Abstract
Our objective was to contrast the effect of apolipoprotein (apo) A-I mimetic peptides, such as 4F and 4F-Pro-4F (Pro), on nascent and mature atherosclerotic lesions and on levels of antibodies against oxidation-specific epitopes. Chow-fed apoE−/− mice were injected intraperitoneally with either the 4F peptide or a tandem helix apoA-I mimetic peptide (Pro) every other day. Mice treated with 4F, but not Pro, for 4 wk starting at 10 wk of age showed a dramatic decrease in atherosclerosis at 2 arterial sites. However, neither peptide was effective in mice treated for 8 wk starting at 20 wk of age; lesions were larger and more mature at this time point. Peptide treatment caused increased production of antibodies against oxidation-specific epitopes, including a disproportionate induction of the IgM natural antibody (NAb) E06/T15 to oxidized phospholipids. In summary, 4F, but not the tandem peptide Pro, effectively inhibited early atherogenesis but was ineffective against more mature lesions. Two different apoA-I mimetic peptides increased titers of natural antibodies against oxidation-specific epitopes.—Wool, G. D., Cabana, V. G., Lukens, J., Shaw, P. X., Binder, C. J., Witztum, J. L., Reardon, C. A., Getz, G. S. 4F Peptide reduces nascent atherosclerosis and induces natural antibody production in apolipoprotein E-null mice.
Keywords: mimetic, apoA-I, oxidation, innate immunity, E06/T15
Despite proper medical treatment, dyslipidemic patients continue to face elevated cardiovascular disease risk if their plasma HDL is low or nonfunctional (1, 2). HDL has several antiatherosclerotic mechanisms of action, including the inhibition of lipoprotein oxidation (3) and the removal of cholesterol from atherosclerotic lesions (4). ApoA-I mimetic peptides that boost various antiatherogenic properties of HDL have been under intense investigation as possible therapeutics (5–7). These peptides offer an easier therapeutic strategy than directly infusing HDL or its major component apoprotein, apoA-I.
4F is an 18-aa peptide containing 4 phenylalanine residues, which contribute to its hydrophobic properties. 4F can alleviate chronic inflammation in several animal disease models, including atherosclerosis (6, 7). Like HDL, apoA-I mimetic peptides have a variety of putative atheroprotective mechanisms, including acting as an antioxidant (8), mediating cholesterol efflux from foam cells (8), and direct antiinflammatory effects (5, 9). One of the most promising peptide mechanisms, which could explain the in vivo efficacy of 4F at low concentrations, is its capacity to bind oxidized phospholipids with high affinity (10). This finding led us to explore whether mimetic peptides might influence the levels of antibodies against oxidized lipids. Oxidized LDL and phospholipids are important for atherogenesis (11), and antibodies against these lipids could modulate atherogenesis.
In our prior studies (8, 9), we investigated the in vitro and short-term in vivo properties of 4F and several tandem peptides. These tandem peptides involved two 4F 18-mer α-helices separated by various linkers (proline, alanine, and others). The tandem peptide containing a proline linker (referred to as Pro peptide) was chosen for these in vivo studies in light of the conserved proline residues in the interhelical regions of apoA-I (12). The properties of the single-helix 4F and tandem-helix Pro peptide differed from each other in several respects (8, 9). 4F remodeled mouse HDL, promoted cholesterol efflux from foam cells, and prevented LDL oxidation in vitro. 4F did not associate with HDL in vivo. The Pro peptide showed increased ability to remodel mouse HDL and promote cholesterol efflux relative to the 4F peptide but did not prevent LDL oxidation in vitro. The Pro peptide associated with HDL in vivo. These results suggest that 4F and Pro peptide might have differing effects on atherosclerosis.
The ability of apoA-I mimetic peptides to influence atherosclerotic lesions at various points of temporal development has not been adequately investigated. Monotherapy with the peptides D4F or 5F is antiatherogenic for the small, early lesions of 9-wk-old chow-fed apoE−/− mice (13) or 24-wk-old cholate-diet-fed wild-type mice (14). The data conflict in indicating how effectively 4F, using either l- or d-amino acids, halts growth or regresses already established lesions (15, 16). The effectiveness of apoA-I mimetic peptides on established lesions is very important if 4F is to be used as a human therapeutic (17). Here we investigated the effects of 4F and Pro in a single animal model at two different time points of lesion development. We have described previously the large, cellular lesions found in 27-wk-old chow-fed apoE−/− mice at the aortic root and innominate artery (18). Lesions from these mice better model clinically significant atherosclerosis than do the small scattered foam cell lesions of extremely young apoE−/− mice.
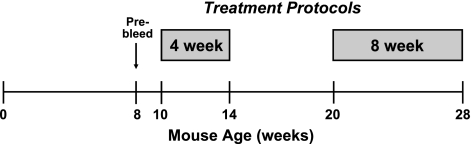
We assayed the ability of 4F and Pro to modify plasma lipid levels, influence titers of antibodies against oxidation-specific epitopes such as those found on oxidized LDL, and to reduce atherosclerotic lesion area in female apoE−/− mice fed a chow diet. Two protocols of treatment were used (Fig. 1). These lengths of treatment coincide with previous reports in which peptides had antiatherosclerotic effects after 4–5 wk (13, 16, 19) or 16 wk of treatment (14).
Figure 1.
Timeline of peptide treatment. Female apoE−/− mice fed chow diet with total cholesterol between 300 to 500 mg/dl at 8 wk of age were selected for the study. Chow-fed mice were either treated for 4 wk starting at 10 wk of age or were treated for 8 wk starting at 20 wk of age.
Based on previous reports, we anticipated that 4F would have an antiatherosclerotic effect on young, but not mature, atherosclerotic lesions (13–16). With the different properties of the monomeric and tandem helix peptides in mind, we have tested the relative efficacy of 4F and Pro peptide on both early and established atherosclerosis. If HDL remodeling and cholesterol efflux properties are more important for attenuating atherosclerosis, the Pro peptide should exhibit greater antiatherosclerotic effects (8). However, if antioxidant ability predominates for peptide efficacy, 4F should be more atheroprotective (8).
MATERIALS AND METHODS
Peptides
Peptides containing l-amino acids were synthesized and purified as described previously (9), or purified peptides (>90% purity) were purchased from BioSynthesis (Lewisville, TX, USA). All peptides were N-terminally acetylated and C-terminally amidated (9). We adhere to the standard used by previous reports in the apoA-I mimetic peptide literature that the denomination “4F” denotes the following peptide: Ac-DWFKAFYDKVAEKFKEAF-NH2 (13). “Pro” denotes the following peptide: Ac-DWFKAFYDKVAEKFKEAF-P-DWFKAFYDKVAEKFKEAF-NH2. Peptides administered to mice were solubilized in sterile PBS.
Mice
All mice were in the C57BL/6 genetic background and were bred in-house. They were housed in microisolators in a specific pathogen-free barrier facility at the University of Chicago, and experimental procedures were performed in accordance with U.S. National Institutes of Health guidelines under protocols approved by the University of Chicago Animal Care and Use Committee.
Female apoE−/− mice were maintained on a standard laboratory chow diet (6.25% fat; TD7913; Harlan Teklad, Indianapolis, IN, USA). At 7–8 wk, mice whose total plasma cholesterol fell within the 300- to 500-mg/dl range were selected for the study. Peptides were administered to mice under 2 protocols: beginning at 10 wk of age for 4 wk, or beginning at 20 wk of age for 8 wk (Fig. 1). The total number of mice in each treatment group is shown in Table 1. All mice completed the study, with no deaths. For those mice whose atherosclerotic lesions were quantified, group sizes are as follows: 4-wk PBS, 8 mice; 4-wk 4F, 8 mice; 4-wk Pro, 9 mice; 8-wk PBS, 12 mice; 8-wk 4F, 13 mice; and 8-wk Pro, 11 mice.
Table 1.
Mouse body and spleen weights at study termination
| Treatment | Body weight (g) | Spleen weight (g) |
|---|---|---|
| 4 wk | ||
| PBS (10 mice) | 20.48 ± 1.15 | 0.1 ± 0.02 |
| 4F (8 mice) | 19.49 ± 0.98* | 0.08 ± 0.01* |
| Pro (9 mice) | 20.94 ± 1.50 | 0.08 ± 0.02* |
| 8 wk | ||
| PBS (15 mice) | 21.3 ± 1.7 | 0.11 ± 0.03 |
| 4F (15 mice) | 21.0 ± 1.3 | 0.10 ± 0.01 |
| Pro (14 mice) | 20.9 ± 1.3 | 0.10 ± 0.01 |
P < 0.05 vs. 4 wk PBS treatment.
Mice received intraperitoneal peptide or PBS injections every other day and were given acidified drinking water with trimethoprim-sulfamethoxazole. The mice were injected with 50 μg 4F or 100 μg Pro peptide in PBS, with a total injection volume of 200 μl. This translates as ∼1.19 μg 4F peptide/g body weight (bw)/d or ∼2.38 μg tandem peptide/g bw/d; this represents ∼0.5 nmol peptide/g bw/d of either peptide.
After 4 or 8 wk of peptide treatment, mice were sacrificed 2 h after the last peptide injection and removal of chow. Anesthetized mice were exsanguinated and perfused transcardially under physiological pressure with PBS for 2 min followed by a 10-min perfusion with buffered formalin solution (4% paraformaldehyde/5% sucrose in PBS). Selected organs were isolated prior to perfusion, their wet weight was determined, and they were stored in RNAlater (Qiagen, Valencia, CA, USA) at −20°C.
Serum paraoxonase (PON) activity
PON activity was assayed as described previously (9) at the conclusion of the 8-wk treatment. Using phenyl acetate substrate, the arylesterase activity of 6 concentrations of unfractionated serum was determined for each mouse. The activity was monitored as the change in absorbance over 2 min on the spectrophotometer. The activities for each serum concentration were fit to a linear regression line. The worst-fit line had a value of r2 = 0.9938. The slope of the regression line (change in OD per minute per microliter serum) was used as the readout of mouse serum activity. This was converted to PON arylesterase units (U), or micromoles phenyl acetate hydrolyzed per minute per mililiter: (ΔOD min−1 ml−1)/(1310 M−1 cm−1).
Plasma SAA levels
At the conclusion of the 8-wk study, whole plasma was separated by SDS-PAGE and immunoblotted for SAA and apoA-I, as described previously (9). Relative SAA level was quantitated by enhanced chemiluminescence (ECL) signal intensity.
Lipoproteins
Lipoprotein distribution was analyzed by fast protein liquid chromatography (FPLC), as described previously (9).
Atherosclerotic lesion size quantitation
The isolated murine heart with the upper arterial vasculature and major branches intact was infused with Optimum Cutting Temperature (OCT) compound (Sakura Finetek, Torrence, CA, USA), and the superior portion of the heart was embedded in OCT, with a liver fragment placed on the heart's ascending aorta side to provide orientation, and frozen at −80°C.
The frozen tissue was sectioned serially into 10-μm sections on a Leica cryostat (Leica Microsystems, Bannockburn, IL, USA). Specific portions of the innominate artery, ascending aorta, and aortic root were saved according to the laboratory's protocol (18, 20). Atherosclerotic lesion size in the innominate artery was assessed using 4 sections between 150 to 450 μm above the junction of the innominate artery with the greater curvature of the aortic arch. Atherosclerotic lesion size in the ascending aorta was assessed using 3 sections at the apex of the lesser curvature of the aortic arch. Atherosclerotic lesion size in the aortic root was assessed using 3 sections beginning at the appearance of a coronary artery and the aortic valve leaflets. The sections in all three sites were separated by 100 μm from each other. Those sections were stained with Oil Red-O and Fast Green. Digital images were captured, and the atherosclerotic lesion size was quantified from those images using OpenLab 3.1.5 software (PerkinElmer, Waltham, MA, USA).
Real-time quantitative PCR analysis
Spleens in RNAlater were disrupted using a polytron homogenizer, and the mRNA was isolated using the Qiagen Mini spin column kit according to the manufacturer's protocol. Up to 5 μg RNA was used to generate cDNA using oligo dT primers and the SuperScript III system (Invitrogen, San Diego, CA, USA) according to the manufacturer's protocol. Real-time PCR was performed using SYBR green on an Applied Biosystems Step One machine (Applied Biosystems, Foster City, CA, USA). The relative mRNA levels were determined by the ΔΔCT method, and HPRT was used as the endogenous control transcript. E06/T15 primers are specific for the known VH complementarity determining region 3 (CDR3) of the E06/T15 idiotype.
Oligonucleotides
EO6 VH: forward, CAGAGACACTTCCCAAAGCA; reverse, CCCAGACATCGAAGTACCAG.
HPRT: forward, ACCTCTCGAAGTGTTGGATA; reverse, CAACAACAAACTTGTCTGGA.
Natural antibody ELISA
Titers were assayed by a chemiluminescent immunoassay, as described previously (21). Each condition represents 5–7 individual plasma samples harvested at the end of the study. The values are in relative light units (RLU) per 100 ms × 103.
To determine titers of IgM and IgG antibodies to OxLDL, 50 μl of antigens at 5 μg/ml in PBS (containing 0.25 mM EDTA) was coated onto white, round-bottomed High Binding Microfluor microtitration plates (Dynex Technologies, Chantilly, VA, USA) overnight at 4°C. After washing 4 times with PBS, using an automated plate washer (Biotek, Winooski, VT, USA), wells containing antigens were blocked with 75 μl TBS buffer (containing 150 mM NaCl, 50 mM Tris base, 0.25 mM EDTA, and 1% BSA) for 30 min. Wells were washed again, and 50 μl of murine sera diluted 1:100 in TBS buffer was incubated for 2 h at room temperature. After four more wash steps, the amount of murine antibodies bound was detected by 1 h incubation with 50 μl of either alkaline phosphatase-labeled goat-anti-mouse IgM or goat-anti-mouse IgG (both Sigma, St Louis, MO, USA) diluted in TBS buffer at 1:35,000 and 1:30,000, respectively. After further washing, 25 μl of a 50% solution of LumiPhos (Lumigen Inc., Southfield, MI, USA) was incubated for 90 min at room temperature in darkness, and antibody binding was measured in RLU per 100 ms using a Victor2 (Perkin Elmer). Unless otherwise indicated, the incubation times, volumes, and concentrations were the same as above. For the determination of total IgM or IgG levels, wells were coated with goat-anti-mouse-IgM or goat-anti-mouse-IgG (Sigma) at 5 μg/ml. Serum samples were diluted at 1:10,000, a concentration previously determined to be within the linear detection range of this assay.
For the detection of EO6-type antibodies in the plasma of mice, 2 μg/ml of the monoclonal anti-idiotypic (αT15 idiotype) antibody AB1–2 in PBS was coated onto microtitration plates overnight at 4°C. After blocking, wells were washed again, and 50 μl of murine sera diluted 1:100 was incubated for 2 h at room temperature. After further washing, bound T15id+ IgM (EO6) was detected using AP-conjugated goat-anti-mouse IgM diluted in BSA-PBS followed by LumiPhos. Purified EO6 and EO14 (an IgM NAb specific for MDA-LDL; e.g., a non-T15 idiotype) were used as positive or negative controls, respectively.
Statistics
Data are presented as averages ± sd. Student's t test was used for determination of significant differences. Values of P < 0.05 were considered significant.
RESULTS
Effect of peptide treatment on body and spleen weight, serum PON activity, and plasma SAA level
The terminal weights of mice undergoing peptide treatment did not differ grossly compared with healthy untreated age-matched mice from our laboratory (data not shown). Four weeks of 4F, but not Pro peptide, treatment significantly decreased mouse body weight by 1 g (Table 1); however, neither the 4F nor Pro peptide affected weight in the 8-wk-treated animals.
Weight loss can indicate an inflammatory response. We measured spleen weight as a marker of systemic inflammation. Neither control nor peptide injections increased spleen weight relative to healthy untreated mice from our laboratory (data not shown). In fact, the 4-wk 4F and Pro treatments significantly reduced spleen weight by 0.02 g, while the 8-wk treatments had no effect (Table 1). The drinking water contained trimethoprim-sulfamethoxazole in order to lessen the risk of bacterial peritonitis. Our laboratory's prior studies have also used this antibiotic (18). No animals died during the current study, and no animals had evidence of purulent peritonitis on sacrifice.
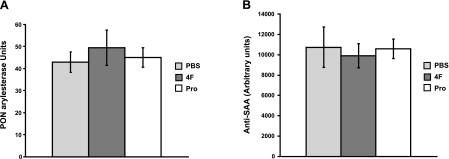
PON1 degrades proinflammatory oxidized phospholipids, and others have reported that D4F up-regulates PON1 activity (5). We did not previously observe an effect of 1 wk of 4F treatment on serum PON activity (9). In this study, 8 wk of 4F treatment caused a nonsignificant trend toward increased PON activity (Fig. 2A).
Figure 2.
A) Total serum PON activity is not modified by peptide treatment in 8-wk study. Arylesterase specific activity of serum (micromoles phenylacetate hydrolyzed per minute per milliliter) was determined for each individual mouse. No significant differences were seen among PBS (5 mice), 4F (7 mice), or Pro treatment (7 mice). B) Total plasma SAA levels are not modified by peptide treatment in 8-wk study. Whole plasma was separated by SDS-PAGE and immunoblotted. No significant differences were seen between PBS (3 mice), 4F (3 mice), or Pro treatment (3 mice).
Though we previously observed that a 1-wk treatment of apoE−/− mice with the Pro peptide significantly decreased the plasma acute-phase reactant SAA (9), a nonsignificant decrease in SAA levels was seen for 4F-treated, but not Pro-treated, mice after 8 wk (Fig. 2B).
4F had no effect on plasma cholesterol levels; Pro peptide increased LDL-C
We did not expect either 4F or Pro peptides to influence plasma lipid levels, given previous results in our laboratory (9) and others (13–16, 19).
In the 4-wk treatment group, 4F had no significant effect on plasma total cholesterol (TC) compared to controls, while Pro peptide significantly increased TC (45%, P<0.0001) (Table 2). Plasma triglyceride (TG) levels were decreased significantly by PBS and Pro treatment, when comparing pre- and posttreatment levels. 4F treatment caused no significant change in posttreatment TG values, relative to pretreatment values. Combined, these effects caused the post-4F-treatment TG values to be significantly higher than post-PBS-treatment TG values.
Table 2.
Plasma lipid and lipoprotein levels
| Lipid or lipoprotein | 4-wk study |
8-wk study |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PBS, n = 10 |
4F, n = 8 |
Pro, n = 9 |
PBS, n = 15 |
4F, n = 14 |
Pro, n = 14 |
|||||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| TC | 395 ± 55 | 438 ± 95 | 384 ± 41 | 479 ± 132 | 373 ± 34 | 635 ± 62^, % | 399 ± 59 | 526 ± 156 | 387 ± 52 | 584 ± 83 | 391 ± 40 | 612 ± 72@ |
| TG | 62 ± 12 | 36 ± 6 | 53 ± 13 | 48 ± 4* | 49 ± 12& | 26 ± 14*,# | ||||||
| Chylo remnant/VLDL-C | 363 ± 122 | 426 ± 97 | 413 ± 83 | |||||||||
| LDL-C | 142 ± 29 | 139 ± 17 | 188 ± 28$ | |||||||||
| HDL-C | 18 ± 5 | 14 ± 8 | 21 ± 9 | |||||||||
Values are average ± sd (mg/dl). Pre, pretreatment at 8–9 wk; post, posttreatment at conclusion of experiment; TC, total cholesterol; TG, triglyceride; VLDL-C, very low density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
P < 0.0001 vs. 4-wk post-PBS-treatment;
P < 0.005 vs. 4-wk post-4F-treatment;
P < 0.05 vs. 8-wk post-PBS-treatment;
P < 0.0005 vs. 4-wk post-PBS-treatment;
P < 0.02 vs. 4 wk pre-PBS-treatment;
P < 0.0005 vs. 4-wk post-4F-treatment;
P < 0.005 vs. 8-wk post-PBS- and post-4F-treatment.
In the 8-wk treatment group, 4F also had no significant effect on plasma TC compared to controls (Table 2). Pro-treated mice had significantly increased plasma cholesterol relative to PBS-treated mice (16%, P<0.04), which was due to an increase in LDL as determined by FPLC (Table 2). HDL-C did not differ significantly.
4F and Pro peptide increased E06/T15 mRNA levels and natural antibody titers
The development of atherosclerosis is accompanied by increases in titers of adaptive (IgG) and innate IgM natural antibodies (NAbs) against oxidation-specific epitopes (22). This response can be monitored by antibody titers against experimentally modified LDL: copper-oxidized LDL (Cu-OxLDL) and malondialdehyde-modified LDL (MDA-LDL). Dyslipidemia is also associated with increased plasma titers and splenic expression of the specific IgM NAb E06/T15, an idiotype that specifically binds to the phosphocholine headgroup of oxidized phospholipids, inhibits oxLDL uptake by macrophages, and can be found within atherosclerotic lesions (22, 23). To determine whether peptide administration affected the production of these antibodies, we measured splenic transcript levels and plasma titers.
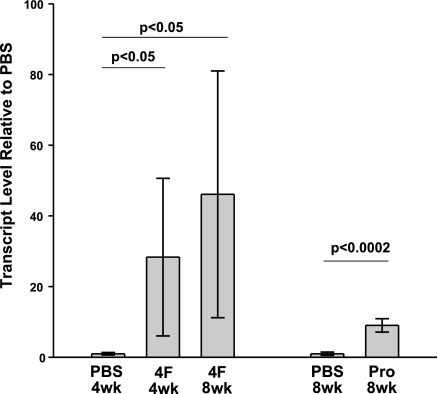
In the 4-wk treatment group, 4F significantly increased the level of E06/T15 mRNA 28-fold compared to PBS controls (Fig. 3). Plasma antibody titers of E06/T15 IgM from the 4-wk treatment group showed no significant effect of 4F administration (data not shown), despite the elevated mRNA level of E06/T15.
Figure 3.
Influence of peptides on splenic E06/T15 mRNA level. E06/T15 transcript levels were determined by real-time PCR using primers specific for the known VH CDR3 region of the E06/T15 idiotype. E06/T15 PCR signal was normalized against HPRT and expressed as levels found in peptide-treated mice relative to those found in PBS-treated control mice.
In the 8-wk treatment group, 4F was associated with a 46-fold increase in E06/T15 mRNA relative to controls (Fig. 3). Eight weeks of Pro peptide treatment caused a significant 9-fold increase in splenic E06/T15 mRNA level (Fig. 3). 4F treatment significantly elevated E06/T15 IgM titers, IgM titers to MDA-LDL and Cu-oxLDL, and IgG titers to Cu-oxLDL (Table 3). E06/T15 showed the greatest increase, roughly 10-fold. Pro peptide treatment caused significantly elevated E06/T15 IgM titers, IgM titers to Cu-oxLDL, and IgG to Cu-oxLDL. Once again, E06/T15 showed the greatest increase of the antibodies: a roughly 6-fold increase with the Pro peptide. The induction of IgM NAb titers was quite specific, as total IgM levels did not differ between PBS-, 4F-, or Pro-treated animals. In the case of adaptive IgG, total IgG levels were the same between PBS and 4F mice, though significantly increased in Pro-treated mice (P<0.05).
Table 3.
Plasma antibodies against oxidized epitopes in 8 wk mice
| Antibody specificity | Antibody class | PBS, n = 5 | 4F, n = 5 | Pro, n = 8 |
|---|---|---|---|---|
| MDA-LDL | IgM | 235.43 ± 19.2 | 287.22 ± 26.1* | 242.77 ± 37.3 |
| IgG | 35.03 ± 26.9 | 17.38 ± 12.8 | 19.17 ± 3.7 | |
| Cu-oxLDL | IgM | 101.81 ± 33.0 | 249.85 ± 20.0* | 162.08 ± 52.6* |
| IgG | 1.29 ± 0.2 | 4.49 ± 0.6* | 3.0 ± 1.0* | |
| E06/T15 | IgM | 25.7 ± 32.1 | 251.32 ± 6.0* | 165.0 ± 76.3* |
| Plasma total IgM | 149.95 ± 38.8 | 143.22 ± 15.5 | 119.73 ± 25.9 | |
| Plasma total IgG | 21.43 ± 12.2 | 29.45 ± 5.4 | 43.51 ± 12.6* |
Values are average ± sd luminescence (103 RLU/100 ms); intratiter comparisons.
P < 0.05 vs. PBS treatment.
Four-week 4F treatment significantly reduced nascent atherosclerosis
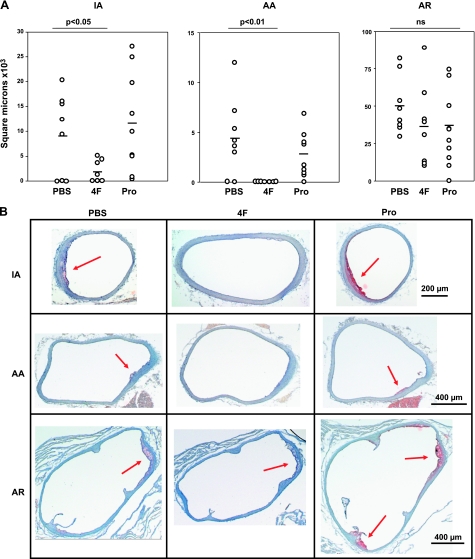
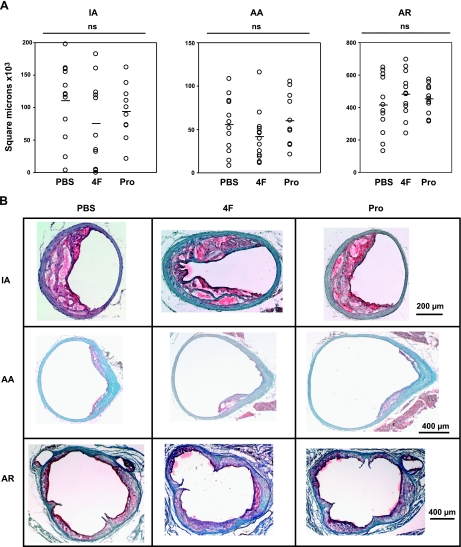
In mice treated with PBS for 4 wk starting at 10 wk of age, the innominate artery (IA) lesions were small (Fig. 4A). 4F-treated mice had 79% smaller IA lesions than PBS controls (P<0.05). Pro-treated mice had nonsignificantly larger lesions than PBS controls. In PBS controls as well as Pro-treated mice, foam cells were present on both the intimal surface as well as between the innermost medial lamellae (Fig. 4B). In 4F-treated mice, scattered foam cells were only noted on the intimal surface. No fibrous caps or acellular gruel were present in the IA regardless of treatment.
Figure 4.
A) Reduction of atherosclerotic lesion size in 4-wk protocol. Lesions were significantly reduced in the innominate artery (IA) and aortic arch (AA) in 4F-treated mice. Reduction of lesion size in the aortic root (AR) did not reach significance. B) Photomicrographs of representative lesions from the 4-wk study. Arrows indicate the small atherosclerotic lesions present at this time point.
4F-treated mice had no atherosclerotic lesion in the aortic arch (AA) (Fig. 4A). Lesions from the AA in PBS control mice averaged roughly 4400 μm2 in size (P<0.01 as compared to 4F-treated mice) and were made up of intimal surface foam cells (Fig. 4B). AA lesions from Pro-treated mice averaged 2600 μm2, which did not differ significantly from controls; those lesions were also made up of intimal surface foam cells.
The aortic root (AR) demonstrated foam-cell rich lesions in both peptide-treated mice and controls (Fig. 4B). 4F and Pro-treated mice had ∼25% smaller AR lesions than PBS controls, but these differences did not reach significance (Fig. 4A).
Overall, 4F significantly reduced nascent atherosclerosis at 2 of 3 vascular sites studied in 14-wk-old female apoE−/− mice treated for 4 wk. Pro peptide caused no significant change in lesion size under this treatment protocol.
Eight-week peptide treatment did not decrease atherosclerotic lesion size in older mice
In mice treated for 8 wk starting at 20 wk of age, atherosclerotic lesions were much larger and more mature (Fig. 5) than those from the 4-wk treatment group (Fig. 4); please note the differing scales for quantitation of the lesions.
Figure 5.
A) Lack of peptide effect in 8-wk protocol. 4F or Pro peptide did not significantly influence lesion size at any vascular site in the 8-wk protocol. B) Photomicrographs of representative lesions from the 8-wk study.
4F- and Pro-treated mice had smaller IA lesions than controls, but these differences were not significant (Fig. 5A). The IA lesions were large, complex, and cellular. No consistent morphological differences were seen between any treatment groups. Lesions typically contained multiple layers, and acellular necrotic gruel was prominent. Fibrous caps over the lesions were generally quite thin, only 1–2 cells thick (Fig. 5B).
4F-treated mice had smaller AA lesions than PBS controls, but again, this difference was not significant (Fig. 5A). AA lesion size in Pro-treated mice was comparable to controls. The AA lesions in the 8-wk treatment group had fibrous caps and areas of acellular material (Fig. 5B).
The AR demonstrated quite robust lesions for each treatment group (Fig. 5A). 4F- and Pro-treated mice had slightly larger AR lesions than controls, but neither result reached significance. The AR lesions were large, complex, and cellular. No consistent morphological differences were seen between any treatments. Lesions encompassed all three sinuses, though the left coronary sinus (LCS) had the largest lesion size, as expected (24). Cholesterol clefts and areas of amorphous necrotic gruel were ubiquitous. The coronary ostia frequently showed lesion formation (Fig. 5B).
Overall, neither apoA-I mimetic peptide significantly affected atherosclerotic lesion size or morphology in chow-fed 28-wk-old female apoE−/− mice.
DISCUSSION
This is the first demonstration of the differential effectiveness of 4F on atherosclerosis depending on the stage of lesion development within the same murine model and experimental conditions. Navab et al. (25) previously showed that D4F combined with a statin was atheroprotective regardless of lesion maturity. We show here that intraperitoneal administration of l-amino acid-based 4F alone can inhibit early lesion formation but does not influence the progression of more mature lesions. Pro peptide, however, was not effective at preventing atherosclerosis at either time point.
A 4-wk treatment of 4F dramatically reduced atherosclerosis in 14-wk-old chow-fed apoE−/− mice in the innominate artery and aortic arch, but not the aortic root. As expected, this reduction in atherosclerosis was not associated with any change in plasma TC or HDL-C. 4F treatment did not significantly affect TG levels, while PBS- and Pro-treated mice had significantly decreased TG levels. This decrease in TG levels may be due to a transient mild sterile inflammatory response (40) related to injection, as lipids were measured 2 h after the last peptide injection. If this transient inflammatory response had truly occurred, it was not great enough to cause splenic enlargement. 4F-treated mice had no change in TG levels, which might indicate that 4F blocked an inflammatory reaction.
A 4-wk treatment with Pro peptide had no effect on lesion size but did significantly increase plasma TC levels. Eight weeks of 4F or Pro peptide did not influence atherosclerosis in chow-fed 28-wk-old apoE−/− mice, though Pro peptide treatment significantly increased LDL-C levels. We have shown that biotinylated Pro peptide associates with LDL (9), an effect that might interfere with LDL clearance and cause increased LDL-C. Potential antiatherogenic effects of Pro peptide could have been overridden by the elevated LDL levels.
Previous reports have suggested that the atheroprotective ability of apoA-I mimetic peptides is sensitive to the stage of lesion evolution. Li et al. (15) have examined the effect of d-amino acid 4F on the accelerated lesions of 20-week old apoE−/− mice fed 21% fat/ 0.15% cholesterol diet. Four weeks of D4F did not reverse atherosclerosis in established aortic root lesions. These lesions were large (∼4×105 μm2), similar to the aortic root lesions described in our 8-wk treatment group. In contrast to this, the peptides D4F and 5F were antiatherogenic in the small, early lesions of 9-wk-old chow-fed apoE−/− mice (13) or 24-wk-old cholate-diet-fed wild-type mice (14). Here, we have confirmed an important temporal limitation to peptide efficacy within the same mouse model and vascular site.
The Garber et al. (14) study showed an antiatherogenic effect of 5F after injecting mice intraperitoneally daily for 16 wk. The treatment groups were controls (200 μl PBS) or 5F (20 μg 5F in 200 μl PBS). We used the Garber et al. (14) study as a guide for our chosen methods. To lessen peritoneal trauma, we halved the injection frequency and increased the peptide dose 2.5-fold (50 μg). Whereas we injected ∼1.19 μg 4F/g bw/d, Garber et al. injected roughly ∼0.952 μg 5F/g bw/d. We administered Pro peptide at an equal molar dose compared to 4F (2.38 μg/g bw/d). In the Li et al. (15) study, despite IP injection of 50 μg of D4F daily (in 200 μl saline) for 4 wk, established aortic root lesions were not reversed.
Lipoprotein oxidation creates a cascade of oxidized products, including conjugated dienes, hydroperoxides, aldehydes such as malondialdehyde, and aldehyde adducts with apoB (26). These adducts serve as neoepitopes for both adaptive (IgG) as well as natural (IgM) T-cell-independent antibodies. NAbs are predominantly produced by B-1 cells (27), which initially reside in body cavities such as the peritoneum before migrating to the spleen and differentiating into plasma cells. The prototypic NAb, E06/T15, recognizes the phosphocholine headgroup of oxidized phospholipids, but not of native phospholipids (28). The titers of such oxidation-specific antibodies appear to reflect a response to the ongoing generation of oxidative epitopes, as occurs in atherogenesis. Typically, the titers of anti-oxLDL antibodies correlate with plasma lipid elevation and atherosclerotic lesion progression in mice (29). Substantially increasing anti-oxLDL antibody titers via immunization is atheroprotective in animal models (30, 31).
We had expected that 4F, known to inhibit lipoprotein oxidation and scavenge oxidized lipids (5, 6), would decrease oxidation-specific NAb titers. Unexpectedly, we found that both 4F and Pro peptides significantly increased transcript levels (after 4 and 8 wk of treatment) and plasma titers (8 wk of treatment) of NAbs against oxidation-specific epitopes. However, total IgM levels were not increased in these mice. This finding suggests that the apoA-I mimetic peptides caused the selective expansion of B-1 cells with antibody specificity for oxidation-specific epitopes. This expansion was not consistently associated with increased plasma lipid levels.
The mechanism by which apoA-I mimetic peptides increase NAbs is currently unknown. However, the fact that the peptides were much more effective at increasing the titer of the oxidized phospholipid-specific NAb E06/T15 than the titers of other IgM antibodies against modified LDLs, which are presumably predominantly NAbs as well, suggests a potential mechanism. E06/T15 recognizes the phosphocholine headgroup of oxidized phospholipids. Others have reported that 4F selectively binds oxidized phospholipid with high affinity (10), and apoA-I mimetic peptides can extract oxidized phospholipid hydroperoxides from lipoproteins and cells (32). If the apoA-I mimetics preferentially bind oxidized phospholipid in vivo, they might then preferentially present to professional antigen presenting cells and/or B-1 cells leading to enhanced generation of E06/T15, similar to lipid antigen presentation via CD1 molecules activating natural killer T cells (33, 34). Indeed, E06/T15 plasma titers increased ten-fold or more with both mimetic peptides, whereas the titers of IgM to OxLDL and MDA-LDL (which comprise a wide variety of oxidized epitopes other than oxidized phospholipid) increased only 2-fold. Future studies will be needed to test this hypothesis.
The increase in the splenic transcripts for E06/T15 at the end of the 4-wk intervention reflects the early expansion of E06/T15 generating B-1 cells, but increases in plasma E06/T15 titers were not yet observed. The difference between splenic transcript and titer could reflect the production of membrane-bound IgM before switching to secreted IgM, causing a delay in the elevation of plasma titers (35, 36). The real-time primers span the E06/T15 VH CDR3 region only. Therefore, transcript levels would be increased during synthesis of membrane-bound E06/T15 while the titer would remain low. Previously we found that it took at least 6 wk following the transfer of B-1 cells into the peritoneum of RAG1−/− mice before plasma titers of IgM could be observed (unpublished observations). In this interval, the B-1 cells must migrate to the spleen, proliferate, and differentiate into plasma cells before IgM Ab can be released into the plasma in sufficient titers to be measured. Indeed, observable increases in E06/T15 titers of nearly 10-fold were seen 8 wk after the introduction of the apoA-I peptides.
Previous studies have shown that marked elevations of E06/T15 titers achieved by exogenous immunizations (up to 10,000-fold over that found in nonimmunized mice) over 6 mo to 1 yr provided atheroprotection (27, 37). In the current studies, the much more modest change in E06/T15 titers (only 10-fold) over a relatively short duration precludes any definitive conclusion about the relationship between the titer of these antibodies and the progression of atherosclerosis. The minimum level of NAb that affords atheroprotection is currently unknown and needs further study.
The Li et al. (15) study is the only previous report to examine the influence of apoA-I mimetic peptides on antibodies against oxidized epitopes. The titer of IgG against Cu-oxLDL was not changed by D4F. In contrast to the Li et al. (15) study, we have shown that 4F treatment increased the titers of IgG against Cu-oxLDL as well as several natural IgM antibodies. The reason for this difference is unknown.
Our results correlate peptide atheroprotection with the antioxidative capacities of 4F and its high affinity for oxidized lipids. The putative mechanisms by which apoA-I mimetic peptides function as atheroprotective agents have recently been reviewed (6, 7). Besides its antioxidative mechanisms of action, 4F might have additional important effects on cytokine production by macrophages or reverse cholesterol transport (RCT). Navab and colleagues (5, 39) have shown that D4F administration to mice increased pre-β HDL levels. That lipoprotein subclass can initiate RCT (38), and D4F increased the cholesterol efflux acceptance capacity of HDL ex vivo (5, 39). We have shown previously that Pro peptide more potently effluxes cholesterol from cultured foam cells and generates pre-β HDL in vitro, as compared to 4F (8). However, we saw no increase in pre-β HDL after a 1-wk 4F treatment (9), nor any change in the lipoprotein distribution after any treatment presented here (Table 2). It is also unlikely that even an undetectable increase in pre-β HDL took place in our study, as the ex vivo cholesterol efflux acceptance capacity of plasma from peptide treated mice was not increased (data not shown). In our hands, neither 4F nor Pro caused an increase in HDL-C, shift in plasma FPLC cholesterol profile consistent with pre-β HDL formation, nor a change in the ex vivo cholesterol efflux acceptance capacity of the plasma.
Despite the more potent in vitro formation of pre-β HDL and cholesterol efflux by the Pro peptide, it affords no atheroprotection against early atherogenesis. Whether higher levels of Pro peptide would be more effective remains to be determined. However, the lower levels of NAbs seen with the Pro peptide treatment could indicate that this peptide does not bind or present oxidized lipids as effectively as does 4F.
In summary, intraperitoneal l-amino acid 4F peptide can reduce nascent, but not mature, atherosclerotic lesions. The effects of 4F are unique to its sequence, as the tandem helix peptide Pro was ineffective at atheroprevention. However, both 4F and Pro peptide treatment induced production of NAbs against oxidized lipids, with 4F being more effective than the Pro peptide.
Acknowledgments
This work was supported by the U.S. National Institutes of Health/National Heart, Lung, and Blood Institute (HL68661 to GSG) and the Foundation Leducq (to G.S.G., C.J.B., and J.L.W.). G.D.W. has received support from the National Institute of Child Health and Human Development (HD007009 Graduate Training in Growth and Development), the Francis L. Lederer Foundation Scholarship Fund, and an American Heart Association predoctoral fellowship. J.L.W. is named as inventor in patents and patent applications from the University of California–San Diego for the potential commercial use of antibodies to oxidized LDL.
REFERENCES
- 1. Kontush A., Chapman M. J. (2006) Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol. Rev. 58, 342–374 [DOI] [PubMed] [Google Scholar]
- 2. Duffy D., Rader D. J. (2009) Update on strategies to increase HDL quantity and function. Nat. Rev. Cardiol. 6, 455–463 [DOI] [PubMed] [Google Scholar]
- 3. Shao B., Heinecke J. W. (2009) HDL, lipid peroxidation, and atherosclerosis. J. Lipid Res. 50, 599–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rader D. J., Alexander E .T., Weibel G. L., Billheimer J., Rothblat G. H. (2009). The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J. Lipid Res. 50(Suppl.), S189–S194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Navab M., Anantharamaiah G. M., Reddy S. T., Hama S., Hough G., Grijalva V. R., Yu N., Ansell B. J., Datta G., Garber D. W., Fogelman A. M. (2005) Apolipoprotein A-I mimetic peptides. Arterioscler. Thromb. Vasc. Biol. 25, 1325–1331 [DOI] [PubMed] [Google Scholar]
- 6. Getz G. S., Wool G. D., Reardon C. A. (2009) Apoprotein A-I mimetic peptides and their potential anti-atherogenic mechanisms of action. Curr. Opin. Lipidol. 20, 171–175 [DOI] [PubMed] [Google Scholar]
- 7. Navab M., Shechter I., Anantharamaiah G. M., Reddy S. T., Van Lenten B. J., Fogelman A. M. (2010) Structure and function of HDL mimetics. Arterioscler. Thromb. Vasc. Biol. 30, 164–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wool G. D., Reardon C. A., Getz G. S. (2008) Apolipoprotein A-I mimetic peptide helix number and helix linker influence potentially anti-atherogenic properties. J. Lipid Res. 49, 1268–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wool G. D., Vaisar T., Reardon C. A., Getz G. S. (2009) An apoA-I mimetic peptide containing a proline residue has greater in vivo HDL binding and anti-inflammatory ability than the 4F peptide. J. Lipid Res. 50, 1889–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Lenten B. J., Wagner A. C., Jung C. L., Ruchala P., Waring A. J., Lehrer R. I., Watson A. D., Hama S., Navab M., Anantharamaiah G. M., Fogelman A. M. (2008) Anti-inflammatory apoA-I-mimetic peptides bind oxidized lipids with much higher affinity than human apoA-I. J. Lipid Res. 49, 2302–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Glass C. K., Witztum J. L. (2001) Atherosclerosis: the road ahead. Cell 104, 503–516 [DOI] [PubMed] [Google Scholar]
- 12. Carnemolla R., Ren X., Biswas T. K., Meredith S. C., Reardon C. A., Wang J., Getz G. S. (2008) The specific amino acid sequence between helices 7 and 8 influences the binding specificity of human apolipoprotein A-I for high density lipoprotein (HDL) subclasses: a potential for HDL preferential generation. J. Biol. Chem. 283, 15779–15788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Navab M., Anantharamaiah G. M., Hama S., Garber D. W., Chaddha M., Hough G., Lallone R., Fogelman A. M. (2002) Oral administration of an Apo A-I mimetic Peptide synthesized from D-amino acids dramatically reduces atherosclerosis in mice independent of plasma cholesterol. Circulation 105, 290–292 [DOI] [PubMed] [Google Scholar]
- 14. Garber D. W., Datta G., Chaddha M., Palgunachari M. N., Hama S. Y., Navab M., Fogelman A. M., Segrest J. P., Anantharamaiah G. M. (2001) A new synthetic class A amphipathic peptide analogue protects mice from diet-induced atherosclerosis. J. Lipid Res. 42, 545–552 [PubMed] [Google Scholar]
- 15. Li X., Chyu K. Y., Faria Neto J. R., Yano J., Nathwani N., Ferreira C., Dimayuga P. C., Cercek B., Kaul S., Shah P. K. (2004) Differential effects of apolipoprotein A-I-mimetic peptide on evolving and established atherosclerosis in apolipoprotein E-null mice. Circulation 110, 1701–1705 [DOI] [PubMed] [Google Scholar]
- 16. Van Lenten B. J., Wagner A. C., Navab M., Anantharamaiah G. M., Hama S., Reddy S. T., Fogelman A. M. (2007) Lipoprotein inflammatory properties and serum amyloid A levels but not cholesterol levels predict lesion area in cholesterol- fed rabbits. J. Lipid Res. 48, 2344–2353 [DOI] [PubMed] [Google Scholar]
- 17. Bloedon L. T., Dunbar R., Duffy D., Pinell-Salles P., Norris R., DeGroot B. J., Movva R., Navab M., Fogelman A. M., Rader D. J. (2008) Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J. Lipid Res. 49, 1344–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reardon C. A., Blachowicz L., White T., Cabana V., Wang Y., Lukens J., Bluestone J., Getz G. S. (2001) Effect of immune deficiency on lipoproteins and atherosclerosis in male apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 21, 1011–1016 [DOI] [PubMed] [Google Scholar]
- 19. Ou J., Wang J., Xu H., Ou Z., Sorci-Thomas M. G., Jones D. W., Signorino P., Densmore J. C., Kaul S., Oldham K. T., Pritchard K.A., Jr. (2005) Effects of D-4F on vasodilation and vessel wall thickness in hypercholesterolemic LDL receptor-null and LDL receptor/apolipoprotein A-I double-knockout mice on Western diet. Circ. Res. 97, 1190–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. VanderLaan P. A., Reardon C. A., Sagiv Y., Blachowicz L., Lukens J., Nissenbaum M., Wang C.-R., Getz G. S. (2007) Characterization of the natural killer T-cell response in an adoptive transfer model of atherosclerosis. Am. J. Pathol. 170, 1100–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chou M. Y., Fogelstrand L., Hartvigsen K., Hansen L. F., Woelkers D., Shaw P. X., Choi J., Perkmann T., Bäckhed F., Miller Y. I., Hörkkö S., Corr M., Witztum J. L., Binder C. J. (2009) Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J. Clin. Invest. 119, 1335–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chou M. Y., Hartvigsen K., Hansen L. F., Fogelstrand L., Shaw P. X., Boullier A., Binder C. J., Witztum J. L. (2008) Oxidation-specific epitopes are important targets of innate immunity. J. Intern. Med. 26 3, 479–488 [DOI] [PubMed] [Google Scholar]
- 23. Shaw P. X., Hörkkö S., Chang M. K., Curtiss L. K., Palinski W., Silverman G. J., Witztum J. L. (2000) Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J. Clin. Invest. 105, 1731–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. VanderLaan P. A., Reardon C. A., Getz G. S. (2004) Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler. Thomb. Vasc. Biol. 24, 12–22 [DOI] [PubMed] [Google Scholar]
- 25. Navab M., Anantharamaiah G. M., Hama S., Hough G., Reddy S. T., Frank J. S., Garber D. W., Handattu S., Fogelman A. M. (2005) D-4F and statins synergize to render HDL antiinflammatory in mice and monkeys and cause lesion regression in old apolipoprotein E-null mice. Arterioscler. Thromb. Vasc. Biol. 25, 1426–1432 [DOI] [PubMed] [Google Scholar]
- 26. Stocker R., Keaney J.F., Jr. (2004) Role of oxidative modifications in atherosclerosis. Physiol. Rev. 84, 1381–1478 [DOI] [PubMed] [Google Scholar]
- 27. Binder C. J., Hartvigsen K., Chang M. K., Miller M., Broide D., Palinski W., Curtiss L. K., Corr M., Witztum J. L. (2004) IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J. Clin. Invest. 114, 427–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Friedman P., Hörkkö S., Steinberg D., Witztum J. L., Dennis E. A. (2002) Correlation of antiphospholipid antibody recognition with the structure of synthetic oxidized phospholipids. Importance of Schiff base formation and aldol condensation. J. Biol. Chem. 277, 7010–7020 [DOI] [PubMed] [Google Scholar]
- 29. Tsimikas S., Palinski W., Witztum J. L. (2001) Circulating autoantibodies to oxidized LDL correlate with arterial accumulation and depletion of oxidized LDL in LDL receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 21, 95–100 [DOI] [PubMed] [Google Scholar]
- 30. Palinski W., Miller E., Witztum J. L. (1995) Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc. Natl. Acad. Sci. U. S. A. 92, 821–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Caligiuri G., Khallou-Laschet J., Vandaele M., Gaston A. T., Delignat S., Mandet C., Kohler H. V., Kaveri S. V., Nicoletti A. (2007) Phosphorylcholine-targeting immunization reduces atherosclerosis. J. Am. Coll. Cardiol. 50, 540–546 [DOI] [PubMed] [Google Scholar]
- 32. Navab M., Hama S. Y., Cooke C. J., Anantharamaiah G. M., Chaddha M., Jin L., Subbanagounder G., Faull K. F., Reddy S. T., Miller N. E., Fogelman A. M. (2000) Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: step 1. J. Lipid Res. 41, 1481–1494 [PubMed] [Google Scholar]
- 33. Salio M., Silk J. D., Cerundolo V. (2010) Recent advances in processing and presentation of CD1 bound lipid antigens. Curr. Opin. Immunol. 22, 81–88 [DOI] [PubMed] [Google Scholar]
- 34. Barral P., Eckl-Dorna J., Harwood N. E., De Santo C., Salio M., Illarionov P., Besra G. S., Cerundolo V., Batista F. D. (2008) B cell receptor-mediated uptake of CD1d-restricted antigen augments antibody responses by recruiting invariant NKT cell help in vivo. Proc. Natl. Acad. Sci. U. S. A. 105, 8345–8350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Danner D., Leder P. (1985) Role of an RNA cleavage/poly(A) addition site in the production of membrane-bound and secreted IgM mRNA. Proc. Natl. Acad. Sci. U. S. A. 82, 8658–8662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ross D. A., Wilson M. R., Miller N. W., Clem L. W., Warr G. W. (1998) Evolutionary variation of immunoglobulin mu heavy chain RNA processing pathways: origins, effects, and implications. Immunol. Rev. 166, 143–151 [DOI] [PubMed] [Google Scholar]
- 37. Binder C. J., Hörkkö S., Dewan A., Chang M. K., Kieu E. P., Goodyear C. S., Shaw P. X., Palinski W., Witztum J. L., Silverman G. J. (2003) Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat. Med. 9, 736–743 [DOI] [PubMed] [Google Scholar]
- 38. Nanjee M. N., Cooke C. J., Garvin R., Semeria F., Lewis G., Olszewski W. L., Miller N. E. (2001) Intravenous apoA-I/lecithin discs increase pre-beta-HDL concentration in tissue fluid and stimulate reverse cholesterol transport in humans. J. Lipid Res. 42, 1586–1593 [PubMed] [Google Scholar]
- 39. Navab M., Anantharamaiah G. M., Reddy S. T., Hama S., Hough G., Grijalva V. R., Wagner A. C., Frank J. S., Datta G., Garber D., Fogelman A. M. (2004) Oral D-4F causes formation of pre-beta high-density lipoprotein and improves high-density lipoprotein-mediated cholesterol efflux and reverse cholesterol transport from macrophages in apolipoprotein E-null mice. Circulation 109, 3215–3220 [DOI] [PubMed] [Google Scholar]
- 40. Cabana V. G., Lukens J. R., Rice K. S., Hawkins T. J., Getz G. S. (1996) HDL content and composition in acute phase response in three species: triglyceride enrichment of HDL a factor in its decrease. J. Lipid Res. 37, 2662–2674 [PubMed] [Google Scholar]