Abstract
Heterochromatin is a form of highly compacted chromatin associated with epigenetic gene silencing and chromosome organization. We have previously shown that unphosphorylated nuclear signal transducer and activator of transcription (STAT) physically interacts with heterochromatin protein 1 (HP1) to promote heterochromatin stability. To understand whether STAT and heterochromatin are important for maintenance of genome stability, we genetically manipulated the levels of unphosphorylated STAT and HP1 [encoded by Su(var)205] in Drosophila and examined the effects on chromosomal morphology and resistance to DNA damage under conditions of genotoxic stress. Here we show that, compared with wild-type controls, Drosophila mutants with reduced levels of unphosphorylated STAT or heterochromatin are more sensitive to radiation-induced cell cycle arrest, have higher levels of spontaneous and radiation-induced DNA damage, and exhibit defects in chromosomal compaction and segregation during mitosis. Conversely, animals with increased levels of heterochromatin exhibit less DNA damage and increased survival rate after irradiation. These results suggest that maintaining genome stability by heterochromatin formation and correct chromosomal packaging is essential for normal cellular functions and for survival of animals under genotoxic stress.—Yan, S.-J., Lim, S. J., Shi, S., Dutta, P., Li, W. X. Unphosphorylated STAT and heterochromatin protect genome stability.
Keywords: Drosophila, DNA damage, JAK, HP1, Su(var)205
Genome stability is a prerequisite for normal cell function. Loss of genome stability can lead to premature aging, cancer, or cell death (1, 2). Cells have developed multiple strategies, such as cell cycle checkpoints and stress responses, to protect their DNA against insults and to safeguard genome stability. In addition, it has become increasingly clear that heterochromatin plays a role in maintaining genome stability (3). It has recently been shown that heterochromatin formation is essential for maintaining the stability of repeat DNA sequences and of the rDNA locus in particular, presumably by suppressing illegitimate homologous recombination (4), and that mutations in the histone H3 lys9 methyltransferase gene Su(var)3–9 cause an increase in DNA damage to heterochromatin (5). The importance of heterochromatin formation in genome stability has previously been demonstrated in mice, where it has been demonstrated that loss of the Suv39h histone methyltransferases impairs genome stability (6). However, it has remained unclear whether and to what extent heterochromatin formation is an important conserved mechanism for protecting genome stability generally in multicellular organisms and for promoting organism survival under conditions of genotoxic stress, and whether signal transducer and activator of transcription (STAT), also a key regulator of heterochromatin formation/stability (see below), plays an important role in this aspect of heterochromatin function.
STAT is an essential component of the JAK/STAT pathway, which is a highly conserved intracellular signaling pathway in animals (7–10). The canonical JAK/STAT pathway consists of extracellular ligands, transmembrane receptors, a Janus kinase [JAK; encoded by the hopscotch (hop) gene in Drosophila], and STAT (encoded by the Stat92E gene in Drosophila). Unphosphorylated (inactive) STAT resides in the cytoplasm. On activation via phosphorylation by JAK, STAT proteins dimerize and translocate from the cytoplasm to the nucleus, where they recognize a consensus DNA sequence at target promoters and induce gene expression (7–10).
We have previously demonstrated the existence of another, “noncanonical,” mode of JAK/STAT signaling, in which unphosphorylated (transcriptionally inactive) STAT is required for directly maintaining heterochromatin stability (10–12). At least a portion of the unphosphorylated STAT pool is localized on heterochromatin in association with HP1 instead of in the cytoplasm, and the heterochromatin-associated unphosphorylated STAT is essential for maintaining HP1 localization and heterochromatin stability. When JAK activates STAT by phosphorylation, the levels of unphosphorylated STAT are reduced, which leads to HP1 delocalization and heterochromatin instability (10–12). Thus, in the context of noncanonical JAK/STAT signaling, both JAK activation and loss of STAT cause heterochromatin disruption, whereas an increase in the levels of unphosphorylated STAT leads to heterochromatin stabilization (11, 13).
To understand whether unphosphorylated STAT is generally required for heterochromatin stability, and whether STAT and heterochromatin are important for maintenance of genome stability, we genetically manipulated the levels of unphosphorylated STAT and of HP1 [encoded by Su(var)205] and used different genetic and cell biological assays to examine the effects on chromosomal morphology and resistance to DNA damage under genotoxic stress. We found that either reducing the levels of unphosphorylated STAT, as in Stat92E loss-of-function or hop gain-of-function mutants, or reducing heterochromatin levels, as in Su(var)205 mutants, leads to chromosomal compaction defects and increased sensitivity to DNA damage. In contrast, overexpression of unphosphorylated STAT or HP1 reduces radiation-induced DNA damage and promotes survival/health of organisms under genotoxic stress. These results suggest that unphosphorylated STAT plays a role in heterochromatin stability in general and that heterochromatin formation is essential for genome stability. To our knowledge, this is the first in vivo evidence that unphosphorylated STAT, in conjunction with heterochromatin, protects organisms from genotoxic stress by maintaining genome stability through correct chromosomal packaging.
MATERIALS AND METHODS
Fly stocks and genetics
All crosses were carried out at 25°C on standard cornmeal/agar medium unless otherwise specified. Fly stocks of w1118, ry506, hopTum-l, hop3, hop2, Stat92E06346, Su(var)2054, and Su(var)2055 were from the Bloomington Drosophila Stock Center (Bloomington, IN, USA). The UAS-HP1-RNAi (stock no. 31994 and 31995) and UAS-Stat92E-RNAi (73866 and 43867) stocks were from the Vienna Drosophila RNAi Center (Vienna, Austria). The Stat92EF (Charles Dearolf, Massachusetts General Hospital, Boston, MA, USA, via Steven Hou, National Cancer Institute–Frederick, Frederick, MD, USA), hsp70-HP1 (Lori Wallrath, University of Iowa, Iowa City, IA, USA, and Gunter Reuter, Martin Luther University Halle, Halle, Germany), and UAS-Stat92EY704F (Martin Zeidler, The University of Sheffield, Western Bank, Sheffield, UK) stocks were generous gifts. To generate embryos lacking the maternal Stat92E gene product (germ-line clones), we crossed hsp70-flp; FRT82B Stat92E06346/TM3 females to hsp70-Flp; FRT82B ovoD1, w+/TM3 males. The progeny were heat-shocked at 37°C for 2 h daily during larval stages. Embryos from hsp70-flp; FRT82B Stat92E06346/FRT82B [ovoD1, w+] females lack maternal STAT92E protein.
Larval brain neuroblast squashes
Crawling third-instar larvae were dissected in 0.7% sodium chloride, and the brain complex was incubated in colchicine (10−5 M) solution for 1 h at room temperature. Brain tissues were then fixed in 3.7% formaldehyde solution for 15 min (room temperature). Fixed brains were transferred to 45% acetic acid for 30 s and then to 60% acetic acid for 3 min. Brains were squashed by placing them on a glass slide and pressing a coverslip against them. Slides with squashed brains were immersed in liquid nitrogen, and the coverslip was peeled off. Squashed brain tissues were then stained with DAPI and examined using an epifluorescence microscope.
Antibodies and immunostaining
Rabbit anti-phospho-histone H3 (Upstate Biotechnology, Lake Placid, NY, USA; 1:1000 dilution); rabbit antibodies specific for Drosophila γ-H2AX (rabbit antibodies against Drosophila phosphorylated H2Av on Ser-137, equivalent to mammalian γ-H2AX; Rockland, Gilbertsville, PA, USA; 1:500 dilution; ref. 14); rabbit anti-cleaved-caspase-3 (Asp175; Cell Signalling Technology, Danvers, MA, USA; 1:250 dilution), and mouse anti-α-tubulin (Sigma-Aldrich, St. Louis, MO, USA) were used as primary antibodies. HRP-conjugated and fluorescent secondary antibodies (Molecular Probes, Eugene, OR, USA) were used in Western blots and whole-mount immunostaining, respectively. Stained tissues were photographed with a Leica confocal microscope (Leica Microsystems, Wetzlar, Germany). Images were cropped and minimally processed with Adobe Photoshop (Adobe Systems, San Jose, CA, USA). Quantification of γ-H2AX and α-tubulin levels was done using ImageJ (U.S. National Institutes of Health, Bethesda, MD, USA).
Pulsed-field gel electrophoresis
Third-instar larvae of appropriate genotypes were treated or not treated (controls) with 500 rad of X-rays, transferred to ice, and dissected. Dissected tissues (mainly brain and imaginal discs) were treated with 0.5 mg/ml collagenase I for 30 min at 37°C. Approximately 3 × 106 dissociated cells/genotype were imbedded in a low-melting agarose (1%) gel block. The gel blocks with embedded cells were digested with proteinase K at 50°C for 48 h and then were placed in the wells of a 0.7% agarose gel. The gel was run at 50 V and 14–15°C for 48–72 h on a pulsed-field electrophoresis apparatus and was stained with ethydium bromide (EtBr).
Ionizing irradiation
To assay for cell cycle checkpoint sensitivity to X-ray/γ-ray irradiation, third-instar larvae (those that had just started crawling out of the food) of appropriate genotypes were treated or not treated (controls) with 500 rad of X-rays/γ-rays (similar results were observed with 250 rad; data not shown). After irradiation, they were immediately transferred to ice and then dissected and fixed with 4% paraformaldehyde. Fixed wing imaginal discs were immunostained with rabbit anti-phospho-histone H3 (pH3; Upstate) and a fluorescent secondary antibody. Stained discs were imaged using confocal microscopy. The number of pH3-positive nuclei was counted for each genotype. See Yan and Li (15) for a detailed protocol.
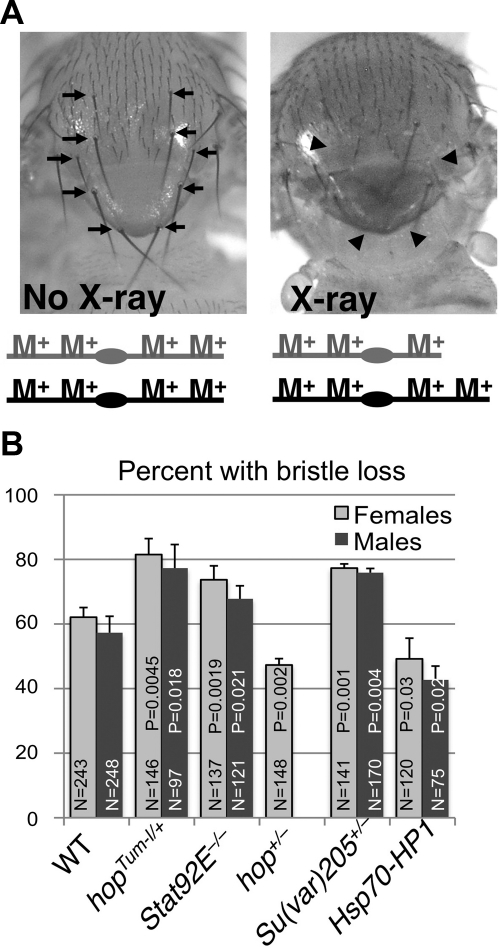
To assay for X-ray-induced chromosomal loss, irradiated larvae were allowed to develop into adult flies or late-stage pupae and examined for the loss of macrochaetes using a light microscope. Missing, shorter, or thinner macrochaetes (reduced in length by ≥50%) were scored at 5 positions: 2 pairs of dorsoventral setaes, 1 pair of postalar setaes, and 2 pairs of scutellar setaes (see Fig. 6A, arrows).
Figure 6.
STAT and heterochromatin protects from radiation-induced chromosomal loss. A, B) Flies of indicated genotypes were treated with 500 rad X-rays at the late third-instar larval stage, and the loss of macrochaetes was quantified in adults. To minimize possible effects of genetic background, larvae were F1 progeny of flies of the various genotypes crossed to wild-type (w1118) flies. A) Left panel: notum of a wild-type adult fly has macrochaetes (large bristles; arrows) that have a characteristic length, are paired, and are distributed in a characteristic position. Right panel: adult fly that was irradiated with 500 rad X-rays during larval growth (crawling third-instar larvae). Arrowheads indicate positions of missing macrochaetes. Schematic diagrams at bottom represent a pair of autosomes with the Minute+ (M+) loci (endogenous wild-type genes encoding ribosomal proteins) distributed along the chromosome arms. Diagram at right represents one incidence of partial chromosomal loss. Note that several machrochaetes are missing (arrowheads) as a result of X-ray-induced chromosomal loss. B) Quantification of percentage of irradiated flies of indicated genotypes with Minute macrochaetes (see Materials and Methods for details). There were no Minute bristles found (0% with Minute bristles) in all genotypes before irradiation. At least 3 independent experiments were done for each genotype. Mutant alleles used were Stat92E06346, Su(var)2055, hop3, or as indicated. N represents total number of adult flies or late-stage pupae (for hopTum-l/Y males) scored. Error bars = sd. P values were calculated using Student's t test by comparing the effect of each mutant to wild-type controls.
To assay for levels of γ-ray-induced DNA damage, embryos were irradiated with 4000 rad and collected at different time points for Western blots; the hopTum-l/+ animals were F1 progeny of hopTum-l/+ females crossed to w1118 males; the Su(var)2055/+ animals were selected GFP-negative F1 progeny of Su(var)2055/CyO, GFP females crossed to w1118 males; wild-type controls were F1 progeny of ry506 females crossed to w1118 males.
For analysis of DNA damage in imaginal discs, third-instar larvae were irradiated with 250 rad (similar results were observed with 500 rad) of γ-rays and then 1 h later were dissected, fixed, and immunostained with rabbit γ-H2AX antibodies against Drosophila-phosphorylated H2Av (Rockland). To assay for the effect of γ-ray-induced DNA damage on animal development and survival, third-instar larvae of various genotypes were irradiated with 2000 rad. Animals were then allowed to develop, and eclosion and survival rates were recorded.
RESULTS
Loss of unphosphorylated STAT or heterochromatin increases sensitivity to radiation-induced cell cycle arrest
Damage to genomic DNA triggers checkpoint responses, which arrest cell cycle progression to allow for DNA damage repair (16, 17). Indeed, when Drosophila larvae are subjected to ionizing irradiation (e.g., γ-rays or X-rays), they respond by checkpoint activation and cell cycle arrest, which can be detected as a dramatic reduction in the number of mitotic cells in developing larval imaginal discs (18). Mitotic cells can be identified by immunostaining for the mitotic marker phosphohistone H3 (pH3) (18). We found that in control animals without transgene expression, the numbers of pH3-positive cells were reduced from >200 before irradiation to ∼30 after irradiation (Fig. 1A, B; left panels; also see ref. 18), with an equal distribution between the anterior and posterior compartments (Fig. 1B, left panels).
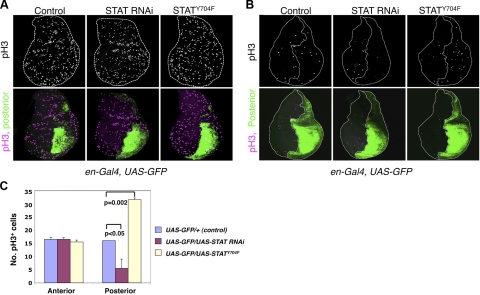
Figure 1.
Transcription-independent role of STAT in radiation-induced cell cycle arrest. A, B) Crawling third-instar larvae obtained by crossing en-Gal4, UAS-GFP/+ to flies carrying the indicated transgenes were not irradiated (A, controls) or were irradiated with 500 rad of γ-rays (B). Larvae were fixed 60 min postirradiation and were immunostained with anti-phospho-histone H3 (pH3). Wing imaginal discs are shown with anterior to the left and dorsal up. Posterior compartment is marked by GFP expression (green). pH3-positive cells are shown as white (top) or magenta (bottom) dots. Dashed lines outline the wing discs or demarcate the anterior-posterior boundaries. C) Quantification of pH3-positive cells per anterior or posterior compartment in panel B. Note that irradiation causes a reduction in the number of pH3-positive cells from >200 (A) to ∼30 (B). Also note that the total number of pH3-positive cells was similar in the anterior and posterior compartments without irradiation (A) or when only GFP was expressed (B, left), whereas after irradiation, fewer and more pH3-positive cells were found in the posterior compartments expressing Stat92E RNAi and Stat92EY704F, respectively (B, right panels), than those of controls. P values were calculated using Student's t test by comparing the effect of Stat92E RNAi or Stat92EY704 to controls. Two independent RNAi lines were used, and results were similar. At least 3 discs were scored for each genotype. Error bars = sd.
We have previously investigated whether canonical or noncanonical JAK/STAT signaling mediates cell cycle checkpoint activation in response to irradiation. In these experiments, we knocked down Stat92E by expressing a UAS-Stat92E RNAi transgene under the control of an en-Gal4 driver, which is expressed in the posterior compartment of Drosophila limb imaginal discs; the anterior compartment of the discs served as an internal control (Fig. 1A, B). After exposing the larvae to low-dose X-rays (500 rad), which cause moderate DNA damage and checkpoint activation (19, 20), we found that the posterior region of the wing discs exhibited more sensitivity to radiation-induced cell cycle arrest than the anterior compartment, with considerably fewer cells continuing to cycle in the posterior compartment (Fig. 1B, middle panels; C). However, expression with the same en-Gal4 driver of an unphosphorylatable mutant form of STAT, STAT92EY704F, which is transcriptionally inactive (21), led to a reduction in radiation-induced cell cycle arrest, such that many more pH3-positive cells were found in the posterior than in the anterior compartment (Fig. 1B, right panels; C). These results, where STAT knockdown and expression of inactive STAT seem to have opposite effects on cell cycle arrest, were inconsistent with a role for canonical JAK/STAT signaling in checkpoint activation, but instead pointed to a role for noncanonical JAK/STAT signaling in this process. In noncanonical JAK/STAT signaling, loss of STAT has an effect opposite to that an increase in transcriptionally inactive STAT on heterochromatin formation (11).
To investigate whether noncanonical JAK/STAT signaling plays a role in radiation-induced cell cycle arrest via regulating heterochromatin formation, we examined the effects of altering heterochromatin levels on radiation-induced cell cycle arrest. Heterochromatin levels are sensitive to the levels of nonhistone proteins such as HP1 and unphosphorylated STAT, and heterozygosity for either HP1 (22) or STAT92E (11) significantly reduces heterochromatin, as measured by suppression of position-effect variegation (PEV), a heterochromatin-dependent phenomenon (3, 23). We found that heterozygosity of Stat92E indeed caused delocalization of HP1 from centromeric and telomeric heterochromatin in polytene chromosomes (not shown), consistent with the suppression of PEV (11). Moreover, we have previously shown that JAK overactivation (as in hopTum-l heterozygotes) causes HP1 delocalization, thus heterochromatin disruption, whereas hop+/− flies have increased heterochromatin levels (13).
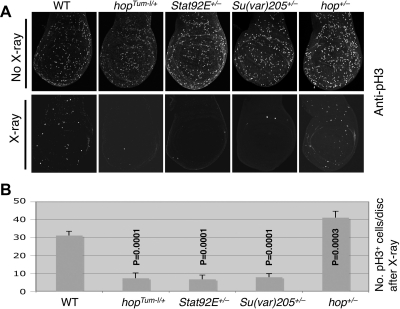
By altering heterochromatin levels using various heterozygous mutants, we found that effects on resistance to irradiation were similar to their effects on PEV. Larvae with reduced levels of heterochromatin, such as hopTum-l/+, Stat92E+/−, or Su(var)205+/−, exhibited less radiation resistance and a more complete cell cycle arrest than wild-type animals, as determined by the number of pH3-positive mitotic nuclei in each wing disc, whereas larvae with increased heterochromatin, such as hop+/−, showed a slight but significant increase in resistance to irradiation (Fig. 2). These results are consistent with a role for heterochromatin formation in cell cycle response to irradiation and implicate noncanonical JAK/STAT signaling, as opposed to canonical JAK/STAT-induced target gene transcription, in this process.
Figure 2.
Unphosphorylated STAT and heterochromatin impart resistance to radiation-induced cell cycle arrest. Crawling third-instar larvae were treated or not treated (controls) with 500 rad of X-rays and were dissected (60 min later) and immunostained with anti-pH3. Stat92E06346, Su(var)2055, and hop3 were used as null-mutant alleles. To minimize possible effects of genetic background, larvae were F1 progeny of flies of various genotypes crossed to wild-type (w1118) flies. A) Representative control (top) and irradiated (bottom) early third-instar larval wing imaginal discs. Mitotic cells were recognized as those positive for pH3 (white spots). B) Quantification of pH3-positive cells per wing disc after X-ray irradiation. At least 5 discs were scored for each genotype. Error bars = sd. P values were calculated using Student's t test by comparing the effect of each mutant to wild type.
Unphosphorylated STAT and heterochromatin protect the genome against DNA damage
The differences shown above in the cellular response to irradiation when heterochromatin levels are altered could reflect differences either in the extent of DNA damage or in the ability of the animals to respond to DNA damaging agents. In other words, reducing unphosphorylated STAT or heterochromatin levels could lead either to increased DNA damage or simply to exaggerated cell cycle checkpoint response. We wished to distinguish between these 2 possibilities and began by examining a more direct marker for DNA damage, phosphorylated H2AX (γ-H2AX). We assessed the levels of γ-H2AX by immunostaining of whole-mount tissues (nonirradiated) in genetic backgrounds giving reduced levels of STAT or HP1. Trans-heterozygotes of 2 strong or null alleles of Stat92E or Su(var)205 were used instead of simple heterozygotes to obtain greater reduction in the levels of these 2 proteins. Su(var)205−/− larvae did not grow imaginal discs, so we examined larval brains instead.
We found that larval brains from nonirradiated animals with reduced heterochromatin [as in hopTum-l/+, Stat92E−/−, or Su(var)205−/− animals] exhibited higher levels of γ-H2AX staining when compared with controls (Fig. 3A). Furthermore, the increased γ-H2AX levels associated with hopTum-l/+ were completely suppressed by overexpression of HP1 or STAT92EY704F (Fig. 3A). Su(var)205−/− animals exhibited the most pronounced increase in γ-H2AX signal, consistent with the idea that loss of heterochromatin leads to increased DNA damage. The increase of γ-H2AX signal in Su(var)205−/− animals was not simply due to apoptosis, as these animals had only a few apoptotic cells, identified by positive staining with antisera against activated caspase 3 (Supplemental Fig. S1). Interestingly, at high magnification and single nucleus resolution, we found that the heterochromatin-rich regions of wild-type nuclei were completely devoid of γ-H2AX signal, whereas in mutant (hopTum-l/+; Su(var)205+/−) nuclei, there were high levels of γ-H2AX signal throughout (Supplemental Fig. S2). These data support the idea that heterochromatin formation suppresses DNA damage.
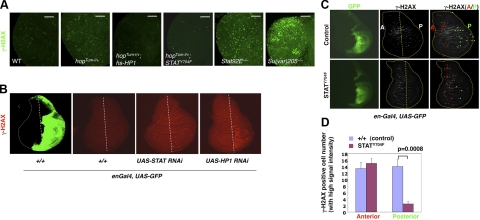
Figure 3.
Effects of unphosphorylated STAT and heterochromatin levels on DNA damage. A, B) Third-instar larval brains (A) or wing imaginal discs (B) of indicated genotypes were immunostained with antibodies specific for Drosophila phosphorylated H2Av (equivalent to mammalian γ-H2AX). Images are from confocal scans obtained at identical settings. A) Note the higher levels of γ-H2AX signals in hopTum-l/+ (29°C), Su(var)205−/− (Su(var)2054/5), and Stat92E−/− (Stat92E06346/F) brains and the reduced γ-H2AX signals in the presence of one copy of hsp70-HP1 (at 29°C) or STATY704F, which was expressed as an Actin-Gal4-driven UAS-Stat92EY704 transgene. First- and second-instar larvae (2–3 d old) of all genotypes were initially reared at 25°C and then were kept at 29°C until dissection. B) Third-instar wing discs are shown with anterior to the left and dorsal up. Indicated transgenes or GFP (control; green) were expressed using en-Gal4 in the posterior compartment of the wing disc. Note the appearance of cells with high levels of γ-H2AX signal (red dots) mainly in the posterior compartment of the wing disc where STAT or HP1 was knocked down by RNAi expression. Two independent RNAi lines were used, and results were similar. Dotted straight lines mark the approximately anterior-posterior boundary. C) Third-instar en-Gal4, UAS-GFP/+ (top; control) and en-Gal4, UAS-GFP/UAS-Stat92EY704F (bottom) larvae were irradiated with 250 rad of γ-rays and immunostained with anti-γ-H2AX sera. Similar results were observed with 500 rad (data not shown). Cells with high γ-H2AX signal are white dots marked by arrowheads in red (anterior) or green (posterior). The anterior-posterior boundary is marked by a dotted line (anterior, to the left). D) Quantification of cells with high γ-H2AX signal per anterior or posterior compartment 1 hr after γ-ray irradiation. Note that fewer cells with high γ-H2AX signal are found in the posterior compartment expressing Stat92EY704F than in the control. P values were calculated using Student's t test by comparing the effect of Stat92EY704F to controls. At least 3 discs were scored for each genotype. Error bars = sd.
To confirm that reducing STAT or heterochromatin levels leads to a spontaneous increase in DNA damage, we used the en-Gal4 driver to express RNAi transgenes targeting Stat92E or Su(var)205 in the posterior compartment of the wing disc and examined the levels of γ-H2AX signal in the anterior and posterior compartments of the wing disc (Fig. 3B). Without transgene expression, few spontaneous γ-H2AX positive cells were detectable in wild-type discs (Fig. 3B, second panel). Consistent with our data on irradiated animals, we found that knocking down of STAT or HP1 resulted in an increase in the number of γ-H2AX-positive cells in the posterior compartment (Fig. 3B, right panels). Furthermore, the increased γ-H2AX levels associated with expressing Su(var)205 or Stat92E RNAi transgenes were not suppressed by coexpressing the apoptotic protein P35 (data not shown), consistent with the idea that increased DNA damage is caused by loss of heterochromatin, rather than apotosis (Fig. 3A and Supplemental Fig. S1). Thus, reducing the levels of STAT or heterochromatin leads to spontaneous DNA damage, consistent with a role for heterochromatin in protecting genome stability.
To directly test whether unphosphorylated STAT92E protects the genome against radiation-induced DNA damage, we expressed the unphosphorylatable mutant form of STAT92E, STAT92EY704F, in the posterior compartment of discs by using the en-Gal4 driver and examined DNA damage after irradiation. Without irradiation, little γ-H2AX signal was detected in the imaginal discs (Supplemental Fig. S3). One hour after exposing larvae to X-rays, γ-H2AX signal was detected in control discs (which expressed GFP only) both as a uniformly low level signal and as discrete high-level foci (Fig. 3C, D). We found that STAT92EY704F expression indeed reduced the number of high-level γ-H2AX foci in the posterior compartment as compared with the anterior compartment (Fig. 3C, D). These results suggest that increasing the levels of unphosphorylated STAT reduces radiation-induced DNA damage and further support the idea that unphosphorylated STAT and heterochromatin protect the genome against DNA damage.
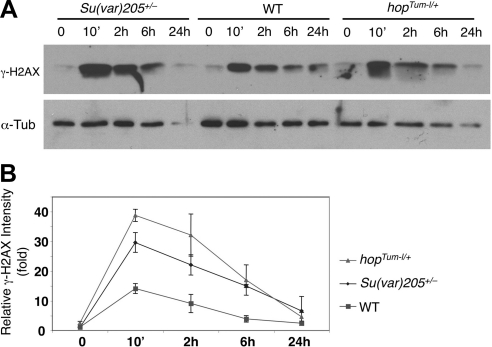
We proceeded to investigate whether the increase in the number of γ-H2AX-positive cells seen on reductions in STAT or heterochromatin levels was due to increased DNA damage or to a decrease in DNA damage repair. We carried out a time-course analysis of γ-H2AX signal, examining γ-H2AX levels in total protein extracts from wild-type, hopTum-l/+, and Su(var)205+/− embryos at different times after irradiation. Here γ-H2AX was at low basal levels in wild-type embryos before irradiation (Fig. 4, time 0). Its levels were increased immediately after γ-ray irradiation (Fig. 4, 10 min) and then gradually decreased over 24 h (Fig. 4A, lanes 8–10; B), presumably because of DNA damage repair (14). In contrast, 10 min after γ-ray irradiation, γ-H2AX levels in extracts from hopTum-l/+ or Su(var)205+/− embryos were >2-fold greater than those of wild-type controls at the same time point (Fig. 4, 10 min). At 24 h after irradiation, however, γ-H2AX levels in all genotypes had returned to basal levels (Fig. 4, 24 h). These results suggest that after irradiation, hopTum-l/+ and Su(var)205+/− animals accumulated more DNA damage than wild-type control animals, but that the ability to repair DNA damage was similar in all genotypes. These data support the idea that unphosphorylated STAT and heterochromatin protect genome stability by preventing DNA damage rather than by promoting DNA damage repair.
Figure 4.
Time course of radiation-induced DNA damage. A) Embryos of indicated genotypes were irradiated with 4000 rad of γ-rays or not irradiated. Total protein was extracted at different time points after irradiation (indicated), subjected to SDS-PAGE, and blotted with anti-γ-H2AX (14) and anti-α-tubulin (loading control). Results shown are representative of 3 independent experiments. B) Quantification of γ-H2AX vs. tubulin (loading control) for the indicated genotypes at different time points after irradiation as shown in panel A from 3 independent experiments. Note that Su(var)205+/− (Su(var)2055/+) and hopTum-l/+ animals exhibit greater levels of DNA damage than wild-type control animals (ry506)/w1118) at 10 min after irradiation. Error bars = sd.
STAT and heterochromatin are essential for proper chromosomal compaction during mitosis
Having demonstrated that loss of unphosphorylated STAT or heterochromatin increases damage to genomic DNA and thus lowers the threshold for the cell cycle checkpoint response, we next investigated how this DNA damage might occur. Heterochromatin is known to function in chromosomal compaction and segregation during mitosis (3, 24). A lack of proper compaction could lead to mitotic defects, which might be the cause of increased DNA damage.
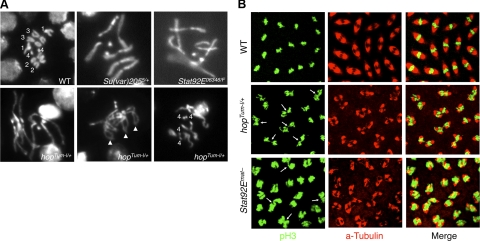
To investigate the importance of unphosphorylated STAT as well as heterochromatin in the proper condensation of mitotic chromosomes, we examined the morphology of metaphase chromosomes, which can be observed most conveniently in the large diploid neuroblasts of squashed third-instar larval brain. Other groups have shown that transheterozygous as well as heterozygous mutations in Su(var)205 result in a range of defects, including uncondensed chromosomes, telomere fusion, and segregation failure (25, 26). We found that compared with wild-type controls (Fig. 5A, top left panel), Su(var)205+/− larvae, which have reduced heterochromatin levels (22), exhibited abnormally long mitotic chromosomes that were 1.5- to 6-fold longer than the mean length of wild-type chromosomes (Fig. 5A, top middle panel, and Table 1). These results are consistent with a condensation failure due to global heterochromatin loss.
Figure 5.
Reducing heterochromatin affects mitotic chromosome condensation and segregation. A) Squashed third-instar larval brains of indicated genotypes were stained with DAPI and photographed at the same magnification. Chromosome numbers are indicated. Arrowheads point to fused telomeres. Note that the metaphase chromosomes in hopTum-l/+, Su(var)205+/−, and Stat92E−/− (Stat92E06346/F) brains are abnormally long, suggesting a lack of proper condensation. B) Embryos (0–2 h after egg deposition) of indicated maternal genotypes were fixed and immunostained with anti-pH3 (green) to reveal mitotic chromosomes and with anti-α-tubulin (red) to reveal mitotic spindles. Note the condensed metaphase chromosomes in wild-type embryos and lack of condensation in hopTum-l/+ and Stat92Emat− embryos. Arrows indicate chromosomal bridges.
Table 1.
Percentage mitotic nuclei with defective chromosomal condensation
| X chromosome length | Wild-type | hopTum-l/+ | Stat92E06346/F | Su(var)2–55/+ |
|---|---|---|---|---|
| >1.5× longer | 1.7% (n=2) | 11.8% (n=28) | 14.4% (n=13) | 22.5% (n=27) |
| >2.0× longer | 0% (n=0) | 5.1% (n=12) | 11.1% (n=10) | 9.2% (n=11) |
| Total nuclei counted | 118 | 237 | 90 | 120 |
Chromosome length was measured for the X chromosomes in metaphase nuclei of neuroblasts in squashed third-instar larval brain of indicated genotypes and was compared to the mean X chromosome length of the wild-type nuclei. At least 5 different larval brains of each genotype were examined. The frequencies in each category for each genotype were similar, and the results were combined.
Furthermore, we found that brain neuroblasts from both hopTum-l/+ and Stat92E−/− larvae, both of which have reduced heterochromatin levels (11, 13), also exhibited abnormally long mitotic chromosomes (Fig. 5A and Table 1) that were similar in appearance to those from Su(var)205 heterozygous animals. Telomere fusion and polyploidy (Fig. 5A, bottom middle and right panels) were also observed in hopTum-l heterozygote larval brain neuroblasts but never in wild-type control larvae. Extremely long chromosomes constitute only a minority of all chromosomes observed in these mutants (Table 1), presumably because these abnormal chromosomes do not undergo mitosis successfully and thus are not propagated. This interpretation is consistent with the observation that mutants with reduced heterochromatin levels had higher levels of spontaneous DNA damage (see Fig. 3).
To further investigate whether a lack of proper chromosomal condensation during mitosis affects normal cell cycle progression and causes DNA damage, we examined mitotic nuclei in syncytially dividing early embryos. Chromosomal condensation and segregation defects have previously been observed in early Drosophila embryos produced by Su(var)205+/− mothers, with the embryos exhibiting chromosomal bridges and uncondensed metaphase chromosomes with high frequency (25). Indeed, compared with wild-type control embryos, in which the chromosomes at metaphase are invariably highly condensed (Fig. 5B, top panels), we found that neither hopTum-l/+ nor Stat92Emat− mutant embryos showed proper condensation of metaphase chromosomes, and both types of mutant embryos frequently exhibited chromosomal bridges (Fig. 5B, arrows). Stat92Emat− mutant embryos lack the maternal supply of STAT92E (see Methods) and can be considered as Stat92E−/− at the syncytial dividing stages before transcription of the zygotic genome begins. Thus, results from studies of 2 different cell types and 2 developmental stages suggest that unphosphorylated STAT and heterochromatin are important for proper chromosomal condensation during mitosis. These data support the notion that the DNA damage observed when unphosphorylated STAT or heterochromatin was reduced (Fig. 3) was due to breakage of unresolved mitotic chromosomes.
STAT and heterochromatin protect against chromosomal breakage
We have shown that cells with reduced levels of unphosphorylated STAT or heterochromatin are prone to cell cycle arrest (Figs. 1 and 2) and exhibit increased DNA damage (Figs. 3 and 4), and we have proposed that the DNA damage is caused by chromosomal breakage resulting from improper chromosomal compaction during mitosis (Fig. 5). Accordingly, we sought evidence for the presence of broken chromosomes in cells with reduced heterochromatin. We indeed found chromosomal bridges between mitotic nuclei in such cells (Fig. 5B, arrows). However, direct observation of significant numbers of broken chromosomes presents a considerable technical challenge. We thus resorted to an indirect method for assessing chromosomal breakage.
It has been shown that low-dose X-ray irradiation during larval growth can cause random chromosomal breakage, resulting in the loss of any of the 65 Minute (M+) loci (genes encoding ribosomal proteins) that are more or less evenly distributed along chromosomal arms throughout the genome (Fig. 6A and ref. 27). Radiation-induced chromosomal breakage during larval growth most frequently causes missing and/or shortening of macrochaetes, the large bristles on the thorax of the surviving adults (Fig. 6A and ref. 28). This is because the loss of any portion of a chromosome will likely take away a M+ locus, and the M+ loci are haploin-sufficient for bristle development during larval growth. Notably, the radiation-induced Minute bristle phenotype is caused rather specifically by chromosomal breakage and not by other types of cellular damage (16, 29, 30). Thus the bristle phenotype after irradiation provides an indirect method for assessing chromosomal breakage.
We assessed the effects of altering STAT and heterochromatin levels on X-ray-induced Minute bristles as a means of quantifying aneuploidy (chromosomal loss) due to chromosomal breakage. We irradiated third-instar larvae of different genetic backgrounds and examined the adult flies for loss or size reduction of macrochaetes. We found that larvae with decreased heterochromatin, such as those with hopTum-l/+, Stat92E+/−, or Su(var)205+/− genotypes, were indeed prone to X-ray induced chromosomal loss, exhibiting Minute macrochaetes in more instances than did wild-type controls (Fig. 6B). In contrast, larvae with increased heterochromatin content, such as hop+/− or hsp70-HP1 larvae, were more resistant to X-ray-induced chromosomal breakage, showing a lower incidence of Minute macrochaetes than controls (Fig. 6B). Without irradiation, none of the genotypes exhibited bristle loss phenotypes (not shown).
To confirm that the Minute macrochaete phenotype was indeed due to chromosomal breakage, we used pulsed-field gel electrophoresis to directly examine the levels of broken genomic DNA after irradiation with X-rays. Broken genomic DNA can be visualized on a pulsed-field electrophoresis gel after staining with EtBr. We found that in flies with reduced levels of heterochromatin, such as Su(var)205+/− or hopTum-l/+ flies, irradiation with X-rays resulted in a larger amount of broken genomic DNA, which was visualized as fast-migrating fragments in the gel (Supplemental Fig. S4).
Unphosphorylated STAT and heterochromatin impart resistance to radiation-induced lethality
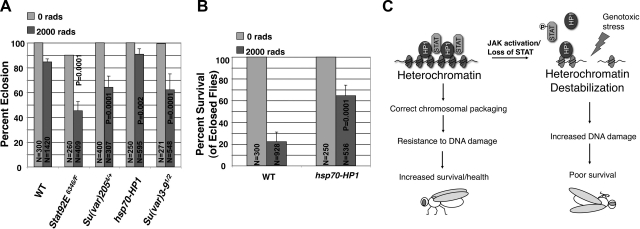
Finally, we investigated the biological functions of unphosphorylated STAT and heterochromatin in protecting animals from radiation-induced lethality. We irradiated third-instar larvae of different genetic backgrounds with γ-rays, which allow for even penetrance in a relatively large population of larvae. We counted the number of eclosed adult flies of each genotype exposed to 2000 rad of γ-ray irradiation, or not exposed, and found that animals with reduced heterochromatin, such as Stat92E transheterozygotes, Su(var)205 heterozygotes, or Su(var)3–9 transheterozygotes, were less viable than wild-type controls, whereas larvae expressing more HP1 were more resistant to γ-ray irradiation (Fig. 7A). Moreover, of eclosed adult flies, 64.4% of the hsp70-HP1 flies remained alive 2 d later, a 3-fold increase in survival compared to wild-type control flies, of which only 22.2% remained alive (Fig. 7B). Taken together, these results not only are consistent with the idea that tissues with reduced heterochromatin levels are more sensitive to radiation-induced DNA damage, but also suggest that increased heterochromatin levels dramatically enhance animal survival under conditions of genotoxic stress (Fig. 7C).
Figure 7.
Unphosphorylated STAT and heterochromatin confer resistance to radiation-induced lethality. A) Third-instar larvae of indicated genotypes were irradiated with 2000 rad of γ-rays, and eclosion rates were calculated. At least 3 independent experiments were done for each genotype. P values were calculated using Student's t test by comparing the effect of each mutant/transgene to wild-type controls. N represents total number of larvae counted. Error bars = sd. B) Calculated survival rates for irradiated flies of the indicated genotypes 2 d after eclosion. At least 7 independent experiments were done for each genotype. P values were calculated using Student's t test by comparing the effect of hsp70-HP1 expression to wild-type controls. Error bars = sd. C) Model for protecting genome stability by STAT and heterochromatin: Unphorsphorylated STAT interacts with HP1 to stabilize heterochromatin and protects genomic DNA from damage and imparts resistance to radiation-induced lethality. Activation of JAK leads to decreased levels of unphosphorylated STAT, which in turn causes loss of heterochromatin stability, thus increasing DNA damage and lethality under conditions of genotoxic stress.
DISCUSSION
By means of genetic manipulations, we have investigated the roles of STAT and HP1 in maintaining genome stability in Drosophila. Our results suggest that unphosphorylated STAT and heterochromatin are important for proper chromosomal compaction and for protecting DNA from damage.
Heterochromatin is a form of highly compacted chromatin important for epigenetic gene silencing and for chromosome organization and movement. Recently it has been shown that proper heterochromatin formation is essential for maintaining the stability of repeated DNA, including the rDNA locus and constitutive heterochromatin regions (4, 5), and is therefore important for protecting genome integrity. Our results extend these observations and demonstrate that heterochromatin plays multiple important roles in preserving genome stability. Specifically, we propose that heterochromatin is essential for proper chromosomal condensation during mitosis, confers resistance to radiation-induced chromosomal breakage, and protects animals against genotoxic stress (Fig. 7C).
We have previously shown that unphosphorylated STAT associates with HP1, and whereby stabilizes HP1 on heterochromatin, whereas overactivation of JAK destabilizes heterochromatin formation (10, 11, 13). We have proposed that JAK overactivation leads to a decrease of unphosphorylated STAT, which in turn causes delocalization of HP1 and heterochromatin instability. This scenario explains the observation that both the gain-of-function mutant hopTum-l and loss of STAT lead to similar chromosomal packaging defects and an increase in DNA damage. We show that overexpression of the transcriptionally inactive STATY704F mutant can rescue the increased spontaneous DNA damage due to hopTum-l and can confer resistance to DNA damage (Fig. 3). These observations support a role of unphosphorylated STAT in protecting genome stability and argue against an involvement of STAT transcriptional activity. Interestingly, we found that overexpressing HP1 also rescues the hopTum-l phenotype (Fig. 3A). This suggests that in the context of JAK activation, that is, decreased unphosphorylated STAT, overexpression of HP1 can overcome the deficiency in unphosphorylated STAT to promote heterochromatin formation.
In summary, we have extended our previous finding that unphosphorylated STAT stabilizes HP1 localization and participates in heterochromatin formation. We provide evidence in support of the idea that heterochromatin and unphosphorylated STAT protect genome stability in general.
Supplementary Material
Acknowledgments
The authors are grateful to Dr. Robert Glaser (Wadsworth Center, New York, NY, USA) for the gift of, and advice on, the phospho-Ser-137 H2Av (γ-H2AX) antibodies; the Goldberg lab (Cornell University, Ithaca, NY, USA) for help and advice on larval brain squash preparations, and E. Rustchenko for help with pulsed field gel electrophoresis. The authors thank D. Guo, K. Larson, A. Tsurumi, and J. Liu for technical assistance and Lori Wallrath (University of Iowa, Iowa City, IA, USA), Gunter Reuter (Martin Luther University Halle, Halle, Germany), James Birchler (University of Missouri, Columbia, MO, USA), the Developmental Hybridoma Bank (Iowa City, IA, USA), and the Bloomington Drosophila Stock Center (Bloomington, IN, USA) for various Drosophila strains and reagents. The authors thank the Edith Lord laboratory (University of Rochester, Rochester, NY, USA) for a Cs-137 irradiator. S.J.Y. was supported in part by a postdoctoral training grant (T32CA009363) from the U.S. National Institutes of Health (NIH).
This study was supported, in part, by grants from the NIH, an American Cancer Society Research Scholar grant, and a Leukemia and Lymphoma Society Research Scholar grant to W.X.L. The authors declare no competing interests.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Jefford C. E., Irminger-Finger I. (2006) Mechanisms of chromosome instability in cancers. Crit. Rev. Oncol. Hematol. 59, 1–14 [DOI] [PubMed] [Google Scholar]
- 2. Lombard D. B., Chua K. F., Mostoslavsky R., Franco S., Gostissa M., Alt F. W. (2005) DNA repair, genome stability, and aging. Cell 120, 497–512 [DOI] [PubMed] [Google Scholar]
- 3. Grewal S. I., Jia S. (2007) Heterochromatin revisited. Nat. Rev. Genet. 8, 35–46 [DOI] [PubMed] [Google Scholar]
- 4. Peng J. C., Karpen G. H. (2007) H3K9 methylation and RNA interference regulate nucleolar organization and repeated DNA stability. Nat. Cell Biol. 9, 25–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peng J. C., Karpen G. H. (2009) Heterochromatic genome stability requires regulators of histone H3 K9 methylation. PLoS Genet. 5, e1000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peters A. H., O'Carroll D., Scherthan H., Mechtler K., Sauer S., Schofer C., Weipoltshammer K., Pagani M., Lachner M., Kohlmaier A., Opravil S., Doyle M., Sibilia M., Jenuwein T. (2001) Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell 107, 323–337 [DOI] [PubMed] [Google Scholar]
- 7. Arbouzova N. I., Zeidler M. P. (2006) JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions. Development 133, 2605–2616 [DOI] [PubMed] [Google Scholar]
- 8. Rawlings J. S., Rosler K. M., Harrison D. A. (2004) The JAK/STAT signaling pathway. J. Cell Sci. 117, 1281–1283 [DOI] [PubMed] [Google Scholar]
- 9. Hou S. X., Zheng Z., Chen X., Perrimon N. (2002) The Jak/STAT pathway in model organisms: emerging roles in cell movement. Dev. Cell. 3, 765–778 [DOI] [PubMed] [Google Scholar]
- 10. Li W. X. (2008) Canonical and non-canonical JAK-STAT signaling. Trends Cell Biol. 18, 545–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shi S., Larson K., Guo D., Lim S. J., Dutta P., Yan S. J., Li W. X. (2008) Drosophila STAT is required for directly maintaining HP1 localization and heterochromatin stability. Nat. Cell Biol. 10, 489–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown S., Zeidler M. P. (2008) Unphosphorylated STATs go nuclear. Curr. Opin. Genet. Dev. 18, 455–460 [DOI] [PubMed] [Google Scholar]
- 13. Shi S., Calhoun H. C., Xia F., Li J., Le L., Li W. X. (2006) JAK signaling globally counteracts heterochromatic gene silencing. Nat. Genet. 38, 1071–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Madigan J. P., Chotkowski H. L., Glaser R. L. (2002) DNA double-strand break-induced phosphorylation of Drosophila histone variant H2Av helps prevent radiation-induced apoptosis. Nucleic Acids Res. 30, 3698–3705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yan S. J., Li W. X. (2011) Using Drosophila larval imaginal discs to study low dose radiation-induced cell cycle arrest. Methods Mol. Biol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Elledge S. J. (1996) Cell cycle checkpoints: preventing an identity crisis. Science 274, 1664–1672 [DOI] [PubMed] [Google Scholar]
- 17. Mogila V., Xia F., Li W. X. (2006) An intrinsic cell cycle checkpoint pathway mediated by MEK and ERK in Drosophila. Dev. Cell. 11, 575–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brodsky M. H., Sekelsky J. J., Tsang G., Hawley R. S., Rubin G. M. (2000) mus304 encodes a novel DNA damage checkpoint protein required during Drosophila development. Genes Dev. 14, 666–678 [PMC free article] [PubMed] [Google Scholar]
- 19. Bi X., Gong M., Srikanta D., Rong Y. S. (2005) Drosophila ATM and Mre11 are essential for the G2/M checkpoint induced by low-dose irradiation. Genetics 171, 845–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Laurencon A., Purdy A., Sekelsky J., Hawley R. S., Su T. T. (2003) Phenotypic analysis of separation-of-function alleles of MEI-41, Drosophila ATM/ATR. Genetics 164, 589–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karsten P., Plischke I., Perrimon N., Zeidler M. P. (2006) Mutational analysis reveals separable DNA binding and trans-activation of Drosophila STAT92E. Cell. Signal. 18, 819–829 [DOI] [PubMed] [Google Scholar]
- 22. Eissenberg J. C., Morris G. D., Reuter G., Hartnett T. (1992) The heterochromatin-associated protein HP-1 is an essential protein in Drosophila with dosage-dependent effects on position-effect variegation. Genetics 131, 345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. James T. C., Elgin S. C. (1986) Identification of a nonhistone chromosomal protein associated with heterochromatin in Drosophila melanogaster and its gene. Mol. Cell. Biol. 6, 3862–3872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grewal S. I., Elgin S. C. (2002) Heterochromatin: new possibilities for the inheritance of structure. Curr. Opin. Genet. Dev. 12, 178–187 [DOI] [PubMed] [Google Scholar]
- 25. Kellum R., Alberts B. M. (1995) Heterochromatin protein 1 is required for correct chromosome segregation in Drosophila embryos. J. Cell Sci. 108(Pt 4), 1419–1431 [DOI] [PubMed] [Google Scholar]
- 26. Perrini B., Piacentini L., Fanti L., Altieri F., Chichiarelli S., Berloco M., Turano C., Ferraro A., Pimpinelli S. (2004) HP1 controls telomere capping, telomere elongation, and telomere silencing by two different mechanisms in Drosophila. Mol. Cell. 15, 467–476 [DOI] [PubMed] [Google Scholar]
- 27. Marygold S. J., Roote J., Reuter G., Lambertsson A., Ashburner M., Millburn G. H., Harrison P. M., Yu Z., Kenmochi N., Kaufman T. C., Leevers S. J., Cook K. R. (2007) The ribosomal protein genes and Minute loci of Drosophila melanogaster. Genome Biol. 8, R216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ahmad K., Golic K. G. (1999) Telomere loss in somatic cells of Drosophila causes cell cycle arrest and apoptosis. Genetics 151, 1041–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McNamee L. M., Brodsky M. H. (2009) p53-independent apoptosis limits DNA damage-induced aneuploidy. Genetics 182, 423–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johnson-Schlitz D. M., Flores C., Engels W. R. (2007) Multiple-pathway analysis of double-strand break repair mutations in Drosophila. PLoS Genet. 3, e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.