Abstract
HO-2 oxidizes heme to CO and biliverdin; the latter is reduced to bilirubin by biliverdin reductase (BVR). In addition, HO-2 is a redox-sensitive K/Ca2-associated protein, and BVR is an S/T/Y kinase. The two enzymes are components of cellular defense mechanisms. This is the first reporting of regulation of HO-2 by BVR and that their coordinated increase in isolated myocytes and intact heart protects against cardiotoxicity of β-adrenergic receptor activation by isoproterenol (ISO). The induction of BVR mRNA, protein, and activity and HO-2 protein was maintained for ≥96 h; increase in HO-1 was modest and transient. In isolated cardiomyocytes, experiments with cycloheximide, proteasome inhibitor MG-132, and siBVR suggested BVR-mediated stabilization of HO-2. In both models, activation of BVR offered protection against the ligand's stimulation of apoptosis. Two human BVR-based peptides known to inhibit and activate the reductase, KKRILHC281 and KYCCSRK296, respectively, were tested in the intact heart. Perfusion of the heart with the inhibitory peptide blocked ISO-mediated BVR activation and augmented apoptosis; conversely, perfusion with the activating peptide inhibited apoptosis. At the functional level, peptide-mediated inhibition of BVR was accompanied by dysfunction of the left ventricle and decrease in HO-2 protein levels. Perfusion of the organ with the activating peptide preserved the left ventricular contractile function and was accompanied by increased levels of HO-2 protein. Finding that BVR and HO-2 levels, myocyte apoptosis, and contractile function of the heart can be modulated by small human BVR-based peptides offers a promising therapeutic approach for treatment of cardiac dysfunctions.—Ding, B., Gibbs, P. E. M., Brookes, P. S., Maines, M. D. The coordinated increased expression of biliverdin reductase and heme oxygenase-2 promotes cardiomyocyte survival; a reductase-based peptide counters β-adrenergic receptor ligand-mediated cardiac dysfunction.
Keywords: apoptosis, antioxidants, heart failure
The end stage of cardiac disease is heart failure, which is characterized by myocardium overload and defects in coronary blood flow. These, in turn, lead to death and apoptosis of cadiomyocytes. Apoptosis has been observed in failing human hearts that have been removed from recipients undergoing transplantation (1).
Apoptosis and inotropic heart modification can be triggered by a sudden surge in levels of catecholamines, the activators of the β-adrenergic receptors (ARs), which include the naturally occurring catecholamines, epinephrine and norepinephrine, as well as drugs such as isoproterenol (ISO) (2–3). Cardiomyocyte apoptosis is primarily dependent on activation of the β2-AR subtype in vitro, although both β1- and β2-AR subtypes coexist in cardiomyocytes (4–5). Reportedly, ISO-mediated activation in vitro of β-AR can be used as a useful model for examination of the response of cardiomyocytes to stimuli (6–9). A previously unknown activity of the ligand, as revealed by this investigation, in cardiomyocytes is increased cellular levels of two heme metabolic enzymes, biliverdin reductase (BVR) and heme oxygenase (HO)-2, an activity that, as shown here, is cardioprotective. The increases appear to self-limit the deleterious actions of the β-AR ligand on the cardiomyocytes and cardiac functions.
Three enzymes, HO-1 and HO-2 (also known as the HSP32 family of proteins) and BVR, are active participants in catalytic conversion of the heme molecule to bile pigments. A number of studies have suggested the cardioprotective action of the stress-inducible cognate of HSP32 family of proteins, HO-1 (10–17). HO-2 is generally considered to be the constitutively expressed member of the family, with cell type- and tissue-dependent levels of expression (18). BVR has been recently identified to be a multifunctional enzyme, one function of which is reduction of biliverdin, the product of heme oxidation, to bilirubin. The other functions of BVR that have been identified with the human form of the enzyme include its dual-specificity kinase activity, it being a transcription factor and molecular scaffold and cellular transporter of kinases and regulatory factors (19–20). HO-2 is prominently expressed in the cardiovascular system, including in the endothelial cells of the carotid artery (21) and the glomus cells of the carotid body (22); the brain and the nervous system, including the hippocampus (18, 23) and the spinal cord (24); and the interstitial cell network of the small intestine (25).
Like BVR, HO-2 also plays a pivotal role in cellular homeostasis. Both isozymes of HO (HO-1 and HO-2) catalyze oxidative cleavage of the heme molecule (Fe-protoporphyrin-IX) to CO and biliverdin, two biologically active molecules. It has been observed, however, that HO-1 and HO-2 exert distinct cytoprotective mechanisms to oxidative stress induced by hydrogen peroxide (26). Carbon monoxide, similar to NO, is a signaling molecule in the brain and the cardiovascular system (21–22, 27–28), while biliverdin is a precursor to the formation of potent intracellular antioxidant bilirubin (29–30). Among functions that are ascribed to BVR is countering apoptosis (31). In context of the cytoprotective mechanism available to the cell, BVR ranks alongside GSH (32–33).
In addition to its activity in the heme degradation pathway, HO-2 performs functions that are exclusive to this form of heme oxygenase; this, in turn, reflects the primary structural features of the protein. HO-2 is among a select group of proteins that possess the so-called heme-regulating motifs (HRMs), which have the core sequence of Cys-Pro. The dipeptide is flanked upstream with positively charged residues and downstream by hydrophobic amino acids; two copies of HRM motifs are preset in HO-2 (34). The amino acid composition provides a suitable configuration for heme binding (35–36). The two HRMs, which are separated by 17 aa, can also form a disulfide bond and, as such, provide a redox-sensitive molecular target (37), as well as a site of interaction with NO. HO-2 is associated with regulation of the calcium-dependent potassium channels, and potentially serves as the oxygen sensor (38–40). The oxygenase is also a phosphoprotein and a substrate for BVR (41).
The human (h) form of BVR (hBVR) activates and is activated by members of the Ca2+-dependent conventional PKC-βII and atypical PKC-ζ kinases (42–43). hBVR is directly phosphorylated by the activated insulin/IGF-1 receptor kinase (IRK) and is an activator of MAPK/PI3K arms of the IRK signaling cascade (reviewed in refs. 19–20). PKC-βII is a component of the multiprotein complex that integrates PKA signaling and Ca2+ regulation in the heart (44). The Ser/Thr PKA that is activated by cAMP functions in various cell types and regulates a host of functions, including cardiac functions (45). CREB, the PKA downstream translocation factor, is activated by Ca2+ and mitogen activation of the MAPK signaling pathway, and its phosphorylation and expression are regulated by BVR in the human kidney cell line, HEK 293A (46). Activated CREB (Su133p) recruits cotranscriptional activators for stress-activated genes that include HO-1, c-jun, and c-fos (46).
Because BVR is the upstream activator of HO-2 and both entities are intimately linked to cell survival and defense mechanisms, in the present study, the hypothesis was tested that the BVR-HO-2 link offers protection against cardiomyocyte death by apoptosis and heart failure. This investigation has uncovered a function of BVR in regulation of HO-2 in cardiomyocytes that results in cardiac protection. The study, which used ISO, a β-AR ligand and promoter of cardiac hypertrophy, has generated data that are supportive of the concept that there is regulatory interaction between the two enzymes; and that, this interaction may be gainfully exploited to control untoward changes observed in functions of the heart, such as the pressure and volume overloads, that the result in heart failure secondary to cardiomyocyte apoptosis. Findings with small BVR-derived peptides offer a positive outlook to this prospect.
MATERIALS AND METHODS
Neonatal rat cardiomyocytes
Primary cultures of neonatal rat cardiomyocytes were prepared as described previously (47). Briefly, fragmented ventricular tissue from 1- to 2-d-old Sprague-Dawley rats (Charles River, Boston, MA, USA) was subjected to multiple rounds of enzymatic digestion with collagenase II (Worthington, Lakewood, NJ, USA). Cells were then collected by low-speed centrifugation at 4°C. Nonmyocytes were removed by 2 rounds of preplating the cells on culture dishes. The enriched cardiomyocytes were cultured in DMEM with 10% FBS and 10% horse serum. The day after cell adhesion, 10 μM cytosine 1-β-d-arabinofuranoside (Sigma, St. Louis, MO, USA) was added to inhibit the growth of contaminating nonmyocytes. Cells were treated with 10 mM ISO (Sigma) for the times indicated in the figures.
Plasmids and constructs
Adenovirus for expression of a dominant-negative PKA inhibitor (PKI) was prepared as described before (48). The BVR siRNA targeting site is at nt 94–114 and the siRNA targeting site for HO-2 is at nt 73–93 downstream of the start codon in each case. Oligonucleotides containing the sequence of a 21-nt siRNA were synthesized as follows: BVR siRNA, 5′-GATCCCC(TCTGCAGCATTCCTGAACCTG)TTCAAGAG(ACAGGTTCAGGAATGCTGCAGA)TTTTTGGAAA and 5′-AGCTTTTCCAAAAA(TCTGCAGCATTCCTGAACCTG)TCTCTTGAA(CAGGTTCAGGAATGCTGCAGA)GGG; HO-2 siRNA, 5′-GATCCCC(GAAAACCATACCAAAATGGCA)TTCAAGAGA(TGCCATTTTGGTATGGTTTTC)TTTTTGGAAA and 3′-AGCTTTTCCAAAAA(GAAAACCATACCAAAATGGCA)TCTCTTGAA(TGCCATTTTGGTATGGTTTTC)GGG. Complementary oligonucleotides were annealed, and then ligated into pSuper-Retro vector (OligoEngine Co., Seattle, WA, USA). Escherichia coli clones containing the desired constructs were identified by restriction analysis and verified by DNA sequencing. The retroviral DNA vectors for generating rat BVR siRNA and rat HO-2 siRNA were transfected into HEK293A packaging cells obtained from Invitrogen, (Carlsbad, CA, USA), and the supernatant containing the expressed siBVR or siHO-2 retrovirus was then titrated in NIH3T3 cells. Retrovirus-expressing siRNAs were used to infect cardiomyocytes at a multiplicity of infection of 4 plaque-forming units/cell. The construction of the human pcDNA-BVR was described before (41); the human pcDNA-HO-2 plasmid was constructed by cloning the HO-2 open reading frame into pcDNA3. BVR scRNA was prepared as described previously (49) by randomizing the hBVR siRNA; because the base compositions of the human and rat BVR siRNAs are the same, the human sc construct was used, and the rat sc construct was not prepared .
Cell culture and transfection of HEK293A cells with hBVR or hHO-2 expression vectors and siBVR
Because of less than adequate stability of cardiomyocytes in culture, the HEK293A cells were used in long-term experiments (0–96 h). Cells were grown in 10-cm plates with DMEM containing 10% FBS and penicillin-G/streptomycin for 24 h or until 70% confluency was reached. Cells were subsequently transfected with 4 μg of plasmid in each 10-cm dish using Transfectin reagent (Bio-Rad, Hercules, CA, USA), according to the manufacturer's protocol. At 24 h after DNA addition, and if appropriate, infection with culture supernatant containing retrovirus expressing human siBVR (at a multiplicity of infection of 4), the cycloheximide (10 μg/ml) was added, and samples were removed at the appropriate times for protein analysis. Additional siRNA virus was added every 12 h during the experiment.
Western blot analysis
Cell lysates were prepared by homogenization in RIPA buffer (47) followed by centrifugation at 800 g for 10 min at 4°C. Supernatant proteins were resolved by SDS-PAGE and transferred to nitrocellulose. Western blots were probed using antibodies against β-actin (Cell Signaling, Beverly, MA, USA), BVR (50), HO-1, HO-2 (24), and cleaved caspase-3 (Cell Signaling). The secondary antibody was horseradish peroxidase-conjugated goat anti-rabbit IgG (1:2000, Bio-Rad).
Quantitative RT-PCR
Total RNA was isolated using TRIzol reagent (Invitrogen). The first-strand cDNA synthesis and quantitative RT-PCR were performed as described previously (51). The following primers were used for PCR analysis: GAPDH, 5′-TGATGCTGGTGCTGAGTATGTCGT (antisense), 5′-TTGTCATTGAGAGCAATGCCAGCC (sense); BVR, 5′-TGCCGAGCCAAAGAGGAAATTTGG (sense), 5′-AGCTGTGAAGCGAAGAGACCCTTT (antisense); HO-1, 5′ TTAAGCTGGTGATGGCCTCCTTGT (sense), 5′-CATGGCCTTCTGCGCAATCTTCT (antisense); HO-2 5′-AATGGCAGACCTTTCTGAGCTCCT (sense), 5′-GGGCATTGTCCACATGCTCAAACA (antisense). PCR products were resolved by electrophoresis and quantified by image analysis of stained gels.
Analysis of apoptosis
The terminal deoxyribonucleotide transferase-mediated dUTP nick-end labeling (TUNEL) assay was used to detect in situ DNA fragmentation, using the in situ cell death detection kit (Roche, Mannheim, Germany), as described previously (47). In addition, cells were stained for cardiomyocyte-specific sarcomeric α-actinin with the antibody EF-53 to distinguish cardiomyocytes from contaminating fibroblasts; and only EF-53-positive cells were counted. Three independent experiments were performed and an average of 1000 EF-53-positive cells from random fields were analyzed.
DNA electrophoresis
Total cellular DNA was isolated from untreated and treated cells by lysis in 10 mM Tris-HCl, pH 7.8; 1 mM, EDTA; 10 mM NaCl; 1% (w/v) SDS; and 1 mg/ml proteinase K at 60°C for 2 h. The lysate was extracted twice each with phenol-chloroform (1:1, v/v) and chloroform, followed by precipitation with ethanol. The precipitate was collected by centrifugation, washed once in 70% ethanol, and resuspended in TE containing 1 mg/ml RNase at 37°C for 30 min. DNA was resolved by electrophoresis in 2.0% agarose gels containing ethidium bromide.
Perfused heart model
Male Sprague-Dawley rats weighing 250–300 g (Charles River) were used in this study; the protocol is based on that described by Tompkins et al. (52) with minor modifications. Rats were fed a standard rat chow and had water ad libitum. All experiments were approved by the University of Rochester Institutional Animal Care and Use Committee. Rats were given two subcutaneous injections of ISO (0.01 mg/kg), 6 h apart, then anesthetized with freshly prepared Avertin (2,2,2-tribromoethanol, 0.5 mg/kg i.p), and treated with heparin (5000 U/ kg i.p). The aorta was cannulated in situ with a 16-gauge needle filled with warm Krebs-Henseleit (KH) buffer. The heart was rapidly transferred (<10 s) to a perfusion apparatus, and retrograde (Langendorff) perfusion was begun with 37°C KH gassed with 95% O2 plus 5% CO2, in constant flow mode (12 ml/min). Perfusion was performed using 0.1 μM ISO or KH buffer as a control; the hBVR-derived peptides KKRILHC281 (25 μM), KYCCSRK296 (25 μM), or FGFPAFSG169 (25 μM) were included in perfusion medium with or without ISO. The peptides were synthesized (EZBiolab, Westfield, IN, USA) as N-myristoylated derivatives to facilitate uptake from the perfusate. Hemodynamics and heart rate were recorded by inserting a water-filled balloon connected to a transducer (159904B; Radnoti, Monrovia, CA, USA) into the left ventricle. The transducer was connected to a data acquisition system (DI-205; DataQ, Akron, OH, USA) At the end of each experiment, the heart was cut into 5-μm sections for TUNEL staining, or homogenized for Western blot analysis.
Implanting ISO miniosmotic pump
Miniosmotic pumps (Alzet model 2001; Durect Co., Cupertino, CA, USA) were filled under sterile conditions with ISO dissolved in 0.001 N HCl, primed in sterile saline at 37°C overnight, and aseptically implanted subcutaneously under halothane anesthesia. The ISO concentration was calculated to allow the pumps to deliver the drug at an infusion rate of 400 μg ISO base/kg/h, essentially as described by Hayes et al. (53).
Measurement of BVR activity
Cardiomyocytes and heart samples were lysed in buffer containing 100 mM sodium phosphate, pH 7.4; 1% Nonidet-P-40; 10% glycerol; 0.2 mM dithiothreitol; 10 mM NaF; and protease inhibitor mixture (10 μg/ml aprotinin, 10 μg/ml leupeptin, 10 μg/ml pepstatin, and 0.1 mM phenylmethylsulfonyl fluoride). BVR activity was measured at pH 6.7 as described previously (50) The rate of conversion of biliverdin to bilirubin was determined as the increase in absorbance at 450 nm at 25°C. Specific activity is expressed as nanomoles of bilirubin formed per minute per milligram of protein.
RESULTS
ISO increases BVR and HO-2 protein expression in vitro
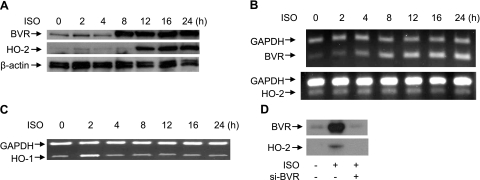
Isolated rat neonatal cardiomyocytes were treated with 10 μM ISO, and the expression of BVR and HO-2 was examined. BVR protein expression was elevated within 8 h of initiating the treatment, while the HO-2 protein level began to increase by 12 h, reaching a plateau by 24 h (Fig. 1A). The increased levels of both proteins remained stable for up to 96 h of treatment with ISO (data not shown).
Figure 1.
ISO increases expression of BVR and HO-2 in rat neonatal cardiomyocytes. A) Effects of ISO on HO-2 and BVR protein levels. Cells were treated with 10 μM ISO (ISO) for the times indicated and lysed; proteins were resolved by SDS-PAGE. Western blot was probed sequentially with antibodies to rat BVR, rat HO-2, and β-actin, as the control. B) HO-2 and BVR mRNA expression (RT-PCR) in ISO-stimulated rat neonatal cardiomyocytes. RNA was isolated at the indicated times and reverse transcribed. The rat BVR and HO-2 sequences were amplified by PCR, and products were resolved by agarose gel electrophoresis. GAPDH was included as a control. C) ISO causes a transient induction of HO-1. Cardiomyocytes were treated with 10 μM ISO for the times indicated; RNA was isolated, and the levels of HO-1 and GAPDH mRNAs determined as in B. D) Induction of HO-2 synthesis by ISO requires induction of BVR. Cardiomyocytes were treated with siBVR retrovirus, and 12 h later with 10 μM ISO for 24 h; siBVR was replenished every 12 h; proteins were resolved by gel electrophoresis and analyzed by Western blot.
The question of whether the increased synthesis of each protein required elevated levels of BVR and HO-2 mRNAs was investigated by RT-PCR. It was found that the level of BVR mRNA increased in advance of the increase in protein, suggesting that the increase was due to elevated protein synthesis, whereas the HO-2 mRNA content remained unchanged during ISO stimulation (Fig. 1B). The BVR mRNA content of the cells remained elevated for up to 4 d—even prolonged exposure of the cells to ISO did not alter the levels of HO-2 mRNA (data not shown). Expression of the HO-1 gene was also examined, since it is believed to be a likely mediator of cardioprotection (10–17). There was a very brief induction of HO-1 mRNA expression, which increased perceptibly after 2 h of treatment, while by 4 h, the level of the mRNA was indistinguishable from that of untreated cells. It is therefore unlikely that HO-1 exerts a significant protective function in this model.
The foregoing suggests that increased BVR levels reflect, and are dependent on, the availability of elevated mRNA, while a mechanism that is independent of transcription is responsible for the enhanced levels of HO-2. To test the requirement for BVR mRNA, cardiomyocytes were treated with virus expressing siBVR, followed 12 h later by 10 μM ISO treatment for 24 h. In untreated cells (Fig. 1D), ISO caused the expected induction of BVR, whereas this was efficiently blocked by siBVR treatment. Interestingly, siBVR treatment also effectively blocked the induction of HO-2. Thus the observed, time-dependent increase in HO-2 (Fig. 1A) is dependent on the prior induction of BVR.
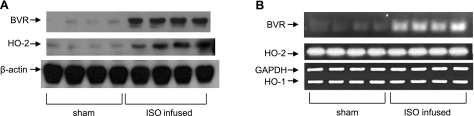
ISO increases BVR and HO-2 expression in the rat heart during ISO infusion
It might be argued that the above data arose as an artifact of cell culture. To test whether those findings also apply in vivo, 4 rats were continuously infused with ISO for 4 d, after which cardiac tissue was harvested for examination of BVR and HO-2 expression. Again, the HO-2 and BVR protein expressions were up-regulated compared to those seen in sham-treated animals (Fig. 2A). These data are consistent with the in vitro observations. Also, as expected from in vitro observations, there was no change in the level of HO-2 mRNA during infusion with ISO (Fig. 2B), whereas the BVR mRNA was increased. Again, there was no apparent change in the level of HO-1 mRNA in the tissue, although any transient early transcriptional response of the HO-1 gene could easily have been missed in these experiments.
Figure 2.
Increased expression of BVR and HO-2 after continuous infusion of ISO in vivo. A) Levels of HO-2 and BVR protein in hearts from 4 individual rats after 4 d of continuous infusion with 400 μg ISO/kg/h, or from 4 sham-operated rats were determined by Western blot. β-Actin is included as control. B) HO-2 and BVR mRNA expression in ISO-infused rat heart in vivo, determined by RT-PCR, as in Fig. 1. Animals were infused with ISO, as in A. GAPDH is included as a control.
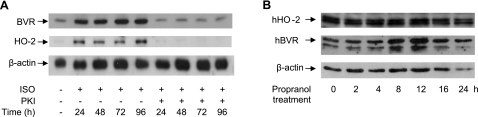
Induction of BVR and HO-2 is mediated by the cAMP-PKA pathway
Because the effects of ISO occur via the cAMP-PKA pathway, we tested the effect of inhibiting PKA on the induction of BVR and HO-2 proteins in cultured cardiomyocytes. Adenovirus-mediated expression of the 75-aa dominant-negative PKA, PKI, blocked the ISO-induced up-regulation of both BVR and HO-2 (Fig. 3A). Thus, the increased levels of both BVR and HO-2 protein after ISO treatment are dependent on the activity of the cAMP-PKA pathway. We also examined whether the events observed here might reflect a nonspecific response to catecholamines. For this, HEK293A cells treated with the β-adrenergic antagonist, propranolol, were examined. A transient increase in hBVR at 8–12 h posttreatment was detected, which returned to the initial level by 16–24 h (Fig. 3B). There was no detectable change in hHO-2 protein during treatment with the antagonist. This suggests that the increase in BVR must be sustained to permit HO-2 levels in the cell to rise. Because both BVR and HO-2 are regulated by the same pathway, and activation of this pathway first results in induction of BVR, with HO-2 induction being dependent on BVR, the question was raised: how does elevated BVR cause enhanced HO-2 expression?
Figure 3.
Increase in BVR and HO-2 protein levels is mediated via PKA. A) Cardiomyocytes were infected with an adenovirus expressing PKA-I or a control gene (lacZ), then treated with 10 μM ISO for the indicated times. BVR and HO-2 were detected in cell lysates by a Western blot, using β-actin as a control. B) HEK293A cells were treated with 10 μM propranolol for the times indicated and lysed; cell lysates were analyzed by gel electrophoresis and Western blotting; blot was sequentially probed with antibodies to HO-2, BVR, and the control β-actin.
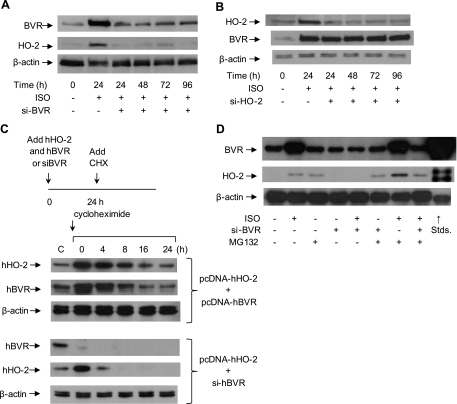
Blockage of BVR protein expression inhibits the HO-2 protein induction but not vice versa
To test further whether expression of HO-2 and BVR proteins are dependent on one another, siRNAs (siBVR or siHO-2) were used to inhibit the expression of one of the proteins, after which the expression of both proteins was assayed. As expected from the data shown in Fig. 1D, when retroviral siBVR was used to infect rat neonatal cardiomyocytes, the expression of BVR was blocked, even after stimulation by ISO; moreover, the expression of HO-2 protein was also blocked (Fig. 4A). In the converse experiment, inhibition of HO-2 protein expression with siHO-2 had no effect on the BVR protein level (Fig. 4B), although, as expected, HO-2 expression was ablated. These experiments confirm that the increase in HO-2 protein expression after stimulation by ISO is dependent on prior induction of BVR, which, by definition, occurs independently of HO-2. On the basis of this observation, further exploration was made concerning the mechanism mediating the interaction of HO-2 and BVR.
Figure 4.
Ablation of BVR protein prevents ISO-induced HO-2 protein expression. A) Cardiomyocytes were infected with retrovirus expressing siBVR, 6 h later, cells were treated with 10 μM ISO. Samples were collected at 24, 48, 72, and 96 h posttreatment. Cell lysates were analyzed by gel electrophoresis and Western blot analysis. Blots were probed with antibodies against rat BVR and HO-2. β-Actin served as a control. B) Cardiomyocytes were infected with a retrovirus expressing siHO-2. Expression of BVR and HO-2 was measured as in A. C) BVR expression regulates the stability of the HO-2 protein. In the first experiment, HEK293A cells were cotransfected with pcDNA-hBVR and pcDNA-hHO-2, then stimulated with ISO for 24 h, followed by treatment with the protein synthesis inhibitor, cycloheximide (10 μg/ml), for the indicated intervals. Expression of hHO-2 and hBVR in HEK293A cells was measured by Western blot. In the second series, the cells were transfected with pcDNA-hHO-2 and treated with retrovirus expressing sihBVR. These data were replicated in ≥2 independent experiments. D) Cardiomyocytes were treated with 10 μM ISO, infected with siBVR virus, or treated with the proteasome inhibitor MG132 (50 μg/ml). HO-2 and BVR protein expression were detected by Western blot. β-Actin served as a control.
ISO stimulation of BVR levels enhanced the stability of HO-2
It is apparent that the level of HO-2 protein observed in ISO-stimulated cardiomyocytes increases in the absence of increased HO-2 mRNA synthesis (Fig. 1). The regulation of protein levels in the cell is dependent on at least two factors: synthesis, which is broadly correlated with the mRNA level, and degradation by either of two main pathways, the lysosome or by ubquitination and subsequent targeting to the proteasome. The elevated HO-2 protein level in the absence of increased mRNA suggests that there is little likelihood of an increased rate of protein synthesis, although this cannot entirely be ruled out—a shift in distribution between the poorly translated 1.9-kb HO-2 mRNA and the well-translated 1.3-kb species (34) might lead to the observed result. The more likely possibility is altered protein degradation; thus, we hypothesized that the enhanced HO-2 protein level was due to increased protein stability. We designed two experiments to examine the dependency of HO-2 protein stability on the level of BVR protein. To facilitate detection of the proteins, these experiments were carried out in human cells, which are readily transfected to allow high starting levels of the proteins. In the experiment shown in Fig. 4C, one set of HEK293A cells was cotransfected with pcDNA-hBVR and pcDNA-hHO-2, while a second set was transfected with pcDNA-hHO-2 and concomitantly infected with retrovirus expressing sihBVR. After 24 h, cells were treated with the protein synthesis inhibitor, cycloheximide, for up to 24 h. In cells overexpressing BVR, HO-2 decayed relatively slowly, demonstrating a half-life of ∼8 h, similar to that of BVR. In cells where BVR expression was ablated, however, hHO-2 was highly unstable, with little remaining at 4 h; decay was essentially complete at 8 h. Thus, the stability of HO-2 is critically dependent on the availability of BVR; that this occurs in human cells indicates that the dependence of HO-2 stability on BVR expression is conserved in evolution. The enhanced BVR-dependent stability of HO-2, due to ISO stimulation, could be a consequence of reduced ubiquitination of HO-2; this possibility was tested by using the proteasome inhibitor MG132 to block ubiquitin-dependent degradation in rat cardiomyocytes, and examination of the BVR and HO-2 protein levels (Fig. 4D). Interestingly, it was found that the HO-2 protein level was increased in MG132-treated cells in the absence of ISO stimulation. Moreover, MG132 increased the HO-2 protein level, even if BVR gene expression was inhibited by siBVR. These results suggest that the ISO-stimulated increase in BVR protein promotes the stability of HO-2 and that this is mediated by the inhibition of proteosomal degradation of presumably ubiquitinated HO-2.
Interaction between HO-2 and BVR is a survival factor for cardiomyocytes during ISO stimulation
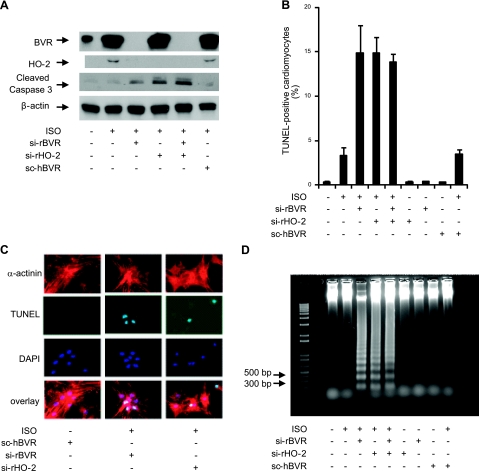
ISO increases cardiomyocyte apoptosis; pathological conditions such as cardiac overload produced by valvular disease and coronary heart failure also increase cardiomyocyte apoptosis (54). It was noted that the level of cleaved caspase-3 protein, a marker for apoptosis, was increased by the inhibition of ISO-induced expression of either BVR or HO-2 using siBVR or sirHO-2, respectively (Fig. 5A). The BVR-mediated response to ISO was clearly blocked by inclusion of the BVR siRNA, whereas addition of a scrambled version of this siRNA allowed stabilization of HO-2. Moreover, the scRNA did not allow the same increase in cleaved caspase observed with the siRNAs, indicating the specificity of the siRNAs and the role of both BVR and HO-2 in preventing apoptosis. We further found that inhibition of protein expression of either BVR or HO-2 by the appropriate siRNA, followed by treatment with ISO, resulted in an increase in the number of cardiac apoptotic cells (Fig. 5B), relative to the extent of apoptosis observed in the absence of ISO stimulation. These data suggest that HO-2 is acting as a heart protective factor during the ISO treatment. That apoptosis was occurring in cardiomyocytes, per se, was determined by triple staining of the cells with anti-α-actinin antibody to detect cardiomyocytes, TUNEL staining for cells undergoing apoptosis, and DAPI to detect nuclei (Fig. 5C). The merged images in Fig. 5C clearly indicate that the cardiomyocytes were undergoing apoptosis. ISO treatment coupled with siBVR and siHO-2 increased apoptosis to such an extent that DNA fragmentation could be detected by electrophoresis of genomic DNA (Fig. 5D). Simultaneously treating cardiomyocytes with siBVR and siHO-2 did not result in an additive increase in apoptosis compared to treating cardiomyocytes with siBVR or siHO-2 alone. This epistatic effect indicates that the activation of BVR and HO-2 are linked in the same pathway, wherein BVR acts upstream of HO-2, as indicated by the results from the HO-2 stability study (Fig. 4) and the temporal relationship between induction of BVR and HO-2 (Fig. 1). These results suggest that overexpression of HO-2 and BVR could, by reducing apoptosis in cardiomyocytes, be beneficial in preventing heart failure.
Figure 5.
Inhibition of BVR and HO-2 protein expression during ISO stimulation increases cardiomyocyte apoptosis. A) Cardiomyocytes were treated with siRNA against rat BVR, HO-2, or both, after which ISO was added at a final concentration of 10 μM, and the cells were incubated for 24 h. A randomized form of sihBVR (scBVR) was added to some cells as a control. Expression of BVR, HO-2, and cleaved caspase-3 was determined by Western blot. β-Actin served as a loading control. B) Quantitative analysis of the number of apoptotic cells detected, in cultures treated with siBVR, siHO-2, or both; scBVR served as control. Number of TUNEL-positive cells that also stained with EA-53 anti-α-actinin antibody was measured as a fraction of all cardiomyocytes. C) Detection of apoptosis in cardiomyocytes by microscopy. Cardiomyocytes were characterized by their staining with antibody against α-actinin. Apoptosis was detected by TUNEL staining; nuclei were visualized with DAPI. D) Total genomic DNA was isolated from cardiomyocytes that had been treated with ISO and siBVR, siHO-2, or both. Fragmentation of nuclear DNA is detectable in ISO-treated cardiomyocytes that were also treated with siBVR, siHO-2, or both. The 1-kb-plus ladder was used as DNA marker.
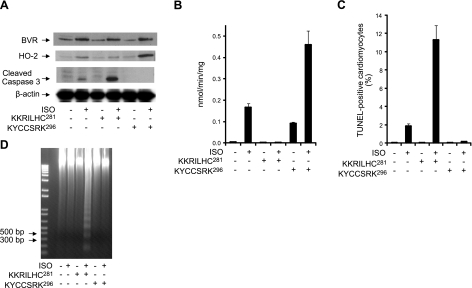
Our results suggest that BVR protein expression affects HO-2 protein expression through the regulation of HO-2 stability, which poses the question of whether there is a functional role for enhanced HO-2 expression after BVR activation. ISO can mimic the β-adrenergic activation associated with cardiac overload; this phenomenon is universally observed when the heart experiences pathophysiological stress, such as hypertension, valvular diseases, and myocardial infarction (6–9). Previously, our studies have shown that there are specific sequences of residues in hBVR, which effectively modulate BVR reductase and kinase activities. The peptide KKRILHC281 is a potent inhibitor of BVR activity, whereas the peptide KYCCSRK296 is an activator (43). These peptides may mimic domains in PKC-ζ, resulting in either inhibition or activation, respectively. The peptides were therefore tested for their effects on the intact heart. The Langendorff perfusion model was used to examine the interaction between BVR and HO-2. Prior to the preparation of the heart for perfusion, rats received 2 subcutaneous injections of a low dose of ISO (0.01 mg/kg) separated by a 6-h interval, to induce BVR activation and protein expression (Fig. 6A, B). The heart was then isolated and perfused with peptide KKRILHC281 or peptide KYCCSRK296, in the presence or absence of ISO.
Figure 6.
Peptide-mediated inhibition of BVR activation in perfused heart increases cardiomyocyte apoptosis, whereas BVR activation is protective. A) Three groups of 4 rats were injected subcutaneously with ISO (0.01 mg/kg), twice, with a 6-h interval between injections prior to perfusion of the isolated hearts. As indicated, isolated hearts were perfused with the peptides (25 μM) KKRILHC281 or KYCCSRK296 in the presence or absence of 0.1 μM ISO, for 3 h. Levels of BVR, HO-2, and cleaved caspase-3 were determined by Western blot, using β-actin as a control for equal loading. B) BVR activity was measured at pH 6.7, using NADH as cofactor. Rate of conversion of biliverdin to bilirubin was determined from the increase in absorbance at 450 nm at 25°C. Specific activity is expressed as nanomoles of bilirubin per minute per milligram of protein. C) Detection of apoptosis in perfused heart cardiomyocytes. Tissue from the perfused hearts was analyzed using the TUNEL assay, as described in Fig. 5B. D) Genomic DNA was isolated from perfused heart, and resolved by agarose gel electrophoresis; fragmentation is detectable only in hearts perfused with ISO and the inhibitory peptide, KKRILHC281. The 1-kb-plus ladder DNA was used as a marker.
As seen in Fig. 6, the peptides KKRILHC281 and KYCCSRK296 affect the activity of BVR, but they did not affect protein expression. However, as shown in Fig. 6A, peptide KKRILHC281 inhibited the BVR-induced HO-2 increase, whereas the peptide KYCCSRK296 increased HO-2 protein expression in ISO-treated heart, above that observed with ISO alone. As shown in Fig. 6B, peptide KKRILHC281 blocked the activity of BVR regardless of the presence of ISO. Treatment of the heart with peptide KYCCSRK296 alone increased BVR activity; there was, however, a significant activation of BVR reductase activity in hearts treated with both ISO and peptide KYCCSRK296. As shown in Fig. 6C, ISO increased apoptosis, as measured by the TUNEL assay, in cardiomyocytes of the intact heart; inhibition of BVR during stimulation by ISO resulted in a significant further increase in apoptosis. The observed increase in apoptosis was further demonstrated by the elevated levels of cleaved caspase-3 seen in Fig. 6A and the detection of cleavage of genomic DNA by formation of a DNA ladder (Fig. 6D).
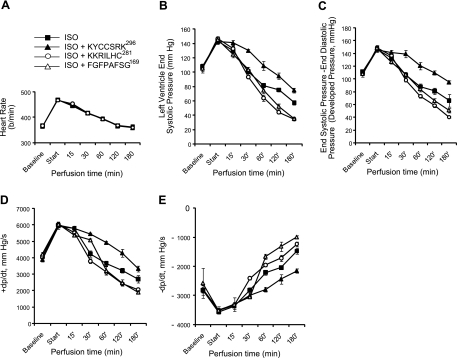
The effect of peptides on the integrity of the heart was also examined in the perfusion system, by measuring left ventricle contractile function in the presence of ISO as a function of time. The heart rate (Fig. 7A), left ventricular systolic pressure (Fig. 7B), developed pressure (Fig. 7C), and the first derivates of systolic pressure (dp/dt, −dp/dt; Fig. 7D, E) all declined over the course of the experiment. If peptide KKRILHC281 was included with ISO in the perfusion buffer, the rate of decline was increased, which could be observed by 30 min after initiating perfusion (Fig. 7). This decline in function occurred independently of the heart rate, which was identical in the peptide-treated and ISO-treated hearts. This decline in heart function is consistent with the increase in apoptosis (Fig. 6) observed with this peptide in the presence of ISO.
Figure 7.
Inhibition of BVR activation exacerbates ISO-mediated decline in the contractile function of the heart; BVR activation protects against loss of heart function. A) Heart rate was measured during perfusion with ISO and peptides (25 μM) KKRILHC281 (n=4), KYCCSRK296 (n=4) or FGFPAFSG169 (n=3), or with ISO alone (n=7). B) Left ventricular systolic pressure was measured simultaneously with heart rate shown in A. C) Developed pressure, calculated as the difference between the end systolic pressure and end diastolic pressure for the left ventricle, for the same animals as in A. D, E) First derivatives of left ventricular systolic pressure during compression, dp/dt (D), and relaxation −dp/dt (E), for the data shown in A–C.
On the other hand, perfusion with the peptide KYCCSRK296 together with ISO slowed the decrease in contractile function observed with ISO alone (Fig. 7), although as for the peptide KKRILHC281, there was no effect on heart rate. This observation is consistent with decreased apoptosis and increased BVR activation followed by elevated HO-2 protein expression compared to the ISO control (Figs. 6 and 7). A third peptide, FGFPAFSG169, derived from human BVR was also examined in these experiments. This peptide has been shown to disrupt hBVR-dependent activation of ERK1/2 (55); however, it had little effect on systolic or developed pressure for the first hour of perfusion, and only later did it lead to some weakening of heart function.
Taken together, the data presented here suggest that BVR activity and its subsequent effects on cellular HO-2 protein levels, plays an important role in the prevention of heart damage that results from β-AR activation.
DISCUSSION
Activation of the β-AR mediates inotropic heart modification and the activation of the apoptotic cell death pathway (2–3). Both β1-AR and β2-AR subtypes coexist in cardiomyocytes (4–5); however, the β-AR generated apoptotic signaling is largely dissociated from the β1-AR subtype and is selectively mediated by the β2-AR subtype in adult rat ventricular myocytes in vitro (56–57). Response of isolated cardiomyocytes to ISO-cultured cells can be utilized as a model to assess activation of β-ARs under pathological conditions, such as overloading and ischemia (6–9).
Presently, we have uncovered that ISO exposure increases the levels of two enzymes in myocytes, in the intact perfused heart and isolated cells, which are intimately linked to cellular defense mechanisms—BVR and HO-2. We have found that, in the intact heart and isolated cardiomyocytes, ISO treatment induces BVR at transcript and protein levels, which, in turn, leads to an increase in the content of HO-2 protein (Figs. 1 and 2), and that BVR induction is a prerequisite for increased myocyte levels of HO-2 (Fig. 4). Data suggest that the increase resulted from a decrease in the turnover of HO-2 protein, rather than an increase in HO-2 transcription (Fig. 4). The refractory response of HO-2 transcription to ISO is similar to its response to the host of stimuli that activate HO-1 gene expression. To date, only the adrenal glucocorticoids have been shown to induce HO-2 transcription; the only functional regulatory elements identified in the HO-2 promoter are GREs (58). HO-2 is expressed in the endothelial cells of the blood vessels and the sensory cells of the heart (21–22). Notably, glucocorticoid receptors are expressed in the heart and arterial walls and act directly to maintain vascular tone and response to inflammation and injury (59).
The HO system, which refers to HO-1, HO-2, and BVR, is a key component of the cellular defense mechanisms against free radical-mediated tissue injury, including apoptosis. The system is the sole biological mechanism for the conversion of the prooxidant heme to bilirubin, a potent intracellular antioxidant (30). The second product of heme catabolism, CO, is known for its ability to dilate blood vessels as well to display anti-inflammatory effects (60–66); induction of HO-1, and by inference generation of CO, also offers protection to cardiomyocytes against cold storage damage (67). HO-1 and HO-2 both catalyze the formation of CO and biliverdin, which is subsequently converted by BVR to bilirubin; clearly, additional functions of HO-2, its tissue distribution in the heart, as well as its regulatory link to BVR, are likely factors in its BVR-dependent selective and sustained increase and underscore the physiological significance of this increase. Specifically, HO-2 is a substrate for BVR kinase activity (41), it functions as an oxygen sensor (38–40), and it is expressed in cell types that control response of the organ to oxygen tension (22). HO-2 has the distinctive ability to bind heme to motifs that are not involved in catalytic activity of the oxygenase (36), and hence enables heme binding of gaseous ligands—O2, CO, and NO. Notably, chronic treatment in vivo with ISO is associated with activation of iNOS, elevated NO levels, and nitrosative stress (54). Perceivably, sequestration of the proinflammatory NO by the membrane-bound HO-2 (68) could be considered as a component of cytoprotection offered by increase in HO-2 levels in cardiomyocytes. However, because NO-mediated mitochondrial dysfunction leads to Ca2+ flux, which, in turn, activates p50/ATF6 and up-regulates the ER-resident molecular chaperone, Grp78, increased NO production can be considered to be cytoprotective (69–70).
On the basis of the current study, it is apparent that HO-2 plays an important role in protecting cardiomyocytes against apoptosis, which correlates with the increase in its cellular content. However, using the currently applied experimental protocols, ISO-modulated HO-1 expression very modestly, and transiently; therefore, our data do not define the molecular basis for the report by Kinobe et al. (71), who reported that HO-1 attenuates ISO-induced cardiac hypertrophy. This, however, is not to question HO-1-mediated attenuation of cardiac hypertrophy induced by angiotensin II (72) or oxidative stress (73). In addition, induction of HO-1 decreases apoptosis of the cardiomyocytes in ischemia/reperfusion model of heart injury, which has been suggested to reflect inhibition of the transcriptional factors AP-1 and NF-κB (74–75). HO-1 induction may also be a contributing factor to ischemic preconditioning as a protection against reperfusion injury, likely by limiting ROS generation; other antioxidant enzymes are also induced during this process (76).
It has been postulated that there is a necessity for a balance between apoptosis and hypertrophy (77). While myocardial hypertrophy can normalize wall tension, it also initiates apoptosis; it is thus an unfavorable outcome for the heart. Apoptosis of cardiomyocytes is significantly elevated in cardiac transplant patients with heart failure arising from a variety of causes (1), despite significant increases in the antiapoptotic signaling protein Bcl-2 (1). Also, apoptosis is rapidly manifested during myocardial infarction, while lower levels of apoptotic cells are observed in generalized heart failure (reviewed in ref. 78). In either instance, reduction in apoptosis to basal levels might ultimately be of benefit to patients, although it has been argued that a complete elimination of apoptosis might not be advantageous in the long term (79), as it could result in neoplasia and/or autoimmunity complications.
Activation of BVR and HO-2 by ISO protects the heart; inhibition of BVR or HO-2 induction with siRNA increases cardiomyocyte apoptosis. However, while ISO increases expression of BVR and HO-2, which serves to limit apoptosis, the induction of these proteins does not eliminate apoptosis entirely. Since apoptosis leads to the loss of the contractile function of the heart, the cumulative effect of ISO treatment is eventual heart failure. It was previously reported that ISO increases the activity of the apoptotic PDE3-ICER pathway (47–48). Thus, we hypothesize that even though apoptosis is decreased by activation of BVR and HO-2, the alternative PDE3-ICER pathway allows for ongoing cell death, assuming that function of this pathway is largely independent of BVR and HO-2. This does not, however, take into account the possibility that further activation of BVR, above that which is achieved by increased synthesis, might circumvent the alternate pathway and completely protect the cell. The findings with hBVR-based peptides shed light on this possibility. Inhibition of BVR kinase and reductase activities with the peptide KKRILHC281 resulted in a series of detrimental effects on both cardiomyocyte survival and heart function. In these experiments, inhibition of BVR activity virtually eliminated the induction of HO-2, led to significant increases in apoptosis in cultured cells, and resulted in significantly diminished cardiac function in the isolated heart perfusion system (Figs. 6 and 7). On the other hand, activation of BVR could be observed with the peptide KYCCSRK296, and the net effect of this treatment was a greatly enhanced increase of HO-2 protein in the cell, a nearly complete reduction in apoptosis, and improved cardiac function. The effect of the peptide on apoptosis suggests that BVR and/or HO-2 exert their antiapoptotic effects in cardiomyocytes primarily by interfering with the PDE3-ICER pathway.
Blockade of ISO-mediated induction of BVR by PKI indicates involvement of the PKA signaling pathway; at this time, however, the molecular mechanism by which BVR promotes increased cellular content of HO-2 protein is not evident, nor is it clear whether BVR acts directly or by means of an intermediary pathway. Nonetheless, a number of possibilities can be considered. Involvement of the human BVR in regulation of several signaling entities has been documented. To elaborate, studies in this laboratory, and others, have demonstrated that BVR is multifunctional and can act both as a kinase and as a kinase-kinase (19, 41, 43, 49, 55, 80–81). For example, BVR has been shown to activate PKC-βII—indeed, there is a distinct possibility that BVR is PKC-βII-kinase (49). BVR also functions in the TNF-α-activated PKC-ζ signaling by binding to and thus activating the PKC (43). BVR can function as an intermediary to link the MAPK and PI3K pathways (42, 55). In addition, using purified preparations of HO-2 and BVR, the reductase has been shown to be a kinase for the oxygenase (41). Accordingly, any of the noted signaling pathways may be involved in HO-2 increase.
In the United States, cardiac failure is a major health problem, despite advances in treatment regimens, and it affects as many as 4.8 million people. Should increase in HO-2 be among the assembly of benefits offered by the glucocorticoids to the cardiovascular system, then the finding that activation of BVR leads to the increase in HO-2 levels in the heart may provide a novel approach for development of therapeutic agents to combat cardiovascular disease; the further elevation of BVR levels in the cardiomyocytes achieved using a 7-residue hBVR-based peptide resulted in a nearly complete reduction in apoptosis and improved cardiac function (Figs. 6 and 7). Although the utility of peptides identified in this report may well be constrained by issues surrounding targeted delivery and antigenicity, the unique chemistry of the peptides could be exploited in design of small molecule therapeutics that would be expected to be more suitable for longer-term treatment with fewer complications.
Acknowledgments
This work was supported by the National Institute of Environmental Health Sciences grants ES04066 and ES012187 and American Heart Association grant AHA0635312N. Work in the laboratory of P.S.B. is supported by National Institutes of Health grant RO1-HL071158.
The assistance and advice of Dr. Sergiy Nadtochiy in setting up the heart perfusion experiments are gratefully acknowledged.
REFERENCES
- 1. Olivetti G., Abbi R., Quaini F., Kajstura J., Cheng W., Nitahara J. A., Quaini E., Di Loreto C., Beltrami C. A., Krajewski S., Reed J. C., Anversa P. (1997) Apoptosis in the failing human heart. N. Engl. J. Med. 336, 1131–1141 [DOI] [PubMed] [Google Scholar]
- 2. Tomita H., Nazmy M., Kajimoto K., Yehia G., Molina C. A., Sadoshima J. (2003) Inducible cAMP early repressor (ICER) is a negative-feedback regulator of cardiac hypertrophy and an important mediator of cardiac myocyte apoptosis in response to beta-adrenergic receptor stimulation. Circ. Res. 93, 12–22 [DOI] [PubMed] [Google Scholar]
- 3. Zaugg M., Xu W., Lucchinetti E., Shafiq S. A., Jamali N. Z., Siddiqui M. A. (2000) Beta-adrenergic receptor subtypes differentially affect apoptosis in adult rat ventricular myocytes. Circulation 102, 344–350 [DOI] [PubMed] [Google Scholar]
- 4. Bristow M. R., Ginsburg R., Umans V., Fowler M., Minobe W., Rasmussen R., Zera P., Menlove R., Shah P., Jamieson S., Stinson E. B. (1986) Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ. Res. 59, 297–309 [DOI] [PubMed] [Google Scholar]
- 5. Del Monte F., Kaumann A. J., Poole-Wilson P. A., Wynne D. G., Pepper J., Harding S. E. (1993) Coexistence of functioning beta 1- and beta 2-adrenoceptors in single myocytes from human ventricle. Circulation 88, 854–863 [DOI] [PubMed] [Google Scholar]
- 6. Sethi R., Dhalla N. S. (1995) Inotropic responses to isoproterenol in congestive heart failure subsequent to myocardial infarction in rats. J. Card. Fail. 1, 391–399 [DOI] [PubMed] [Google Scholar]
- 7. Grimm D., Elsner D., Schunkert H., Pfeifer M., Griese D., Bruckschlegel G., Muders F., Riegger G. A., Kromer E. P. (1998) Development of heart failure following isoproterenol administration in the rat: role of the renin-angiotensin system. Cardiovasc. Res. 37, 91–100 [DOI] [PubMed] [Google Scholar]
- 8. Oudit G. Y., Crackower M. A., Eriksson U., Sarao R., Kozieradzki I., Sasaki T., Irie-Sasaki J., Gidrewicz D., Rybin V. O., Wada T., Steinberg S. F., Backx P. H., Penninger J. M. (2003) Phosphoinositide 3-kinase gamma-deficient mice are protected from isoproterenol-induced heart failure. Circulation 108, 2147–2152 [DOI] [PubMed] [Google Scholar]
- 9. Ferreira A. J., Oliveira T. L., Castro M. C., Almeida A. P., Castro C. H., Caliari M. V., Gava E., Kitten G. T., Santos R. A. (2007) Isoproterenol-induced impairment of heart function and remodeling are attenuated by the nonpeptide angiotensin-(1–7) analogue AVE 0991. Life Sci. 81, 916–923 [DOI] [PubMed] [Google Scholar]
- 10. Choi A. M. (2001) Heme oxygenase-1 protects the heart. Circ. Res. 89, 105–107 [PubMed] [Google Scholar]
- 11. Masini E., Vannacci A., Marzocca C., Pierpaoli S., Giannini L., Fantappie O., Mazzanti R., Mannaioni P. F. (2003) Heme oxygenase-1 and the ischemia-reperfusion injury in the rat heart. Exp. Biol. Med. (Maywood) 228, 546–549 [DOI] [PubMed] [Google Scholar]
- 12. Pachori A. S., Melo L. G., Hart M. L., Noiseux N., Zhang L., Morello F., Solomon S. D., Stahl G. L., Pratt R. E., Dzau V. J. (2004) Hypoxia-regulated therapeutic gene as a preemptive treatment strategy against ischemia/reperfusion tissue injury. Proc. Natl. Acad. Sci. U. S. A. 101, 12282–12287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakao A., Neto J. S., Kanno S., Stolz D. B., Kimizuka K., Liu F., Bach F. H., Billiar T. R., Choi A. M., Otterbein L. E., Murase N. (2005) Protection against ischemia/reperfusion injury in cardiac and renal transplantation with carbon monoxide, biliverdin and both. Am. J. Transplant. 5, 282–291 [DOI] [PubMed] [Google Scholar]
- 14. Giannini L., Vannacci A., Fabrizi F., Uliva C., Bani D., Masini E., Mannaioni P. F. (2005) Protection from cardiac injury by induction of heme oxygenase-1 and nitric oxide synthase in a focal ischaemia-reperfusion model. Cell. Mol. Biol. (Noisy-le-grand) 51, 393–401 [PubMed] [Google Scholar]
- 15. Yano Y., Ozono R., Oishi Y., Kambe M., Yoshizumi M., Ishida T., Omura S., Oshima T., Igarashi K. (2006) Genetic ablation of the transcription repressor Bach1 leads to myocardial protection against ischemia/reperfusion in mice. Genes Cells 11, 791–803 [DOI] [PubMed] [Google Scholar]
- 16. Yamashita K., Ollinger R., McDaid J., Sakahama H., Wang H., Tyagi S., Csizmadia E., Smith N. R., Soares M. P., Bach F. H. (2006) Heme oxygenase-1 is essential for and promotes tolerance to transplanted organs. FASEB J. 20, 776–778 [DOI] [PubMed] [Google Scholar]
- 17. Immenschuh S., Schroder H. (2006) Heme oxygenase-1 and cardiovascular disease. Histol. Histopathol. 21, 679–685 [DOI] [PubMed] [Google Scholar]
- 18. Ewing J. F., Maines M. D. (1991) Rapid induction of heme oxygenase 1 mRNA and protein by hyperthermia in rat brain: heme oxygenase 2 is not a heat shock protein. Proc. Natl. Acad. Sci. U. S. A. 88, 5364–5368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Florczyk U. M., Jozkowicz A., Dulak J. (2008) Biliverdin reductase: new features of an old enzyme and its potential therapeutic significance. Pharmacol. Rep. 60, 38–48 [PMC free article] [PubMed] [Google Scholar]
- 20. Kapitulnik J., Maines M. D. (2009) Pleiotropic functions of biliverdin reductase: cellular signaling and generation of cytoprotective and cytotoxic bilirubin. Trends Pharmacol. Sci. 30, 129–137 [DOI] [PubMed] [Google Scholar]
- 21. Ewing J. F., Raju V. S., Maines M. D. (1994) Induction of heart heme oxygenase-1 (HSP32) by hyperthermia: possible role in stress-mediated elevation of cyclic 3′:5′-guanosine monophosphate. J. Pharmacol. Exp. Ther. 271, 408–414 [PubMed] [Google Scholar]
- 22. Prabhakar N. R., Dinerman J. L., Agani F. H., Snyder S. H. (1995) Carbon monoxide: a role in carotid body chemoreception. Proc. Natl. Acad. Sci. U. S. A. 92, 1994–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ewing J. F., Maines M. D. (1992) In situ hybridization and immunohistochemical localization of heme oxygenase-2 mRNA and protein in normal rat brain: Differential distribution of isozyme 1 and 2. Mol. Cell. Neurosci. 3, 559–570 [DOI] [PubMed] [Google Scholar]
- 24. Panahian N., Maines M. D. (2001) Site of injury-directed induction of heme oxygenase-1 and -2 in experimental spinal cord injury: differential functions in neuronal defense mechanisms? J. Neurochem. 76, 539–554 [DOI] [PubMed] [Google Scholar]
- 25. Miller S. M., Farrugia G., Schmalz P. F., Ermilov L. G., Maines M. D., Szurszewski J. H. (1998) Heme oxygenase 2 is present in interstitial cell networks of the mouse small intestine. Gastroenterology 114, 239–244 [DOI] [PubMed] [Google Scholar]
- 26. Kim Y. S., Zhuang H., Koehler R. C., Dore S. (2005) Distinct protective mechanisms of HO-1 and HO-2 against hydroperoxide-induced cytotoxicity. Free Radic. Biol. Med. 38, 85–92 [DOI] [PubMed] [Google Scholar]
- 27. Verma A., Hirsch D. J., Glatt C. E., Ronnett G. V., Snyder S. H. (1993) Carbon monoxide: a putative neural messenger. Science 259, 381–384 [DOI] [PubMed] [Google Scholar]
- 28. Prabhakar N. R. (1999) NO and CO as second messengers in oxygen sensing in the carotid body. Respir. Physiol. 115, 161–168 [DOI] [PubMed] [Google Scholar]
- 29. Ryter S. W., Morse D., Choi A. M. (2007) Carbon monoxide and bilirubin: potential therapies for pulmonary/vascular injury and disease. Am. J. Respir. Cell. Mol. Biol. 36, 175–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maghzal G. J., Leck M. C., Collinson E., Li C., Stocker R. (2009) Limited role for the bilirubin-biliverdin redox amplification cycle in the cellular antioxidant protection by biliverdin reductase. J. Biol. Chem. 284, 29251–29259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miralem T., Hu Z., Torno M. D., Lelli K. M., Maines M. D. (2005) Small interference RNA-mediated gene silencing of human biliverdin reductase, but not that of heme oxygenase-1, attenuates arsenite-mediated induction of the oxygenase and increases apoptosis in 293A kidney cells. J. Biol. Chem. 280, 17084–17092 [DOI] [PubMed] [Google Scholar]
- 32. Baranano D. E., Rao M., Ferris C. D., Snyder S. H. (2002) Biliverdin reductase: a major physiologic cytoprotectant. Proc. Natl. Acad. Sci. U. S. A. 99, 16093–16098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sedlak T. W., Saleh M., Higginson D. S., Paul B. D., Juluri K. R., Snyder S. H. (2009) Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc. Natl. Acad. Sci. U. S. A. 106, 5171–5176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rotenberg M. O., Maines M. D. (1990) Isolation, characterization, and expression in Escherichia coli of a cDNA encoding rat heme oxygenase-2. J. Biol. Chem. 265, 7501–7506 [PubMed] [Google Scholar]
- 35. Rublevskaya I., Maines M. D. (1994) Interaction of Fe-protoporphyrin IX and heme analogues with purified recombinant heme oxygenase-2, the constitutive isozyme of the brain and testes. J. Biol. Chem. 269, 26390–26395 [PubMed] [Google Scholar]
- 36. McCoubrey W. K., Jr., Huang T. J., Maines M. D. (1997) Heme oxygenase-2 is a hemoprotein and binds heme through heme regulatory motifs that are not involved in heme catalysis. J. Biol. Chem. 272, 12568–12574 [DOI] [PubMed] [Google Scholar]
- 37. Yi L., Jenkins P. M., Leichert L. I., Jakob U., Martens J. R., Ragsdale S. W. (2009) Heme regulatory motifs in heme oxygenase-2 form a thiol/disulfide redox switch that responds to the cellular redox state. J. Biol. Chem. 284, 20556–20561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Maines M. D. (1997) The heme oxygenase system: a regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 37, 517–554 [DOI] [PubMed] [Google Scholar]
- 39. Williams S. E., Wootton P., Mason H. S., Bould J., Iles D. E., Riccardi D., Peers C., Kemp P. J. (2004) Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel. Science 306, 2093–2097 [DOI] [PubMed] [Google Scholar]
- 40. Kemp P. J. (2005) Hemeoxygenase-2 as an O2 sensor in K+ channel-dependent chemotransduction. Biochem. Biophys. Res. Commun. 338, 648–652 [DOI] [PubMed] [Google Scholar]
- 41. Lerner-Marmarosh N., Shen J., Torno M. D., Kravets A., Hu Z., Maines M. D. (2005) Human biliverdin reductase: a member of the insulin receptor substrate family with serine/threonine/tyrosine kinase activity. Proc. Natl. Acad. Sci. U. S. A. 102, 7109–7114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Maines M. D. (2007) Biliverdin reductase: PKC interaction at the cross-talk of MAPK and PI3K signaling pathways. Antioxid. Redox Signal. 9, 2187–2195 [DOI] [PubMed] [Google Scholar]
- 43. Lerner-Marmarosh N., Miralem T., Gibbs P. E., Maines M. D. (2007) Regulation of TNF-alpha-activated PKC-zeta signaling by the human biliverdin reductase: identification of activating and inhibitory domains of the reductase. FASEB J. 21, 3949–3962 [DOI] [PubMed] [Google Scholar]
- 44. Carnegie G. K., Means C. K., Scott J. D. (2009) A-kinase anchoring proteins: from protein complexes to physiology and disease. IUBMB Life 61, 394–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McKnight G. S., Cummings D. E., Amieux P. S., Sikorski M. A., Brandon E. P., Planas J. V., Motamed K., Idzerda R. L. (1998) Cyclic AMP, PKA, and the physiological regulation of adiposity. Recent Prog. Horm. Res. 53, 139–159; discussion 160–131 [PubMed] [Google Scholar]
- 46. Kravets A., Hu Z., Miralem T., Torno M. D., Maines M. D. (2004) Biliverdin reductase, a novel regulator for induction of activating transcription factor-2 and heme oxygenase-1. J. Biol. Chem. 279, 19916–19923 [DOI] [PubMed] [Google Scholar]
- 47. Ding B., Abe J., Wei H., Huang Q., Walsh R. A., Molina C. A., Zhao A., Sadoshima J., Blaxall B. C., Berk B. C., Yan C. (2005) Functional role of phosphodiesterase 3 in cardiomyocyte apoptosis: implication in heart failure. Circulation 111, 2469–2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ding B., Abe J., Wei H., Xu H., Che W., Aizawa T., Liu W., Molina C. A., Sadoshima J., Blaxall B. C., Berk B. C., Yan C. (2005) A positive feedback loop of phosphodiesterase 3 (PDE3) and inducible cAMP early repressor (ICER) leads to cardiomyocyte apoptosis. Proc. Natl. Acad. Sci. U. S. A. 102, 14771–14776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Maines M. D., Miralem T., Lerner-Marmarosh N., Shen J., Gibbs P. E. (2007) Human biliverdin reductase, a previously unknown activator of protein kinase C betaII. J. Biol. Chem. 282, 8110–8122 [DOI] [PubMed] [Google Scholar]
- 50. Huang T. J., Trakshel G. M., Maines M. D. (1989) Detection of 10 variants of biliverdin reductase in rat liver by two-dimensional gel electrophoresis. J. Biol. Chem. 264, 7844–7849 [PubMed] [Google Scholar]
- 51. Tudor C., Lerner-Marmarosh N., Engelborghs Y., Gibbs P. E., Maines M. D. (2008) Biliverdin reductase is a transporter of haem into the nucleus and is essential for regulation of HO-1 gene expression by haematin. Biochem. J. 413, 405–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tompkins A. J., Burwell L. S., Digerness S. B., Zaragoza C., Holman W. L., Brookes P. S. (2006) Mitochondrial dysfunction in cardiac ischemia-reperfusion injury: ROS from complex I, without inhibition. Biochim. Biophys. Acta 1762, 223–231 [DOI] [PubMed] [Google Scholar]
- 53. Hayes J. S., Wyss V. L., Schenck K. S., Cohen M. L. (1986) Effects of prolonged isoproterenol infusion on cardiac and vascular responses to adrenoceptor agonists. J. Pharmacol. Exp. Ther. 237, 757–763 [PubMed] [Google Scholar]
- 54. Hu A., Jiao X., Gao E., Koch W. J., Sharifi-Azad S., Grunwald Z., Ma X. L., Sun J. Z. (2006) Chronic beta-adrenergic receptor stimulation induces cardiac apoptosis and aggravates myocardial ischemia/reperfusion injury by provoking inducible nitric-oxide synthase-mediated nitrative stress. J. Pharmacol. Exp. Ther. 318, 469–475 [DOI] [PubMed] [Google Scholar]
- 55. Lerner-Marmarosh N., Miralem T., Gibbs P. E., Maines M. D. (2008) Human biliverdin reductase is an ERK activator; hBVR is an ERK nuclear transporter and is required for MAPK signaling. Proc. Natl. Acad. Sci. U. S. A. 105, 6870–6875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Steinberg S. F. (1999) The molecular basis for distinct beta-adrenergic receptor subtype actions in cardiomyocytes. Circ. Res. 85, 1101–1111 [DOI] [PubMed] [Google Scholar]
- 57. Nikolaev V. O., Moshkov A., Lyon A. R., Miragoli M., Novak P., Paur H., Lohse M. J., Korchev Y. E., Harding S. E., Gorelik J. (2010) Beta2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science 327, 1653–1657 [DOI] [PubMed] [Google Scholar]
- 58. Raju V. S., McCoubrey W. K., Jr., Maines M. D. (1997) Regulation of heme oxygenase-2 by glucocorticoids in neonatal rat brain: characterization of a functional glucocorticoid response element. Biochim. Biophys. Acta 1351, 89–104 [DOI] [PubMed] [Google Scholar]
- 59. Walker B. R. (2007) Glucocorticoids and cardiovascular disease. Eur. J. Endocrinol. 157, 545–559 [DOI] [PubMed] [Google Scholar]
- 60. Slebos D. J., Ryter S. W., Choi A. M. (2003) Heme oxygenase-1 and carbon monoxide in pulmonary medicine. Respir. Res. 4, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li Volti G., Rodella L. F., Di Giacomo C., Rezzani R., Bianchi R., Borsani E., Gazzolo D., Motterlini R. (2006) Role of carbon monoxide and biliverdin in renal ischemia/reperfusion injury. Nephron Exp. Nephrol. 104, e135–139 [DOI] [PubMed] [Google Scholar]
- 62. Li Volti G., Sorrenti V., Murabito P., Galvano F., Veroux M., Gullo A., Acquaviva R., Stacchiotti A., Bonomini F., Vanella L., Di Giacomo C. (2007) Pharmacological induction of heme oxygenase-1 inhibits iNOS and oxidative stress in renal ischemia-reperfusion injury. Transplant. Proc. 39, 2986–2991 [DOI] [PubMed] [Google Scholar]
- 63. Bolognesi M., Sacerdoti D., Piva A., Di Pascoli M., Zampieri F., Quarta S., Motterlini R., Angeli P., Merkel C., Gatta A. (2007) Carbon monoxide-mediated activation of large-conductance calcium-activated potassium channels contributes to mesenteric vasodilatation in cirrhotic rats. J. Pharmacol. Exp. Ther. 321, 187–194 [DOI] [PubMed] [Google Scholar]
- 64. Chung H. T., Choi B. M., Kwon Y. G., Kim Y. M. (2008) Interactive relations between nitric oxide (NO) and carbon monoxide (CO): heme oxygenase-1/CO pathway is a key modulator in NO-mediated antiapoptosis and anti-inflammation. Methods Enzymol. 441, 329–338 [DOI] [PubMed] [Google Scholar]
- 65. Ryter S. W., Choi A. M. (2009) Heme oxygenase-1/carbon monoxide: from metabolism to molecular therapy. Am. J. Respir. Cell Mol. Biol. 41, 251–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gozzelino R., Jeney V., Soares M. P. (2010) Mechanisms of cell protection by heme oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 50, 323–354 [DOI] [PubMed] [Google Scholar]
- 67. Abuarqoub H., Green C. J., Foresti R., Motterlini R. (2007) Curcumin reduces cold storage-induced damage in human cardiac myoblasts. Exp. Mol. Med. 39, 139–148 [DOI] [PubMed] [Google Scholar]
- 68. Ding Y., McCoubrey W. K., Jr., Maines M. D. (1999) Interaction of heme oxygenase-2 with nitric oxide donors. Is the oxygenase an intracellular ‘sink’ for NO? Eur. J. Biochem. 264, 854–861 [DOI] [PubMed] [Google Scholar]
- 69. Xu W., Liu L., Charles I. G., Moncada S. (2004) Nitric oxide induces coupling of mitochondrial signalling with the endoplasmic reticulum stress response. Nat. Cell Biol. 6, 1129–1134 [DOI] [PubMed] [Google Scholar]
- 70. Erusalimsky J. D., Moncada S. (2007) Nitric oxide and mitochondrial signaling: from physiology to pathophysiology. Arterioscler. Thromb. Vasc. Biol. 27, 2524–2531 [DOI] [PubMed] [Google Scholar]
- 71. Kinobe R. T., Dercho R. A., Nakatsu K. (2008) Inhibitors of the heme oxygenase-carbon monoxide system: on the doorstep of the clinic? Can. J. Physiol. Pharmacol. 86, 577–599 [DOI] [PubMed] [Google Scholar]
- 72. Hu C. M., Chen Y. H., Chiang M. T., Chau L. Y. (2004) Heme oxygenase-1 inhibits angiotensin II-induced cardiac hypertrophy in vitro and in vivo. Circulation 110, 309–316 [DOI] [PubMed] [Google Scholar]
- 73. Brunt K. R., Tsuji M. R., Lai J. H., Kinobe R. T., Durante W., Claycomb W. C., Ward C. A., Melo L. G. (2009) Heme oxygenase-1 inhibits pro-oxidant induced hypertrophy in HL-1 cardiomyocytes. Exp. Biol. Med. (Maywood) 234, 582–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jadhav A., Torlakovic E., Ndisang J. F. (2008) Interaction among heme oxygenase, nuclear factor-κB, and transcription activating factors in cardiac hypertrophy in hypertension. Hypertension 52, 910–917 [DOI] [PubMed] [Google Scholar]
- 75. Yeh C. H., Chen T. P., Wang Y. C., Lin Y. M., Lin P. J. (2009) HO-1 activation can attenuate cardiomyocytic apoptosis via inhibition of NF-κB and AP-1 translocation following cardiac global ischemia and reperfusion. J. Surg. Res. 155, 147–156 [DOI] [PubMed] [Google Scholar]
- 76. Das D. K., Maulik N. (2006) Cardiac genomic response following preconditioning stimulus. Cardiovasc. Res. 70, 254–263 [DOI] [PubMed] [Google Scholar]
- 77. van Empel V. P., De Windt L. J. (2004) Myocyte hypertrophy and apoptosis: a balancing act. Cardiovasc. Res. 63, 487–499 [DOI] [PubMed] [Google Scholar]
- 78. Whelan R. S., Kaplinskiy V., Kitsis R. N. (2010) Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu. Rev. Physiol. 72, 19–44 [DOI] [PubMed] [Google Scholar]
- 79. Marshall D., Sack M. N. (2000) Apoptosis: a pivotal event or an epiphenomenon in the pathophysiology of heart failure? Heart 84, 355–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pachori A. S., Smith A., McDonald P., Zhang L., Dzau V. J., Melo L. G. (2007) Heme-oxygenase-1-induced protection against hypoxia/reoxygenation is dependent on biliverdin reductase and its interaction with PI3K/Akt pathway. J. Mol. Cell. Cardiol. 43, 580–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wegiel B., Baty C. J., Gallo D., Csizmadia E., Scott J. R., Akhavan A., Chin B. Y., Kaczmarek E., Alam J., Bach F. H., Zuckerbraun B. S., Otterbein L. E. (2009) Cell surface biliverdin reductase mediates biliverdin-induced anti-inflammatory effects via phosphatidylinositol 3-kinase and Akt. J. Biol. Chem. 284, 21369–21378 [DOI] [PMC free article] [PubMed] [Google Scholar]