Abstract
Both overuse and disuse of joints up-regulate matrix metalloproteinases (MMPs) in articular cartilage and cause tissue degradation; however, moderate (physiological) loading maintains cartilage integrity. Here, we test whether CBP/p300-interacting transactivator with ED-rich tail 2 (CITED2), a mechanosensitive transcriptional coregulator, mediates this chondroprotective effect of moderate mechanical loading. In vivo, hind-limb immobilization of Sprague-Dawley rats up-regulates MMP-1 and causes rapid, histologically detectable articular cartilage degradation. One hour of daily passive joint motion prevents these changes and up-regulates articular cartilage CITED2. In vitro, moderate (2.5 MPa, 1 Hz) intermittent hydrostatic pressure (IHP) treatment suppresses basal MMP-1 expression and up-regulates CITED2 in human chondrocytes, whereas high IHP (10 MPa) down-regulates CITED2 and increases MMP-1. Competitive binding and transcription assays demonstrate that CITED2 suppresses MMP-1 expression by competing with MMP transactivator, Ets-1 for its coactivator p300. Furthermore, CITED2 up-regulation in vitro requires the p38δ isoform, which is specifically phosphorylated by moderate IHP. Together, these studies identify a novel regulatory pathway involving CITED2 and p38δ, which may be critical for the maintenance of articular cartilage integrity under normal physical activity levels.—Leong, D. J., Li, Y. H., Gu, X. I., Sun, L., Zhou, Z., Nasser, P., Laudier, D. M., Iqbal, J., Majeska, R. J., Schaffler, M. B., Goldring, M. B., Cardoso, L., Zaidi, M., Sun, H. B. Physiological loading of joints prevents cartilage degradation through CITED2.
Keywords: matrix metalloproteinases; mechanotransduction, chondrocyte regulation
Mechanical loading is one of the most important environmental factors responsible for joint homeostasis, and there is tremendous concern about how joint motion and load bearing may impact joint health. Articular cartilage maintains its integrity throughout life under moderate loading conditions experienced during routine daily activity. In contrast, when cartilage is subjected either to excessive or insufficient mechanical loading, degeneration occurs. Exposure of joints to acute and chronic (repetitive) high-intensity loading eventually leads to osteoarthritis (OA) (1, 2). On the other hand, the absence of normal mechanical stimulation, as occurs in paralysis following spinal cord injury, can also cause articular cartilage breakdown through the induction of a similar catabolic response (3, 4). To date, we have begun to define many of the cellular and molecular processes responsible for these catabolic changes (5). However, the mechanism through which moderate loads prevent cartilage degradation and maintain functional capacity remains unknown.
Articular cartilage destruction results from the breakdown of extracellular matrix (ECM) constituents, primarily type II collagen and aggrecan, whose proteolysis are mediated by matrix metalloproteinases (MMPs) and a disintegrin-metalloproteinases with thrombospondin motifs (ADAMTS), respectively. MMPs comprise a large family of structurally related endopeptidases and are considered to be the principal mediators of both normal ECM remodeling and pathological degradation (6). Of the 26 MMPs identified, MMP 1, 8, and 13 represent the secreted neutral proteinases capable of cleaving type II collagen (6). MMPs 1 and 13 are highly expressed in the cartilage and synovium of OA and rheumatoid arthritis patients (7–10), though MMP-8 is not appreciably elevated in OA (11). Other MMPs, including MMPs 2, 3, and 9 have also been reported to play a role in the degradation of cartilage ECM (5, 12).
MMP expression and function are controlled, both positively and negatively, at multiple levels, including transcription and inactivation by specific inhibitors (tissue inhibitors of metalloproteinases, or TIMPs) (13). Up-regulation of MMPs in response to mechanical stimuli has been documented in a wide range of tissues and cells (14), via multiple signal transduction pathways often involving members of MAP kinase families, e.g., p38 and p42/44 (15), but much less is known about the down-regulation of MMP expression by mechanical loading. In a previous study, we showed that moderate levels of flow shear down-regulated expression of MMP-1 and MMP-13 in human C28/I2 chondrocytes and that this down-regulation coincided with up-regulation of the transcriptional regulator CITED2 (CBP/p300-interacting transactivator with ED-rich tail 2) (16), suggesting that CITED2 may contribute to MMP regulation in chondrocytes. In the present study, we sought to define the role of CITED2 in the regulation of MMP-1 and more importantly, the underlying mechanisms by using an in vivo immobilization/remobilization model, and a cartilage explant and cell culture system in which intermittent hydrostatic pressure was used to mimic the loading environment experienced by chondrocytes in vivo.
MATERIALS AND METHODS
Animal experiments
Male Sprague-Dawley rats (5–6 mo old, 580±35 g) were housed with access to food and water ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of Mount Sinai School of Medicine. The right hind limbs were immobilized as described previously (17). Briefly, rats were anesthetized with isoflurane and were fitted with a cast made of steel mesh and cotton materials, which fixed the knee in full flexion (115°). Animals were either left immobilized for the duration of the experiment, released from casting for 1 h under anesthesia (sham treatment) or subjected to motion loading at a frequency of 2 cycles/min with a range of motion between 65° and 115° using a custom-designed loading device (18). After motion loading or release from immobilization under anesthesia (sham treatment group), the rats were sacrificed or recast until the next motion loading session. The short and extended protocols are described in Results. In each protocol, a control group was allowed free cage activity throughout the experiment. Following sacrifice, cartilage from immobilized knee joints (distal femur, proximal tibia) was rapidly removed and prepared for MMP activity assays, histology or RNA isolation.
MMP activity assay
Articular cartilage was dissected, flash-frozen in liquid nitrogen, and stored at −80°C. MMP-1 activity was assayed by using the Senosolyte Plus 520 MMP-1 assay kit (Anaspec, Fremont, CA, USA). Frozen tissue samples were pulverized (Dismembrator; B Braun Biotech, Burladingen, Germany) in MMP activity assay buffer (Anaspec) containing 0.1% (v/v) Triton-X 100, and then centrifuged for 15 min at 10,000 g at 4°C. The supernatant was added to a 96-well plate containing immobilized MMP-1 antibody in the presence of 4-aminophenylmercuric acetate (APMA) for 3 h, and its proteolytic activity was measured by the 5-FAM/QXL 520 FRET peptide (Anaspec). The fluorescence of 5-FAM (fluorophore) was quenched by QXL 520 in the intact FRET peptide. Fluorescence signal was monitored at Ex/Em = 490 nm/520 nm on MMP-1-induced cleavage of the QXL FRET substrate. Fluorescent intensity was measured using the SpectraMax M5 spectrofluorometer (Molecular Devices, Sunnyvale, CA, USA).
Immunohistochemistry and Safranin O staining
Total knee joints were fixed in formalin, decalcified, paraffin-embedded, and sectioned. For immunostaining, endogenous peroxidase activity was blocked with 3% (v/v) H2O2 for 5 min. Tissues were incubated overnight at 4°C with either anti-CITED2 (1:300; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-MMP-1 (1:100; Abcam, Cambridge, MA, USA), or Col2–3/4M (1:200; Ibex Pharmaceuticals, Montreal, QC, Canada), followed by a 30-min incubation with anti-mouse or anti-rabbit secondary antibody (Dako, Carpinteria, CA, USA) and visualization with DAB chromagen for 3 min. Negative control sections were prepared using irrelevant isotype-matched antibodies in place of the primary antibody. Safranin O-fast green staining was carried out to demonstrate glycosaminoglycans in the articular cartilage.
Cartilage explants, cell culture, and intermittent hydrostatic pressure loading
Articular cartilage from the rat distal femur and proximal tibia were carefully separated from the underlying bone under a dissecting microscope. The cartilage explants and C28/I2 cells (cultured as high-density monolayers) were each cultured in DMEM supplemented with 10% FBS, 100 μg/ml ascorbic acid, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C (19). Both the rat articular cartilage explants and C28/I2 cells were starved in serum-free DMEM 18 h prior to intermittent hydrostatic pressure (IHP) loading in a custom-made device (20). After loading, cartilage explants pulverized in liquid nitrogen and C28/I2 cells were lysed for total RNA or protein isolation, for quantitative PCR or Western blot, respectively.
Transfection of siRNA and plasmids
C28/I2 cells were incubated with siRNA for CITED2 (Santa Cruz Biotechnology) or transfected with pcDNA3.1 plasmids constructed to encode cDNA sequences for wild-type (WT) human CITED2, Ets-1, or mutant forms of CITED2 and Ets-1 with selected sequences deleted using restriction enzyme digestion and PCR cloning strategies as described previously (16). In other studies, WT or mutated human CITED2 cDNA was cotransfected with either WT p300 or a dominant-negative (DN) p300 form, in which the CH1 domain was deleted to prevent CITED2-binding (Upstate Biotechnology, Lake Placid, NY, USA).
RNA isolation and quantitative PCR
Total RNA from flash-frozen articular cartilage or C28/I2 cells was extracted using the RNeasy mini kit (Qiagen, Valencia, CA, USA) with DNase treatment, according to manufacturer's instructions. RNA was quantified with a Nanodrop spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA), then reverse transcribed (RT) using oligo(dT) primers. Two nanograms of total RNA was analyzed by quantitative PCR with SYBR Green to assess relative gene expression, as well as GAPDH and β-actin as housekeepers. PCR primer pairs used were as follows: rat-MMP-1A, forward 5′-ACAGTTTCCCCGTGTTTCAG-3′, reverse 5′-CCCACACCTAGGTTTCCTCA-3′; human-MMP1, forward 5′-GGTGACACCAGTGACTGCAC-3′, reverse 5′-TCCACAAATGGTGGGTACAA-3′; rat/human-CITED2, forward 5′-TGCCGCCCAATGTCATAG-3′, reverse 5′-CTGCTGTTTGCACACGAAG-3′; rat/human-GAPDH, forward 5′-GAGGACCAGGTTGTCTCCTG-3′, reverse 5′-ATGTAGGCCATGAGGTCCAC-3′; rat/human-β-actin, forward 5′-TTGCTGACAGGATGCAGAAG-3′, and reverse 5′-ACATCTGCTGGAAGGTGGAC-3′. Expression values of GAPDH and β-actin for each treatment condition were averaged and used as a denominator to determine the relative expression of target genes using the 2−ΔΔCt method.
Western blot/immunoprecipitation assay
Western blot analysis was performed by using antibodies specific for MMP-1 (EMD Chemicals, Gibbstown, NJ, USA) CITED2 (Santa Cruz Biotechnology). Ets-1 (Santa Cruz Biotechnology), and p300 (Upstate Biotechnology). Approximately 10 μg of the extracted proteins was separated by electrophoresis (SDS-PAGE) in a 10% polyacrylamide gel, and the separated proteins were transferred to Immun-Blot PVDF membranes (Bio-Rad, Hercules, CA, USA). Membranes were incubated with primary antibodies followed by incubation with secondary antibodies conjugated to horseradish peroxidase (ECL Western blotting analysis system; GE Life Sciences, Piscataway, NJ, USA). A mouse antibody specific for β-actin (Sigma-Aldrich, St. Louis, MO, USA) was used as loading control.
For immunoprecipitation, 100 μg of nuclear extracts was mixed with 8 μg of p300-specific antibody (Upstate Biotechnology) and incubated with gentle rocking at 4°C overnight. The immunocomplexes were captured by mixing with protein G-agarose beads for 2 h at 4°C. The collected agarose beads were washed 4 times with an ice-cold cell lysis buffer, and the precipitated proteins were eluted by boiling the beads for 5 min in the SDS sample buffer. Fractions of the supernatant were analyzed by SDS-PAGE and immunoblotting using antibodies against p300, CITED2, and Ets-1.
Promoter analysis and luciferase assay
A 510-bp region from the MMP-1 promoter (−468 to +42 relative to the transcription start site, GenBank M16567) was cloned by amplifying human genomic DNA by PCR and subcloned into the pGL3-Basic Vector (Promega, Madison, WI, USA) immediately upstream of the luciferase reporter gene. WT and serial deletions of the 5′ end of the CITED2 promoter region were generated in separate reactions and subcloned into pGL3-basic vector. For the MMP-1 promoter assay, chondrocytes were transfected or cotransfected with MMP-1 promoter reporter construct, pcDNACITED2, pCMVp300 (Upstate Biotechnology), pKD-p300-v1 (p300 siRNA), p300 siRNA scramble, and empty vector controls, and/or subjected to IHP loading (2.5 MPa, 1 Hz, 1 h). For the CITED2 transactivation assay, the cells were transfected or cotransfected with a CITED2 promoter reporter construct, pCMVp38δ WT, pCMVp38δ DN, pCMVp38α WT, pCMVp38α DN (Cell Biolabs, San Diego, CA, USA), p38δsiRNA (Ambion, Austin, TX), and/or subjected to IHP loading (2.5 MPa, 1 Hz, 1 h). For the CITED2 promoter serial deletion analysis, WT and deleted CITED2 promoter reporters were cotransfected with pCMVp38δ WT into chondrocytes. DNA constructs were transfected into cells using Lipofectamine Plus Reagent (Life Technologies, Carlsbad, CA, USA). For luciferase assay, cells were harvested in Passive Lysis Buffer (Promega). Luciferase activity in the cell lysates was determined using the Dual-Luciferase Reporter Assay System (Promega). All firefly luciferase values were normalized for transfection efficiency using the pRL-TK, Renilla luciferase value.
Peptide competition assay
Ets-1 displacement from GST-p300 CH1 (aa 302–423) domain by the synthetic CITED2 transactivation domain (TAD) peptide (aa 224–255), and CITED2 TAD displacement from the GST-p300 CH1 domain by Ets-1 TAD peptide (aa 153–210) were measured by an ELISA-based peptide competition assay, as described previously (21). Briefly, increasing amounts of CITED2 or Ets-1 TAD peptide were added to immobilized biotinylated Ets-1 peptide or biotinylated CITED2 TAD peptide, respectively. A constant amount of GST-p300 CH1 was then added. Bound GST-p300 CH1 was quantified by time-resolved fluorescence using europium-labeled anti-GST antibody (Perkin Elmer, Waltham, MA, USA). Mean half-maximal inhibitor concentration (IC50) values were calculated for 25 separate experiments after fitting data using a nonlinear regression (Prism; GraphPad, La Jolla, CA, USA).
Kinase assay
The phosphorylation of p38 isoforms were conducted using a nonisotopic p38 MAPK assay kit (Cell Signaling, Danvers, MA, USA), as described previously (22). Briefly, total cell lysates were prepared from chondrocytes and equal amounts of total protein (100 μg) were precipitated using p38α or p38δ MAPK antibody, respectively. The activities of precipitated kinases were then allowed to phosphorylate the p38 substrate, ATF2, in the presence of ATP. Phosphorylation of ATF2 was analyzed by immunoblotting with an antibody specific for phospho-ATF2. The expression of p38α or p38δ was confirmed by immunoblotting with the isoform-specific antibody.
Statistical analysis
Results are expressed as means ± sd. Statistical analysis was carried out using a 1-way ANOVA and Tukey's test for post hoc analysis with significance set at P < 0.05.
RESULTS
CITED2 induction in articular cartilage in vivo is associated with MMP-1 down-regulation and anticatabolic effects of joint motion
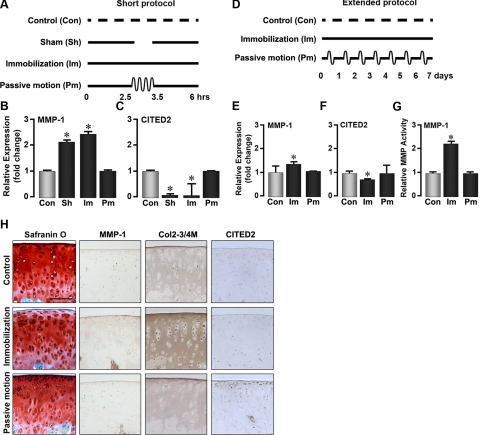
To test whether CITED2 mediates the protective effects of physiological joint motion on cartilage, we first determined the relationship between of CITED2 and MMP-1 expression under different mechanical loading regimens. Two protocols were used. First, in the short protocol, 4 groups of rats were treated as follows: 1) no immobilization and no passive motion, 2) continuous immobilization for 6 h, 3) immobilization for 6 h interrupted by 1-h passive motion, and 4) immobilization for 6 h interrupted by a 1-h release from the cast under anesthesia, with no passive motion (Fig. 1A).
Figure 1.
Passive motion loading prevents cartilage degradation. Schematics: representations of the short protocol (A), where the rat hind limb is immobilized for 6 h interrupted by 1 h of passive motion or release from immobilization under anesthesia, or the extended protocol (D), where the rat limb is immobilized for 7 d with or without a 1-h/d passive motion protocol. Graphs: qPCR showing fold-change in mRNA levels of MMP-1 (B, E) or CITED2 (C, F), and MMP-1 (enzyme) activity (G) after immobilization (Im), passive motion loading (Pm), sham treatment (Sh), or no treatment (Con). Images: Safranin O staining and immunohistochemical localization of MMP-1, type II collagen denaturation (Col2–3/4M antibody), and CITED2 in articular cartilage from rats undergoing the extended protocol (H). For statistical analysis, 1-way ANOVA and Tukey's post hoc test were performed; n = 5 rats/group. Scale bar = 100 μm. *P < 0.05 vs. control.
Quantitative PCR (qPCR) analysis of articular cartilage samples dissected immediately after the immobilization period revealed that MMP-1 was up-regulated in the immobilization-only group (Fig. 1B). The up-regulation of MMP-1 was accompanied by a decline in CITED2 mRNA (Fig. 1C). In contrast, passive motion loading applied for 1 h in the midst of the 6-h immobilization period completely reversed these changes (Fig. 1B, C). Notably, simply removing the immobilization stimulus for 1 h without accompanying passive motion (sham treatment group), did not reverse the immobilization-induced up-regulation of MMP-1.
We next extended the immobilization protocol to 7 d and inserted a 1-h passive motion loading period each day (extended protocol; Fig. 1D). qPCR analysis (Fig. 1E, F) revealed that immobilization stimulated MMP-1 while attenuating CITED2 expression, but both of these changes were prevented on passive motion loading. We also measured the activity of MMP-1 and found that it mirrored MMP-1 mRNA levels under all conditions examined (Fig. 1G).
Histological examination of distal femoral articular cartilage revealed evidence of tissue degradation in immobilized rats, and also indicated that these changes were largely prevented by passive motion loading (Fig. 1H). In particular, immobilization led to decreased Safranin O staining (indicating reduced proteoglycan content) in the superficial zone of articular cartilage; this decrease was accompanied by increases in the number of MMP-1-positive cells, stronger staining for denatured type II collagen in the superficial and middle zones of the articular cartilage, and reduction in the number of cells with positive staining for CITED2. By contrast, passive motion loading of immobilized limbs prevented loss of proteoglycans, limited the increases in immunohistochemically detectable MMP-1 and denatured type II collagen, and also increased the number of cells staining positive for CITED2 (Fig. 1H, middle and bottom panels). Taken together, these results indicate that in vivo moderate loading suppresses the catabolic response to immobilization by inducing CITED2 and down-regulating MMP-1.
CITED2 is required for moderate loading-induced down-regulation of MMP-1
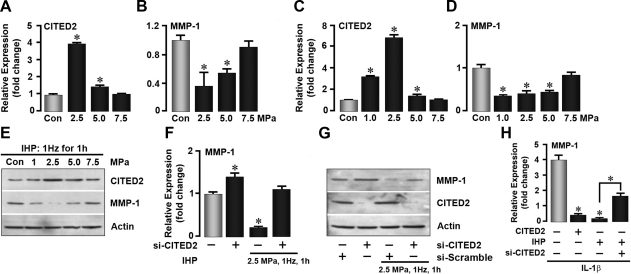
To assess the regulatory role of CITED2 in MMP-1 expression in vitro and ex vivo, IHP, which is thought to best mimic the loading environment experienced by articular cartilage in vivo (23), was utilized to mechanically stimulate freshly recovered rat cartilage explants or cultured chondrocytes. As shown in Fig. 2A, B, the application of 2.5 and 5.0 MPa, pressures consistent with those experienced by joints during walking (10), caused a profound increase in CITED2 and a decrease in MMP-1 mRNA expression in rat articular cartilage explants. Parallel in vitro studies with chondrocytes showed qualitatively similar results to those with cartilage explants, namely elevation in CITED2 mRNA and reduction in MMP-1 mRNA seen best at 2.5 MPa (Fig. 2C, D), as well as corresponding changes in protein expression on Western blot analysis (Fig. 2E).
Figure 2.
CITED2 is required for load-induced down-regulation of MMP-1. A–E) qPCR showing the effect of IHP loads (MPa as shown, 1 Hz, 1 h) applied to articular cartilage explants (n=5 rats/group) (A, B) or to chondrocytes on CITED2 and MMP-1 mRNA expression (C, D) and CITED2 and MMP-1 protein expression (E) detected by Western blot. F–H) qPCR (F, H) and Western blots (G) showing, respectively, the effect of transfecting chondrocytes with pcDNA3.1-CITED2 or small interfering RNAs for CITED2 (si-CITED2) or scrambled RNA (si-Scramble) on MMP-1 mRNA and protein expression in response to IHP (2.5 MPa, 1 Hz, 1 h) in the absence or presence of IL-1β. Experiments were performed in triplicate. *P < 0.05 vs. control.
To determine whether CITED2 was indeed required for MMP-1 down-regulation in response to mechanical stimuli, we used CITED2 loss- and gain-of-function strategies. A modest, but significant, increase in MMP-1 mRNA was noted when CITED2 was suppressed by siRNA knockdown in the basal state (Fig. 2F). As expected, loading lowered MMP-1 mRNA but, notably, failed to do so in si-CITED2-transfected cells (Fig. 2F). Western blotting showed an effective reduction of CITED2 protein in the si-CITED2 transfectants and also demonstrated their resistance to load-induced MMP-1 down-regulation (Fig. 2G). Consistently, moderate loading, as well as CITED2 overexpression, also dramatically inhibited the expression of MMP-1 induced by IL-1β, a key mediator of cartilage degradation in both OA and rheumatoid arthritis (24) (Fig. 2H). This load-induced suppression of MMP-1 was again abolished in si-CITED2 transfected cells (Fig. 2H). These experiments provide clear evidence that, at least in vitro, the up-regulation of CITED2 is required for the load-induced attenuation of MMP-1.
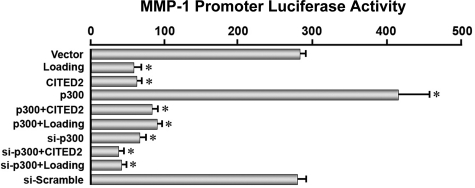
To further demonstrate the critical involvement of CITED2 in the moderate loading-induced down-regulation of MMP-1, modulation of MMP-1 at the transcriptional level by CITED2 was examined by transactivation assays. CITED2 is a transcriptional regulator that does not bind directly to DNA promoter regions, but functions by interacting with other transcription factors or cofactors, such as p300 (25). For this reason, chondrocytes were cotransfected with a 2.3-kb MMP-1 promoter-luciferase construct and either empty vector or constructs containing CITED2, p300, or si-p300, and the promoter activity was measured using a luciferase assay. In certain conditions, cells were subjected to moderate loading at 2.5 MPa, 1 Hz, for 1 h. As shown in Fig. 3, cells transfected with empty vector or si-scramble displayed potent luciferase activity (in agreement with the MMP-1 mRNA results in Fig. 2), and the activity level was further stimulated by p300 overexpression. In contrast, the MMP-1 transactivation was substantially reduced in all groups of cells overexpressing CITED2, and the suppressive effect of CITED2 was comparable to those with either loading (2.5 MPa) or p300 knockdown (Fig. 3). These findings indicate that MMP-1 transcription is directly mediated by CITED2 and p300.
Figure 3.
Direct effects of CITED2 and p300 on MMP-1 promoter transactivation. Effect of cotransfecting chondrocytes with CITED2, Ets-1, or p300 in various combinations (shown) with or without siRNA for p300 (si-p300) or IHP (2.5 MPa, 1 Hz, 1 h) on MMP-1 promoter activity in luciferase assays. Experiments were performed in triplicate. *P < 0.05 vs. vector-transfected cells.
CITED2 down-regulates MMP-1 by competing with Ets-1 for p300 binding
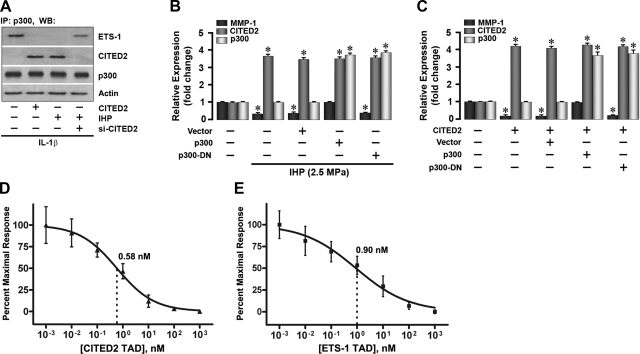
We next took further steps to dissect the detailed molecular events surrounding the CITED2 regulation of MMP-1 expression. The transcriptional coregulator p300 is the principal binding partner of both CITED2 and MMP-1 transactivator Ets-1 (21, 26–28). We speculated that CITED2 may function to down-regulate MMP-1 by competing with Ets-1 for p300 binding. To test this hypothesis, we characterized the p300 complexes in cells with altered expression of CITED2 by immunoprecipitation assays. As shown in Fig. 4A, the formation of CITED2-p300 complexes due to either loading (which up-regulated CITED2) or CITED2 overexpression was accompanied by complete displacement of Ets-1 from its p300 binding site. Conversely, suppression of CITED2 by transfection with si-CITED2 enhanced Ets-1 binding to p300 (Fig. 4A). These results support our premise that the p300-CITED2 complex formed on loading is responsible for the effects of CITED2 on MMP-1 expression.
Figure 4.
CITED2 competes with Ets-1 for p300 binding. A) Immunoprecipitation with anti-p300 antibody and Western blot (WB) with either anti-CITED2 or anti-Ets-1 antibodies to reveal protein-protein interactions between Ets-1, CITED2, and p300. B, C) qPCR showing the effects of overexpressing p300 or dominant-negative p300 (p300-DN) on the relative expression of MMP-1, CITED2, and p300 in response to IHP (2.5 MPa, 1 Hz, 1 h; B) or cotransfection with WT CITED2 without IHP (C). D, E) Mean IC50 concentrations (nM) for CITED2 on Ets-1-p300 (D) or Ets-1 on CITED2-p300 (E).
The direct competition between CITED2 and Ets-1 for binding to p300 was supported by two additional observations. First, an examination of a dominant-negative (DN) p300 lacking the CH1 domain that binds to CITED2 and Ets-1 indicated that p300 was absolutely required for the regulation of MMP-1 by CITED2 or Ets-1. As expected, loading at 2.5 MPa reduced MMP-1 and increased CITED2 expression without affecting p300, both in untransfected and vector-transfected chondrocytes. In contrast, overexpression of WT p300, which led to increased complex formation between p300 and Ets-1, blocked the load-induced attenuation of MMP-1 without altering CITED2 levels (Fig. 4B). However, p300-DN was unable to correct the load-induced down-regulation of MMP-1. Overexpression of CITED2 under these conditions mimicked the effects of loading (Fig. 4C). Second, bound GST-p300 CH1 quantified by time-resolved fluorescence measurements of a Europium-labeled anti-GST antibody in ELISA-based competition assays revealed a subtle difference between the IC50 concentrations for reduction of the Ets-1-p300 complex by CITED2 (0.58 nM) and of the CITED2-p300 complex by Ets-1 (0.90 nM) (Fig. 4D, E). This difference suggests a potential chondroprotective mechanism that p300, under physiological conditions, may exhibit a slight preference for binding to its partner, CITED2, over its catabolic coregulator Ets-1.
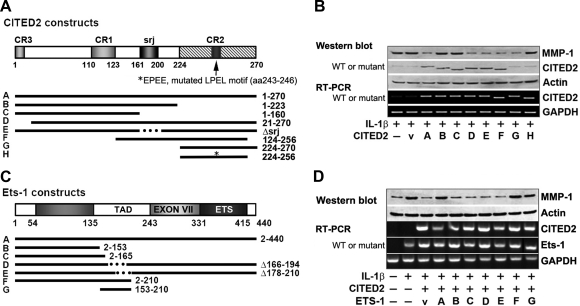
To identify specific regions within the binding domains of CITED2 and Ets-1 required for MMP-1 regulation, we carried out a series of transfection experiments using deletions and point mutations introduced into each molecule (Fig. 5A). In the first set of experiments, we treated chondrocytes transfected with WT or mutant CITED2 with IL-1β and examined MMP-1 mRNA and protein expression by PCR and Western blot analysis, respectively. We found that the CITED2 fragment containing aa 224 to 256 was both necessary and sufficient for MMP-1 down-regulation, whereas a CITED2 fragment lacking this region (i.e., fragment 1–223) did not (Fig. 5B). More specifically, EPEE, a mutation of the LPEL motif found within the 224–256 aa region, completely abolished the down-regulation of MMP-1 expression, suggesting that LPEL was critical for load-induced MMP-1 regulation.
Figure 5.
CITED2 regulates MMP-1 through specific motif. A, B) Effect of transfecting chondrocytes with plasmids encoding either WT CITED2, or CITED2 deleted, truncated, or point mutants on MMP-1 and CITED2 mRNA (PCR; A) and/or protein (Western blot) expression in the presence of IL-1β (B). CR, conserved region; Δsrj, CITED2 WT with deletion of 39-aa serine-glycine rich junction; EPEE, mutated LPEL (aa 243–246) motif. C, D) Effect of cotransfecting WT Ets-1 or Ets-1 deleted or truncated mutants with CITED2 (C) on MMP-1 and CITED2 mRNA (PCR) and/or protein (Western blot) expression (D) in the presence of IL-1β.
Cotransfection of chondrocytes with WT CITED2 and mutated forms of Ets-1 (Fig. 5C) showed that overexpression of CITED2 suppressed IL-1β-induced expression of MMP-1 and that overexpression of WT Ets-1 partially reversed the MMP-1 suppression caused by CITED2. However, this rescue was abolished in the transfectants overexpressing Ets-1 fragments, in which the TAD (aa 135–243) was removed either by truncation beyond aa 153 and 165, respectively, or through its deletion, as in the fragments Δ166–194 and Δ178–210 (Fig. 5D). Most importantly, however, the fragment 153–210 fully reversed the effects of CITED2 overexpression, suggesting that the p300-binding domain of Ets-1, which competes with CITED2, is indeed the critical component of the TAD.
p38δ Activation mediates the loading-induced transactivation of CITED2
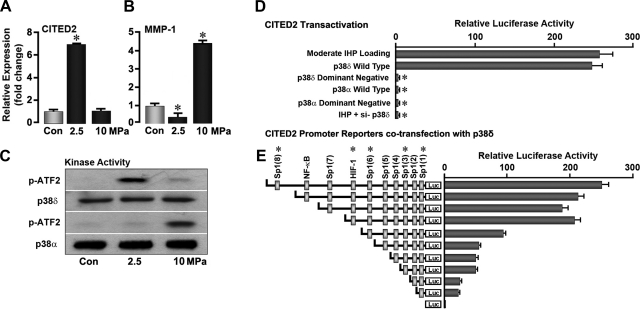
Finally, we determined the upstream signals necessary for moderate mechanical loads to induce CITED2 expression. Among several known candidates that act as potential mechanotransducers, the MAP kinases, particularly p38, have received much attention (29, 30). However, the roles played by individual p38 isoforms (p38α, p38β, p38γ, p38δ) in cartilage mechanotransduction have not been investigated. Moderate loads of 2.5 MPa, which up-regulate CITED2 and down-regulate MMP-1 (Fig. 6A, B), did not stimulate phosphorylation of p38α but specifically stimulated the phosphorylation of a p38δ (Fig. 6C). In contrast, chondrocytes loaded at 10 MPa resulted in the phosphorylation of p38α (Fig. 6C), which coincides with the up-regulation of MMP-1 (Fig. 6B), a change that typifies a catabolic response to nonphysiological loads. We therefore explored how p38δ regulates CITED2 expression in response to moderate loading by examining the transactivation of the 3.3-kb CITED2 promoter. Promoter-reporter assays using luciferase constructs transfected into chondrocytes confirmed the stimulation of CITED2 transactivation by both loading and p38δ overexpression, proving that p38δ is upstream of CITED2 and downstream of loading (Fig. 6D). Furthermore, this response was abolished by dominant-negative p38δ and si-p38δ.
Figure 6.
MAP kinase p38δ is the upstream mediator of CITED2 in response to moderate loading. A, B) qPCR showing the effect of IHP (MPa as shown, 1 Hz, 1 h) on the expression of CITED2 (A) and MMP-1 (B). C) Western blot showing p38δ and p38α activation, measured as phosphorylation of the p38 substrate, ATF2. D) Luciferase activity in chondrocytes transfected with the CITED2 promoter-reporter construct after application of IHP (2.5 MPa, 1 Hz, 1 h). E) Cotransfection with WT or dominant-negative p38δ or p38α, or with sequential deletion constructs of CITED2 cotransfected with p38δ. Experiments were performed in triplicate. *P < 0.05 vs. control, WT, or previous deletion construct.
Analysis of CITED2 promoter responsiveness to p38δ by means of progressive deletion constructs showed that removal of the NF-κB-binding site did not appreciably reduce promoter transactivation (Fig. 6E). Deletion of the HIF-1α response element in the (−940) CITED2 promoter region attenuated transactivation by ∼50% (Fig. 6E). However, while removal of one Sp1 site in the −2300-bp CITED2 fragment produced no change in transactivation, the subsequent deletion of 3 further Sp1 sites almost completely abolished promoter responsiveness to p38δ (Fig. 6E). Together, these data suggest that the effect of p38δ on CITED2 is, at least in part, mediated by HIF-1α and Sp1.
DISCUSSION
The idea that musculoskeletal tissue integrity is best maintained at moderate (physiological) levels of mechanical loading has long been appreciated. In muscle and bone, for example, repair of minor tissue damage from overuse entails breakdown and removal of the damaged tissue and replacement with newly formed matrix (31, 32). On the other hand, tissue breakdown also occurs as a result of loading insufficiency, resulting in muscle atrophy (33) and osteopenia or osteoporosis (34). While an understanding of the molecular mechanisms that transduce mechanical stimuli into increases in bone and muscle mass is emerging, less is known of how mechanical stimuli generated by joint movement, and the frequency and intensity of such loads, help to maintain cartilage integrity. Accumulating clinical evidence indicates that, in contrast to excessive or inadequate loads, continuous passive motion along with other forms of physiological joint loading is necessary for proper joint maintenance, and joint motion can protect cartilage from cartilage degradation (35–37). In this study, we show for the first time that physiologically relevant passive loading of normal joints prevents cartilage degradation through the up-regulation of CITED2 using the p38δ pathway.
MMPs are proteolytic enzymes responsible for the destruction of articular cartilage. This study focused on a single MMP, MMP-1, and on its transcriptional control by CITED2. MMP-1 was selected for this study as a prototypical catabolic enzyme because it directly cleaves type II collagen and is highly expressed in arthritic articular cartilage (8, 38). Moreover, since MMP-1 has only been identified recently in mice and rats (39, 40), the role of MMP-1 is less understood in rodent models. We believe that the results obtained with MMP-1, however, will be reflected in other MMPs as well. We recently showed in this experimental system that MMP-3 is also sensitive to immobilization and passive motion (41). In addition, we have established that expression of MMP-13 in vitro follows the same inverse relationship with CITED2 demonstrated for MMP-1 (16).
Our study provides several lines of evidence that CITED2 is essential in order to mediate the transcriptional suppression of MMP-1 by moderate hydrostatic pressure loading, and elucidate mechanistic features of this novel pathway. Analysis of CITED2 loss and gain of function revealed that under moderate levels of intermittent hydrostatic pressure, si-CITED2 abolished the reduction in MMP-1 expression and forced overexpression of CITED2 mimicked the effects of moderate loading by down-regulating MMP-1. CITED2 competed with a MMP transactivator, Ets-1, and displaced it from the p300-Ets-1 complex to reduce MMP-1 transactivation. The CITED2 nucleotide region encoding the fragment between aa 224 and 270 was absolutely required and sufficient to down-regulate MMP-1. CITED2 suppressed MMP-1 promoter activation in a process requiring p300. Taken together, these observations strongly support our hypothesis that CITED2 mediates a chondroprotective signaling pathway by competing with Ets-1 for p300 binding to down-regulate MMP-1 expression. That our results in vivo and in vitro were in excellent agreement with respect to the inverse relationship between expression of CITED2 and MMP-1 argues strongly that the mechanistic relationships we established in vitro are likely to hold in vivo.
The proposed mechanism of MMP-1 regulation by CITED2, involving competition with Ets-1 for binding to p300, was consistent with expectations based on published binding characteristics of CITED2 and HIF-1α for p300 (21). Interestingly, however, the competition binding studies indicated a slightly greater affinity of p300 for CITED2 than for Ets-1. To the extent that these relationships translate to in vivo circumstances, the result suggests that this potential regulatory switching mechanism may be slightly biased toward suppression, rather than activation, of MMP transcription. Such a bias might be useful to prevent overaggressive catabolic responses to changes in the mechanical environment.
While our results demonstrated direct transcriptional regulation of MMP-1 by CITED2 in chondrocytes, it is likely that additional regulatory mechanisms may also operate, particularly in vivo. Virtually all cell types present in joint tissues (cartilage, bone, synovium) are mechanoresponsive (16, 42–44). Furthermore, mechanical stimulation is known to alter the production of, as well as response to, cytokines known to regulate MMP expression, including IL-1β and TNF-α (35–37).
Another novel finding of this study was that the mechanosensitive up-regulation of CITED2, which occurred in response to moderate levels of mechanical loading in vitro and in vivo, was associated with the selective activation of a specific isoform of p38, p38δ. Members of the p38 MAP kinase family are activated in response to mechanical stresses and a wide range of chemical stimuli (30, 45) and have been implicated in the up-regulation of MMPs (46). We have observed that high levels of loading led to neither phosphorylation of p38δ nor CITED2 up-regulation. This may partially explain why CITED2 is functional only under moderate loading with regard to the regulation of MMP expression. Conversely, excessive loading induced MMP-1 and phosphorylation of p38α, suggesting that the latter is likely involved in the regulation of MMP-1. Together, these data indicate that distinct members of the p38 family can act within the same cell population to promote and to suppress MMP-based matrix breakdown, depending on the mechanical loading environment.
In summary, we have identified a novel CITED2-mediated pathway in which CITED2, induced by moderate loads through the activation of p38δ, suppresses MMP-1 transcription by displacing transactivator Ets-1 from coactivator p300. The identification of this CITED2 pathway as a means to regulate MMP expression in cartilage not only provides a molecular basis for the prevention of cartilage degradation by physiological loads, but also offers the possibility that this pathway could be exploited therapeutically in diseases like arthritis.
Acknowledgments
This study was supported by grants from the National Institutes of Health to H.S. (AR47628 and AR52743), L.S. (DK804590), M.Z (AG23176, DK70526, and DK804590), and M.B.G. (AG022021).
REFERENCES
- 1. Buckwalter J. A., Martin J. A., Brown T. D. (2006) Perspectives on chondrocyte mechanobiology and osteoarthritis. Biorheology 43, 603–609 [PubMed] [Google Scholar]
- 2. Helmick C. G., Felson D. T., Lawrence R. C., Gabriel S., Hirsch R., Kwoh C. K., Liang M. H., Kremers H. M., Mayes M. D., Merkel P. A., Pillemer S. R., Reveille J. D., Stone J. H. (2008) Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 58, 15–25 [DOI] [PubMed] [Google Scholar]
- 3. Jurvelin J., Kiviranta I., Tammi M., Helminen J. H. (1986) Softening of canine articular cartilage after immobilization of the knee joint. Clin. Orthop. Relat. Res. 246–252 [PubMed] [Google Scholar]
- 4. Vanwanseele B., Eckstein F., Knecht H., Stussi E., Spaepen A. (2002) Knee cartilage of spinal cord-injured patients displays progressive thinning in the absence of normal joint loading and movement. Arthritis Rheum. 46, 2073–2078 [DOI] [PubMed] [Google Scholar]
- 5. Goldring M. B., Marcu K. B. (2009) Cartilage homeostasis in health and rheumatic diseases. Arthritis Res. Ther. 11, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burrage P. S., Mix K. S., Brinckerhoff C. E. (2006) Matrix metalloproteinases: role in arthritis. Front. Biosci. 11, 529–543 [DOI] [PubMed] [Google Scholar]
- 7. Mengshol J. A., Mix K. S., Brinckerhoff C. E. (2002) Matrix metalloproteinases as therapeutic targets in arthritic diseases: bull's-eye or missing the mark? Arthritis Rheum. 46, 13–20 [DOI] [PubMed] [Google Scholar]
- 8. Vincenti M. P., Brinckerhoff C. E. (2002) Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 4, 157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu W., Billinghurst R. C., Pidoux I., Antoniou J., Zukor D., Tanzer M., Poole A. R. (2002) Sites of collagenase cleavage and denaturation of type II collagen in aging and osteoarthritic articular cartilage and their relationship to the distribution of matrix metalloproteinase 1 and matrix metalloproteinase 13. Arthritis Rheum. 46, 2087–2094 [DOI] [PubMed] [Google Scholar]
- 10. Yoshida H., Faust A., Wilckens J., Kitagawa M., Fetto J., Chao E. Y. (2006) Three-dimensional dynamic hip contact area and pressure distribution during activities of daily living. J. Biomech. 39, 1996–2004 [DOI] [PubMed] [Google Scholar]
- 11. Stremme S., Duerr S., Bau B., Schmid E., Aigner T. (2003) MMP-8 is only a minor gene product of human adult articular chondrocytes of the knee. Clin. Exp. Rheumatol. 21, 205–209 [PubMed] [Google Scholar]
- 12. Echtermeyer F., Bertrand J., Dreier R., Meinecke I., Neugebauer K., Fuerst M., Lee Y. J., Song Y. W., Herzog C., Theilmeier G., Pap T. (2009) Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat. Med. 15, 1072–1076 [DOI] [PubMed] [Google Scholar]
- 13. Cawston T. E., Wilson A. J. (2006) Understanding the role of tissue degrading enzymes and their inhibitors in development and disease. Best Pract. Res. Clin. Rheumatol. 20, 983–1002 [DOI] [PubMed] [Google Scholar]
- 14. Blain E. J. (2007) Mechanical regulation of matrix metalloproteinases. Front. Biosci. 12, 507–527 [DOI] [PubMed] [Google Scholar]
- 15. Ziegler N., Alonso A., Steinberg T., Woodnutt D., Kohl A., Mussig E., Schulz S., Tomakidi P. Mechano-transduction in periodontal ligament cells identifies activated states of MAP-kinases p42/44 and p38-stress kinase as a mechanism for MMP-13 expression. BMC Cell Biol. 11, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yokota H., Goldring M. B., Sun H. B. (2003) CITED2-mediated regulation of MMP-1 and MMP-13 in human chondrocytes under flow shear. J. Biol. Chem. 278, 47275–47280 [DOI] [PubMed] [Google Scholar]
- 17. Coutinho E. L., Gomes A. R., Franca C. N., Salvini T. F. (2002) A new model for the immobilization of the rat hind limb. Braz. J. Med. Biol. Res. 35, 1329–1332 [DOI] [PubMed] [Google Scholar]
- 18. Gu X. I., Leong D. J., Guzman F., Mahamud R., Li Y. H., Majeska R. J., Schaffler M. B., Sun H. B., Cardoso L. (2010) Development and validation of a motion and loading system for a rat knee joint in vivo. Annals Biomed. Eng. 38, 621–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fuentes M. A., Opperman L. A., Bellinger L. L., Carlson D. S., Hinton R. J. (2002) Regulation of cell proliferation in rat mandibular condylar cartilage in explant culture by insulin-like growth factor-1 and fibroblast growth factor-2. Arch. Oral Biol. 47, 643–654 [DOI] [PubMed] [Google Scholar]
- 20. Trindade M. C., Shida J., Ikenoue T., Lee M. S., Lin E. Y., Yaszay B., Yerby S., Goodman S. B., Schurman D. J., Smith R. L. (2004) Intermittent hydrostatic pressure inhibits matrix metalloproteinase and pro-inflammatory mediator release from human osteoarthritic chondrocytes in vitro. Osteoarthritis Cartilage 12, 729–735 [DOI] [PubMed] [Google Scholar]
- 21. Freedman S. J., Sun Z. Y., Kung A. L., France D. S., Wagner G., Eck M. J. (2003) Structural basis for negative regulation of hypoxia-inducible factor-1alpha by CITED2. Nat. Struct. Biol. 10, 504–512 [DOI] [PubMed] [Google Scholar]
- 22. Efimova T., Broome A. M., Eckert R. L. (2004) Protein kinase Cdelta regulates keratinocyte death and survival by regulating activity and subcellular localization of a p38delta-extracellular signal-regulated kinase 1/2 complex. Mol. Cell. Biol. 24, 8167–8183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wong M., Carter D. R. (2003) Articular cartilage functional histomorphology and mechanobiology: a research perspective. Bone 33, 1–13 [DOI] [PubMed] [Google Scholar]
- 24. Shlopov B. V., Lie W. R., Mainardi C. L., Cole A. A., Chubinskaya S., Hasty K. A. (1997) Osteoarthritic lesions: involvement of three different collagenases. Arthritis Rheum. 40, 2065–2074 [DOI] [PubMed] [Google Scholar]
- 25. Sun H. B. CITED2 mechanoregulation of matrix metalloproteinases. Ann. N. Y. Acad. Sci. 1192, 429–436 [DOI] [PubMed] [Google Scholar]
- 26. Bhattacharya S., Michels C. L., Leung M. K., Arany Z. P., Kung A. L., Livingston D. M. (1999) Functional role of p35srj, a novel p300/CBP binding protein, during transactivation by HIF-1. Genes Dev. 13, 64–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gutman A., Wasylyk B. (1990) The collagenase gene promoter contains a TPA and oncogene-responsive unit encompassing the PEA3 and AP-1 binding sites. EMBO J. 9, 2241–2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matrisian L. M. (1994) Matrix metalloproteinase gene expression. Ann. N. Y. Acad. Sci. 732, 42–50 [DOI] [PubMed] [Google Scholar]
- 29. Fanning P. J., Emkey G., Smith R. J., Grodzinsky A. J., Szasz N., Trippel S. B. (2003) Mechanical regulation of mitogen-activated protein kinase signaling in articular cartilage. J. Biol. Chem. 278, 50940–50948 [DOI] [PubMed] [Google Scholar]
- 30. Fitzgerald J. B., Jin M., Chai D. H., Siparsky P., Fanning P., Grodzinsky A. J. (2008) Shear- and compression-induced chondrocyte transcription requires MAPK activation in cartilage explants. J. Biol. Chem. 283, 6735–6743 [DOI] [PubMed] [Google Scholar]
- 31. Karalaki M., Fili S., Philippou A., Koutsilieris M. (2009) Muscle regeneration: cellular and molecular events. In Vivo 23, 779–796 [PubMed] [Google Scholar]
- 32. Schaffler M. B. (2003) Role of bone turnover in microdamage. Osteoporos. Int. 14(Suppl. 5), S73–S77; discussion S77–S80 [DOI] [PubMed] [Google Scholar]
- 33. Favier F. B., Benoit H., Freyssenet D. (2008) Cellular and molecular events controlling skeletal muscle mass in response to altered use. Pflügers Arch. 456, 587–600 [DOI] [PubMed] [Google Scholar]
- 34. Ferretti J. L., Cointry G. R., Capozza R. F., Frost H. M. (2003) Bone mass, bone strength, muscle-bone interactions, osteopenias and osteoporoses. Mech. Ageing Dev. 124, 269–279 [DOI] [PubMed] [Google Scholar]
- 35. Ferretti M., Gassner R., Wang Z., Perera P., Deschner J., Sowa G., Salter R. B., Agarwal S. (2006) Biomechanical signals suppress proinflammatory responses in cartilage: early events in experimental antigen-induced arthritis. J. Immunol. 177, 8757–8766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ferretti M., Srinivasan A., Deschner J., Gassner R., Baliko F., Piesco N., Salter R., Agarwal S. (2005) Anti-inflammatory effects of continuous passive motion on meniscal fibrocartilage. J. Orthop. Res. 23, 1165–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Torzilli P. A., Bhargava M., Park S., Chen C. T. (2010) Mechanical load inhibits IL-1 induced matrix degradation in articular cartilage. Osteoarthritis Cartilage 18, 97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yoshida M., Tsuji M., Funasaki H., Kan I., Fujii K. (2005) Analysis for the major contributor of collagenase to the primary cleavage of type II collagens in cartilage degradation. Mod. Rheumatol. 15, 180–186 [DOI] [PubMed] [Google Scholar]
- 39. Balbin M., Fueyo A., Knauper V., Lopez J. M., Alvarez J., Sanchez L. M., Quesada V., Bordallo J., Murphy G., Lopez-Otin C. (2001) Identification and enzymatic characterization of two diverging murine counterparts of human interstitial collagenase (MMP-1) expressed at sites of embryo implantation. J. Biol. Chem. 276, 10253–10262 [DOI] [PubMed] [Google Scholar]
- 40. Fanjul-Fernandez M., Folgueras A. R., Cabrera S., Lopez-Otin C. (2010) Matrix metalloproteinases: evolution, gene regulation, and functional analysis in mouse models. Biochim. Biophys. Acta 1803, 3–19 [DOI] [PubMed] [Google Scholar]
- 41. Leong D. J., Gu X. I., Li Y., Lee J. Y., Laudier D. M., Majeska R. J., Schaffler M. B., Cardoso L., Sun H. B. (2010) Matrix metalloproteinase-3 in articular cartilage is upregulated by joint immobilization and suppressed by passive joint motion. Matrix Biol. 29, 420–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Grodzinsky A. J., Levenston M. E., Jin M., Frank E. H. (2000) Cartilage tissue remodeling in response to mechanical forces. Annu. Rev. Biomed. Eng. 2, 691–713 [DOI] [PubMed] [Google Scholar]
- 43. Robling A. G., Castillo A. B., Turner C. H. (2006) Biomechanical and molecular regulation of bone remodeling. Annu. Rev. Biomed. Eng. 8, 455–498 [DOI] [PubMed] [Google Scholar]
- 44. Sun H. B., Yokota H. (2001) Altered mRNA level of matrix metalloproteinase-13 in MH7A synovial cells under mechanical loading and unloading. Bone 28, 399–403 [DOI] [PubMed] [Google Scholar]
- 45. Kim E. K., Choi E. J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta 1802, 396–405 [DOI] [PubMed] [Google Scholar]
- 46. Reuben P. M., Cheung H. S. (2006) Regulation of matrix metalloproteinase (MMP) gene expression by protein kinases. Front. Biosci. 11, 1199–1215 [DOI] [PubMed] [Google Scholar]