. C., Albelda S. M., Harrison D. G., Muzykantov V. R. (2009) Targeted detoxification of selected reactive oxygen species in the vascular endothelium. J. Pharmacol. Exp. Ther. 331, 404–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scherpereel A., Rome J. J., Wiewrodt R., Watkins S. C., Harshaw D. W., Alder S., Christofidou-Solomidou M., Haut E., Murciano J. C., Nakada M., Albelda S. M., Muzykantov V. R. (2002) Platelet-endothelial cell adhesion molecule-1-directed immunotargeting to cardiopulmonary vasculature. J. Pharmacol. Exp. Ther. 300, 777–786 [DOI] [PubMed] [Google Scholar]
- 30. Muro S., Muzykantov V. R., Murciano J. C. (2004) Characterization of endothelial internalization and targeting of antibody-enzyme conjugates in cell cultures and in laboratory animals. Methods Mol. Biol. 283, 21–36 [DOI] [PubMed] [Google Scholar]
- 31. Cook-Mills J. M. (2006) Hydrogen peroxide activation of endothelial cell-associated MMPs during VCAM-1-dependent leukocyte migration. Cell. Mol. Biol. (Noisy-le-grand) 52, 8–16 [PMC free article] [PubMed] [Google Scholar]
- 32. Sana T. R., Janatpour M. J., Sathe M., McEvoy L. M., McClanahan T. K. (2005) Microarray analysis of primary endothelial cells challenged with different inflammatory and immune cytokines. Cytokine 29, 256–269 [DOI] [PubMed] [Google Scholar]
- 33. Muro S., Wiewrodt R., Thomas A., Koniaris L., Albelda S. M., Muzykantov V. R., Koval M. (2003) A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1. J. Cell Sci. 116, 1599–1609 [DOI] [PubMed] [Google Scholar]
- 34. Beckman J. S., Minor R. L., Jr., White C. W., Repine J. E., Rosen G. M., Freeman B. A. (1988) Superoxide dismutase and catalase conjugated to polyethylene glycol increases endothelial enzyme activity and oxidant resistance. J. Biol. Chem. 263, 6884–6892 [PubMed] [Google Scholar]
- 35. Hagiwara S., Iwasaka H., Matsumoto S., Hasegawa A., Yasuda N., Noguchi T. (2010) In vivo and in vitro effects of the anticoagulant, thrombomodulin, on the inflammatory response in rodent models. Shock 33, 282–288 [DOI] [PubMed] [Google Scholar]
- 36. Van Buul J. D., Fernandez-Borja M., Anthony E. C., Hordijk P. L. (2005) Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid. Redox Signal. 7, 308–317 [DOI] [PubMed] [Google Scholar]
- 37. Oakley F. D., Smith R. L., Engelhardt J. F. (2009) Lipid rafts and caveolin-1 coordinate interleukin-1β (IL-1β)-dependent activation of NF-κB by controlling endocytosis of Nox2 and IL-1β receptor 1 from the plasma membrane. J. Biol. Chem. 284, 33255–33264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Botos I., Liu L., Wang Y., Segal D. M., Davies D. R. (2009) The toll-like receptor 3:dsRNA signaling complex. Biochim. Biophys. Acta 1789, 667–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Matsui T., Yamagishi S., Nakamura K., Inoue H. (2007) Bay w 9798, a dihydropyridine structurally related to nifedipine with no calcium channel-blocking properties, inhibits tumour necrosis factor-alpha-induced vascular cell adhesion molecule-1 expression in endothelial cells by suppressing reactive oxygen species generation. J. Int. Med. Res. 35, 886–891 [DOI] [PubMed] [Google Scholar]
- 40. Yamagishi S., Nakamura K., Matsui T. (2008) Role of oxidative stress in the development of vascular injury and its therapeutic intervention by nifedipine. Curr. Med. Chem. 15, 172–177 [DOI] [PubMed] [Google Scholar]
- 41. Gao B., Flores S. C., Leff J. A., Bose S. K., McCord J. M. (2003) Synthesis and anti-inflammatory activity of a chimeric recombinant superoxide dismutase: SOD2/3. Am. J. Physiol. Lung Cell. Mol. Physiol. 284, L917–L925 [DOI] [PubMed] [Google Scholar]
- 42. Lin S. J., Shyue S. K., Shih M. C., Chu T. H., Chen Y. H., Ku H. H., Chen J. W., Tam K. B., Chen Y. L. (2007) Superoxide dismutase and catalase inhibit oxidized low-density lipoprotein-induced human aortic smooth muscle cell proliferation: role of cell-cycle regulation, mitogen-activated protein kinases, and transcription factors. Atherosclerosis 190, 124–134 [DOI] [PubMed] [Google Scholar]
- 43. Watanabe N., Iwamoto T., Bowen K. D., Dickinson D. A., Torres M., Forman H. J. (2003) Bio-effectiveness of Tat-catalase conjugate: a potential tool for the identification of H2O2-dependent cellular signal transduction pathways. Biochem. Biophys. Res. Commun. 303, 287–293 [DOI] [PubMed] [Google Scholar]
- 44. Kim D. W., Kim S. Y., Lee S. H., Lee Y. P., Lee M. J., Jeong M. S., Jang S. H., Park J., Lee K. S., Kang T. C., Won M. H., Cho S. W., Kwon O. S., Eum W. S., Choi S. Y. (2008) Protein transduction of an antioxidant enzyme: subcellular localization of superoxide dismutase fusion protein in cells. BMB Rep. 41, 170–175 [DOI] [PubMed] [Google Scholar]
- 45. Yao H., Yang S. R., Kode A., Rajendrasozhan S., Caito S., Adenuga D., Henry R., Edirisinghe I., Rahman I. (2007) Redox regulation of lung inflammation: role of NADPH oxidase and NF-κB signalling. Biochem. Soc. Trans. 35, 1151–1155 [DOI] [PubMed] [Google Scholar]
- 46. White C. W., Jackson J. H., Abuchowski A., Kazo G. M., Mimmack R. F., Berger E. M., Freeman B. A., McCord J. M., Repine J. E. (1989) Polyethylene glycol-attached antioxidant enzymes decrease pulmonary oxygen toxicity in rats. J. Appl. Physiol. 66, 584–590 [DOI] [PubMed] [Google Scholar]
- 47. Yi X., Zimmerman M. C., Yang R., Tong J., Vinogradov S., Kabanov A. V. (2010) Pluronic-modified superoxide dismutase 1 attenuates angiotensin II-induced increase in intracellular superoxide in neurons. Free Radic. Biol. Med. 49, 548–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee S., Yang S. C., Heffernan M. J., Taylor W. R., Murthy N. (2007) Polyketal microparticles: a new delivery vehicle for superoxide dismutase. Bioconjug. Chem. 18, 4–7 [DOI] [PubMed] [Google Scholar]
- 49. Reddy M. K., Labhasetwar V. (2009) Nanoparticle-mediated delivery of superoxide dismutase to the brain: an effective strategy to reduce ischemia-reperfusion injury. FASEB J. 23, 1384–1395 [DOI] [PubMed] [Google Scholar]
- 50. Rosenbaugh E. G., Roat J. W., Gao L., Yang R. F., Manickam D. S., Yin J. X., Schultz H. D., Bronich T. K., Batrakova E. V., Kabanov A. V., Zucker I. H., Zimmerman M. C. (2010) The attenuation of central angiotensin II-dependent pressor response and intra-neuronal signaling by intracarotid injection of nanoformulated copper/zinc superoxide dismutase. Biomaterials 31, 5218–5226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Matsuda J. J., Filali M. S., Collins M. M., Volk K. A., Lamb F. S. (2010) The ClC-3 Cl−/H+ antiporter becomes uncoupled at low extracellular pH. J. Biol. Chem. 285, 2569–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li Q., Spencer N. Y., Oakley F. D., Buettner G. R., Engelhardt J. F. (2009) Endosomal Nox2 facilitates redox-dependent induction of NF-κB by TNF-alpha. Antioxid. Redox Signal. 11, 1249–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Buetler T. M., Krauskopf A., Ruegg U. T. (2004) Role of superoxide as a signaling molecule. News Physiol. Sci. 19, 120–123 [DOI] [PubMed] [Google Scholar]
- 54. Carver L. A., Schnitzer J. E. (2003) Caveolae: mining little caves for new cancer targets. Nat. Rev. Cancer 3, 571–581 [DOI] [PubMed] [Google Scholar]
- 55. Wang Z., Tiruppathi C., Minshall R. D., Malik A. B. (2009) Size and dynamics of caveolae studied using nanoparticles in living endothelial cells. ACS Nano 3, 4110–4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.