Abstract
OBJECTIVE
Serial measurements of the fetal abdominal circumference have been used to guide metabolic management of pregnancies complicated by gestational diabetes mellitus (GDM). A reduction in the number of repeat ultrasound examinations would save resources. Our purpose was to determine the number of serial abdominal circumference measurements per patient necessary to reliably predict the absence of fetal overgrowth.
RESEARCH DESIGN AND METHODS
Women who had GDM were asked to return for repeat ultrasound at 3- to 4-week intervals starting at initiation of care (mean 26.9 ± 5.7 weeks). Maternal risk factors associated with fetal overgrowth were determined.
RESULTS
A total of 4,478 ultrasound examinations were performed on 1,914 subjects (2.3 ± 1.2 per pregnancy). Of the 518 women with fetal abdominal circumference >90th percentile, it was diagnosed in 73.9% with the first ultrasound examination at entry and in 13.1% with the second ultrasound examination. Of the fetuses, 85.9 and 86.9% of the fetuses were born non-large for gestational age (LGA) when abdominal circumference was <90th percentile at 24–27 weeks and 28–32 weeks, respectively, and 88.0% were born non-LGA when both scans showed normal growth. For those women who had no risk factors for fetal overgrowth (risk factors: BMI >30 kg/m2, history of macrosomia, and fasting glucose > 100 mg/dl), the accuracy of prediction of a non-LGA neonate was 90.0, 89.5, and 95.2%. The predictive ability did not increase with more than two normal scans.
CONCLUSIONS
The yield of sonographic diagnosis of a large fetus drops markedly after the finding of a fetal abdominal circumference <90th percentile on two sonograms, which excludes with high reliability the risk of a LGA newborn. The ability was enhanced in women who had no risk factors for neonatal macrosomia.
The recommendations for diagnosis and treatment of gestational diabetes mellitus (GDM) of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus (1) suggest consideration of fetal growth patterns to guide metabolic management of pregnant women with GDM. Estimation of fetal weight, particularly at term and in fetuses with high neonatal weight, is not as precise as is desirable (2). However, enlarged size (3–6) and accelerated growth velocity of the fetal abdominal circumference in the third trimester is known to predict large-for-gestational-age (LGA) birth weight (7). Previous randomized studies have demonstrated that measurement of the fetal abdominal circumference throughout pregnancy in women who have GDM is useful to identify pregnancies at high risk for fetal overgrowth and therefore in need of intensified intervention (8–11). On the other side, relaxed glycemic goals had been allowed in women with sonographic evidence of normal fetal growth. Besides saving insulin therapy, this approach reduced the rate of fetal growth restriction in the fetuses of those women. Published protocols for fetal growth–based management require sonographic determination of fetal abdominal circumference at the time of diagnosis of GDM (8–11) followed by repeat examinations at 2 (11)- to 4-week intervals (9,10). Serial sonographic examinations are costly and require the time and expertise of experienced ultrasonographers and/or physicians.
The purpose of our study was to determine the number of sequential ultrasound examinations necessary not to miss development of an enlarged abdominal circumference during pregnancy and to assure a low risk for a LGA neonate with a great degree of certainty when the scans suggest normal fetal growth. In addition, we wished to evaluate whether the absence of maternal risk factors for neonatal macrosomia would enhance the accuracy of the ultrasound examination predicting a non-LGA neonate.
RESEARCH DESIGN AND METHODS
Women with historical risk factors for diabetes (e.g., family history of diabetes, GDM in a prior pregnancy, and obesity) were tested in the first trimester. All other patients were tested for GDM either at 24–28 weeks or whenever risk factors (e.g., glycosuria or sonographic suspicion of macrosomia) first appeared during the course of pregnancy. In ∼20% of the women an elevated 50-g glucose challenge test with a 1-h value >140 mg/dl preceded the oral glucose tolerance test (OGTT).
GDM was defined by the criteria of Carpenter and Coustan (12). Data for women who had GDM and who attended the Diabetes Prenatal Care Clinic of the Department of Obstetrics of Vivantes Medical Center between 1 January 2001 and 31 December 2007 were analyzed. These data had been entered prospectively. This study was approved by the ethics committee of the Charité Hospital, Humboldt University of Berlin. Study inclusion criteria were 1) documented GDM, 2) accurate gestational age, confirmed by an ultrasound examination before 20 weeks of gestation, 3) a singleton pregnancy, 4) a fetal biometry determined by ultrasound at initiation of therapy for GDM, 5) at least one repeat ultrasound biometry examination, 6) the absence of fetal anomalies identified at birth, and 7) availability of data regarding maternal obstetrical history and anthropometry.
Care for GDM was delivered according to German guidelines for diagnosis and therapy of GDM (13). The latter were largely derived from the recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus (14). All women were given individualized diets for the treatment of diabetes in pregnancy. Those requiring treatment with only diet were instructed to self-monitor blood glucose four times a day (fasting and 2-h postprandial) twice a week using memory-based reflectance meters (Advantage Glucose Meter, Roche Diagnostics, Mannheim, Germany). Women with sporadically elevated glucose values but not meeting the criteria for insulin therapy were tested more often. Insulin therapy was recommended when fasting glucose values repeatedly were ≥90 mg/dl (5.0 mmol/l) and/or 2-h postprandial values were ≥120 mg/dl (6.6 mmol/l). The term “profile at entry,” which is used in Table 1 and 2, includes the mean of all fasting glucose measurements and the mean of the postprandial glucose measurements of the glucose profiles performed by the women within the first 2 weeks after diet education (before initiation of insulin therapy).
Table 1.
Maternal demographics and delivery data
| Abdominal circumference >90th percentile |
P value | ||
|---|---|---|---|
| None | ≥1 | ||
| n | 1,399 | 518 | |
| Clinical parameter | |||
| History of GDM (%) | 14.9% | 20.7% | 0.006 |
| History of macrosomia (%) | 10.2% | 19.8% | <0.0001 |
| Parity | 1.9 ± 1.4 | 2,3 ± 1.6 | <0.0001 |
| Age (years) | 30.6 ± 5.6 | 31.8 ± 5.3 | <0.0001 |
| Prepregnancy BMI (kg/m2) | 27.0 ± 5.8 | 28.9 ± 6.6 | <0.0001 |
| BMI >30 kg/m2 (%) | 26.6 | 36.6 | <0.0001 |
| Delivery of newborn with birth weight >90th percentage (%) | 11.1 | 37.4 | <0.0001 |
| Measures of maternal glucose | |||
| Gestational age at diagnosis | 27.0 ± 5.3 | 26.7 ± 6.6 | 0.218 |
| OGTT fasting (mg/dl) | 92.1 ± 20,8 | 94.1 ± 24.0 | <0.0001 |
| 1 h | 194.4 ± 36.2 | 198.0 ± 34.7 | 0.66 |
| 2 h | 146.3 ± 40,4 | 150.6 ± 40.0 | 0.055 |
| Glucose on initiation of treatment for GDM: | |||
| Fasting of profile at entry (mg/dl) | 83.1 ± 14.4 | 87.1 ± 16.6 | <0.0001 |
| Postprandial of profile at entry (mg/dl) | 117.4 ± 20.7 | 119.4 ± 21.5 | 0.17 |
| Insulin use (%) | 18.9 | 22.1 | 0.7 |
| Delivery | |||
| Gestational age at delivery (weeks) | 38.8 ± 1.8 | 38.3 ± 2.0 | 0.001 |
| Cesarean section (%) | 14.4 | 21.1 | 0.001 |
| Birth weight(g) | 3,309.8 ± 550.8 | 3,639.6 ± 559.4 | 0.001 |
| LGA (%) | 18.4 | 38.1 | <0.0001 |
| SGA (%) | 9.2 | 2.7 | <0.0001 |
Data are means ± SD or %. LGA, birthweight ≥90th percentile; small for gestational age (SGA), birthweight ≤10th percentile.
Ultrasound examinations
An initial ultrasound examination with complete fetal biometry was scheduled at the first visit for diabetes care and at 3- to 4-week intervals thereafter, in conjunction with clinic visits. Examinations were performed by physicians experienced in fetal ultrasound. The fetal abdominal circumference was measured in the standard cross-sectional view of the abdomen. An abdominal circumference ≥90th percentile for gestational age (15) was considered predictive of fetal macrosomia (this term is used interchangeably with enlarged abdominal circumference in the rest of this report). All scans were classified into five categories according to gestational ages at performance: <24, 24/0–27/6, 28/0–31/6, 32/0–35/6, and 36/0–39/6 weeks. LGA neonates were defined as those whose birth weight was ≥90th percentile for gestational age using a standard German birth weight reference (16) (LGA is used interchangeably with neonatal macrosomia).
Statistical analysis
Differences between pregnant women whose fetuses had at least one abdominal circumference ≥90th percentile during pregnancy and those with no fetal abdominal circumference ≥90th percentile were tested for statistical significance by the Mann-Whitney U test (continuous variables) or by χ2 analysis (for categorical variables). Data are presented as means ± SD.
The frequency of newly diagnosed abdominal circumference ≥90th percentile for each ultrasound examination and the cumulative detection rate were determined for each additional ultrasound examination. To evaluate whether the absence of maternal risk factors improves the ability of the sonographic prediction of a normal-sized baby, forward stepwise multivariate logistic regression analysis including all parameters (except delivery of newborn with birth weight ≥90th percentile) was performed, putting those independent variables found to be statistically significantly associated with a fetal abdominal circumference ≥90th percentile in the univariate analyses (Table 1) into the multivariable model. Continuous variables were dichotomized according to established clinical thresholds (e.g., BMI ≥30 kg/m2 and gestational age at diagnosis <24 weeks) and/or statistical considerations (fasting glucose at first profile >100 mg/dl). Determination of the prevalence of LGA was done by frequency analysis. All statistical analyses were performed with the statistical program SPSS 16.0 (SPSS, Chicago, IL). Statistical significance was set at P < 0.05.
RESULTS
A total of 1,914 women were eligible for inclusion in the study. An OGTT was performed in 32.7% because of historical risk factors, in 33.9% as part of routine screening, and in 33.4% for risk factors first noted during pregnancy (e.g., excessive weight gain or excessive fetal growth). Patient demographics are reported in Table 1. Insulin therapy was started at a mean of 25.4 ± 10.6 weeks of gestation.
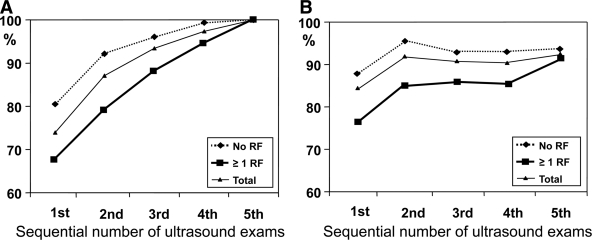
A total of 4,478 ultrasound examinations were reviewed. The mean ± SD number of examinations performed per patient was 2.3 ± 1.2 with a range from two to six scans per patient. The gestational age at the first ultrasound examination was 26.6 ± 6.5 weeks. In 518 women (27.0%), there was at least one abdominal circumference measurement ≥90th percentile during pregnancy, whereas in 1,399 (73.0%) women, no fetal abdominal circumference measurement was ≥90th percentile for gestational age. Of the 518 women with at least one abdominal circumference measurement ≥90th percentile, 383 (73.9%) of enlarged fetal abdominal circumferences were found on the first ultrasound examination, 68 (13.1%) were found on the second sonogram, 33 (6.4%) on the third, 20 (3.9%) on the fourth, and 14 (2.7%) on the fifth. Subanalysis in the 318 women without a need for insulin therapy (an additional 40 women refused therapy) showed very similar percentages: 77.4% found with the first scan, 13.8% with the second scan, 6.3% with the third scan, and 1.3% with the fourth and fifth scan each. Figure 1A displays the rate of identification of fetuses whose abdominal circumference ≥90th percentile was identified at the initial or each subsequent ultrasound examination. With two sequential scans, 87.1% of fetuses with an abdominal circumference ≥90th percentile for gestational age were detected (Fig. 1), while a third scan added only 3% new cases. Women whose fetuses were found to have an abdominal circumference ≥90th percentile were of greater parity, more frequently had a history of GDM and of neonatal macrosomia in at least one previous pregnancy, and had prepregnancy BMIs and fasting glucose values both at OGTT and throughout the duration of their current pregnancies that were significantly greater than those of women with no abdominal circumference measurement >90th percentile (Table 1).
Figure 1.
A: Cumulative detection rate of an abdominal circumference ≥90th percentile in all 518 pregnancies with at least one event of fetal abdominal circumference >90th percentile according to the number of the scan when abdominal circumference >90th percentile was diagnosed for the first time (50.4% had two, 26.3% had three, 15.3% had four, and 8.1% had five or six ultrasound examinations). B: Rate of non-LGA newborns (sensitivity) according to number of scans with abdominal circumference <90th percentile. Data are given for the total population and for women with and without maternal risk factors (RF).
The multivariable regression analysis identified three variables as independent predictors for development of neonatal macrosomia: history of LGA newborn, BMI >30 kg/m2, and fasting glucose values >100 mg/dl (Table 2). A fetal abdominal circumference >90th percentile was less frequently found in the absence of risk factors (n = 1,133; 59.2%) than in the presence of risk factors for neonatal macrosomia (20.4 vs. 34.1%; P < 0.001). In women without any of these risk factors, fetal abdominal circumference >90th percentile was more frequently found with the first scan at entry than in women with the presence of risk factors (80.2 vs. 67.8% (Fig. 1), and with the second scan 92.2% of all cases of fetal macrosomia had been detected in contrast with 79.1%. In women without risk factors the slope of the curve (Fig. 1A) became markedly flat after the second scan, indicating that there is a minor increase in the diagnostic rate of newly developed abdominal circumference >90th percentile with further ultrasound examinations. Gestational age at the first scan was not significantly different in women without or with risk factors (26.7 ± 6.4 vs. 26.3 ± 6.4 weeks; P = 0.2).
Table 2.
Independent risk factors for LGA birth weight in pregnancies with abdominal circumference <90th percentile at first ultrasound (n = 589 women with at least one risk factors of 1,443 subjects with maternal data)
| OR (95% CI) | P value | |
|---|---|---|
| History of LGA newborn | 2.2 (1.2–3.8) | 0.004 |
| Prepregnancy BMI >30 kg/m2 | 1.6 (1.04–2.5) | 0.032 |
| Mean fasting glucose at profile at entry >100 mg/dl (5.5 mmol/l) | 2.1 (1.2–3.3) | 0.003 |
The probability of delivering a non-LGA neonate with an abdominal circumference <90th percentile according to the number of ultrasound examinations is shown in Fig. 1B. Besides in women with risk factors, the rate of correctly predicted non-LGA newborns did not further increase when more than two ultrasound examinations were performed. In women in whom all ultrasound examinations showed a normal abdominal circumference, the probability was 88.9%, and probability increased to 92.2% in the absence of maternal risk factors for neonatal macrosomia (Table 3). Among women with no risk factors, the probability of a non-LGA neonate was not statistically significantly different between women who had only two and those who had more than two fetal abdominal circumference measurements <90th percentile (P = 0.6). In women with risk factors, the probability for a non-LGA newborn was significantly lower at all gestational ages and combination of scans. The sensitivity to predict LGA birth weight was 42.4% with abdominal circumference >90th percentile at 24–27 weeks, 40.0% at 28–31 weeks, 49.1% at 32–35 weeks, and 56.6% at 36–39 weeks of gestation.
Table 3.
Ability of an abdominal circumference measurement <90th percentile to predict a normally grown neonate depending on the gestational age at performance of the scan and the absence or presence of maternal risk factors for LGA birth weight
| Gestational age at abdominal circumference <90th percentile | Non-LGA birth weight (%) |
||
|---|---|---|---|
| Total population | No maternal risk factor | With maternal risk factor | |
| All U.S. (= never abdominal circumference >90th percentile) | 88.9 | 92.2 | 83.2* |
| 24–27 weeks (n = 313) | 85.9 | 90.0 | 81.8* |
| 28–31 weeks (n = 663) | 86.9 | 89.5 | 79.7* |
| 32–35 weeks (n = 817) | 87.9 | 92.5 | 81.6* |
| 36–39 weeks (n = 703) | 89.8 | 93.7 | 81.0* |
| In both, 24–27 and 28–31 weeks (n = 280) | 88.0 | 92.5 | 84.0* |
*Significantly different from percentage in pregnancies without maternal risk factors.
CONCLUSIONS
An intensive insulin regimen was proven to reduce the rate of LGA newborns (17); however, only a minority of women are at risk for accelerated fetal growth. Since the early 1990s, trials incorporating sonographic measurement of the fetal abdomen into the care of women who had GDM have been reported. Intensified care, meaning insulin therapy and a high number of glucose profiles, was offered only to women whose ultrasound examinations suggested accelerated fetal growth. A remarkable decrease in newborns with neonatal macrosomia as well as with growth restriction was found among women who followed the sonographically determined fetal growth–based plan of management that incorporated performance of serial sonograms from entry to delivery to select women in need of intensive care (8–11,18). Repeated ultrasound examinations are costly and require transfer to an experienced ultrasonographer. The question addressed by the current study is the number of sequential fetal abdominal circumference measurements needed to detect all fetuses with a tendency for fetal overgrowth and to exclude those fetuses at low risk for being LGA at birth with a high degree of certainty. The findings of our study suggest that the yield of sonographic diagnosis of a large fetus drops markedly after the finding of a fetal abdominal circumference <90th percentile. The probability of neonatal macrosomia when both the scan at diagnosis of GDM and a subsequent scan 3 weeks later showed normal growth is relatively low. To be specific, we found that 74% of sonographically large babies were diagnosed with the first scan and 50% of the remainder with the second ultrasound. With two consecutive ultrasound findings of normal fetal growth, non-LGA neonates of women who had GDM were predicted with good accuracy. The predictive ability did not improve with more scans. These findings are consistent with a previous study showing that multiple measurements in the third trimester seem to provide little improvement in prediction of birth weight compared with a single scan (19). When the fetal abdominal circumference was <90th percentile at both 24–27 and 28–31 weeks of gestation, fetal growth stayed normal throughout the remainder of pregnancy in 88% of the women. The predictive ability for non-LGA birth weight of two normal scans at 24–27 and 28–31 weeks of gestation was identical with those of all scans with fetal abdominal circumference <90th percentile. Thus, limitation to an ultrasound examination at time of diagnosis of GDM at 24–27 weeks of gestation followed by a subsequent scan at 28–31 weeks might be reasonable, particularly in women with no risk factors for neonatal macrosomia.
We identified three independent maternal risk factors for predicting an LGA newborn (history of a previous LGA newborn, maternal obesity, and a fasting blood glucose>100 mg/dl on the initial profile after diagnosis of GDM). Consideration of these risk factors seems to be useful to decide how many additional scans are necessary to identify pregnancies at risk for fetal overgrowth. The number of scans needed to detect the majority of all cases of sonographic fetal overgrowth and the ability to predict normal-weight neonates differ in women with and without the presence of maternal risk factors. The prediction of non-LGA birth weight was enhanced in women without risk factors. In the absence of maternal risk factors, two normal serial scans in the early third trimester predict non-LGA newborns with a reliability of 94% in comparison with 79% in women with risk factors, indicating that there might be a lower probability that macrosomia occurs later in pregnancy in low-risk women.
The major limitation of every study involving ultrasound is the unsatisfying reliability of the method per se. Ultrasound became an essential tool in obstetrics, but unfortunately the accuracy of the prediction of birth weight depends on many factors such as the position of the fetus, maternal obesity, the timing of the scan, and others. Even with experienced ultrasonographers, there is a considerable interobserver error. Reports of positive predictive value and sensitivity range from 52 to 67 and from 55 to 100%, respectively, with a median positive predictive value of 67% and a sensitivity of 62% (2). This is a problem, particularly in newborns with high birth weights, because the percent error seems to increase with birth weight (20), and this is true for most variations of formulas used for calculation of estimated fetal weight. When formulas for composite estimated fetal weight based on three measures, head and abdomen circumference and femur length, were compared against those using abdominal circumference alone, a systematic review including 19,000 women indicated similar overall accuracy (21). Abdominal circumference measurements in the third trimester, either the actual size or the growth velocity, respectively, have been known for a long time to identify fetuses with a risk of neonatal macrosomia in a diabetic pregnancy quite well (3–7).
A limitation of our study data was that in 10% of the women the reason for performing an OGTT was suspected fetal macrosomia diagnosed on the routine scan at 28–32 weeks of gestation. In patient populations in which all pregnant women are tested for GDM, the rate of fetal overgrowth that is detected with the first ultrasound examination on initiation of diabetes care might be slightly lower. A practical limitation of our data is that there are missing scans in the longitudinal ultrasound follow-up. The latter was due to either women not keeping appointments or a lack of qualified sonographers on a given day or both. However, we can reasonably infer from our data that additional fetal ultrasound examinations after the first two fetal ultrasound examinations suggesting normal fetal growth are unlikely to improve the ability to exclude the risk of fetal overgrowth. Furthermore, the absence of maternal risk factors improves the predictive ability. These findings await confirmation by randomized controlled trials.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
U.M.S.-G. was responsible for study design and data analysis, was involved in patient care and performed the ultrasound examinations, and wrote the manuscript. L.W. extracted the data from the clinic charts and entered them into the database. D.A.S. and M.A.-D. contributed to analysis and discussion of the data and reviewed/edited the manuscript. Ö.K., B.G., S.M., and K.V. were involved in patient care and performed the ultrasound examinations.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Metzger B, Buchanan T, Coustan D, De Leiva A, Dunger D, Hod M, Kitzmiller J, Kjos S, Oats J, Pettitt D, Sacks D, Zoupas C: Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 2007;30(Suppl.):S251–S260 [DOI] [PubMed] [Google Scholar]
- 2.Sacks DA, Chen W: Estimating fetal weight in the management of macrosomia. Obstet Gynecol Surv 2000;55:229–239 [DOI] [PubMed] [Google Scholar]
- 3.Jazayeri A, Heffron JA, Phillips R, Spellacy WN: Macrosomia prediction using ultrasound fetal abdominal circumference of 35 centimeters or more. Obstet Gynecol 1999;93:523–526 [DOI] [PubMed] [Google Scholar]
- 4.De Reu PA, Smits LJ, Oosterbaan HP, Nijhuis JG: Value of a single early third trimester fetal biometry for the prediction of birth weight deviations in a low risk population. J Perinat Med 2008;36:324–329 [DOI] [PubMed] [Google Scholar]
- 5.Bochner CJ, Medearis AL, Williams J, 3rd, Castro L, Hobel CJ, Wade ME: Early third-trimester ultrasound screening in gestational diabetes to determine the risk of macrosomia and labor dystocia at term. Am J Obstet Gynecol 1987;157:703–708 [DOI] [PubMed] [Google Scholar]
- 6.Landon MB, Mintz MC, Gabbe SG: Sonographic evaluation of fetal abdominal growth: predictor of the large-for-gestational-age infant in pregnancies complicated by diabetes mellitus. Am J Obstet Gynecol 1989;160:115–121 [DOI] [PubMed] [Google Scholar]
- 7.Kehl RJ, Krew MA, Thomas A, Catalano PM: Fetal growth and body composition in infants of women with diabetes mellitus during pregnancy. J Matern Fetal Med 1996;5:273–280 [DOI] [PubMed] [Google Scholar]
- 8.Buchanan TA, Kjos SL, Montoro MN, Wu PY, Madrilejo NG, Gonzalez M, Nunez V, Pantoja PM, Xiang A: Use of fetal ultrasound to select metabolic therapy for pregnancies complicated by mild gestational diabetes. Diabetes Care 1994;17:275–283 [DOI] [PubMed] [Google Scholar]
- 9.Kjos SL, Schaefer-Graf U, Sardesi S, Peters RK, Buley A, Xiang AH, Bryne JD, Sutherland C, Montoro MN, Buchanan TA: A randomized controlled trial using glycemic plus fetal ultrasound parameters versus glycemic parameters to determine insulin therapy in gestational diabetes with fasting hyperglycemia. Diabetes Care 2001;24:1904–1910 [DOI] [PubMed] [Google Scholar]
- 10.Schaefer-Graf UM, Kjos SL, Fauzan OH, Bühling KJ, Siebert G, Bührer C, Ladendorf B, Dudenhausen JW, Vetter K: A randomized trial evaluating a predominantly fetal growth-based strategy to guide management of gestational diabetes in Caucasian women. Diabetes Care 2004;27:297–302 [DOI] [PubMed] [Google Scholar]
- 11.Bonomo M, Cetin I, Pisoni MP, Faden D, Mion E, Taricco E, Nobile de Santis M, Radaelli T, Motta G, Costa M, Solerte L, Morabito A: Flexible treatment of gestational diabetes modulated on ultrasound evaluation of intrauterine growth: a controlled randomized clinical trial. Diabete Metab 2004;30:237–244 [DOI] [PubMed] [Google Scholar]
- 12.Carpenter MW, Coustan DR: Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol 1982;144:768–773 [DOI] [PubMed] [Google Scholar]
- 13.Arbeitsgemeinschaft Diabetes und Schwangerschaft der deutschen Diabetesgesellschaft (DDG), Arbeitsgemeinschaft für Materno-Fetale Medizin (AGMFM) der DGGG und Deutsche Gesellschaft für Perinatale Medizin Empfehlungen zu Diagnostik und Therapie des Gestationsdiabetes (GDM). Frauenarzt 2001;42:891–899 [Google Scholar]
- 14.Metzger BE, Coustan DR: Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 1998;21(Suppl.):161–167 [PubMed] [Google Scholar]
- 15.Hadlock FP, Deter RL, Harrist RB, Park SK: Estimating fetal age: computer-assisted analysis of multiple fetal growth parameters. Radiology 1984;152:497–501 [DOI] [PubMed] [Google Scholar]
- 16.Voigt M, Schneider KT, Jährig K: Analysis of a 1992 birth sample in Germany. 1: New percentile values of the body weight of newborn infants. Geburtshilfe Frauenheilkd 1996;56:550–558[in German] [DOI] [PubMed] [Google Scholar]
- 17.Alwan N, Tuffnell DJ, West J: Treatments for gestational diabetes. Cochrane Database Syst Rev 2010;3:CD003395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kjos SL, Schaefer-Graf UM: Modified therapy for gestational diabetes using high-risk and low-risk fetal abdominal circumference growth to select strict versus relaxed maternal glycemic target. Diabetes Care 2007;30:S200–S205 [DOI] [PubMed] [Google Scholar]
- 19.Hedriana H, Moore T: A comparison of single versus multiple growth ultrasound examinations in predicting birth weight. Am J Obstet Gynecol 1994;1600–1604 [DOI] [PubMed] [Google Scholar]
- 20.Kurmanavicius J, Burkhardt T, Wisser J, Huch R: Ultrasonographic fetal weight estimation: accuracy of formulas and accuracy of examiners by birth weight from 500 to 5000 g. J Perinat Med 2004;32:155–161 [DOI] [PubMed] [Google Scholar]
- 21.Coomarasamy A, Connock M, Thornton J, Khan KS: Accuracy of ultrasound biometry in the prediction of macrosomia: a systematic quantitative review. BJOG 2005;112:1461–1466 [DOI] [PubMed] [Google Scholar]