Abstract
CC chemokine receptor 2 (CCR2) is essential to acute skeletal muscle injury repair. We studied the subpopulation of inflammatory cells recruited via CCR2 signaling and their cellular functions with respect to muscle regeneration. Mobilization of monocytes/macrophages (MOs/MPs), but not lymphocytes or neutrophils, was impaired from bone marrow to blood and from blood to injured muscle in Ccr2−/− mice. While the Ly-6C+ but not the Ly-6C− subset of MOs/MPs was significantly reduced in blood, both subsets were drastically reduced in injured muscle of Ccr2−/− mice. Expression of insulin-like growth factor-1 (IGF-I) was markedly up-regulated in injured muscle of wild-type but not Ccr2−/− mice. IGF-I was strongly expressed by macrophages within injured muscle, more prominently by the Ly-6C− subset. A single injection of IGF-I, but not PBS, into injured muscle to replace IGF-I remarkably improved muscle regeneration in Ccr2−/− mice. CCR2 was not detected in myogenic cells or capillary endothelial cells in injured muscle to suggest its direct involvement in muscle regeneration or angiogenesis. We conclude that CCR2 is essential to acute skeletal muscle injury repair primarily by recruiting Ly-6C+ MOs/MPs. Within injured muscle, these cells conduct phagocytosis, contribute to accumulation of intramuscular Ly-6C− macrophages, and produce a high level of IGF-I to promote muscle regeneration.—Lu, H., Huang, D., Saederupm, N., Charo, I. F., Ransohoff, R. M., Zhou, L. Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury.
Keywords: chemokine, chemokine receptor, inflammation, IGF-I, skeletal muscle regeneration
CC chemokine receptor 2 (CCR2) is essential to mount an inflammatory response during acute skeletal muscle injury repair. Ccr2−/− mice showed markedly reduced macrophage infiltration in response to acute muscle injuries induced by ischemia (1) or myotoxic agents, including netoxin and cardiotxin (2, 3). The diminished inflammatory response was accompanied by poor muscle regeneration, suggesting that muscle inflammation is beneficial and blocking CCR2 signaling is detrimental to acute skeletal muscle injury repair.
Skeletal muscle injury repair is a complex process, which mainly consists of inflammation, myofiber regeneration, and angiogenesis (4). Inflammation predominantly involves monocytes/macrophages (MOs/MPs), which remove necrotic debris (4) and may also produce soluble factors to promote regeneration (5–7). Myogenic precursor cells, also referred to as muscle satellite cells, normally reside between basal lamina and sarcolemma of muscle fibers in a quiescent state. During muscle regeneration, satellite cells become activated and grow to become myoblasts. Myoblasts migrate to contact and fuse with each other or with existing myofibers to form myotubes. Myotubes further grow and differentiate into mature new muscle fibers (8, 9). Angiogenesis to reestablish perfusion to injured muscle is also important to muscle regeneration (8).
Although lack of an adequate inflammatory response contributes to poor muscle regeneration, the mechanisms by which CCR2 deficiency leads to a defective inflammatory response and poor muscle regeneration are not fully understood. It has been shown that wild-type bone marrow-derived cells restored muscle inflammation and improved regeneration in Ccr2−/− mice (3). However, which cells are recruited from bone marrow through CCR2 signaling and what functions of these cells are important to muscle regeneration have not been clearly addressed. Previous studies (3, 10, 11) also suggested that CCR2 might be expressed on myogenic cells and capillary endothelial cells to directly affect muscle regeneration and angiogenesis. However, the CCR2 protein expression studies in mice have been technically difficult and unreliable due to lack of validated antibodies for immunostaining and commercial antibodies for flow cytometry. It thus remains unclear whether CCR2 protein is expressed on myogenic cells or endothelial cells in vivo. CCR2 plays a significant pathogenic role in several chronic inflammatory diseases, including multiple sclerosis (12, 13), atherosclerosis (14), and rheumatoid arthritis (15). CCR2 signaling is regarded as a salient target for treating inflammatory disorders (16, 17), and CCR2 antagonists have been under active preclinical and clinical development (16–18). Poor muscle injury repair might be a significant side effect of this line of therapies. Therefore, it is important to understand the mechanisms by which CCR2 deficiency leads to poor muscle regeneration, so that we can develop a targeted approach to prevent or control this muscle side effect.
In the present study, we identified the subpopulation of inflammatory cells recruited via CCR2 signaling in response to acute muscle injury and characterized their functional properties with respect to muscle regeneration. We also used a novel CCR2-red fluorescent protein knock-in mouse model (Ccr2RFP/+; ref. 19) to address whether CCR2 was expressed by myogenic cells or endothelial cells to directly affect muscle regeneration or angiogenesis during acute muscle injury repair.
MATERIALS AND METHODS
Animals
C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Ccr2−/− mice were derived and crossed into C57BL/6J background as described previously (20, 21). In Ccr2RFP/+ mice, the RFP cDNA was inserted into the Ccr2 locus to replace the first 279 bp of the amino terminus of the Ccr2 open reading frame, so that the expression of RPF was driven by the Ccr2 gene promoter. The RFP gene construct included a poly-A termination signal to exclude the remainder of the Ccr2 sequence from the mature transcript, so the expression of Ccr2 on the same allele was disrupted by the RFP insertion (19). These mice were extensively backcrossed to C57BL/6J background, and biallelic expression of CCR2 and RFP was confirmed by flow cytometry (19). We followed the Guide for the Care and Use of Laboratory Animals of Cleveland Clinic, and the study was approved by the Cleveland Clinic Institutional Animal Care and Use Committee.
Acute muscle injury, peritonitis, and IGF-I treatment
To induce acute skeletal muscle injury, 100 μl barium chloride (BaCl2; 1%) was injected into the right quadriceps muscle of each mouse (age 8–10 wk). To induce acute sterile peritonitis, each mouse received an intraperitoneal injection of 2 ml 4% thioglycollate. For IGF-I treatment, each mouse received an injection of IGF-I (0.5 μg/100 μl/muscle) or PBS into the right quadriceps muscle 3 d after the BaCl2 injection.
Histopathological analysis
Mice were killed at d 1, 3, 5, 7, 14, 21, and 42 after BaCl2 injections. Quadriceps muscles were collected and fresh frozen in liquid nitrogen-cooled isopentane, sectioned at 8 μm, stained with hematoxylin and eosin, and viewed under a bright-field microscope at ×20.
Immunostaining
Immunostaining was performed using the protocols previously described (22, 23). Briefly, frozen sections were blocked in 5% serum for 2 h, followed by overnight incubation in primary antibodies, including rat-anti-mouse CD31 (1:500; BD Biosciences, San Jose, CA, USA), rat-anti-mouse NCAM (1:200; Millipore, Bedford, MA, USA), or rabbit-anti-mouse RFP (1:50; Abcam, Cambridge, MA, USA). The sections were then incubated with secondary antibodies for 1 h, followed by incubation with ABC for 30 min. Antibody binding was visualized with DAB substrate (Vector Laboratories, Burlingame, CA, USA) and viewed under a light microscope. For fluorescent staining, following the primary antibody incubation, the sections were incubated with fluorescence-conjugated secondary antibodies (Invitrogen, Carlsbad, CA, USA) for 2 h. Antibody binding was visualized under a fluorescent microscope.
Quantification of muscle regeneration and capillary densities
Muscle regeneration was quantified by measuring muscle volume, percentage of regenerative area, and muscle fiber cross-sectional area (CSA) in muscle 21 and 42 d after BaCl2 injections using the method previously described (1). Briefly, for muscle volume measurements, the length (L), width (W), and height (H) of each quadriceps muscle were measured. The volume of each quadriceps muscle was calculated using the formula π/6 × (L×W×H). For regenerative area and muscle fiber CSA measurements, 5 sections from each specimen separated by ≥50 μm were stained with hematoxylin-eosin, and 5 nonoverlapping areas of each section were digitally captured. These images were analyzed using ImageJ software (http://rsbweb.nih.gov/ij/) to measure the total area, area of regeneration, and CSA of regenerated muscle fibers.
Intramuscular capillary density was quantified in muscle 21 d after BaCl2 injections using the methods previously described (24). Briefly, 5 sections from each specimen separated by ≥50 μm were immunostained with CD31 antibody, and 5 nonoverlapping areas of each section were digitally captured. CD31+ cells were counted, and the capillary density was expressed as the number of CD31+ cells per section area.
Flow cytometry and macrophage isolation from inflamed muscle and peritoneum
Flow cytomety was performed using muscle single-cell suspension, blood, and bone marrow. Muscle single-cell suspension was prepared as described previously (25). Briefly, muscles were removed from mice, minced in 10 ml of collagenase solution (0.5 mg/ml type IA collagenase and 0.5 mg/ml type IV collagenase; Invitrogen), and incubated at 37°C for 90 min. The top liquid layers were filtered through a 70-μm basket, brought up to 40 ml with Dulbecco's PBS, and centrifuged at 670 g for 10 min. The pallets were resuspended in 15 ml of Dulbecco's PBS, filtered through a 40-μm cell strainer twice, layered on 15 ml of Lympholyte-M (Cedarlane, Burlington, NC, USA), and centrifuged at 2095 g for 45 min. Cells at the interface were collected, centrifuged at 965 g for 10 min, and resuspended in PBS. The percentage of cells from each population was determined by flow cytometry, and the absolute number of each cell population was calculated by using the percentage number multiplied by the isolated cells per gram of tissue. Blood was collected from heart, lysed using red blood cell lysis buffer (Biolegend, San Diego, CA, USA), centrifuged at 350 g, and resuspended in FACS buffer (PBS containing 1% BSA and 0.1% NaF). The absolute number of each cell population in blood was calculated by using the percentage number multiplied by the number of cells per milliliter of blood. Bone marrow was harvested from the femurs, centrifuged at 350 g, and resuspended in FACS buffer. The absolute number of each cell population in bone marrow was calculated using the percentage number multiplied by the isolated cells per femur. Flow cytometry was carried out using the following antibodies; APC-F4/80 (1:200; Biolegend,), FITC-Ly-6G (1:1000; Biolegend), PerCP-Cy5.5-CD3 (1:100; eBioscience, San Diego, CA, USA), APC-Cy7-CD19 (1:200; Biolegend), PE-7/4 (1:10; Serotec, Raleigh, NC, USA), PE-Cy7-Ly-6C (1:200; Biolegend), and the corresponding isotype control IgGs. All analyses were done on a LSR II (BD Biosciences) using Flowjo 8.2.6 (Tree Star, Inc., Ashland, OR, USA). We used 2 definitions for MOs/MPs: 7/4+ Ly-6G− cells (26) and F4/80+ cells (27). As bone marrow MO/MP precursor cells do not express high levels of F4/80, we used 7/4+ Ly-6G− to indicate MOs/MPs in bone marrow or blood. As MOs/MPs do not universally express 7/4, we also used F4/80 as a marker for MOs/MPs when studying blood and muscle tissue. We used Ly-6G+ as a marker for neutrophils (27), CD3+ for T cells, and CD19+ for B cells. We also analyzed F4/80+ Ly-6C+ and F4/80+ Ly-6C− cells to study 2 subsets of MOs/MPs (28, 29).
For tissue macrophage isolation, single-cell suspensions of muscle 3 d after intramuscular injections of BaCl2 or cells extracted from peritoneal exudates in mice 3 d after intraperitoneal injections of thioglycollate were subjected to sorting of F4/80+ Ly-6C+ and F4/80+ Ly-6C− cells using a FACSAria cell sorter (BD Biosciences).
Adoptive transfer
Bone marrow cells were harvested from the femur of Ccr2RFP/+ and Ccr2RFP/RFP mice. Red blood cells were lysed, and the remaining cells were washed with PBS, resuspended in RPMI 1640, and injected intravenously into wild-type recipient mice. Each mouse received 3.0 × 107 cells at the time of receiving BaCl2 injection.
Quantitative RT-PCR
Quantitative RT-PCR was performed using the protocol previously described (30). Briefly, muscle tissues or sorted cells were lysed using Trizol (Gibco-BRL, Rockville, MD, USA), and total RNA was extracted and treated with RNase-free DNase I (Roche Diagnostics). The RNA samples were then used for reverse transcription using the SuperScript TM II RT kit (Invitrogen) and the protocol provided by the manufacturer. Quantitative PCR was performed using the ABI Prism 7500 sequence detector (Applied Biosystems, Inc., Foster City, CA, USA) and the following primers: IGF-I: forward, 5′-CTACAAAAGCAGCCCGCTCT-3′ and reverse, 5′-CTTCTGAGTCTTGGGCATGTCA-3′; hepatocyte growth factor (HGF): forward, 5′-AAGAGTGGCATCAAGTGCCAG-3′ and reverse, 5′-CTGGATTGCTTGTGAAACACC-3′; fibroblast growth factor 2 (FGF-2): forward, 5′-CCCACCAGGCCACTTCAA-3′ and reverse, 5′-GATGGATGCGCAGGAAGAA-3′; platelet derived growth factor-BB (PDGF-BB): forward, 5′-TGTTCCAGATCTCGCGGAAC-3′ and reverse, 5′-GCGGCCACACCAGGAAG-3′; leukemia inhibitory factor (LIF): forward, 5′-AATGCCACCTGTGCCATACG-3′ and reverse, 5′-CAACTTGGTCTTCTCTGTCCCG-3′; and GAPDH: forward, 5′-CATGGCCTTCCGTGTTCCTA-3′ and reverse, 5′-ATGCCTGCTTCACCACCTTCT-3′. Mouse GAPDH was used as an internal control. Reaction specificity was determined by product melting curves. The PCR products were verified by running 3% agarose gels.
ELISA
Murine IGF-I ELISA was performed using the kit purchased from R&D Systems (MG100; R&D Systems, Minneapolis, MN, USA) and the protocol provided by the manufacturer.
Statistical analyses
SPSS11.5 software (SPSS, Inc. Chicago, IL, USA) was used for statistical analyses. Results are expressed as means ± sd. Differences in various parameters among or between different experimental groups were evaluated by ANOVA analysis with least significant difference and Student-Newman-Keul's as post hoc tests or by Student's t test after testing for normality. Values of P < 0.05 were considered significant.
RESULTS
Ccr2−/− mice showed markedly reduced muscle inflammation, poor phagocytosis, and impaired muscle regeneration after acute injury induced by BaCl2
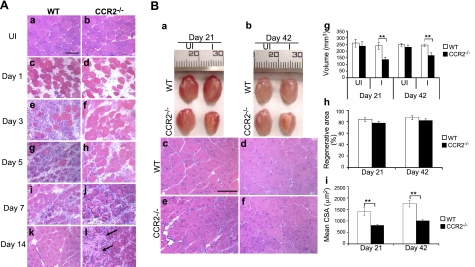
In wild-type mice (Fig. 1A), 1 d after BaCl2 injection, most of the muscle fibers were necrotic, and the muscle tissue was edematous. Robust inflammatory cell infiltrates along with necrotic fibers were noted at d 3, and many mononucleated and polynucleated myoblasts/myotubes were seen in injured areas at d 5. By d 7, necrotic fibers were largely cleared and replaced by central-nucleated regenerating fibers with few scattered endomysial inflammatory cells. By d 14, inflammation was resolved, and regenerated fibers were predominant in the injured areas. At d 21 and 42 (Fig. 1B), regenerated fibers became more mature, and the injured areas recovered their normal architecture. In summary, muscle inflammation peaked at d 3, and muscle regeneration was largely completed by d 21. After BaCl2 injection, Ccr2−/− mice exhibited minimal inflammatory cell infiltrates at d 3 and 5 (Fig. 1A). Modest inflammation was seen at d 7, and there were still scattered inflammatory cells at d 14. Abundant necrotic fibers surrounded by activated satellite cells and small central-nucleated myoblasts remained at d 7. Small necrotic areas were still seen and the regenerated fibers were small at d 14. At d 21 and 42 (Fig. 1B), although the injured areas were filled with central-nucleated regenerated fibers, the size of these fibers was significantly smaller than those in wild-type mice, and scattered fat accumulation was noted as well. Muscle regeneration as quantified by the volume of injured muscle (mm3) after regeneration (d 21: 137.2±18.8 in Ccr2−/− mice, 239.1±23.8 in wild-type mice, P<0.01; d 42: 168.4±20.9 in Ccr2−/− mice, 244.6 ± 20.9 mm3 in wild-type mice, P<0.01) and mean CSA (um2) of regenerated muscle fibers (d 21: 809.3±44.6 in Ccr2−/− mice, 1412.9±166.2 in wild-type mice, P<0.01; d 42: 1016.3±57.4 in Ccr2−/− mice, 1763.6±142.7 in wild-type mice, P<0.01) was poor in Ccr2−/− mice as compared with wild-type controls (Fig. 1B).
Figure 1.
Ccr2−/− mice showed markedly reduced muscle inflammation, delayed phagocytosis, and impaired muscle regeneration after acute injury induced by BaCl2. A) Hematoxylin-eosin staining showed myofiber necrosis and tissue edema in injured muscle at d 1 of both wild-type (c) and Ccr2−/− mice (d). While robust inflammatory infiltrates were seen at d 3 in injured muscle of wild-type mice (e), very few inflammatory cells were seen in Ccr2−/− mice (f). Necrotic fibers were gradually replaced by mononucleated and multinucleated myoblasts and myotubes at d 5 (g) and 7 (i) in wild-type mice; they remained in Ccr2−/− mice with scattered endomysial inflammation (h, j). While the injured areas were filled with central-nucleated regenerated myofibers with no necrotic fibers or inflammatory cells seen in wild-type mice at d 14 (k), scattered necrotic fibers (arrows) and inflammatory cells were still present in Ccr2−/− mice along with smaller regenerated fibers (l). B) At d 21 and 42, the injured muscle (a, b) and regenerated muscle fibers (c-f) were smaller in Ccr2−/− mice (a, b, e, f) than in wild-type mice (a–d). Quantitative analyses showed that the volume of injured muscle (g) and the mean CSA of regenerated fibers (i) were significantly reduced in Ccr2−/− mice as compared with wild-type controls. There was no difference in the percentage of regenerative area (h). n = 5–7 mice/group/time point. WT, wild-type; UI, uninjured; I, injured. Scale bars = 50 μm. **P < 0.01.
CCR2 was not expressed on myogenic cells in injured muscle
CCR2 mRNA was detected in myoblasts (11) in vitro and in human muscle cells (31). However, CCR2 mRNA expression does not uniformly correlate with CCR2 protein expression (unpublished results), and it has been difficult to localize murine CCR2 protein due to lack of validated antibodies for CCR2 immunostaining. To circumvent this difficulty, Ccr2RFP/+ mice were recently generated and characterized, enabling us to address whether the CCR2 protein was expressed in myogenic cells in vivo.
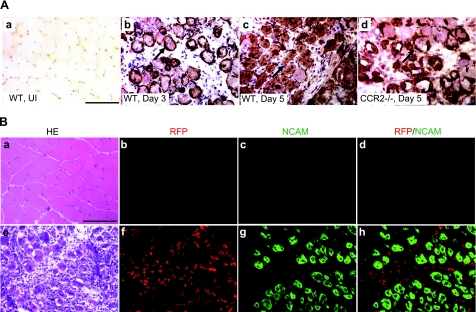
We evaluated the distribution of NCAM immunoreactivity to characterize myogenic elements in tissues from wild-type and Ccr2−/− mice after BaCl2 injection (Fig. 2A). NCAM, which marks activated satellite cells, myoblasts, and myotubes, was not detected in uninjured muscle. NCAM immunostaining highlighted activated satellite cells surrounding necrotic fibers at d 3 in wild-type mice. At d 5, some necrotic fibers were cleared and replaced by NCAM+ mononucleated myoblasts and polynucleated myotubes. In Ccr2−/− mice at d 5, however, the majority of necrotic fibers still remained, which were surrounded by activated satellite cells and small mononucleated myoblasts. The findings indicated that the necrotic fiber phagocytosis and early muscle regeneration were impaired in Ccr2−/− mice. In Ccr2 RFP/+ mice at d 5, the pattern of NCAM immunostaining was equivalent to wild-type mice. Double immunostaining of RFP and NCAM in injured muscle showed no detectable RFP+/NCAM+ cells to indicate expression of CCR2 on myogenic cells (Fig. 2B).
Figure 2.
CCR2 was not expressed on myogenic cells in injured muscle.. A) Immunostaining of NCAM showed no positive staining in normal muscle (a). In injured muscle of wild-type mice at d 3, satellite cells were activated expressing NCAM (b, arrow). At d 5, many NCAM+ myoblasts and multinucleated myotubes were also seen (c, arrow). In Ccr2−/− mice at d 5, only activated satellite cells and small myoblasts were seen surrounding necrotic fibers (d, arrow). B) NCAM/RFP double immunostaining of injured muscle of Ccr2RFP/+ mice at d 5 showed RFP+ inflammatory cells (f) and NCAM membrane staining of myogenic cells (g). No RFP+/NCAM+ cells were detected (h) to indicate expression of CCR2 on myogenic cells. RFP or NCAM was not detected in uninjured muscle of Ccr2RPP/+ mice (b–d). Adjacent sections of uninjured muscle (a) and injured muscle at d 5 (e) were stained with hematoxylin-eosin (HE). n = 3 mice/group. Scale bars = 50 μm.
CCR2 was not expressed on capillary endothelial cells in injured muscle
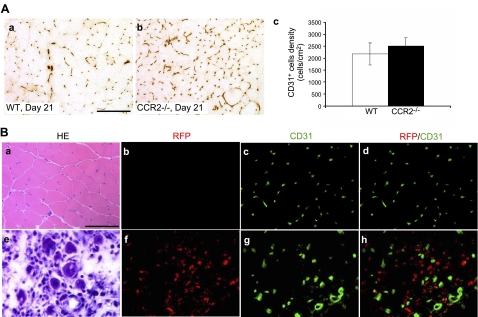
Previous studies (10) suggested that CCR2 might be expressed by vascular endothelial cells and that CCR2 deficiency might directly influence angiogenesis during acute muscle injury repair. We used CD31 immunostaining to evaluate postinjury capillary density and performed RFP/CD31 double immunostaining in Ccr2RFP/+ mice to address whether CCR2 was expressed on capillary endothelial cells. The capillary density did not differ significantly in wild-type and Ccr2−/− mice at d 21 (Fig. 3A), so angiogenesis was not significantly affected in Ccr2−/− mice, consistent with the findings by others (1, 32). No RFP+/CD31+ cells were detected in injured muscle of Ccr2RFP/+ mice to indicate expression of CCR2 on capillary endothelial cells (Fig. 3B).
Figure 3.
CCR2 was not expressed on capillary endothelial cells in injured muscle.. A) CD31+ cell densities were not significantly different in wild-type (a, c) and Ccr2−/− mice (b, c) at d 21. B) RFP/CD31 double immunostaining of uninjured (b–d) and injured (f–h) muscle of Ccr2RFP/+ mice showed that no RFP+/CD31+ cells were detected in uninjured (d) or injured muscle (h) at d 5 to indicate expression of CCR2 on capillary endothelial cells. Adjacent sections of uninjured muscle (a) and injured muscle at d 5 (e) were stained with hematoxylin-eosin (HE). Bar = 50 μm; n = 3 mice/group.
CCR2 deficiency was associated with impaired MO/MP recruitment following acute muscle injury
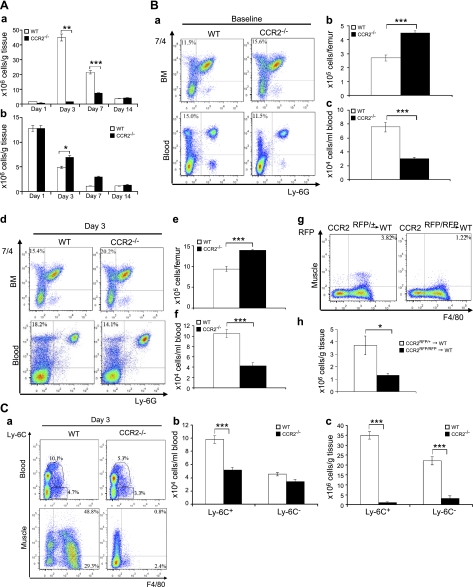
Since CCR2 was not expressed on myogenic cells or capillary endothelial cells to directly affect muscle regeneration or angiogenesis, it regulated muscle regeneration primarily by altering muscle inflammation. We first addressed which subpopulations of inflammatory cells were affected by CCR2 deficiency. We measured the numbers of intramuscular macrophages, neutrophils, T cells, and B cells in injured muscle by flow cytometry (Fig. 4A). In wild-type mice, the numbers of macrophages were remarkably elevated at d 3, to a lesser degree at d 7, and almost returned to baseline at d 14. The accumulation of intramuscular macrophages was diminished in Ccr2−/− mice with a small peak at d 7 rather than a large peak at d 3. The numbers of neutrophils in injured muscle of Ccr2−/− mice increased modestly but significantly only at d 3, as compared with wild-type controls. There was no significant difference in the numbers of intramuscular T cells or B cells between Ccr2−/− mice and wild-type mice (data not shown). Therefore, CCR2 deficiency primarily limited muscle recruitment of MOs/MPs in response to acute injury.
Figure 4.
CCR2 deficiency blocked MO/MP recruitment into injured muscle.. A) Flow cytometry showed a markedly increased number of intramuscular macrophages in wild-type mice at d 3 and 7, with the peak at d 3 (a). The number of intramuscular macrophages in Ccr2−/− mice was also increased after injury (a) but was significantly reduced at d 3 and 7 as compared with wild-type mice. The number of neutrophils in Ccr2−/− mice was increased at d 3 as compared with wild-type mice (b). B) Flow cytometry at baseline showed that the percentage of 7/4+Ly-6G− MOs/MPs (a) was increased in bone marrow but reduced in blood in Ccr2−/− mice as compared with wild-type controls. At d 3 postinjury, the percentage of 7/4+Ly-6G− MOs/MPs (d) was also increased in bone marrow and reduced in blood in Ccr2−/− mice. The numbers of 7/4+Ly-6G− MOs/MPs per femur were significantly higher in bone marrow (BM; b, e) but lower in blood (c, f) at baseline (b, c) and d 3 (e, f) in Ccr2−/− mice than those in wild-type mice. Bone marrow monocyte transfer showed that the number of Ccr2RFP/+ monocytes recruited into wild-type injured muscle at d 3 was significantly higher than that of Ccr2RFP/RFP monocytes (lacking CCR2) (g, h). C) Flow cytometry at d 3 showed that the numbers of F4/80+ MOs/MPs were reduced in blood and in injured muscle of Ccr2−/− mice (a), among which the Ly-6C+ but not the Ly-6C− subset was significantly reduced in blood (b). However, both Ly-6C+ and Ly-6C− subsets were significantly reduced in injured muscle of Ccr2−/− mice (c). n = 5–7 mice/group/experiment. *P < 0.05; **P < 0.01; ***P < 0.001.
Recruitment of MOs/MPs to injured tissues consists of mobilization of these cells from bone marrow to blood and further from blood to injured tissues. There are 2 main subsets of MOs/MPs in blood based on the expression of Ly-6C: Ly-6C+ cells, which infiltrate injured tissues, and Ly-6C− cells, which mainly contribute to tissue-resident macrophages (28, 29). We addressed at which level the recruitment of MOs/MPs was blocked and which subset of MOs/MPs was mainly affected in Ccr2−/− mice during acute muscle injury repair. To this end, we performed flow cytometry using bone marrow, blood, and single-cell suspensions of injured muscle 3 d after BaCl2 injections, which corresponded to the peak of inflammation in wild-type mice.
To address at which level the mobilization of MOs/MPs was blocked, we used 7/4+Ly-6G− to identify MOs/MPs (26). At baseline and d 3 postinjury, 7/4+Ly-6G− cells were increased in bone marrow and reduced in blood in Ccr2−/− mice as compared with wild-type controls (Fig. 4B). The findings indicted that CCR2 deficiency impaired the mobilization of 7/4+Ly-6G− MOs/MPs from bone marrow to blood at baseline and in response to acute muscle injury.
To examine monocyte recruitment from blood to injured muscle tissue, we intravenously injected Ccr2RFP/+ or Ccr2RFP/RFP bone marrow cells into wild-type mice on injury and monitored their accumulation in injured muscle at d 3. Muscle recruitment of circulating MOs/MPs from Ccr2RFP/RFP mice (lacking CCR2) was impaired [(1.3±0.2)×106 cells/g tissue] as compared with those from Ccr2RFP/+ mice [(3.7±0.7)×106 cells/g tissue; P<0.05; Fig. 4B].
We further addressed which subset of MOs/MPs was primarily affected by CCR2 deficiency (Fig. 4C). At d 3, F4/80+ MOs/MPs were significantly reduced in blood and in injured muscle of Ccr2−/− mice, among which the Ly-6C+ but not the Ly-6C− subset was significantly reduced in blood. However, both Ly-6C+ and Ly-6C− macrophages were significantly reduced in injured muscle of Ccr2−/− mice.
Macrophages expressed a high level of IGF-I in injured muscle
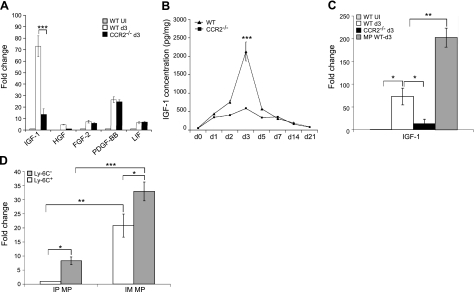
Since macrophage infiltration in injured muscle is crucial to muscle regeneration, we addressed whether intramuscular macrophages played a role in muscle regeneration beyond phagocytosis. Macrophages produce growth factors to promote myogenic cell growth and differentiation (5–7). IGF-I, HGF, FGF-2, PDGF-BB, and LIF have been reported to influence myogenesis (33–37). We first determined whether the mRNA expression of these growth factors was increased in injured muscle as compared with uninjured muscle (Fig. 5A). Quantitative RT-PCR showed that the expression of all 5 growth factors was up-regulated in injured muscle. The level of expression of FGF-2, PDGF-BB, and LIF was not different between wild-type and Ccr2−/− mice. However, the expression of IGF-I was significantly higher in wild-type than in Ccr2−/− mice, suggesting that its deficiency might partially account for the poor regeneration seen in Ccr2−/− mice. The mRNA expression of HGF was undetectable at baseline but was detected and increased in injured muscle of wild-type mice as compared with those of Ccr2−/− mice. However, the overall expression level of HGF was low in both types of muscle, suggesting a minor role for this growth factor. ELISA for IGF-I (Fig. 5B) showed up-regulation at d 1 in both wild-type and Ccr2−/− mice, with peak levels at d 3, being remarkably higher in wild-type than in Ccr2−/− mice. IGF-I protein level gradually came down and was mildly elevated at d 21 as compared with uninjured muscle. The surge of IGF-I protein levels appeared temporally correlated with macrophage infiltration in wild-type mice.
Figure 5.
Macrophages (MPs) expressed a high level of IGF-I in injured muscle. A) Quantitative RT-PCR showed that the IGF-I mRNA expression was significantly higher in injured muscle of wild-type mice than in those of Ccr2−/− mice at d 3. B) ELISA analysis showed that the level of IGF-I was increased in injured muscle of both wild-type and Ccr2−/− mice, with the peak at d 3, at which point the IGF-I level was remarkably higher in wild-type mice than in Ccr2−/− mice. C) Quantitative RT-PCR showed that the MPs from injured muscle of wild-type mice at d 3 expressed a remarkably higher level of IGF-I mRNA than the injured muscle itself from wild-type or Ccr2−/− mice. D) Quantitative RT-PCR showed that both Ly-6C+ and Ly-6C− intramuscular MPs (IM MP) expressed IGF-I mRNA, the level being significantly higher in the Ly-6C− subset than in the Ly-6C+ subset. Although the intraperitoneal MPs (IP MP) also expressed IGF-I mRNA, the level was much lower than that in the IM MPs. n = 5–7 mice/group/experiment. Fold change refers to the comparison to wild-type uninjured (UI) muscle (A, C) and to Ly-6C+ IP MPs (D). *P < 0.05; ** P < 0.01; ***P < 0.001.
Since the major difference in injured muscle at d 3 between Ccr2−/− mice and wild-type mice was macrophage infiltration and the protein expression of IGF-I correlated with macrophage infiltration in wild-type mice, we addressed whether macrophages expressed a high level of IGF-I. We sorted out F4/80+ intramuscular macrophages and found that the macrophages from injured muscle of wild-type mice expressed a much higher level of IGF-I mRNA than the injured muscle themselves from wild-type or Ccr2−/− mice (Fig. 5C). The finding indicates that the intramuscular macrophage in injured muscle is the major cellular source of IGF-I.
We then addressed which subset of intramuscular macrophages expressed a high level of IGF-I mRNA. Both Ly-6C+ and Ly-6C− subsets expressed IGF-I mRNA, the level being significantly higher in the Ly-6C− subset than in the Ly-6C+ subset (Fig. 5D). We further addressed whether a high level of IGF-I expression by macrophages within inflamed tissues is relatively specific to skeletal muscle. We compared the expression of IGF-I mRNA between intramuscular macrophages collected during muscle inflammation and intraperitoneal macrophages collected during acute sterile peritonitis. We found that although intraperitoneal macrophages also expressed IGF-I mRNA, the level was much lower than that in intramuscular macrophages (Fig. 5D), suggesting that IGF-I produced by intramuscular macrophages might play an important and relatively unique role in the skeletal muscle injury repair.
Local IGF-I replacement improved muscle injury repair in Ccr2−/− mice
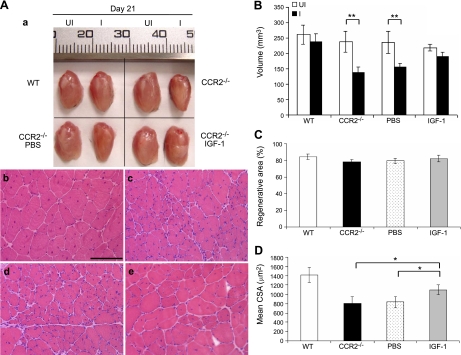
Given the high level of IGF-I expression by intramuscular macrophages during acute muscle injury repair and the importance of IGF-I in myogenesis, we further addressed whether IGF-I replacement could restore muscle regeneration in Ccr2−/− mice (Fig. 6). A single injection of IGF-I (0.5 μg/muscle), but not PBS, into injured muscle at d 3 remarkably improved muscle regeneration in Ccr2−/− mice, as evidenced by increased volume of regenerated quadriceps and increased mean CSA of regenerated muscle fibers (untreated: 809.3±44.6 μm2; PBS treated: 833.2±49.8 μm2; IGF-I treated:1098.8±102.9 μm2; P<0.05) at d 21. The dose of IGF-I was determined based on the IGF-I protein level in injured muscle of wild-type mice at d 3, as measured by ELISA (Fig. 5B).
Figure 6.
Local IGF-I treatment improved muscle regeneration in Ccr2−/− mice. At d 21, while the injured quadriceps muscle of wild-type mice regenerated to reach the size of contralateral uninjured controls, the size (volume) of injured quadriceps in Ccr2−/− mice was significantly smaller than the contralateral uninjured controls (Aa, B). Regenerated muscle fibers were also smaller in Ccr2−/− mice (Ac) than in wild-type mice (Ab). Intramuscular injection of IGF-I (Aa, e; B; D), but not PBS (Aa, d; B; D), improved muscle regeneration with increased muscle volume and muscle fiber size. There was no difference in the percentage of regenerative area in these muscles (C). n = 5 mice/group/experiment. Scale bar = 50 μm. *P < 0.05; **P < 0.01.
DISCUSSION
CCR2 is essential to acute skeletal muscle injury repair, as Ccr2−/− mice displayed markedly reduced muscle inflammation and poor muscle regeneration. In the present study, we demonstrate that CCR2 exerts its regulatory function primarily by recruiting Ly-6C+ MOs/MPs to injured muscle to conduct phagocytosis, contribute to the accumulation of intramuscular Ly-6C− macrophages, and produce a high level of IGF-I to promote muscle regeneration (Table 1). CCR2 is not expressed by myogenic cells or muscle capillary endothelia cells to directly affect muscle regeneration or angiogenesis.
Table 1.
Differences between wild-type and Ccr2−/− mice during acute muscle injury repair
| Genotype | Inflammation | Phagocytosis | Regeneration | IGF-1 production |
|---|---|---|---|---|
| Wild type | Large peak at d 3 | Efficient at d 5 and 7 | Active at d 5 and 7; regenerated well by d 21 and 42 | Large peak at d 3 |
| Ccr2−/− | Small peak at d 7; ↓Ly-6C+ MOs/MPs in PB and IM; ↓Ly-6C− MOs/MPs in IM | Impaired at d5 and d7 | Impaired at d 5 and 7; small regenerated fibers by d 21 and 42 | Small peak at d 7 |
PB, peripheral blood; IM, injured muscle.
MOs/MPs are the predominant inflammatory cells infiltrating acutely injured skeletal muscle. Ccr2−/− mice showed markedly reduced macrophages in injured muscle (1–3, 38). The present study further showed that on acute muscle injury, CCR2 deficiency blocked mobilization of MOs/MPs but not T cells, B cells, or neutrophils, from bone marrow to blood and from blood to injured muscle. The number of Ly-6C+ but not Ly-6C− MOs/MPs were significantly reduced in blood, indicating that CCR2 deficiency affected the ability of bone marrow precursor cells of this subset to enter blood. These findings are consistent with the previous reports (28) that blood monocytes mainly consist of 2 subsets, CX3CR1loCCR2+Ly-6C+ and CX3CR1hiCCR2−Ly-6C− cells. CX3CR1loCCR2+Ly-6C+ cells are inflammatory monocytes that enter tissues in response to injury or infection and differentiate into inflammatory macrophages or dendritic cells within inflamed tissues (28, 29). CX3CR1hiCCR2−Ly-6C− monocytes patrol the vascular space and contribute to resident macrophages (28, 39). The preferential block of Ly-6C+ MO/MP recruitment in Ccr2−/− mice was also reported in the study of other tissue inflammation, including atherosclerosis and acute sterile peritonitis (26). Our transfer studies further showed that recruitment of CCR2-deficient MOs/MPs from blood to injured muscle was also impaired. As a result, Ly-6C+ macrophages were diminished in injured muscle of Ccr2−/− mice. However, the number of Ly-6C− macrophages was also significantly reduced as compared with wild-type mice, suggesting either that the recruitment of both subsets from blood to injured muscle might be suppressed by CCR2 deficiency or that initial accumulation of Ly-6C+ cells was followed by their differentiation into Ly-6C− phenotype. It also remained possible that Ly-6C+ MOs/MPs facilitated recruitment of Ly-6C− cells. These findings may be clarified by a recent report (2) of notexin-induced acute muscle injury that showed that injured muscle recruited only Ly-6C+ but not Ly-6C− MOs/MPs. Phagocytosis of necrotic muscle fibers by Ly-6C+ macrophages could switch the phenotype of these cells into Ly-6C− macrophages. Therefore, the reduced number of Ly-6C− macrophages in injured muscle of Ccr2−/− mice is likely due, at least in part, to the impairment of muscle recruitment of Ly-6C+ MOs/MPs, which we suggest is the primary defect in Ccr2−/− mice.
Although CCR2-mediated inflammation plays a significant pathogenic role in chronic inflammatory diseases, it is clearly beneficial and essential to muscle regeneration during acute muscle injury repair. The findings support the concept that MOs/MPs are phenotypically heterogeneous and can be influenced by tissue environment (40). MOs/MPs are essential to acute skeletal muscle injury repair (41–43).
Phagocytosis is an important function of macrophages. In Ccr2−/− mice, phagocytosis of necrotic fibers is greatly impaired. Necrotic fibers may act as a physical barrier to prevent myoblasts from efficiently contacting and fusing with each other. Whether necrotic fibers also produce toxic factors to suppress myoblast growth and fusion remains unknown. It is unlikely that increased neutrophil accumulation in the injured muscle of Ccr2−/− mice represents a major block to regeneration given their modest numbers and transient presence, only at d 3. Increased neutrophil accumulation was probably due both to increased neutrophil recruitment in Ccr2−/− mice and reduced neutrophil phagocytosis by intramuscular macrophages.
Although phagocytosis represents an important function of infiltrating macrophages during acute muscle injury repair, it is not the only positive role played by these cells in muscle regeneration. In acute muscle injury induced by notexin, the size of regenerated muscle fibers was reduced by depletion of intramuscular macrophages after phagocytosis was complete (2), indicating that macrophages could exert functions to promote muscle fiber regeneration independent of phagocytosis. The finding supports the notion that macrophages may play multiple constructive roles in muscle regeneration, including the production of muscle trophic factors to promote regeneration (5, 6), chemotactic factors to attract myogenic cells to injured areas (7), and anti-inflammatory cytokines to resolve inflammation (2).
We evaluated 5 growth factors that have been reported to affect myogenic cell growth and differentiation, including IGF-I, HGF, FGF-2, PDGF-BB, and LIF (33–37). IGF-I became the focus as its mRNA and protein expressions were markedly up-regulated in injured muscle, which correlated with macrophage infiltration. IGF-I, an anabolic growth factor that exerts its function by increasing protein synthesis and decreasing protein degradation via activation of the Akt pathway, stimulated myoblast proliferation and differentiation in vitro (44). IGF-I deficient mice died immediately after birth with multiple tissue defects, including skeletal muscle atrophy (45, 46). Transgenic mice overexpressing IGF-I in skeletal muscle displayed marked muscle hypertrophy and sustained hypertrophy and regeneration in senescent muscle (47). IGF-I mRNA was markedly increased in regenerating muscle following various injuries (48–50). Transgenic expression of IGF-I in skeletal muscle accelerated the recovery of muscle regeneration after cardiotoxin injury (51). These findings demonstrate that IGF-I is a key regulator of skeletal muscle growth and size during development and regeneration (37).
The link between macrophages and IGF-I has been implicated by several studies. Following muscle unloading and reloading, IGF-I mRNA correlated with macrophage accumulation (49), whereas macrophage depletion was associated with a major decrease in IGF-I mRNA and impairment of muscle regeneration following freeze injury (42). A study using in vitro myotube/macrophage cocultures showed that macrophages protected against muscle atrophy and that the protection was abolished by anti-IGF-I antibody treatment (41). However, whether IGF-I is expressed by intramuscular infiltrating macrophages in vivo has not been directly addressed. In the present study, our findings provided direct evidence that IGF-I mRNA was strongly expressed by intramuscular macrophages, especially by the Ly-6C− subset. It has been shown by in vitro experiments that Ly-6C+ inflammatory macrophages stimulated myogenic cell proliferation, while anti-inflammatory Ly-6C− macrophages promoted myogenic cell growth and differentiation (2). IGF-I is also known to exert anti-inflammatory functions. Transgenic expression of IGF-I in skeletal muscle accelerated muscle injury repair and also modulated inflammation, as evidenced by significantly reduced inflammatory cytokine and chemokine expression (51). Taken in this context, it is plausible that the high level production of IGF-I contributes to the anti-inflammatory and myogenic functions of intramuscular Ly-6C− macrophages during acute muscle injury repair. Our study also showed that the expression of IGF-I mRNA was significantly higher in macrophages derived from inflamed muscle than in those derived from inflamed peritoneum, which supports an important and relatively tissue-specific role for IGF-I-producing macrophages in acute muscle injury repair. The essential role of IGF-I in skeletal muscle regeneration associated with injury repair is further demonstrated by our finding that a single intramuscular injection of IGF-I in injured muscle at d 3 markedly improved muscle regeneration in Ccr2−/− mice, especially as this time point corresponds to the peak of macrophage infiltration and IGF-I protein production in wild-type mice. The finding also strongly suggests that the early surge of IGF-I from infiltrating macrophages is critical to drive the normal skeletal muscle regeneration program during acute injury repair. The increased IGF-I production correlated with macrophage infiltration in wild-type mice but not in Ccr2−/− mice, as IGF-I production was less than expected at d 7 in Ccr2−/− muscle with a small peak of macrophage accumulation. This could be due to a different muscle environment at d 3 in wild-type mice and at d 7 in Ccr2−/− mice and/or due to the CCR2 deficiency in intramuscular macrophages in Ccr2−/− mice. Whether CCR2 signaling regulates IGF-I production by infiltrating macrophages remains to be determined.
Since satellite cells and endothelial cells are important cellular players in muscle regeneration, it has been questioned whether CCR2 is expressed on these cells in injured muscle to directly affect muscle regeneration and angiogenesis. Studies (38) using commercial antibodies and in situ hybridization suggested that CCR2 was expressed by myogenic cells. However, there are no validated antibodies for localizing CCR2 protein in murine tissues and we have found that the available reagents equally label cells in Ccr2+/+ and Ccr2−/− tissues (unpublished results). To circumvent this technical difficulty, Ccr2RFP/+ mice were generated and characterized and showed that expressions of CCR2 mRNA and RFP mRNA were tightly correlated in all tissues. CCR2 protein invariably correlated with RFP protein and CCR2 and RFP mRNA in monocytes but not in T cells or NK cells, in which RFP and CCR2 mRNA but not CCR2 protein was detected (19). Therefore, CCR2 mRNA does not always correlate with CCR2 protein. In the present study, by using the Ccr2RFP/+ mice, we unequivocally showed that CCR2 was not expressed by activated satellite cells, myoblasts, myotubes, or capillary endothelial cells in injured muscle. The findings indicate that myogenic cells or endothelial cells do not utilize CCR2 signaling to regulate muscle regeneration or angiogenesis during acute skeletal muscle injury repair.
Inflammation is a natural response to acute skeletal muscle injury. Up-regulation of IGF-I expression has been reported in regenerating muscle in different injury models caused by notexin (50), freeze damage (48), mechanical damage (52), and muscle unloading and reloading (49). Blocking muscle inflammation by genetic ablation of Ccr2 or macrophage depletion invariably caused poor muscle regeneration after acute injuries (1–3, 42, 43). Therefore, the essential role of muscle inflammation and IGF-I production by intramuscular macrophages seen in our BaCl2 model may also apply to the other injury models. CCR2 function is highly conserved between mice and humans, and CCR2 signaling may regulate acute skeletal muscle injury repair in a similar fashion in humans. Our finding that local IGF-I treatment significantly improved the muscle regeneration in Ccr2−/− mice is clinically relevant and strongly suggests that IGF-I may represent a useful therapy to promote acute skeletal muscle injury repair and control the potential skeletal muscle regenerative defect in the context of therapeutic CCR2 blockade.
Acknowledgments
This study is supported by U.S. National Institutes of Health grants K08 NS049346 (to L.Z.), MDA#91682 (to L.Z.), and K24 51400 (to R.M.R.). The authors thank Drs. Liping Liu and Georgiana Cheng and the Flow Cytometry Core of the Cleveland Clinic for superb technical support.
REFERENCES
- 1. Contreras-Shannon V., Ochoa O., Reyes-Reyna S. M., Sun D., Michalek J. E., Kuziel W. A., McManus L. M., Shireman P. K. (2007) Fat accumulation with altered inflammation and regeneration in skeletal muscle of CCR2−/− mice following ischemic injury. Am. J. Physiol. Cell Physiol. 292, C953–C967 [DOI] [PubMed] [Google Scholar]
- 2. Arnold L., Henry A., Poron F., Baba-Amer Y., van Rooijen N., Plonquet A., Gherardi R. K., Chazaud B. (2007) Inflammatory monocytes recruited after skeletal muscle injury switch into anti-inflammatory macrophages to support myogenesis. J. Exp. Med. 204, 1057–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sun D., Martinez C. O., Ochoa O., Ruiz-Willhite L., Bonilla J. R., Centonze V. E., Waite L. L., Michalek J. E., McManus L. M., Shireman P. K. (2009) Bone marrow-derived cell regulation of skeletal muscle regeneration. FASEB J. 23, 382–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tidball J. G. (2005) Inflammatory processes in muscle injury and repair. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R345–353 [DOI] [PubMed] [Google Scholar]
- 5. Bischoff R., Heintz C. (1994) Enhancement of skeletal muscle regeneration. Dev. Dyn. 201, 41–54 [DOI] [PubMed] [Google Scholar]
- 6. Cantini M., Giurisato E., Radu C., Tiozzo S., Pampinella F., Senigaglia D., Zaniolo G., Mazzoleni F., Vitiello L. (2002) Macrophage-secreted myogenic factors: a promising tool for greatly enhancing the proliferative capacity of myoblasts in vitro and in vivo. Neurol. Sci. 23, 189–194 [DOI] [PubMed] [Google Scholar]
- 7. Robertson T. A., Maley M. A., Grounds M. D., Papadimitriou J. M. (1993) The role of macrophages in skeletal muscle regeneration with particular reference to chemotaxis. Exp. Cell. Res. 207, 321–331 [DOI] [PubMed] [Google Scholar]
- 8. Carlson B. M., Faulkner J. A. (1983) The regeneration of skeletal muscle fibers following injury: a review. Med. Sci. Sports Exerc. 15, 187–198 [PubMed] [Google Scholar]
- 9. Hawke T. J., Garry D. J. (2001) Myogenic satellite cells: physiology to molecular biology. J. Appl. Physiol. 91, 534–551 [DOI] [PubMed] [Google Scholar]
- 10. Ochoa O., Sun D., Reyes-Reyna S. M., Waite L. L., Michalek J. E., McManus L. M., Shireman P. K. (2007) Delayed angiogenesis and VEGF production in CCR2−/− mice during impaired skeletal muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R651–R661 [DOI] [PubMed] [Google Scholar]
- 11. Yahiaoui L., Gvozdic D., Danialou G., Mack M., Petrof B. J. (2008) CC family chemokines directly regulate myoblast responses to skeletal muscle injury. J. Physiol. 586, 3991–4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fife B. T., Huffnagle G. B., Kuziel W. A., Karpus W. J. (2000) CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J. Exp. Med. 192, 899–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Izikson L., Klein R. S., Charo I. F., Weiner H. L., Luster A. D. (2000) Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J. Exp. Med. 192, 1075–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boring L., Gosling J., Cleary M., Charo I. F. (1998) Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 394, 894–897 [DOI] [PubMed] [Google Scholar]
- 15. Quinones M. P., Ahuja S. K., Jimenez F., Schaefer J., Garavito E., Rao A., Chenaux G., Reddick R. L., Kuziel W. A., Ahuja S. S. (2004) Experimental arthritis in CC chemokine receptor 2-null mice closely mimics severe human rheumatoid arthritis. J. Clin. Invest. 113, 856–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Charo I. F., Ransohoff R. M. (2006) The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 354, 610–621 [DOI] [PubMed] [Google Scholar]
- 17. Proudfoot A. E. (2002) Chemokine receptors: multifaceted therapeutic targets. Nat. Rev. Immunol. 2, 106–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xia M., Sui Z. (2009) Recent developments in CCR2 antagonists. Expert Opin. Ther. Pat. 19, 295–303 [DOI] [PubMed] [Google Scholar]
- 19. Saederup N., Cardona A. E., Croft K., Cotleur A. C., Mizutani M., Tsou T. L., Ransohoff R. M., Charo I. F. (2010) Analysis of CCR2 expression and function in multiple sclerosis by creation of red fluorescent protein knock-in mice. PLoS One In press [Google Scholar]
- 20. Cardona A. E., Sasse M. E., Liu L., Cardona S. M., Mizutani M., Savarin C., Hu T., Ransohoff R. M. (2008) Scavenging roles of chemokine receptors: chemokine receptor deficiency is associated with increased levels of ligand in circulation and tissues. Blood 112, 256–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuziel W. A., Morgan S. J., Dawson T. C., Griffin S., Smithies O., Ley K., Maeda N. (1997) Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc. Natl. Acad. Sci. U. S. A. 94, 12053–12058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang P., Zhao X. S., Fields M., Ransohoff R. M., Zhou L. (2009) Imatinib attenuates skeletal muscle dystrophy in mdx mice. FASEB J. 23, 2539–2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou L., Rafael-Fortney J. A., Huang P., Zhao X. S., Cheng G., Zhou X., Kaminski H. J., Liu L., Ransohoff R. M. (2008) Haploinsufficiency of utrophin gene worsens skeletal muscle inflammation and fibrosis in mdx mice. J. Neurol. Sci. 264, 106–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wagatsuma A. (2007) Endogenous expression of angiogenesis-related factors in response to muscle injury. Mol. Cell. Biochem. 298, 151–159 [DOI] [PubMed] [Google Scholar]
- 25. Vetrone S. A., Montecino-Rodriguez E., Kudryashova E., Kramerova I., Hoffman E. P., Liu S. D., Miceli M. C., Spencer M. J. (2009) Osteopontin promotes fibrosis in dystrophic mouse muscle by modulating immune cell subsets and intramuscular TGF-beta. J. Clin. Invest. 119, 1583–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsou C. L., Peters W., Si Y., Slaymaker S., Aslanian A. M., Weisberg S. P., Mack M., Charo I. F. (2007) Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J. Clin. Invest. 117, 902–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lagasse E., Weissman I. L. (1996) Flow cytometric identification of murine neutrophils and monocytes. J. Immunol. Methods 197, 139–150 [DOI] [PubMed] [Google Scholar]
- 28. Geissmann F., Jung S., Littman D. R. (2003) Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82 [DOI] [PubMed] [Google Scholar]
- 29. Gordon S., Taylor P. R. (2005) Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 [DOI] [PubMed] [Google Scholar]
- 30. Zhou L., Porter J. D., Cheng G., Gong B., Hatala D. A., Merriam A. P., Zhou X., Rafael J. A., Kaminski H. J. (2006) Temporal and spatial mRNA expression patterns of TGF-beta1, 2, 3 and TbetaRI, II, III in skeletal muscles of mdx mice. Neuromuscul. Disord. 16, 32–38 [DOI] [PubMed] [Google Scholar]
- 31. Bartoli C., Civatte M., Pellissier J. F., Figarella-Branger D. (2001) CCR2A and CCR2B, the two isoforms of the monocyte chemoattractant protein-1 receptor are up-regulated and expressed by different cell subsets in idiopathic inflammatory myopathies. Acta. Neuropathol. 102, 385–392 [DOI] [PubMed] [Google Scholar]
- 32. Tang G., Charo D. N., Wang R., Charo I. F., Messina L. (2004) CCR2-/- knockout mice revascularize normally in response to severe hindlimb ischemia. J. Vasc. Surg. 40, 786–795 [DOI] [PubMed] [Google Scholar]
- 33. Husmann I., Soulet L., Gautron J., Martelly I., Barritault D. (1996) Growth factors in skeletal muscle regeneration. Cytokine Growth Factor Rev. 7, 249–258 [DOI] [PubMed] [Google Scholar]
- 34. Kurek J. B., Bower J. J., Romanella M., Koentgen F., Murphy M., Austin L. (1997) The role of leukemia inhibitory factor in skeletal muscle regeneration. Muscle Nerve 20, 815–822 [DOI] [PubMed] [Google Scholar]
- 35. Menetrey J., Kasemkijwattana C., Day C. S., Bosch P., Vogt M., Fu F. H., Moreland M. S., Huard J. (2000) Growth factors improve muscle healing in vivo. J. Bone Joint Surg. Br. 82, 131–137 [DOI] [PubMed] [Google Scholar]
- 36. Miller K. J., Thaloor D., Matteson S., Pavlath G. K. (2000) Hepatocyte growth factor affects satellite cell activation and differentiation in regenerating skeletal muscle. Am. J. Physiol. Cell Physiol. 278, C174–181 [DOI] [PubMed] [Google Scholar]
- 37. Musaro A. (2005) Growth factor enhancement of muscle regeneration: a central role of IGF-1. Arch. Ital. Biol. 143, 243–248 [PubMed] [Google Scholar]
- 38. Warren G. L., Hulderman T., Mishra D., Gao X., Millecchia L., O'Farrell L., Kuziel W. A., Simeonova P. P. (2005) Chemokine receptor CCR2 involvement in skeletal muscle regeneration. FASEB J. 19, 413–415 [DOI] [PubMed] [Google Scholar]
- 39. Auffray C., Fogg D., Garfa M., Elain G., Join-Lambert O., Kayal S., Sarnacki S., Cumano A., Lauvau G., Geissmann F. (2007) Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317, 666–670 [DOI] [PubMed] [Google Scholar]
- 40. Auffray C., Sieweke M. H., Geissmann F. (2009) Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 27, 669–692 [DOI] [PubMed] [Google Scholar]
- 41. Dumont N., Frenette J. Macrophages protect against muscle atrophy and promote muscle recovery in vivo and in vitro: a mechanism partly dependent on the insulin-like growth factor-1 signaling molecule. Am. J. Pathol. 176, 2228–2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Summan M., Warren G. L., Mercer R. R., Chapman R., Hulderman T., Van Rooijen N., Simeonova P. P. (2006) Macrophages and skeletal muscle regeneration: a clodronate-containing liposome depletion study. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R1488–1495 [DOI] [PubMed] [Google Scholar]
- 43. Tidball J. G., Wehling-Henricks M. (2007) Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J. Physiol. 578, 327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Allen R. E., Boxhorn L. K. (1989) Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J. Cell. Physiol. 138, 311–315 [DOI] [PubMed] [Google Scholar]
- 45. Liu J. P., Baker J., Perkins A. S., Robertson E. J., Efstratiadis A. (1993) Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75, 59–72 [PubMed] [Google Scholar]
- 46. Powell-Braxton L., Hollingshead P., Warburton C., Dowd M., Pitts-Meek S., Dalton D., Gillett N., Stewart T. A. (1993) IGF-I is required for normal embryonic growth in mice. Genes Dev. 7, 2609–2617 [DOI] [PubMed] [Google Scholar]
- 47. Musaro A., McCullagh K., Paul A., Houghton L., Dobrowolny G., Molinaro M., Barton E. R., Sweeney H. L., Rosenthal N. (2001) Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat. Genet. 27, 195–200 [DOI] [PubMed] [Google Scholar]
- 48. Hayashi S., Aso H., Watanabe K., Nara H., Rose M. T., Ohwada S., Yamaguchi T. (2004) Sequence of IGF-I, IGF-II, and HGF expression in regenerating skeletal muscle. Histochem. Cell Biol. 122, 427–434 [DOI] [PubMed] [Google Scholar]
- 49. Heinemeier K. M., Olesen J. L., Haddad F., Schjerling P., Baldwin K. M., Kjaer M. (2009) Effect of unloading followed by reloading on expression of collagen and related growth factors in rat tendon and muscle. J. Appl. Physiol. 106, 178–186 [DOI] [PubMed] [Google Scholar]
- 50. Sacco A., Doyonnas R., LaBarge M. A., Hammer M. M., Kraft P., Blau H. M. (2005) IGF-I increases bone marrow contribution to adult skeletal muscle and enhances the fusion of myelomonocytic precursors. J. Cell Biol. 171, 483–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pelosi L., Giacinti C., Nardis C., Borsellino G., Rizzuto E., Nicoletti C., Wannenes F., Battistini L., Rosenthal N., Molinaro M., Musaro A. (2007) Local expression of IGF-1 accelerates muscle regeneration by rapidly modulating inflammatory cytokines and chemokines. FASEB J. 21, 1393–1402 [DOI] [PubMed] [Google Scholar]
- 52. Hill M., Goldspink G. (2003) Expression and splicing of the insulin-like growth factor gene in rodent muscle is associated with muscle satellite (stem) cell activation following local tissue damage. J. Physiol. 549, 409–418 [DOI] [PMC free article] [PubMed] [Google Scholar]