Abstract
Multiple stress resistance pathways were evaluated in the liver of Ames dwarf mice before and after exposure to the oxidative toxin diquat, seeking clues to the exceptional longevity conferred by this mutation. Before diquat treatment, Ames dwarf mice, compared with nonmutant littermate controls, had 2- to 6-fold higher levels of expression of mRNAs for immediate early genes and 2- to 5-fold higher levels of mRNAs for genes dependent on the transcription factor Nrf2. Diquat led to a 2-fold increase in phosphorylation of the stress kinase ERK in control (but not Ames dwarf) mice and to a 50% increase in phosphorylation of the kinase JNK2 in Ames dwarf (but not control) mice. Diquat induction of Nrf2 protein was higher in dwarf mice than in controls. Of 6 Nrf2-responsive genes evaluated, 4 (HMOX, NQO-1, MT-1, and MT-2) remained 2- to 10-fold lower in control than in dwarf liver after diquat, and the other 2 (GCLM and TXNRD) reached levels already seen in dwarf liver at baseline. Thus, livers of Ames dwarf mice differ systematically from controls in multiple stress resistance pathways before and after exposure to diquat, suggesting mechanisms for stress resistance and extended longevity in Ames dwarf mice.—Sun, L. Y., Bokov, A. F., Richardson, A., Miller, R. A. Hepatic response to oxidative injury in long-lived Ames dwarf mice.
Keywords: ERK, Nrf2, aging, stress
Enhanced stress resistance is associated with extended longevity in worms, flies, and yeast (1–3), raising the hypothesis that longevity in mammals might also be associated with, and perhaps a consequence of, increased resistance to multiple forms of stress. The pituitary mutants Prop-1df and Pitdw (the Ames and Snell dwarf mice, respectively) provide models that can be used to test the relationship of stress resistance to life-span extension in mammals (4, 5). Each of these mutations leads to defects in the development of the embryonic pituitary, characterized by drastically lower production of growth hormone (GH), thyroid stimulating hormone, and prolactin, with secondary deficits in IGF-1 and the thyroid hormones T3 and T4. Both mutations lead to an increase in mean and maximal life span of both males and females of ∼40%, and both have shown these antiaging effects on multiple genetic backgrounds (4–6). These mice show delay in a wide spectrum of age-related phenotypes, including collagen cross-linking, cataracts, kidney diseases, fatal neoplastic disease and declines in immune function, locomotor activity, learning, and memory (5–8).
Stress resistance has been evaluated in Snell and Ames dwarf mice both in vivo and in vitro. For example, fibroblast cells expanded from biopsy of adult skin samples from Snell dwarf mice are resistant to lethal injury induced by exposure to oxidative agents such as H2O2, and paraquat, as well as to agents that lead to cell death by nonoxidative processes, such as ultraviolet irradiation and the DNA alkylating agent MMS. Snell dwarf fibroblasts are also resistant to the lethal effects of heat and to the heavy metal cadmium (9, 10). Similar patterns of resistance have been seen in fibroblasts from Ames dwarf mice and from long-lived mice deficient in the receptor for GH (10). Cells from Snell dwarf mice are resistant to the effects of atmospheric oxygen on long-term proliferation (11) and are faster to resume RNA transcription after exposure to nonlethal levels of ultraviolet irradiation (12).
Recent work (13) has shown that Ames dwarf mice are resistant, compared with nonmutant littermates, to the lethal effects of exposure to the oxidative toxins diquat and paraquat in vivo. Diquat and paraquat are often used as standard agents to induce oxidative stress in cells and whole animals (14, 15). Paraquat induces oxidative stress by catalyzing the generation of the superoxide anion and the oxidation of cellular NADPH (15). Diquat is a potent redox cycler and is readily converted to a free radical that, in reaction with molecular oxygen, generates superoxide anions and subsequently other redox products. These products can induce lipid peroxidation in cell membranes and potentially cause cell death (14). As measured by overall survival after a lethal dose of diquat or paraquat, Ames dwarf mice, both males and females, were much more resistant to both stressors than were normal littermates, and this resistance to paraquat toxicity was maintained with age in the Ames dwarf mice (13).
Studies in cell culture as well as in intact mice have begun to delineate biochemical pathways that might contribute to stress resistance in long-lived mutant mice. The mitogen-activated protein kinases (MAPK) comprise a ubiquitous group of signaling proteins that play a prominent role in regulating cell proliferation, differentiation, and adaptation, emerging as important regulators of cellular responses to various stimuli (16). There are 3 major MAPK subfamilies, namely the extracellular signal-regulated protein kinases (ERK), the c-Jun N-terminal kinases (JNK), and p38 MAPK, and each set of kinases has been implicated in cell injury and disease pathogenesis (16, 17). Exposure of primary skin-derived fibroblasts to peroxide, cadmium, or paraquat leads, within 60 min, to phosphorylation of the stress-activated kinases ERK1 and ERK2 (18). The level of ERK phosphorylation induced by these stresses is, however, significantly lower in cells from Snell dwarf mice, suggesting defects in the pathways that sense cellular injury and lead to activation of the kinase MEK, responsible for phosphorylation of ERK (18). Paradoxically, despite the relatively low induction of ERK phosphorylation, cells from Snell dwarf mice show abnormally high levels of mRNA accumulation for a series of ERK-sensitive genes, including Egr-1, Fos, and Jun (18). Moreover, recently p38 phosphorylation was reported to occur in fibroblasts from Ames mice in response to the mitochondrial inhibitor rotenone, and cells from dwarf mice had an attenuated response compared with control cells (19).
Other work has begun to dissect the role of Nrf2 in stress resistance and longevity of Snell dwarf mice. Nrf2 is a transcription factor that binds to the antioxidant response element (ARE) of target genes in response to oxidative stress (20). Under normal conditions, Nrf2 is bound to Keap1 in the cytoplasm, where it undergoes proteolytic degradation and rapid turnover (20). When cells are exposed to stress, Nrf2 is released and moves to the nucleus, where it binds to AREs to induce expression of multiple cytoprotective enzymes, including NAD(P)H-quinone oxidoreductase 1 (NQO1), glutathione S-transferases (GSTs), and heme oxygenase 1 (HO-1; refs. 20, 21). Recent experiments using Snell dwarf fibroblasts have shown elevated mRNA levels for several Nrf2-dependent genes, as well as cellular traits, such as elevated glutathione levels, resistance to lipid peroxidation, and increased activity of plasma membrane redox transport pump, that are known to be modulated by Nrf2 function (22). Stress resistance of normal fibroblasts was found to be increased by the Nrf2 activator arsenite to the level of resistance seen in cells from dwarf mice, consistent with the idea that higher levels of Nrf2 function and transcription of ARE-sensitive genes might play a role in stress resistance of cells from the long-lived mutants. A set of Nrf2-dependent mRNAs was also shown to be elevated in the liver, heart, and brain from young adult dwarf mice, suggesting that elevated Nrf2 activity might characterize at least some cell types in Snell dwarf mice under normal housing conditions (22).
The current study used Ames dwarf mice to see whether this mutation leads to differences in hepatic stress response pathways related to MAPK signaling and Nrf2-dependent gene transcription, either before or after exposure to the oxidative toxin diquat.
MATERIALS AND METHODS
Animals and treatment
Ames heterozygous (Prop-1+/−) mice were kindly provided by Dr. Holly Brown-Borg (University of North Dakota, Grand Forks, ND, USA). To establish our Ames colony, Prop-1+/− male and female mice were mated. The offspring were Prop-1−/− (dwarf), Prop-1+/+, and Prop-1+/− (the latter 2, which are phenotypically indistinguishable, were used as the control group). All mice were fed a standard NIH-31 chow and maintained in microisolator cages on a 12-h light-dark cycle, and age-matched groups were chosen for the experiments reported here. All animal procedures were approved by the subcommittee for Animal Studies at the Audie L. Murphy Veterans Administration Hospital and the University of Texas Health Science Center Institutional Animal Care and Use Committee.
Diquat (Chem Service, Westchester, NY, USA) was dissolved in 0.9% saline (25 mg/ml) and injected intraperitoneally in a volume adjusted to deliver 50 mg/kg body weight, as described previously (13). Six hours after injection, the diquat-treated mice were anesthetized with ketamine/acepromazine/xylazine cocktail and then euthanized by cervical dislocation. Livers were immediately removed and snap-frozen in liquid nitrogen.
Antibodies
The following antibodies were obtained for immunoblotting: p38 MAPK, phospho-p38 MAPK (Thr180/Tyr182), ERK, phospho-ERK (Thr202/Tyr204), JNK, phospho-JNK (Thr183/Tyr185), phospho-Akt (Ser-473), and Akt from Cell Signaling Technology (Beverly, MA, USA); Nrf2 antibody from Novus Biologicals (Littleton, CO, USA); β-actin from Sigma-Aldrich (St. Louis, MO, USA); and goat anti-rabbit and goat anti-mouse antibodies from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Real-time RT-PCR
Quantitative real-time PCR was performed using a Rotor-Gene 3000 system (Corbett Research, Cambridge, UK) with a QuantiTect SYBR Green RT-PCR kit (Bio-Rad Laboratories, Hercules, CA, USA) as described previously (23). In brief, cells were homogenized with RNA extraction buffer (TRIZOL reagent; Invitrogen Life Technologies, Carlsbad, CA, USA) to yield total RNA following the manufacturer's instructions. Total RNA was reverse transcribed with polydT oligodeoxynucleotide and SuperScript II (Invitrogen Life Technologies), again using the manufacturer's recommended conditions. After an initial denaturation step (95°C for 90 s), amplification was performed over 40–45 cycles of denaturation (95°C for 10 s), annealing (60°C for 5 s), and elongation (72°C for 13 s). Amplification was monitored by measuring the fluorometric intensity of SYBR Green I at the end of each elongation phase. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or β-actin expression was quantified to normalize the amount of cDNA in each sample. The change in threshold cycle number (ΔCt) was normalized to the GAPDH reference gene by subtracting ΔCt GAPDH from ΔCt gene. The effect of treatment (ΔΔCt) was calculated by subtracting ΔCt normal from ΔCt Tg. Fold induction was determined by calculating 25ΔΔCt.
Western blot analysis
After exposure to various forms of stress, cells were washed in ice-cold PBS and then harvested in 1 ml of lysis buffer (20 mM Tris, pH 7.5; 150 mM NaCl; 1% Triton 100; with protease inhibitor cocktail and phosphatase inhibitor cocktails; Sigma-Aldrich) and centrifuged at 12,000 rpm for 40 min. The supernatants were removed and stored at −70°C. Protein concentrations were determined using the bicinchoninic acid assay (Pierce, Rockford, IL, USA) according to the manufacturer's instructions. For Western blotting, protein extracts were mixed with sample buffer (Bio-Rad Laboratories) and boiled for 5 min. Forty micrograms of total protein was separated electrophoretically according to size by SDS-PAGE using Criterion XT Precast Gel (Bio-Rad Laboratories) for 60 min at 120 V. Proteins were then wet transferred for 1 h at 100 V onto nitrocellulose membranes. Membranes were rinsed briefly in TBS (pH 7.6) and blocked with 5% dry milk (or 3% BSA for phosphorylated proteins) in TBS plus 0.05% Tween 20 (TBST) for 1 h at room temperature. Blots were washed with TBST and incubated with the primary antibody diluted in the appropriate blocking solution at 4°C overnight with shaking. After incubation, blots were washed 3 times (15 min each) with TBST and incubated with an appropriate secondary antibody. For visualization of specific bands in the chemiluminescence assays, the membrane was exposed to X-OMAT film; for chemifluorescence, the membrane was incubated with enhanced chemifluorescence substrate and a digital image was generated with the Molecular Dynamics Storm system (Molecular Dynamics, Sunnyvale, CA, USA). Quantification of immunoblot signals was performed using the ImageQuant software package (Molecular Dynamics).
Statistical analyses
Statistical analyses were performed using unpaired t tests for comparison between dwarf and control mice. Graphs show the mean of combined experiments; error bars represent the se. Differences between treatments were calculated using repeated-measures ANOVA by NCSS software (NCSS, Kaysville, UT, USA).
RESULTS
Phosphorylation of ERK1/2, JNK1/2, p38, and Akt in the liver of Ames dwarf mice before and after exposure to diquat
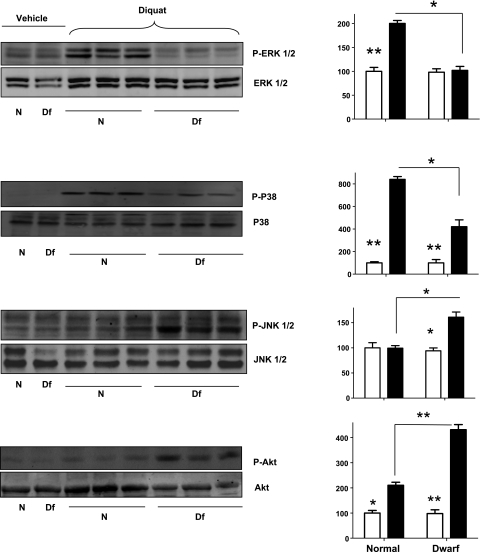
Our previous studies (18) of skin-derived fibroblasts have shown that cells from dwarf mice respond less strongly than control cells in stress-induced phosphorylation of ERK1 and ERK2. We therefore speculated that differential activation of ERK signaling might contribute to diquat resistance in Ames dwarf mice. We first examined the levels of the phosphorylation of various members of the MAPK family by Western blotting using liver lysates. As shown in Fig. 1, we found that Ames dwarf and control mice had similar levels of hepatic p-ERK before treatment and that diquat treatment led to robust increases in phosphorylation of ERK1/2 in the liver of normal mice but no significant increase in dwarf mice. Both findings are consistent with our previous studies of Snell dwarf skin fibroblasts in culture (18).
Figure 1.
Phosphorylation of ERK1/2, p38, JNK1/2, and Akt in the liver of Ames dwarf (Df) and normal (N) mice in response to diquat stress. Left panels: representative results of Western blots for phosphorylated and total forms of ERK1/2, p38, JNK1/2, and Akt protein in dwarf and control mice. Right panels: quantification of results of Western blots, as means ± se for 4 untreated or 6 diquat-treated mice of each genotype. Values represent ratios of phosphoprotein to total protein for each enzyme, relative to the value in the control mice, with vehicle-treated mice set at 100. Open bars represent vehicle-treated mice; solid bars represent diquat-treated mice. *P < 0.05; **P < 0.01.
We next examined the effect of diquat on phosphorylation of p38 and JNK kinases. Phosphorylation of p38 was very low in the liver of untreated mice but was significantly elevated in both normal and dwarf mice after diquat exposure. The increase in p-p38 was ∼2-fold higher in the control mice than in the Ames dwarfs. In contrast, diquat-induced JNK phosphorylation was seen only in the Ames dwarf mice but not in controls. Thus, responses of Ames dwarf and control mice to diquat were very different: controls responded by increases in ERK and p38 phosphorylation, while dwarf mice responded by increases in p38 and JNK activation. We also evaluated diquat-induced phosphorylation of Akt, an index of induction of PI3K-Akt signaling pathway. We found (Fig. 1) that phosphorylation of Akt was induced by diquat in both normal and dwarf liver, but that the response was significantly (∼2×) higher in the liver of dwarf mice.
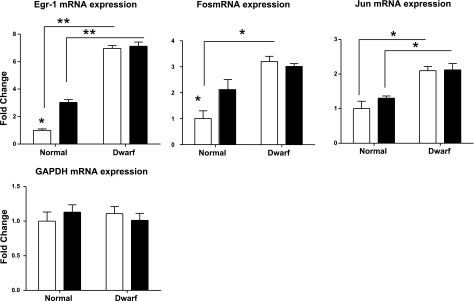
Stress-induced expression of immediate early gene (IEG) family members
The IEG family includes Egr-1, Fos, and Jun, each of which encodes a zinc-finger-containing DNA-binding protein. Levels of these IEGs rise very rapidly in response to various forms of stress (24), and activation of PI3K-Akt and MAPK signaling pathways is involved in these responses (25, 26). Our studies of Snell dwarf fibroblasts in culture (18) have shown, surprisingly, that dwarf cells showed stronger induction of ERK-dependent IEG mRNAs despite the relatively low levels of p-ERK induction. In these earlier studies, we also found that IEG activation was blocked in control and dwarf cells by MEK inhibition, showing that ERK phosphorylation is required for IEG activation. Figure 2 shows mRNA levels for Egr-1, Fos, and Jun (with Gapdh as a loading control) from Ames dwarf and control mice. Before diquat exposure, the livers of dwarf mice have higher levels of each of these IEG mRNAs, ranging from 6× for Egr-1 to 2× for Jun. The levels of Egr-1 and Fos (but not Jun) rise significantly in normal liver after diquat exposure. None of these genes responds in dwarf mice to the diquat treatment, but because the dwarf baseline levels are higher, the resulting levels of Egr-1 and Jun are higher in dwarf than in control liver, similar to the result previously seen in Snell dwarf fibroblasts after oxidative stress. Although the present data are limited to a single dose, a single time point, and a single organ, they suggest that mRNA levels for some IEGs are higher in dwarf than in control liver both before and after diquat exposure.
Figure 2.
Expression of Egr-1, Fos, and Jun mRNA levels in the liver of Ames dwarf and control mice after exposure to diquat. mRNA was measured using real-time RT-PCR. Data for Egr-1, Fos, and Jun are normalized to GAPDH values and were expressed as a ratio to levels of mRNA in control mice with vehicle treatment. GAPDH values are normalized to β-actin as a loading control. Bars represent means ± se for 4 untreated or 6 diquat-treated mice of each genotype. *P < 0.05; **P < 0.01.
Nrf2-dependent responses in Ames dwarf liver
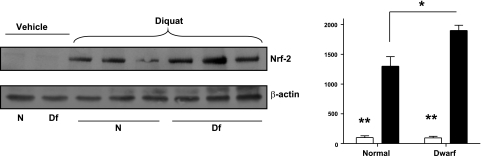
Previous work (22) has shown elevation of many Nrf2/ARE-dependent mRNAs in the heart and liver of Snell dwarf mice, as well as in fibroblasts derived from Snell dwarf mice; these analyses, using the fibroblast system, also showed elevated levels of Nrf2 protein and several cellular traits (glutathione levels, resistance to lipid peroxidation, and components of the plasma membrane redox pump) known to be regulated by Nrf2. To investigate whether augmented activity of Nrf2- and ARE-responsive genes could contribute to the resistance of Ames dwarf mice to diquat stress, we evaluated protein levels of Nrf2. As shown in Fig. 3, analysis of liver lysates showed that Nrf2 protein levels increased in both normal and dwarf mice after diquat treatment. The resulting level of Nrf2 protein was ∼30% higher in dwarf than in control mice.
Figure 3.
Nrf2 protein levels in the liver of Ames dwarf and normal mice before (open bars) and after (solid bars) diquat exposure. Left panels: representative results of Western blots for Nrf2 (110 kDa) and actin protein liver of dwarf and control mice. Right panel: bars represent mean ± se Nrf2/actin ratio in 4 untreated or 6 diquat-treated mice of each genotype, relative to the value for the control mice, with vehicle-treated mice set at 100. *P < 0.05; **P < 0.01.
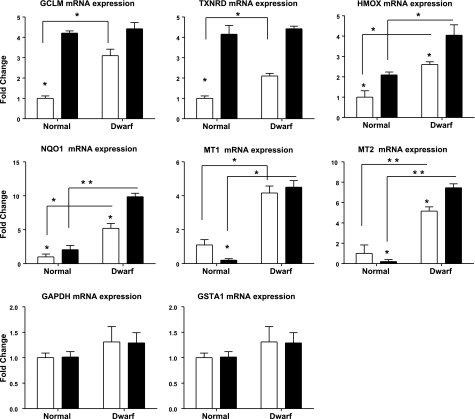
To determine whether the increase in Nrf2 protein levels was accompanied by a corresponding increase in ARE-responsive genes, we evaluated 7 such genes by quantitative RT-PCR, using Gadph as a loading control (Fig. 4). Before diquat exposure, all the Nrf2-dependent genes tested (except GSTA1) were expressed at higher levels in liver from dwarf mice than in control liver. These 6 mRNAs included those encoding glutamate cysteine ligase modifier subunit 1 (GCLM), an important rate-limiting enzyme of glutathione synthesis (27); heme oxygenase (HMOX; refs. 28, 29); thioredoxin reductase (TXNRD), an important enzyme for ascorbate recycling and protection from oxidative stress (30, 31); quinone oxidoreductase 1 (NQO1; ref. 32); and metallothioneins 1 and 2, both of which participate in protection against heavy metal toxicity (32, 33). These results in Ames dwarf mice housed in Texas are consistent with prior observations in the liver of Snell dwarf from the University of Michigan colony (22), suggesting that up-regulation of Nrf2-dependent genes is seen on different genetic backgrounds and in at least 2 distinct environments.
Figure 4.
Expression of GCLM, TXNRD, HMOX, NQO1, MT1, MT2, and GSTA1 mRNA levels in the liver of Ames and control mice before (open bars) and after (solid bars) exposure to diquat treatment. mRNA was measured using real-time RT-PCR. Data are normalized to GAPDH values and expressed as a ratio to level seen in liver of control mice with vehicle treatment. GAPDH values are normalized to β-actin as a loading control. Bars represent means ± se for 4 untreated or 6 diquat-treated mice of each genotype. *P < 0.05; **P < 0.01.
Although each of these 6 genes was expressed at higher levels in untreated Ames dwarf mice, their responses to diquat exposure revealed several distinct patterns. GCLM and TXNRD, for example, increased in both normal and dwarf mice after diquat treatment, and responses in the normal mice produced levels equivalent to those of Ames dwarfs at the diquat dose used. In contrast, HMOX and NQO1 also increased in both normal and dwarf mice after diquat, but the levels in the normal mice remained well below those seen in the Ames dwarf animals after exposure to this oxidative stress. Levels of the 2 metallothionein genes, MT1 and MT2, showed a third pattern: each of these metallothionein genes decreased after diquat in normal mouse liver but increased in dwarf liver. Evaluation of other time points, other doses, other tissues, and other sources of stress will be needed to develop a more comprehensive picture of the role of Nrf2-dependent genes in stress resistance and longevity of long-lived mice, but the current results, like those for the MAPK study, suggest that the responses of Ames dwarf mice to stress are dramatically different from those of control animals.
Expression of apoptotic control genes in the liver of Ames dwarf mice
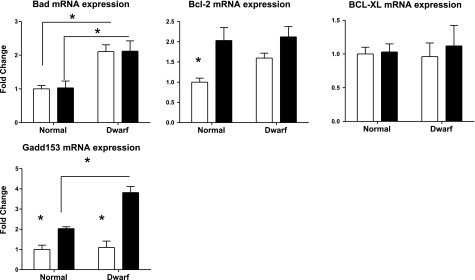
Although Ames dwarf mice are relatively resistant to the lethal effects of diquat, they also, paradoxically, have higher levels of liver cell injury, as measured by alanine aminotransferase levels in serum, after diquat exposure (13). To shed light on the molecular controls of apoptosis in the liver of diquat-treated mice, we evaluated mRNA expression of apoptosis-related genes, including Bad, Bcl-2, Bcl-XL, and Gadd153. Gadd153 [also known as C/Ebp-homologous protein (CHOP)] is an indicator of commitment to apoptosis (34, 35). As shown in Fig. 5, hepatic levels of Gadd153 mRNA are similar in untreated dwarf and control mice. Diquat leads to a significant induction of Gadd153 mRNA in controls but a much higher increase in Ames dwarf. The difference between dwarf and littermate controls is significant at P = 0.04, consistent with the previous report of increased hepatotoxicity in the Ames dwarf mice (13, 36). Levels of mRNA for Bad, another proapoptotic protein (37, 38), were higher at baseline in dwarf liver and did not respond to diquat in either dwarf or control mice. Thus elevation of Bad might also contribute to higher levels of liver cell apoptosis in dwarf mice. In contrast, Bcl2 and Bcl-xl are both antiapoptotic (38, 39), and although Bcl2 mRNA increases slightly after diquat exposure, neither Bcl-2 nor Bcl-xl mRNAs differ between dwarf and control mice after diquat treatment.
Figure 5.
Expression of Bad, Bcl-2, Bcl-XL, and Gadd153 mRNA levels in the liver of Ames dwarf and control mice before (open bars) or after (solid bars) exposure to diquat. mRNA was measured using real-time RT-PCR. Data are normalized to GAPDH values and expressed as a ratio to level seen in liver of control mice with vehicle treatment. Bars represent means ± se for 4 untreated or 6 diquat-treated mice of each genotype. *P < 0.05; **P < 0.01.
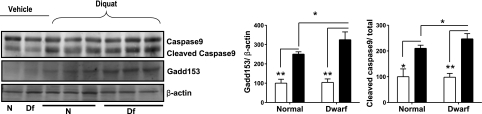
We also evaluated Gadd153 protein levels by immunoblotting. As shown in Fig. 6, both control and dwarf mice respond to diquat by elevation of Gadd153, with a significantly higher level (P<0.05) in the liver of the dwarf mice, consistent with the mRNA data of Fig. 5. Figure 6 also shows results from a study of cleavage of caspase 9, an indicator of cellular commitment to apoptosis. Both normal and dwarf livers show diquat stimulation of caspase 9 cleavage, with a significantly higher response in the dwarf mice, providing further indication of higher apoptotic activity in liver from dwarf mice in response to a single injection of diquat.
Figure 6.
Gadd153 and cleavage of caspase 9 in liver of Ames dwarf and normal mice before (open bars) and after (solid bars) diquat exposure. Left panels: representative results of Western blots for caspase 9, showing both uncleaved and cleaved forms, and for Gadd153 protein, as well as β-actin as a loading control, in liver of dwarf and control mice. Right panels: bars represent means ± se in 4 untreated or 6 diquat-treated mice of each genotype. Values are expressed relative to value for vehicle-treated control mice, set at 100. *P < 0.05; **P < 0.01.
DISCUSSION
The observations that mutations that extend nematode life span render the worms resistant to multiple forms of lethal stress (40) suggested the appealing idea that the longevity seen in these mutants was a consequence of their stress resistance. Evaluation of changes in gene expression patterns (41, 42) has begun to show how modulation of a few key regulatory circuits by antiaging mutations can promote coordinated resistance to multiple forms of cellular injury, including oxidative damage and damage to DNA. The idea that longevity in mammals, whether caused by dietary manipulation (43, 44), single-gene mutants (4, 5, 45), or evolutionary divergence might also reflect modulation of cellular stress resistance properties is a tempting hypothesis (46) but stands, so far, on a shakier platform of evidence. Fibroblasts from long-lived mutant mice are resistant in culture to multiple forms of injury (9, 10) and so are fibroblasts from longer lived species of mammals (12, 47, 48), but it is not clear to what extent the stress resistance of skin-derived cells in culture reflects the properties in live animals of cell types more relevant to aging and late-life diseases. Caloric restriction and methionine restriction render mice resistant to the hepatotoxic effects of acetaminophen (48, 49), but Snell dwarf, GHR-KO (48), and Ames dwarf (13) mice are each more sensitive than controls to hepatotoxic injury. Despite their sensitivity to hepatotoxic effects of diquat, Ames dwarf mice survive exposure to doses of this toxin that are lethal to wild-type controls (13), suggesting that liver cell vulnerability is not the key determinant to survival of the organism after diquat exposure.
Studies of cultured fibroblasts from Snell dwarf mice have shown multiple differences in stress resistance pathways, including differences in expression of Nrf2-dependent genes (22), in MEK-mediated phosphorylation of ERK and in activation of ERK-sensitive genes (18), in removal of ultraviolet-induced DNA lesions (12), and in resistance to O2-mediated growth crisis on longer term culture (11). The current study was intended to evaluate some of these pathways in intact mice before and after exposure to diquat. We found that phosphorylation of the stress-activated ERK1/2 kinases induced by diquat was attenuated in the liver of Ames dwarf mice. This result is consistent with our previous findings in cell culture. Interpretation of this result is not straightforward, however. In the cultured Snell dwarf fibroblasts, the lower levels of ERK phosphorylation are seen in cells that are relatively resistant to lethal injury. Diminished ERK phosphorylation is found in Ames dwarf liver, but in the in vivo model the dwarf hepatocytes are more susceptible to cell death than controls, whether this is evaluated by serum levels of liver enzymes (13) or by induction of apoptotic genes (see Fig. 5).
ERKs are integrally involved in regulating pivotal processes, including proliferation, differentiation, adaptation (i.e., cell motility, long-term potentiation), survival, and cell death. The ERK signaling module involves sequential activation of Raf, MEK1/2, and ERK1/2 (16). ERK1/2 signaling has been shown to be crucial for response to oxidative stress, and the cascade is known to be activated by oxidative injury (16). Several recent studies (50, 51) have suggested that that inhibition of ERK1/2 activation is associated with resistance to oxidative damage in vivo and in vitro. Our results (Fig. 1) show dramatic differences in the pattern of diquat-induced MAPK activation in the liver, with controls showing elevated phosphorylation of ERK and p38 and Ames dwarf mice showing increased phosphorylation of Akt and JNK. It is possible that the relatively lower levels of diquat-induced P-ERK and P-p38 in dwarf mice, or the higher levels of JNK phosphorylation, may be part of the mechanism of resistance to oxidative stress. Interestingly, recent studies (52–54) have indicated that activation of p38 MAPK is also a key signaling step in inflammation-related cell death. Inhibition of p38 MAPK signaling has been shown to be an effective cellular protective intervention following ischemia by decreasing levels of proinflammatory cytokine release (52–54). Experiments that evaluate multiple forms of stress and that include tests for other cells and tissues in vivo will be required to test hypotheses about the relative importance of ERK, JNK, and p38 in preventing late-life illnesses and extending life span in Ames and Snell dwarf mice.
A major mediator of the cellular stress response is the IEG, including egr-1, fos, and jun, whose transcription can be induced by stress, injury, mitogens, and cytokines (55, 56). Modulation of gene expression by stress has been suggested to play an important role in regulating susceptibility to apoptosis when cells are challenged with oxidative agents (56). These genes are regulated by a highly interconnected network of transcription factors, including jun, myc, elk-1, atf-2, atf-4, and creb, most of which are substrates for various combinations of JNK, p38, and ERK. In the current study, we have found that stress-induced elevation of mRNA for members of the IEG family is higher in dwarf than in control liver both under baseline conditions and after diquat exposure. In parallel to the data on MAPK activation, we think it possible that increased activation of IEGs, in some cell types, may contribute to the resistance of long-lived dwarf mice in response to lethal injury in vivo and in vitro, but we cannot at present reconcile these observations with the vulnerability of Ames and Snell dwarf hepatocytes to injury induced by acetaminophen and by diquat. It is also puzzling that baseline expression levels of egr-1, fos, and jun were higher in untreated dwarf mice, compared with untreated controls, despite similar levels of phosphorylation of ERK, JNK, and p38. Perhaps investigation of other MAP kinases (such as MAPK6, MAPK7, and MAPK15) will reveal an additional pathway that is basally up-regulated in Ames dwarf mice and accounts for the basal gene expression patterns.
The basic leucine-zipper transcription factor-Nrf2 has been shown to play a vital role in protecting cells from oxidative stress (57). In response to oxidative stress, Nrf2 translocates to the nucleus and binds to AREs in the promoters of target genes, leading to transcriptional induction of several cellular defense genes, including GCLM and GCLC, glutathione peroxidase, GST, NQO1, HO-1, and glutathione reductase (57). Nrf2 is thought to be critical for maintaining the GSH redox state via transcriptional regulation of ARE genes, thus protecting cells against oxidative stress (57). Nrf2 is a highly unstable protein, with a half-life of ∼15 min (58), and its levels are modulated acutely by a balance between new synthesis and degradation by the ubiquitin-proteasome system. Although hepatic Nrf2 protein levels are similar in unstressed Ames dwarf and control mice, 6 of the 7 Nrf2 target genes we evaluated were elevated in the Ames dwarf mice. These results are mostly consistent with our previous observations in the liver of Snell mice (22), which had higher mRNA levels of MT1, HMOX, and TXNRD, though not of GCLM. The general pattern, also noted in Snell dwarf fibroblasts and heart and brain tissue of Snell dwarf mice (22), suggests chronic elevation of many (though not all) Nrf2 targets in long-lived pituitary dwarf mice. Our results showed that hepatic Nrf2 levels are induced dramatically by diquat (Fig. 3), more so in dwarf than in control mice. This finding may reflect phosphorylation of Nrf2 by JNK (59), which responds to diquat more strongly in dwarf than in control liver. Diquat-induced transcription of Nrf2 target genes was, however, variable from gene to gene, with a different pattern in Ames dwarf and control mice. In controls, 2 genes reached levels similar to those seen at baseline in dwarf mice, 2 others were elevated but did not reach the dwarf baseline level, and 2 others, the metallothionein genes, actually dropped after diquat treatment in control mice, while showing no such diminution in the dwarf liver samples. The overall trend seems to be one of up-regulation in dwarf livers in response to diquat and more varied patterns of expression in the control livers. Elevation of PI3K activity, suggested by higher levels of Akt phosphorylation in dwarf mice, might also lead to phosphorylation of C/EBPβ and thus to activation of xenobiotic response element sites on the same genes where Nrf2 stimulates through ARE sites. This model is, however, too simple to explain all of the observations; in particular, elevated pAKT activity is expected to inhibit both ASK1 (which is upstream from p38) and MKK4 (which is upstream from JNK), contrary to our data showing enhanced phosphorylation of both p38 and JNK in dwarf mice. Moreover, AKT is an inhibitor of apoptosis, but increased levels of BAD and CHOP expression are observed in diquat-treated dwarf mice and our previous study reported increased liver cell death in diquat-treated dwarf mice. There are 3 different AKT isoforms in mice, and the increased AKT phosphorylation we see in dwarf mouse livers might be limited to AKT2 and perhaps AKT3, which play less of a role in apoptosis than AKT1 (60).
In the nematode Caenorhabditis elegans, the Nrf ortholog SKN-1 has been shown to play a key role in oxidative stress resistance and acts in multiple longevity pathways (61). SKN-1 has also been reported to be required for diet restriction-induced longevity (62) in a neuronal-specific manner. Furthermore, enhanced Nrf2 signaling also increases oxidative stress resistance and extends life span in fruit flies (63). A study of mice lacking the Nrf2 gene has suggested (64) that Nrf2 may play a role in the effects of a calorie-restricted diet on susceptibility to tumors induced by 7,12-dimethylbenz(a)anthracene, but Nrf2 was not needed for the calorie restriction diet to induce life-span extension. Our results are consistent with the hypothesis that augmented Nrf2 signals and elevated transcription of ARE genes might contribute to stress resistance and life extension in Ames dwarf mice but provide no information about the cell types in which Nrf2-mediated gene expression changes might contribute to disease resistance and longevity.
One surprising result from the previous study (13) was that even though the Ames dwarf mice showed increased resistance to diquat at the whole-animal level, their livers appeared to show greater diquat-induced damage than those of normal mice. Livers from the dwarf mice had a 2.8-fold increase in apoptosis as measured by double-strand DNA breaks after diquat treatment (13). Consistent with these observations, we found an elevated basal level of proapoptotic Bad mRNA expression in Ames dwarf mouse liver. Interestingly, another group (36) has also reported increased basal levels of Bax, another proapoptotic Bcl-2 family member, in cultured hepatocytes from Ames dwarf mice. Gadd153, also known as CHOP, is highly inducible by oxidative stress and plays a crucial role in endoplasmic reticulum stress-induced programmed cell death (34, 65, 66). The dwarf liver showed much higher induction of Gadd153 mRNA relative to normal control mice after diquat exposure, also consistent with the idea that liver from dwarf mice may undergo apoptosis in response to lethal stress more readily than controls. It has been suggested (67) that a propensity for increased apoptosis of damaged tissues might help long-lived mice regenerate new, undamaged cells after an oxidative challenge. Up-regulated apoptosis within proliferative tissues, such as the liver, may provide an advantage to the dwarf mouse by eliminating damaged and potentially harmful neoplastic cells more efficiently.
Our current data show convincingly that liver in Ames dwarf mice differs from controls in multiple aspects of stress resistance defense, both before and within 6 h after exposure to a dose of diquat that is sufficient to kill many control mice but survivable by most Ames dwarf mice. The present data are limited, however, to a single dose, a single time point, and a single organ, and thus are just a first step toward developing a comprehensive picture of how Ames dwarf and control mice differ in their responses to oxidative and other forms of lethal stress. Furthermore, interpretation is complicated by the surprising vulnerability of Ames dwarf hepatocytes to cell death after diquat exposure, in contrast to the resistance of these mice to diquat-induced death. The differential activation of signaling components in the network may reflect only one aspect of a more complex set of homeostatic circuits, with animal survival depending not just on initial levels of cellular injury but also on compensatory repair processes, inflammatory responses, availability of metabolic reserves, and pharmacodynamic differences (68) that may regulate detoxification and excretion of the toxic agent. To address some of these questions, we are now carrying out a series of experiments to analyze multiple stress-responsive signaling pathways in different tissues of Snell dwarf mice and mice of other long-lived stocks in response to different forms of stress. Experiments of this sort should provide insights into the paths by which altered production of pituitary hormones renders dwarf mice resistant to multiple late-life diseases and degenerative changes.
Acknowledgments
This work was supported by National Institute on Aging grants AG-031736, AG-024824, and AG-013283 (to R.A.M.); AG-021890 (to A.F.B.); and San Antonio Nathan Shock Aging Center 1P30-AG-13319 (to A.R.). The authors thank Marian Sabia and Vivian Diaz for assistance.
REFERENCES
- 1. Larsen P. L. (1993) Aging and resistance to oxidative damage in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A. 90, 8905–8909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lin Y. J., Seroude L., Benzer S. (1998) Extended life-span and stress resistance in the Drosophila mutant methuselah. Science 282, 943–946 [DOI] [PubMed] [Google Scholar]
- 3. Martin G. M., Austad S. N., Johnson T. E. (1996) Genetic analysis of ageing: role of oxidative damage and environmental stresses. Nat. Genet. 13, 25–34 [DOI] [PubMed] [Google Scholar]
- 4. Brown-Borg H. M., Borg K. E., Meliska C. J., Bartke A. (1996) Dwarf mice and the ageing process. Nature 384, 33. [DOI] [PubMed] [Google Scholar]
- 5. Flurkey K., Papaconstantinou J., Miller R. A., Harrison D. E. (2001) Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc. Natl. Acad. Sci. U. S. A. 98, 6736–6741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bartke A. (2005) Minireview: role of the growth hormone/insulin-like growth factor system in mammalian aging. Endocrinology 146, 3718–3723 [DOI] [PubMed] [Google Scholar]
- 7. Ikeno Y., Bronson R. T., Hubbard G. B., Lee S., Bartke A. (2003) Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: correlation to extended longevity. J. Gerontol. A Biol. Sci. Med. Sci. 58, 291–296 [DOI] [PubMed] [Google Scholar]
- 8. Kinney B. A., Meliska C. J., Steger R. W., Bartke A. (2001) Evidence that Ames dwarf mice age differently from their normal siblings in behavioral and learning and memory parameters. Horm. Behav. 39, 277–284 [DOI] [PubMed] [Google Scholar]
- 9. Murakami S., Salmon A., Miller R. A. (2003) Multiplex stress resistance in cells from long-lived dwarf mice. FASEB J. 17, 1565–1566 [DOI] [PubMed] [Google Scholar]
- 10. Salmon A. B., Murakami S., Bartke A., Kopchick J., Yasumura K., Miller R. A. (2005) Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am. J. Physiol. Endocrinol. Metab. 289, E23–29 [DOI] [PubMed] [Google Scholar]
- 11. Maynard S. P., Miller R. A. (2006) Fibroblasts from long-lived Snell dwarf mice are resistant to oxygen-induced in vitro growth arrest. Aging Cell 5, 89–96 [DOI] [PubMed] [Google Scholar]
- 12. Salmon A. B., Ljungman M., Miller R. A. (2008) Cells from long-lived mutant mice exhibit enhanced repair of ultraviolet lesions. J. Gerontol. A Biol. Sci. Med. Sci. 63, 219–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bokov A. F., Lindsey M. L., Khodr C., Sabia M. R., Richardson A. (2009) Long-lived ames dwarf mice are resistant to chemical stressors. J. Gerontol. A Biol. Sci. Med. Sci. 64, 819–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jones G. M., Vale J. A. (2000) Mechanisms of toxicity, clinical features, and management of diquat poisoning: a review. J. Toxicol. Clin. Toxicol. 38, 123–128 [DOI] [PubMed] [Google Scholar]
- 15. Farrington J. A., Ebert M., Land E. J., Fletcher K. (1973) Bipyridylium quaternary salts and related compounds. V. Pulse radiolysis studies of the reaction of paraquat radical with oxygen. Implications for the mode of action of bipyridyl herbicides. Biochim. Biophys. Acta 314, 372–381 [DOI] [PubMed] [Google Scholar]
- 16. Chang L., Karin M. (2001) Mammalian MAP kinase signalling cascades. Nature 410, 37–40 [DOI] [PubMed] [Google Scholar]
- 17. Junttila M. R., Li S. P., Westermarck J. (2008) Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 22, 954–965 [DOI] [PubMed] [Google Scholar]
- 18. Sun L. Y., Steinbaugh M. J., Masternak M. M., Bartke A., Miller R. A. (2009) Fibroblasts from long-lived mutant mice show diminished ERK1/2 phosphorylation but exaggerated induction of immediate early genes. Free Radic. Biol. Med. 47, 1753–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hsieh C. C., Papaconstantinou J. (2009) Dermal fibroblasts from long-lived Ames dwarf mice maintain their in vivo resistance to mitochondrial generated reactive oxygen species (ROS). Aging 1, 784–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaspar J. W., Niture S. K., Jaiswal A. K. (2009) Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 47, 1304–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kobayashi M., Yamamoto M. (2005) Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid. Redox Signal. 7, 385–394 [DOI] [PubMed] [Google Scholar]
- 22. Leiser S. F., Miller R. A. Nrf2 signaling, a mechanism for cellular stress resistance in long-lived mice. Mol. Cell. Biol. 30, 871–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun L. Y., D'Ercole A. J. (2006) Insulin-like growth factor-I stimulates histone H3 and H4 acetylation in the brain in vivo. Endocrinology 147, 5480–5490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thiel G., Cibelli G. (2002) Regulation of life and death by the zinc finger transcription factor Egr-1. J. Cell. Physiol. 193, 287–292 [DOI] [PubMed] [Google Scholar]
- 25. Beidelschies M. A., Huang H., McMullen M. R., Smith M. V., Islam A. S., Goldberg V. M., Chen X., Nagy L. E., Greenfield E. M. (2008) Stimulation of macrophage TNFalpha production by orthopaedic wear particles requires activation of the ERK1/2/Egr-1 and NF-kappaB pathways but is independent of p38 and JNK. J. Cell. Physiol. 217, 652–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cabodi S., Morello V., Masi A., Cicchi R., Broggio C., Distefano P., Brunelli E., Silengo L., Pavone F., Arcangeli A., Turco E., Tarone G., Moro L., Defilippi P. (2009) Convergence of integrins and EGF receptor signaling via PI3K/Akt/FoxO pathway in early gene Egr-1 expression. J. Cell. Physiol. 218, 294–303 [DOI] [PubMed] [Google Scholar]
- 27. Dickinson D. A., Levonen A. L., Moellering D. R., Arnold E. K., Zhang H., Darley-Usmar V. M., Forman H. J. (2004) Human glutamate cysteine ligase gene regulation through the electrophile response element. Free Radic. Biol. Med. 37, 1152–1159 [DOI] [PubMed] [Google Scholar]
- 28. Morse D., Lin L., Choi A. M., Ryter S. W. (2009) Heme oxygenase-1, a critical arbitrator of cell death pathways in lung injury and disease. Free Radic. Biol. Med. 47, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Piantadosi C. A. (2008) Carbon monoxide, reactive oxygen signaling, and oxidative stress. Free Radic. Biol. Med. 45, 562–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bianchet M. A., Erdemli S. B., Amzel L. M. (2008) Structure, function, and mechanism of cytosolic quinone reductases. Vitam. Horm. 78, 63–84 [DOI] [PubMed] [Google Scholar]
- 31. Meyer Y., Buchanan B. B., Vignols F., Reichheld J. P. (2009) Thioredoxins and glutaredoxins: unifying elements in redox biology. Annu. Rev. Genet. 43, 335–367 [DOI] [PubMed] [Google Scholar]
- 32. Oppermann U. (2007) Carbonyl reductases: the complex relationships of mammalian carbonyl- and quinone-reducing enzymes and their role in physiology. Annu. Rev. Pharmacol. Toxicol. 47, 293–322 [DOI] [PubMed] [Google Scholar]
- 33. Cobbett C., Goldsbrough P. (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu. Rev. Plant Biol. 53, 159–182 [DOI] [PubMed] [Google Scholar]
- 34. McCullough K. D., Martindale J. L., Klotz L. O., Aw T. Y., Holbrook N. J. (2001) Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell. Biol. 21, 1249–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oyadomari S., Mori M. (2004) Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 11, 381–389 [DOI] [PubMed] [Google Scholar]
- 36. Kennedy M. A., Rakoczy S. G., Brown-Borg H. M. (2003) Long-living Ames dwarf mouse hepatocytes readily undergo apoptosis. Exp. Gerontol. 38, 997–1008 [DOI] [PubMed] [Google Scholar]
- 37. Levine B., Sinha S., Kroemer G. (2008) Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy 4, 600–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zinkel S., Gross A., Yang E. (2006) BCL2 family in DNA damage and cell cycle control. Cell Death Differ. 13, 1351–1359 [DOI] [PubMed] [Google Scholar]
- 39. Yang E., Korsmeyer S. J. (1996) Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood 88, 386–401 [PubMed] [Google Scholar]
- 40. Lithgow G. J., Miller R. A. (2008) The determination of aging rate by coordinated resistance to multiple forms of stress. In The Molecular Biology of Aging (Guarente L., Partridge L., D., Wallace D. eds.), Cold Spring Harbor Press, Cold Spring, NY, USA [Google Scholar]
- 41. Weindruch R., Kayo T., Lee C. K., Prolla T. A. (2001) Microarray profiling of gene expression in aging and its alteration by caloric restriction in mice. J. Nutr. 131, 918S–923S [DOI] [PubMed] [Google Scholar]
- 42. Lee C. K., Klopp R. G., Weindruch R., Prolla T. A. (1999) Gene expression profile of aging and its retardation by caloric restriction. Science 285, 1390–1393 [DOI] [PubMed] [Google Scholar]
- 43. Weindruch R., Sohal R. S. (1997) Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N. Engl. J. Med. 337, 986–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Orentreich N., Matias J. R., DeFelice A., Zimmerman J. A. (1993) Low methionine ingestion by rats extends life span. J. Nutr. 123, 269–274 [DOI] [PubMed] [Google Scholar]
- 45. Coschigano K. T., Clemmons D., Bellush L. L., Kopchick J. J. (2000) Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology 141, 2608–2613 [DOI] [PubMed] [Google Scholar]
- 46. Miller R. A. (2009) Cell stress and aging: new emphasis on multiplex resistance mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 64, 179–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kapahi P., Boulton M. E., Kirkwood T. B. (1999) Positive correlation between mammalian life span and cellular resistance to stress. Free Radic. Biol. Med. 26, 495–500 [DOI] [PubMed] [Google Scholar]
- 48. Harper J. M., Salmon A. B., Chang Y., Bonkowski M., Bartke A., Miller R. A. (2006) Stress resistance and aging: influence of genes and nutrition. Mech. Ageing Dev. 127, 687–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miller R. A., Buehner G., Chang Y., Harper J. M., Sigler R., Smith-Wheelock M. (2005) Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell 4, 119–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li Y., Xu W., McBurney M. W., Longo V. D. (2008) SirT1 inhibition reduces IGF-I/IRS-2/Ras/ERK1/2 signaling and protects neurons. Cell. Metab. 8, 38–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Torres F., Quintana J., Estevez F. (2010) 5,7,3′-trihydroxy-3,4′-dimethoxyflavone-induced cell death in human Leukemia cells is dependent on caspases and activates the MAPK pathway. Mol. Carcinog. 49, 464–475 [DOI] [PubMed] [Google Scholar]
- 52. Korb A., Tohidast-Akrad M., Cetin E., Axmann R., Smolen J., Schett G. (2006) Differential tissue expression and activation of p38 MAPK alpha, beta, gamma, and delta isoforms in rheumatoid arthritis. Arthritis Rheum. 54, 2745–2756 [DOI] [PubMed] [Google Scholar]
- 53. Legos J. J., Erhardt J. A., White R. F., Lenhard S. C., Chandra S., Parsons A. A., Tuma R. F., Barone F. C. (2001) SB 239063, a novel p38 inhibitor, attenuates early neuronal injury following ischemia. Brain Res. 892, 70–77 [DOI] [PubMed] [Google Scholar]
- 54. Rabkin S. W., Lodhia P., Luong M. W. (2009) P38 MAP kinase in valve interstitial cells is activated by angiotensin II or nitric oxide/peroxynitrite, but reduced by Toll-like receptor-2 stimulation. J. Heart Valve Dis. 18, 653–661 [PubMed] [Google Scholar]
- 55. Sukhatme V. P., Cao X. M., Chang L. C., Tsai-Morris C. H., Stamenkovich D., Ferreira P. C., Cohen D. R., Edwards S. A., Shows T. B., Curran T., Le Beau M. M., Adamson E. D. (1988) A zinc finger-encoding gene coregulated with c-fos during growth and differentiation, and after cellular depolarization. Cell 53, 37–43 [DOI] [PubMed] [Google Scholar]
- 56. Adamson E. D., Mercola D. (2002) Egr1 transcription factor: multiple roles in prostate tumor cell growth and survival. Tumour Biol. 23, 93–102 [DOI] [PubMed] [Google Scholar]
- 57. Kensler T. W., Wakabayashi N., Biswal S. (2007) Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 47, 89–116 [DOI] [PubMed] [Google Scholar]
- 58. Nguyen T., Nioi P., Pickett C. B. (2009) The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 284, 13291–13295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Surh Y. J. (2003) Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 3, 768–780 [DOI] [PubMed] [Google Scholar]
- 60. Garofalo R. S., Orena S. J., Rafidi K., Torchia A. J., Stock J. L., Hildebrandt A. L., Coskran T., Black S. C., Brees D. J., Wicks J. R., McNeish J. D., Coleman K. G. (2003) Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J. Clin. Invest. 112, 197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tullet J. M., Hertweck M., An J. H., Baker J., Hwang J. Y., Liu S., Oliveira R. P., Baumeister R., Blackwell T. K. (2008) Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell 132, 1025–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bishop N. A., Guarente L. (2007) Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature 447, 545–549 [DOI] [PubMed] [Google Scholar]
- 63. Sykiotis G. P., Bohmann D. (2008) Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev. Cell 14, 76–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pearson K. J., Lewis K. N., Price N. L., Chang J. W., Perez E., Cascajo M. V., Tamashiro K. L., Poosala S., Csiszar A., Ungvari Z., Kensler T. W., Yamamoto M., Egan J. M., Longo D. L., Ingram D. K., Navas P., de Cabo R. (2008) Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc. Natl. Acad. Sci. U. S. A. 105, 2325–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Li J., Holbrook N. J. (2004) Elevated gadd153/chop expression and enhanced c-Jun N-terminal protein kinase activation sensitizes aged cells to ER stress. Exp. Gerontol. 39, 735–744 [DOI] [PubMed] [Google Scholar]
- 66. Song B., Scheuner D., Ron D., Pennathur S., Kaufman R. J. (2008) Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J. Clin. Invest. 118, 3378–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sierra F. (2006) Is (your cellular response to) stress killing you? J. Gerontol. A Biol. Sci. Med. Sci. 61, 557–561 [DOI] [PubMed] [Google Scholar]
- 68. Amador-Noguez D., Yagi K., Venable S., Darlington G. (2004) Gene expression profile of long-lived Ames dwarf mice and Little mice. Aging Cell 3, 423–441 [DOI] [PubMed] [Google Scholar]